Abstract
A novel type of 5-oxoprolinase was found in a cell extract of strain N-38A, which was later identified as Alcaligenes faecalis. The enzyme in the cell extract was purified to a homogeneous state with a yield of 16.6%. The molecular weight of the purified enzyme was estimated to be 47,000 by both sodium dodecyl sulfate-polyacrylamide gel electrophoresis and gel filtration, suggesting that the enzyme is a monomeric protein. The enzyme specifically catalyzed a decyclization of l-pyroglutamate without hydrolyzing ATP and also without any requirements for metal ions such as Mg2+ and K+. The optimal pH for the decyclization was 7.4. The reaction was reversible. The equilibrium constant of the reaction, Keq = [l-glutamate]/[l-pyroglutamate], was evaluated to be approximately 0.035, which indicates that the reaction tends to form l-pyroglutamate. The amino-terminal amino acid sequence of the enzyme was H-Glu-Pro-Arg-Leu-Asp-Thr-Ser-Gln-Leu-Tyr-Ala-Asp-Val-His-Phe-. No protein with a similar sequence was found in the DNASIS database. Based on these data, it was strongly suggested that the enzyme described here is a novel type of 5-oxoprolinase.
l-Monosodium glutamate is used as a flavor enhancer in food. It is recognized in food technology that the concentration of glutamate in a given product is very important for its quality when proteins are hydrolyzed enzymatically to produce seasonings such as soy sauce.
It is well known that in the early stages of brewing soy sauce, glutamine and glutamate are released from proteinous materials by the proteinase-peptidase system of soy sauce koji (25, 26). Glutamine is changed to glutamate by glutaminase from Aspergillus. On the other hand, nonenzymatic formation of pyroglutamate from glutamine and glutamate occurs during the production of soy sauce (11). In general, the formation of pyroglutamate is much faster than that of glutamate by glutaminase. The pyroglutamate has no flavor and cannot be converted to glutamine or glutamate in the fermentation of soy sauce. This conversion of glutamate to pyroglutamate eliminates the flavor potentiator.
5-Oxoprolinase (ATP hydrolyzing) (EC 3.5.2.9) catalyzes the ATP-dependent decyclization of l-pyroglutamate to form l-glutamate. These enzymes have been found in animal tissue (7, 18, 21, 22), plants (13), and microorganisms (9, 19, 23). 5-Oxoprolinase from rat kidneys was purified by Williamson and Meister (24). The molecular weight of the enzyme is 325,000. It requires ATP, Mg2+, and K+ for its activity. Its amino acid sequence was determined from the gene (8). 5-Oxoprolinase from Pseudomonas putida is also an ATP-, Mg2+-, and K+-dependent enzyme and is composed of two protein components (A and B) (19). Thus, the 5-oxoprolinases reported so far are ATP dependent. This feature has prevented the practical use of 5-oxoprolinase. If an ATP-independent 5-oxoprolinase were available, it would be useful in the food industry.
As reported previously (17), a large number of microorganisms have been screened for a strain which produces an ATP-independent 5-oxoprolinase, and strain N-38A was successfully isolated from a soil sample. In this paper, identification of N-38A and purification and characterization of this new type of 5-oxoprolinase are described.
MATERIALS AND METHODS
Materials.
Phenyl-Toyopearl 650M, DEAE-Toyopearl 650M, Butyl-Toyopearl 650M, and TSKgel G2000SW were from Tosoh Co., Tokyo, Japan. Sephadex G-100 was from Pharmacia Fine Chemical Co., Uppsala, Sweden. Shim-pack SCR-101H was from Shimadzu Co., Kyoto, Japan. l-Pyroglutamate, l-glutamate, and l-pyroglutamyl-l-alanine were from Peptide Institute, Inc. d-Pyroglutamate and l-glutamyl-l-alanine were from the Sigma Chemical Co., St. Louis, Mo. Bio-Lyte 3/10 for isoelectric focusing was from Bio-Rad Laboratories, Richmond, Calif.
Screening of microorganisms that produce 5-oxoprolinase.
The soil samples were collected mainly from rice fields, vegetable gardens, and forests in Kumamoto Prefecture. They were suspended in distilled water, and the suspension was streaked on an agar plate containing a medium of 1% dl-pyroglutamate, 0.1% yeast extract, 0.1% KH2PO4, 0.1% K2HPO4, 0.05% MgSO4 · 7H2O, and 0.01% FeSO4 · 7H2O (pH 7.0). The isolated microorganisms were cultivated aerobically at 30°C for 2 to 7 days in the medium described above. After cultivation, the culture broths were centrifuged (19,700 × g for 5 min at 4°C) and the resulting supernatants were used for the assay of decyclization with l-pyroglutamate as a substrate. On the other hand, the collected cells suspended in 0.1 M Tris-HCl buffer (pH 8.0) were disrupted with an Ultrasonic Disruptor UD-20 (output, 50 W) (TOMY Co., Tokyo, Japan) four times for 1 min at intervals of 30 s at 0 to 4°C. The resulting supernatants were also assayed for decyclization activity.
