Abstract
To describe the contribution of garden dormice to the epizootiology of Lyme disease, we compared their reservoir capacity for these pathogens to that of other sympatric hosts. Garden dormice are trapped most abundantly during early spring and again during midsummer, when their offspring forage. They are closely associated with moist forests. Garden dormice serve as hosts to nymphal ticks far more frequently than do other small mammals. Spirochetal infection is most prevalent in dormice, and many more larval ticks acquire infection in the course of feeding on these than on other rodents in the study site. Mature dormice appear to contribute more infections to the vector population than juveniles do. Replete larval ticks generally detach while their dormouse hosts remain within their nests. The population of garden dormice contributes five- to sevenfold more infections to the vector population than the mouse population does. Their competence, nymphal feeding density, and preference for a tick-permissive habitat combine to favor garden dormice over other putative reservoir hosts of Lyme disease spirochetes.
Although mice of the species Apodemus flavicollis and Apodemus sylvaticus appear to be the main reservoir hosts for Lyme disease spirochetes (Borrelia burgdorferi sensu lato, hereafter termed spirochetes) in much of central Europe (3), edible dormice, Glis glis, may contribute at least twice as many such spirochetal infections to the local population of Ixodes ricinus vector ticks (5). This differential capacity of edible dormice as reservoirs for the agent of Lyme disease derives from the numerous nymphal ticks that feed on them and their longer life spans. A larger tick load implies that infectivity for vector ticks begins earlier in life, and greater longevity implies a relatively extended duration of infectivity. The prevalence of infection in edible dormice, therefore, greatly exceeds that in mice.
Garden dormice, Eliomys quercinus, are about as long-lived as edible dormice are (10, 11), and the similarity in their sizes suggests that both may be parasitized by similar densities of nymphal ticks. These animals differ in an additional potentially reservoir-relevant behavioral trait. Edible dormice inhabit relatively dry forests that tend to be mature, whereas garden dormice become abundant where the forest is more humid and transitional; these kinds of dormice rarely coexist (10, 13). Because vector ticks tend to be most abundant in such relatively humid sites, the capacity of garden dormice as hosts for the Lyme disease spirochete may correspondingly exceed that of edible dormice. The epizootiological significance of the correlation between the niche of reservoir and vector hosts, however, has not systematically been examined.
It may be that the capacity of garden dormice as reservoir hosts for the Lyme disease spirochete is far greater than that of other small mammals in sites in which vector ticks most readily thrive. To describe the characteristics of garden dormice that promote their contribution of spirochetal infections to the vector population, we related the epizootiology of these spirochetes transmitted among garden dormice to that in mice and other small mammals present in the same site.
(Portions of this research were conducted in partial fulfillment of the requirements for a doctoral degree from the Humboldt-Universität zu Berlin, Berlin, Germany.)
MATERIALS AND METHODS
Description of study site.
Our study site was located in the Petite Carmarque, a forested region in France, near the German and Swiss borders at an altitude of 240 m above sea level and within the flood plain of the Rhein River. This 224-ha region had been excavated extensively and otherwise exploited during the 1800s and early 1900s. Segments began to revert to forest after 1908. A key bastion of the Maginot line had been located there. The Petite Carmarque was declared a nature preserve in 1982. The trees covering the study site itself appear to be between 15 and 30 years of age and include oak, alder, and ash; dense brush covers the forest floor.
Trapping method.
Small mammals were captured in live traps (Longworth Scientific Instruments, Abingdon, United Kingdom) baited with apple, grain, and cotton. A total of 60 traps were placed in the site for four successive nights each month from April through October 1995. Traps were spaced 8 to 10 m apart in linear arrays straddling the ecotone on either side. Captured small mammals were taken to the laboratory, where they were weighed and identified. They were caged over water until all naturally attached ticks had detached. The water was inspected twice daily, and ticks were promptly removed, counted, and identified. After subsequent xenodiagnosis, the animals were released at the point of capture. Ticks were collected once a month at the site by means of a flannel flag. They were confined in screened vials and stored at 15 ± 1°C until they were identified as to stage and species and examined for spirochetes.
