Abstract
In Chronic Obstructive Pulmonary Disease (COPD), hypoxemia is associated with multiple underlying mechanisms, of which one of the most significant is ventilation-perfusion (V/Q) mismatch, which is correctable with supplemental oxygen (O2) therapy. Hypoxemia that is refractory to very high concentration of inspired O2 can be indicative of cardiac defect with shunt, e.g., a patent foramen ovale (PFO) with right-to-left (R-T-L) shunt. In hypoxemic COPD patients, the diagnosis of a PFO requires a heightened sense of clinical suspicion along with careful assessment of other underlying possibilities. Platypnea-orthodeoxia and a non-response to the hyperoxia test, while not diagnostic, increase suspicion. A correct diagnosis of interatrial bypass needs to be confirmed with transthoracic echocardiogram and contrast transesophageal echocardiography. Presently, no data are available supporting the effectiveness of PFO closure in COPD patients to relieve symptoms and correct hypoxemia.
We report a case of hypoxemic COPD with platypnea-orthodeoxia syndrome due to PFO. The decision of its closure with device after echocardiographic evaluation of right ventricular function has completely corrected refractory hypoxemia with improvement of SpO2 and functional capacity. Thus, in selected COPD with refractory hypoxemia, closure of PFO should be considered as novel therapeutic target with improvement of quality of life and less likelihood of hospitalization.
Keywords: COPD, Hypoxemia, Cardiac shunt, PFO
1. Introduction
Hypoxemia is defined as the decrease in partial pressure of oxygen (O2) in the arterial blood generated by four pathophysiological mechanisms: 1) ventilation/perfusion mismatch, 2) increased shunt, 3) diffusion impairment, and 4) alveolar hypoventilation. These mechanisms individually are associated with a wide variety of diseases leading to hypoxemia with normocapnia or hypocapnia (hypoxemic or type I respiratory failure/lung failure) and hypoxemia with hypercapnic (or type II respiratory failure/pump failure) [1].
In chronic obstructive pulmonary disease (COPD) ventilation/perfusion (V/Q) mismatching is the most common mechanism. It develops when there is a decreased ventilation to normally perfused regions or regions with a more significant reduction in ventilation than in perfusion occurs in the lung. On the other hand, intrapulmonary or intracardiac shunting causes deoxygenated mixed venous blood to bypasses ventilated alveoli, resulting in “venous admixture”. While, hypoxemia resulting from V/Q inequality or diffusion abnormalities can easily be corrected by supplementing inspired oxygen, even very high concentrations of inspired oxygen cannot correct hypoxemia induced by a pure shunt.
Hypoxemia caused by R-T-L shunt could respond to percutaneous PFO closure but no data are available regarding PFO closure in COPD patients. An important impediment to successful treatment is the lack of awareness of the potential role of PFO in this condition.
We present an unusual case of a COPD patient with hypoxemia refractory to O2 therapy found to have with a cardiovascular right-to-left (R-T-L) shunt with improvement after closure: percutaneous closure of PFO revealed stable relief of symptoms, with improvement of pulse oxygen saturation (SpO2), functional capacity, quality of life and reduction of health care costs.
2. Case report
An 80-year-old man with COPD was admitted to the hospital with chronic cough and breathlessness. The patient, a former smoker, had a history of hypertension, deep vein thrombosis (DVT), and paroxysmal arterial fibrillation thought to be the cause of a previous stroke. His medications at the time included amiodarone, perindopril, amlodipine, citalopram, inhaled bronchodilators, and warfarin without monitoring of the international normalized ratio (INR) target.
