Abstract
Introduction
Cubital fossa wounds can be complicated by the non-availability of reliable, well-vascularized donor tissue. Closure with pliable and readily available donor tissue for cubital defect and early mobilization of the elbow joint is essential for better results. The authors did this study to see how best the results of cubital fossa defect cover can be achieved by pedicle flaps in a single stage without compromising the donor areas.
Material and method
Patients having deep elbow wounds in which vital structures were lying exposed in the cubital region were included in this study. The patients were assessed for the availability of tissue for cover, reliability of flaps, flap pliability, the functional outcome of the elbow and donor site morbidity.
Results
A total of 17 cases of cubital region defects are presented wherein closure of the wound by means of primary closure was not possible. Out of these, eight were covered with Pedicled Thoracodorsal Artery Perforator (TDAP) flaps, five with Pedicled split Latissimus Dorsi Muscle (SLDM) flaps and four with reversed lateral arm flaps (RLA). Post-operatively all the flaps were healthy, patients attained a good range of elbow joint movements with no clinically evident morbidity of the donor site.
Conclusion
Cubital fossa defect coverage needs dedicated planning to obtain a sturdy tissue for cover. In the presence of local tissue damage or scarring, we have looked elsewhere to bring pliable and well-vascularized tissue which is reliable. The flaps we used have allowed single-stage reconstruction and early mobilization of the elbow joint with good functional recovery.
Keywords: Cubital fossa defect, Reverse lateral arm flap, Thoracodorsal artery perforator flap
1. Introduction
A normally functioning elbow joint is essential for an individual to go about his daily activities. A deformity or decreased range of motion at the elbow joint can significantly affect his lifestyle, as simple as bringing a glass of water to his mouth or lifting himself from a recumbent position. To achieve maximum range of motion at the elbow joint, the tissue cover provided should be adequate, lax, and have excellent elastic properties. While selecting the donor tissue for flap cover, an assessment regarding its vascularity and the resultant morbidity of the donor area, if any, should also be carried out. Elbow wounds that are encountered, varied from those caused by congenital anomalies, contracture release, tumour excision, and burns, to autoimmune disease, trauma, infection, and exposed prostheses. Regardless of the cause, wounds about the elbow region and forearm have profound disabling effects.
The cubital region contains important structures (subaponeurotic) which lie relatively superficial with a propensity to get exposed. In elbow defects, local tissue is frequently involved so the reconstructive surgeon has to look for regional or distant tissue sources.1 Coverage by fasciocutaneous flaps such as a proximally based radial forearm flap has its morbidity with the sacrifice of the major artery2 and cannot be utilized if there are concurrent ulnar or radial vessels injury. Commonly there is an associated tissue damage limiting our choice of usage of local muscles for cover, such as Flexor Carpi Ulnaris or Brachioradialis,3 this could also lead to further loss of some degree of hand movement in an already injured hand. However, these options are also not suitable for large elbow defects. Abdominal flaps and Intercostal artery perforator flaps can give cover for large wounds4 without sacrificing any muscle but need prolonged immobilization in an uncomfortable position and multiple interventions. Free flap coverage is a good option5 but requires special skills training, extensive operative hours and in many instances not feasible in polytrauma cases. Keeping all these requirements in mind we chose to cover elbow defects by pedicled flaps like Reverse Lateral arm flap (RLA), Pedicled Thoracodorsal artery perforator flap (TDAP) and pedicled Split Latissimus Dorsi muscle flap (SLD).
2. Material and methods
This retrospective study was conducted after getting approval from Institutional Ethical Committee. It includes 17 patients over a period of three years from September 2016 to August 2019 with deep defects in the cubital region. These patients were followed up for a period of six months or more. All patients underwent resurfacing with flaps, namely Reverse Lateral arm flap (RLA), Pedicled Thoracodorsal Artery Perforator flap (TDAP) and Pedicled Split Latissimus Dorsi Muscle Flap (SLDM). The cubital fossa defects with unexposed vitals structures and those managed with flaps requiring two/multiple stage/free flaps procedures were excluded.
