Abstract
Background
The primary purpose of this study was to determine if cannabis use decreases narcotic consumption in patients undergoing total joint arthroplasty (TJA).
Material and methods
Forty-six patients undergoing a primary unilateral TJA, who self-reported the use of cannabis, were prospectively enrolled and completed this study between July 2015 and November 2019. This cohort was prospectively matched to patients who did not report cannabis use. Morphine equivalents (MEs) were averaged and recorded at 1 and 2 weeks postoperatively. Secondary outcomes and complications were recorded and reported.
Results
There were no differences noted in ME during the hospitalization between the user (78.7 ± 58.5) and nonusers (70.4 ± 46.3), P = .455. ME daily average did not differ between the cohorts (user [36.8 ± 30.7] and nonuser [31.7 ± 25.6] at 1 week (P = .389) or user [22.5 ± 26.3] and nonusers [15.9 ± 18.3] at 2 weeks, P = .164, postoperatively). The total ME at 2 weeks did not differ between the user and nonuser groups (415 ± 375 vs 333 ± 275, P = .235). Pain scores at 1 week were significantly higher in patients who used cannabis (4.1 ± 1.9 vs 3.4 ± 1.6, P = .05). No differences in pain were noted during the patient’s hospitalization or at 2- (P = .071) or 6-week (P = .111) follow-up. No differences in secondary outcomes or complications were noted.
Conclusion
We were unable to show a decrease in narcotic consumption in patients who use cannabis undergoing primary unilateral joint replacement. These findings do not support the routine use of cannabis to decrease or supplement narcotic use after primary TJA.
Level of evidence
Level II therapeutic.
Keywords: Total knee arthroplasty, Marijuana, Cannabinoids, Opioid, Narcotics
Introduction
Despite widespread use of multimodal pain protocols, opioids are often needed to obtain adequate pain management after total joint arthroplasty (TJA). However, narcotic use has been associated with increased morbidity and mortality in elective orthopedic procedures [[1], [2], [3]]. For these reasons, alternatives to decrease or negate the need of opioids for pain management are being explored.
The recent legalization of cannabis (recreational and/or medicinal) in many states has led to an increase in interest in its potential use for pain management after orthopedic procedures [[4], [5], [6], [7], [8], [9]]. The self-reported use in patients undergoing TJA increased from 1% to 11% after its legalization in Colorado [10]. Cannabis may function to reduce sensitization of the nociceptive sensory pathways and induce alterations in cognitive and autonomic processing in selective chronic pain conditions [11,12]. However, the evidence to date does not support its use in patients undergoing orthopedic procedures [[8], [9], [13], [14], [15]]. Therefore, the primary purpose of this prospective study was to determine if cannabis use decreases narcotic consumption in patients undergoing TJA. We hypothesize that patients using cannabis would require fewer morphine equivalents (MEs) postoperatively than patients who do not use cannabis. Secondary outcomes included the evaluation of pain, range of motion (ROM), postoperative complications, Knee Society Scores (KSS) or Hip Society Scores (HSS), The Veterans RAND 12 Item Health Survey (VR-12) mental component score (MCS), and VR-12 physical component score (PCS).
