Abstract
Background:
Advanced age is associated with a decline in the natural oocytes, low oocyte yield, and also increases the assisted reproductive technology (ART) failure rate, and consequently resulted in a pregnancy rate decrease. Platelet-rich plasma (PRP) is one of the proposed therapeutic strategies for women with poor ovarian response (POR). Because of the autologous source of PRP, the lowest risks of disease transmission, immunogenic and allergic reactions have been expected. This study aimed to evaluate the single-dose intraovarian injection of autologous PRP in poor ovarian reserve.
Materials and Methods:
We conducted a clinical trial study in the Al-Zahra hospital and Milad Infertility Clinic, Tabriz, Iran (April and May, 2021). A total of thirty-five women with a POR and mean age 40.68 ± 0.34 enrolled in this study. After injection of autologous PRP into the ovaries, the number of oocytes, antral follicles, and level of estradiol, anti-Müllerian hormone (AMH), follicle-stimulating hormone (FSH), luteal hormone (LH), FSH/LH ratio also were evaluated while, these parameters were evaluated before PRP administration.
Results:
At the 2-month follow-up, women treated with PRP showed a significant elevation in the number of oocytes (3.68 ± 0.24, P=0.0043) and embryos (3.17 ± 0.14, P=0.0001), as well as in the estradiol levels (404.1 ± 16.76 vs. 237.7 ± 13.14, P=0.0003).
Conclusion:
Single PRP injection is effective and might be a promising therapeutic approach in the patients with POR to conceive with their own oocytes, although further evidence is required to assess the influence of PRP on the live birth rate.
Keywords: Infertility, Ovary, Platelet-Rich Plasma, Pregnancy
Introduction
The tendency of women to postpone their childbearing was increased because of the professional investment and looking for better living conditions. Today, we observe an increase in the first-time mothers' mean age, approximately 30-40 (1). This issue is accompanied by ovarian aging; physiological alterations that may lead to oocyte quantity and quality decline critical problem (2). In both ovaries, the total number of oocytes at birth is 1-2 million, and before reaching puberty, more than half of the oocytes will undergo atresia (3). The remarkable increase in the follicle degeneration was observed in the above 37 years old women, and on average, about 1000 oocytes are present at the menopause stage (4).
It is estimated that the prevalence of poor responses to gonadotropin stimulation among women undergoing in vitro fertilization (IVF) is about 9-25% of this population (5). Management of these patients is challenging, and to improve the pregnancy rate, different therapeutic approaches must be tried (6). Furthermore, it was not reported until now an effective and beneficial treatment to prevent, postpone, or reverse ovarian senescence. Various environmental factors can irreversibly decline oocyte quality and numbers, such as unhealthy dietary habits (7), cigarette smoking (8), and exposure to both the chemo and radiotherapy (9). Different treatment strategies have been used in reproductive medicine such as coenzyme Q10, dehydroepiandrosterone (DHEA) (10), antioxidant dietary supplements containing vitamins C and E (11), and melatonin to decrease oxidative stress and also improve ovarian reserve. However, the approval studies of the effectiveness of these therapies remain unclear, also meta-analysis studies have been inconclusive (12).
The role of platelets in triggering cell proliferation and tissue differentiation is therefore considered a promising strategy in regenerative medicine (13). Following activation of platelets by external stimuli like hemorrhage and tissue damage, they can release bioactive molecules and various growth factors that subsequently induce inflammation, severe neovascularization, clotting, and local tissue repair (14). The most prominent healing properties of platelet have led to using platelet-rich plasma (PRP) in all aspects of regenerative medicine (15). However, its concentration is a powerful factor, it has a concentration of 7 times than current circulating serum, The effectiveness of PRP as a critical therapeutic strategy in different illnesses such as eye disease, myocardial infarction, nerve injuries, and cosmetic surgery is considerable (16). Here, we aimed to investigate the single intraovarian injection of PRP effects on the inducing fertility in poor responders to ovarian reserve.