If elbow injuries were associated with fracture or dislocation, the pre-operative planning was done along with orthopaedic surgeons, regarding the location of external fixator pin insertion to avoid damage of perforators and to achieve appropriate immobilization. In cases of elbow dislocation, the slab was used as a splint for a period of two weeks and when associated with fractures, external fixators were applied and kept in-situ for a period of 6 weeks. Further postoperative management included early physiotherapy until the maximum range of movement was achieved.
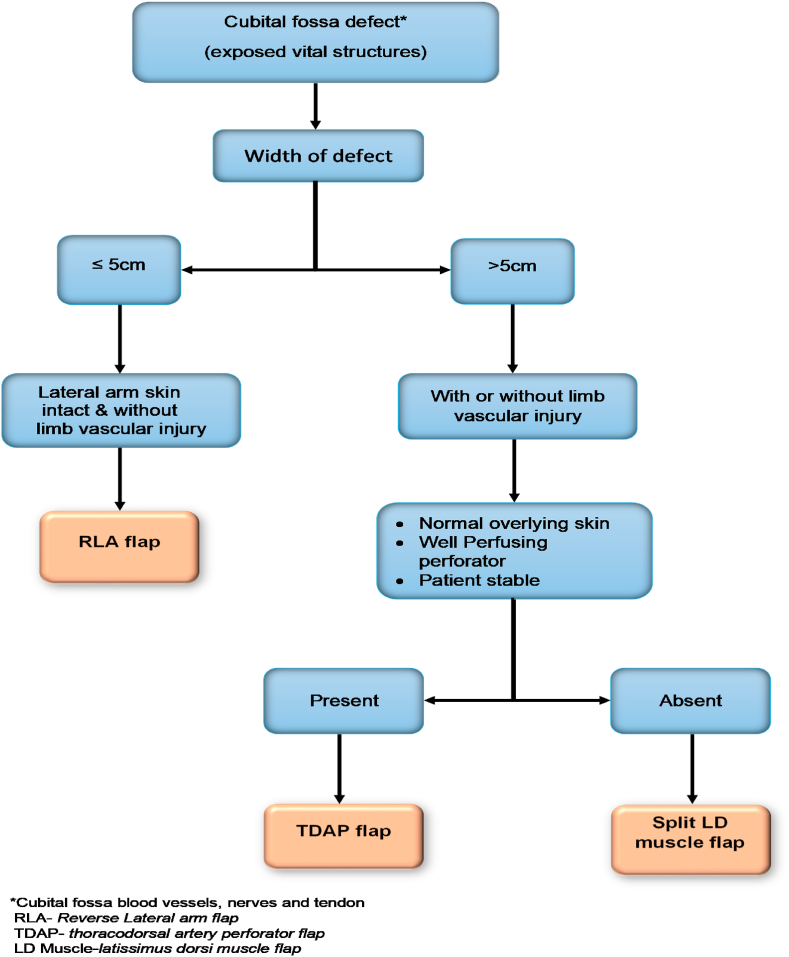
In burn contractures and cubital fossa defect without associated bony injury, post-operative immobilization of elbow joint was done by splinting with a slab, for a period of 7–10 days followed by early mobilization and physiotherapy. While choosing a flap for the cubital fossa defect cover, a scheme was developed, and the main factor taken into consideration was the size of the defect, condition of overlying skin over the lateral arm or at the back and status of skin perforators preoperatively and during intraoperative dissection (Fig. 1).
Fig. 1.
Flowchart for Preoperative Planning of various flaps in a patient with deep cubital fossa defect.
