Graphical abstract
Keywords: Aroma compounds, Huangjiu, Post-fermentation, Metabolomics, Differential metabolites
Highlights
-
•
The differential metabolites of manual (mechanized) Huangjiu were determined during post-fermentation stage.
-
•
The metabolic pathways associated with the differential metabolites were identified.
-
•
The contribution of different metabolites to the flavor of Huangjiu was analyzed.
Abstract
In order to understand the differences of metabolites and their key metabolic pathways between traditional manual and mechanized Huangjiu, gas chromatography-mass spectrometry (GC–MS) combined with non targeted metabolomics was used to track and monitor Huangjiu in the whole post-fermentation stage. The results showed that 25 metabolites and 14 metabolites were identified as differential metabolites in manual and mechanized Huangjiu, respectively (VIP > 1, P < 0.05); three metabolic pathways had significant effects on differential metabolites (−log (P) > 1, impact > 0.01). Compared with the two kinds of Huangjiu, 21 kinds of metabolites were identified as differential metabolites (VIP > 1, P < 0.05); four metabolic pathways had significant effects on differential metabolites (−log (P) > 1, impact > 0.01). This study is helpful to gain insight into the underlying mechanism of flavor formation during the post-fermentation process of Huangjiu and provide a theoretical basis for the industrial development.
1. Introduction
Chinese Huangjiu (yellow rice wine), a traditional Chinese alcohol beverage, is popular among Chinese customers. Huangjiu is honored as national banquet alcohol beverage due to a large number of flavor substances. The brewing technology of Huangjiu is divided into manual Huangjiu and mechanized Huangjiu (Fig. S1). The mechanized Huangjiu is fermented in a large stainless-steel tank with yeast and wheat starter as saccharifying and fermenting agents, and the fermentation cycle is generally 24 days. The distiller's yeast is cultivated by pure breed, and the wheat Qu is mixed by natural culture and pure breed. Traditional manual Huangjiu uses naturally cultivated wheat Qu and yeast as saccharifying and fermenting agents, and the fermentation cycle is generally 72 days. In the first fermentation stage, pottery jar is used as fermentation container, and in the second fermentation stage (post-fermentation), pottery jar is used as fermentation container (Xie et al., 2021). The fermentation of Huangjiu requires a post-fermentation process with a long time at low temperature. In the post-fermentation stage, besides the further action of yeast to produce ethanol, the main purpose is to produce the special flavor substances of Huangjiu and make the flavor of Huangjiu more harmonious. Therefore, it is of great significance to deeply understand differences in metabolites and metabolic pathways in post-fermentation stage between traditional manual and mechanized Huangjiu for improving the brewing technology. As an important part of systems biology, metabolomics, which is able to detect dozens or even hundreds of endogenous metabolites, has been widely applied in food research (Aihua et al., 2011, Pang et al., 2019, Saigusa et al., 2020, Wishart and David, 2011). Analytical techniques used in metabolomics mainly include nuclear magnetic resonance (NMR), gas chromatography-mass spectrometry (GC–MS) and liquid chromatography-mass spectrometry (HPLC-MS) (Pang et al., 2019). Compared with other methods, GC–MS has the advantages of low cost, good repeatability, high resolution and small matrix effect (Aihua et al., 2011, Saigusa et al., 2020, Wishart and David, 2011), and it is still the main analysis method of metabolomics. Such as Jang et al.(2014), the metabolites of seven industrial vinegar products and two traditional vinegar products were analyzed by gas chromatography time of flight mass spectrometry (GC-TOF-MS). Chuangye et al. (2018) analyzed the metabolite changes of B.macrocephala at different growth rates by GC-TOF-MS technique and explored the mechanism of their different growth. Zeng et al. (2019) used GC-TOF-MS technique to study the metabolic fingerprinting of rats after dietary intake of kiwifruit wine, revealing that kiwifruit wine may have a positive effect on health.
Metabolomics combined with stoichiometry has been widely used in food quality analysis and origin identification in recent years. Peng et al. (2015) successfully identified the origin and age of Tongshan liquor by using stoichiometry combined with the analysis technology of volatile components of liquor products; and used stoichiometry combined with electronic nose technology to identify the geographical origin of camellia oil (Peng et al., 2020).
However, the current reports on Chinese Huangjiu mainly focus on the changes of flavor substances during fermentation. There are few studies on the differences, dynamic changes and key metabolic pathways of metabolites between traditional manual and mechanized Chinese Huangjiu. To analyze the differences that existed between traditional manual Huangjiu and mechanized Huangjiu, the key differential volatile substances and key differential metabolic pathways were identified. How to determine key differential volatile compounds and key differential metabolic pathways to analyze the differences between traditional manual and mechanized Huangjiu?
In this study, GC–MS combined with chemometric analysis was used to explore the key metabolic pathways of the differential metabolites between the two kinds of Huangjiu in the post-fermentation process, which would help to gain insight into the underlying mechanism of flavor formation and provide a theoretical basis for the improvement of quality during the industrial development of Huangjiu.
2. Materials and methods
2.1. Samples
The manual Huangjiu was obtained from Gu Yue Long Shan, Zhejiang Province, and the sampling time points were days 2, 4, 6, 16, 26, 36, 54,72. One batch was taken for each process, and 250 mL was taken after mixing the bottles and recorded once.
The mechanized Huangjiu was obtained from Gu Yue Long Shan in Zhejiang Province, the sampling time points were days 1, 2, 3, 4, 13, 21. One batch was taken for each process, and 250 mL was collected after mixing in bottles and recorded once.
All samples were aseptically sealed and put into −80 °C ultra low temperature freezer for further use.
2.2. Samples preparation
The 100 μL manual Huangjiu sample was taken in a 1.5 mL EP tube, and then added 300 μL of extraction solution (methanol) followed by addition of 5 μL ribitol, vortexed for 30 s; sonication for 10 min (ice water bath); samples were centrifuged at 12,000 r/min for 15 min at 4 °C; the supernatant 30 μL was pipetted into a 1.5 mL EP tube, and 10 μL of each sample was mixed to form a QC sample; the extract was dried in a vacuum concentrator; to the dried metabolites, 100 μL methoxyamine salt reagent (methoxyamine hydrochloride dissolved in pyridine 20 mg/mL), mixed gently, and placed in the oven for 30 min at 80 °C; methoxamine salt reagent 100 μL (methoxamine hydrochloride was dissolved in pyridine 20 mg/mL) was added to the dried metabolites, mixed gently, and incubated at 80 °C for 30 min in the oven. To each sample, 100 μL bis (trimethylsilyl) trifluoroacetamide, incubating the mixture at 70 °C for 1.5 h; the 100 μL bis (trimethylsilyl) trifluoroacetamide was added to each sample, and incubated the mixture at 70 °C for 1.5 h. The samples were cooled to room temperature, and 5 μL of saturated fatty acid methyl ester (dissolved in chloroform) was added to the mixed samples. Samples were randomly and sequentially tested on the machine. This method was improved on the basis of Kanani et al. (2008) and Zeki et al. (2020).
