Abstract
Background:
Today, vitamin D deficiency (VDD) is one of the major health issues around the world and VDD is associated with several diseases. This study was conducted to find the relationship between vitamin D status in male’s serum with sperm function and clinical outcomes in infertile men candidate for intracytoplasmic sperm injection (ICSI).
Materials and Methods:
In this cohort study, different parameters of male fertility such as sperm parameters, oxidative stress, and sperm chromatin status were evaluated in sperm samples of 30 infertile couples candidate for ICSI. Clinical outcomes like fertilization, embryo quality, and implantation were also assessed. Data were analyzed using SPSS Statistics 25.0 software. Besides, assessment of the correlation between aforementioned parameters with the level of serum vitamin D, in this study, ICSI candidates were divided into three groups [individuals with sufficient vitamin D levels (>30 ng/ml), insufficient vitamin D levels (between 20-29 ng/ml), and VDD (<20 ng/ml)]. The aforementioned parametesr were also compared between these study groups.
Results:
Analysis of all the data revealed a significant correlation between the level of vitamin D with sperm concentration (P=0.000, r=0.5), sperm count (P=0.03, r=0.31) and sperm reactive oxygen species (ROS) level (P=0.000, r=-0.77). Moreover, comparing clinical outcomes within study groups showed a significant difference in implantation rate between sufficient and other groups (insufficient and deficient) (P=0.02).
Conclusion:
Considering the association between sperm concentration and level of ROS with vitamin D and, higher implantation rate in individuals with vitamin D sufficient group compared to other two groups, our data call for vitamin D supplementation as part of male infertility treatment. But considering our sample size, further research is needed to verify these findings.
Keywords: DNA Fragmentation, Infertility, Oxidative Stress, Sperm Motility, Vitamin D
Introduction
Vitamin D deficiency (VDD) is known as a major health issue and affects the normal functions of many organs (1). It is believed that the effect of VDD is more profound in organs in which vitamin D metabolizing enzymes and vitamin D receptors (VDRs) are present (2, 3). Given the presence of VDRs as well as its metabolizing enzymes in male and female reproductive systems, VDD is also likely to affect human fertility (4). The presence of VDRs and several cytochrome P450 enzymes (CYPs) which are known as vitamin D metabolizing enzymes such as CYP2R1, CYP27R1, CYP24A1 in Sertoli cells, germinal cells, Leydig cells, spermatozoa, and epithelial cells of the male reproductive tubules highlights the importance of vitamin D in male fertility (5). It is clear that expression of CYP24A1 is positively correlated with total sperm count, concentration, motility, and morphology (5) while the presence of vitamin D,unlike in follicular fluid, is limited in the semen. So, researchers believe vitamin D released during ovulation via follicular fluid, may act as chemoattractant, and facilitate the process of in vivo fertilization (6). So, activation of VDRs in sperm could increase intracellular calcium in the human sperm and mediate sperm motility, sperm capacitation, acrosomal reaction, and sperm attachment to the oocyte (7). In addition, vitamin D increases lipase activity by lowering triglycerides, providing the energy needed for sperm (8). Furthermore, a recent study by our group showed that dietary VDD not only may affect spermatogenesis but also could impact chromatin status and DNA integrity subsequently reduction of fertility potential in men (9). These findings also validate the results of some studies showing the association between VDD and male infertility in humans (7, 10).
While the limited number of studies assessing the association between vitamin D levels and sperm parameters, chromatin status, DNA integrity, oxidative stress, and fertility in humans, a previous study on rats showed the importance of the level of vitamin D in their diet for sperm DNA integrity and fertility potential (11). Lack of vitamin D could be associated with infertility in mammals. Moreover, another study by Azizi et al. (12) revealed that sperm DNA fragmentation and ROS do not have a significant relationship with vitamin D. Therefore, this study aims to assess sperm quality, chromatin integrity, and ROS in individual candidates for intracytoplasmic sperm injection (ICSI) with different levels of vitamin D deficiency.
Materials and Methods
This cohort study was conducted on 30 couple candidates of ICSI that referred to Shiraz Infertility Treatment Center between April 2019 and October 2019. This study was approved by the Ethics Committee of Azad University, Science and Research Branch of Tehran, Iran (IR.IAU. SRB.REC.1397.102). Prior to participation, individuals were informed about the study, and they were asked to sign an informed consent form.
Serum vitamin D
For assessment of serum vitamin D levels, a vitamin D total kit (Roche Diognosis, USA) was used. This assay is intended for the quantitative determination of a total 25-hydroxyvitamin D in human serum and plasma. The functional sensitivity of this test is determined to be 4.01 ng/ml. The limits and measurement ranges are between 3.00-70.00 ng/ml. The value below the limit of detection is reported as <3 ng/ml and values above the measurement range are reported as >70 ng/ml. Accordingly, individuals were divided into three groups based on previous literature cut off values (13): individuals with sufficient vitamin D levels (>30 ng/ml), insufficient vitamin D levels (between 20-29 ng/ml), and VDD (<20 ng/ml) (14, 15).