3. Results
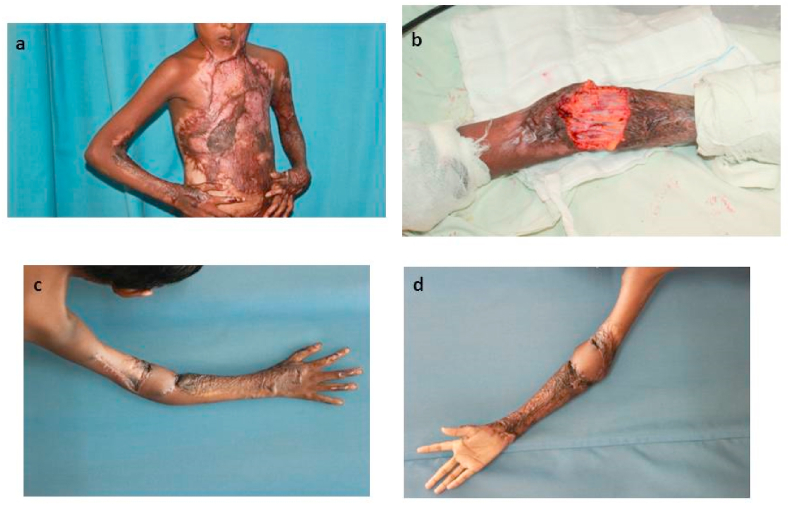
Out of the 17 patients included in the study, four underwent Reverse Lateral Arm (RLA) flap (Fig. 2),
Fig. 2.
A 9-year-old girl with post-burn contracture, neck, right elbow and left hand. a. Right elbow burn contracture, b. Contracture release and elbow defect, c. Defect covered by Reverse Lateral Arm (RLA) flap with healed donor site, d. Anterior view after 8 months.
Eight pedicled Thoracodorsal Artery Perforator (TDAP) flap and the rest five pedicled split Latissimus Dorsi muscle (LD) flap coverage. Of the 17 patients, 11 was male and 4 were female. The age of the patients ranged from 7 years to 59 years. The most common reason for cubital defects in our study was post-trauma either by a road traffic accident or fall from height (n = 11, Table 1).
Table 1.
Demographic Profile of patients.
| S·N. | Age/Gender | Area Involved | Etiology of Defect | Exposed Vital Structures | Defect Size (cm) | Flap size (cm) | Flap Transferred | Complication | Donor site morbidity | Elbow range at last follow up | Follow Up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 43Y/M | Lt elbow | FFH | Brachial artery repair with GSV | 13 × 8 | 15 × 9 | TDAP | None | None | 10°-130° | 14 months |
| 2. | 7Y/M | Lt Elbow | Burn | Exposed vessels | 6 × 4 | 8 × 5 | RLA | None | Hypertrophic scar | 5°-135° | 36 months |
| 3. | 40Y/M | Lt Elbow | FFH | Brachial artery repair with GSV | 15 × 6 | 17 × 7 | Split LD | Skin Graft loss in some area | Hypertrophic scar | 10°-125° | 12months |
| 4. | 9Y/F | Rt elbow | RTA | Exposed tendon & Brachial artery | 6 × 5 | 8 × 6 | RLA | None | None | 10°-130° | 8 months |
| 5. | 38Y/F | Rt Elbow | RTA | Exposed Tendon | 8 × 5 | 10 × 6 | TDAP | 2 cm distal partial necrosis | None | 5°-135° | 18 months |
| 6. | 20Y/M | Rt Elbow | RTA | Exposed vessels | 8 × 4 | 10 × 5 | TDAP | None | None | 10°-125° | 16 months |
| 7. | 44Y/M | Lt Elbow | Burn | Exposed tendon & Brachial artery | 10 × 5 | 12 × 6 | RLA | None | None | 15°-130° | 24 months |
| 8. | 25Y/F | Rt Elbow | FFH | Brachial artery repair with GSV | 8 × 5 | 10 × 6 | TDAP | None | None | 10°-130° | 13 months |
| 9. | 40Y/M | Lt Elbow | Burn | Exposed vessels | 9 × 6 | 10 × 6 | TDAP | None | None | 5°-125° | 17 months |
| 10. | 39Y/F | Lt Elbow | RTA | Brachial artery repair with GSV | 12 × 7 | 13 × 8 | Split LD | Skin graft loss in some area | None | 10°–130° | 12months |
| 11. | 41Y/F | Left Elbow | FFH (compartment syndrome) | Thrombosed Brachial artery. Interposition GSV graft and median Nerve | 15 × 8 | 20 × 8 | Split LD | None | None | 10°–125° | 6 months |
| 12. | 42Y/M | Right Arm and elbow | Crush injury (stone crusher) | Brachial artery repair with GSV | 14 × 6 | 18 × 6 | Split LD | None | None | 5°–125° | 19 months |