Materials and Methods
Patient selection
Current before-after study was conducted between April and May 2021. Women with a poor ovarian response (POR) were referred to the infertility clinic of AlZahra hospital and Milad infertility clinic, Tabriz, Iran were invited to this study. In this study, the total number of participants was 49 women; 7 individuals were excluded because only one ovary was injected and access to another ovary was impossible. The other 7 participants were reluctant to further treatment after PRP injection because of personal reasons. The inclusion criteria were as follows; infertile women aged 30 to 42 years with at least one ovary and poor response criteria, including antiMüllerian hormone (AMH)<1.1 ng/mL, number of antral follicles less <5-7, a history of cycle cancellation due to low follicular growth or oocytes obtained <3, and also persons willing to cooperate in this research. Exclusion criteria included; FSH >25, current or previous IgA deficiency, genital or non-genital cancers, treatment with anticoagulants, ovarian failure due to abnormal sex chromosomes, prior pelvic surgery resulting in pelvic adhesions, and most importantly, no willingness to cooperate in the current study. Patients with anemia, hemoglobin ˂10 g/dl, signs of thrombocytopenia, platelet count ˂10 5/μl, did not receive the PRP injection.
Informed written consents were obtained from the participants. Also, was thoroughly approved by the Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (IR.TBZMED.REC.1399.794).
The sample size was determined based on the number of oocytes; in line, a previous study conducted by Farimani et al. (5) in 2019 also used this oocytes number. Using G Power software, version 3.01 (Christian-Albrechts-Universität Kiel, Kiel, Germany) selected the family of test such as t tests. Statistical test was calculated as mean: difference between two dependent means (match mean) with α error: 0.05, power: 0.8, and effect sized=0.66. Totally, 22 patients were invited to this study, two cases more than calculation due to patients with incomplete data and/or early drop out.
To avoid any laboratory error and bias in the data, all of the experimental examinations were performed in the same laboratory.
Ovarian stimulation
All participants will undergo minimal stimulation protocol involving Letrozole. The patients were referred for undergone sonography on the first to the third day of their menstrual period. Ovarian stimulation was started with Letrozole (Femara, Novartis, Dorval, Quebec, Canada) PO administered daily (5 mg/kg) from the second or third day of menstruation to the day of ovulation triggering.Recombinant human FSH (rhFSH, Cat. No. sc7798, Cinal-F, Cinagen, Tehran, Iran) was started from the third day of Letrozole administration subcutaneously (225 IU). Human menopausal gonadotropin (hMG) ampule (Cat. No. ab200726, Abcam Co., USA) at a dose of 75 mg/kg was intramuscularly injected daily from the fourth day of Letrozole administration. Transvaginal ultrasound was carried out on the 7th or 8th day of stimulation. When one or more follicles reached a diameter of 14 mm, gonadotropin-releasing hormone (GnRH) antagonist (Merck Serono, Germany), and Cetrotide (ASTA Medica AG, Frankfurt am Main, Germany) 0.25 mg, was administrated to avoid premature ovulation and continued daily until the day of human chorionic gonadotropin (hCG) injection. After reaching the mean diameter of the follicle to 17-18 mm, the intramuscular hCG (10,000 IU, Cat. No. C5297, Sigma, USA) administrated to induce follicular maturation. Serum estradiol levels were measured on the day of hCG injection. Transvaginal ultrasound-guided oocyte retrieval was done under general anesthesia 35-36 hours after hCG injection.
Platelet-rich plasma preparation
PRP preparation has been done by segregation of PRP following the centrifugation. About 20 ml of blood sample was carefully collected under sterile conditions, and subsequently, PRP was prepared using a Royagen kit (Co. SN: 312569, Arya Mabna Tashkis, Iran) based on the manufacturer’s instructions. Briefly, centrifugation was done at 830 g for 8 minutes after blood sample collection. Then, a 16 G needle was connected to a 5 ml syringe, inserted into the tube, and advanced to the buffy coat layer. Through rotating the needle tip, all of the PRP was collected. After collecting about 2-4 cc from one sample, PRP from the first tube applied the same process for the second tube (the total collecting PRP was 4-8 cc).
The prepared solution was shifted to the re-suspension tube and then shaken slightly for 30 seconds-1 minute.