Inclusion criteria
Couples with at least one parameter below the cutoff values defined by World Health Organization (WHO, 2010), were considered as a male factor and were included in the study. In these cases, females presented normal menstrual cycles with normal hysterosonography. In addition, to reducing female confounding factors, females with age higher than 35 years old, and women with infertility causes such as low levels of anti-mullerian hormone (AMH), polycystic ovary syndrome (PCOS), and endometriosis were excluded in the study. Besides, men under vitamin D supplement treatments within the past 3 months, women with vitamin D deficiency, men with seminal infections, systematic disease or endocrine disorders, and men with azoospermia and severe oligozoospermia were excluded in this study.
Semen analysis
Semen samples were collected into sterile containers after 3-7 days of sexual abstinence, and were assessed according to WHO (2010) criteria or as described below. Sperm concentration was assessed by a sperm counting chamber with 10 µm depth (Sperm meter, sperm processor, Garkheda, Aurangabad, India). Ten micro-liters of liquefied semen were loaded on the chamber and the number of sperm were counted and expressed as million per ml. Sperm motility was assessed by light microscopy as sperm were considered progressive, non-progressive, and immotile. For assessment of sperm morphology, Papanicolaou staining was used according to WHO (2010) protocol. In this method, at least 200 sperm were counted in each sample at ×100 magnification. A portion of the remaining semen sample was processed by density gradient centrifugation (DGC) method for ICSI technique, and the remainder was used for assessment of functional tests such as intracellular ROS, sperm DNA fragmentation, and protamine deficiency.
Cytosolic reactive oxygen species
Cytosolic ROS was assessed using Dichlorofluorescin (DCF) staining by a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) according to a previous study (16). Briefly, two million sperm per ml phosphate-buffered saline (PBS, Sigma, Louis, MO, USA) was separated from the semen sample and incubated with 0.5 µl DCFH-DA for 40 minutes at room temperature in a dark condition. Then, sperm DCF was evaluated by flow cytometry. For verification of the procedure, a positive control tube was considered. Initially, ROS was induced by adding H2 O2 to sperm samples before incubation with DCFH-DA stain, and then 0.5 µM DCFH-DA was added to the sperm sample. The result was expressed as the percentage of DCF positive spermatozoa.
Protamine status
Sperm protamine deficiency was assessed using Chromomycin A3 (CMA3) staining according to Iranpour et al. (17). Briefly, 100 microliters of semen samples were washed by PBS buffer and then fixed in Carnoy’s fixative solution at 4°C for 5 minutes. After preparing smears, slides were treated with 100 microliters of 0.25 mg/ml CMA3 (Sigma, St. Louis, MO, USA) in McIlvaine buffer. The slides were then rinsed in PBS buffer. Finally, microscopic analysis was performed using an epifluorescence microscope (Olympus, Japan) equipped with appropriate filters (460-470 nm) at ×100 magnification. For each sample, at least 200 sperm were assessed and sperm with bright yellow color were considered as positive or protamine deficient sperm, while sperm with dim yellow color were considered as negative or sperm with normal protamine content.
Sperm DNA fragmentation (TUNEL assay)
Sperm DNA fragmentation was assessed by Terminal Deoxynucleotidyl Transferase dUTP nick end labeling (TUNEL) commercial kit (Promega, Germany) according to manufacturer’s instructions (18). Briefly, the semen sample was washed with PBS and fixed with paraformaldehyde. Subsequently, cells were permeabilized with Triton x100 solution in PBS for 5 minutes, and then washed with PBS and the procedure was continued according to GMP stated in the kit. The status of sperm DNA integrity was analyzed by a FACS-Calibur flow cytometer (BD Biosciences, San Jose, CA, USA) and at least 10,000 sperm were counted. The result for each case was expressed as a percentage of DNA fragmentation.
Ovulation induction and intracytoplasmic sperm injection
For ovarian stimulation, gonadotropin-releasing hormone (GnRH) antagonist was used for superovulation, using Cinal-F (Sinagen, Iran) and Menogon (Ferring, Germany) along with Cetrotide (Serono, Germany). The cycle was monitored using vaginal ultrasound. Ovulation was triggered by the administration of 10000IU human chorionic gonadotropin (HCG, Poyesh Daro, Iran). G-V series Vitrolife culture media (Vitrolife, Sweden) was used for performing ICSI and culturing embryos. Following vaginal aspiration of the follicle, ICSI was carried out according to standard protocols. Briefly, follicles were aspirated with the aid of transvaginal guided ultrasound. The aspirated cumulus oocyte complex was treated with hyaluronidase to remove cumulus cells. Maturity of oocyte was defined and MII oocyte was inseminated with a motile and morphologically normal sperm under 200 magnification. Inseminated oocytes and embryos were cultured at 37ºC in 6% CO2 and 6% O2 under humidified conditions.