| 13. | 59Y/M | Left Elbow | Infected pseudoaneurysm | Brachial artery repair with GSV | 15 × 7 | 17 × 8 | Split LD | None | None | 10°–125° | 22 months |
| 14. | 30Y/F | Right elbow | Post Burn contracture | Exposed vessels | 6 × 5 | 10 × 5 | RLA | None | None | 0°–125° | 17 months |
| 15. | 46Y/M | Right humerus epicondyle and elbow | RTA | Exposed vessels and implant | 8 × 6 | 26 × 5 | TDAP | 2 cm distal Flap necrosis | 5°–125° | 15 months | |
| 16. | 25Y/M | Right elbow | Infected pseudoaneurysm | Exposed vessels & Brachial Artery | 7 × 7 | 18 × 7 | TDAP | None | None | 0°–125° | 9 months |
| 17. | 35Y/M | Left elbow | Post - trauma | Exposed vessels | 10 × 6 | 12 × 6 | TDAP | None | None | 10°–125° | 11 months |
M − Male, F- Female, Y- Year, RTA- Road Traffic Accident, FFH- Fall from height, TDAP- Pedicled Thoracodorsal Artery perforator, RLA-Reverse Lateral Arm, Split LD- Split Latissimus Dorsi Muscle.
Others causes were post burn contracture, and two cases of infected pseudoaneurysm in brachio-cephalic (A-V fistula). Seven of the patients had brachial artery repair with interposition great saphenous vein graft.
The most common flap used by us was pedicled TDAP (n = 8) and the largest defect covered was 120 cm.2 The largest flap harvested was TDAP having an area of 130 cm.2
All the patients were discharged within 3–5 days except for two patients with pedicled TDAP flap cover who suffered distal tip necrosis of 2 cm. The patient with tip necrosis of the flap was managed with debridement and secondary suturing. In two patients of pedicled split LD muscle flap cover with minimal graft loss were discharged on 5th day and continued with paraffin gauze dressing as an outpatient. Small residual raw areas due to graft loss healed by conservative treatment within 7–10 days. At the time of discharge, the mobilized flaps of all patients were well settled, and donor sites showed no morbidity.
4. Discussion
The cubital fossa contains vital structures such as a brachial artery, brachialis tendon and median nerve of upper limb superficially and hence prone to injury. Coverage of cubital defect should be planned by keeping in mind the need for a reliable, elastic, and flap with good vascularity. Due attention must be given to the possibility of resultant donor site morbidity. The defect in this region can also be attempted for closure by flexing the joint and bringing the defect margin together. However, such approach should be avoided as it may result in contracture of the elbow joint which may require secondary release. Such approach also hampers the achievement of a good range of motion in the long run even by starting early mobilization of the joint.