Cultivation conditions for enzyme production and preparation of cell extract.
5-Oxoprolinase was produced at 30°C by Alcaligenes faecalis N-38A in a 30-liter jar fermentor containing 20 liters of medium consisting of 0.3% meat extract, 1.0% polypeptone, 0.5% NaCl, and 0.015% Adecanol, with an initial pH of 7.0. A. faecalis N-38A was cultivated for 16 h. The cells were collected by centrifugation (13,200 × g for 10 min at 4°C) and washed with 0.1 M Tris-HCl buffer (pH 8.0). The washed cells (285 g [wet weight]) were suspended in 2 liters of 0.1 M Tris-HCl buffer (pH 8.0) and disrupted with a Dyno-mill type KDL (Willy A. Bachofen AG, Maschinenfabrik, Basel, Switzerland), using 0.1-mm-diameter glass bead at 0 to 4°C. The suspension of disrupted cells was centrifuged at 17,500 × g for 10 min at 4°C. The cell extract was used as the starting material for purification of the enzyme.
Assay of decyclization activity with l-pyroglutamate as a substrate.
Decyclization activity was assayed with l-pyroglutamate as a substrate by the method of Beutler (3). The reaction was done as follows. A reaction mixture (1.0 ml) containing 39 μmol of l-pyroglutamate and enzyme in 0.1 M Tris-HCl buffer (pH 8.0) was incubated at 30°C for 10 min. One unit of enzyme activity was defined as the amount of enzyme required to form 1 μmol of l-glutamate from l-pyroglutamate per min.
Assay of cyclization activity with l-glutamate as a substrate.
Cyclization activity against l-glutamate was assayed by high-pressure liquid chromatography (HPLC) Shim-pack SCR-101H column. The flow rate of the mobile phase (2 mM aqueous perchloric acid) was 1.0 ml/min. Pyroglutamate was detected with a CDD-6A conductivity detector (Shimadzu Co.). The concentration of pyroglutamate was calculated by the area normalization method. For the reaction, a mixture (1.0 ml) containing 30 μmol of l-glutamate and enzyme in 0.1 M Tris-HCl buffer (pH 8.0) was incubated at 30°C for 10 min.
Substrate specificity.
A 1-ml volume of substrate solution (60 μmol in 50 mM Tris-HCl buffer [pH 8.0]) and 5 μl of enzyme solution (containing 66 ng of enzyme [1.4 pmol]) were mixed. After 60 min, the reaction was stopped by boiling for 10 min. The decrease in amount of the substrates was analyzed by an amino acid analyzer. Pyroglutamate was measured by HPLC (Shim-pack SCR-101H column). The decyclization of l-pyroglutamyl-l-alanine was judged by measuring the increase in the amount of the NH2 group by the ninhydrin reaction, and the cyclization of l-glutamyl-l-alanine was judged by measuring the decrease in the amount of the NH2 group by the ninhydrin reaction.
ATP, Mg2+, and K+ requirement for decyclization and cyclization activity.
The ATP, Mg2+, and K+ requirements of the 5-oxoprolinase for decyclization activity were assayed as follows. The reaction mixture (1 ml) containing 5 μmol of MgSO4, 80 μmol of KCl, 5 μmol of ATP, and 39 μmol of l-pyroglutamate in 0.1 M Tris-HCl buffer (pH 8.0) and the enzyme (1.4 pmol) were incubated at 30°C for 60 min. l-Glutamate generated from l-pyroglutamate was measured by the method described above. For the assay of ADP and inorganic phosphate, 5 ml of the reaction mixture was lyophilized. ADP was measured in a pyruvate kinase-lactate dehydrogenase coupled assay (1). Inorganic phosphate (Pi) was measured by the method of Fiske and SubbaRow (6).
The ATP, Mg2+, and K+ requirements of the 5-oxoprolinase for cyclization were assayed as follows. The reaction mixture (1 ml) containing 5 μmol of MgSO4, 80 μmol of KCl, 5 μmol of ATP, and 73 μmol of l-glutamate in 0.1 M Tris-HCl buffer (pH 8.0) and the enzyme (1.4 pmol) were incubated at 30°C for 60 min. The l-pyroglutamate, ADP, and Pi generated were measured by the methods described above.