Ticks used for xenodiagnosis were derived from laboratory-bred adult I. ricinus ticks. Subadult and adult ticks were reared by feeding on spirochete-free jirds and on rabbits, respectively. These ticks were in their third generation of continuous laboratory rearing and had never been exposed to infected hosts. A portion of each larval cohort was routinely examined for the presence of spirochetes by dark-field microscopy and also by feeding them on mice.
For xenodiagnosis (7) and studies on time of detachment, about 50 laboratory-reared larval ticks were brushed onto each dormouse at 1800 h. Infested hosts were enclosed in wire mesh tubes that were wrapped in absorbent paper for 2 to 3 h. The dormice were subsequently removed and caged over water; the water was inspected every 2 h, and replete ticks were removed. The photophase began at 0800 h, and the scotophase began at 2200 h.
After detachment, engorged ticks (both laboratory reared and those naturally attached) were enclosed in screened vials and kept at 20 ± 2°C in sealed desiccator jars over supersaturated MgSO4 under a light-dark (16 h–8 h) regime. Molted ticks remained in the original vials until they were examined for the presence of spirochetes.
Field-collected questing ticks as well as molted ticks that had engorged on small mammals were dissected, and their midguts were examined for spirochetal infection by dark-field microscopy (4).
RESULTS
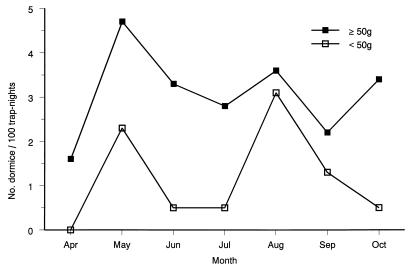
To help describe the natural history of the garden dormouse in our study site, we collected these animals by using arrays of traps deployed from April through October. Dormice entered these traps most frequently in May (Fig. 1). We used weight as an indicator of age of these dormice, but solely during late summer. About a third of these animals weighed less than <50 g in May, presumably because they had not yet recovered from hibernation. Indeed, the pelage of these underweight dormice confirmed their maturity. The trapping frequency of mature dormice subsequently declined, reflecting a change in activity rather than in abundance. The first immature dormice were captured in August; these animals weighed <50 g and were covered with grey juvenile fur. By October, virtually all dormice weighed at least 50 g. We conclude that garden dormice move most abundantly over the forest floor in April and May and again in midsummer, when the offspring of the year begin to forage.
FIG. 1.
Seasonal distribution of garden dormice, E. quercinus, that weigh more than 50 g and those that weigh less.
To evaluate the contribution of various small mammals as hosts for Lyme disease spirochetes in the Petite Carmarque study site, we determined the relative densities of the small mammals endemic there and the densities of subadult vector ticks feeding on them. Of the five kinds of small mammals that were captured, garden dormice and wood mice, A. sylvaticus, entered these traps most frequently (Table 1). Subadult I. ricinus ticks were the only kind of ticks parasitizing these animals. Although larvae infested about as many dormice as they did other small mammals, the densities of larvae feeding on dormice and mice were greater than those on bank voles, Clethrionomys glareolus, and much greater than those on greater white-toothed shrews, Crocidura russula. Appreciable numbers of nymphal ticks, however, were present solely on dormice. Dormice, therefore, serve as hosts to nymphal ticks far more frequently than do other endemic small mammals.
TABLE 1.
Density of the various small mammals in the Petite Carmarque study site and of attached subadult I. ricinus ticks on hosts
| Mammals captured
|
Larval ticks
|
Nymphal ticks
|
Larva/nymph ratio | ||||
|---|---|---|---|---|---|---|---|
| Kinda | Total no. | No./100 trap nights | % of hosts infested | Mean no./host | % of hosts infested | Mean no./host | |
| E. quercinus | 63 | 4.2 | 95 | 14.3 | 70 | 4.5 | 3 |
| A. sylvaticus | 62 | 4.5 | 89 | 15.1 | 28 | 0.3 | 50 |
| A. flavicollis | 31 | 2.2 | 86 | 11.9 | 0 | 0 | ∞ |
| C. glareolus | 20 | 1.3 | 75 | 6.9 | 7 | 0.1 | 69 |
| C. russula | 12 | 0.9 | 60 | 1.6 | 0 | 0 | ∞ |
Eliomys quercinus, garden dormouse; Apodemus sylvaticus, wood mouse; Apodemus flavicollis, yellow-necked mouse; Clethrionomys glareolus, bank vole; Crocidura russula, greater white-toothed shrew.
