Abstract
miR-15b-5p is encoded by MIR15B gene. This gene is located on cytogenetic band 3q25.33. This miRNA participates in the pathogenesis of several cancers as well as non-malignant conditions, such as abdominal aortic aneurysm, Alzheimer’s and Parkinson’s diseases, cerebral ischemia reperfusion injury, coronary artery disease, dexamethasone induced steatosis, diabetic complications and doxorubicin-induced cardiotoxicity. In malignant conditions, both oncogenic and tumor suppressor impacts have been described for miR-15b-5p. Dysregulation of miR-15b-5p in clinical samples has been associated with poor outcome in different kinds of cancers. In this review, we discuss the role of miR-15b-5p in malignant and non-malignant conditions.
Keywords: miR-15b-5p, cancer, biomarker, expression, malignance
Introduction
microRNAs (miRNAs) are a category of non-coding RNA with sizes about 20-24 nucleotide which participate in post-transcriptional control of gene expression (1). This effect is exerted through modulation of stability and translation of mRNAs. The primary transcripts produced by RNA polymerase II have 5’-cap and 3’-polyadenylated tail. Then, Drosha ribonuclease III enzyme cleaves this transcript to make the stem-loop precursor miRNA with an estimated size of 70 nucleotides (2). Finally, this transcript is processed by the Dicer ribonuclease to make the mature miRNA which can be combined into the RNA-induced silencing complex. Through incorporation into this complex, miRNAs can recognize their target transcript in a base pairing-dependent process resulting in suppression of translation or destabilization of transcript (3).
MIR15B gene is located on cytogenetic band 3q25.33 and encodes hsa-mir-15b. This miRNA participates in the pathogenesis of several cancers as well as non-malignant conditions, including cardiovascular disorders, neuropsychiatric diseases and metabolic conditions. This miRNA has been reported to exert oncogenic or tumor suppressor effects in different malignancies. We have searched the literature and discussed the role of miR-15b-5p in malignant and non-malignant conditions.
miR-15b-5p in Cancers
Cell Line Studies
In bladder cancer cell lines, the long non-coding RNA (lncRNA) MAGI2-AS3 acts as a molecular sponge for miR-15b-5p. In fact, MAGI2-AS3 exerts its tumor suppressor role in bladder cancer through decreasing level of this miRNA. Meanwhile, miR-15b-5p has been found to target the tumor suppressor gene CCDC19. Taken together, MAGI2-AS3/miR-15b-5p/CCDC19 axis has been revealed to regulate progression of bladder cancer (4).
An in vitro experiment in breast cancer cells has shown that miR-15b-5p silencing could restrain cell proliferation and invasiveness and induce apoptosis, while its up-regulation has exerted the opposite impacts. Notably, heparanase-2 (HPSE2) has been acknowledged as the target of miR-15b-5p in breast cancer cells, through which this miRNA applies its effect (5).
In cervical cancer cells, level of the tumor suppressor lncRNA FENDRR has been shown to be decreased. This lncRNA has binding sites for miR-15a-5p and miR-15b-5p, two miRNAs that can down-regulate expression of Tubulin alpha1A (TUBA1A). Taken together, FENDRR/miR-15a/b-5p/TUBA1A molecular route has been proved to regulate progression of cervical cancer (6).
Expression of miR-15b-5p has been reported to be surged in colon cancer cells. Treatment of HT-29 cells with a PNA against miR-15b-5p has been shown to reduce cell proliferation and activate the pro-apoptotic pathway (7). Another research in colon cancer cells has displayed that SIRT1 suppresses metastatic ability of cells through decreasing expression of miR-15b-5p. In fact, SIRT1 disrupts the regulatory effect of AP-1 on activation of expression of miR-15b-5p via deacetylating this activation factor. miR-15b-5p can target the transcript of a central enzyme in the fatty acid oxidation, namely acyl-CoA oxidase 1 (ACOX1). Taken together, SIRT1/miR-15b-5p/ACOX1 axis has been identified as a functional route in regulation of metastatic ability of colorectal cancer cells (8).
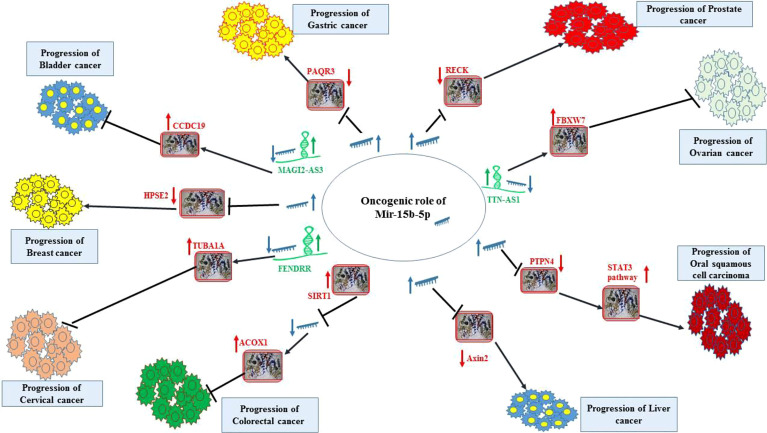
Figure 1 displays the oncogenic role of miR-15b-5p in bladder, breast, cervical, colorectal, liver, oral, ovarian, prostate and gastric cancers.
Figure 1.
Oncogenic effect of miR-15b-5p in bladder, breast, cervical, colorectal, liver, oral, ovarian, prostate and gastric cancers. Detailed information about the conducted experiments is shown in Table 1 .
Table 1.
Summary of cell line studies on the role of miR-15b-5p in cancers (Δ, knock-down or deletion; MET, mesenchymal-epithelial transition).
| Tumors | Interactions | Cell line | Function | Reference |
|---|---|---|---|---|
| Bladder cancer | MAGI2-AS3 and CCDC19 | EJ, T24 and RT4, SV-HUC-1 | ↑↑ MAGI2-AS3 (which sponges mir-15b-5p): ↓ Proliferation, ↓ migration and ↓ invasion | (4) |
| Breast cancer | HPSE2 | MDA-MB-231, MCF-7, 293T | Δ miR-15b-5p: ↓ proliferation, ↓ colony formation, ↓ migration and ↓ invasion, ↑ apoptosis | (5) |
| Cervical cancer | FENDRR, TUBA1A | HeLa, SiHa, CaSki, C33A, Ect1-E6E7 | ↑↑ FENDRR (which sponges mir-15b-5p): ↓ proliferation, ↓ migration and ↓ invasion, and ↓ cell viability, and ↑ apoptosis | (6) |
| ↑↑ mir-15b-5p: ↑ proliferation, ↑ migration and ↑ invasion, and ↑ cell viability, and ↓ apoptosis | ||||
| Colorectal cancer | NF-κB1 and IKK-α | NCM460, SW620, HCT116, DLD1, SW1116 | ↑↑ miR-15b-5p: ↑ sensitivity to 5-FU and ↑ apoptosis | (9) |
| _ | HT-29 cell line | R8-PNA-a15b molecule treatment: ↓ miR-15b-5p levels and ↑ inhibition of HT-29 cell growth associated with pro-apoptotic effects | (7) | |
| SIRT1, AP-1, ACOX1 | HCT116, SW480, SW620, LoVo, Caco-2, HT-29 | ↑↑ SIRT1: ↓ migration and invasion and suppresses mir-15b-5p transcription via AP-1 | (8) | |
| IL-17A, PD-L1, P65, NRF1 | CT26, MC38, SW1116, HT29, SW480, SW620 | ↑↑ miR-15b-5p: ↓ PD-L1 protein level and ↑ anti-PD-1 sensitivity | (10) | |
| CERS6-AS1 | FHC, Caco-2, T84, HCT-15 | Δ CERS6-AS1 (whish sponges miR-15b-5p): ↓ proliferation, ↓ migration, ↓ invasion, ↓ EMT, and ↓ stemness | (11) | |
| Gastric cancer | PAQR3 | AGS, BGC-823, SGC-7901, MGC-803 | ↑↑ miR-15b-5p: ↑ migration and ↑ invasion | (12) |
| Glioblastoma multiforme | _ | U251 | Combo-therapy using PNA-a15b and SFN via interfering with miR-15b-5p could be used as a treatment for Glioblastoma multiforme to stimulate apoptosis. | (13) |
| Hepatocellular carcinoma | OIP5, AKT/mTORC1 and β-catenin signaling pathways | HepG2, Hep3B, SK-HEP-1, Chang liver and THLE2, Huh7 | Δ OIP5 (a target of mir-15b-5p): ↓ migration, ↓ invasion and ↓ EMT process via mTORC1 and GSK-3β/β-catenin signaling | (12) |
| H19 and CDC42/PAK1 signaling pathway | HepG2, SMMC-7721, Bel-7402, Huh-7, WRL-68, 293T | Δ H19 (which sponges mir-15b-5p): ↓ proliferation, migration, invasion, EMT and CDC42/PAK1 signaling pathway and ↑ apoptosis | (14) | |
| Rab1A | SMMC-7721, HepG2, Hep3B, HL-7702 | ↑↑ miR-15b-5p: ↓ cell growth, ↑ endoplasmic reticulum stress and apoptosis | (15) | |
| Δ miR-15b-5p: ↑ proliferation and ↓ apoptosis | ||||
| Laryngeal cancer | TXNIP | HEP-2 | ↑↑ miR-15b-5p: ↑ cell growth via targeting TXNIP | (16) |
| Liver cancer | Axin2 | HepG2 and Huh7, Hep3B and HCCLM3 | ↑↑ miR-15b-5p: ↑ | (14) |
| Proliferation and ↑ invasion | ||||
| Neuroblastoma | MYCN | SK‐N‐BE (2), NB‐19, SH‐EP Tet21N, CHLA‐136 | ↑↑ miR-15b-5p: ↓ proliferation, ↓ migration, and ↓ invasion of NB cells | (17) |
| SNHG16, PRPS1 | neuroblastoma cells | Δ SNHG16 (which sponges mir-15b-5p): ↓ proliferation, and ↑ G0/G1 phase arrest | (18) | |
| Non-small cell lung cancer | MEG8 and PSAT1 | 16HBE, A549, H1299, H1975, SPC-A1, and PC-9 | Δ MEG8 (which sponges mir-15b-5p): ↓ proliferation, ↓ migration, and ↓ invasion | (19) |
| Oral squamous cell carcinoma | PTPN4, STAT3 pathway | SCC-4, UM-1, CAL-27, OSC-4 | Δ mir-15b-5p: ↓ proliferation, ↓ migration, and ↓ invasion and ↑ apoptosis | (20) |
| Oral tongue squamous cell carcinoma | TRIM14 | SCC25 | ↑↑ miR-15b: ↑ MET phenotypes and ↓ cisplatin-resistance via targeting TRIM14 | (21) |
| Osteosarcoma | PDK4 | hFOB1.19, MNNG-HOS, Saos-2, MG63, U-2OS | ↑↑ miR-15b-5p: ↓ proliferation and the Warburg effect by suppressing PDK4 expression | (22) |
| TRPM2-AS and PPM1D | OS cells | Δ TRPM2-AS (which sponges mir-15b-5p): ↓ viability, ↓ proliferation, ↓ migration and ↑ apoptosis | (23) | |
| Ovarian cancer | TTN-AS1, FBXW7 | A2780, OVCA429, IOSE80 | ↑↑ TTN-AS (which sponges mir-15b-5p): ↓ proliferation and ↓ colony formation, ↑ apoptosis | (24) |
| Prostate cancer | RECK | PCa cell lines (PC3 and 22RV1) | Δ miR-15b-5p: ↓ cell growth and invasion | (25) |
| PVT1 and NOP2 | DU 145, PC-3, RWPE-1 | ↑↑ PVT1 (which sponges mir-15b-5p): ↑ migration and ↑ invasion | (26) | |
| Thyroid carcinoma | GDI2, MMP2 and MMP9 | FTC133, SW1736, K1, Nthy-ori3-1 | ↑↑ mir-15b-5p: ↓ proliferation and ↓ invasion | (27) |
↑ Up-regulation; ↓ Down-regulation.