Electrophoretic analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done by the method of Laemmli (12), with a 7.5% polyacrylamide gel in 0.375 M Tris-HCl buffer (pH 8.8) containing 7.3% acrylamide, 0.2% N,N′-methylenebisacrylamide, and 0.1% SDS. The enzyme preparation (30 μg) was electrophoresed at a constant current of 20 mA at room temperature. The gel was stained with Coomassie brilliant blue R-250 to detect protein bands and periodic acid-Schiff to detect glycoprotein bands. Isoelectric focusing was done with a preparative isoelectric focusing cell Rotofor (Bio-Rad Laboratories) containing 1% Bio-Lyte (pH range from 3 to 10) at 4°C.
Measurement of molecular weight.
Gel filtration chromatography was done with a TSKgel G2000SW column (diameter, 0.75 cm; length, 30 cm; Tosoh Co.) by the method of Andrews (2). Bovine serum albumin (molecular weight, 67,000), ovalbumin (43,000), and carbonic anhydrase (30,000) were used as calibration proteins. For SDS-PAGE analysis (12), phosphorylase b, bovine serum albumin, ovalbumin, and carbonic anhydrase with molecular weights of 94,000, 67,000, 43,000, and 30,000, respectively, were used as the reference proteins.
Enzyme concentration.
The lyophilized enzyme was dried in an Abderhalden drying oven at 40°C. The absorption spectrum of the enzyme solution (1.85 mg of purified enzyme in 2.5 ml of 50 mM phosphate buffer [pH 7.0]) was measured with a UV-3100 recording spectrophotometer (Shimadzu Co.). The concentration of enzyme was determined based on the specific absorption coefficient, E1 cm1% = 13.9.
Amino acid composition.
The 5-oxoprolinase was hydrolyzed by constant-boiling HCl at 110°C in an evacuated sealed tube for 24, 48, and 72 h. The resulting hydrolysates were assayed with a Hitachi 835 amino acid analyzer. The content of the cystine and/or cysteine residue was estimated after performic acid oxidation followed by HCl hydrolysis by Moore’s method (16). The content of the tryptophan residue was estimated by Edelhoch’s method (5).
N-terminal amino acid sequence.
The amino-terminal amino acid sequence of the enzyme was analyzed by the automated Edman degradation method with a Shimadzu PSQ-1 gas-phase sequencer.
Identification of l-pyroglutamate formed by the enzyme reaction.
The melting point was measured with a micro-melting-point apparatus (Yanaco, Tokyo, Japan). The infrared spectrum was measured by the KBr tablet method with an IR-810 infrared spectrophotometer (Japan Spectroscopic Co. Ltd., Tokyo, Japan). The specific rotation was measured by using a DIP-370 digital polarimeter (Japan Spectroscopic Co. Ltd.). The fast atom bombardment mass spectrum was measured by with a JMS-AX 505W mass spectrometer (JEOL, Tokyo, Japan).
RESULTS
Identification of strain N-38A, which produced a 5-oxoprolinase.
In the course of screening for 5-oxoprolinase-producing microorganisms, strain N-38A was isolated. N-38A was rod shaped (length, ∼1.5 μm; width ∼0.6 μm); it grew under aerobic conditions and produced no special pigments. N-38A was motile with one peritrichous flagellum. Gram staining was negative. Catalase and oxidase tests were both positive. N-38A could reduce nitrite, and it was capable of anaerobic respiration in the presence of nitrite. It could also assimilate some organic acids, such as acetate, citrate, malate, and phenyl acetate. According to the taxonomic criteria in Bergey’s Manual of Systematic Bacteriology (8a), strain N-38A was allocated to the genus Alcaligenes faecalis due to its morphological and physiological properties (Table 1). Thus, we named this strain Alcaligenes faecalis N-38A.
TABLE 1.
Some morphological and physiological properties of the N-38A strain
| Property | Value |
|---|---|
| Gram staining test | Negative |
| Cell | Rod |
| Flagellum | Peritrichous |
| Pigmentsa | − |
| Oxidase | + |
| Catalase | + |
| O-F test | − |
| Nitrate reduction testb | − |
| Nitrite reduction testc | + |
| Growth under anaerobic conditions | |
| Nitrateb | − |
| Nitritec | + |
| Hydrolysis of gelatin | − |
| Carbon sources for growth | |
| d-Glucose | − |
| l-Arabinose | − |
| d-Xylose | − |
| d-Fructose | − |
| d-Mannitol | − |
| d-Mannose | − |
| d-Gluconate | − |
| Acetate | + |
| Adipate | − |
| Pimelate | − |
| Sebacate | − |
| meso-Tartrate | − |
| Itaconate | − |
Nutrient agar.
Nutrient broth–1% NaNO3.
Nutrient broth–1% NaNO2.
Production of 5-oxoprolinase by A. faecalis N-38A.
A. faecalis N-38A was cultivated as described in Materials and Methods. Production of 5-oxoprolinase in the cell extract reached its maximum after 16 h of cultivation (data not shown).
Purification of 5-oxoprolinase.