The lateral arm flap (LAF), a septofasciocutaneous flap is a good option for coverage of elbow defect if the defect is small and is associated with the local vascular injury which precludes the usage of the Radial Artery Flap. Use of local muscle flap may further decrease the power of an already injured limb especially if nerves are also involved in the injury. The abdominal flap can also be used in such a situation but has its disadvantages of uncomfortable positioning, multiple secondary procedures, and joint stiffness due to prolong immobilization.6
LAF was first described by Song et al.7 in 1982 for treatment of a burn scar contracture of the face and neck. Cormack and Lamberty8 and Katsaros et al.9 elaborated the advantages of the conventional LAF in succeeding years. The Katsaros et al.10 and Kuek and Chuan11 were first to report the clinical use of an “extended” lateral arm flap (ELAF) in 1991. Katsaros et al.9 shown that skin paddle based on the reverse blood supply of posterior radial collateral artery and Interosseous Recurrent Artery can be harvested which is the vascular basis of the RLA (Reverse Lateral Arm) flap. This flap has numerous advantages including constant vascular anatomy, minimal donor site morbidity12 and easy access for harvesting the flap for complex elbow defect.13 Surgical planning with an Orthopaedic surgeon is advisable in cases associated with bony injuries requiring an external fixator because their appliance may come in the territory of RLA (Reverse Lateral Arm) flap. In children, we do not recommend skin grafting at the elbow joint because graft contracture proceeds as the child grows even though the regular application of splints was carried on.
In situations with neurovascular injuries along with degloving of adjacent skin in the upper limb, local fasciocutaneous cannot be used, pedicled TDAP (thoracodorsal artery perforator) flap was found to be a good choice. Coninck et al.14 described the detailed anatomy of musculocutaneous branches arising from the thoracodorsal and lateral thoracic artery supplying the area of skin. Later Angrigiani15 did a study in cadavers to demonstrate a cutaneous supply of thoracodorsal artery. He stated that the first proximal perforator was present 8 cm caudal to the posterior axillary fold and 2–3 cm posterior to the lateral border of LD muscle. More work on the TDAP flap was done by Kim et al.16 who demonstrated the suprafascial dissection of the flap to decrease the thickness of the flap by including only skin and subcutaneous tissue in 12 clinical cases without flap necrosis. In their study on cadavers, Binu et al.17 showed that the most proximal perforator of the thoracodorsal artery was about 3 cm medial to the anterior border of muscle and in line with the angle of the scapula while the second perforator was 3 cm distal to proximal one, these two perforators were found relatively consistent in their location while rest of the perforators were variable in their location. Most of the perforators were musculocutaneous in nature with an average number of 5.5 ± 1.8. The mean diameter of perforator he found was 0.9 ± 0.3 mm. The advantage of the TDAP flap is its vascular reliability and minimal seroma formation and donor site morbidity. In our series of Eight cases of pTDAP flap, routine use of pre-operative handheld doppler of 8 MHz was used to locate the perforators. We found atleast one good perforator, approximately at 8–10 cm cephalad from the dome of axilla and 3 cm medial to the anterior border of Latissimus Dorsi muscle on which the flap was reliably based. The longest pedicle length reported till now is 24 cm while in our study it varied from 16 to 20 cm, this is also one of the major benefits of doing TDAP flap.18 In all of our cases but one, we found at least two nice perforators supplying the flap. Perforators that were initially TDAP flap, during dissection below the 12th rib, came out to be the intercostal perforators after further dissection. To avoid confusion between these two it is advisable to choose the perforators found above the 12th rib and check the direction in which the perforator enters the muscle. Pedicled TDAP was found to be a sturdy flap with a distal edge reaching to the proximal third of the forearm. In two patients, we observed necrosis of distal 2 cm, which was debrided and re-sutured. The flap was raised on the perforator which were preoperatively identified using handheld Doppler (8Hz).19 Instead of tunnelling the pedicle under the axillary skin, we chose to open the axillary region, by an incision and prefer to prevent compression of the delicate pedicle (Fig. 3). In the post-operative period, patients were positioned in a 45-degree shoulder abduction splint continuously for a week and then at nights only for another week. In our opinion, the TDAP flap is a good choice for large elbow defects with locally traumatized tissue. There is a significant initial learning curve for the surgeon, due to the required meticulous and gentle dissection prolonging the operative time.