2.3. GC–MS analysis
The extraction head was removed from the sample bottle and quickly inserted into the gas chromatograph inlet (240 °C, 5 min) to start GC/MS detection and analysis (Li et al., 2008). Gas Chromatography (Ancín-Azpilicueta, 2008) conditions: The carrier gas is high-purity helium (flow rate 1 mL/min); the temperature of the injection port was set at 40 °C for 5 min, then increased to 120 °C at 5 °C/min and 240 °C at 10 °C/min (Xian et al., 2019), and then its operating temperature is 240 °C; connecting rod 150 °C; sample injection in splitless mode. MS conditions: ion source (EI) temperature 230 °C; EI ionization source 70 eV, quadrupole temperature 150 °C, mass scanning range m/z 35∼450; transmission line temperature 250 °C (Chen et al., 2019).
2.4. Bioinformatic analysis of the untargeted metabolomics dataset
Mass spectral data of both liquor samples were analyzed using chromatof software (V 4.3xleco) for peak extraction, baseline correction, deconvolution, peak integration, peak alignment, and so on. For the qualitative work on substances, the LECO fiehn rtx5 database was used, including mass spectral matches and retention time index matches. Peaks with a call rate below 50% or a relative standard deviation greater than 30% in QC samples were finally removed. Peaks below 50% call rate or greater than 30% relative standard deviation in QC samples were removed after filling.
2.5. Statistical analysis
SPSS 25 was used to analyze variance of fermentation process data of manual and mechanized Huangjiu samples (n = 20), and only metabolites with P value less than 0.05 were defined as significant differences. SIMCA 14.1 software was used for data conversion and centralized formatting, and then modeling and analyzing the data of two kinds of Huangjiu samples (Fig. 3).
Fig. 3.
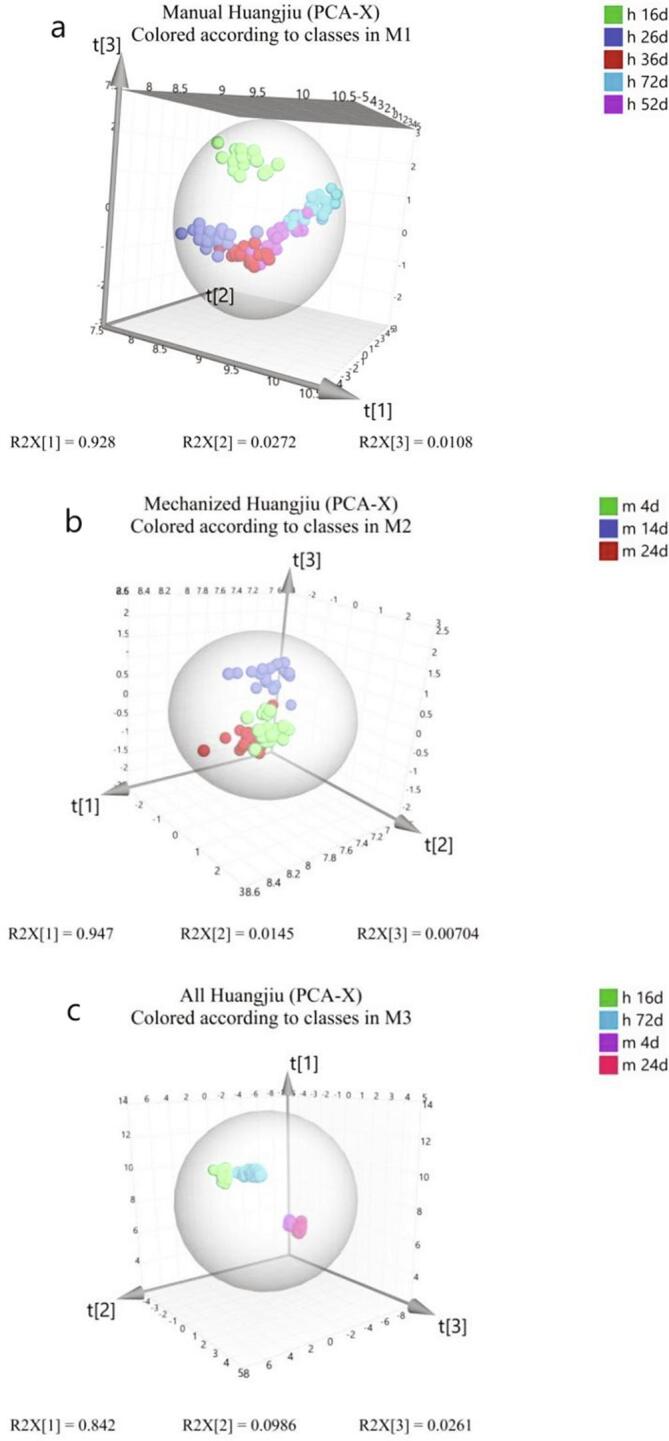
a. PCA-X diagrams of different stages of fermentation of manual Huangjiu (h) samples; b. PCA-X diagrams of different stages of fermentation of mechanized Huangjiu (m) samples; c. PCA-X diagram of Huangjiu samples taken from the initial and end stages of post-fermentation.
2.6. Stoicheiometry analysis
The measured data (peak values, sample names and relative substance contents are included) were subjected to principal components analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA). The quality of the constructed PCA was assessed by R2X and Q2 and the quality of the constructed OPLS-DA model was assessed by R2X, R2Y and Q2. The metabolites with VIP value greater than 1 were selected as candidate differential metabolites by OPLS-DA model. The relative contents of candidate differential metabolites were subjected to t-test, and only metabolites with a p-value less than 0.05 were defined as differential metabolites.
To further elucidate the biological significance of the differential metabolites, the differential metabolites were imported into metaboanalyst 5.0 (https://www.metaboanalyst.ca) Middle, the key metabolic pathways were screened based on - log (P) > 1 and impact > 0.01 (Pang et al., 2021).