All the procedures were done at 5°C. Enzyme activity was assayed for the decyclization with l-pyroglutamate as a substrate. Purification was performed as follows.
(i) Ammonium sulfate precipitation.
Finely powdered ammonium sulfate was added to the cell extract to produce 40% saturation. The precipitate was removed by centrifugation at 15,750 × g for 10 min. To the supernatant, ammonium sulfate was added to produce 80% saturation. The precipitate was dissolved in 0.1 M Tris-HCl buffer (pH 8.0) and dialyzed against 5 mM Tris-HCl buffer (pH 8.0) overnight.
(ii) Phenyl-Toyopearl 650M column chromatography.
Ammonium sulfate was added to the dialyzed solution to produce 20% saturation. The resulting solution was loaded onto a Phenyl-Toyopearl 650M column (diameter, 2.6 cm; length, 24 cm) equilibrated with 50 mM Tris-HCl buffer (pH 8.0) saturated with 20% ammonium sulfate. The adsorbed enzyme was eluted with a linear gradient from 20 to 0% ammonium sulfate-saturated buffer. The active fractions were pooled and dialyzed against the 5 mM Tris-HCl buffer (pH 8.0) overnight.
(iii) DEAE-Toyopearl 650M column chromatography.
The dialyzed solution was loaded onto a DEAE-Toyopearl 650M column (diameter, 2.6 cm; length, 24 cm) equilibrated with 50 mM Tris-HCl buffer (pH 8.0). The adsorbed enzyme was eluted with a linear gradient in the same buffer from 0 to 0.5 M NaCl. The active fractions were pooled and dialyzed against the 5 mM Tris-HCl buffer (pH 8.0) overnight.
(iv) Second DEAE-Toyopearl 650M column chromatography.
The dialyzed solution was loaded onto a DEAE-Toyopearl 650M column (diameter, 2.2 cm; length, 18 cm) equilibrated with the same buffer. The adsorbed enzyme was eluted with a linear gradient from 0 to 0.3 M NaCl. The active fractions were pooled, and ammonium sulfate was added to produce 20% saturation.
(v) Butyl-Toyopearl 650M column chromatography.
The resulting solution from step iv was loaded onto a Butyl-Toyopearl 650M column (diameter, 2.2 cm; length, 18 cm) equilibrated with 50 mM Tris-HCl buffer (pH 8.0) saturated with 20% ammonium sulfate. The adsorbed enzyme was eluted with a linear gradient from 20 to 0% ammonium sulfate-saturated buffer. The active fractions were pooled and dialyzed against 5 mM Tris-HCl buffer (pH 7.0).
(vi) Third DEAE-Toyopearl 650M column chromatography.
The dialyzed solution was loaded onto a DEAE-Toyopearl 650M column (diameter, 1.6 cm; length, 10 cm) equilibrated with 50 mM Tris-HCl buffer (pH 7.0). The adsorbed enzyme was eluted with a linear gradient in the same buffer from 0 to 0.25 M NaCl. The active fractions were pooled and concentrated by ultrafiltration.
(vii) Sephadex G-100 gel filtration.
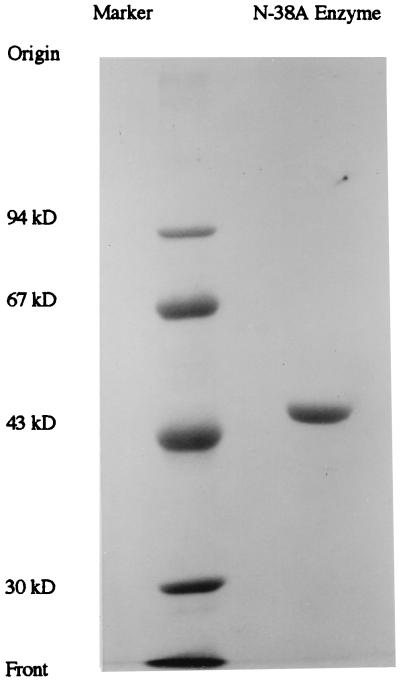
The concentrated enzyme solution was loaded onto a Sephadex G-100 column (diameter, 2.4 cm; length, 54 cm) equilibrated with 50 mM Tris-HCl buffer (pH 8.0) containing 50 mM NaCl. The active fractions were dialyzed against distilled water and lyophilized. The purification of 5-oxoprolinase is summarized in Table 2. From the cell extract of 20 liters of culture, 10.3 mg of protein was obtained with a yield of 16.6%. Specific activity was increased 9,770-fold from the cell extract. As shown in Fig. 1, the purified enzyme exhibited a single band on SDS-PAGE, indicating that this enzyme preparation is homogeneous. The purified enzyme contained no carbohydrates, because the periodic acid-Schiff staining was negative (data not shown).
TABLE 2.
