Abstract
Infertility is one of the major problems faced in medicine. There are numerous factors that play a role in infertility. For example, numerous studies mention the impact of the quantity and quality of mitochondria in sexual gametes. This is a narrative review of the effects of the mitochondrial genome on fertility. We searched the PubMed, Science Direct, SID, Google Scholar, and Scopus databases for articles related to “Fertility, Infertility, Miscarriage, Mitochondria, Sperm, mtDNA, Oocytes” and other synonymous keywords from 2000 to 2020. The mitochondrial genome affects infertility in both male and female gametes; in sperm, it mainly releases free radicals. In the oocyte, a mutation in this genome can affect the amount of energy required after fertilisation, leading to gestation failure. In both cases, infertile cells have substantially less mitochondrial DNA (mtDNA) copies. The effects of mtDNA on gamete fertility occur via changes in oxidative phosphorylation and cellular energy production. Also, a reduction in the number of mtDNA copies is directly associated with sex cell infertility. Therefore, evaluation of the mitochondrial genome can be an excellent diagnostic option for couples who have children with neonatal disorders, infertile couples who seek assisted reproductive treatment, and those in whom assisted reproductive techniques have failed.
Keywords: Infertility, In vitro Fertilization, Hormones, Genetics
Introduction
Infertility, which is defined as being unable to conceive after having unprotected sex for more than one year, affects approximately 20% of couples worldwide and 25% of couples in developing countries. It affects diverse aspects of infertile couples’ lives (mental, physical, sexual, and social aspects) and thus requires appropriate interventions (1, 2). Accordingly, understanding the main cause of infertility and choosing the right treatment method within the patient's affordability and availability is very important for designing treatment methods and programs (2, 3).
Assisted reproductive technologies (ART) are usually used as strategies to manage infertility with multifactorial causes (including genetically predisposed diseases). ART has evolved rapidly since 1976, and scientists have been trying to establish the proper approach option for each infertile couple. Among assisted reproductive technologies, in vitro fertilization (IVF) is the most popular (4).
IVF is a multi-step procedure meant to help conception or counteract genetic issues. In the first step of IVF, mature eggs are obtained from ovaries. These are fertilized by sperm in the laboratory. The next step is transference of the fertilized egg (embryo) to the uterus. In general, the full IVF course requires about three weeks depending on stimulation protocols, and sometimes these levels are divided into different sublevels, and the process may take more time (5).
Despite continuous advances in IVF and increasing success, only about one-third of women undergoing IVF will become pregnant, and about 60% of cases fail (6). Various factors are considered as causes of infertility, such as infertility duration, lifestyle, recovered eggs, endometrial thickness, number of transferred embryos, and quality of blastocyst, as well as demographic factors, like ethnicity (7).
The efficacy of IVF depends on the treating clinic’s overall success rate and the infertile couple’s characteristics. Accordingly, among the factors thought to affect IVF success rate are the physical environment, genetics, psychological factors, serum levels of some hormones, sperm and egg characteristics, as well as age and body mass index (BMI) of couples (8-10).
The objective of this review is to investigate the factors that affect IVF failure and success rates.
This review is focused on studies of multiple factors and their influence on the outcome of IVF technology. Articles were eligible if they evaluated the association between any factor and IVF outcome. Articles were selected if the target population consisted of patients undergoing IVF and intra-cytoplasmic sperm injection (ICSI) procedures.
A thorough search of four databases (PubMed, Embase, Cinahl, and Cochrane) was conducted from their inception until March 2021. The reference lists of review articles and relevant studies were hand-searched to identify other potentially eligible studies. Abstracts from conference proceedings were also considered. No language filters or any other restrictions were applied. Keywords used for the searchers were: IVF, age, BMI, psychological factors, sperm features, ovarian stimulation, hormonal profile, ICSI, IVF, single-nucleotide polymorphism (SNP), and genetic. We downloaded all references identified into EndNote software (version X7). Two authors (Radin Dabbagh Rezaeiyeh, and Arian Mehrara) agreed on the inclusion criteria. Articles were incorporated if they were: original, review, or peer-reviewed research. One author (Amin Mohammad Ali Pour) conducted the initial screening analysis. After removing duplicates and screening the titles and abstracts of the articles, those meeting the inclusion criteria were reviewed. The reference list of every selected article was carefully checked to identify other potentially eligible studies.
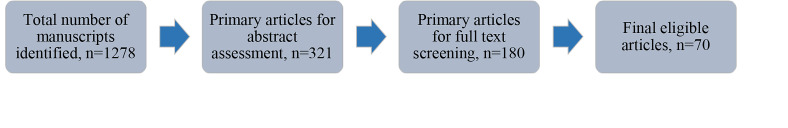
Our search resulted in 1278 articles (excluding duplicates). The article selection process was such that articles that did not qualify and did not meet the inclusion criteria were removed at the title screening. Three hundred twenty-one articles met inclusion criteria and were chosen for abstract screening. Out of those 321 articles, 180 were found eligible for full text screening. After assessing the full text of these 180 papers, we selected 70 articles for further reading (Fig .1).
Fig.1.
Initial to final search steps for selecting manuscripts.
Factors affecting the success or failure of in vitro fertilization
In Table 1, we present a summary of factors that affect the failure and success of IVF. Each factor is explained and discussed at the end of the Table.
Table 1.