Fertilization rate and embryos quality
After 16-18 hours post-ICSI, oocytes were assessed for the presence or absence of two pronuclei (2PN). The fertilization rate was calculated by dividing the ratio of fertilized oocytes by the total number of injected oocytes multiplied by 100. Embryos were graded on days 2 and 3 after insemination based on the 3 scoring system. Grade A: equal-sized blastomeres with blastomeric fragmentation less than 5%, and having at least 4 blastomeres on day 2 and 8 blastomeres on day 3. Grade B: 5-15% blastomeric fragmentation, having at least 4 blastomeres on day 2 and 8 blastomeres on day 3. Grade C: unequal blastomeric size with the fragmentation of more than 15%, and having less than 4 blastomeres on day 2 and 8 blastomeres on day 3. For the assessment of chemical pregnancy, the level of βHCG was measured. Clinical pregnancy was defined by ultrasonography findings showing at least one embryo with a fetal heartbeat, 5 weeks after embryo transfer. Implantation was defined by the number of observed gestational sacs divided by the number of transferred embryos.
Statistical analysis
Obtained data from sperm parameters, sperm functional tests, and clinical outcomes of participating couples were analyzed by IBM SPSS Statistics 25.0 software (SPSS.Inc., Chicago, USA) and the graphics were designed by GraphPad Prism (GraphPad Software,San Diego, California, version 8.00). The data represented were reported as mean ± SD. The one-way ANOVA (Tukey’s post hoc test) was used to compare study parameters within groups. In addition, for assessment of the relationship between vitamin D with other parameters, two-tailed Pearson correlation test was used. P<0.05 was considered significant. Furthermore, a chisquare test was used for analyzing the mean of chemical and clinical pregnancy. The Kolmogorov-Smirnov test and Shapiro-Wilk test were utilized to evaluate the normality of data.
Results
In this study, semen samples of 30 infertile couple candidates for ICSI were analyzed. The mean of sperm concentration, motility, abnormal morphology, and semen volume were 44.0 ± 23.77 (106/ ml), 40.83 ± 16.19 (%), 95.5 ± 3.24 (%), and 3.7 ± 1.81 (ml), respectively. The mean of male and female ages was 35.25 ± 4.78 and 28.25 ± 4.47, respectively.
Vitamin D level in subgroups
The mean value of measured serum vitamin D in couples within sufficient, insufficient, and deficient groups and other demographic characteristics are presented in Table 1 respectively; the difference of male serum vitamin D between the three groups was statically significant (P≤0.001). The number of couples in the sufficient, insufficient group, and deficient groups were 11, 8, and 11, receptively (Table 1).
Table 1.
Comparison of couples vitamin D and ages between study groups
|
| ||||
|---|---|---|---|---|
| Age and vitamin D supplement | Sufficient vitamin D (>30 ng/ml) (n=11) | Insufficient vitamin D (20-29 ng/ml) (n=8) | Deficient vitamin D (<20 ng/ml) (n=11) | P value between groups |
|
| ||||
| Male age (Y) | 35 ± 5.38 | 35 ± 3.11 | 35 ± 5.51 | 0.94 |
| Female age (Y) | 27.45 ± 5.31 | 28.37 ± 4.56 | 28.9 ± 3.75 | 0.75 |
| Male vitamin D (ng/ml) | 39.3 ± 6a | 26.36 ± 3.3 | 15.02 ± 3.65a | <0.001 |
| Female vitamin D (ng/ml) | 39.86 ± 7.85 | 37.56 ± 6.5 | 42.29 ± 9 | 0.47 |
|
| ||||
All data were presented as mean ± SD. Common letters indicate a significant difference between the two groups in each column (ANOVA followed by Tukey's multiple comparisons). ROS; Reactive oxygen species.
Comparison of age, sperm parameters, and functional tests within study groups
In this study, the ages of the males and females in the sufficient, insufficient, and deficient vitamin D groups were compared, and no significant difference was observed. In addition, semen volume, sperm concentration, sperm total count, motility, and abnormal morphology were compared within these groups, only sperm concentration was significantly lower in the deficient vitamin D group compared to sufficient vitamin D group (P=0.000, Table 2).
Table 2.