3. Results and discussion
3.1. Analysis of the difference of two kinds of Huangjiu
3.1.1. Basic physicochemical characteristics
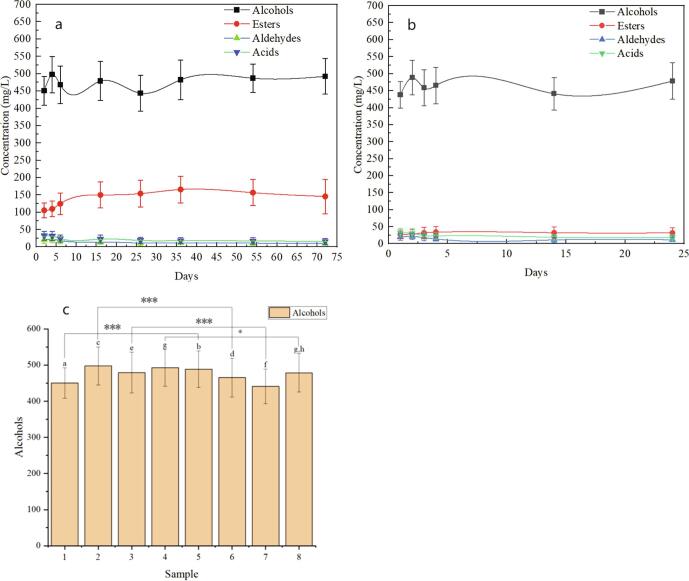
The dynamic changes of metabolite differences between manual and mechanized Huangjiu during the post-fermentation stages are shown in Fig. 1. The variation trend of some metabolites in manual and mechanized Huangjiu was similar in post-fermentation. The metabolite differences between manual and mechanized Huangjiu in the post-fermentation stages were indicated by the significant differences in the contents of esters. A total of 26 esters were detected in manual Huangjiu, and 9 esters were detected in mechanized Huangjiu. This may be one of the important reasons why mechanized Huangjiu mouthfeel lacks “flavor” compared with manual Huangjiu.
Fig. 1.
a. Changes of physical and chemical indicators in each stage of manual Huangjiu fermentation; b. Changes of physical and chemical indexes in each stage of mechanized Huangjiu fermentation; c. Content difference of Alcohols in two kinds of Huangjiu (1-manual Huangjiu 2d; 2-manual Huangjiu 4d; 3-manual Huangjiu 16d; 4-manual Huangjiu 16d; 5-mechanized Huangjiu 2d; 6-mechanized Huangjiu 4d; 7-mechanized Huangjiu 14d; 8-mechanized Huangjiu 24d).
The contents of esters in the two kinds of Huangjiu were significantly different, which was mainly related to the different types and dosage of wheat Qu. By optimizing the wheat Qu formula, Zhang et al. (2016) significantly improved the content of esters in mechanized Huangjiu, thus improving the flavor quality of Huangjiu, and solved the bitter taste problem commonly reported in mechanized Huangjiu. The species and abundance of microorganisms in different fermented mash were significantly different due to the different types and dosage of wheat Qu. The preliminary study of our laboratory found that there were more bacterial species in well-fermented manual Huangjiu, while the bacterial species in mechanized Huangjiu were relatively few (Xie et al., 2021), and the quality of fermented mash was directly related to the content of Lactobacillus.
3.1.2. The difference of acids in two kinds of Huangjiu
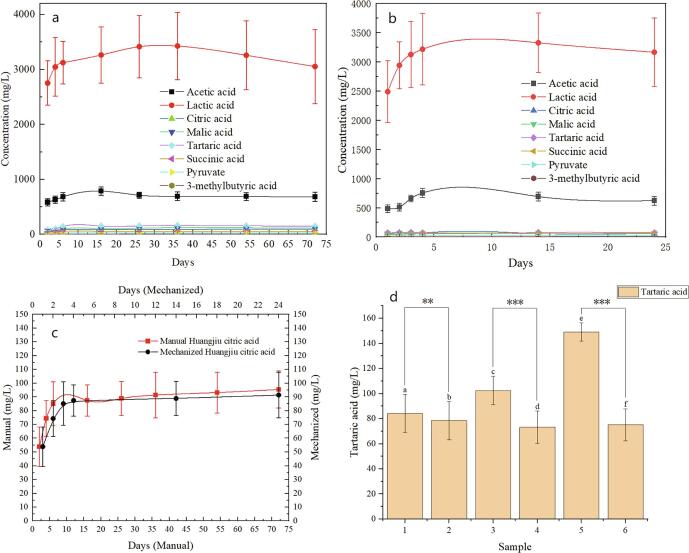
At the post-fermentation stage, the content changes of organic acids in the two kinds of Huangjiu are shown in the Fig. 2. The content of tartaric acid was significantly different (P < 0.05), and the content of artificial yellow rice wine was higher. Since the solubility of tartaric acid in ethanol is less than that in water, and ethanol promotes the decomposition of tartaric acid, the rapid accumulation of ethanol content in mechanized rice wine at the early stage of fermentation accelerates the loss of tartaric acid (Amerine et al., 1980).
Fig. 2.
a. Changes of organic acid content in each stage of manual Huangjiu fermentation; b. Changes of organic acid content in each stage of mechanized Huangjiu fermentation; c. Changes of citric acid content in each stage of all Huangjiu fermentation. d. Content difference of tartaric acid in two kinds of Huangjiu (1-manual Huangjiu 2d; 2-mechanized Huangjiu 2d; 3-manual Huangjiu 4d; 4-mechanized Huangjiu 4d; 5-manual Huangjiu 72d; 6-mechanized Huangjiu 24d).
Citric acid was more abundant in the fermentation after manual Huangjiu, and the fermentation ability of the naturally cultured yeast used in manual Huangjiu was stronger than that of the pure cultivated yeast in mechanized Huangjiu. The content of citric acid was higher in the post-fermentation of manual Huangjiu (Mao, 2008, Mao, 2004, Wang et al., 2012). Manual Huangjiu reached 93.216 (±14.8) on 72 days, while mechanized Huangjiu reached 91.4 (±16.6) on 21 days (Fig. 2 c.). A. G. Reynolds studies have shown that different yeasts vary in their ability to produce citric acid in the fermentation of wine (Reynolds et al., 2001).