Physical examination was significant for central cyanosis with SpO2 of less than 90%, muffled basal crackles on lung auscultation, and regular cardiac tones with a normal blood pressure of 110/70 mmHg. His previous spirometry showed an FEV1/FVC of 68% with an FEV1 of less than 60%. A plain chest radiograph was unremarkable except for atelectatic streaks at the lung bases. Electrocardiography revealed normal sinus rhythm, a right conduction delay, and left ventricular hypertrophy. Bedside transthoracic echocardiography (TTE) revealed mild left ventricular hypertrophy with preserved ejection fraction, a dilated aortic root, a normal pericardium, and no pleural effusion. Right ventricular systolic function was unable to be assessed due to poor visualization, but systolic pulmonary artery pressure (PAP) was estimated to be 40 mmHg.
Upon further examination, his SpO2 dropped from 90% to 78% on room air, moving from the upright to supine position, clinically compatible with platypnea-orthodeoxia syndrome (POS) [2]. Arterial blood gas analyses confirmed severe hypoxemia with a partial pressure of oxygen (PaO2) of 37 mmHg, partial pressure of carbon dioxide (PaCO2) of 25 mmHg, and a pH of 7.50 while upright, which improved to a PaO2 of 54.8 mmHg when supine. Supplemental O2 via Venturi mask at a fraction of inspired oxygen (FiO2) of 50% was insufficient for correcting this hypoxemia. Even the application of non-invasive ventilation with continuous positive airways pressure (CPAP) with a pressure of 8 cmH2O on an FiO2 of 65% was ineffective.
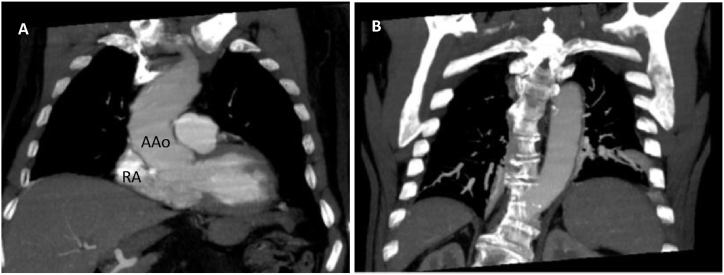
Both a V/Q scan and chest computed tomography (CT) angiography excluded thromboembolism, dilation of pulmonary vessels, and other vascular abnormalities in the lungs and confirmed atelectasis present at the pulmonary bases; furthermore, a spinal deviation and ectatic aorta were evident that associated with an overdeveloped Eustachian valve are conditions facilitating PFO shunting (Fig. 1). A hyperoxia test was performed (HT) [3], showing merely a slight increase in the PaO2 from 37 to 48 mmHg and a widened alveolar-arterial gradient (A-aO2) of 195 mmHg, indicative of an R-T-L shunt.
Fig. 1.
Thorax CT scan (coronal view): (A) Dilated ascending aorta (Aao) with right atrial (RA) shape deformation causing interatrial septal distortion. (B) thoracic spine kyphoscoliosis is an accompanying pathologic condition.
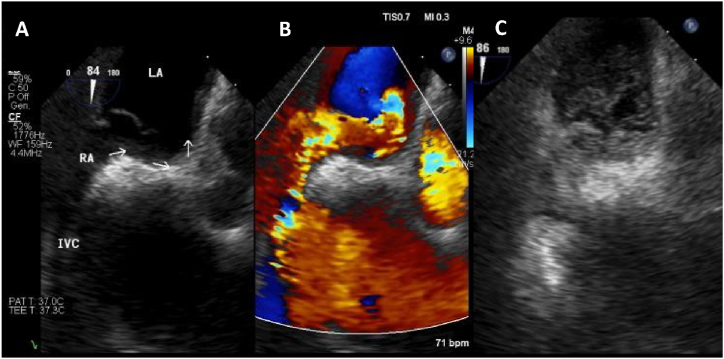
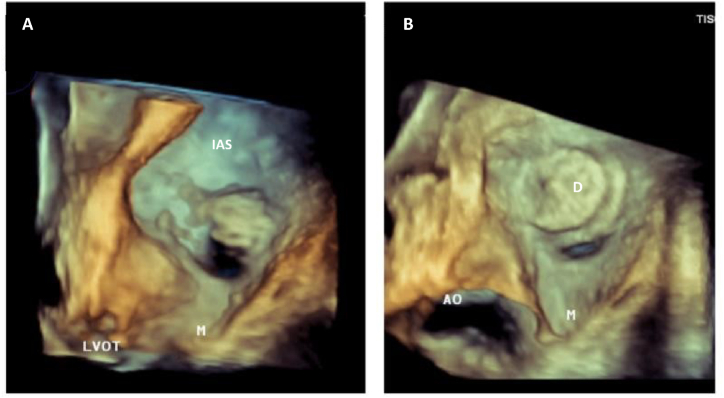
The patient underwent a contrast transesophageal echocardiography (c-TEE), revealing a significant R-T-L shunt through a 1.1 cm patent foramen oval (PFO) and deviation of the interatrial septum to the left (Fig. 2). The PFO was percutaneously closed using an 18–25 Amplatzer septal occlusion device. Post-procedure three-dimensional transthoracic echocardiography (3D-TTE) showed minimum residual shunting (Fig. 3). At the time of discharge, the patient's SpO2 was at 96% on room air with the ability to maintain moderate exercise. On follow-up, his SpO2 remained normal on room air, and a TTE showed preserved left and right ventricular function.
Fig. 2.