Comparison of sperm parameters and functional tests between study groups
|
| ||||
|---|---|---|---|---|
| Parameters | Sufficient vitamin D (>30 ng/ml) (n=11) | Insufficient vitamin D (20-29 ng/ml) (n=8) | Deficient vitamin D (<20 ng/ml) (n=11) | P value between groups |
|
| ||||
| Volume (ml) | 3.63 ± 1.75 | 401 ± 2.03 | 3.54 ± 1.86 | 0.8 |
| Sperm concentration (106/ml) | 63.36 ± 12.66 ͣ | 41.12 ± 28.63 | 26.81 ± 12.70 ͣ | <0.001 |
| Sperm total count (106/ejaculate) | 234.72 ± 138 | 164 ± 161.78 | 102.72 ± 88.52 | 0.08 |
| Abnormal sperm morphology (%) | 96.1 ± 2.45 | 96.37 ± 1.84 | 94.36 ± 4.43 | 0.32 |
| Sperm motility (%) | 46.36 ± 15.32 | 40.62 ± 15.22 | 35.45 ± 17.24 | 0.3 |
| Sperm DNA fragmentation (%) | 7.72 ± 3.31 | 7.42 ± 1.98 | 9.45 ± 4.52 | 0.41 |
| ROS positive sperm (%) | 22.12 ± 10.07 ͣ | 27.66 ± 10.04 | 58.59 ± 11.28 ͣ | <0.001 |
| Sperm Protamine deficiency (%) | 16.81 ± 6.09 | 21 ± 5.47 | 22.45 ± 15.8 | 0.45 |
|
| ||||
All data were presented as mean ± SD. Common letters indicate a significant difference between the two groups in each column (ANOVA followed by Tukey's multiple comparisons). ROS; Reactive oxygen species.
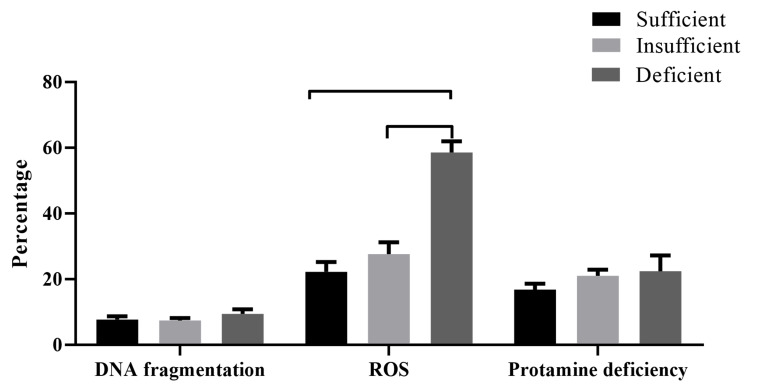
Mean of sperm DNA fragmentation, ROS, and protamine deficiency were also compared within groups (Fig .1). The means of sperm DNA fragmentation were 7.72 ± 3.3, 7.42 ± 2.0, and 9.45 ± 4.5, in the sufficient, insufficient, and deficient vitamin D groups, respectively. The mean of this parameter was insignificantly higher in the deficient group compared to sufficient and deficient vitamin D groups (P>0.05). Regarding sperm ROS, the mean of this parameter was significantly higher in the deficient group compared to sufficient and deficient vitamin D groups (P=0.000). Moreover, we did not observe any significant difference in sperm protamine deficiency within sufficient, insufficient, and deficient vitamin D groups (Fig .1).
Fig.1.
Comparison of sperm DNA fragmentation, reactive oxygen species (ROS), and protamine deficiency between sufficient, insufficient, and deficient vitamin D, groups. Mean value compared using ANOVA followed by Tukey’s multiple comparisons. The number of participants in the sufficient, insufficient, and deficient vitamin D, group were 11, 8, and 11, respectively (P<0.001).
Correlation of vitamin D with sperm parameters
Among sperm parameters and sperm functional tests, only sperm concentration, sperm total count, and ROS level showed significant correlations with vitamin D level (Table 3).
Table 3.
The relationship between vitamin D level and sperm parameters and sperm functional tests
|
| ||
|---|---|---|
| Sperm parameters | Correlation coefficient (r) | P value |
|
| ||
| Sperm concentration (106/ml) | 0.5 | <0.001 |
| Sperm total count (106/ejaculate) | 0.31 | 0.03 |
| Abnormal sperm morphology (%) | 0.24 | 0.2 |
| Sperm motility (%) | 0.24 | 0.2 |
| Sperm protamine deficiency (%) | -0.26 | 0.15 |
| Sperm DNA fragmentation (%) | -0.17 | 0.36 |
| ROS positive sperm (%) | -0.77 | <0.001 |
|
| ||
P≤0.05 was considered significant, using two-tailed Pearson correlation, n=30.