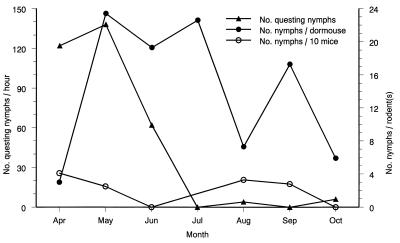
To determine when rodents in our study site were most likely to acquire spirochetal infection, we systematically sampled questing ticks from ecotonal vegetation while simultaneously determining their feeding densities on captured rodents. Nymphal vector ticks were collected from vegetation most frequently during spring and early summer (Fig. 2). Their density declined during midsummer and became nil by July. In contrast, such ticks continued to parasitize dormice throughout the summer and into the fall. The somewhat reduced tick burden that was observed on these animals during midsummer coincided with an increase in the density of the dormice following the birth of their offspring. Comparatively few nymphs attached to wood mice, and these were distributed throughout the transmission season. In an additional series of observations on 332 nymphs that were swept from vegetation, we found that 27% contained spirochetes and infectivity was constant during April through June (data not shown). We concluded that dormice experience intense exposure to nymphal vector ticks beginning in April and continuing through October.
FIG. 2.
Seasonal distribution of nymphal I. ricinus vector ticks questing on vegetation and attached to garden dormice, E. quercinus, and wood mice, A. sylvaticus.
We then compared the small-mammal fauna that was active along the forested side of the Petite Carmarque ecotone with that which was active along its grassy border. Garden dormice were far more frequently trapped in the forest than were any other animals sampled, and they were trapped in the forest about five times as frequently as they were in the meadow (Table 2). Wood mice, however, were dominant among the rodents captured in the meadow but virtually absent in the woods. The yellow-necked mouse, A. flavicollis, occurred about three times as frequently in the meadow as in the forest, and bank voles were caught exclusively in the meadow. Garden dormice are associated more closely with the forest than are any other of the abundant small mammals in the study site.
TABLE 2.
Distribution across the ecotone of the most abundant small rodents endemic to the Petite Carmarque study site
| Habitat and kind of rodenta | No. of rodents/100 trap nights | Ratio in each habitatb |
|---|---|---|
| Forest | ||
| E. quercinus | 7.8 | 85 |
| A. sylvaticus | 0.2 | 2 |
| A. flavicollis | 1.2 | 13 |
| C. glareolus | 0 | 0 |
| Meadow | ||
| E. quercinus | 1.6 | 9 |
| A. sylvaticus | 9.0 | 53 |
| A. flavicollis | 3.6 | 21 |
| C. glareolus | 3.0 | 17 |
For details, see Table 1, footnote a.
Relative to the other rodent species.
We compared the infectivity of these small mammals to the larval vector ticks that infested them in nature. Although ticks acquired spirochetal infection from virtually all dormice, infection was evident in less than half of the other rodents and absent in the shrews that were captured (Table 3). Each dormouse contributed about five times as many infections to the vector population as did mice, and voles contributed relatively few infections. Spirochetal infection, therefore, is most prevalent in dormice, and many more ticks acquire infection in the course of feeding on these than on other small mammals in the study site.
TABLE 3.
Prevalence of spirochetal infection in small mammals captured in the Petite Carmarque study site and infectivity of these hosts for larval I. ricinus ticks
| Mammals captured
|
Spirochetal infection in nymphs derived from field-infesting larvae
|
|||
|---|---|---|---|---|
| Kinda | No. | No. tested | % of hosts with ≥1 infected nymph | % infected |
| E. quercinus | 60 | 240 | 91 | 77 |
| A. sylvaticus | 45 | 146 | 31 | 12 |
| A. flavicollis | 29 | 79 | 47 | 20 |
| C. glareolus | 20 | 48 | 22 | 8 |
| C. russula | 5 | 12 | 0 | 0 |
For details, see Table 1, footnote a.