Summary of purification of the 5-oxoprolinase from A. faecalis N-38A
| Purification step | Total amt of protein (mg) | Total activity (mU) | Sp act (mU/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Cell extract | 612,000 | 133,000 | 0.22 | 1 | 100 |
| 40–80% (NH4)2SO4 precipitation | 391,000 | 119,000 | 0.30 | 1.4 | 89.5 |
| Phenyl-Toyopearl 650M | 1,350 | 70,800 | 52.4 | 238 | 53.2 |
| DEAE-Toyopearl 650M | 99.7 | 111,000 | 1,110 | 5,050 | 83.5 |
| DEAE-Toyopearl 650M | 65.8 | 97,500 | 1,480 | 6,730 | 73.3 |
| Butyl-Toyopearl 650M | 28.5 | 62,600 | 2,200 | 10,000 | 47.1 |
| DEAE-Toyopearl 650M | 15.9 | 35,000 | 2,200 | 10,000 | 26.3 |
| Sephadex G-100 | 10.3 | 22,100 | 2,150 | 9,770 | 16.6 |
FIG. 1.
SDS-PAGE of 5-Oxoprolinase. Electrophoresis was done under the following conditions. A sample (about 30 μg protein) was loaded onto a 7.5% polyacrylamide gel after denaturation with 4% SDS and 10% mercaptoethanol. After electrophoresis, the protein was stained with Coomassie brilliant blue R-250. Markers: phosphorylase b (94 kDa), bovine serum albumin (67 kDa), ovalbumin (43 kDa), and carbonic anhydrase (30 kDa).
Some physicochemical properties of the 5-oxoprolinase.
The molecular weight of the 5-oxoprolinase was estimated to be 47,000 by both SDS-PAGE and gel filtration, suggesting that the enzyme is a monomer. The isoelectric point of the enzyme was determined to be 5.0. The specific absorption coefficient, E1 cm1%, (absorbance at 280 nm of a 1% solution in a 1-cm optical path length), was estimated to be 13.9. The amino acid composition of the enzyme is shown in Table 3. The amino-terminal amino acid sequence was determined to be H-Glu-Pro-Arg-Leu-Asp-Thr-Ser-Gln-Leu-Tyr-Ala-Asp-Val-His-Phe-. No protein with a significant similarity to the sequence was found in the DNASIS database.
TABLE 3.
Amino acid composition of the 5-oxoprolinase
| Amino acid | No. of amino acids (nearest integral)a |
|---|---|
| Asx | 42 |
| Thr | 15b |
| Ser | 27b |
| Glx | 53 |
| Pro | 28 |
| Gly | 23 |
| Ala | 27 |
| Val | 29 |
| Met | 10 |
| Ile | 19 |
| Leu | 31 |
| Tyr | 24 |
| Phe | 17 |
| Lys | 17 |
| His | 12 |
| Arg | 20 |
| Cys | 2c |
| Trp | 8d |
| Total | 404 |
Values are average of 24-, 48-, and 72-h hydrolyses, except as otherwise indicated.
Values extraporated to zero hydrolysis time.
Measured as cysteinic acid on a 24-h hydrolysate after performic acid oxidation.
Measured spectrophotometrically.
Some enzymatic properties of the 5-oxoprolinase.
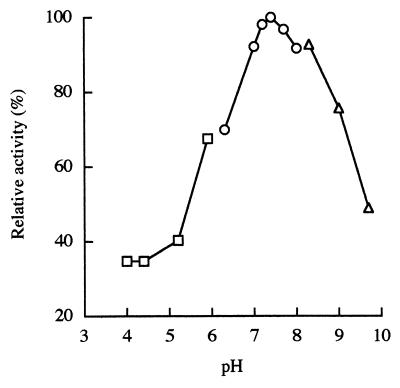
The effects of pH on the activity and stability of the enzyme were examined with respect to decyclization. The optimum pH was 7.4 (Fig. 2), and the enzyme did not show any decrease of activity in a pH range from 6.5 to 10.5 after treatment at 30°C for 30 min. The effects of temperature on the activity and stability of the enzyme were also examined with respect to decyclization. The optimum temperature was 45°C (assay at pH 7.4). The enzyme was stable up to 55°C after treatment for 30 min at pH 7.4.
FIG. 2.
Effect of pH on 5-oxoprolinase activity. Enzyme activities were assayed under standard conditions (see Materials and Methods). □, 100 mM acetate-sodium acetate; ○, 100 mM Tris-HCl; ▵, 100 mM glycine–NaOH–NaOH.
Substrate specificity of the 5-oxoprolinase.
The substrate specificity of the enzyme was analyzed by using various substrates. The enzyme could act on l-pyroglutamate and l-glutamate. It was inert toward d-pyroglutamate, l-proline, l-hydroxyproline, d-glutamate, l-glutamine, l-aspartic acid, d-aspartic acid, l-asparagine, l-pyroglutamyl-alanine, and l-glutamyl-alanine.