Comparison of intracytoplasmic sperm injection outcomes between study groups
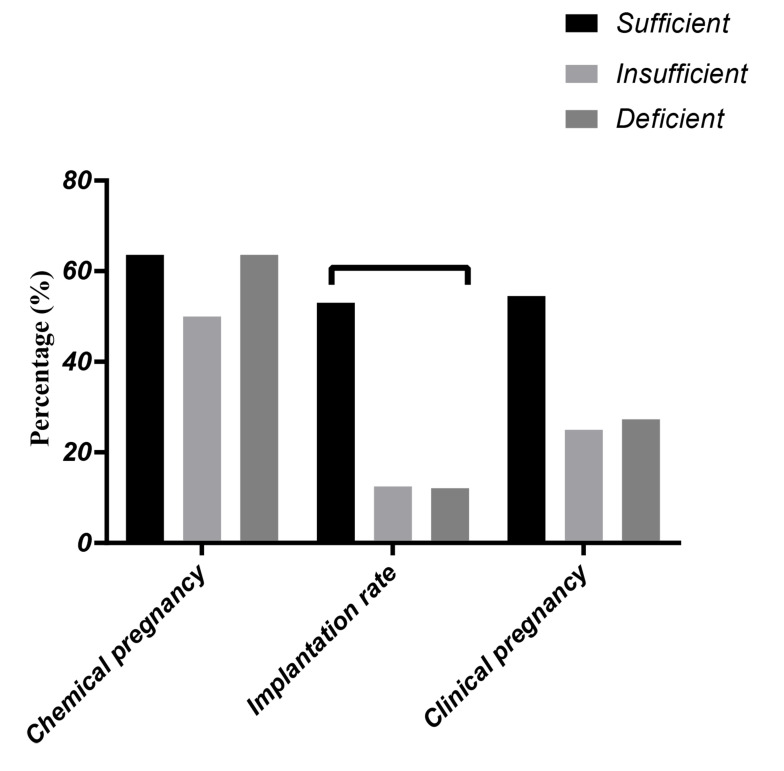
The mean number of oocytes, matured oocytes, percentage of fertilization, embryo quality with grade A on day 3 and mean number of embryos transferred were compared within sufficient, insufficient, and deficient vitamin D groups (Table 4). No significant difference was not observed in these parameters within groups. Besides, the outcomes of clinical and chemical pregnancy and implantation rates in all cases were followed (Fig .2), and the results showed the higher mean percentage of chemical and clinical pregnancy in the sufficient group (63.6% for chemical pregnancy and 54.5% for a clinical pregnancy), compared to insufficient (50% for chemical pregnancy and 25% for clinical pregnancy), and deficient (63.6% for chemical pregnancy and 27% for clinical pregnancy) vitamin D groups, respectively. The mean for implantation rate was significantly higher in the sufficient group (53.03 ± 47.6) compared to insufficient (12.5 ± 35.35), and deficient (12.12 ± 21.2) vitamin D groups, respectively (P=0.02).
Table 4.
Comparison of intracytoplasmic sperm injection (ICSI) outcomes between study groups
|
| ||||
|---|---|---|---|---|
| ICSI outcomes | Sufficient vitamin D (>30 ng/ml) (n=11) | Insufficient vitamin D (20-29 ng/ml) (n=8) | Deficient vitamin D (<20 ng/ml) (n=11) | P value between groups |
|
| ||||
| Retrieved oocytes (No.) | 12.63 ± 6.56 | 16.37 ± 12.12 | 13.36 ± 7.6 | 0.63 |
| Mature oocytes (No.) | 7.36 ± 3.82 | 9.75 ± 7.32 | 8.9 ± 6.15 | 0.65 |
| Fertilization rate (%) | 75.98 ± 26.97 | 69.07 ± 30.65 | 78.49 ± 22.42 | 0.75 |
| Embryo quality with grade A (%) | 49.94 ± 37.87 | 29.28 ± 33.35 | 53.72 ± 27.56 | 0.43 |
| Transferred embryo (No.) | 2.1 ± 0.55 | 1.87 ± 0.85 | 2.18 ± 0.75 | 0.64 |
|
| ||||
All data were presented as mean ± SD (ANOVA followed by Tukey’s multiple comparisons).
Fig.2.
Comparison of chemical and clinical pregnancy and implantation rates within sufficient, insufficient, and deficient vitamin D, groups. for chemical and clinical pregnancy Mean value compared using chi-square test. No significant difference was shown within groups (P=0.8 for chemical pregnancy, P=0.3 for clinical pregnancy), for implantation rate Mean value compared using ANOVA followed by Tukey's multiple comparisons which shows a significant correlation between sufficient and deficient groups (number of participants in sufficient group=11, in insufficient group=8, in deficient group=11) (P=0.02).