The spirochete infectivity of young garden dormice for vector ticks was compared to that of mature animals. About half as many immature (<50-g) dormice as mature (≥50-g) dormice were captured, and larval ticks infested all but a few (Table 4). Spirochetes were detected in virtually all cohorts of ticks that were found naturally attached to these rodents; infection was nearly universal in these hosts (82% of small animals and 93% of larger animals; chi-square test, P = 0.23). Laboratory-reared larvae placed on these rodents, however, acquired spirochetal infection more frequently when feeding on larger than on smaller dormice (chi-square test, P = 0.004). Assuming that their tick loads were similar, each mature dormouse would therefore contribute about half again as many infections to the vector population as would an immature dormouse.
TABLE 4.
Effect of age of garden dormice captured in the Petite Carmarque study site on spirochete infectivity for I. ricinus ticks
| Host wt (g) | No. of hosts | Hosts naturally with ≥1 larva
|
Spirochetes in laboratory-reared larvae fed on infected hosts
|
||
|---|---|---|---|---|---|
| No. | % Infected | No. | % Infected | ||
| <50 | 18 | 17 | 82 | 108 | 68 |
| ≥50 | 44 | 42 | 93 | 270 | 81 |
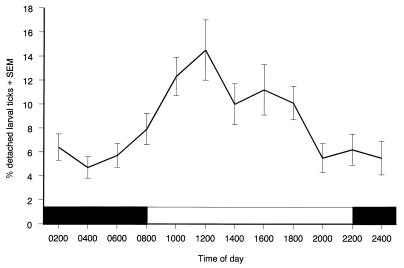
We determined when replete larval vector ticks most frequently detach from garden dormice. More than 98% of ticks infesting 47 such animals detached within 2 days after detachment began and were included in our analysis. About four-fifths of replete ticks detached during the dormouse’s diurnal period of sleep (Fig. 3). Ticks began to detach at about 2 h after the dormice became dormant. Because these animals sleep for 16 h each day, we estimate that all but a few replete ticks detach while their dormouse hosts remain within their nests.
FIG. 3.
Diurnal pattern of detachment of replete larval I. ricinus ticks from garden dormice, E. quercinus.
Finally, we derived an overall estimate of the contribution of the various abundant small rodents in the Petite Carmarque study site to the frequency of spirochetal infection in the vector population. We synthesized estimates of the trapping density of the various candidate rodents, the feeding density of larval ticks on these hosts, their prevalence of spirochetal infection, and the degree of infectivity for vector ticks. The product of these estimates provided the basis for this epizootiological comparison (Table 5). Garden dormice were far more frequently infected and more infectious to vector ticks than were any other rodent that was tested. The population of garden dormice contributes five- to sevenfold more infections to the vector population than the mouse population does.
TABLE 5.
Relative contribution of various reservoir hosts to spirochetal infection in the I. ricinus tick population in the Petite Carmarque study site
| Kind of rodenta | No. of hosts/trap
|
No. of larvae/host
|
Prevalence of infection in host
|
Infectivity of infected host
|
Reservoir inoculation index (102)c | ||||
|---|---|---|---|---|---|---|---|---|---|
| Observed | Ratiob | Observed | Ratio | Observed | Ratio | Observed | Ratio | ||
| E. quercinus | 4.2 | 0.34 | 14.3 | 0.30 | 0.91 | 0.48 | 0.79 | 0.40 | 1.96 |
| A. sylvaticus | 4.5 | 0.37 | 15.1 | 0.31 | 0.31 | 0.16 | 0.41 | 0.21 | 0.39 |
| A. flavicollis | 2.2 | 0.18 | 11.9 | 0.25 | 0.47 | 0.25 | 0.47 | 0.24 | 0.27 |
| C. glareolus | 1.3 | 0.11 | 6.9 | 0.14 | 0.22 | 0.12 | 0.31 | 0.15 | 0.03 |
For details, see Table 1, footnote a.
Relative to other rodent species.
The resulting reservoir inoculation index represents the product of the relative densities of each potential rodent host, the relative feeding densities of larval ticks on these hosts, the relative infectivity to ticks of each infected rodent host, and the prevalence of infection in each reservoir population.