Material and methods
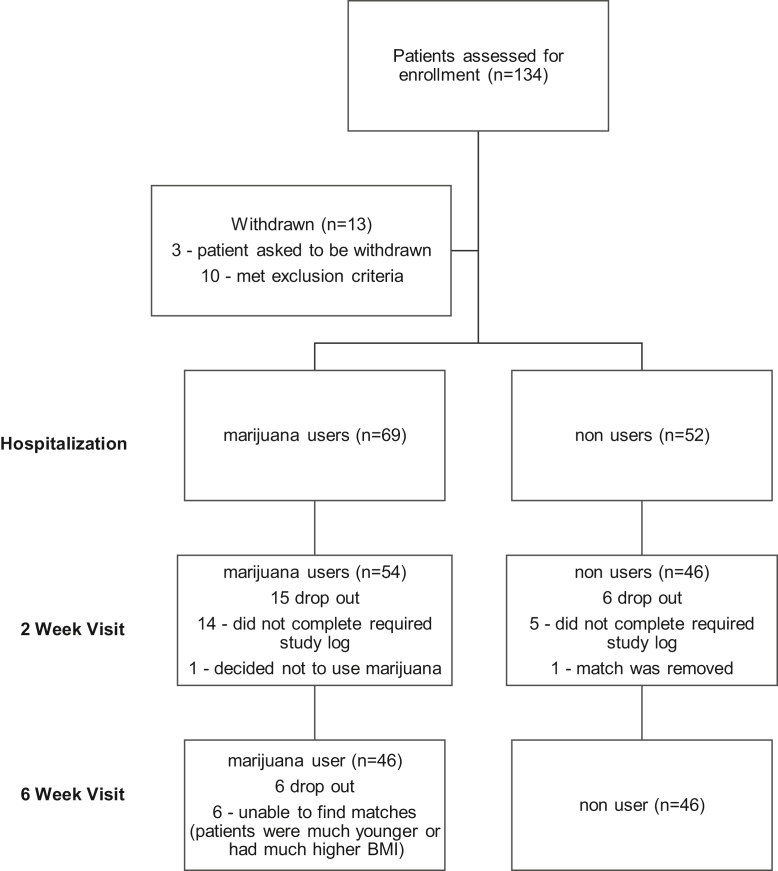
This study was approved by our institutional review board prior to its initiation. Colorado Amendment 64 legalized cannabis in 2012, with the commercial sale of cannabis to the general public initiated on January 1, 2014, at establishments licensed under the regulatory framework. After the legalization, our patient intake form reflected cannabis as a drug, but not as an illegal drug. We therefore have inquired and asked all new and existing patients in our clinic about its use as the standard of care in our practice. We prospectively identified patients undergoing primary unilateral TJA at our institution who self-reported cannabis use on a regular basis prior to their surgical intervention. The study period was continuous from July 2015 to November 2019. All patients were active users at the time of surgery. Patients were excluded with a history of narcotic use, alcohol (>7 drinks per week) or drug abuse, tobacco use, depression, anxiety, or a previous surgical intervention within the past 6 months. These patients were excluded in an attempt to limit postoperative variables that have been associated with adverse outcomes. This cohort was prospectively matched (1:1) (age, sex, body mass index) to patients who did not report cannabis use having a primary unilateral TJA during this study period. Additionally, none of the matched patients had a history of narcotic use, alcohol or drug abuse, tobacco use, depression, anxiety, or previous surgical intervention within the past 6 months. Patients that met the inclusion criteria were approached at their preoperative appointment by a member of our research team for potential recruitment (Fig. 1). All patients completed a preoperative questionnaire regarding cannabis, alcohol, and tobacco use.
Figure 1.
CONsolidated Stands of reporting Trials diagram.
All TJAs were performed by 1 of 5 surgeons at our institution. Preoperative adductor canal blocks and spinal anesthesia were utilized in each patient for knee arthroplasty surgery. Spinal anesthesia was performed for all patients undergoing total hip arthroplasty. Patients received 1 gram of tranexamic acid prior to incision and 1 gram prior to closure. Before wound closure, all patients received an intraarticular injection of a cocktail containing ropivacaine, epinephrine, clonidine, and ketorolac to enhance postoperative analgesia control. Patients were provided with the same standard rehabilitation protocol during their inpatient stay. In addition, outpatient physical therapy was performed without variation from our routine postoperative protocol between the cohorts.
The primary outcome of narcotic use via ME was recorded during the patients' hospitalization and averaged at 1 and 2 weeks postoperatively. Patients were given detailed instructions preoperatively and postoperatively regarding recording narcotic use and pain at selective time intervals. Additionally, phone calls were made to each subject on discharge day 1, 3, and 7 to assure compliance with recording these variables. Secondary outcomes included pain (visual analog scale), ROM, KSS or HSS, VR-12 MCS, and VR-12 PCS, all of which were evaluated preoperatively and postoperatively). Length of stay, ME while hospitalized, and complications were recorded and reported.