In contrast to the previously mentioned experiment in colorectal cancer cells (7), Zhao et al. have shown that miR-15b-5p has a tumor suppressor impact in this cancer. Notably, miR-15b-5p can enhance 5-fluorouracil (5-FU)-induced apoptosis in these cells and reversed the resistance of colorectal cancer cells to this therapeutic agent. Mechanistically, miR-15b-5p exerts this impact through modulating activity of the NF-κB signaling via decreasing NF-κB1 and IKK-α levels. miR-15b-5p has been found to target the anti-apoptosis transcript XIAP (9).
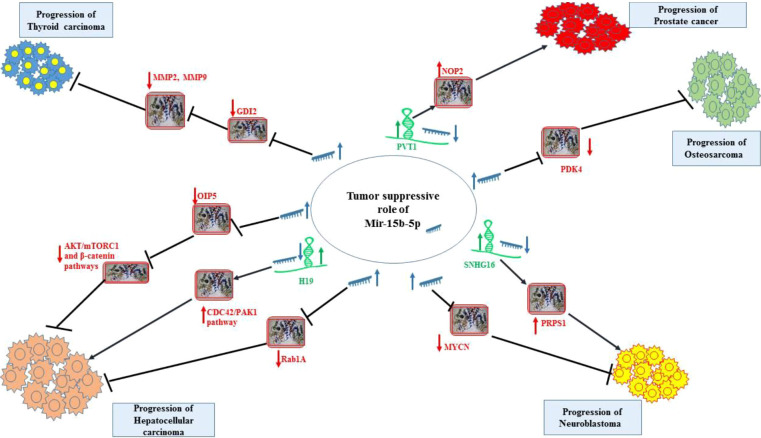
In vitro experiments in neuroblastoma cells have shown that up-regulation of miR-15a-5p, miR-15b-5p or miR-16-5p can reduce expression of MYCN transcript and N-Myc protein. On the other hand, suppression of these miRNAs could lead to enhancement of MYCN transcripts and N-Myc protein level, along with increasing half-life of its mRNA. The interaction between these miRNAs and MYCN mRNA has been proved through conducting immunoprecipitation and luciferase reporter assays. Notably, up-regulation of these miRNAs has diminished proliferation, migration, and invasiveness of neuroblastoma cells (17). Figure 2 shows tumor suppressor role of miR-15b-5p in thyroid cancer, hepatocellular carcinoma, neuroblastoma, osteosarcoma and prostate cancer.
Figure 2.
Tumor suppressor role of miR-15b-5p in thyroid cancer, hepatocellular carcinoma, neuroblastoma, osteosarcoma and prostate cancer. Detailed information about the conducted experiments is shown in Table 1 .
Animal Studies
Lovat et al. have produced miR-15b/16-2 knockout mice for the purpose of identification of the role of this cluster. This intervention has led to development of B-cell lymphomas by age 15–18 month possibly though modulation of expression of Cyclins D2 and D1, and IGF1R. These genes participate in the regulation of proliferation and antiapoptotic pathways. Taken together, this cluster has been shown to have a tumor suppressor role in mice models of B-cell lymphoma (28).
In xenograft models of bladder cancer, up-regulation of MAGI2-AS3 has reduced tumor volume possibly through decreasing expression of miR-15b-5p (4). Up-regulation of FENDRR, another miR-15b-5p-sponging lncRNA has exerted similar effects in xenograft models of cervical cancer (6). In colorectal cancer cells, a single study has shown that over-expression of miR-15b-5p improves sensitivity of cells to 5-FU (9). On the other hand, another study has indicated that SIRT1 decreases metastasis through suppression of miR-15b-5p transcription (8). Moreover, miR-15b-5p has been demonstrated to decrease expression of PD-L1, suppress tumorigenic potential of colorectal cancer cells and increase anti-PD-1 sensitivity in colitis-associated cancer and APCmin/+ models of colorectal cancer (10).
In an animal model of osteosarcoma, over-expression of miR-15b-5p has been associated with reduced cell proliferation (22). Table 2 shows summary of animal studies on the role of miR-15b-5p in cancers.
Table 2.
Summary of animal studies on the role of miR-15b-5p in cancers (Δ, knock-down or deletion).
| Tumors | Animals | Results | Reference |
|---|---|---|---|
| Bladder cancer | 4-week-old female BALB/C nude mic | ↑↑ MAGI2-AS3: ↓ tumor volume and↓ tumor weight | (4) |
| Breast cancer | 5-week-old female BALB/C nude mice | Δ miR-15b-5p: ↓ tumorigenic ability | (5) |
| Cervical cancer | 6-week-old male BALB/C nude mice | ↑↑ FENDRR (which sponges mir-15b-5p): ↓ tumor volume and ↓ tumor weight | (6) |
| Colorectal cancer | Four-week-old female athymic nude mice | ↑↑ miR-15b-5p: ↑ sensitivity of colon cancer cells to 5-FU and ↑ apoptosis via the NF-κB pathway | (9) |
| 4-6 weeks old BALB/c nude mice | ↑↑ SIRT1: ↓ metastasis by suppressing mir-15b-5p transcription via AP-1 | (8) | |
| female BALB/c mice with two different groups control and blocking miR-15b-5p groups | Δ miR-15b-5p: ↑ tumorigenesis and ↑ PD-L1 levels | (10) | |
| BALB/c nude mice | Δ CERS6-AS1 (whish sponges miR-15b-5p): ↓ tumor growth | (11) | |
| Hepatocellular carcinoma | Four-week-old female BALB/c nude mice | Δ OIP5 (a target of mir-15b-5p): ↓ tumor growth and ↓ metastasis | (12) |
| Four-week-old male BALB/C nude mice | ↑↑ miR-15b-5p: ↓ tumor growth, ↓ tumor volume and ↓ tumor weight | (15) | |
| Neuroblastoma | Six‐week‐old NOD mice | ↑↑ miR-15b-5p: ↓ tumor size and ↓ tumor weight | (17) |
| Non-small cell lung cancer | Balb/c nude mice | Δ MEG8 (which sponges mir-15b-5p): ↓ tumor growth | (19) |
| Oral squamous cell carcinoma | 5-week-old female specific-pathogen-free mice | Δ mir-15b-5p: ↓ tumor growth and ↓ metastasis | (20) |
| Osteosarcoma | 5-week-old male BALB/C nude mice | ↑↑ miR-15b-5p: ↓ proliferation | (22) |
| Prostate cancer | PC3 xenograft tumor model | Δ miR-15b-5p: ↓ tumor volume and ↓ tumor weight | (25) |
↑ Up-regulation; ↓ Down-regulation.