Summary of the factors that have been identified as influencing IVF outcomes and their effects on fertility
|
| ||||
|---|---|---|---|---|
| Factors | IVF outcomes | Role | ||
| Fertilization rate | Pregnancy rate | Life birth rate | ||
|
| ||||
| Demographic | ||||
| Age (higher) | ||||
| BMI (higher) | ↓ | |||
| Psychologic | ↓ | ↓ | ||
| Depression | ||||
| Anxiety | ↓ | |||
| Distress | ↓ | Most women undergoing ART are anxious and depressed due to infertility. | ||
| Hormonal | ↓ | Some studies show that the pregnancy rate is lower among distressed women before and during treatment. | ||
| LH | ||||
| FSH (high level on day 2 or 3 of cycle) | LH prepares the uterine environment for the fertilized egg to grow. | |||
| Estradiol (elevated abnormal levels) | ↓ | Women with higher blood FSH concentration have a lower chance of pregnancy in contrast to women of the same age with lower FSH levels. | ||
| Progesterone | ↓ | Increased abnormal levels of Estradiol are associated with decreased response to ovulation-inducing drugs resulting in reduced IVF success. | ||
| AMH | Progesterone prepares the uterus for the arrival of a fertilized egg. | |||
| Sperm characteristic (abnormalities) | AMH levels shows a good correlation with ART outcomes, thus it is considered to be the most accurate biomarker for ovarian storage. | |||
| Motility | ||||
| Number | ↑ | ↑ | Higher sperm motility results in higher chance of pregnancy and fertilization | |
| Morphological | ↑ | ↑ | Higher Sperm total number increases the chance of oocyte-sperm fusion. | |
| Genetic factors | ↓ | ↓ | ↓ | Sperm deformity is a reliable predictor of fertility success in patients undergoing IVF. |
| PGD | ||||
| Analysis of genetic variants | ↑ | PGD methods are designed to minimize the possibility of transmitting genetically abnormal embryos after IVF. Theoretically, choosing genetically normal embryos for transmission leads to more successful pregnancies and fewer miscarriages. | ||
| Genetic analysis involved in ART can be based on methods of PGD and analysis of genetic variants affecting the success rate of IVF. | ||||
|
| ||||
BMI; Body mass index, LH; Luteinizing hormone, FSH; Follicle-stimulating hormone, AMH; Anti-müllerian hormone, PGD; Preimplantation genetic testing, ART; Assisted reproductive technology, and IVF; In vitro fertilization.
Age
Women's age is considered among the most important elements affecting the likelihood of achieving pregnancy in ART programs. As age increases, women’s fertility and live birth rates decrease significantly (especially after 35 years). Increasing maternal age leads to a decrease in pregnancy rate (although this is similar between IVF and ICSI), fertilization rate, and number of recovered eggs (11, 12). Accordingly, decreased female reproductive capacity increases with age due to the gradual reduction of eggs from the ovaries and a significant reduction in egg quality. Morphological evaluation methods for human embryos have proven that maternal age has a significant effect on the quality of human embryos. In addition, the rate of aneuploidy in human embryos increases with age (13, 14).
The results of the study by Yan et al. (15) showed that older women had a weaker response during controlled ovarian hyper-stimulation (COH), fewer retrieved eggs, low oocyte fertilization rate, low-quality embryo rates, low embryo implantation rate, low delivery, high abortion, and high preterm delivery. Goldman et al. (16) stated that age-related fertility decline had much more effect on the live birth rate at older ages than BMI. As age can’t be altered, it can be concluded that spending more time on lowering BMI before IVF is of significant benefit in older women.
Body mass index
In addition to age, the obesity factor is also very important in reproductive support programs. The harmful consequences of obesity on the reproductive system include maternal complications, infertility, and menstrual disorders. Decreased fertility is attributed to various parameters in obese women, such as endocrine and metabolic dysfunctions, which sequentially may affect follicular proliferation, implantation, and the growth of clinical pregnancy (17). Hence, monitoring the effects of body weight during IVF is of paramount importance. Many studies have demonstrated that pregnancies and live birth rates in overweight and obese individuals are reduced in comparison to women of normal weight (18, 19). Possible causes of these discrepancies include increased gonadotropin requirements during ovarian stimulation, fewer recovered oocytes, decreased serum estradiol concentrations, and low fertilization rates. In contrast, other studies have shown that obesity has no effect on gonadotropin requirement, and the number of ovarian stimulation days does not affect estradiol levels (20, 21).
It has been found that obese women who become pregnant after IVF are at risk of miscarriage and obstetric difficulties generally. But it must be taken into consideration that whether achieving a specific BMI and spending a lot of time losing weight before the beginning of the IVF cycle is detrimental to the possibility of live birth, given that the woman is constantly ageing. Obesity increases ovulation induction time, decrease edtradiol peak, and decrease the number of mature follicles. In addition, obesity may adversely affect the quality of eggs and embryos (22, 23).
Dokras et al. (22) observed approximately 1,300 patients in one study, the IVF failure rate was 25% in obese women in comparison to 10.9% in normal weight women. In addition, IVF failure in obese women with polycystic ovary syndrome (PCOS) is more probable than in normal women. Contrary to these findings, many studies, including the study by Kim et al. (24), did not find any considerable gap in clinical pregnancy rates between obese and normal weight women. In a recent study Maged et al. (25) found that implantation, chemical pregnancy, and clinical pregnancy rates were inversely related to increasing BMI. From January 2013 to February 2018, Hallisey et al. (26) performed a cohort study. Their population included women aged over 45 years who underwent IVF with PGT (preimplantation genetic testing). Five hundred thirtythree cycles were separated into 3 groups of women categorized by BMI as normal, overweight, and obese. Euploidy rate was the primary outcome. Their study showed that a higher miscarriage possibility in women with higher BMI and a lower probability of having a live baby after IVF. They stated that the root cause of these discrepancies is currently unknown and it is accepted that obesity may be associated with higher rates of aneuploidy, which can lead to worsening pregnancy outcomes. The dosage of gonadotrophin required for ovarian stimulation is higher in women with BMI more than 25 kg/m2 is also but not the ovarian stimulation duration (27).