DISCUSSION
The term “vectorial capacity” has been coined to express the relative contribution of diverse vector mosquitoes to the force of transmission of malaria pathogens (1). The concept derives directly from Macdonald’s (2) landmark model analyzing malaria transmission and ultimately from Ross’ seminal work (9). These authors identified and ranked various properties of vector populations that together specify the number of new infections deriving from each original infection per day. The vector-related dynamics of tick-borne pathogens have been compared to those that are insect-borne (8). The corresponding properties of the reservoir hosts in which such cycles are completed, however, have not similarly been analyzed. Our observations provide a basis for identifying such components in the case of a tick-borne zoonosis, Lyme disease.
The contribution of reservoir density to the force of transmission of a pathogen may be complex. In the traditional malariological use of vectorial capacity, the frequency of vector-reservoir contact would vary inversely with reservoir density. A dense human population, for example, would impede transmission, at least during the course of feeding of those vectors then present in the site. In the case of the agent of Lyme disease, the presence of numerous mice would facilitate development of vector ticks by ensuring that they find hosts while reducing the feeding density of the vector population relative to that of the reservoir. This would delay the time that pathogens first are introduced into individual reservoir hosts, thereby reducing the cumulative proportion of infectious members of the reservoir population. Spirochete transmission, therefore, would be most efficient at some optimal density of reservoir hosts relative to that of vector ticks.
The tendency of larval I. ricinus vector ticks to feed on a broader variety of mammals than do nymphal ticks increases the complexity inherent in these relationships. Although we find that the density of larval ticks feeding on mice is similar to that on garden dormice, far more nymphs feed on dormice than on mice. This implies that dormice become infected by Ixodes-borne pathogens earlier in their lives than mice do, an effect that becomes exaggerated by the far greater life span of dormice. Infection would be proportionately more prevalent in dormice; mice are relatively “zooprophylactic” against Lyme disease spirochetes because the many larval ticks that feed on these animals would fail to acquire spirochetal infection.
The seasonality of the corresponding vector and reservoir hosts may also affect transmission. Indeed, I. ricinus ticks are most abundant in early summer, before the next generation of garden dormice is born, and infectivity for ticks increases with age of this host. This separation of generations is less pronounced in mice than in dormice because mice are born earlier in the year (6), before these ticks cease questing. Such a correspondence of vector density with reservoir infectivity potentiates transmission.
The venue of spirochete transmission remains poorly defined. Human hosts become infected most frequently during late spring and early summer (12, 14), when nymphal vector ticks can most frequently be sampled from vegetation. Young dormice, however, appear to be infected in our study site soon after they are born. We trapped infected juveniles in mid-summer, when they begin foraging and when only few ticks can be swept from vegetation. Numerous nymphal ticks infest these animals, and this suggests that the juvenile dormice are encountering ticks within their nests. Indeed, we found that replete larval ticks detach from their hosts at a time of day when dormice would be sleeping in their nests. Our finding of spirochetal infection in virtually all garden dormice, regardless of age, confirms the inference that the dormouse nest constitutes the main venue of spirochete transmission.
The tendency of garden dormice to be parasitized by numerous nymphal vector ticks combines with their long life span to render these animals particularly effective as reservoir hosts for the agent of Lyme disease. The multitude of nymphs that feed on them, in comparison to those feeding on Apodemus mice, ensures that each of these animals is infected early in life. These animals may encounter infected nymphal ticks even before they are weaned, and once infected, such animals remain infected for as long as 3 years, the mean life span of garden dormice (10, 13). Indeed, their superior capacity as reservoir hosts is increased by their spirochete competence; we find that vector ticks become infected in the course of feeding on infected garden dormice at least twice as frequently as they do while feeding on other small rodents.
Each garden dormouse contributes about six times as many infections to the vector population as does either kind of mice that we tested, in part because they are more intensely exposed to nymphal vector ticks. The resulting pattern of early and repeated infection may enhance spirochete competence. Edible dormice, on the other hand, are only about twice as effective as reservoir hosts as mice are (5). Although both kinds of dormice infect virtually all ticks that feed on them, garden dormice tend to be far more abundant than edible dormice in their favored forested sites. In addition, the various rodents in our relatively moist study site are parasitized by many more larval ticks than are those in the relatively dry site in which edible dormice have been studied. Their competence, nymphal feeding density, and preference for a tick-permissive habitat combine to favor garden dormice over other putative reservoir hosts of Lyme disease spirochetes.