Intraovarian injection
An antibiotic was administrated before oocyte pickup and PRP injection. Furthermore, intravenous a single dose of Cefazolin (Jaber-eben Hayan, Tehran, Iran) administrated one hour prior to injection. During the first stage puncture, after oocyte pick up about 2 cc of autologous obtained PRP injected into the cortex of both ovaries using a 35 cm 17 G single lumen needle. During PRP injection, we used color Doppler [DW-F3 (DW-C80), Jiangsu, China] to prevent damage to large blood vessels. After this process, the patients were transferred to the recovery room and carefully observed for about 30-40 minutes, and then discharged home on the same day. All embryos were frozen. Two months later, or after the third menses post PRP injection, and the lab test and transvaginal sonography were performed, then patients received a new ovarian stimulation cycle in the same way and with the same dose of previous pattern. Patients were discharged from the hospital and received Azithromycin (as Dihydrate, Abouraihan Pharma. Co., Iran) for five days. Patients, who were referred for a second ovarian stimulation, received a low dose estrogen (LD, OVOCEPT-LD, Abouraihan Pharma. Co., Iran) pill for a month if they have an ovarian cyst on the first, second, and third day of the ultrasound and are referred again in the next menses. This population was excluded and omitted from the study due to the prolonged interval between PRP injection and subsequent ovarian stimulation and the possibility of bias in this research.
The number of obtained metaphase II (MII) oocytes, mature oocytes, and the estradiol level on the day of human placental gonadotropin injection was evaluated. The level of ovarian reserve markers, including AMH, follicle-stimulating hormone (FSH), luteal hormone (LH), FSH/LH ratio. Also, these measurements were performed before and after treatment.
Statistical analysis
Data were carefully assessed for normality using the Shapiro-Wilk test, and results were expressed as a standard error of mean (SEM). A Paired-samples t test was performed in normal data to compare the effects of PRP injection in the both of the steps. A Wilcoxon signed-rank test was used to establish non normal data and univariate comparisons before and after of PRP treatment. Differences were considered significant where the P<0.05. The statistical SPSS software, version 22 (IBM SPSS, Armonk, NY, USA), was used for data analysis.
Results