Psychological factors
Despite the prevalence of infertility, most infertile women do not share their stories with family or friends, thus increasing their psychological vulnerability. Inability to reproduce normally can result in low self-esteem and feelings of shame and guilt. These negative emotions can lead to different levels of depression, nervousness, distress, and poor quality of life. Most women undergoing ART are frequently anxious and depressed due to infertility. Nearly 32% of women in the early stages of infertility treatment are at risk of mental disorders (28-30). Several studies have examined the relationship between psychological symptoms before and during the ART cycle and subsequent pregnancy. These have provided conflicting results. Some have shown that the pregnancy rate is lower among distressed women before and during treatment, while other studies have not found such a result (31). Several plausible psychological pathways play a role in the likelihood that a woman's distress will affect her fertility or may disrupt infertility treatment success. These pathways include the hypothalamic-pituitary adrenal (HPA) axis, which plays a role in stress response regulation, and the hypothalamic-pituitary gonadal axis (HPG), which regulates reproduction (32). The physiological pathways that influence psychological factors involved in pregnancy are still generally unknown, but these factors can be associated with incomplete ovulation, secondary amenorrhea, and irregular menstrual periods. Various mechanisms have been proposed for the negative effect of psychological factors on infertility, including impaired gonadotropin secretion, local effect of catecholamine on the uterus and fallopian tubes, and impaired immune processes involved in maintaining fertility (33). Cesta et al. (34) studied women who received infertility treatment from September 2011 to December 2013 and followed them until December 2014. Before IVF initiation, data was gathered through an online questionnaire as well as clinical charts. Cortisol from saliva samples was measured and the correlation between stress and cycle outcomes (embryo and oocyte quality parameters and clinical pregnancy) was examined. Unexpectedly, it was revealed that women with higher salivary cortisol concentration had no different IVF outcome than women with normal cortisol levels. Psychosocial care could be helpful for couples experiencing infertility treatment. It has been established that psychosocial care can be effective in decreasing plasma cortisol levels and psychological distress and can improve the clinical rate of pregnancy significantly. Another study by Cui et al. (35) showed that depression during IVF has adverse effects on pregnancy outcomes. Accordingly, measurement of angiotensin II and salivary amylase may be a reference indicator for patients' psychological status during IVF.
Hormonal profiles
Simultaneous combined endocrine events involving the anterior pituitary, hypothalamus, and ovaries are a reflection of the menstrual cycle. These events are important for successful ovulation, egg growth, implantation, and fertilization. In general, the levels of follicle-stimulating hormone, estradiol, luteinizing hormone, and anti-mullerian hormone affect the success or failure of IVF, so it is necessary to check these hormones before performing any ART. Gonadotrophins [follicle-stimulating hormone (FSH) and luteinizing hormone (LH)] are used for ovulation during IVF procedure (36). The functions of some of these hormones are listed below:
Follicle-stimulating hormone
FSH helps in regulating the menstrual cycle and producing eggs. On day 2 or 3 of the menstrual cycle, FSH levels are tested to determine ovarian function and assess egg quality. Generally, women with high levels of blood FSH on day 2 or 3 of the menstrual cycle have a smaller chance of having a live baby than other women of the same age, even with ovulation induction and IVF (37). Abdalla and Thum (38) studied all patients who were candidates for IVF/ICSI treatment between January 1997 and December 2001 in Lister hospital, London. Patients were divided into four groups by FSH level. Follicle maturity, miscarriage rate, pregnancy rate, live birth rate, were defined as outcome measures. The authors concluded that an increase in basal FSH levels did not indicate a deterioration in egg and embryo quality and did not lead to a decrease in fertilization or an increase in abortion. The findings of this study showed that the decrease in pregnancy rate is due to the reduction in the number of eggs collected and consequently the limited selection of available embryos for transfer.
Estradiol
Besides the quantification of FSH, ovarian function and egg condition can be evaluated by measuring estradiol (an important form of estrogen). Estradiol is also examined on day 2 or 3 of the menstrual cycle. These test results are not definite indicators of infertility, but increased abnormal levels are associated with decreased response to ovulation-inducing drugs resulting in reduced IVF success. The human corpus luteum (CL) produces significant amounts of progesterone (P4), estradiol (E2), androgens, growth factors, and nonsteroidal hormones. The overall maintenance of CL function depends entirely on the regular stimulation of pituitary luteinizing hormone (LH) or human placental gonadotropin (hCG) to maintain steroidogenesis in granulosa cells (39). Drakakis et al. (40) assessed the effect of estradiol support on IVF success. They performed their prospective study in the assisted reproduction unit of the First Department of Obstetrics and Gynecology of the Athens University Medical School, from August 2004 to February 2005. They examined patients who were under IVF/ICSI treatment. Implantation and pregnancy rates assessed in the two groups were considered as major outcome measures. They found a steep elevation in implantation rate and pregnancy rate in women who received luteal phase estradiol support compared to women who did not. However, the mean number of fertilized oocytes, transferred embryos, and retrieved oocytes approximately remained the same.
Luteinizing hormone
LH stimulates the ovaries to release eggs and begin to produce progesterone (a hormone that prepares the uterine environment for the fertilized egg to grow). LH can be detected in a woman’s urine just before ovulation. Urine LH tests are frequently conducted to help with the timing of intercourse to raise the chance of pregnancy (41).
Abbara et al. (42) showed that there was an unexpected negative association between increased progesterone and LH levels during egg maturation. In addition, elevated progesterone appears to be the most reliable biochemical predictor of oocyte maturation following all stimulation factors.
Progesterone
Right after ovulation, the ovaries produce progesterone. Progesterone prepares the uterus for the arrival of a fertilized egg approximately in the middle of the cycle - 12 to 16 days after the first day of the menstrual cycle. Progesterone concentration generally peaks within 7 days of ovulation, and the amount of blood progesterone can be measured through blood tests. When a basal level of blood progesterone is established, the doctor will order a mid-luteal serum progesterone test around day 21 of the menstrual cycle (43). Tulic et al. (44) at the Gynecology and Obstetrics Clinic Center of Serbia conducted a cohort study. The study included all patients who met the criteria of inclusion (infertility diagnosed, 18-40 years, regular menstrual cycle, 18-30 kg/m2 BMI, 18-40 years, without a legal guardian), enrolled in the ART procedure during the study period (January 2015 to December 2015). Embryos were classified into four classes: class A (perfect symmetry), class B (moderate asymmetry), class C (pronounced asymmetry). Main outcome measures included pregnancy outcome and procedure success. They stated that low levels of progesterone on oocyte retrieval day (<2.0 ng) in an ART procedure is associated with high levels of FSH and low levels of AMH and lead to the delivery of healthy infants in more than 50% of cases. However, many researchers have found no significant difference between high levels of progesterone and a reduced pregnancy rate due to different data assessment protocols (45).