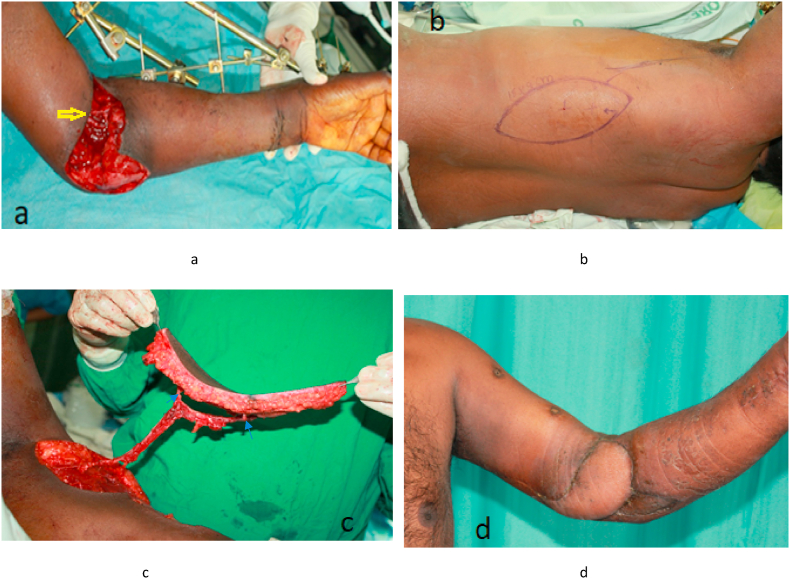
Fig. 3.
A case of 43-year male, sustained injury to left elbow due to accidental fall from a tree. a. An ulnar view of Left elbow defect with repaired brachial artery by GSV (Great Saphenous Vein) interposition graft (yellow arrow), b. Marking of TDAP flap, c. Elevated TDAP flap on two perforators (Blue arrows), d. Post-operative, flap cover of the elbow defect. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In a clinical setting of a large defect, during TDAP flap elevation, wherein the perforator was deemed inadequate or with damaged skin at the back, a split LD muscle flap was chosen to cover the wound (Fig. 4). Split LD was first described by Tobin et al.20 in 1981. The Splitting of the Latissimus dorsi muscle was based on a vertical branch of the thoracodorsal vessel. SLDM flap provides a reduced bulk at the recipient site along with decreased donor site morbidity.21The flap harvest is less time consuming, less intraoperative bleeding, decreased chances of postoperative seroma formation at the back, and a short hospital stay in comparison to using a full Latissimus Dorsi muscle flap.22 The Split LD is easy to dissect, reliable and can easily reach the proximal third of the forearm.
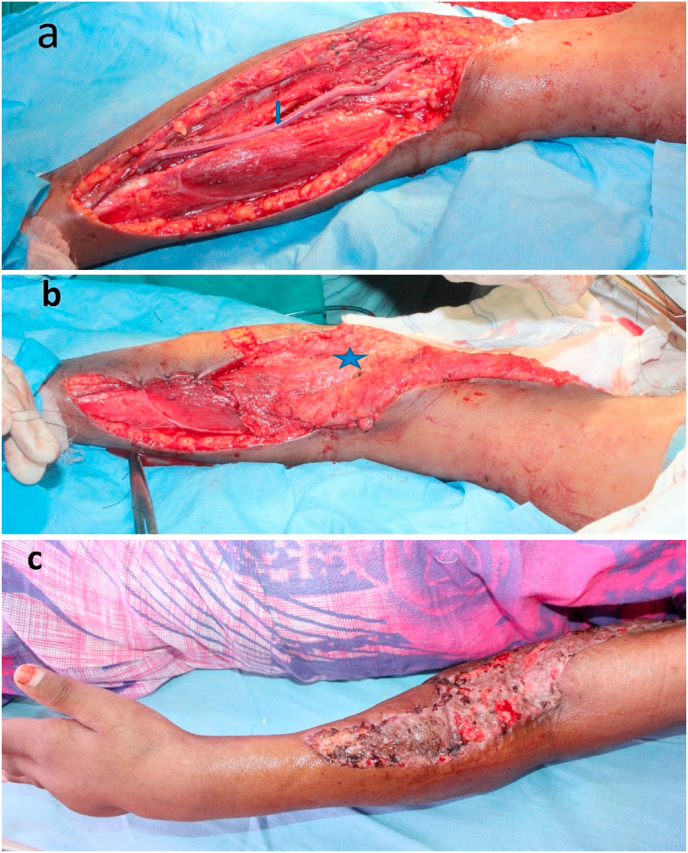
Fig. 4.