TEE 2D: (A) Interatrial septal with PFO. (B) Same position demonstrating right left shunting with color doppler. (C) Non transpulmonary echocontrast. LA: Left Atrial; RA: Right Atrial; IVC: Inferior Vena Cava. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
TTE 3D: Left atrial view of interatrial septal (IAS). (A) Large patent foramen ovale (arrow). (B) Effective transcatheter PFO closure with device and residual minimal hole (arrow). LVOT: Left Ventricular Outflow Tract; M: Mitral valve.
3. Discussion
Presentation of a PFO can be subtle and clues to the disorder can be obtained from meticulous history. The first step in diagnosing PFO is a clinical assessment of the patient's SpO2 that must be in the upright and lying positions. A presumptive diagnosis can be considered if there is a drop in PaO2 > 4 mmHg in the arterial blood or SpO2 > 5% from supine to an upright position. Suspicion can be further confirmed by performing HT, showing no significant improvement in hypoxemia when a large R-T-L shunt is present. However, the clinical picture may be harder to distinguish when significant lung disease coexists, such as COPD. Diagnosis is obtained with TTE or transcranial doppler ultrasound (TCD), but the most straightforward examination is c-TEE [4].
For R-T-L shunt to occur in patients with PFO, anatomic and functional defects must be present to allow deoxygenated blood from the lower-pressure venous system into a higher-pressure systemic circulation. In most patients with PFO, functional defects lead to a transient increase of right atrial pressures permitting deoxygenated blood (usually from the inferior vena cava) to flow directly across the PFO into the left atrium. This phenomenon can be present due to an isolated/combination of the dilation of the proximal ascending aorta, cardiac surgery, atrial septal aneurysms, intracardiac lipomas, and/or the redirection of blood flow from the inferior vena cava through a prominent Eustachian valve. This transience increases in frequency with the duration of disease, lowered arterial oxygen content, pulmonary arterial hypertension (PAH), and age [5].
In COPD, hypoxemia is usually attributed to prevalent V/Q defects and results in PAH. Thus, the relationship between PFO, PAH, and hypoxemia in patients with COPD remains complex [[6], [7], [8]].
At present, few studies exist examining the prevalence of PFO in COPD, but they support that PFO may contribute to hypoxemia in these patients [9,10]. It has also been considered that PFO protects patients with PAH against additional increases in pulmonary artery pressure and in the concomitant deterioration of cardiac function [11]. Shaikh et al. reported no differences between the degree of shunt among hypoxemic and less-hypoxemic patients at rest; a significantly larger PFO and higher shunt fraction were noted in patients with COPD following Valsalva than in the control group [12]. Kilic et al. also concluded in a study of 21 COPD patients with hypoxemia were not linked to a PFO. However, significantly higher PAP levels were detected in patients with PFOs [13]. On the other hand, Martolini et al. discovered in 12 out of 22 patients with COPD, patients with a PFO had a significantly greater degree of hypoxemia than their non-PFO counterparts. The authors, however, did not report PAP values [14].