Anti-mullerian hormone
AMH is a glycoprotein and known as a member of the growth factors of the β family. This hormone is produced by the antral and small antral follicles in the ovary and plays a significant part in folliculogenesis and determining the number of primary follicles. In addition, AMH levels shows a good correlation with ART outcomes and are thus considered as the most accurate biomarkers for ovarian storage (46). In general, current ovarian stimulation protocols are performed during IVF treatments to personalize protocols based on female AMH levels (47). Although there is no definitive value for normal and abnormal AMH, it is generally accepted that AMH >0.8-1.0 ng/ml indicates normal ovarian reserve (48). It is well accepted that young women with high AMH levels have significant fertility performance, while older women with low AMH levels have poor IVF outcomes. However, due to high individual heterogeneity, there are differences in some patients (49). Güngör and Gürbüz (50) carried out a retrospective study between November 2014 and September 2019 at the Gynecology and IVF Department. They chose a logistic regression model rather than linear regression and negative binomial regression due to better dataset fit. Patients were separated into three groups (15 oocytes or more=excessive ovarian response, 6 to 15 oocytes=normal ovarian response, and 5 oocytes or less=weak ovarian response). They considered the number and the quality of the retrieved oocytes as a means of ovarian response quality. They found that higher levels of serum AMH is associated with higher quality of ovarian response (especially the number and quality of eggs).
Sperm characteristics
In the study of infertile couples, sperm testing is the most important and basic method to assess the cause of infertility and choose the treatment method. Evaluation of sperm characteristics such as motility, total number, and morphological abnormalities of sperm seems to be very important to predict successful fertilization, implantation, fetal growth, and continuous pregnancy. Various studies have been performed on the role of different semen parameters on fertilization and pregnancies after IVF. Higher sperm motility and sperm total number are associated with higher chance of pregnancy and fertilization (51).
Sperm morphology is one of the most important parameters. Sperm deformity is a reliable predictor of fertility success in patients undergoing IVF (52). According to this criterion, when less than 14% of sperm are of normal morphology the pregnancy rate decreases. In cases where normal morphology is less than 4%, the treatment result may be very poor (53).
Another parameter that has been investigated for its effect on reproductive fertility techniques is the age of men. Older age is significantly associated with decreased semen volume, sperm count, motility, and normal morphology (54). Male genital infection is one of the leading causes of male infertility worldwide. Bacterial invasion of the reproductive system has often been shown to be associated with decreased sperm function and lead to infertility (55). The quality of semen is so important for the IVF outcome and nutrients are among the most important factors, affecting the quality of semen (56).
Silea et al. (54) examined 500 semen samples from of patients who sought infertility treatment over 5 years (April 2013-April 2017). They evaluated semen samples both macroscopically and microscopically using WHO criteria published in 2010 as the standard threshold. Their findings showed that sperm volume and pH were not affected by age but that sperm viability and progressive motility decrease with age. According to the results of the study by Morin et al. (57), total motile sperm count (TMSC) is the most significant of all the parameters used to assess sperm quality. In addition, this study, along with other studies, has shown that TMSC counts are superior to WHO criteria in predicting the success of IVF cycles. There is a significant difference between the samples of semen contaminated with bacteria compared to the control group in terms of reduced sperm concentration and a significant reduction in sperm motility (55).
Genetic factors
In recent years, seemingly ineffective genetic differences, known as genetic polymorphisms among healthy people in the community, have been the focus of studies in many multifactorial diseases, including miscarriage and implant failure. In general, the genetic analyses involved in ART are based on methods of pre-implantation genetic diagnosis (PGD) and analysis of genetic variants affecting the success or failure of IVF.
Genetic analysis to increase the success rate of in vitro gertilization outcome
Since IVF technology has emerged, multiple attempts have been made to increase efficiency and success. Accordingly, selecting a healthy fetus is considered one of the most important strategies, every aspect of which has been evaluated. Staessen et al. (58) reported that in women over the age of 37, just 35% of day 3 embryos with over eight cells and 65% of proliferating blastocysts were normal. PGD methods are designed to minimize the possibility of transmitting genetically abnormal embryos after IVF. Theoretically, choosing genetically normal embryos for transmission leads to more successful pregnancies and fewer miscarriages (59). Genetic techniques used to select genetically normal embryos include fluorescence in situ hybridization (FISH), comparative genomic hybridization (CGH), whole genome amplification (WGA), array-CGH, next-generation sequencing, real-time quantitative polymerase chain reaction (RT-qPCR), and SNP arrays.
Single-nucleotide polymorphism affecting in vitro fertilization outcome
So far, several studies have been performed on the effects of different SNPs on various aspects of human reproduction, including recurrent miscarriage, infertility, and fetal implant failure. The association of SNPs in various genes, including MTHFR, Leiden factor V, progesterone receptor, FSH receptor, plasminogen activating factor (PAI-1), prothrombin, and estrogen receptor gene, with different aspects of fertility has been observed.
Several studies show that thrombophilia leads to repeated implantation failure. Leiden factor V genetic mutations and prothrombin G20210A mutations have generally been shown to lead to failure of ART (60).
The P53 gene is considered to be one of the genes most integral to the efficient regulation of different physiological processes, including fertility. This gene interacts with the LIF-1 gene and plays a crucial role in controlling and regulating the implantation process. LIF levels are seriously reduced in most females with infertility of unknown cause. Some studies suggest that the prevalence of the codon 72 polymorphism in the P53 gene has a significant effect on implantation rejection rate in IVF cycles (61).
Growing evidence suggests that vitamin B status may modulate infertility treatment outcomes. Water-soluble B vitamin folate is believed to be vital for biosynthetic and epigenetic processes and furthermore, regulate the synthesis and methylation of nucleic acids and proteins. As a result, folate has been proven to be essential during follicular and embryonic developmental periods (62). The MTHFR gene is one of the key genes in the folate pathway. The MTHFR: c.677C>T polymorphism results in a significant change in folate concentration. Because enzyme activity and serum folate concentrations are highest in people with CC wild genotype, this is considered to be the most effective genotype for health. However, current discoveries suggest that, according to the IVF treatment outcome, the heterozygous CT genotype of MTHFR in nucleotide 677 of the mother results in a higher percentage of good quality embryos and a major chance of clinical pregnancy compared to the homozygous CC and TT genotypes. Consistent with these outcomes, it has been shown that the CT genotype, instead of the CC genotype in the woman significantly increases the chances of getting pregnant with IVF treatment (63). Another polymorphism studied is the MTHFR: c.1298A>C, which is associated with higher concentrations of basal FSH and a reduced reaction to ovarian stimulation. The study by Rosen et al. showed that the CC genotype reduced the ovarian response to FSH stimulation compared to the AA and AC genotypes (64).