Discussion
Vitamin D has been found to have various impacts on fertility, levels of sex hormones, and various organs including the uterus, prostate, and testis (19). Vitamin D levels higher than 30 ng/ml have been reported to be associated with improved fertility rates (20). In this study, we investigated the effect of vitamin D on sperm parameters, sperm chromatin status, oxidative stress, DNA damage, in infertile men candidates for ICSI. The findings of this study showed that individuals with higher levels of vitamin D had a significant difference in sperm count and sperm concentration compared to subjects with low levels of vitamin D.
Regarding the relation between vitamin D levels and sperm parameters, considerable disagreement exists among studies. Similar to the results of this study, Hammoud et al. (21) showed a significant correlation between vitamin D level and sperm concentration. A significant difference for sperm concentration between vitamin insufficient/deficient with vitamin D sufficient group was also reported in that study. In contrast, Abbasihormozi et al. (9) showed a significant correlation between vitamin D level and sperm concentration. Considering sperm morphology, Blomberg et al. (5) did not observe a relationship between sperm morphology and vitamin D. Contrary to this, de Angelis et al. (22) reported a significant correlation between these two parameters. Several differences can account for the controversies observed between different research groups, these include sample size, type of patient selected, vitamin D supplementation, different social, economic backgrounds. Indeed, it has been shown that there is a relation between vitamin D level, vitamin B6, and acid folic. In this study, we selected individual candidates for ICSI, while other studies may have included general infertile and fertile groups (23).
It is of note that in the animal model these factors are accounted for, VDD has been reported to reduce fertility rate and reduced sperm count, and vitamin D repletion can rectify this shortcoming (11). In this regard, it has been shown that VDR knock-out mice show a decrease in sperm count and motility and histological abnormalities of the testis (19). These results indicate that VDD may play an important role in spermatogenesis and sperm maturation.
In this study, we also assessed the relationship between vitamin D level with protamine deficiency, DNA fragmentation, and ROS production. Only a significant negative relation between sperm ROS with vitamin D level was observed in this study. In this regard, VDR is closely related to the nuclear matrix, and it is believed that VDD plays a significant role in stabilizing chromosomal structure and thereby protecting DNA from insults and breaks (24). Interestingly, it has been proposed that the sperm nuclear matrix is crucial in the regulation of DNA fragmentation and degradation and therefore, one may speculate that vitamin D and its receptor may act as guardians of genomic in sperm. Therefore, a significant negative relation between vitamin D and sperm protamine deficiency as well as DNA fragmentation was expected to observe in the present study (24, 25). This could be due to the small sample size which is the main limitation of this study. However, what was interesting in this study is the strong significant relationship observed between ROS production and vitamin D level. Aquila et al. (8) reported that vitamin D has a direct effect on many sperm functions including sperm motility, capacitation, acrosomal activity, and even the metabolic performance of sperm. Indeed, there exists significant evidence that shows a key role for ROS in these events (26). Considering the fact that ROS is increased in the vitamin D deficient individuals, it may be suggested that the capacity of sperm in these individuals to decrease ROS production is reduced. Indeed, vitamin D plays an enhanced role in promotors regions of many enzymes involved in spermatogenesis and in enzymes with anti-oxidative activity (7). ROS decreases fluidity of plasma membrane by lipid peroxidation of unsaturated fatty acids in sperm which can decline sperm’s function (27). It has been thought that vitamin D can protect protein and cell membranes from oxidative stress by preventing pro-oxidative insults (28). Lack of antioxidant protection and free radical productions in sperm can cause oxidative stress (29). Therefore, the increase in ROS production could be related to the reduced antioxidant capacity of these sperm, related to the diminished vitamin D level in these individuals.
Increased ROS production is commonly associated with an increase in DNA fragmentation (30). In this study, a trend toward increased DNA fragmentation in individuals with reduced vitamin D levels was observed, however, the increase was not significant. Aitken et al. studies suggest a lag of several hours between ROS increase and DNA oxidation in sperm (29, 31). It is also suggested that vitamin D could reduce chromosomal aberrations, prevent telomere shortening, inhibit telomerase activity and decline biological damages which are induced by oxidative stress (32). So probably, it is vitamin D itself that protects DNA from being damaged by ROS.