Human Studies
Expression assays in clinical samples obtained from patients with bladder cancer, breast cancer, gastric cancer, oral squamous cell carcinoma and prostate cancer have shown up-regulation of miR-15b-5p. On the other hand, this miRNA has been found to be down-regulated in head and neck cancer squamous cell carcinomas, neublastoma and thyroid cancer samples. Different studies in colorectal cancer and hepatocellular carcinoma sample have shown contradictory expression patterns ( Table 3 ). Moreover, dysregulation of expression of miR-15b-5p has been associated with poor clinical outcome in bladder cancer, breast cancer, head and neck/oral squamous cell carcinoma, hepatocellular carcinoma and neuroblastoma.
Table 3.
Summary of human studies on the role of miR-15b-5p in cancers (NB, Neuroblastoma; OS, Overall survival; ANCTs, adjacent non-cancerous tissues; TNM, tumor‐node‐metastasis; MSS, microsatellite stable; CRC, colorectal cancer; RFS, relapse-free survival; HCC, Hepatocellular carcinoma).
| Tumors | Specimens | Expression (Tumor vs. Normal) | Kaplan-Meier analysis (as a result of dysregulation in mir-15b-5p) | Multivariate/Univariate cox regression | Clinicopathologic characteristics | Method by which RNA was detected | Reference |
|---|---|---|---|---|---|---|---|
| Bladder cancer | 10 patients with and without BC included 3 healthy persons and 7 patients with other urologic diseases | upregulated | _ | _ | _ | ExiLENT SYBR® Green master mix | (29) |
| TCGA database 58 pairs of tumor tissues and ANCTs | upregulated | Poorer OS | _ | _ | PrimeScript RT-PCR kit | (4) | |
| Breast cancer | 6 pairs of tumor tissues and ANCTs TCGA databases | upregulated | Poorer OS | _ | _ | _ | (5) |
| Cervical cancer | 53 pairs of tumor tissues and ANCTs | Downregulation of FENDRR (which sponges mir-15b-5p) | _ | _ | _ | SYBR Green kit | (6) |
| Colorectal cancer | 23 pairs of tumor tissues and ANCTs TCGA database | downregulated | _ | _ | _ | TransStart SYBR Green supermix | (9) |
| Colorectal cancer | 94 tumor tissues | downregulation in SIRT1 which suppresses mir-15b-5p transcription via AP-1 | _ | _ | _ | _ | (8) |
| 110 pairs of tumor tissues and ANCTs TCGA database: MSS CRC samples | downregulated | _ | _ | _ | _ | (10) | |
| GEPIA database | upregulation of CERS6-AS1 (which sponges mir-15b-5p) | _ | _ | _ | _ | (11) | |
| Gastric cancer | 40 pairs of tumor tissues and ANCTs 100 patients and 100 healthy controls | upregulated | _ | _ | degree of tumor invasion and lymph node metastasis and distant metastasis | PrimeScript™ RT reagent kit | (12) |
| Head and neck cancer squamous cell carcinomas | 43 HNSCC patient in explorative phase | downregulated | Shorter locoregional RFS | miR-15b-5p was found to be an independent predictive factor of LRC in HNSCC patients. | _ | TaqMan stem-loop | (30) |
| 51 HNSCC patient in validation phase | |||||||
| Hepatocellular carcinoma | TCGA and GEO databases | upregulated | _ | _ | _ | _ | (31) |
| 991 HCC and 456 adjacent non-HCC tissue samples | |||||||
| GEO database (GSE36411: 42 pairs of tumor tissues and ANCTs) | Upregulation of OIP5 (a target of miR-15b-5p) | _ | _ | _ | _ | (12) | |
| 46 pairs of tumor tissues and ANCTs | downregulated | _ | _ | _ | SYBR Green | (14) | |
| Phase I: 6 pairs of tumor tissues and ANCTs (from 6 HCC patients) | Overexpression in tumor tissues and preoperative plasmas, and downregulation in postoperative plasma | _ | _ | _ | ALL-in-One™ miRNA qRT-PCR Detection Kit | (32) | |
| Phase II: 10 patients | |||||||
| Phase III: 37 HCC patients, 29 cirrhosis patients, and 31 healthy controls | |||||||
| 28 pairs of tumor tissues and ANCTs | upregulated | _ | _ | _ | SYBR Premix Ex Taq II on an FTC-3000TM System | (15) | |
| Hepatocellular carcinoma (HBV-related type) | GEO database GSE27462 (5 pairs of tumor tissues and ANCTs) GSE76903 (20 pairs of tumor tissues and ANCTs) GSE121248 (70 pairs of tumor tissues and ANCTs) | upregulated | Poorer OS | _ | _ | _ | (33) |
| Liver cancer | 69 pairs of tumor tissues and ANCTs | upregulated | Poorer OS | _ | TNM stage and tumor capsular infiltration | SYBR Premix Ex Taq | (14) |
| Neuroblastoma | Two cohort: | downregulated | Poorer OS | _ | _ | SYBR green mix (Bio-Rad) for mRNA expression or TaqMan Universal Fast PCR master mix | (17) |
| 88 NB patients and 105 NB patients | |||||||
| 46 neuroblastoma samples and 28 normal tissues | downregulated | _ | _ | _ | _ | (18) | |
| Non-small cell lung cancer | 37 pairs of tumor tissues and ANCTs | downregulated | _ | _ | _ | _ | (19) |
| Oral squamous cell carcinoma | TCGA database | upregulated | Poorer OS | _ | tumor stage, TNM stage, and tumor metastasis | SYBR Premix Ex Taq II | (20) |
| 37 pairs of tumor tissues and ANCTs | |||||||
| Ovarian cancer | TCGA and genotype-tissue expression (GTEx) databases | downregulation in TTN-AS1 which sponges mir-15b-5p | _ | _ | _ | _ | (24) |
| Prostate cancer | TCGA database: | upregulated | _ | _ | age and Gleason score of patients with PCa | _ | (25) |
| 495 patients and 52 pairs of tumor tissues and ANCTs | |||||||
| Squamous cell carcinoma | 10 patients and 30 healthy controls | downregulated | _ | _ | _ | _ | (34) |
| Thyroid carcinoma | Cancer Genome Atlas project database: 509 patients and 58 healthy controls | downregulated | Poorer OS | _ | _ | _ | (27) |
Role of miR-15b-5p in Non-Malignant Conditions
Cell Line Studies
In vitro experiments in vascular smooth muscle cells (VSMCs) have shown that up-regulation of miR-15b-5p suppresses cell proliferation and induces apoptosis, while its knock down leads to opposite results. These effects are possibly mediated through suppression of ACSS2. Transfection of these cells with miR-15b-5p mimic or inhibitor has led to down-regulation and up-regulation of ACSS2 and PTGS2, respectively. Taken together, miR-15b-5p may increase apoptosis of aortic VSMCs and suppress their proliferation through influencing ACSS2/PTGS2 axis, thus participating in the pathoetiology of abdominal aortic aneurysm (35).
miR-15b-5p has also been shown to mediate the anti-amyloid effect of curcumin in an in vitro model of Alzheimer’s disease through influencing expression of the amyloid precursor protein (36). Moreover, the antiangiogenic effect of isopimpinellin has been attributed to its impact on induction of miR-15b-5p expression and subsequent down-regulation of angiogenic stimulators (37).
In addition, miR-15b-5p has been shown to mediate the effects of LINC00473 in cerebral I/R injury. Experiments in a cellular model of cerebral I/R injury has shown down-regulation of LINC00473 in these cells. Up-regulation of this lncRNA has reversed the effects of oxygen glucose deprivation/reperfusion on cell viability and apoptosis as well as ROS levels. Mechanistically, LINC00473 acts as a molecular sponge for miR-15b-5p and miR-15a-5p and regulates expression of SRPK1 (38). Table 4 shows summary of cell line studies on the role of miR-15b-5p in non-malignant conditions.
Table 4.
Summary of cell line studies on the role of miR-15b-5p in non-malignant conditions (Δ, knock-down or deletion; DOX, doxorubicin; H2S, Hydrogen sulfide; HG, High glucose; SHF, secondary hair follicle; ER, endoplasmic reticulum; EVs, extracellular vesicles).