Thirty-five women with mean age ± SEM (40.43 ± 0.26) and POR enrolled in this study (Table 1). The mean numbers of oocytes before and after PRP injection were 2.22 and 3.68, respectively. Our results showed that intraovarian injection of PRP led to a significant increase in the oocytes numbers (P=0.0043). Moreover, the number of embryos (3.17 ± 0.14) significantly was increased following PRP injection (P=0.0001). For women before the injection of PRP, the average number of oocytes (mean ± SD) was (0.64 ± 0.92) and for the same women after injection of PRP was (2.5 ± 2.1).
Table 1.
Clinical features of our participants
|
| |||||
|---|---|---|---|---|---|
| Cases | Age (Y) | Oocyte number(Before PRP) | Oocyte number(After PRP) | Embryo number(Before PRP) | Embryonumber (AfterPRP) |
|
| |||||
| Case 1 | 42 | 2 | 2 | 1 | 2 |
| Case 2 | 39 | 1 | 3 | 1 | 2 |
| Case 3 | 40 | 2 | 3 | 1 | 3 |
| Case 4 | 41 | 1 | 3 | 0 | 2 |
| Case 5 | 41 | 2 | 4 | 2 | 4 |
| Case 6 | 41 | 2 | 4 | 1 | 3 |
| Case 7 | 39 | 2 | 3 | 1 | 3 |
| Case 8 | 38 | 3 | 5 | 2 | 4 |
| Case 9 | 43 | 1 | 3 | 0 | 2 |
| Case 10 | 40 | 2 | 2 | 0 | 2 |
| Case 11 | 41 | 2 | 3 | 1 | 3 |
| Case12 | 39 | 3 | 5 | 3 | 4 |
| Case 13 | 40 | 4 | 5 | 3 | 4 |
| Case 14 | 38 | 3 | 4 | 1 | 3 |
| Case 15 | 41 | 2 | 4 | 2 | 3 |
| Case 16 | 41 | 2 | 2 | 1 | 2 |
| Case 17 | 43 | 3 | 8 | 2 | 5 |
| Case 18 | 42 | 3 | 3 | 2 | 3 |
| Case 19 | 39 | 3 | 4 | 3 | 3 |
| Case 20 | 42 | 1 | 3 | 1 | 2 |
| Case 21 | 41 | 2 | 5 | 2 | 3 |
| Case 22 | 44 | 2 | 4 | 1 | 3 |
| Case 23 | 40 | 2 | 2 | 1 | 4 |
| Case 24 | 37 | 2 | 2 | 1 | 3 |
| Case 25 | 41 | 3 | 3 | 1 | 1 |
| Case 26 | 38 | 4 | 4 | 2 | 1 |
| Case 27 | 39 | 3 | 3 | 1 | 2 |
| Case 28 | 41 | 2 | 2 | 1 | 1 |
| Case 29 | 40 | 2 | 2 | 1 | 1 |
| Case 30 | 39 | 2 | 3 | 2 | 2 |
| Case 31 | 41 | 2 | 2 | 1 | 1 |
| Case 32 | 40 | 2 | 2 | 1 | 1 |
| Case 33 | 42 | 1 | 1 | 2 | 2 |
| Case 34 | 42 | 2 | 2 | 3 | 3 |
| Case 35 | 40 | 2 | 2 | 1 | 1 |
| Mean ± SEM | 40.43 ± 0.26 | 2.22 ± 0.13 | 3.68 ± 0.24*** | 1.41 ± 0.13 | 3.17 ± 0.14*** |
|
| |||||
Data expressed as mean ± standard error of the mean (SEM). PRP; Platelet-rich plasma and ***; P<0.001.
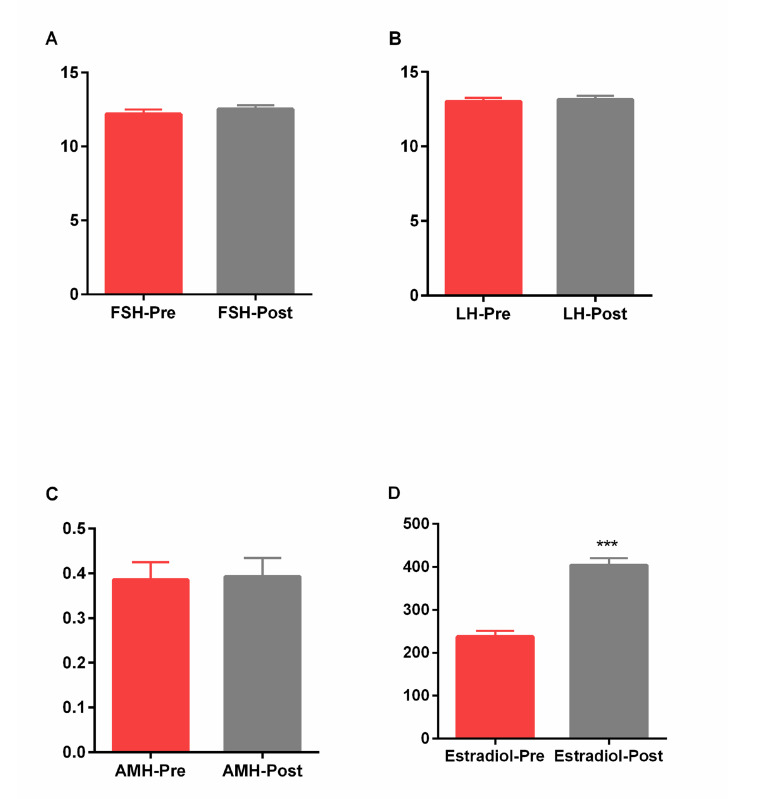
As shown in Figure 1A, B, there was no significant difference in the serum FSH before and after PRP treatment (12.2 ± 0.31 and 12.51 ± 0.28, respectively) and also, LH (13.00 ± 0.25 and. 13.14 ± 0.26, respectively). Also, AMH level was not statistically significantly different after PRP treatment (0.38 ± 0.039) in comparison with before of treatment (0.39 ± 0.04, Fig .1C). We observed a statistically significant increase in the estradiol levels following PRP treatment (404.1 ± 16.76), while it showed lower levels before treatment (237.7 ±13.14, P=0.0003, Fig .1D).