The solute-carrier gene (SLC) superfamily encodes membrane-bound transporters. A prospective study by Haggarty et al. (63) carried out from October 2000 to September 2004 included 602 women undergoing fertility treatment. Plasma and red-blood-cell concentrations were measured by radioimmunoassay and the absorbed amount of vitamin B12 and folate were evaluated through a questionnaire. Five B-vitamin-associated-gene variants were measured in women who were treated, as well as 932 women who conceived naturally. They found that the SLC19A1 c.80G>A polymorphism increased homocysteine (Hcy) concentration in heterozygous GA people compared to patients with wild-type genotype. Higher concentrations of Hcy usually lead to detrimental effects on IVF outcomes.
In addition, other variants involved in the success rate of IVF include MTHFR: c.677C>T and CTH (cystathionine gamma-lyase) c.1208G>T. Accordingly, heterozygous individuals have favorable IVF results for these variants compared to wild-type homozygous individuals (65).
The LHB gene (Luteinizing hormone beta) is located in the 11p13 chromosome region and has three exons. Trp8Arg, Ile15Thr, and Gly102Ser polymorphisms lead to menstrual irregularities, infertility, and recurrent miscarriages. Furthermore, in women undergoing IVF treatment, these variants have been shown to play a marked role in the IVF success rate (66).
Growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) gene are expressed in oocytes from primary phase follicles. Both proteins play a key role in specifying follicle growth and ovulation rate. Accordingly, polymorphisms in these genes (GDF9: c.546G>A, BMP15: c.2673C>T, c.29C>G, IVS1+905A>G) are also associated with fertility success rates and increased occurrence of dizygotic twins (67).
One of the important genes in the pathway of ovarian metabolism is the aromatase gene (CYP19A1). Aromatase is viewed as one of the main enzymes in ovarian steroidogenesis, which catalyzes the ultimate stage of conversion of testosterone and androstenedione androgens to estradiol and estrone. Tetranucleotide repeat polymorphism (TTTA) n in intron 4 of the CYP19A1 gene leads to aromatase hyperactivity. In general, women with fewer (TTTA) repeats in this gene show lower estrogen concentrations which results in susceptibility to unexplained infertility (68).
Conclusion
The purpose of this review was to summarize the factors that have been identified as influencing IVF outcomes. We have reviewed 70 studies that have reported multiple factors involved in IVF treatment. Prior research has focused on a few factors involved in the IVF success rate.
Predicting the likelihood of pregnancy after IVF can help stop over-treatment and equilibrate the chances of IVF success. In this review, we evaluate 6 predictors, especially genetic factors that can help predict the success of IVF. Based on the available literature, we conclude that female age, BMI, psychological factors, hormonal profiles, sperm characteristics, and genetic factors are predictive factors of IVF success.
In this regard, a wide range of factors could be taken into consideration on which a particular emphasis should be placed. As a matter of fact, nutrients are viewed as one of the essential factors in IVF success rate. With regard to semen quality which is believed to be a vital factor, it proves efficient in IVF outcomes. As nutrients play a massive role in the quality of semen, it is of major significance. Therefore, the consumption of nutritional supplements could have innumerable constructive impacts on IVF. What is more, Females’ BMI is considered to be an integral element in the serum level of hormones. Consequently, once BMI decreases and takes place in a normal range, the IVF process could have more productivity and efficiency in obese women. Due to the fact that sperm characteristics are regarded as one of the most basic ways in order to evaluate the cause of infertility among couples. Hence, when visiting an infertility center, this factor is of more importance compared to other elements, and it is better to check sperm-related characteristics first. Moreover, due to unique genetic sequences that vary from person to person, based on their mutations, a unique personal approach could be taken into account. This process could give a tremendous boost to the level of IVF success rate.
Acknowledgements
The authors thank Mr H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance with the editing of the manuscript. This study is considered to be self-funded. The authors declare that they have no conflicts of interest.
Authors’ Contributions
R.D.R., A.M.; Performed the research. A.M.A.P., J.F., S.F.; Designed the research study. S.F., R.D.R, A.M.A.P.; Analyzed the data. R.D.R, A.M., A.M.A.P., J.F.; Wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.Coussa A, Hasan HA, Barber TM. Impact of contraception and IVF hormones on metabolic, endocrine, and inflammatory status. J Assist Reprod Genet. 2020;37(6):1267–1272. doi: 10.1007/s10815-020-01756-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friis Wang N, Skouby SO, Humaidan P, Andersen CY. Response to ovulation trigger is correlated to late follicular phase progesterone levels: a hypothesis explaining reduced reproductive outcomes caused by increased late follicular progesterone rise. Hum Reprod. 2019;34(5):942–948. doi: 10.1093/humrep/dez023. [DOI] [PubMed] [Google Scholar]
- 3.Azmoudeh A, Shahraki Z, Hoseini FS, Akbari-Asbagh F, DavariTanha F, Mortazavi F. In vitro fertilization success and associated factors: a prospective cohort study. Int J Women's Health Reprod Sci. 2018;6(3):350–355. [Google Scholar]