ATP, Mg2+, and K+ requirements of the 5-oxoprolinase.
The ATP, Mg2+, and K+ requirements of the enzyme for decyclization were examined. Without any ATP, Mg2+, or K+ supplements, 1.5 μmol of l-glutamate was formed from 39 μmol of l-pyroglutamate. ADP and Pi were not detected. Thus, the decyclization activity of the enzyme was independent of ATP, Mg2+, and K+. The ATP, Mg2+, and K+ requirements of the enzyme for cyclization were also examined. Without any additions of ATP, Mg2+, or K+, to the reaction mixture, 70 μmol of l-pyroglutamate was formed from 73 μmol of l-glutamate. ADP and Pi were not detected. Thus, the cyclization activity of the enzyme was also independent of ATP, Mg2+, and K+.
Stoichiometry of the 5-oxoprolinase reaction.
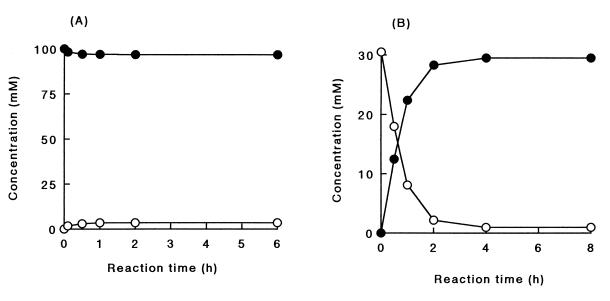
The stoichiometry of the decyclization was examined with l-pyroglutamate as a substrate. Pyroglutamate was measured by HPLC, while glutamate was measured by amino acid analysis and an enzymatic method with l-glutamate dehydrogenase. The glutamate content obtained by the amino acid analysis was identical to that obtained by the enzymatic method. It was suggested that the product of the decyclization reaction was l-glutamate. The molar ratio of l-pyroglutamate consumed to l-glutamate formed was 1:1 (Fig. 3A).
FIG. 3.
Stoichiometry of the 5-oxoprolinase reaction. (A) Decyclization reaction. The reaction mixture (100 mM l-pyroglutamate and the purified enzyme in 0.1 M Tris-HCl buffer [pH 8.0]) was incubated at 30°C. At set intervals, aliquots were withdrawn and analyzed by the methods described in Materials and Methods. ○, l-glutamate; •, l-pyroglutamate. (B) Cyclization reaction. The reaction mixture (30 mM l-glutamate and the purified enzyme in 0.1 M Tris-HCl buffer [pH 8.0]) was incubated at 30°C. At set intervals, aliquots were withdrawn and analyzed by the methods described in Materials and Methods. ○, l-glutamate; •, l-pyroglutamate.
The stoichiometry of the cyclization reaction was also studied with l-glutamate as a substrate. The molar ratio of l-glutamate consumed to pyroglutamate formed was also 1:1 (Fig. 3B).
Reaction equilibrium.
Prolonged incubation of the enzyme with l-pyroglutamate at pH 8.0 led to a maximum conversion of about 3.6% of the l-pyroglutamate to l-glutamate. When the enzyme was incubated with l-glutamate under the same conditions, 96.7% of the l-glutamate was converted to l-pyroglutamate. Accordingly, the equilibrium constant, Keq = [l-glutamate]/[l-pyroglutamate], was evaluated as approximately 0.035.
Identification of l-pyroglutamate formed by the enzyme reaction.
The enzyme was incubated with 3.0 g of l-glutamate in a total volume of 100 ml at pH 7.4 and 30°C. After incubation for 16 h, approximately 90% of the l-glutamate had disappeared. The reaction mixture was applied to an Amberlite IRA-124 column (H+ form; diameter, 2.2 cm; length, 15 cm) and washed with 150 ml of water. The eluent was evaporated to dryness in vacuo, and the residue was dissolved in warm ethanol. Ether was added to the solution. Crystals were obtained by chilling this solution. The yield of the twice-recrystallized product was 1.39 g and 52.9%. Element analysis was done. Found: C, 46.44; H, 5.35; N, 10.75. Calculated for C5H7O3N: C, 46.51; H, 5.46; N, 10.85%. The crystals melted at 162 to 163°C, and the melting point of a mixed sample with an authentic l-pyroglutamate showed no depression. The specific rotation, [α]d20, of the crystal was −11.9° (c 2, H2O). All of these data were identical to those of the authentic l-pyroglutamate. The infrared spectrum and fast atom bombardment mass spectrum were in good agreement with those of the authentic l-pyroglutamate (data not shown). According to these data, the reaction product was identified as l-pyroglutamate.