Cannabis use was not recommended to users (or nonusers) at any point in time during this study. Patients continued their regular use at their discretion preoperatively and postoperatively. The type (ie, edible vs inhalation) and frequency of cannabis use were recorded by patients postoperatively. No attempt was made to regulate the usage (ie, frequency, type, or amount) of cannabis. Despite being legal in the authors state, cannabis is still considered a Scheduled I drug, and its regulation falls under federal law. As such, health-care providers who recommend its use may be at risk for losing their Medicare participation status. This justified the design of the current study based on inherent limitations of this treatment.
Postoperatively all patients were placed on similar multimodal pain-control regimens including the use of tramadol, nonsteroidal anti-inflammatory drugs, gabapentin, acetaminophen, and rescue oral narcotic (hydrocodone/apap, oxycodone, or hydromorphone). MEs were averaged for day 7 and day 14 postoperatively. Pain scores via the visual analog scale were obtained at 6, 12, 24, 36, and 48 hours postoperatively. Additional time points for pain monitoring at 1, 2, and 6 weeks postoperatively were obtained. ROM measurements were measured at 6 weeks. KSS and VR-12 (MCS and PCS) were filled out by the patient at their 6-week follow-up appointment.
Descriptive statistics were provided using means and standard deviations for continuous variables while frequencies and relative percentages are reported for categorical variables. The paired Student’s t-test was used to test the difference between the users and nonusers for continuous variable outcomes including pain, function, ROM, KSS or HSS, VR-12 scores, length of stay, and narcotic usage. Statistical analyses were performed using Minitab version 17.0 (Statistical Analysis Software, State College, PA). P values of less than 0.05 indicated statistical significance. In a prior study, the average ME dose at 24 hours postoperatively were 17 ± 5.5 mg and at 48 hours 18.4 ± 5.5 mg [16]. Using the 48-hour average, an a priori power analysis with 80% power, P of <.5, and 25% reduction in the ME dose in the cannabis use group demonstrated a need for 22 participants in each cohort.
Results
Ninety-two patients completed the study (46 in each cohort) (Fig. 1). The mean age was 61.3 ± 8.4 years, and there were 24 males (52%) and 22 females (48%) enrolled. The preoperative VR-12 (MCS) revealed that the cannabis users had a significant decrease in their score compared with the nonuser cohort (51.5 ± 11.4 vs 57.2 ± 10.8, P = .017). Otherwise, there were no differences in preoperative secondary outcome measures between the cohorts (Table 1). The mean length (hours) of hospital stay in the user group was 31.5 ± 7.6, and for nonusers, 29.2 ± 7.4 (P = .143). Pain scores during the hospitalization did not differ between the 2 groups (Table 2). There were no differences noted in ME during the hospitalization between the users (78.7 ± 58.5) and nonusers (70.4 ± 46.3), P = .455. ME daily average did not differ between the cohorts (user [36.8 ± 30.7] and nonuser [31.7 ± 25.6] at 1 week (P = .389) or user [22.5 ± 26.3] and nonusers [15.9 ± 18.3] at 2 weeks, P = .164, postoperatively). The total ME at 2 weeks did not differ between the user and nonuser groups (415 ± 375 vs 333 ± 275, P = .235). The cannabis users had a significantly higher pain score average at 1 week than the nonuser cohort (4.1 ± 1.9 vs 3.4 ± 1.6, P = .05). These differences were not seen with the 2-week ME average between users and nonusers (3.5 ± 1.9 vs 2.9 ± 1.5, P = .071). No differences in secondary outcomes were noted at 6 weeks (ROM, KSS or HSS, VR-12 [MCS or PCS]) (Table 3). The overall change from baseline (secondary outcomes) with regard to pain, KSS or HSS, and VR-12 scores did not reveal differences between the cohorts. The types and frequency of cannabis consumed are outlined in Table 4. Lastly, no postoperative complications were noted in either cohort.