After evaluating clinical outcomes, we observed a significant difference in implantation rate within study groups, despite a similar number of embryos transferred between the groups. The implantation rate was significantly higher in the vitamin D sufficient group than the other two groups. Indeed, effect of male on embryo development appeared after day 3 when the embryo also becomes dependent on gene expression from male genome (33). Interestingly, Ozkan et al. (34) showed an association between vitamin D and implantation rate. But another study showed negative effect of vitamin D in IVF outcomes (35). Due to these controversies, the importance of vitamin D in clinical outcomes remain to be clarified. According to the results of this study, it could be concluded that vitamin D has the potential to increase fertility potential by improving the sperm parameters. It may be suggested that infertile men could be checked for vitamin D level and if necessary, vitamin D could be supplemented in their diet for two to three months before ICSI, to improve sperm parameters as well as both fertilization and implantation rates.
Conclusion
In this study, we assessed the relationship between serum vitamin D levels with sperm parameters, sperm function, and clinical outcomes of infertile male candidates for ICSI. The result of the present study shows that there is a significant negative and positive relation between sperm ROS and sperm concentration with serum vitamin D level, respectively. Also, the implantation rates were significantly lower in the vitamin D insufficient and deficient groups compared to the sufficient group. In light of our results, it can be concluded that vitamin D has the potential to improve fertility in infertile men by improving sperm concentration and reducing ROS level, which consequently may account for an improved implantation rate. But, based on our sample size further trials are needed to bonify these observations.
Acknowledgements
The authors are thankful to Dr. Marziyeh Tavalaee for her administrative support, and Dr. Sirous Rostami manager of Shiraz Fertility Center and its entire staff, and to Esfahan Royan Institute. There is no financial support and conflict of interest in this study.
Authors’ Contributions
M.H.N.-E., M.H.-M.; Conceived the idea, planned and designed the manuscript. M.H.N.-E., M.H.-M., F.S.; Contributed extensively in interpretation of the data and the conclusion. M.H.N.-E., F.S.; Were responsible for overall supervision. M.H.-M.; Wrote the first draft, which was then revised by F.S., M.H.N-E. All authors read and approved the final manuscript.
References
- 1.Pludowski P, Holick M, Pilz S, Wagner CL, Hollis BW, Grant WB, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—a review of recent evidence. Autoimmun Rev. 2013;12(10):976–989. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595–113595. doi: 10.1016/j.bcp.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Ciccone IM, Costa EM, Pariz JR, Teixeira TA, Drevet JR, Gharagozloo P, et al. Serum vitamin D content is associated with semen parameters and serum testosterone levels in men. Asian J Androl. 2021;23(1):52–58. doi: 10.4103/aja.aja_9_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nandi A, Sinha N, Ong E, Sonmez H, Poretsky L. Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Investig. 2016;25(1):15–28. doi: 10.1515/hmbci-2015-0051. [DOI] [PubMed] [Google Scholar]
- 5.Blomberg Jensen M, Jørgensen A, Nielsen JE, Bjerrum PJ, Skalkam M, Petersen JH, et al. Expression of the vitamin D metabolizing enzyme CYP24A1 at the annulus of human spermatozoa may serve as a novel marker of semen quality. Int J Androl. 2012;35(4):499–510. doi: 10.1111/j.1365-2605.2012.01256.x. [DOI] [PubMed] [Google Scholar]
- 6.Aleyasin A, Hosseini MA, Mahdavi A, Safdarian L, Fallahi P, Mohajeri MR, et al. Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):132–137. doi: 10.1016/j.ejogrb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Cito G, Cocci A, Micelli E, Gabutti A, Russo GI, Coccia ME, et al. Vitamin D and male fertility: an updated review. World J Mens Health. 2020;38(2):164–177. doi: 10.5534/wjmh.190057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aquila S, Guido C, Middea E, Perrotta I, Bruno R, Pellegrino M, et al. Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism. Reprod Biol Endocrinol. 2009;7:140–140. doi: 10.1186/1477-7827-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbasihormozi Sh, Kouhkan A, Alizadeh AR, Shahverdi AH, Nasr- Esfahani MH, Sadighi Gilani MA, et al. Association of vitamin D status with semen quality and reproductive hormones in Iranian subfertile men. Andrology. 2017;5(1):113–118. doi: 10.1111/andr.12280. [DOI] [PubMed] [Google Scholar]
- 10.Rehman R, Lalani S, Baig M, Nizami I, Rana Z, Gazzaz ZJ. Association between vitamin D, reproductive hormones and sperm parameters in infertile male subjects. Front Endocrinol (Lausanne). 2018;9:607–607. doi: 10.3389/fendo.2018.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahreza FD, Hajian M, Gharagozloo P, Drevet JR, Nasr-Esfahani MH. Impact of vitamin D deficiency on mouse sperm structure and function. Andrology. 2020;8(5):1442–1455. doi: 10.1111/andr.12820. [DOI] [PubMed] [Google Scholar]