| Disease type | Interactions | Cell line | Function | Reference |
|---|---|---|---|---|
| Abdominal aortic aneurysm | ACSS2 and PTGS2 | Human aortic VSMCs (T/G HA-VSMC cell line) | ↑↑ miR-15b-5p: ↓ proliferation and ↑ apoptosis of aortic VSMCs via targeting the ACSS2/PTGS2 axis | (35) |
| Alzheimer’s disease | amyloid precursor protein and amyloid-β | swAPP695-HEK293 cells and HEK293 | Curcumin treatment: ↑ mir-15b-5p and ↓ amyloid precursor protein and ↓ amyloid-β | (36) |
| Angiogenesis | _ | Human Umblical Vein Endothelial Cell (HUVEC) | Isopimpinellin: ↓ proliferation, ↓ invasion, ↓ migration, and tube formation via increasing mir-15b-5p levels and decreasing angiogenic stimulators | (37) |
| Asthma | YAP1 | ASM cells | ↑↑ miR-15b-5p: ↓ proliferation, migration, inflammatory response, and ECM deposition of TNF-α-induced ASM cells | (39) |
| Atherosclerosis | circCHFR and GADD45G | HUVECs | Upregulation of miR-15b-5p was found to reduce apoptosis, proinflammatory cytokine secretion, and improved cell survival via targeting GADD45G. | (40) |
| Cerebral I/R injury | LINC00473, SRPK1 | Neuro-2a (N2a) cells | ↑↑ LINC00473 (which sponges mir-15b-5p): ↑ cell viability, ↓ apoptosis and ↓ ROS level induced by OGD/R | (38) |
| Clopidogrel-induced liver injury | TLK1 | HepG2 cells | Clopidogrel treatment: ↓ miR-15b and its target TLK1, which shows other molecules are involved in the regulation of TLK1 expression as a result of exposure to clopidogrel. | (41) |
| Coronary artery disease | AKT3 | Human umbilical vein endothelial cells (HUVECs) | ↑↑ miR-15b-5p: ↓ migration and ↓ proliferation of endothelial cells | (42) |
| Δ miR-15b-5p: ↑ migration and ↑ proliferation of endothelial cells | ||||
| Coronary atherosclerotic heart disease | MALAT1 and MAPK1, mTOR signaling pathway | HEK 293T cells | Δ MALAT1 (which sponges mir-15b-5p): ↑ cell viability, ↑ autophagy and ↓ development of CAD | (43) |
| Dexamethasone induced steatosis | ENST00000608794, PDK4 | dexamethasone treated HepG2 cell lines | Δ ENST00000608794 (which sponges miR-15b-5p): ↓ dexamethasone induced steatosis | (44) |
| ↑↑ miR-15b-5p: ↓ dexamethasone induced steatosis | ||||
| Diabetic foot ulcers | IKBKB and WEE1 | human keratinocytes | S. aureus: ↑ miR-15b-5p levels | (45) |
| ↑↑ miR-15b-5p: ↓ DNA repair and ↓ inflammatory response | ||||
| Diabetic nephropathy | JNK and Akt/mTOR pathway | HK-2 and HKC-5 cells | High glucose treatment: ↓ expression of miR-15b-5p in HK-2 cells | (46) |
| ↑↑ miR-15b-5p: ↓ High glucose-induced apoptosis in HK-2 cells | ||||
| BCL-2 | Mouse MCs (CRL1927) and human embryonic kidney (HEK) 293 cells | High glucose treatment: ↑ miR-15b-5p expression in mouse MCs, so ↑ mouse MC apoptosis by targeting BCL-2 | (47) | |
| Diabetic nephropathy | CDKN2B-AS1 and WNT2B | HMCs | Δ miR-15b-5p: ↑ viability, ↑ cell cycle progression, ↑ ECM accumulation, ↑ inflammatory response | (48) |
| PDK4 and VEGFA | MPC5 cells | High-glucose treatment: ↓ mir-15b-5p in podocytes | (49) | |
| ↑↑ EVs-derived miR-15b-5p: ↓ MPC5 cell apoptosis and ↓ inflammation via reducing PDK4 and VEGFA | ||||
| Diabetic retinopathy | circ_001209, COL12A1 | human retinal vascular endothelial cells (HRVECs) | High-glucose treatment: ↑ circ_001209 (which sponges miR-15b-5p) levels, thus ↑ COL12A1 (a target of miR-15b-5p) levels | (50) |
| ↑↑ miR-15b-5p: ↓ invasion, ↓ migration and ↓ tubular formation induced by HG | ||||
| Diabetic retinopathy | TNFα, SOCS3 and IGFBP-3 l | Human REC | miR-15b was found to have a role in the inhibition of insulin resistance by decreased TNFα and SOCS3 signaling and increased IGFBP-3 levels, resulting in REC protection from hyperglycemia-induced apoptosis. | (51) |
| DOX-induced cardiotoxicity | Bmpr1a | H9c2 cardiomyocytes | ↑↑ miR-15b-5p: ↑ DOX-induced apoptosis, ↑ oxidative stress and ↑ mitochondria damage | (52) |
| Endoplasmic reticulum stress mediated neurons apoptosis | Rab1A | HT22 cells | Sevoflurane exposure: ↓ cell viability, and ↑ apoptosis and ↑ ER stress via increasing mir-15b-5p levels, thus inhibiting Rab1A | (53) |
| Fracture | HCAR, VEGF and MMP13 | BMSCs | HCAR sponges miR-15b-5p to regulate VEGF and MMP13, so induces endochondral bone repair in hypertrophic chondrocyte. | (54) |
| High glucose-induced podocyte injury | Sema3A | mouse podocytes | ↑↑ mir-15b-5p: ↓ apoptosis, ↓ oxidative stress, and ↓ inflammatory response | (55) |
| Inductive property of DPCs in cashmere goat | lncRNA-599547, Wnt10b | dermal papilla cells (DPCs) of passage 3 of cashmere goat SHF | lncRNA-599547 (which sponges miR-15b-5p) showed strongly high levels in dermal papilla of cashmere goat SHF. | (56) |
| Myocardial infarction | circ-Ttc3, Arl2 | cardiomyocytes and cardiac fibroblasts | High levels of f circ-Ttc3 (which sponges miR-15b) was found to protect cardiomyocytes against ischemia-related apoptotic death. | (57) |
| Necroptosis and inflammation | TGFBR3, TGF-β pathway | HD11 and DT40 | H2S exposure: ↑ oxidative stress and activates the TGF-β pathway by regulating miR-15b-5p/TGFBR3 axis miR-15b-5p is upregulated in H2S-induced necroptosis and inflammation. | (58) |
| Obstructive sleep apnea | PTGS1-NF-κB-SP1 signaling | human THP-1, HUVEC, and SH-SY5Y cell lines | Δ miR-15b-5p: ↑ IHR-induced oxidative stress and ↑ MAOA hyperactivity via targeting PTGS1-NF-κB-SP1 signaling in OSA patients | (59) |
| Osteoarthritis | LINC00662, GPR120 | rat chondrocytes | LINC00662 is downregulated in osteoarthritis, so mir-15b-5p is upregulated and GPR120 is suppressed, thus inflammatory responses and apoptosis are induced. | (60) |
| Parkinson’s disease | LINC00943 and RAB3IP | SK-N-SH cells | Δ LINC00943 (which sponges miR-15b-5p): ↓ MPP+-caused decrease of cell viability so reduced MPP+-induced neuronal damage | (61) |
| SNHG1 and GSK3β | 1-methyl-4-phenylpyridinium ion (MPP+)-treated SH-SY5Y cells | ↑↑ SNHG1 (which sponges miR-15b-5p): ↑ MPP+ -induced cellular toxicity, ↓ cell viability via miR-15b-5p/GSK3β axis | (62) | |
| Akt3 | 293T cells and the human dopaminergic neuroblastoma SH-SY5Y cells | ↑↑ miR-15b-5p: ↑ apoptosis by targeting Akt3 in an MPP+-induced PD cell model | (63) | |
| SNHG1, SIAH1 | SH-SY5Y | ↑↑ miR-15b-5p: ↓ α-synuclein aggregation and ↓ apoptosis via targeting SIAH1 | (64) | |
| Severe acute respiratory syndrome coronavirus 2 | viral RdRp | _ | ↑↑ miR-15b-5p: ↓ viral infection and ↓ proliferation by targeting the RNA template component of SARS-CoV-2 RdRp | (65) |
| Skeletal muscle atrophy | lncIRS1 and IRS1 | DF‐1 cells | LncIRS1 (which sponges mir-15b-5p) was found to regulate myoblast proliferation and differentiation in vitro via increasing IRS1. | (66) |
| Tendon injury | circRNA-Ep400, FGF-1/7/9 | 293 T cells, fibroblasts and tenocytes | ↑↑ M2 macrophage-derived circRNA-Ep400 (which sponges mir-15b-5p): ↑ fibrosis, ↑ proliferation, and ↑ migration | (67) |
↑ Up-regulation; ↓ Down-regulation.
Animal Studies
Animal studies have highlighted the role of miR-15b-5p in different cellular processes and disorders such as angiogenesis, coronary artery disease, diabetic nephropathy, diabetic retinopathy, myocardial I/R injury, necroptosis and inflammation, Parkinson’s disease and trachea inflammatory injury ( Table 5 ). For instance, overexpression of miR-15b-5p has considerably suppressed arteriogenesis and angiogenesis in animal models through targeting AKT3. Remarkably, siRNA-mediated silencing of AKT3 has inhibited arteriogenesis and the rescue of blood perfusion following femoral ligation in animals (42). Another animal study has shown that silencing of the miR-15b-5p-sponging lncRNA MALAT1 decreases atherosclerotic process (43). miR-15b-5p has also been shown to affect diabetic nephropathy and retinopathy in animals. Assessment of transcriptome of high glucose-exposed mouse mesangial cells has shown the effect of miR-15b-5p and its downstream target BCL-2 in regulation of high glocose-induced apoptosis. Besides, db/db mice has been shown to have higher levels of urinary miR-15b-5p (47).
Table 5.