A 41-year female with a history of fall from a height with fractured radius & ulna with compartment syndrome left forearm. a. She underwent exploration fasciotomy cubital fossa and forearm, and repair of the brachial and radial artery with interposition GSV (Blue Arrow). b Split Latissimus dorsi muscle (blue star) was utilized to cover the cubital fossa and mid-forearm. c. GSV covered with Split LD muscle flap and skin graft. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In this study, we had covered cubital fossa defects by using only pedicle flaps. Though using free flap in such situations is also a good option for coverage but it is seen to be not feasible in patients having multiple comorbidities, as in such cases failure rates are found to be high. In those trauma patients, who are having concomitant life threatening injuries, they cannot be made to withstand long hours of microvascular surgery.
5. Conclusion
Deep cubital fossa wound coverage needs dedicated planning with sturdy and reliable tissue to cover. We have described coverage of such wounds with different types of flaps and the resultant minimal donor site morbidity. This allows the surgeon to tackle such difficult wounds in a prime area. Coverage with these flaps provides single-stage reconstruction and the promise of an early mobilization of the elbow joint to achieve the required good range of motion.
Ethical approval
This study was approved from Institutional Ethical Board.
Patients consent
Taken.
Funding
None Declared.
Declaration of competing interest
None.
Acknowledgement
To Dr. Kanika Suhag for grammar correction.
Contributor Information
Gaurav Chaturvedi, Email: drgauravchaturvedi2012@gmail.com.
Elvino Barreto, Email: elvino7@gmail.com.
References
- 1.Stevanovic M., Sharpe F. Soft-tissue coverage of the elbow. Plast Reconstr Surg. 2013 Sep;132(3):387e–402e. doi: 10.1097/PRS.0b013e31829ae29f. [DOI] [PubMed] [Google Scholar]
- 2.Jones N.F., Jarrahy R., Kaufman M.R. Pedicled and free radial forearm flaps for reconstruction of the elbow, wrist, and hand. Plast Reconstr Surg. 2008 Mar;121(3):887–898. doi: 10.1097/01.prs.0000299924.69019.57. [DOI] [PubMed] [Google Scholar]
- 3.Adkinson J.M., Chung K.C. Flap reconstruction of the elbow and forearm: a case-based approach. Hand Clin. 2014 May;30(2):153–163. doi: 10.1016/j.hcl.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farber G.L., Taylor K.F., Smith A.C. Pedicled thoracoabdominal flap coverage about the elbow in traumatic war injuries. Handb Neuropsychol N. 2010 Mar;5(1):43–48. doi: 10.1007/s11552-009-9213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SpindlerN AlBennaS., RingA, et al. Free anterolateral thigh flaps for upper extremity soft tissue reconstruction. GMS Interdiscip Plast Reconstr Surg. 2015;4 doi: 10.3205/iprs000064. Doc05. DGPW, URN: urn:nbn:de:0183-iprs000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabapathy S.R., Venkatramani H., Martin Playa P. The use of pedicled abdominal flaps for coverage of acute bilateral circumferential degloving injuries of the hand. Trauma Case Rep. 2015 Apr;1(3–4):25–31. doi: 10.1016/j.tcr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song R., Song Y., Yu Y., Song Y. The upper arm free flap. Clin Plast Surg. 1982 Jan;9(1):27–35. [PubMed] [Google Scholar]
- 8.Cormack G.C., B G.H. Lamberty. Fasciocutaneous vessels in the upper arm: application to the design of new fasciocutaneous flaps. Plast Reconstr Surg. 1984 Aug;74(2):244–249. [PubMed] [Google Scholar]