3.1.3. The difference of amino acids in two kinds of Huangjiu
Amino acids are important flavor composition components in Huangjiu (Burin et al., 2015). The contents of amino acid nitrogen were increased slowly with the passage of fermentation time during the post fermentation of manual and mechanized Huangjiu (Fig. S2). The gradual death of yeast cells provides a steady source of feed for proteolysis to amino acids (Lysine, arginine, serine, α-alanine, glutamic acid, etc.). The process produced contains long-chain fatty acids (C14-C1 ester 8), terpene alcohol (linalool-musk), isoamino alcohol (benzene 2-ethanol rose smell), aldehyde (methyl 3-butanol-grass smell), Lactone (α-delactone-peach, coconut) (Leskó et al., 2011).
The content of amino acids in the two kinds of Huangjiu is significantly different (P < 0.05), especially the amino acids with umami taste (Serine, glutamic acid, proline, valine, isoleucine, leucine, tyrosine, alanine, lysine, histidine, arginine), as shown in Fig. S2.
3.2. Chemometric analysis of two kinds of Huangjiu
3.2.1. PCA topographic plots of two kinds of Huangjiu
SIMCA 14.1 software was used for data conversion and centralized formatting, and then modeling and analyzing the data of two kinds of Huangjiu samples (Fig. 3). Q2 > 0.5 indicated good fitting degree of PCA model. Q2 < 0.5, indicating poor fitting degree of PCA model. The manual Huangjiu sample model showed that the values of R2X, and Q2 were 0.928, 0.958, respectively (Fig. 3 a); the mechanized Huangjiu sample model showed that the values of R2X, and Q2 were 0.947, 0.916, respectively (Fig. 3 b); the models of all Huangjiu samples at the start and end of post-fermentation stage showed that the values of R2X, and Q2 were 0.975, and 0.961, respectively (Fig. 3 c).
The PCA analysis based on the contribution of metabolites showed that the metabolites of the both Huangjiu were significantly different (Fig. 3 a), and the internal metabolites of the mechanized Huangjiu were quite different (Fig. 3 b), but the difference was smaller than that of the manual Huangjiu (Fig. 3 c).
PCA analysis based on metabolite contribution showed that the metabolites of the two kinds of Huangjiu were significantly different (Fig. 3 a), while the internal metabolites of mechanized Huangjiu were significantly different (Fig. 3 b), but the difference was smaller than that of manual Huangjiu (Fig. 3 c).
3.2.2. Metabolite difference analysis of two kinds of Huangjiu
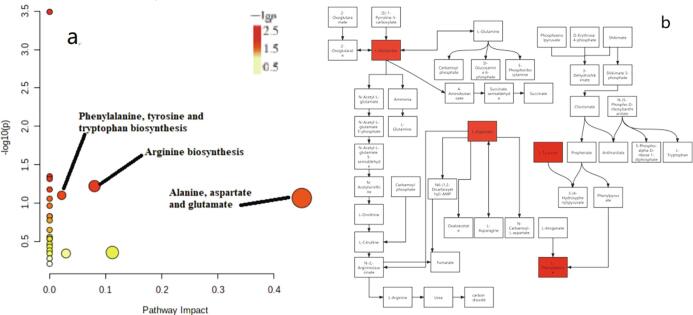
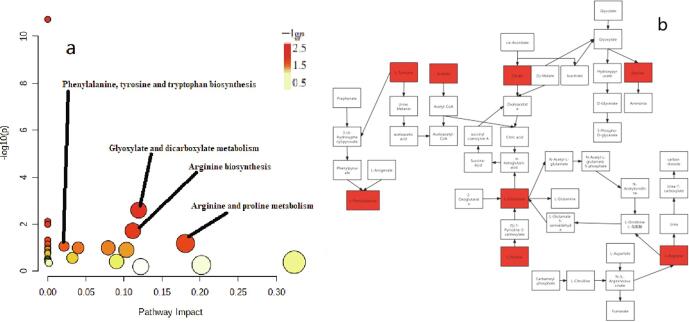
The OPLS-DA model was used to analyze the metabolite data in Huangjiu samples (Fig. S3). Q2 > 0.5 indicated good fitting degree of OPLS-DA model. Q2 < 0.5, indicating poor fitting degree of OPLS-DA model. The model of manual Huangjiu sample showed that the values of R2X, R2Y and Q2 were 0.968, 0.949, 0.928, respectively (Fig. S3 a); the model of mechanized Huangjiu sample showed that the values of R2X, R2Y and Q2 were 0.969, 0.919, 0.9 (Fig. S3 b); the models of all Huangjiu samples at the start and end of post-fermentation stage showed that the values of R2X, R2Y and Q2 were 0.969, 0.735, and 0.703, respectively (Fig. S3 c). Based on the results of OPLS-DA model analysis, metabolites (VIP > 1, P < 0.05) were regarded as differential metabolites in the post-fermentation stage. The results showed that there were 25 different metabolites (VIP > 1, P < 0.05) in the whole post-fermentation stage (prophase vs telophase) of manual Huangjiu, as shown in Table 1; there were 14 different metabolites in the whole post-fermentation stage (prophase vs telophase) of mechanized Huangjiu (VIP > 1, P < 0.05), as shown in Table 2. There were 21 different metabolites between the two kinds of Huangjiu in the whole post-fermentation stage, as shown in Table 3. The s-plots and heat maps clearly showed that there was a significant difference between the metabolites of manual and mechanized Huangjiu, and the metabolites of manual Huangjiu were significantly different in the post-fermentation stage (prophase vs telophase) (Fig. S4, Fig. 4). A total of 25 metabolites in the whole post-fermentation stage of manual Huangjiu were identified as differential metabolites (VIP > 1, P < 0.05); the metabolic pathways with significant effects on the differential metabolites (−log(P) > 1, impact > 0.01) were phenylalanine, tyrosine and tryptophan biosynthesis pathway, alanine, aspartic acid and glutamate metabolism pathway, and arginine biosynthesis pathway (Fig. 5). A total of 14 metabolites in the whole post-fermentation stage of mechanized Huangjiu were identified as differential metabolites (VIP > 1, P < 0.05); the absence of metabolic pathways had a significant effect on differential metabolites (−log(P) > 1, impact > 0.01). A total of 21 metabolites of the two kinds of Huangjiu were identified as differential metabolites during the whole post-fermentation stage (VIP > 1, P < 0.05); the metabolic pathways with significant effects on the differential metabolites (−log(P) > 1, impact > 0.01) were phenylalanine, tyrosine and tryptophan biosynthesis pathway, glyoxylic acid and dicarboxylic acid metabolism pathway, arginine biosynthesis pathway, arginine and proline metabolism pathway (Fig. 6).
Table 1.