Table 1.
Preoperative secondary outcome measures.
| Secondary outcomes | User (average ± SD) N = 46 | Nonuser (average ± SD) N = 46 | Statistical analysis |
|---|---|---|---|
| Preoperative | |||
| VR-12 (MCS) | 51.5 ± 11.4 | 57.2 ± 10.8 | P = .017 |
| VR-12 (PCS) | 35.0 ± 9.2 | 34.3 ± 9.5 | P = .748 |
| Knee patients (N = 25) | |||
| Function | 64.8 ± 20.0 | 69.4 ± 28.0 | P = .508 |
| ROM (Ext./Flex.) | 118.9 ± 7.8 | 117.0 ± 13.5 | P = .533 |
| KSS | 46.3 ± 15.9 | 48.0 ± 17.5 | P = .717 |
| Hip patient (N = 21) | |||
| Function | 34.8 ± 7.6 | 31.8 ± 8.9 | P = .255 |
| ROM | 92.4 ± 8.6 | 93.9 ± 10.7 | P = .636 |
| HSS | 58.9 ± 13.9 | 56.0 ± 16.2 | P = .542 |
Ext., extension; Flex., flexion; SD, standard deviation.
Table 2.
Postoperative (PO) pain control measured by VAS.
| Hospital stay | |||
|---|---|---|---|
| Postoperative pain | User (average ± SD) N = 46 | Nonuser (average ± SD) N = 46 | Statistical analysis |
| Pain, 6 h PO | 2.9 ± 1.9 | 2.9 ± 2.0 | P = .916 |
| Pain, 12 h PO (N) | 3.0 ± 1.8 (45) | 2.9 ± 1.2 (45) | P = .786 |
| Pain, 24 h PO (N) | 3.0 ± 2.2 (42) | 3.0 ± 1.7 (41) | P = .999 |
| Pain, wk 1 average | 4.1 ± 1.9 | 3.4 ± 1.6 | P = .055 |
| Pain, wk 2 average | 3.5 ± 1.9 | 2.9 ± 1.5 | P = .071 |
| Pain (self-reported at visit) | 1.6 ± 1.6 | 1.0 ± 1.6 | P = .111 |
SD, standard deviation; VAS, visual analog scale.
Table 3.
Secondary outcomes at 6-wk follow-up.
| User (average ± SD) N = 46 | Nonuser (average ± SD) N = 46 | Statistical analysis | |
|---|---|---|---|
| 6-Wk follow-up | |||
| VR-12 (MCS) | 52.9 ± 9.5 | 56.2 ± 8.7 | P = .085 |
| VR-12 (PCS) | 38.5 ± 10.0 | 39.1 ± 10.0 | P = .802 |
| Knee patients (N = 25) | |||
| Function | 72.0 ± 20.1 | 78.2 ± 18.1 | P = .258 |
| ROM (Ext./Flex.) | 122.2 ± 10.1 | 118.1 ± 22.4 | P = .408 |
| Knee score | 84.5 ± 12.8 | 85.2 ± 12.0 | P = .245 |
| Hip patients (N = 21) | |||
| Function | 39.8 ± 5.2 | 39.5 ± 6.3 | P = .853 |
| ROM (Ext./Flex.) | 99.5 ± 7.1 | 99.1 ± 8.8 | P = .848 |
| HSS | 86.1 ± 10.3 | 88.5 ± 7.34 | P = .838 |
Ext., extension; Flex., flexion; SD, standard deviation.
Table 4.