- 4.Van Loendersloot L, Van Wely M, Limpens J, Bossuyt P, Repping S, Van Der Veen F. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010;16(6):577–589. doi: 10.1093/humupd/dmq015. [DOI] [PubMed] [Google Scholar]
- 5.Van Landuyt L, De Vos A, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Blastocyst formation in in vitro fertilization versus intracytoplasmic sperm injection cycles: influence of the fertilization procedure. Fertil Steril. 2005;83(5):1397–1403. doi: 10.1016/j.fertnstert.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 6.Gnoth C, Maxrath B, Skonieczny T, Friol K, Godehardt E, Tigges J. Final ART success rates: a 10 years survey. Hum Reprod. 2011;26(8):2239–2246. doi: 10.1093/humrep/der178. [DOI] [PubMed] [Google Scholar]
- 7.Edwards RG, Fishel SB, Cohen J, Fehilly CB, Purdy JM, Slater JM, et al. Factors influencing the success of in vitro fertilization for alleviating human infertility. J In Vitro Fert Embryo Transf. 1984;1(1):3–23. doi: 10.1007/BF01129615. [DOI] [PubMed] [Google Scholar]
- 8.Younglai EV, Holloway AC, Foster WG. Environmental and occupational factors affecting fertility and IVF success. Hum Reprod Update. 2005;11(1):43–57. doi: 10.1093/humupd/dmh055. [DOI] [PubMed] [Google Scholar]
- 9.Sneed ML, Uhler ML, Grotjan HE, Rapisarda JJ, Lederer KJ, Beltsos AN. Body mass index: impact on IVF success appears agerelated. Hum Reprod. 2008;23(8):1835–1839. doi: 10.1093/humrep/den188. [DOI] [PubMed] [Google Scholar]
- 10.Templeton A, Morris JK, Parslow W. Factors that affect outcome of in-vitro fertilisation treatment. Lancet. 1996;348(9039):1402–1406. doi: 10.1016/S0140-6736(96)05291-9. [DOI] [PubMed] [Google Scholar]
- 11.Law YJ, Zhang N, Venetis CA, Chambers GM, Harris K. The number of oocytes associated with maximum cumulative live birth rates per aspiration depends on female age: a population study of 221221 treatment cycles. Hum Reprod. 2019;34(9):1778–1787. doi: 10.1093/humrep/dez100. [DOI] [PubMed] [Google Scholar]
- 12.Kalem Z, Simsir C, Var T. The factors that effect in vitro fertilization success in women of critical ages:“38-40 years”. Cumhur Medical J. 2019;41(1):223–229. [Google Scholar]
- 13.Laopaiboon M, Lumbiganon P, Intarut N, Mori R, Ganchimeg T, Vogel JP, et al. WHO Multicountry Survey on Maternal Newborn Health Research Network.Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG. 2014;121(Suppl 1):49–56. doi: 10.1111/1471-0528.12659. [DOI] [PubMed] [Google Scholar]
- 14.Sebire NJ, Foskett M, Fisher RA, Rees H, Seckl M, Newlands E. Risk of partial and complete hydatidiform molar pregnancy in relation to maternal age. BJOG. 2002;109(1):99–102. doi: 10.1111/j.1471-0528.2002.t01-1-01037.x. [DOI] [PubMed] [Google Scholar]
- 15.Yan J, Wu K, Tang R, Ding L, Chen ZJ. Effect of maternal age on the outcomes of in vitro fertilization and embryo transfer (IVF-ET). Sci China Life Sci. 2012;55(8):694–698. doi: 10.1007/s11427-012-4357-0. [DOI] [PubMed] [Google Scholar]
- 16.Goldman RH, Farland LV, Thomas AM, Zera CA, Ginsburg ES. The combined impact of maternal age and body mass index on cumulative live birth following in vitro fertilization. Am J Obstet Gynecol. 2019;221(6):617–617. doi: 10.1016/j.ajog.2019.05.043. e1-617e13. [DOI] [PubMed] [Google Scholar]
- 17.Mascarenhas M, Kulkarni M, Balen A. Can the ethnic differences in IVF cycle outcome be influenced by the impact of BMI? Hum Fertil (Camb). 2020;23(4):275–281. doi: 10.1080/14647273.2018.1563915. [DOI] [PubMed] [Google Scholar]
- 18.Yazdanpanahi Z, Forouhari S, Parsanezhad ME. Prepregnancy body mass index and gestational weight gain and their association with some pregnancy outcomes (short communication). IRCMJ. 2008;10(4):326–331. [Google Scholar]
- 19.Kasum M, Orešković S, Čehić E, Lila A, Ejubović E, Soldo D. The role of female obesity on in vitro fertilization outcomes. Gynecol Endocrinol. 2018;34(3):184–188. doi: 10.1080/09513590.2017.1391209. [DOI] [PubMed] [Google Scholar]
- 20.Badawy A, Wageah A, El Gharib M, Osman EE. Prediction and diagnosis of poor ovarian response: the dilemma. J Reprod Infertil. 2011;12(4):241–248. [PMC free article] [PubMed] [Google Scholar]
- 21.Dahan MH, Zeadna A, Dahan D, Son WY, Steiner N. The biochemical pregnancy loss rate remains stable up irrespective of age and differs in pattern from clinical miscarriages. Gynecol Endocrinol. 2021;37(1):61–64. doi: 10.1080/09513590.2020.1807931. [DOI] [PubMed] [Google Scholar]
- 22.Dokras A, Baredziak L, Blaine J, Syrop C, VanVoorhis BJ, Sparks A. Obstetric outcomes after in vitro fertilization in obese and morbidly obese women. Obstet Gynecol. 2006;108(1):61–69. doi: 10.1097/01.AOG.0000219768.08249.b6. [DOI] [PubMed] [Google Scholar]
- 23.Abdullahi H, Egwuda K, Suleiman M, Warshu IH, Miko M, Emmanuel ED, et al. Evaluation of age and BMI as predictors of the outcome of art among African population in Kano, Nigeria. DUJOPAS. 2020;6(1):10–16. [Google Scholar]
- 24.Kim J, Juneau C, Patounakis G, Morin S, Neal S, Seli E, et al. The appraisal of body content (ABC) trial: obesity does not significantly impact gamete production in infertile men and women. J Assist Reprod Genet. 2020;37(11):2733–2742. doi: 10.1007/s10815-020-01930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maged AM, Fahmy RM, Rashwan H, Mahmood M, Hassan SM, Nabil H, et al. Effect of body mass index on the outcome of IVF cycles among patients with poor ovarian response. Int J Gynaecol Obstet. 2019;144(2):161–166. doi: 10.1002/ijgo.12706. [DOI] [PubMed] [Google Scholar]