Metabolite content of manual Huangjiu at different post-fermentation stages (+ indicates that after the end of post-fermentation period, the substance content of manual Huangjiu increases; – indicates that the substance content of manual Huangjiu increases after the end of post-fermentation period).
| Var ID (Var. Sec. ID:1) | M10.VIPpred | Probability | Fermentation change trend |
|---|---|---|---|
| Butanol | 1.06307 | 0.000383275 | + |
| Hexanol | 1.14137 | 1.13519E-05 | – |
| Octanol | 3.86508 | 1.06451E-27 | – |
| 2,3-Butanediol | 1.09603 | 2.11185E-11 | + |
| Propanol | 1.14995 | 3.40687E-05 | + |
| Ethyl benzoate | 1.07946 | 1.36587E-08 | + |
| Ethyl phenylacetate | 1.1903 | 1.67303E-07 | + |
| Ethyl lactate | 1.03623 | 0.000100722 | + |
| Ethyl Myristate | 1.04684 | 0.000443796 | – |
| 2-phenethyl acetate | 1.32564 | 4.93293E-07 | – |
| Ethyl butyrate | 1.33323 | 1.9035E-06 | + |
| Ethyl propionate | 1.54982 | 3.64852E-10 | – |
| Ethyl Valerate | 2.14518 | 3.66139E-08 | + |
| Furfural | 1.05775 | 2.18988E-07 | – |
| Nonanal | 2.35651 | 6.3488E-16 | – |
| Decanal | 2.68285 | 2.0444E-12 | – |
| Capric acid | 2.07368 | 1.58686E-10 | – |
| 3-methylbutyric acid | 1.50739 | 7.4959E-07 | – |
| Aspartic acid | 1.63616 | 1.10282E-13 | + |
| Isoleucine | 1.75483 | 2.11589E-19 | + |
| Tyrosine | 1.40572 | 2.63338E-29 | + |
| Phenylalanine | 1.2445 | 8.35209E-23 | + |
| Lysine | 1.75266 | 5.80359E-23 | + |
| Serine | 1.79176 | 1.11464E-18 | + |
| Glutamate | 1.06404 | 1.3974E-24 | – |
Table 2.
Metabolite contents in different fermentation stages of mechanized Huangjiu (+ indicates that after the end of post-fermentation period, the substance content of mechanized Huangjiu increases; – indicates that the substance content of mechanized Huangjiu increases after the end of post-fermentation period).
| Var ID (Var. Sec. ID:1) | M11.VIPpred | Probability | Fermentation change trend |
|---|---|---|---|
| 2-methyl-1-propanol | 1.33808 | 0.00199426 | + |
| Butanol | 1.43532 | 5.77938E-05 | + |
| Octanol | 3.95865 | 4.27897E-23 | – |
| 2,3-Butanediol | 1.23102 | 6.28137E-10 | + |
| 4-methyl-2-pentanol | 1.10731 | 0.00055835 | – |
| Propanol | 1.37468 | 1.90317E-06 | + |
| Methyl octadecanoate | 1.27898 | 0.00290336 | + |
| Ethyl Myristate | 1.68022 | 3.70571E-08 | + |
| 2-phenethyl acetate | 1.58522 | 4.09498E-06 | + |
| Benzaldehyde | 1.17072 | 0.000719877 | – |
| Nonanal | 2.32914 | 4.07877E-09 | – |
| Decanal | 2.38281 | 1.84179E-08 | – |
| Capric acid | 1.82434 | 1.33252E-05 | – |
| 3-methylbutyric acid | 2.44974 | 1.91206E-09 | – |
Table 3.
Different metabolites of two kinds of Huangjiu in the post-fermentation stage (+ indicates that after the end of post-fermentation period, the substance content of manual Huangjiu increases; – indicates that the substance content of mechanical Huangjiu increases after the end of post-fermentation period).
| Var ID (Var. Sec. ID:1) | M14.VIPpred | Probability | Content comparison |
|---|---|---|---|
| Ethyl acetate | 1.34111 | 3.61796E-18 | + |
| Ethyl lactate | 1.46777 | 1.89278E-22 | + |
| Ethyl butyrate | 1.4756 | 2.91E-23 | + |
| Isoleucine | 1.377 | 5.24E-14 | + |
| Leucine | 2.77925 | 7.16145E-32 | + |
| Tyrosine | 2.4386 | 1.21258E-31 | + |
| Phenylalanine | 2.16063 | 9.98335E-28 | + |
| Lysine | 2.57151 | 2.67207E-27 | + |
| Histidine | 1.74284 | 2.28399E-17 | + |
| Arginine | 2.20517 | 2.81916E-19 | + |
| Serine | 1.57135 | 1.38331E-13 | + |
| Glutamate | 1.66034 | 1.00779E-14 | + |
| Proline | 2.4321 | 1.26685E-24 | + |
| Glycine | 1.2428 | 4.91504E-10 | – |
| Valine | 1.93658 | 1.03105E-21 | + |
| Methionine | 1.20071 | 2.9255E-14 | – |
| acetic acid | 1.55697 | 5.23613E-07 | + |
| Lactic acid | 2.3334 | 0.0163673 | + |
| Citric acid | 1.34154 | 1.54733E-15 | + |
| Malic acid | 2.05118 | 1.16062E-27 | + |
| Tartaric acid | 2.42603 | 6.25122E-37 | + |
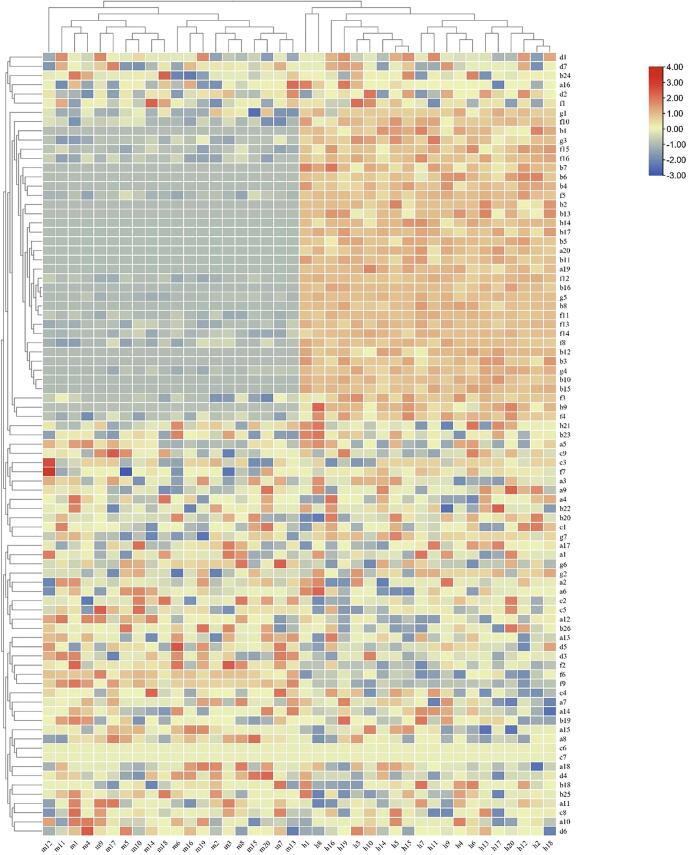
Fig. 4.