Types and frequency of cannabis use.
| Patient ID | Type of marijuana used | How many days used in first 2 wks postop | Average times of use per day in 2 wk |
|---|---|---|---|
| 001 | Edible | 14 | 1.1 |
| 002 | Smoke (bowls smoked) | 7 | 3.6 |
| 003 | Smoke (inhales on a joint) | 6 | 3 |
| 004 | Smoke (inhales on a joint)/Edible | 14 | 1.4 |
| 005 | Edible | 9 | 1.8 |
| 006 | Edible | 7 | 1.3 |
| 007 | Smoke (bowls smoked) | 14 | 5.1 |
| 008 | Smoked (inhales on a joint) | 13 | 7.2 |
| 009 | Edible | 6 | 1.2 |
| 010 | Smoke (whole joints) | 7 | 0.8 |
| 011 | Edible/Patch/Oil tincture | 12 | 2.3 |
| 012 | Edible/Vapor | 10 | 6 |
| 013 | Edible | 14 | 1.1 |
| 014 | Smoked (inhales on a joint) | 14 | 4.6 |
| 015 | Edible | 14 | 5.2 |
| 016 | Smoke (inhales on a vape)/Edible | 11 | 1.6 |
| 017 | Edible | 4 | 1 |
| 018 | Smoked (whole joints)/Edible | 14 | 0.8 |
| 019 | Smoke (inhales on a pipe) | 14 | 2.9 |
| 020 | Smoke (inhales on a joint)/Edible | 11 | 5.3 |
| 021 | Smoke (inhales on a joint) | 14 | 2.6 |
| 022 | Smoke (inhales on a joint)/Oil tincture | 14 | 8.6 |
| 023 | Smoke (inhales on a joint) | 13 | 37.6 |
| 024 | Vapor | 6 | 3.5 |
| 025 | Vapor | 14 | 2.6 |
| 026 | Edible | 12 | 1.2 |
| 027 | Edible/Smoke (inhales on a joint) | 14 | 1.8 |
| 028 | Smoke (inhales on a joint) | 10 | 3 |
| 029 | Edible | 12 | 2.7 |
| 030 | Smoke (inhales on a vape)/Edible | 14 | 4.4 |
| 031 | Smoke (inhales on a joint) | 14 | 18.6 |
| 032 | Smoke (inhales on a vape)/Edible | 14 | 8.64 |
| 033 | Smoke (inhales on a pipe) | 13 | 9.4 |
| 034 | Smoke (whole joints)/Edible | 13 | 1.2 |
| 035 | Edible | 14 | 1.4 |
| 036 | Edible | 13 | 2.6 |
| 037 | Edible | 10 | 2.8 |
| 038 | Smoke (inhales on a vape) | 14 | 2.3 |
| 039 | Edible | 8 | 0.6 |
| 040 | Smoke (inhales on a vape) | 13 | 3.1 |
| 041 | Edible | 10 | 0.9 |
| 042 | Smoke (inhales on a pipe) | 10 | 1.8 |
| 043 | Edible | 14 | 4.1 |
| 044 | Edible | 9 | 0.6 |
| 045 | Smoke (inhales on a bong) | 5 | 0.8 |
| 046 | Smoke (inhales on a joint)/Edible | 9 | 2.4 |
Discussion
To date, there remains a paucity of scientific evidence to support the use of medicinal cannabis for many medical conditions. If a benefit can be demonstrated with regard to pain management, there may be potential to help stem the opioid epidemic. As such, the primary aim of this study was to determine if cannabis use decreases narcotic consumption in patients undergoing TJA. Our prospectively matched cohort study did not show a difference in ME at any of our time intervals. Additionally, patients who used cannabis did not demonstrate adverse effects or a difference in short-term outcomes. To our knowledge, this is the first study exploring the use of cannabis and its relationship to pain management in patients undergoing TJA.
Cannabis use has been associated with decreased opioid consumption in patients with chronic pain (noncancer) conditions. The “opioid-sparing” effect hints to potential synergistic effects between cannabis and opioids in this patient population [[17], [18], [19]]. A recent retrospective review demonstrated no differences in ME in patients using dronabinol vs patients who did not during their hospitalization after TJA [8]. Our data support these findings, and in our cohort, we did not see ME differences during the patients' hospitalization or at the 1- or 2-week postoperative follow-up. While there were no differences in ME at our time intervals, we did find a significant difference in pain scores in the first week with the cannabis users showing an increase in level of their week-1 average scores. With this increase in pain and the same amount of ME needed to obtain adequate pain control, one could argue that the cannabis user group actually required more “medication” between the narcotic use and cannabis to obtain the control. It certainly is possible that the “tolerance” from cannabis may play a role in these patients. Unlike other areas in medicine, we did not observe the “opioid-sparing” effect in our study cohort. We do concede there certainly may be a difference between acute and chronic conditions which may have accounted for these differing findings. Further studies are needed in this area to see if this result is generalizable.