- 12.Azizi E, Naji M, Shabani-Nashtaei M, Aligholi A, Najafi A, Amidi F. Association of serum content of 25-hydroxy vitamin D with semen quality in normozoospermic and oligoasthenoteratozoospermic men. Int J Reprod Biomed. 2018;16(11):689–696. [PMC free article] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16(7):713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 15.Vieth R. Why the minimum desirable serum 25-hydroxyvitamin D level should be 75 nmol/L (30 ng/ml). Best Pract Res Clin Endocrinol Metab. 2011;25(4):681–691. doi: 10.1016/j.beem.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Kiani-Esfahani A, Bahrami S, Tavalaee M, Deemeh MR, Mahjour AA, Nasr-Esfahani MH. Cytosolic and mitochondrial ROS: which one is associated with poor chromatin remodeling? Syst Biol Reprod Med. 2013;59(6):352–359. doi: 10.3109/19396368.2013.829536. [DOI] [PubMed] [Google Scholar]
- 17.Iranpour FG, Nasr-Esfahani MH, Valojerdi MR, al-Taraihi TM. Chromomycin A3 staining as a useful tool for evaluation of male fertility. J Assist Reprod Genet. 2000;17(1):60–66. doi: 10.1023/A:1009406231811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma R, Ahmad G, Esteves SC, Agarwal A. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values, and quality control. J Assist Reprod Genet. 2016;33(2):291–300. doi: 10.1007/s10815-015-0635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol. 2012;166(5):765–778. doi: 10.1530/EJE-11-0984. [DOI] [PubMed] [Google Scholar]
- 20.Sollis SS. Vitamin D deficiency and infertility: a systematic review.Presented for the M.Sc.; Provo.Nursing Brigham Young University. Nursing Brigham Young University; 2015. [Google Scholar]
- 21.Hammoud AO, Meikle AW, Peterson CM, Stanford J, Gibson M, Carrell DT. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J Androl. 2012;14(6):855–859. doi: 10.1038/aja.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Angelis C, Galdiero M, Pivonello C, Garifalos F, Menafra D, Cariati F, et al. The role of vitamin D in male fertility: a focus on the testis. Rev Endocr Metab Disord. 2017;18(3):285–305. doi: 10.1007/s11154-017-9425-0. [DOI] [PubMed] [Google Scholar]
- 23.Shahraki Z, Shahraki Mojahed B, Shahraki A. Comparison of vitamin D levels in fertile and infertile men. Maedica (Buchar). 2020;15(1):96–98. doi: 10.26574/maedica.2020.15.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui X, Pertile R, Eyles DW. The vitamin D receptor (VDR) binds to the nuclear matrix via its hinge domain: a potential mechanism for the reduction in VDR mediated transcription in mitotic cells. Mol Cell Endocrinol. 2018;472:18–25. doi: 10.1016/j.mce.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Merino O, Sánchez R, Gregorio BM, Sampaio FJ, Risopatrón J. Effects of diet-induced obesity and deficient in vitamin D on spermatozoa function and DNA integrity in sprague-dawley rats. Biomed Res Int. 2018;2018:5479057–5479057. doi: 10.1155/2018/5479057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab J Urol. 2019;17(2):87–97. doi: 10.1080/2090598X.2019.1599624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004;2:12–12. doi: 10.1186/1477-7827-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amaral S, Redmann K, Sanchez V, Mallidis C, Ramalho-Santos J, Schlatt S. UVB irradiation as a tool to assess ROS-induced damage in human spermatozoa. Andrology. 2013;1(5):707–714. doi: 10.1111/j.2047-2927.2013.00098.x. [DOI] [PubMed] [Google Scholar]
- 29.Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16(1):3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 30.Iommiello VM, Albani E, Di Rosa A, Marras A, Menduni F, Morreale G, et al. Ejaculate oxidative stress is related with sperm DNA fragmentation and round cells. Int J Endocrinol. 2015;2015:321901–321901. doi: 10.1155/2015/321901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aitken RJ, De Iuliis GN, Finnie JM, Hedges A, McLachlan RI. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod. 2010;25(10):2415–2426. doi: 10.1093/humrep/deq214. [DOI] [PubMed] [Google Scholar]
- 32.Nair-Shalliker V, Armstrong BK, Fenech M. Does vitamin D protect against DNA damage? Mutat Res. 2012;733(1-2):50–57. doi: 10.1016/j.mrfmmm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Colaco S, Sakkas D. Paternal factors contributing to embryo quality. J Assist Reprod Genet. 2018;35(11):1953–1968. doi: 10.1007/s10815-018-1304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94(4):1314–1319. doi: 10.1016/j.fertnstert.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anifandis G, Dafopoulos K, Messini C, Chalvatzas N, Liakos N, Pournaras S, et al. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol. 2010;8:91–91. doi: 10.1186/1477-7827-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]