DISCUSSION
In this study, purification and characterization of a novel type of 5-oxoprolinase from a cell extract of A. faecalis N-38A were performed. The novelty of the 5-oxoprolinase is discussed based on the decyclization reaction of l-pyroglutamate and the cyclization reaction of l-glutamate.
Decyclization of l-pyroglutamate to l-glutamate.
5-Oxoprolinase (ATP hydrolyzing) (EC 3.5.2.9) catalyzes the decyclization of l-pyroglutamate to form l-glutamate. One of the features of the 5-oxoprolinases is that they have a high molecular weight: i.e., rat kidney, 325,000 (24); wheat germ, 230,000 (13); P. putida component A, 750,000; P. putida component, B 650,000 (19); and Alcaligenes sp. strain F-137, 126,000 (9). Another feature is that these enzymes require ATP, Mg2+, and K+ for the enzyme reaction. Compared with these enzymes, the 5-oxoprolinase from A. faecalis N-38A showed similar substrate specificity. However, the following points are clearly different: (i) the molecular weight of the enzyme (47,000) was much lower than those of the known 5-oxoprolinases; (ii) no similarity was observed in the amino-terminal amino acid sequence; and (iii) the decyclization reaction of the enzyme was independent of ATP, Mg2+, and K+. This is the first enzyme that catalyzes the ATP-independent decyclization of l-pyroglutamate to form l-glutamate. Thus, the 5-oxoprolinase from A. faecalis N-38 is apparently distinct from the 5-oxoprolinases (ATP hydrolyzing) reported so far.
Cyclization of l-glutamate to l-pyroglutamate.
The 5-oxoprolinase from A. faecalis has a unique cyclization activity on l-glutamate. Some enzymes that catalyze the cyclization of glutamate have been reported. (i) Glutamine synthetase (EC 6.3.1.2) catalyzes the synthesis of l-glutamine from l-glutamate and ammonia in the presence of ATP; l- and d-pyroglutamate are formed from l- and d-glutamate, respectively, in the absence of ammonia (10, 14). This cyclization reaction requires ATP and Mg2+. (ii) d-Glutamate cyclase (EC 4.2.1.48) catalyzes a conversion of d-glutamate to form d-pyroglutamate (15). The enzyme has been found in the kidneys and liver of mice, rats, and humans. The purified enzyme from mouse kidneys requires Mn2+ or Mg2+ for its activity. (iii) γ-Glutamylcyclotransferase (EC 2.3.2.4) also catalyzes a reaction in which pyroglutamate is formed from γ-glutamyl peptide (4, 20). This enzyme can act on γ-glutamyl peptide, but it is inert toward l-glutamate.
Compared with the enzymes described here, the 5-oxoprolinase from A. faecalis N-38 differs clearly from d-glutamate cyclase and γ-glutamylcyclotransferase based on their substrate specificities, and it also differs from glutamine synthetase because of its ATP requirement. Thus, it was concluded that the 5-oxoprolinase from A. faecalis N-38 is a novel type of 5-oxoprolinase (without ATP-hydrolyzing activity).
The 5-oxoprolinase from A. faecalis N-38 may be useful for enzymatic determination of l-pyroglutamate by coupling l-glutamate oxidase and peroxidase. In addition, it may be useful in the enzymatic fractionation of d-glutamate from a dl-glutamate mixture.
This is the first demonstration of a new type of 5-oxoprolinase which does not require energy from ATP hydrolysis. Cloning of the gene for this enzyme is under investigation. We are also trying to find practical applications.
ACKNOWLEDGMENTS
This research was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (project 08660123).
We are grateful to Mitsuaki Watanabe (Center for Instrumental Analysis, Nagasaki University) for analyzing the elementary analysis of l-pyroglutamate. We thank B. M. Dunn (Department of Biochemistry and Molecular Biology, University of Florida College of Medicine) and Kohei Oda (Department of Applied Biology, Faculty of Textile Science, Kyoto Institute of Technology) for their critical reading of the manuscript.