Patients with a hstory of “drug misuse” have been reported to have increased postoperative complications after TJA [2,3,14,20,21]. In an attempt to decrease the potential variables associated with “worse” patient outcomes (ie, tobacco use, alcohol abuse, illegal drug use), we only included patients with isolated cannabis use. For this reason, our recruitment process was labor-intensive and prolonged to try and control for these variables. Additionally, we are limited by federal regulations which continue to maintain cannabis as Scheduled I drug. Our results in this small cohort suggest no differences in early postoperative complications or outcomes. These findings are similar to those of a previous retrospective study out of our institution regarding short-term outcomes in this patient population [15]. Future studies should focus on long-term outcomes in this patient population.
The legalization of cannabis has led to either more users or more patients willing to self-report use given the lack of legal ramifications [10]. Surgeons should be aware that their patients may be currently using cannabis for medicinal and/or recreational purposes with or without reported use. Many patients are currently self-medicating with cannabis of different types (ie, edible, inhalation), dosage, and frequency of use, and these numbers are likely to increase. Our data show surprising differences between patients with all the variables we looked at in this cohort (Table 4). To our knowledge, this is the first study looking at the variability between type and frequency of use in patients that are using cannabis after TJA. This variability raises many questions for health-care providers. A sound knowledge by the orthopedic community regarding cannabis is imperative given the increased interest in this patient population for both medicinal and recreational purposes. The potential synergistic effects in combination with opioid use must be studied on a larger scale to demonstrate the potential efficacy and safety before recommendations can be made for its perioperative use in patients undergoing TJA.
This study is not without limitations. Our secondary outcome for pain average at 1 week certainly may have been underpowered as this was not our primary outcome. Our cohort was limited to patients who self-reported the use of various types (ie, inhalation vs edible), duration, dosage, and frequency of use both preoperatively and postoperatively. Our postoperative frequency and types of cannabis used varied greatly, but we do feel this is a fair assessment of what patients are doing in the perioperative period. There certainly may have been patients who did not report use in the nonuser cohort that we were unable to account for in this study. We feel we made every effort to control for this by having our orthopedic team, preoperative medical team, and research team screen for use in all patients. The amount of delta-9-tertrahydrocannabinol and cannabidiol is variable, making these differences hard to account for in this patient cohort. Additionally, the quality and reliability between different types of cannabis are well known and are impossible to be controlled for with this particular study design. We still believe that this is a valid way to explore cannabis use in this patient population since there currently is no regulation for these variables. Ideally patients would be randomized and have a specific form of cannabis administered (dose, frequency, type) postoperatively, but this type of study design is currently difficult since cannabis is still considered a Scheduled I drug. We have recently been approved at our institution to study this in a prospective randomized blinded fashion, which we believe can further answer questions that remain with cannabis use and its application after TJA. Part of this approval process was our institution presenting these data to our federal regulators to assist with this process. We concede that the small cohort may not reveal all the benefits that a large cohort would offer. However, recruitment into the study was difficult secondary to the exclusion of a large majority of the prospective population for narcotic use, tobacco use, and the use of other drugs, all of which have been associated with adverse outcomes in patients undergoing TJA. This is not surprising since the self-reported use of cannabis in patients undergoing TJA is more common in patients that are current smokers, use preoperative narcotics, and have a history of substance abuse [10]. We did combine both hip and knee arthroplasty procedures to increase our cohort size. Ideally in the future, this may be done with separate cohorts but may require a multicenter study if all the aforementioned variables are eliminated to only find patients with isolated cannabis use. As we have previously stated, finding patients with isolated cannabis use is very difficult. We did not attempt to distinguish between the reason for cannabis use (recreational vs medicinal). The distinction between the reason for use is often difficult as many “medicinal” users have used cannabis for recreational reasons in the past [22,23]. The frequency and duration of preoperative use were not extensively explored in this study, and therefore, we concede that this certainly could have led to a confounding effect. Future studies should aim to identify cannabis naive patients to see if this may change the narcotic consumption postoperatively as preoperative users may have a “tolerance” that negates the synergistic effects to potentially decrease opioid consumption. Additionally, we stopped looking monitoring ME at 2 weeks postoperatively. It certainly would have been reasonable to determine ME for 6 weeks to see if there were differences in ME or other outcome measures at this time point. Lastly, we did not monitor nausea, sleep patterns, and outcomes which should be an area of future study in this patient population. This is an area we plan to explore in a future study.