Summary of studies on the role of miR-15b-5p in non-malignant conditions (Δ, knock-down or deletion; MDA, malondialdehyde; ECs, endothelial cells; ACR, Albumin-to-Creatinine Ratio; H2S, Hydrogen sulfide).
| Disease Type | Animal models | Results | Reference |
|---|---|---|---|
| Angiogenesis | zebrafish embryos | Isopimpinellin: ↓ intersegmental vessels | (37) |
| Coronary artery disease | 8-10-week-old male C57BL/6 mice Mice were received agomiR-15b, agomiR-NC, or cholesterol-conjugated AKT3 siRNA by multi-point injections. | miR-15b-5p expression was decreased, because of a reduced expression in EC layer of collaterals and miR-15b-5p was mainly derived from ECs. | (42) |
| ↑↑ miR-15b-5p: ↓ arteriogenesis and ↓ angiogenesis | |||
| Coronary atherosclerotic heart disease | Six-week old male ApoE−/−mice | Δ MALAT1 (which sponges mir-15b-5p): ↓ atherosclerosis | (43) |
| Diabetic nephropathy | 5 db/m mice and 5 db/db mice | Higher urine miR-15b-5p levels were found in db/db mice. | (47) |
| Urinary EV miR-15b-5p levels were positively associated with urinary ACR. | |||
| Diabetic retinopathy | 80 Sprague–Dawley male rats | With increased levels of circ_001209 (which sponges miR-15b-5p) retinal thickness was thinner in diabetic rats, and apoptosis was enhanced. | (68) |
| Myocardial ischemia reperfusion injury | 6-8 week-old male C57/B6 mice | Δ mir-15b-5p: ↓ arrhythmia, infarct extent and apoptosis, ↓ MDA content in the myocardial tissue by increasing levels of KCNJ2 (a target of mir-15b-5p) | (69) |
| Necroptosis and inflammation | 40 one-day-old Ross 308 male broilers | H2S exposure: ↑ necroptosis and inflammation | (58) |
| Parkinson’s disease | five-week-old male C57BL/6 mice | Δ miR-15b-5p: ↓ MPTP-induced apoptosis by regulating Akt3 | (63) |
| Skeletal muscle atrophy | 1‐day‐old chicks | LncIRS1 (which sponges mir-15b-5p) was found to regulate muscle mass and muscle fibre cross‐sectional area. | (66) |
| Trachea inflammatory injury | Eighty one-day-old Ross 308 broilers divided into two groups (control group and H2S group) | H2S exposure: ↑ mir-15b-5p miR-15b-5p reduced ATF2 levels to mediate METs release, which induces trachea inflammatory damage | (70) |
↑ Up-regulation; ↓ Down-regulation.
Human Studies
Different experiments in human samples obtained from patients with acute mountain sickness, asthma-COPD overlap, coronary artery disease, diabetic foot ulcers, diabetic nephropathy, late pulmonary complications, obstructive sleep apnea and Parkinson’s disease have shown dysregulation of miR-15b-5p levels ( Table 6 ).
Table 6.
Summary of human studies on the role of miR-15b-5p in non-malignant conditions (CAD, coronary atherosclerotic heart disease; CCC, coronary collateral circulation; ACR, albumin-to-creatinine ratio; eGFR, Estimated Glomerular Filtration Rate; AMS, Acute mountain sickness; COPD, chronic obstructive pulmonary disease; ACO, asthma-COPD overlap; DN, diabetic nephropathy; OSA, obstructive sleep apnea; CPAP, continuous positive airway pressure; DFU, Diabetic foot ulcers; FS, foot skin).
| Disease type | Numbers of clinical samples | Expression (Tumor vs. Normal) | Clinicopathologic characteristics of patients | Method by which RNA was detected | Reference |
|---|---|---|---|---|---|
| Acute mountain sickness | 124 healthy men (75 AMS+ group and 49 AMS– group) | upregulated in AMS- group | _ | iQ™5 Real-Time PCR Detection System | (71) |
| Alzheimer’s disease | 50 AD patients and 50 healthy controls | no significant differences | _ | _ | (72) |
| Asthma-COPD overlap | Cohort 1: 6 patients with ACO and 6 patients with asthma | downregulated in ACO patients | _ | miScript SYBR Green PCR Ki | (73) |
| Cohort 2; 30 patients with asthma, 30 patients with COPD, or 30 patients with ACO | |||||
| Atherosclerosis | 30 patients with atherosclerosis and 30 healthy controls | downregulated | _ | SYBR Green PCR kit | (40) |
| Coronary artery disease | 5 patients with poor CCC and 5 patients with good CCC | upregulated in patients with poor CCC | miR-15b-5p was associated with insufficient coronary collateral artery function. | SYBR Premix Ex Taq qRT-PCR assays | (42) |
| 20 patients with poor CCC and 18 patients with good CCC and 18 healthy controls | |||||
| Coronary atherosclerotic heart disease | GEO database (GSE18608: 10 CAD patients and 4 healthy controls | downregulated | _ | SYBR green | (43) |
| 5 CAD patients and 5 healthy controls | |||||
| Diabetic foot ulcers | 12 DFU and 12 FS specimens | upregulated in DFU | _ | PerfeCTa® SYBR® Green SuperMix | (45) |
| 6 DFU and 6 FS specimens | |||||
| (GEO database GSE80178) | |||||
| Diabetic nephropathy | 85 type 2 diabetic patients and 39 healthy controls | upregulated | Urinary EV miR-15b-5p levels were found to be positively associated with urinary ACR, negatively associated with eGFR, and correlated with rapid decline in kidney function in humans. | _ | (47) |
| 34 DN patients and 34 healthy controls | downregulated | _ | SYBR Green | (48) | |
| Late pulmonary complications | 20 Sulfur mustard-exposed individuals and 20 healthy controls | no differences | _ | _ | (74) |
| Obstructive sleep apnea | Discovery cohort: 16 OSA Patients and 8 healthy controls | downredulated in OSA patients | miR-15b-5p was negatively associated with an apnea hypopnea index | NGS (Illumina MiSeq platform) and SYBR Green PCR kit | (59) |
| Validation cohort: 20 Primary Snoring, 45 Treatment-Naïve | |||||
| OSA Patients, and 13 OSA Patients on CPAP | |||||
| Parkinson’s disease | 10 patients and 5 healthy controls | upregulated | _ | ABI PRISM® 7500 Sequence Detection System | (63) |
This miRNA might participate in the pathoetiology of acute mountain sickness. Levels of miR-15b-5p in the saliva have been found to be higher in individuals being resistant to this condition compared to susceptible ones. Combination of levels of miR-134-3p and miR-15b-5p could discriminate between these two groups. Thus, salivary levels of miR-134-3p and miR-15b-5p have been suggested as non-invasive markers for prediction of acute mountain sickness prior to exposure to high altitude (71).
Although in vitro studies indicated possible role of miR-15b-5p in the pathogenesis of Alzheimer’s disease (36), serum levels of miR-15b-5p were not significantly different between patients with Alzheimer’s disease and healthy subjects (72).
miR-15b-5p has been among miRNA having lower expression in asthma-COPD overlap patients. This miRNA can distinguish between asthma-COPD overlap patients and individuals with either asthma or COPD. In fact, miR-15b-5p has been shown to be superior to other miRNAs in separation of patients with asthma-COPD overlap from similar conditions (73).
In some conditions, dysregulation of this miRNA has been associated with clinicopathological parameters. For instance, in patients with coronary artery disease, dysregulation of miR-15b-5p has been associated with insufficient coronary collateral artery function (42). Moreover, in diabetic nephropathy, Urinary exosomal levels of miR-15b-5p have been positively associated with urinary albumin-to-creatinine ratio, negatively associated with eGFR, and correlated with speedy failure in kidney function (47).
Discussion
miR-15b-5p is an example of miRNAs with dual roels in the carcinogenesis. While it is a putative oncogenic miRNA in bladder cancer, breast cancer, gastric cancer, oral squamous cell carcinoma and prostate cancer, it has been found to be down-regulated in head and neck cancer squamous cell carcinomas, neublastoma and thyroid cancer samples as compared with corresponding non-cancerous samples (75). Moreover, in colorectal cancer and hepatocellular carcinoma, different studies have reported contradictory results.
This miRNA also participates in the pathogenesis of several non-malignant conditions, such as abdominal aortic aneurysm, Alzheimer’s disease, Parkinson’s disease, cerebral I/R injury, coronary artery disease, dexamethasone induced steatosis, diabetic complications and doxorubicin-induced cardiotoxicity.
miR-15b-5p has been shown to be sponged by several lncRNAs, namely MAGI2-AS3, H19, SNHG1, SNHG16, TTN‐AS1, PVT1, FENDRR, SSTR5−AS1, MALAT1, ENST00000608794, CDKN2B-AS1, LINC00473, LINC00662, LINC00943, LncRNA-599547 and CDKN2B-AS1 as well as the circular RNA Circ_001209. Thus, lncRNAs and circRNAs can affect expression of this miRNA. Other possible regulatory mechanisms for modulation of expression levels of miR-15b-5p should be clarified in future studies.
NF-κB, STAT3, AKT/mTORC1, CDC42/PAK1 and β-catenin signaling pathways are signaling pathways that mediate the effects of miR-15b-5p in the carcinogenesis. Notably, this miRNA could regulate response of cancer cells to 5-FU and anti-PD-1 drugs. Thus, therapeutics modalities affecting expression of miR-15b-5p can be considered as possible ways to combat resistance to anti-cancer agents. Evidence from in vitro and in vivo studies indicates that therapeutic intervention with miR-15-5p levels can significantly influence pathological processes. Moreover, disease-associated abnormal expression pattern of this miRNA in the affected tissues potentiates it as a diagnostic biomarkers. Particularly, in bladder cancer, breast cancer, head and neck cancers, liver cancer, neuroblastoma, oral squamous cell carcinoma and thyroid cancer, abnormal expression of miR-15-5p has been associated with poor clinical outcomes indicating the role of this miRNA as a prognostic biomarker. It is expected that therapeutic modalities affect expression of miR-15-5p and amend disease-associated dysregulation of this miRNA. Therefore, expression pattern of miR-15-5p can be used to monitor disease status and response to therapeutic options.