- 26.Hallisey SM, Makhijani RB, Thorne J, Godiwala PN, Nulsen J, Benadiva CA, et al. The effect of obesity on euploidy rates in women undergoing in vitro fertilization (IVF) with preimplantation genetic testing (PGT). Fertil Steril. 2020;114(3):e106–e106. doi: 10.1007/s10815-022-02624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eltoukhi NM, Azzam HF, Hani M, Abdel-Monem AS. Relationship between women's body mass index and success rate of in vitro fertilization. Malays J Med Sci. 2017;1(2):8–17. [Google Scholar]
- 28.Rooney KL, Domar AD. The relationship between stress and infertility. Dialogues Clin Neurosci. 2018;20(1):41–47. doi: 10.31887/DCNS.2018.20.1/klrooney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santa-Cruz DC, Agudo D. Impact of underlying stress in infertility. Curr Opin Obstet Gynecol. 2020;32(3):233–236. doi: 10.1097/GCO.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 30.Beygi Z, Jahromi Bahia N, Nourimand F, Amoozandeh Z, Forouhari S. The relationship between spiritual well-being, mental health, and quality of life in infertile women. Family Medicine and Primary Care Review. 2021;23(4):412–416. [Google Scholar]
- 31.Cousineau TM, Domar AD. Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):293–308. doi: 10.1016/j.bpobgyn.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Miller N, Herzberger EH, Pasternak Y, Klement AH, Shavit T, Yaniv RT, et al. Does stress affect IVF outcomes?. A prospective study of physiological and psychological stress in women undergoing IVF. Reprod Biomed Online. 2019;39(1):93–101. doi: 10.1016/j.rbmo.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Maroufizadeh S, Navid B, Omani-Samani R, Amini P. The effects of depression, anxiety and stress symptoms on the clinical pregnancy rate in women undergoing IVF treatment. BMC Res Notes. 2019;12(1):256–256. doi: 10.1186/s13104-019-4294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cesta CE, Johansson ALV, Hreinsson J, Rodriguez-Wallberg KA, Olofsson JI, Holte J, et al. A prospective investigation of perceived stress, infertility-related stress, and cortisol levels in women undergoing in vitro fertilization: influence on embryo quality and clinical pregnancy rate. Acta Obstet Gynecol Scand. 2018;97(3):258–268. doi: 10.1111/aogs.13280. [DOI] [PubMed] [Google Scholar]
- 35.Cui Y, Yu H, Meng F, Liu J, Yang F. Prospective study of pregnancy outcome between perceived stress and stress-related hormones. J Obstet Gynaecol Res. 2020;46(8):1355–1363. doi: 10.1111/jog.14278. [DOI] [PubMed] [Google Scholar]
- 36.Murto T, Bjuresten K, Landgren BM, Stavreus-Evers A. Predictive value of hormonal parameters for live birth in women with unexplained infertility and male infertility. Reprod Biol Endocrinol. 2013;11(1):61–61. doi: 10.1186/1477-7827-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang LN, Jun SH, Drubach N, Dahan MH. Predictors of in vitro fertilization outcomes in women with highest follicle-stimulating hormone levels ≥ 12 IU/L: a prospective cohort study. PLoS One. 2015;10(4):e0124789–e0124789. doi: 10.1371/journal.pone.0124789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdalla H, Thum MY. An elevated basal FSH reflects a quantitative rather than qualitative decline of the ovarian reserve. Hum Reprod. 2004;19(4):893–898. doi: 10.1093/humrep/deh141. [DOI] [PubMed] [Google Scholar]
- 39.Thomsen LH, Kesmodel US, Andersen CY, Humaidan P. Daytime variation in serum progesterone during the mid-luteal phase in women undergoing in vitro fertilization treatment. Front Endocrinol (Lausanne). 2018;9:92–92. doi: 10.3389/fendo.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drakakis P, Loutradis D, Vomvolaki E, Stefanidis K, Kiapekou E, Anagnostou E, et al. Luteal estrogen supplementation in stimulated cycles may improve the pregnancy rate in patients undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer. Gynecol Endocrinol. 2007;23(11):645–645. doi: 10.1080/09513590701664923. [DOI] [PubMed] [Google Scholar]
- 41.Conrad D, Hughes G, Sacks G. LH supplementation for low LH levels during antagonist IVF cycles improves live birth rates. JFIV Reprod Med Genet. 2015;3(155):22–24. [Google Scholar]
- 42.Abbara A, Hunjan T, Ho VNA, Clarke SA, Comninos AN, Izzi-Engbeaya C, et al. Endocrine requirements for oocyte maturation following hCG, GnRH Agonist, and kisspeptin during IVF treatment. Front Endocrinol (Lausanne). 2020;11:537205–537205. doi: 10.3389/fendo.2020.537205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nayak S, Ochalski ME, Fu B, Wakim KM, Chu TJ, Dong X, et al. Progesterone level at oocyte retrieval predicts in vitro fertilization success in a short-antagonist protocol: a prospective cohort study. Fertil Steril. 2014;101(3):676–682. doi: 10.1016/j.fertnstert.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Tulic L, Tulic I, Bila J, Nikolic L, Dotlic J, Lazarevic-Suntov M, et al. Correlation of progesterone levels on the day of oocyte retrieval with basal hormonal status and the outcome of ART. Sci Rep. 2020;10(1):22291–22291. doi: 10.1038/s41598-020-79347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Wu Y. Progesterone Intramuscularly or vaginally administration may not change live birth rate or neonatal outcomes in artificial frozen-thawed embryo transfer cycles. Front Endocrinol. 2020;11:539427–539427. doi: 10.3389/fendo.2020.539427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhide P, Shah A, Gudi A, Homburg R. The role of anti-müllerian hormone as a predictor of ovarian function. Obstet Gynecol. 2012;14(3):161–166. [Google Scholar]