Conclusions
In conclusion, we were unable to show a decrease in narcotic consumption in patients, who use cannabis, undergoing primary unilateral TJA. One may argue that patients who use cannabis require more for pain management (ie, equivalent opioid + cannabis) than nonusers. Lastly, the cannabis type and frequency of use varied greatly after TJA. These findings do not currently support the routine postoperative use of cannabis to decrease narcotic consumption after primary unilateral TJA.
Conflicts of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: J. M. Jennings receives royalties from Total Joint Orthopedics; is a paid consultant for Total Joint Orthopedics and Xenex; has stock or stock options in Xenex; and receives research support as a principal investigator from DePuy, a Johnson & Johnson Company. D. C. McNabb is a paid consultant for Total Joint Orthopedics. R. H. Kim receives royalties from DJ Orthopaedics and Innomed; is in the speakers' bureau of or gave paid presentations for Ceramtec and Convatec; is a paid consultant for DJ Orthopaedics; and is a board member of International Congress for Joint Reconstruction. D. A. Dennis receives royalties from DePuy, a Johnson & Johnson Company; is in the speakers' bureau of or gave paid presentations for DePuy, a Johnson & Johnson Company; is a paid consultant for Corin USA and DePuy, a Johnson & Johnson Company; has stock or stock options in Joint Vue; receives research support as a principal investigator from DePuy, a Johnson & Johnson Company, and Porter Adventist Hospital; receives material or financial support from Wolters Kluwer Health—Lippincott Williams & Wilkins; and is in the editorial or governing board of Clinical Orthopaedics and Related Research, Journal of Arthroplasty, Journal of Bone and Joint Surgery, and Orthopedics Today.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2022.03.018.
Appendix A. Supplementary data
References
- 1.Morris B. The opioid epidemic: impact on orthopaedic surgery. J Am Acad Orthop Surg. 2015;23:267–271. doi: 10.5435/JAAOS-D-14-00163. [DOI] [PubMed] [Google Scholar]
- 2.Menendez M.E., Ring D., Bateman B.T. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473:2402–2412. doi: 10.1007/s11999-015-4173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancienne J.M., Patel K.J., Browne J.A., Werner B.C. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33:113–118. doi: 10.1016/j.arth.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Chan M.H., Knoepke C.E., Cole M.L., McKinnon J., Matlock D.D. Colorado medical students’ attitudes and beliefs about marijuana. J Gen Intern Med. 2017;32:458–463. doi: 10.1007/s11606-016-3957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khelemsky Y., Goldberg A.T., Hurd Y.L., et al. Perioperative patient beliefs regarding potential effectiveness of marijuana (cannabinoids) for treatment of pain: a prospective population survey. Reg Anesth Pain Med. 2017;42:652–659. doi: 10.1097/AAP.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 6.Heng M., McTague M.F., Lucas R.C., Harris M.B., Vrahas M.S., Weaver M.J. Patient perceptions of the use of medical marijuana in the treatment of pain after musculoskeletal trauma. J Orthop Trauma. 2018;32:e25–e30. doi: 10.1097/BOT.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 7.Hayes M.J., Brown M.S. Legalization of medical marijuana and incidence of opioid mortality. JAMA Intern Med. 2014;174:1673. doi: 10.1001/jamainternmed.2014.2716. [DOI] [PubMed] [Google Scholar]