Fig.1.
Alterations of serum hormone levels pre and post PRP injection. A. FSH, B. LH, C. AMH, and D. Estradiol levels before (pre) and after (post) PRP injection. Data were expressed as mean + standard error of the mean (SEM). ***; P<0.001, FSH; Follicle stimulating hormone, LH; Luteinizing hormone, AMH; Antimullerian hormone, and PRP; Platelet rich plasma.
Furthermore, in the current study, spontaneous pregnancy was observed in the 3 of 30 women within 4 months after PRP treatment. We concluded that PRP injection into the ovary by an expert person is safe. Any complications such as infection, bleeding, and hematoma did not observe during this research.
Discussion
Induction of torsion/detorsion causes histological PRP is a valuable therapeutic approach in the female factor infertility. In the current study, single intra-ovarian PRP administration significantly increased the oocyte numbers and embryos in the women with POR. Also, regarding the single injection of PRP, our results showed an increase in the estradiol levels of our participants.
To give birth to a child is one of problems women of reproductive age who suffered from the decline or loss of ovarian reserve. Ovarian failure is characterized by ovarian atrophy, reduction of follicles, and menopausallevel serum gonadotropins. PRP therapy has become a novel treatment in the multiple aspects of medicine, especially in the reproductive medicine and infertility (17). A systematic review study on the 663 subfertile women who received PRP injections reported that PRP is beneficial in the improving ovarian reserve parameters such as: serum AHM, serum FSH or antral follicle count (AFC) (18). Sills et al. (19) examined the effect of intraovarian injection of PRP on the women with a mean age of 42 ± 4 years. They found that administration of PRP improved ovarian function two months after trans-vaginal calcium gluconate-activated autologous injection PRP in all cases. It has reported that growth and survival rates of follicles in the media supplementation with PRP were significantly higher than those without PRP, indicating PRP may be a practical approach to induce follicular development (20).
Interestingly, the present study showed an number of oocytes and embryos following of a single injection of PRP, which can be a cost-effective and time-consuming approach. Farimani et al. (5) reported the same results. Here, we did not see any changes in the levels of serum FSH, LH, and AMH. In line with our study, Cakiroglu et al. (21) demonstrated that intra-ovarian injection of autologous PRP in the women with primary ovarian insufficiency had no significant effect on the FSH levels, and also, associated with minimal improvement in the AMH levels. Although, Aflatoonian et al. (22) did not observe a significant difference in the hormonal (LH and FSH) profile of women with POR or primary ovarian insufficiency after PRP injection. Previous studies showed an elevation in the Estradiol level, following intra-ovarian PRP treatment. A significant decrease in the FSH levels was observed six weeks after autologous PRP therapy (23). On another hand, Cakiroglu et al. (21) reported that PRP treatment increases the AMH level and AFC in the women with primary ovarian insufficiency. Therefore, regarding these studies, PRP’s long-term effectiveness and serial measurements of FSH, LH, and AMH levels following PRP treatment should be more assessed in the context of this study. In our study, six of thirty-five women had spontaneous pregnancy three months after PRP therapy.
The healing regenerative properties of PRP are due to higher concentrations of various growth factors, including transforming growth factor-β, insulin-like growth factors 1 and 2 (IGF-1 and IGF-2), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), basic fibroblast growth factor and hepatocyte growth factor (HGF) (24). Growth differentiation factor 9 (GDF-9), as an oocyte-derived protein, is one of the PRP factors that its critical role in the oocytes maturation associates with an increase in the primary and preantral follicle number (25). Furthermore, mutation of GDF-9 gene leads to premature ovarian failure (26). On the other hand, injection of PRP can activate postnatal oogenesis in the ovary that results in the new primordial follicle generation in the menopausal women’s ovaries through the activation and stimulation of GnRH receptors (27). Melo et al. (16) reported that ovarian administration of PRP, effectively improved markers of the low ovarian reserve before assisted reproductive technology (ART) in the 83 women (median age 41 years) with low ovarian reserve. Also, they observed that PRP treatment was accompanied by a significant increase in the pregnancy rates, both of biochemical and clinical stages. Several studies suggested that treatment with PRP increases neoangiogenesis of the menopausal ovary and promotes ovarian stem cells development to mature follicles (23, 28).