- 9.Katsaros J., Schusterman Mark, Beppu Moroe. The lateral upper arm flap: anatomy and clinical applications. Ann Plast Surg. 1984;12(6):489–500. doi: 10.1097/00000637-198406000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Katsaros J., Tan E., Zoltie N., Barton M., null Venugopalsrinivasan, null Venkataramakrishnan. Further experience with the lateral arm free flap. Plast Reconstr Surg. 1991 May;87(5):902–910. doi: 10.1097/00006534-199105000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Kuek L.B., Chuan T.L. The extended lateral arm flap: a new modification. J Reconstr Microsurg. 1991 Jul;7(3):167–173. doi: 10.1055/s-2007-1006775. [DOI] [PubMed] [Google Scholar]
- 12.Pranti L., et al. A safe and simple technique using the distal pedicled reversed upper arm flap to cover large elbow defects. J Plast Reconst Aesthet Surg. 2008 May;61(5):546–551. doi: 10.1016/j.bjps.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Morrison C.S., Sullivan S.R., Bhatt R.A., Chang J.T., Taylor H.O. The pedicled reverse-flow lateral arm flap for coverage of complex traumatic elbow injuries. Ann Plast Surg. 2013 Jul;71(1):37–39. doi: 10.1097/SAP.0b013e318248b627. [DOI] [PubMed] [Google Scholar]
- 14.Coninck A de, Vanderlinden E., Boeckx W. The thoracodorsal skin flap: a possible donor site in distant transfer of island flaps by microvascular anastomosis. Chir Plast. 1976 Dec 1;3(4):283–291. [Google Scholar]
- 15.Angrigiani Claudo, Grilli Daniel, Siebert John. Latissimus dorsi musculocutaneous flap without muscle. Plast Reconstr Surg. 1995;96:1608. doi: 10.1097/00006534-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Kim Jeong Tae, Koo B.S., Kim Seok Kwun. The thin latissimus dorsi perforator based free flap for resurfacing. Plast Reconstr Surg. 2001;107:374. doi: 10.1097/00006534-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Thomas BP, Geddes CR, Tang M, Williams J, Morris SF. The vascular basis of the thoracodorsal artery perforator flap.Plast Reconstr Surg. 116(3):818–822. [DOI] [PubMed]
- 18.Oksus S., Ulkur E., Tuncer S., Sever C., Karagoz H. Elbow reconstruction with a pedicled thoracodorsal flap after excision of an upper extremity giant hairy nevus. J Plast Reconstr Aesthetic Surg. 2013 Apr;66(4):566–569. doi: 10.1016/j.bjps.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Sever C., Uygur F., Kulahci Y., Karagoz H., Sahin C. Thoracodorsal artery perforator fasciocutaneous flap: a versatile alternative for coverage of various soft tissue defects. Indian J. Plast. Surg. 2012;45(3):478–484. doi: 10.4103/0970-0358.105956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobin G.R., Moberg A.W., DuBou R.H., Weiner L.J., Bland K.I. The split latissimus dorsi myocutaneous flap. Ann Plast Surg. 1981 Oct;7(4):272–280. doi: 10.1097/00000637-198110000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Alberto Terzi, Luzzi Luca. The split latissimus dorsi muscle flap to protect a branchial stump at risk of bronchial insufficiency. Ann Thorac Surg. 2009;87:329–330. doi: 10.1016/j.athoracsur.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 22.Yoshihiro Sowa, et al. Comparison of morbidity-related seroma formation following conventional latissimus dorsi flap versus muscle-sparing latissimus dorsi flap breast reconstruction. Ann Surg Treat Res. 2017 Sep;93(3):119. doi: 10.4174/astr.2017.93.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]