- 47.Zhang B, Meng Y, Jiang X, Liu C, Zhang H, Cui L, et al. IVF outcomes of women with discrepancies between age and serum antiMüllerian hormone levels. Reprod Biol Endocrinol. 2019;17(1):58–58. doi: 10.1186/s12958-019-0498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang JG, Douglas NC, Nakhuda GS, Choi JM, Park SJ, Thornton MH, et al. The association between anti-Müllerian hormone and IVF pregnancy outcomes is influenced by age. Reprod Biomed Online. 2010;21(6):757–761. doi: 10.1016/j.rbmo.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 49.Hsieh HC, Su JY, Wang S, Huang YT. Age effect on in vitro fertilization pregnancy mediated by anti-Mullerian hormone (AMH) and modified by follicle stimulating hormone (FSH). BMC Pregnancy Childbirth. 2020;20(1):209–209. doi: 10.1186/s12884-020-02875-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Güngör ND, Gürbüz T. Prediction of the number of oocytes based on AMH and FSH levels in IVF candidates. JOSAM. 2020;4(9):733–737. [Google Scholar]
- 51.Alemi M, Omrani R, Tarhani J, Khodadadi B. The effect of semen parameters on the outcome of induced in vitro fertilization (IVF) in infertile couples. Int J Life SCi Pharma Res. 2019;9(1):L34–L44. [Google Scholar]
- 52.Cito G, Picone R, Fucci R, Giachini C, Micelli E, Cocci A, et al. Sperm morphology: what implications on the assisted reproductive outcomes? Andrology. 2020;8(6):1867–1874. doi: 10.1111/andr.12883. [DOI] [PubMed] [Google Scholar]
- 53.Danis RB, Samplaski MK. Sperm morphology: History, challenges, and impact on natural and assisted fertility. Curr Urol Rep. 2019;20(8):43–43. doi: 10.1007/s11934-019-0911-7. [DOI] [PubMed] [Google Scholar]
- 54.Silea C, Cucu I-A, Zarnescu O, Stoian AP, Motofei IG, Bratu OG, et al. Influence of age on sperm parameters in men with suspected infertility. Rom Biotechnol Lett. 2019;24(1):82–90. [Google Scholar]
- 55.Khadim EH, Al-Bermani OK. Poor health parameters of semen associated with bacterial infection in infertile men. EurAsian J Biosci. 2020;14(2):5669–5673. [Google Scholar]
- 56.Sabetian S, Jahromi BN, Vakili S, Forouhari S, Alipour S. The effect of oral vitamin e on semen parameters and IVF outcome: a doubleblinded randomized placebo-controlled clinical trial. Biomed Res Int. 2021;2021:5588275–5588275. doi: 10.1155/2021/5588275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morin S, Juneau C, Neal S, Scott R, Hotaling J. Total motile sperm count is negatively correlated with fertilization rate but not blastulation, euploidy, or implantation in ICSI cycles. Fertil Steril. 2017;108(3):e127–e127. [Google Scholar]
- 58.Staessen C, Platteau P, Van Assche E, Michiels A, Tournaye H, Camus M, et al. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19(12):2849–2858. doi: 10.1093/humrep/deh536. [DOI] [PubMed] [Google Scholar]
- 59.Sciorio R, Tramontano L, Catt J. Preimplantation genetic diagnosis (PGD) and genetic testing for aneuploidy (PGT-A): status and future challenges. Gynecol Endocrinol. 2020;36(1):6–11. doi: 10.1080/09513590.2019.1641194. [DOI] [PubMed] [Google Scholar]
- 60.Patounakis G, Bergh E, Forman EJ, Tao X, Lonczak A, Franasiak JM, et al. Multiple thrombophilic single nucleotide polymorphisms lack a significant effect on outcomes in fresh IVF cycles: an analysis of 1717 patients. J Assist Reprod Genet. 2016;33(1):67–73. doi: 10.1007/s10815-015-0606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang HJ, Feng Z, Sun Y, Atwal G, Murphy ME, Rebbeck TR, et al. Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans. Proc Natl Acad Sci USA. 2009;106(24):9761–9766. doi: 10.1073/pnas.0904280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laanpere M, Altmäe S, Kaart T, Stavreus-Evers A, Nilsson TK, Salumets A. Folate-metabolizing gene variants and pregnancy outcome of IVF. Reprod Biomed Online. 2011;22(6):603–614. doi: 10.1016/j.rbmo.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Haggarty P, McCallum H, McBain H, Andrews K, Duthie S, McNeill G, et al. Effect of B vitamins and genetics on success of in-vitro fertilisation: prospective cohort study. Lancet. 2006;367(9521):1513–1519. doi: 10.1016/S0140-6736(06)68651-0. [DOI] [PubMed] [Google Scholar]
- 64.Rosen MP, Shen S, McCulloch CE, Rinaudo PF, Cedars MI, Dobson AT. Methylenetetrahydrofolate reductase (MTHFR) is associated with ovarian follicular activity. Fertil Steril. 2007;88(3):632–638. doi: 10.1016/j.fertnstert.2006.11.165. [DOI] [PubMed] [Google Scholar]
- 65.Hansson B, Westerberg L. On the correlation between heterozygosity and fitness in natural populations. Mol Ecol. 2002;11(12):2467–2474. doi: 10.1046/j.1365-294x.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- 66.De Placido G, Alviggi C, Mollo A, Strina I, Ranieri A, Alviggi E, et al. Effects of recombinant LH (rLH) supplementation during controlled ovarian hyperstimulation (COH) in normogonadotrophic women with an initial inadequate response to recombinant FSH (rFSH) after pituitary downregulation. Clin Endocrinol (Oxf). 2004;60(5):637–643. doi: 10.1111/j.1365-2265.2004.02027.x. [DOI] [PubMed] [Google Scholar]
- 67.Morón FJ, de Castro F, Royo JL, Montoro L, Mira E, Sáez ME, et al. Bone morphogenetic protein 15 (BMP15) alleles predict overresponse to recombinant follicle stimulation hormone and iatrogenic ovarian hyperstimulation syndrome (OHSS). Pharmacogenet Genomics. 2006;16(7):485–495. doi: 10.1097/01.fpc.0000215073.44589.96. [DOI] [PubMed] [Google Scholar]
- 68.Altmäe S, Haller K, Peters M, Saare M, Hovatta O, Stavreus-Evers A, et al. Aromatase gene (CYP19A1) variants, female infertility and ovarian stimulation outcome: a preliminary report. Reprod Biomed Online. 2009;18(5):651–657. doi: 10.1016/s1472-6483(10)60009-0. [DOI] [PubMed] [Google Scholar]