Since both oncogenic and tumor suppressor roles have been reported for miR-15-5p, different miR-15-5p-targeting therapeutic targets can been applied in the field of cancer therapy. In tissues that this miRNA exerts tumor suppressor roles, exogenous miR-15-5p can be used to inhibit cell proliferation or induce apoptosis. This goal can be achieved by administration of chemically synthesized miR-15-5p mimics to induce expression of endogenous mature double-stranded miR-15-5p to restore function of this miRNA. Viral vectors expressing miR-15-5p are appropriate vectors for delivery of this miRNA to tumor cells. On the other hand, when miR-15-5p exerts oncogenic roles, antisense oligonucleotides and miR-15-5p sponges can be used for suppression of level of this miRNA. Although these strategies are putative therapeutic modalities for treatment of cancer, they have not been applied in the clinical setting yet.
Conclusion
While the prognostic impact of dysregulation of miR-15b-5p has been confirmed in different types of cancer, there is no explicit evidence for application of this miRNA as a diagnostic marker in cancers. Since miRNAs dysregulation in the circulation provides a potential way for early non-invasive diagnosis of cancer, future studies should focus on evaluation of expression levels of miR-15b-5p in different biofluids during the course of cancer to provide insights into diagnostic role of this miRNA in cancer.
Author Contributions
SG-F wrote the manuscript and revised it. MT supervised and designed the study. TK, HJ, MH and BH collected the data and designed the figures and tables. All authors read and approved the submitted version.
Funding
This study was financially supported by Grant from Medical School of Shahid Beheshti University of Medical Sciences.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Hussen BM, Hidayat HJ, Salihi A, Sabir DK, Taheri M, Ghafouri-Fard S. MicroRNA: A Signature for Cancer Progression. Biomed Pharmacother = Biomed Pharmacother (2021) 138:111528. doi: 10.1016/j.biopha.2021.111528 [DOI] [PubMed] [Google Scholar]
- 2. Ghafouri-Fard S, Shaterabadi D, Abak A, Shoorei H, Bahroudi Z, Taheri M, et al. An Update on the Role of miR-379 in Human Disorders. Biomed Pharmacother = Biomed Pharmacother (2021) 139:111553. doi: 10.1016/j.biopha.2021.111553 [DOI] [PubMed] [Google Scholar]
- 3. Ha M, Kim VN. Regulation of microRNA Biogenesis. Nat Rev Mol Cell Biol (2014) 15(8):509–24. doi: 10.1038/nrm3838 [DOI] [PubMed] [Google Scholar]
- 4. Wang F, Zu Y, Zhu S, Yang Y, Huang W, Xie H, et al. Long Noncoding RNA MAGI2-AS3 Regulates CCDC19 Expression by Sponging miR-15b-5p and Suppresses Bladder Cancer Progression. Biochem Biophys Res Commun (2018) 507(1-4):231–5. doi: 10.1016/j.bbrc.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 5. Wu B, Liu G, Jin Y, Yang T, Zhang D, Ding L, et al. miR-15b-5p Promotes Growth and Metastasis in Breast Cancer by Targeting HPSE2. Front Oncol (2020) 10:108. doi: 10.3389/fonc.2020.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu Y, Zhang X, Wang L, Zhu X, Xia Z, Xu L, et al. FENDRR Suppresses Cervical Cancer Proliferation and Invasion by Targeting miR-15a/B-5p and Regulating TUBA1A Expression. Cancer Cell Int (2020) 20(1):1–10. doi: 10.1186/s12935-020-01223-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gasparello J, Gambari L, Papi C, Rozzi A, Manicardi A, Corradini R, et al. High Levels of Apoptosis are Induced in the Human Colon Cancer HT-29 Cell Line by Co-Administration of Sulforaphane and a Peptide Nucleic Acid Targeting miR-15b-5p. Nucleic Acid Ther (2020) 30(3):164–74. doi: 10.1089/nat.2019.0825 [DOI] [PubMed] [Google Scholar]
- 8. Sun L-N, Zhi Z, Chen L-Y, Zhou Q, Li X-M, Gan W-J, et al. SIRT1 Suppresses Colorectal Cancer Metastasis by Transcriptional Repression of miR-15b-5p. Cancer Lett (2017) 409:104–15. doi: 10.1016/j.canlet.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 9. Zhao C, Zhao Q, Zhang C, Wang G, Yao Y, Huang X, et al. miR-15b-5p Resensitizes Colon Cancer Cells to 5-Fluorouracil by Promoting Apoptosis via the NF-κb/XIAP Axis. Sci Rep (2017) 7(1):1–12. doi: 10.1038/s41598-017-04172-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, et al. Blocking IL-17A Enhances Tumor Response to Anti-PD-1 Immunotherapy in Microsatellite Stable Colorectal Cancer. J Immunother Cancer (2021) 9(1):1–14. doi: 10.1136/jitc-2020-001895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao SY, Wang Z, Wu XB, Zhang S, Chen Q, Wang DD, et al. CERS6-AS1 Contributes to the Malignant Phenotypes of Colorectal Cancer Cells by Interacting With miR-15b-5p to Regulate SPTBN2. Kaohsiung J Med Sci (2022) 38(5):403–414. doi: 10.1002/kjm2.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao C, Li Y, Chen G, Wang F, Shen Z, Zhou R. Overexpression of miR-15b-5p Promotes Gastric Cancer Metastasis by Regulating PAQR3. Oncol Rep (2017) 38(1):352–8. doi: 10.3892/or.2017.5673 [DOI] [PubMed] [Google Scholar]
- 13. Gasparello J, Papi C, Zurlo M, Gambari L, Rozzi A, Manicardi A, et al. Treatment of Human Glioblastoma U251 Cells With Sulforaphane and a Peptide Nucleic Acid (PNA) Targeting miR-15b-5p: Synergistic Effects on Induction of Apoptosis. Molecules (2022) 27(4):1299. doi: 10.3390/molecules27041299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou Y, Fan R-G, Qin C-L, Jia J, Wu X-D, Zha W-Z. LncRNA-H19 Activates CDC42/PAK1 Pathway to Promote Cell Proliferation, Migration and Invasion by Targeting miR-15b in Hepatocellular Carcinoma. Genomics (2019) 111(6):1862–72. doi: 10.1016/j.ygeno.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 15. Yang Y, Hou N, Wang X, Wang L, Se C, He K, et al. miR-15b-5p Induces Endoplasmic Reticulum Stress and Apoptosis in Human Hepatocellular Carcinoma, Both In Vitro and In Vivo, by Suppressing Rab1A. Oncotarget (2015) 6(18):16227. doi: 10.18632/oncotarget.3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu F, Lin Y, Tan G, Ai M, Gong H, Liu W, et al. Tumor-Derived Exosomal microRNA-15b-5p Augments Laryngeal Cancer by Targeting TXNIP. Cell Cycle (2022) 7:1–11. doi: 10.1080/15384101.2021.2022845 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Chava S, Reynolds CP, Pathania AS, Gorantla S, Poluektova LY, Coulter DW, et al. miR-15a-5p, miR-15b-5p, and miR-16-5p Inhibit Tumor Progression by Directly Targeting MYCN in Neuroblastoma. Mol Oncol (2020) 14(1):180–96. doi: 10.1002/1878-0261.12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ge Y, Tan S, Bi J, Rao M, Yu Y, Tian L. SNHG16 Knockdown Inhibits Tumorigenicity of Neuroblastoma in Children via miR-15b-5p/PRPS1 Axis. NeuroReport (2020) 31(17):1225–35. doi: 10.1097/WNR.0000000000001537 [DOI] [PubMed] [Google Scholar]
- 19. Guo K, Qi D, Huang B. LncRNA MEG8 Promotes NSCLC Progression by Modulating the miR-15a-5p-miR-15b-5p/PSAT1 Axis. Cancer Cell Int (2021) 21(1):1–16. doi: 10.1186/s12935-021-01772-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu X, Dong Y, Song D. Inhibition of microRNA-15b-5p Attenuates the Progression of Oral Squamous Cell Carcinoma via Modulating the PTPN4/STAT3 Axis. Cancer Manage Res (2020) 12:10559. doi: 10.2147/CMAR.S272498 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Wang X, Guo H, Yao B, Helms J. miR-15b Inhibits Cancer-Initiating Cell Phenotypes and Chemoresistance of Cisplatin by Targeting TRIM14 in Oral Tongue Squamous Cell Cancer. Oncol Rep (2017) 37(5):2720–6. doi: 10.3892/or.2017.5532 [DOI] [PubMed] [Google Scholar]
- 22. Weng Y, Shen Y, He Y, Pan X, Xu J, Jiang Y, et al. The miR-15b-5p/PDK4 Axis Regulates Osteosarcoma Proliferation Through Modulation of the Warburg Effect. Biochem Biophys Res Commun (2018) 503(4):2749–57. doi: 10.1016/j.bbrc.2018.08.035 [DOI] [PubMed] [Google Scholar]