. Heat map of different metabolites in Huangjiu at the end of fermentation. The mechanized Huangjiu is in the lower-adjusted interval on the left, and manual Huangjiu is in the upper-adjusted interval on the right. The horizontal axis corresponds to the sample number, and the vertical axis corresponds to the substance number (refer to the supplementary materials for the substance type corresponding to the number).
Fig. 5.
a. Enrichment analysis of differential metabolite pathways after fermentation of manual Huangjiu; b. Metabolic pathway analysis of different metabolites after fermentation of manual Huangjiu.
Fig. 6.
a. Enrichment analysis of differential metabolite pathways after fermentation of All Huangjiu; b. Metabolic pathway analysis of different metabolites after fermentation of All Huangjiu.
Among the key metabolic difference pathways of post-fermentation of manual Huangjiu, aspartic acid is synthesized by addition reaction of oxaloacetic acid and asparagine in arginine biosynthesis pathway. Aspartic acid is catabolized to produce oxaloacetate to complete a cycle, or to produce arginine, fumarate, etc. Arginine and proline participate in arginine and proline metabolism to produce glutamic acid and α-ketoglutarate; the former participates in the continuous consumption of the urea cycle, and the latter enters the tricarboxylic acid cycle to participate in cell metabolism. Tyrosine is synthesized into phenylalanine through a multi-step enzymatic reaction in the biosynthetic pathway of phenylalanine, tyrosine and tryptophan. In the post-fermentation stage, the content of glutamic acid decreased and the content of phenylalanine, tyrosine and aspartic acid increased with the continuous fermentation. It showed that the arginine biosynthesis pathway was not active with phenylalanine tyrosine and tryptophan biosynthesis pathway. CAR1 encodes arginase, which is used to hydrolyze arginine into ornithine and urea, and is one of the key genes involved in controlling the metabolism of arginine and proline (Carrasco et al., 2003). CAN1 and ALP1 encode arginine permease and high-affinity amino acid permease, respectively (Sychrova & Chevallier (1994)). The overexpression of CAN1, ALP1 and GAP1 is beneficial to the decomposition and transport of arginine (Alberto et al., 2012, Elberry et al., 1993, Jiao, 2016, Zhang et al., 2012, Zhang et al., 2016), and may improve the utilization of arginine in cells. These genes are all associated with the decreased contents of oxaloacetate, asparagine, arginine, fumarate and N-carbamoyl-l-aspartic acid, which were the downstream metabolites of arginine biosynthesis pathway, and are one of the main regulatory genes affecting the metabolic differences in the post-fermentation stage of manual Huangjiu.
Tyrosine is synthesized from phenylalanine through the biosynthetic pathway of phenylalanine, tyrosine and tryptophan in the key differential metabolic pathway between manual and mechanized Huangjiu during post-fermentation stage. Aspartic acid participates in the arginine biosynthesis pathway to metabolize oxaloacetic acid to complete a cycle, or generate arginine, fumarate, etc. Arginine and proline are involved in the metabolism of glutamic acid and α -ketoglutaric acid. Glutamic acid, acetic acid, and citric acid are metabolized to glycine by glyoxylic acid and dicarboxylic acid. In the post-fermentation stage, the contents of tyrosine, alanine, arginine, glutamic acid, proline, acetic acid and citric acid in manual Huangjiu were higher than those of mechanized Huangjiu, but the contents of glycine, aspartic acid and methionine were lower. Since glycine is involved in the synthesis of inosinic acid (IMP) and the synthesis of nucleotide is involved in the growth and metabolism of yeast, it is speculated that the active pathways of arginine biosynthesis and arginine and proline metabolism are higher in manual Hungjiu than those in mechanized Huangjiu, but the active levels of glyoxylic acid and dicarboxylic acid metabolism are lower. Genes such as CAR1, CAN1, ALP1 and GAP1 are involved in the regulation of this process. The strong metabolism of arginine in manual Huangjiu may be related to the expression levels of DAL80, DUR1,2, DUR3, DAL82, and GAP1 (Alberto et al., 2012, Elberry et al., 1993, Jiao, 2016, Zhang et al., 2012, Zhang et al., 2016).
In the post-fermentation stage, the contents of ethyl acetate and isoamyl acetate in manual Huangjiu were higher than those of mechanized Huangjiu, while the contents of higher alcohols such as isoamyl alcohol were lower. Lilly et al. 2006 showed that overexpression of ATF1 gene in wine yeast S.cerevisiae VIN13 could significantly increase the production of ethyl acetate and isoamyl acetate, while the production of higher alcohol was significantly decreased (Lilly et al., 2006). Zhang et al. overexpressed the ATF1 gene in the Huangjiu yeast S.cerevisiae RY1 with low isoamyl acetate production and knocked out the IAH1 gene. The production of isoamyl alcohol was reduced by 49.0%, and the production of ethyl acetate was increased by 20.9 times (Zhang et al., 2012). In the post-fermentation stage, the contents of ethyl acetate, isoamyl acetate and isoamyl alcohol showed significant differences in the two kinds of Huangjiu, which may be closely related to these genes. In the post-fermentation stage, the total amount of higher alcohols in the two kinds of Huangjiu is different. Many genes can affect the production of higher alcohols. In Saccharomyces cerevisiae, aldehydes are hydrogenated to alcohols under the catalysis of gene-coded (ADH1, ADH2, ADH3, ADH4, ADH5, ADH6, ADH7, SFA1, etc.) alcohol dehydrogenase. In addition, there are also differences in the roles of genes encoding decarboxylase and alcohol dehydrogenase in the metabolic pathways of higher alcohols (Hazelwood et al., 2008). The difference of wheat Qu, especially the difference of microorganism in fermented mash, is the main reason that causes the difference between manual and mechanized Huangjiu in post-fermentation stage. The wheat Qu of mechanized Huangjiu is cultivated by pure breed. The wheat Qu of manual Huangjiu is naturally cultivated and mixed with pure breeds. Due to the different types and dosages of wheat Qu, there are significant differences in the types and abundance of microorganisms in different fermented mash (Xie et al., 2021), resulting in differences in metabolic pathways between manual Huangjiu and mechanized Huangjiu in the post-fermentation stage.