- 8.Hickernell T.R., Lakra A., Berg A., Cooper H.J., Geller J.A., Shah R.P. Should cannabinoids be added to multimodal pain regimens after total hip and knee arthroplasty? J Arthroplasty. 2018;33:3637–3641. doi: 10.1016/j.arth.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Kleeman-Forsthuber L.T., Dennis D.A., Jennings J.M. Medicinal cannabis in orthopaedic practice. J Am Acad Orthop Surg. 2019;28:268–277. doi: 10.5435/JAAOS-D-19-00438. [DOI] [PubMed] [Google Scholar]
- 10.Jennings J.M., Williams M.A., Levy D.L., Johnson R.M., Eschen C.L., Dennis D.A. Has self-reported marijuana use changed in patients undergoing total joint arthroplasty after the legalization of marijuana? Clin Orthop Relat Res. 2019;477:95–100. doi: 10.1097/CORR.0000000000000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzcharles M.-A., Häuser W. Cannabinoids in the management of musculoskeletal or rheumatic diseases. Curr Rheumatol Rep. 2016;18:76. doi: 10.1007/s11926-016-0625-5. [DOI] [PubMed] [Google Scholar]
- 12.Di Marzo V., Bifulco M., De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 13.Bhashyam A.R., Heng M., Harris M.B., Vrahas M.S., Weaver M.J. Self-reported marijuana use is associated with increased use of prescription opioids following traumatic musculoskeletal injury. J Bone Joint Surg Am. 2018;100:2095–2102. doi: 10.2106/JBJS.17.01400. [DOI] [PubMed] [Google Scholar]
- 14.Law T.Y., Kurowicki J., Rosas S., et al. Cannabis use increases risk for revision after total knee arthroplasty. J Long Term Eff Med Implants. 2018;28:125–130. doi: 10.1615/JLongTermEffMedImplants.2018027401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jennings J., Angerame M., Eschen C.L., Phocas A., Dennis D.A. Cannabis use does not affect outcomes after TKA. J Arthroplasty. 2019;34:1667–1669. doi: 10.1016/j.arth.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Lamplot J.D., Wagner E.R., Manning D.W. Multimodal pain management in total knee arthroplasty. a prospective randomized controlled trial. J Arthroplasty. 2014;29:329–334. doi: 10.1016/j.arth.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Degenhardt L., Lintzeris N., Campbell G., et al. Experience of adjunctive cannabis use for chronic non-cancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend. 2015;147:144–150. doi: 10.1016/j.drugalcdep.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Boehnke K.F., Litinas E., Clauw D.J. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain. 2016;17:739–744. doi: 10.1016/j.jpain.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Bachhuber M.A., Saloner B., Cunningham C.O., Barry C.L. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010. JAMA Intern Med. 2014;174:1668. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Ari A., Chansky H., Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty. J Bone Joint Surg Am. 2017;99:1–9. doi: 10.2106/JBJS.16.00167. [DOI] [PubMed] [Google Scholar]
- 21.Best M.J., Buller L.T., Klika A.K., Barsoum W.K. Outcomes following primary total hip or knee arthroplasty in substance misusers. J Arthroplasty. 2015;30:1137–1141. doi: 10.1016/j.arth.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 22.Ablin J., Ste-Marie P.A., Schafer M., Hauser W., Fitzcharles M.-A. Medical use of cannabis products: lessons to be learned from Israel and Canada. Schmerz. 2016;30:3–13. doi: 10.1007/s00482-015-0083-4. [DOI] [PubMed] [Google Scholar]
- 23.Ste-Marie P.A., Shir Y., Rampakakis E., et al. Survey of herbal cannabis (marijuana) use in rheumatology clinic attenders with a rheumatologist confirmed diagnosis. Pain. 2016;157:2792–2797. doi: 10.1097/j.pain.0000000000000706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.