REFERENCES
- 1.Adam H. Nucleotide, coenzyme, and related compounds. In: Bergmeyer H U, editor. Methods of enzymatic analysis. 3rd ed. Vol. 8. Weinheim, Germany: VCH Publishers; 1985. pp. 365–370. [Google Scholar]
- 2.Andrews P. The gel filtration behavior of proteins related to their molecular weight over a wide range. Biochem J. 1965;96:595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler H. Amino acids. In: Bergmeyer H U, editor. Methods of enzymatic analysis. 3rd ed. Vol. 8. Weinheim, Germany: VCH Publishers; 1985. pp. 369–376. [Google Scholar]
- 4.Bodnaryk R P, McGirr L. Purification, properties and function of a unique γ-glutamyl cycrotransferase from the housefly, Musca domestica L. Biochim Biophys Acta. 1973;315:352–362. [Google Scholar]
- 5.Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967;6:1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- 6.Fiske C H, SubbaRow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- 7.Griffith O W, Meister A. 5-Oxo-l-prolinase (l-pyroglutamate hydrolase) J Biol Chem. 1981;256:9981–9985. [PubMed] [Google Scholar]
- 8.Guo-jie, Breslow E, Meister A. The amino acid sequence of rat kidney 5-oxo-l-prolinase determined by cDNA cloning. J Biol Chem. 1996;271:32293–32300. doi: 10.1074/jbc.271.50.32293. [DOI] [PubMed] [Google Scholar]
- 8a.Kersters K, De Ley J. Genus Alcaligenes Castellani and Chalmers 1919, 936AL. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins; 1984. pp. 361–373. [Google Scholar]
- 9.Koyama H. Purification and characterization of 5-oxo-l-prolinase (l-pyroglutamate hydrolase) from Alcaligenes sp. F-137. Agric Biol Chem. 1988;52:735–741. [Google Scholar]
- 10.Krishnaswamy P R, Pamiljans V, Meister A. Studies on the mechanism of glutamine synthesis: evidence for the formation of enzyme-bound activated glutamic acid. J Biol Chem. 1962;237:2932–2940. [Google Scholar]
- 11.Kuroshima E, Ohyama Y, Matsuo R, Sugimori T. Biosynthesis and degradation of glutamic acid in microorganisms relating to the Soy sauce brewing. J Ferment Technol. 1969;47:693–698. [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Mazelis M, Creveling R K. 5-Oxoprolinase (l-pyroglutamate hydrolase) in higher plants. Plant Physiol. 1978;62:798–801. doi: 10.1104/pp.62.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meister A, Krishnaswmy P R, Pamiljans V. Mechanism of glutamic acid activation and glutamine systhesis. Fed Proc. 1962;21:1013–1022. [PubMed] [Google Scholar]
- 15.Meister A, Bukenberger M W, Strassburger M. The optically-specific enzymatic cyclization of d-glutamate. Biochem Z. 1963;338:217–229. [PubMed] [Google Scholar]
- 16.Moore S. On the determination of cystine as cysteic acid. J Biol Chem. 1963;238:235–237. [Google Scholar]
- 17.Murao S, Nishimura A, Ozaki Y, Oyama H, Shin T. Isolation and characterization of a novel 5-oxoprolinase (without ATP-hydrolyzing) from Alcaligenes faecalis N-38A. Biosci Biotechnol Biochem. 1995;59:2010–2012. doi: 10.1271/bbb.59.2010. [DOI] [PubMed] [Google Scholar]
- 18.Ross L L, Barber L, Tate S S, Meister A. Enzymes of the γ-glutamyl cycle in the ciliary body and lens. Proc Natl Acad Sci USA. 1973;70:2211–2214. doi: 10.1073/pnas.70.8.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seddon A P, Li L, Meister A. Resolution of 5-oxo-l-prolinase into a 5-oxo-l-proline-dependent ATPase and a coupled protein. J Biol Chem. 1984;259:8091–8094. [PubMed] [Google Scholar]
- 20.Taniguchi N, Meister A. γ-Glutamyl cyclotransferase from rat kidney. J Biol Chem. 1978;253:1799–1806. [PubMed] [Google Scholar]
- 21.Tate S S, Ross L L, Meister A. The γ-glutamyl cycle in the choroid plexus: its possible function in amino acid transport. Proc Natl Acad Sci USA. 1973;70:1447–1449. doi: 10.1073/pnas.70.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Der Werf P, Orlowski M, Meister A. Enzymatic conversion of 5-oxo-l-proline (l-pyrrolidone carboxylate) to l-glutamate coupled with cleavage of adenosine triphosphate to adenosine diphosphate, a reaction in the γ-glutamyl cycle. Proc Natl Acad Sci USA. 1971;68:2982–2985. doi: 10.1073/pnas.68.12.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Der Werf P, Meister A. Isolation of 5-oxoprolinase from a prokaryote. Biochem Biophys Res Commun. 1974;56:90–96. doi: 10.1016/s0006-291x(74)80319-0. [DOI] [PubMed] [Google Scholar]
- 24.Williamson J M, Meister A. Effect of sulfhydryl group modification on the activities of 5-oxo-l-prolinase. J Biol Chem. 1982;257:9161–9172. [PubMed] [Google Scholar]
- 25.Yamamoto S, Hirooka H. Production of glutaminase by Aspergillus sojae. J Ferment Technol. 1974;52:564–569. [Google Scholar]
- 26.Yamamoto S, Hirooka H. Partial purification and properties of glutaminase from Aspergillus sojae. J Ferment Technol. 1974;52:570–578. [Google Scholar]