4. Conclusion
The metabolites of Huangjiu produced by different fermentation processes were studied in this paper. The key metabolic pathways of different metabolites in the post-fermentation process of Huangjiu with two brewing methods were explored. In the whole post-fermentation stage, there are three metabolic pathways which had significant effects on the differential metabolites in the manual Huangjiu. During the whole post-fermentation stage of mechanized Huangjiu, the related metabolic pathways have no significant effect on the differential metabolites. Comparing the two kinds of Huangjiu, a total of 21 metabolites were identified as differential metabolites during the whole post-fermentation stage; four metabolic pathways had a significant impact on the differential metabolites.
In the manual pressing, the moisture and air in the raw wheat Qu are higher (Xie et al., 2021), which is conducive to the early growth of yeast and fungi, and Aspergillus enzyme in the manual Qu quickly established the growth advantage. Manual operation also brought more bacterial infection to raw wheat Qu, resulting in higher microbial diversity in finished manual wheat Qu. Mechanized ripe wheat Qu had a more uniform fungal community, but less water and air, resulting in slower early mold growth (Zhou, 2021). These factors lead to significant differences in the expression levels of many genes in the fermented flora of the two kinds of Huangjiu. The main reason for the significant difference in the metabolite content of the two kinds of Huangjiu in the post-fermentation stage is mainly due to the different strains in the fermentation process. The content of metabolites between the two kinds of Huangjiu in the post-fermentation stage is significantly different, mainly due to the differences in the expression and regulation of arginine biosynthesis pathway and its associated pathways. These differential metabolic pathways are regulated by genes such as CAR1, CAN1, ALP1, GAP1, DAL80 and ATF1.
The exploration of the differences in metabolites in Huangjiu during the post-fermentation process will help to understand the potential mechanism of flavor formation in the post-fermentation process of Huangjiu, and provide a theoretical basis for the industrial development of Huangjiu.
CRediT authorship contribution statement
Qi Peng: Writing – review & editing, Supervision, Project administration, Funding acquisition. Kai Meng: Writing – review & editing, Investigation, Data curation. Huajun Zheng: Conceptualization, Writing – original draft, Visualization, Investigation. Hefeng Yu: Investigation, Methodology, Resources. Yuhao Zhang: Investigation, Formal analysis. Xinyi Yang: Investigation, Data curation. Zichen Lin: Visualization, Investigation. Guangfa Xie: Validation, Resources, Investigation, Formal analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This work was supported by the Foundation of Public Projects of Zhejiang Province, China (No. GN22C205486); National Training Program for College Students’ Innovation and Entrepreneurship Project (No. 202110349031); Scientific and Technological Innovation Activity Plan of College Students in Zhejiang Province (Xinmiao Talents Scheme) (No. 2021R432010); Program Foundation of Public Projects of Shaoxing city, Zhejiang Province, China (No. 2018C30010); Foundation of Public Projects of Zhejiang Province, China (No. 2017C32101); Shaoxing University Fund (No. 08021066).
Conflicts of interest
There are no conflicts of interest in this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100324.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aihua Z., Hui S., Ping W., Ying H., Xijun W. Modern analytical techniques in metabolomics analysis. Analyst. 2011;137 doi: 10.1039/c1an15605e. [DOI] [PubMed] [Google Scholar]
- Alberto M.R., Manca D., Arena M.E. Influence of phenolic compounds on the growth and arginine deiminase system in a wine lactic acid bacterium. Brazilian Journal of Microbiology. 2012;43(1) doi: 10.1590/S1517-83822012000100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerine M.A., Kunkee R.E., Ough C.S., Singleton V.L., Berg H.W. AVI Pub. Co; 1980. The technology of wine making. [Google Scholar]
- Ancín-Azpilicueta, G. (2008). Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. LWT - Food Science and Technology.
- Burin V., Gomes T., Caliari V., Rosier J., Luiz M. Establishment of influence the nitrogen content in musts and volatile profile of white wines associated to chemometric tools. Microchemical Journal. 2015;122:20–28. doi: 10.1016/j.microc.2015.03.011. [DOI] [Google Scholar]
- Carrasco P., Pérez-Ortín J.E., Olmo M.l.d. Arginase activity is a useful marker of nitrogen limitation during alcoholic fermentations. Systematic & Applied Microbiology. 2003;26(3):471–479. doi: 10.1078/072320203322497518. [DOI] [PubMed] [Google Scholar]
- Chen S., Wang C.C., Qian M., Li Z., Xu Y. Characterization of the key aroma compounds in aged chinese rice wine by comparative aroma extract dilution analysis, quantitative measurements, aroma recombination, and omission studies. Journal of Agricultural and Food Chemistry. 2019;67(17):4876–4884. doi: 10.1021/acs.jafc.9b01420. [DOI] [PubMed] [Google Scholar]
- Chuangye Y., Ruijuan H., Xiaodong Q., Ruijiao Response to different dietary carbohydrate and protein levels of pearl oysters (Pinctada fucata martensii) as revealed by GC-TOF/MS-based metabolomics. The Science of the Total Environment. 2018 doi: 10.1016/j.scitotenv.2018.10.023. [DOI] [PubMed] [Google Scholar]
- Elberry H.M., Majumdar M.L., Cunningham T.S., Sumrada R.A., Cooper T.G. Regulation of the urea active transporter gene (DUR3) in Saccharomyces cerevisiae. Journal of Bacteriology. 1993;175(15):4688–4698. doi: 10.1007/BF02182739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelwood L.A., Daran J.M., Maris A., Pronk J.T., Dickinson J.R. The ehrlich pathway for fusel alcohol production: A century of research on saccharomyces cerevisiae metabolism. Applied and Environmental Microbiology. 2008;74(12):2259–2266. doi: 10.1128/AEM.02625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.K., Lee M.Y., Kim H.Y., Lee S., Yeo S.H., Baek S.Y., Lee C. Metabolite profiling and antioxidant activity comparison of traditional and commercial vinegars. Journal of Microbiology & Biotechnology. 2014 doi: 10.4014/jmb.1408.08021. [DOI] [PubMed] [Google Scholar]
- Jiao Z. Zhe Jiang University; 2016. The effect of transcriptome repressor Dal80p on ethyl carbamate formation during Chinese rice wine fermentation. [Google Scholar]
- Kanani H., Chrysanthopoulos P.K., Klapa M.I. Standardizing GC-MS metabolomics. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2008;871(2):191–201. doi: 10.1016/j.jchromb.2008.04.049. [DOI] [PubMed] [Google Scholar]
- Leskó A., Kállay M., Nyúl-Pühra B., Nyitrai-Sárdy D. The change of polyphenolic composition and tyrosol content of the wine as an effect of sur lie method. Acta Alimentaria. 2011;40(Supplement 1):79–90. doi: 10.1556/AAlim.40.2011.Suppl.8. [DOI] [Google Scholar]
- Li H., Tao Y.S., Wang H., Zhang L. Impact odorants of Chardonnay dry white wine from Changli County (China) European Food Research and Technology. 2008;227(1):287–292. doi: 10.1007/s00217-007-0722-9. [DOI] [Google Scholar]
- Lilly M., Bauer F.F., Lambrechts M.G., Swiegers J.H., Cozzolino D., Pretorius I.S. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast. 2006;23 doi: 10.1002/yea.1382. [DOI] [PubMed] [Google Scholar]
- Mao, Q. (2008). Study on the yeast wash separation in traditional Shaoxing yellow rice wine and its biological characteristics. Jiangsu Condiment and Subsidiary Food. https://doi.org/10.16782/j.cnki.32-1235/ts.2008.04.007.