The simultaneous injection of PRP and ovarian puncture surgery made this study principal novelty. These were resulted in a drastic therapy cost reduction, and also, ovarian enlargement that facilitates the injection of PRP in these patients. This research was a before-after study, and we didn’t find a similar previous study till now. Another strength of this study was a single injection of PRP, a low dose, while previous studies repeated the PRP injection more times. This study had its own limitations, such as small study population of mean age of 40 years therefore, it is recommended to conduct a large population over the age of 40.
Conclusion
Our findings showed the beneficial effects of single intra-ovarian injection of autologous PRP in the patients with POR. It could be considered as a cost-effective and time-consuming treatment strategy in the future clinical therapies. Injecting PRP into the ovaries raises the hope that women with a POR, were conceived through themselves eggs. However, further studies with a larger sample size are mandatory to assess the impact of PRP on pregnancy outcomes in infertile women.
Acknowledgements
We express our appreciation to the staff of the Department of Obstetrics and Gynecology, Alzahra Hospital, Tabriz University of Medical Sciences, Tabriz, Iran. Also, the authors greatly appreciated whom participate in this research. This study was funded by Women’s Reproductive Health Research Centre, Tabriz University of Medical Sciences (grant number: 65746). There is no conflicts of interest in this study.
Authors’ Contributions
N.N., L.S.; Participated in study design, clinical data collection, patients screening and selection. L.F., A.Gh.; Conducted patients’ evaluation, molecular experiments and RT-qPCR analysis. K.H., P.H., B.N.; Were responsible for manuscript preparation, collaboration in the data collection, and data analysis. All authors read and approved the final manuscript.
References
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. Natl Vital Stat Rep. 2018;67(1):1–55. [PubMed] [Google Scholar]
- 2.Vo TKC, Tanaka Y, Kawamura K. Ovarian rejuvenation using autologous platelet-rich plasma. Endocrines. 2021;2(1):15–27. [Google Scholar]
- 3.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30(5):465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 4.Fritz R, Jindal S. Reproductive aging and elective fertility preservation. J Ovarian Res. 2018;11(1):66–66. doi: 10.1186/s13048-018-0438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farimani M, Heshmati S, Poorolajal J, Bahmanzadeh M. A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol Biol Rep. 2019;46(2):1611–1616. doi: 10.1007/s11033-019-04609-w. [DOI] [PubMed] [Google Scholar]
- 6.Giovanale V, Pulcinelli FM, Ralli E, Primiero FM, Caserta D. Poor responders in IVF: an update in therapy. Gynecol Endocrinol. 2015;31(4):253–257. doi: 10.3109/09513590.2014.987228. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev. 2015;27(4):716–724. doi: 10.1071/RD14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oboni JB, Marques-Vidal P, Bastardot F, Vollenweider P, Waeber G. Impact of smoking on fertility and age of menopause: a populationbased assessment. BMJ Open. 2016;6(11):e012015–e012015. doi: 10.1136/bmjopen-2016-012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53(4):727–739. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 10.Özcan P, Fıçıcıoğlu C, Kizilkale O, Yesiladali M, Tok OE, Ozkan F, et al. Can Coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J Assist Reprod Genet. 2016;33(9):1223–1230. doi: 10.1007/s10815-016-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahadori MH, Sharami SH, Fakor F, Milani F, Pourmarzi D, DalilHeirati SF. Level of vitamin E in follicular fluid and serum and oocyte morphology and embryo quality in patients undergoing IVF treatment. J Family Reprod Health. 2017;11(2):74–81. [PMC free article] [PubMed] [Google Scholar]