- 23. Cai Y, Yang Y, Zhang X, Ma Q, Li M. TRPM2-AS Promotes the Malignancy of Osteosarcoma Cells by Targeting miR-15b-5p/PPM1D Axis. Cell Cycle (2022) 8:1–16. doi: 10.1080/15384101.2022.2033414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miao S, Wang J, Xuan L, Liu X. LncRNA TTN-AS1 Acts as Sponge for miR-15b-5p to Regulate FBXW7 Expression in Ovarian Cancer. BioFactors (2020) 46(4):600–7. doi: 10.1002/biof.1622 [DOI] [PubMed] [Google Scholar]
- 25. Chen R, Sheng L, Zhang HJ, Ji M, Qian WQ. miR-15b-5p Facilitates the Tumorigenicity by Targeting RECK and Predicts Tumour Recurrence in Prostate Cancer. J Cell Mol Med (2018) 22(3):1855–63. doi: 10.1111/jcmm.13469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun F, Wu K, Yao Z, Mu X, Zheng Z, Sun M, et al. Long Noncoding RNA PVT1 Promotes Prostate Cancer Metastasis by Increasing NOP2 Expression via Targeting Tumor Suppressor MicroRNAs. OncoTargets Ther (2020) 13:6755. doi: 10.2147/OTT.S242441 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Zou J, Qian J, Fu H, Yin F, Zhao W, Xu L. MicroRNA−15b−5p Exerts its Tumor Repressive Role via Targeting GDI2: A Novel Insight Into the Pathogenesis of Thyroid Carcinoma. Mol Med Rep (2020) 22(4):2723–32. doi: 10.3892/mmr.2020.11343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lovat F, Fassan M, Gasparini P, Rizzotto L, Cascione L, Pizzi M, et al. miR-15b/16-2 Deletion Promotes B-Cell Malignancies. Proc Natl Acad Sci USA (2015) 112(37):11636–41. doi: 10.1073/pnas.1514954112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tölle A, Buckendahl L, Jung K. Plasma Mir−15b−5p and Mir−590−5p for Distinguishing Patients With Bladder Cancer From Healthy Individuals. Oncol Rep (2019) 42(4):1609–20. doi: 10.3892/or.2019.7247 [DOI] [PubMed] [Google Scholar]
- 30. Ahmad P, Sana J, Slávik M, Gurín D, Radová L, Gablo NA, et al. MicroRNA-15b-5p Predicts Locoregional Relapse in Head and Neck Carcinoma Patients Treated With Intensity-Modulated Radiotherapy. Cancer Genomics Proteomics (2019) 16(2):139–46. doi: 10.21873/cgp.20119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pan WY, Zeng JH, Wen DY, Wang JY, Wang PP, Chen G, et al. Oncogenic Value of microRNA−15b−5p in Hepatocellular Carcinoma and a Bioinformatics Investigation. Oncol Lett (2019) 17(2):1695–713. doi: 10.3892/ol.2018.9748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Y, Chen J, Liu Y, Li S, Huang P. Plasma miR-15b-5p, miR-338-5p, and miR-764 as Biomarkers for Hepatocellular Carcinoma. Med Sci Monit (2015) 21:1864. doi: 10.12659/MSM.893082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu J, Zhang J, Shan F, Wen J, Wang Y. SSTR5−AS1 Functions as a ceRNA to Regulate CA2 by Sponging Mir−15b−5p for the Development and Prognosis of HBV−Related Hepatocellular Carcinoma. Mol Med Rep (2019) 20(6):5021–31. doi: 10.3892/mmr.2019.10736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jin X, Chen Y, Chen H, Fei S, Chen D, Cai X, et al. Evaluation of Tumor-Derived Exosomal miRNA as Potential Diagnostic Biomarkers for Early-Stage Non–Small Cell Lung Cancer Using Next-Generation Sequencing. Clin Cancer Res (2017) 23(17):5311–9. doi: 10.1158/1078-0432.CCR-17-0577 [DOI] [PubMed] [Google Scholar]
- 35. Gan S, Mao J, Pan Y, Tang J, Qiu Z. Hsa−Mir−15b−5p Regulates the Proliferation and Apoptosis of Human Vascular Smooth Muscle Cells by Targeting the ACSS2/PTGS2 Axis. Exp Ther Med (2021) 22(5):1–8. doi: 10.3892/etm.2021.10642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu H-Y, Fu X, Li Y-F, Li X-L, Ma Z-Y, Zhang Y, et al. miR-15b-5p Targeting Amyloid Precursor Protein Is Involved in the Anti-Amyloid Eflect of Curcumin in Swapp695-HEK293 Cells. Neural Regen Res (2019) 14(9):1603. doi: 10.4103/1673-5374.255979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhagavatheeswaran S, Ramachandran V, Shanmugam S, Balakrishnan A. Isopimpinellin Extends Antiangiogenic Effect Through Overexpression of miR-15b-5p and Downregulating Angiogenic Stimulators. Mol Biol Rep (2021) 49(1):279–91. doi: 10.1007/s11033-021-06870-4. [DOI] [PubMed] [Google Scholar]
- 38. Yao B, Ye L, Chen J, Zhuo S, Lin H. LINC00473 Protects Against Cerebral Ischemia Reperfusion Injury via Sponging miR-15b-5p and miR-15a-5p to Regulate SRPK1 Expression. Brain Injury (2021) 35(11):1462–71. doi: 10.1080/02699052.2021.1972156 [DOI] [PubMed] [Google Scholar]
- 39. Zeng S, Cui J, Zhang Y, Zheng Z, Meng J, Du J. MicroRNA-15b-5p Inhibits Tumor Necrosis Factor Alpha-Induced Proliferation, Migration, and Extracellular Matrix Production of Airway Smooth Muscle Cells via Targeting Yes-Associated Protein 1. Bioengineered (2022) 13(3):5396–406. doi: 10.1080/21655979.2022.2036890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Y, Wang B. Circular RNA circCHFR Downregulation Protects Against Oxidized Low-Density Lipoprotein-Induced Endothelial Injury via Regulation of microRNA-15b-5p/Growth Arrest and DNA Damage Inducible Gamma. Bioengineered (2022) 13(2):4481–92. doi: 10.1080/21655979.2022.2032967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freitas RC, Bortolin RH, Lopes MB, Tamborlin L, Meneguello L, Silbiger VN, et al. Modulation of miR-26a-5p and miR-15b-5p Exosomal Expression Associated With Clopidogrel-Induced Hepatotoxicity in HepG2 Cells. Front Pharmacol (2017) 8:906. doi: 10.3389/fphar.2017.00906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu L-P, Zhou J-P, Zhang J-X, Wang J-Y, Wang Z-Y, Pan M, et al. MiR-15b-5p Regulates Collateral Artery Formation by Targeting AKT3 (Protein Kinase B-3). Arterioscler Thromb Vasc Biol (2017) 37(5):957–68. doi: 10.1161/ATVBAHA.116.308905 [DOI] [PubMed] [Google Scholar]
- 43. Zhu Y, Yang T, Duan J, Mu N, Zhang T. MALAT1/miR-15b-5p/MAPK1 Mediates Endothelial Progenitor Cells Autophagy and Affects Coronary Atherosclerotic Heart Disease via mTOR Signaling Pathway. Aging (Albany NY) (2019) 11(4):1089. doi: 10.18632/aging.101766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu F, Chen Q, Chen F, Wang J, Gong R, He B. The lncRNA ENST00000608794 Acts as a Competing Endogenous RNA to Regulate PDK4 Expression by Sponging miR-15b-5p in Dexamethasone Induced Steatosis. Biochim Biophys Acta (BBA)-Molecular Cell Biol Lipids (2019) 1864(10):1449–57. doi: 10.1016/j.bbalip.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 45. Ramirez HA, Pastar I, Jozic I, Stojadinovic O, Stone RC, Ojeh N, et al. Staphylococcus Aureus Triggers Induction of miR-15B-5P to Diminish DNA Repair and Deregulate Inflammatory Response in Diabetic Foot Ulcers. J Invest Dermatol (2018) 138(5):1187–96. doi: 10.1016/j.jid.2017.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shen H, Fang K, Guo H, Wang G. High Glucose-Induced Apoptosis in Human Kidney Cells was Alleviated by miR-15b-5p Mimics. Biol Pharm Bull (2019) 42(5):758–63. doi: 10.1248/bpb.b18-00951 [DOI] [PubMed] [Google Scholar]
- 47. Tsai Y-C, Kuo M-C, Hung W-W, Wu L-Y, Wu P-H, Chang W-A, et al. High Glucose Induces Mesangial Cell Apoptosis Through miR-15b-5p and Promotes Diabetic Nephropathy by Extracellular Vesicle Delivery. Mol Ther (2020) 28(3):963–74. doi: 10.1016/j.ymthe.2020.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang J, Yu Y, Fang Z, He H, Wang D, Teng J, et al. Long non-Coding RNA CDKN2B-AS1 Regulates High Glucose-Induced Human Mesangial Cell Injury via Regulating the miR-15b-5p/WNT2B Axis. Diabetol Metab Syndrome (2020) 12(1):1–11. doi: 10.1186/s13098-020-00618-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao T, Jin Q, Kong L, Zhang D, Teng Y, Lin L, et al. microRNA-15b-5p Shuttled by Mesenchymal Stem Cell-Derived Extracellular Vesicles Protects Podocytes From Diabetic Nephropathy via Downregulation of VEGF/PDK4 Axis. J Bioenerg Biomembr (2021) 54:17–30. doi: 10.1007/s10863-021-09919-y [DOI] [PubMed] [Google Scholar]