ACKNOWLEDGMENTS
This study was supported by grant Ma 942/7-1 from the Deutsche Forschungsgemeinschaft.
We thank P. Knibiely and B. Scaar at the Reserve Naturelle de la Petite Carmarque Alsacienne for granting permission to capture small mammals and C. Vaterlaus for his valuable help in trapping and marking garden dormice.
REFERENCES
- 1.Garrett-Jones C, Shidrawi G R. Malaria vectorial capacity of a population of Anopheles gambiae. Bull W H O. 1969;40:531–545. [PMC free article] [PubMed] [Google Scholar]
- 2.Macdonald G. The epidemiology and control of malaria. London, United Kingdom: Oxford University Press; 1957. [Google Scholar]
- 3.Matuschka F-R, Fischer P, Heiler M, Richter D, Spielman A. Capacity of European animals as reservoir hosts for the Lyme disease spirochete. J Infect Dis. 1992;165:479–483. doi: 10.1093/infdis/165.3.479. [DOI] [PubMed] [Google Scholar]
- 4.Matuschka F-R, Heiler M, Eiffert H, Fischer P, Lotter H, Spielman A. Diversionary role of hoofed game in the transmission of Lyme disease spirochetes. Am J Trop Hyg. 1993;48:693–699. doi: 10.4269/ajtmh.1993.48.693. [DOI] [PubMed] [Google Scholar]
- 5.Matuschka F-R, Eiffert H, Ohlenbusch A, Spielman A. Amplifying role of edible dormice in Lyme disease transmission in central Europe. J Infect Dis. 1994;170:122–127. doi: 10.1093/infdis/170.1.122. [DOI] [PubMed] [Google Scholar]
- 6.Niethammer J. Apodemus sylvaticus (Linnaeus, 1758)-Waldmaus. In: Niethammer J, Krapp F, editors. Handbuch der Säugetiere Europas. 1/I. Wiesbaden, Germany: Akademische Verlagsgesellschaft; 1978. pp. 337–358. [Google Scholar]
- 7.Piesman J, Mather T N, Sinsky R J, Spielman A. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randolph S E. Ticks are not insects: consequences of contrasting vector biology for transmission potential. Parasitol Today. 1998;14:186–192. doi: 10.1016/s0169-4758(98)01224-1. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. The prevention of malaria. 2nd ed. London, United Kingdom: John Murray; 1911. [Google Scholar]
- 10.Storch G. Eliomys quercinus (Linnaeus, 1766)-Gartenschläfer. In: Niethammer J, Krapp F, editors. Handbuch der Säugetiere Europas. 1/I. Wiesbaden, Germany: Akademische Verlagsgesellschaft; 1978. pp. 208–225. [Google Scholar]
- 11.Storch G. Glis glis (Linnaeus, 1766)-Siebenschläfer. In: Niethammer J, Krapp F, editors. Handbuch der Säugetiere Europas. 1/I. Wiesbaden, Germany: Akademische Verlagsgesellschaft; 1978. pp. 243–258. [Google Scholar]
- 12.Strle F, Maraspin V, Furlan-Lotric S, Cimperman J. Epidemiological study of a cohort of adult patients with erythema migrans registered in Slovenia in 1993. Eur J Epidemiol. 1996;12:503–507. doi: 10.1007/BF00144004. [DOI] [PubMed] [Google Scholar]
- 13.Vaterlaus C. Der Gartenschläfer—Ökologie, Populationsstruktur, Populationsdynamik und die Verbreitung in der Schweiz. Ph.D. thesis. Basel, Switzerland: Universität Basel; 1998. [Google Scholar]
- 14.Wilske B, Steinhuber R, Bergmeister H, Fingerle V, Schierz G, Preac-Mursic V, Lorbeer B. Lyme-Borreliose in Süddeutschland. Dtsch Med Wochenschr. 1987;112:1730–1736. doi: 10.1055/s-2008-1068320. [DOI] [PubMed] [Google Scholar]