- 12.Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Antioxidants for female subfertility. Cochrane Database Syst Rev. 2020;8(8):CD007807–CD007807. doi: 10.1002/14651858.CD007807.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez-González DJ, Méndez-Bolaina E, Trejo-Bahena NI. Platelet-rich plasma peptides: key for regeneration. Int J Pept. 2012;2012:532519–532519. doi: 10.1155/2012/532519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian Y, Han Q, Chen W, Song J, Zhao X, Ouyang Y, et al. Platelet-rich plasma derived growth factors contribute to stem cell differentiation in musculoskeletal regeneration. Front Chem. 2017;5:89–89. doi: 10.3389/fchem.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Corrêa do Amaral RJ, Granjeiro JM, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(3):67–67. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melo P, Navarro C, Jones C, Coward K, Coleman L. The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: a non-randomized interventional study. J Assist Reprod Genet. 2020;37(4):855–863. doi: 10.1007/s10815-020-01710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranjbaran A, Nejabati HR, Ghasemnejad T, Latifi Z, Hamdi K, Hajipour H, et al. Follicular fluid levels of adrenomedullin 2, vascular endothelial growth factor and its soluble receptors are associated with ovarian response during ART cycles. Geburtshilfe Frauenheilkd. 2019;79(1):86–93. doi: 10.1055/a-0764-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panda SR, Sachan S, Hota S. A systematic review evaluating the efficacy of intra-ovarian infusion of autologous platelet-rich plasma in patients with poor ovarian reserve or ovarian insufficiency. Cureus. 2020;12(12):e12037–e12037. doi: 10.7759/cureus.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sills ES, Rickers NS, Li X, Palermo GD. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol. 2018;34(9):756–760. doi: 10.1080/09513590.2018.1445219. [DOI] [PubMed] [Google Scholar]
- 20.Hosseini L, Shirazi A, Naderi MM, Shams-Esfandabadi N, Borjian Boroujeni S, Sarvari A, et al. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod Biomed Online. 2017;35(4):343–350. doi: 10.1016/j.rbmo.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Cakiroglu Y, Saltik A, Yuceturk A, Karaosmanoglu O, Kopuk SY, Scott RT, et al. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany NY). 2020;12(11):10211–10222. doi: 10.18632/aging.103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aflatoonian A, Lotfi M, Saeed L, Tabibnejad N. Effects of intraovarian injection of autologous platelet-rich plasma on ovarian rejuvenation in poor responders and women with primary ovarian insufficiency. Reprod Sci. 2021;28(7):2050–2059. doi: 10.1007/s43032-021-00483-9. [DOI] [PubMed] [Google Scholar]
- 23.Sfakianoudis K, Simopoulou M, Nitsos N, Rapani A, Pappas A, Pantou A, et al. Autologous platelet-rich plasma treatment enables pregnancy for a woman in premature menopause. J Clin Med. 2018;8(1):1–1. doi: 10.3390/jcm8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramaswamy Reddy SH, Reddy R, Babu NC, Ashok GN. Stemcell therapy and platelet-rich plasma in regenerative medicines: a review on pros and cons of the technologies. J Oral Maxillofac Pathol. 2018;22(3):367–374. doi: 10.4103/jomfp.JOMFP_93_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krüger JP, Freymannx U, Vetterlein S, Neumann K, Endres M, Kaps C. Bioactive factors in platelet-rich plasma obtained by apheresis. Transfus Med Hemother. 2013;40(6):432–440. doi: 10.1159/000356329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otsuka F, McTavish KJ, Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev. 2011;78(1):9–21. doi: 10.1002/mrd.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pantos K, Nitsos N, Kokkali G, Vaxevanoglou T, Markomichali C, Pantou A, et al. Ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous plateletrich plasma treatment. 2016 P-401Availabale from: https://sa1s3patientpopcom/assets/docs/111052pdf (07 Jul 2021) [Google Scholar]
- 28.Hsu CC, Hsu L, Hsu I, Chiu YJ, Dorjee S. Live birth in woman with premature ovarian insufficiency receiving ovarian administration of platelet-rich plasma (PRP) in combination with gonadotropin: a case report. Front Endocrinol (Lausanne). 2020;11:50–50. doi: 10.3389/fendo.2020.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]