- 50. Li B, Zhang G, Wang Z, Yang Y, Wang C, Fang D, et al. C-Myc-Activated USP2-AS1 Suppresses Senescence and Promotes Tumor Progression via Stabilization of E2F1 mRNA. Cell Death Dis (2021) 12(11):1–14. doi: 10.1038/s41419-021-04330-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ye E-A, Steinle JJ. miR-15b/16 Protects Primary Human Retinal Microvascular Endothelial Cells Against Hyperglycemia-Induced Increases in Tumor Necrosis Factor Alpha and Suppressor of Cytokine Signaling 3. J Neuroinflammation (2015) 12(1):1–8. doi: 10.1186/s12974-015-0265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gao ZF, Ji XL, Gu J, Wang XY, Ding L, Zhang H. microRNA-107 Protects Against Inflammation and Endoplasmic Reticulum Stress of Vascular Endothelial Cells via KRT1-Dependent Notch Signaling Pathway in a Mouse Model of Coronary Atherosclerosis. J Cell Physiol (2019) 234(7):12029–41. doi: 10.1002/jcp.27864 [DOI] [PubMed] [Google Scholar]
- 53. Li Y, Xia H, Chen L, Zhang X. Sevoflurane Induces Endoplasmic Reticulum Stress Mediated Apoptosis Inmouse Hippocampal Neuronal HT22 Cells via Modulating miR-15b-5p/Rab1A Signaling Pathway. Int J Clin Exp Pathol (2017) 10(8):8270–80. [PMC free article] [PubMed] [Google Scholar]
- 54. Bai Y, Gong X, Dong R, Cao Z, Dou C, Liu C, et al. Long non-Coding RNA HCAR Promotes Endochondral Bone Repair by Upregulating VEGF and MMP13 in Hypertrophic Chondrocyte Through Sponging miR-15b-5p. Genes Dis (2020). doi: 10.1016/j.gendis.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fu Y, Wang C, Zhang D, Chu X, Zhang Y, Li J. miR-15b-5p Ameliorated High Glucose-Induced Podocyte Injury Through Repressing Apoptosis, Oxidative Stress, and Inflammatory Responses by Targeting Sema3A. J Cell Physiol (2019) 234(11):20869–78. doi: 10.1002/jcp.28691 [DOI] [PubMed] [Google Scholar]
- 56. Yin RH, Zhao SJ, Wang ZY, Zhu YB, Yin RL, Bai M, et al. LncRNA-599547 Contributes the Inductive Property of Dermal Papilla Cells in Cashmere Goat Through miR-15b-5p/Wnt10b Axis. Anim Biotechnol (2020) 14:1–15. doi: 10.1080/10495398.2020.1806860 [DOI] [PubMed] [Google Scholar]
- 57. Cai L, Qi B, Wu X, Peng S, Zhou G, Wei Y, et al. Circular RNA Ttc3 Regulates Cardiac Function After Myocardial Infarction by Sponging miR-15b. J Mol Cell Cardiol (2019) 130:10–22. doi: 10.1016/j.yjmcc.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 58. Qianru C, Xueyuan H, Bing Z, Qing Z, Kaixin Z, Shu L. Regulation of H2S-Induced Necroptosis and Inflammation in Broiler Bursa of Fabricius by the miR-15b-5p/TGFBR3 Axis and the Involvement of Oxidative Stress in This Process. J Hazard Mater (2021) 406:124682. doi: 10.1016/j.jhazmat.2020.124682 [DOI] [PubMed] [Google Scholar]
- 59. Chen Y-C, Hsu P-Y, Su M-C, Chen T-W, Hsiao C-C, Chin C-H, et al. MicroRNA Sequencing Analysis in Obstructive Sleep Apnea and Depression: Anti-Oxidant and MAOA-Inhibiting Effects of miR-15b-5p and miR-92b-3p Through Targeting PTGS1-NF-κb-SP1 Signaling. Antioxidants (2021) 10(11):1854. doi: 10.3390/antiox10111854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lu M, Zhou E. Long Noncoding RNA LINC00662-miR-15b-5p Mediated GPR120 Dysregulation Contributes to Osteoarthritis. Pathol Int (2020) 70(3):155–65. doi: 10.1111/pin.12875 [DOI] [PubMed] [Google Scholar]
- 61. Meng C, Gao J, Ma Q, Sun Q, Qiao T. LINC00943 Knockdown Attenuates MPP+-Induced Neuronal Damage via miR-15b-5p/RAB3IP Axis in SK-N-SH Cells. Neurol Res (2021) 43(3):181–90. doi: 10.1080/01616412.2020.1834290 [DOI] [PubMed] [Google Scholar]
- 62. Xie N, Qi J, Li S, Deng J, Chen Y, Lian Y. Upregulated lncRNA Small Nucleolar RNA Host Gene 1 Promotes 1-Methyl-4-Phenylpyridinium Ion-Induced Cytotoxicity and Reactive Oxygen Species Production Through miR-15b-5p/GSK3β Axis in Human Dopaminergic SH-SY5Y Cells. J Cell Biochem (2019) 120(4):5790–801. doi: 10.1002/jcb.27865 [DOI] [PubMed] [Google Scholar]
- 63. Zhu J, Xu X, Liang Y, Zhu R. Downregulation of microRNA-15b-5p Targeting the Akt3-Mediated GSK-3β/β-Catenin Signaling Pathway Inhibits Cell Apoptosis in Parkinson’s Disease. BioMed Res Int (2021) 2021:8814862. doi: 10.1155/2021/8814862 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64. Cory-Slechta D, Allen J, Conrad K, Marvin E, Sobolewski M. Developmental Exposure to Low Level Ambient Ultrafine Particle Air Pollution and Cognitive Dysfunction. Neurotoxicology (2018) 69:217–31. doi: 10.1016/j.neuro.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sato A, Ogino Y, Tanuma S-i, Uchiumi F. Human microRNA hsa-miR-15b-5p Targets the RNA Template Component of the RNA-Dependent RNA Polymerase Structure in Severe Acute Respiratory Syndrome Coronavirus 2. Nucleosides Nucleotides Nucleic Acids (2021) 40(8):790–7. doi: 10.1080/15257770.2021.1950759 [DOI] [PubMed] [Google Scholar]
- 66. Li Z, Cai B, Abdalla BA, Zhu X, Zheng M, Han P, et al. LncIRS1 Controls Muscle Atrophy via Sponging miR-15 Family to Activate IGF1-PI3K/AKT Pathway. J Cachexia Sarcopenia Muscle (2019) 10(2):391–410. doi: 10.1002/jcsm.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yu Y, Sun B, Wang Z, Yang M, Cui Z, Lin S, et al. Exosomes From M2 Macrophage Promote Peritendinous Fibrosis Posterior Tendon Injury via the MiR-15b-5p/FGF-1/7/9 Pathway by Delivery of circRNA-Ep400. Front Cell Dev Biol (2021) 1557. doi: 10.3389/fcell.2021.595911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang F, Zhang M. Circ_001209 Aggravates Diabetic Retinal Vascular Dysfunction Through Regulating miR-15b-5p/COL12A1. J Trans Med (2021) 19(1):1–12. doi: 10.1186/s12967-021-02949-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Niu S, Xu L, Yuan Y, Yang S, Ning H, Qin X, et al. Effect of Down-Regulated miR-15b-5p Expression on Arrhythmia and Myocardial Apoptosis After Myocardial Ischemia Reperfusion Injury in Mice. Biochem Biophys Res Commun (2020) 530(1):54–9. doi: 10.1016/j.bbrc.2020.06.111 [DOI] [PubMed] [Google Scholar]
- 70. Song N, Wang W, Wang Y, Guan Y, Xu S, Guo M-y. Hydrogen Sulfide of Air Induces Macrophage Extracellular Traps to Aggravate Inflammatory Injury via the Regulation of miR-15b-5p on MAPK and Insulin Signals in Trachea of Chickens. Sci Total Environment (2021) 771:145407. doi: 10.1016/j.scitotenv.2021.145407 [DOI] [PubMed] [Google Scholar]
- 71. Huang H, Dong H, Zhang J, Ke X, Li P, Zhang E, et al. The Role of Salivary miR-134-3p and miR-15b-5p as Potential Non-Invasive Predictors for Not Developing Acute Mountain Sickness. Front Physiol (2019) 10:898. doi: 10.3389/fphys.2019.00898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Poursaei E, Abolghasemi M, Bornehdeli S, Shanehbandi D, Asadi M, Sadeghzadeh M, et al. Evaluation of Hsa-Let-7d-P, Hsa-Let-7g-5p and hsa-miR-15b-5p Level in Plasma of Patients With Alzheimer’s Disease Compared to Healthy Controls. Psychiatr Genet (2022). doi: 10.1097/YPG.0000000000000303 [DOI] [PubMed] [Google Scholar]
- 73. Hirai K, Shirai T, Shimoshikiryo T, Ueda M, Gon Y, Maruoka S, et al. Circulating microRNA-15b-5p as a Biomarker for Asthma-COPD Overlap. Allergy (2021) 76(3):766–74. doi: 10.1111/all.14520 [DOI] [PubMed] [Google Scholar]
- 74. Salimi S, Noorbakhsh F, Faghihzadeh S, Ghaffarpour S, Ghazanfari T. Expression of miR-15b-5p, miR-21-5p, and SMAD7 in Lung Tissue of Sulfur Mustard-Exposed Individuals With Long-Term Pulmonary Complications. Iranian J Allergy Asthma Immunol (2019) 8:332–9. doi: 10.18502/ijaai.v18i3.1126 [DOI] [PubMed] [Google Scholar]
- 75. Taheri M, Noroozi R, Rakhshan A, Ghanbari M, Omrani MD, Ghafouri-Fard S. IL-6 Genomic Variants and Risk of Prostate Cancer. Urol J (2019) 16(5):463–8. doi: 10.22037/uj.v0i0.4543 [DOI] [PubMed] [Google Scholar]