- Mao Z. Discussion on the biochemical process of traditional rice wine leaching rice wine mother in the nesting stage. Liquor Making. 2004;31(5):3. doi: 10.3969/j.issn.1002-8110.2004.05.034. [DOI] [Google Scholar]
- Pang H., Jia W., Hu Z. Emerging applications of metabolomics in clinical pharmacology. Clinical Pharmacology & Therapeutics. 2019;106(3) doi: 10.1002/cpt.1538. [DOI] [PubMed] [Google Scholar]
- Pang Z., Chong J., Zhou G., de Lima Morais D.A., Chang L., Barrette M.…Xia J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Research. 2021;49(W1):W388–W396. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q., Tian R., Chen F., Li B., Gao H. Discrimination of producing area of Chinese Tongshan kaoliang spirit using electronic nose sensing characteristics combined with the chemometrics methods. Food Chemistry. 2015;178(jul.1):301–305. doi: 10.1016/j.foodchem.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Peng Q., Xu Q., Dula B.G., Wang J., Ding Y. Discrimination of geographical origin of camellia seed oils using electronic nose characteristics and chemometrics. Journal für Verbraucherschutz und Lebensmittelsicherheit. 2020;15(9) doi: 10.1007/s00003-020-01278-x. [DOI] [Google Scholar]
- Reynolds A.G., Edwards C.G., Cliff M.A., Iii J., Marr J.C. Evaluation of yeast strains during fermentation of Riesling and Chenin blanc musts. American Journal of Enology & Viticulture. 2001;52(4):336–344. [Google Scholar]
- Saigusa D., Matsukawa N., Hishinuma E., Koshiba S. Identification of biomarkers to diagnose diseases and find adverse drug reactions by metabolomics. Drug Metabolism and Pharmacokinetics. 2020 doi: 10.1016/j.dmpk.2020.11.008. [DOI] [PubMed] [Google Scholar]
- Sychrova H., Chevallier M.R. Yeast sequencing reports. APL1, a yeast gene encoding a putative permease for basic amino acids. Yeast. 1994 doi: 10.1002/yea.320100509. [DOI] [PubMed] [Google Scholar]
- Wang J., Shen Y., Lu W., Qian Y. Situation and development trend of Chinese rice wine research. China Brewing. 2012;31(11):6. doi: 10.3969/j.issn.0254-5071.2012.11.005. [DOI] [Google Scholar]
- Wishart, David S. Advances in metabolite identification. Bioanalysis. 2011;3(15):1769–1782. doi: 10.4155/BIO.11.155. [DOI] [PubMed] [Google Scholar]
- Xian Y.P., Wu Y.L., Dong H., Liang M., Wang B., Wang L.…Zhao X.J. Ice-bath assisted sodium hydroxide purification coupled with GC-MS/MS analysis for simultaneous quantification of ethyl carbamate and 12 N-nitrosoamines in yellow rice wine and beer. Food Chemistry. 2019;300:9. doi: 10.1016/j.foodchem.2019.125200. [DOI] [PubMed] [Google Scholar]
- Xie G., Zheng H., Qiu Z., Lin Z., Peng Q., Dula Bealu G.…Liu G. Study on relationship between bacterial diversity and quality of Huangjiu (Chinese Rice Wine) fermentation. Food Science & Nutrition. 2021;9(7):3885–3892. doi: 10.1002/fsn3.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki O.C., Eylem C.C., Recber T., Kir S., Nemutlu E. Integration of GC-MS and LC-MS for untargeted metabolomics profiling. Journal of Pharmaceutical and Biomedical Analysis. 2020;190:17. doi: 10.1016/j.jpba.2020.113509. [DOI] [PubMed] [Google Scholar]
- Zeng Q., Song H., Xu X., Mao W. Health effects of kiwi wine on rats: An untargeted metabolic fingerprint study based on GC-MS/TOF. RSC Advances. 2019;9 doi: 10.1039/c9ra02138h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.W., Zhang C.Y., Dai L.H., Liu Y.L., Guo X.W., Xiao D.G. Effects of overexpression of the alcohol acetyltransferase-encoding gene ATF1 and disruption of the esterase-encoding gene IAH1 on the flavour profiles of Chinese yellow rice wine. International Journal of Food Science & Technology. 2012;47(12):2590–2596. doi: 10.1111/j.1365-2621.2012.03140.x. [DOI] [Google Scholar]
- Zhang P., Du G., Zou H., Chen J., Xie G., Shi Z., Zhou J. Effects of three permeases on arginine utilization in Saccharomyces cerevisiae. Scientific Reports. 2016;6(1):20910. doi: 10.1038/srep20910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z. (2021). Research on driving forces of microbial community succession of Shaoxing Huangjiu raw wheat Qu and optimization of kojimaking process. https://doi.org/10.27169/d.cnki.gwqgu.2021.001403.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.