So, we can hypothesize that in hypoxemic COPD pulmonary hypertension such as Valsalva maneuver may increase R-T-L shunting facilitated by larger PFOs.
Alternative explanations in COPD patients with both PAH and hypoxemia, R-T-L shunting may also result from intrapulmonary arteriovenous pathways. These pathways are due to a rise in microvascular pressure, akin to several mechanisms seen during physiological exercise and in hepatopulmonary syndrome. Alternatively, hypoxemia could also result from an increase in complex anatomic anastomosis of bronchial and/or pleural circulation alongside pulmonary circulation, as in chronic thromboembolic pulmonary hypertension [15,16].
In our case, there were some anatomical and functional conditions facilitating PFO shunting in addition to symptoms and complications inherent to COPD as cough, exacerbations, mild PAH, which may explain the preferential R-T-L shunt. Furthermore, the patient's age and increase in physiological shunt may have also contributed to his hypoxemia.
PFOs are increasingly being recognized as a mediator of paradoxical embolism, allowing the passage of air, thrombus, and fat with subsequent systemic sequelae.
While there remains skepticism about the efficacy of closure of PFO as a preventative measure, this procedure prevents further paradoxical embolism [17] and is effective in correcting hypoxemia [18]. If closure of the PFO is scheduled, an assessment of the residual performance of the right ventricle is necessary. As PFO may be open due to elevated right-sided pressures due to pre-existing pulmonary hypertension, closure may result in right heart failure but no data exists to suggest when PFO closure would be contraindicated in pulmonary hypertension [19].
In our case, preserved right ventricular performance measured using tricuspid annular plane excursion (TAPSE), mild-to-moderate pulmonary pressures, and severe hypoxemia refractory to supplemental O2 supported the closure of this patient's PFO. Ultimately resulting in clinical improvement and follow-up showing preserved right-sided function.
So, we consider that a preserved right ventricular systolic function can be a “conditio sine qua non” for PFO closure in hypoxemic COPD with pulmonary hypertension.
4. Conclusions
In COPD, diagnosis of hypoxemia due to an R-T-L atrial shunt is not a straightforward process. Thus, hypoxemia refractory to high O2 therapy with dyspnea must be thoroughly evaluated with a careful history and focused physical examination. The presence of POS should raise the suspicion of an R-T-L shunt. A positive HT should be obtained before subjecting the patient to a c-TEE. If a PFO is identified, adequate assessment of lung function and right ventricular systolic reserve is necessary. Prior to closure, the optimization and stabilization of other COPD therapies should be achieved. A careful approach to distinguish pathogenic PFOs from incidental and clinically insignificant ones is also mandatory. In our case, PFO closure led to a resolution of symptoms, corrected hypoxemia, and marked improvement in the patient's quality of life.
A paucity of data limits the ability to draw conclusions in patients with PFO and COPD. The development of a multidisciplinary group to lead a multicenter study is needed to further discern the benefits of this novel therapy.
Financial disclosures
The authors have no relevant financial interest in this article.
Authors’ contributions
All authors contributed equally to and read and approved the final version of the manuscript.
Declaration of competing interest
The authors report no conflict of interest.
Acknowledgments
We thank Pr. E. Ryan and Pr. S. Albertini from NYU (USA) who contributed to language revision.
References
- 1.Roussos C., Koutsoukou A. Respiratory failure. Eur. Respir. J. 2003;22:3s–14s. doi: 10.1183/09031936.03.00038503. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal A., Palkar A., Talwar A. The multiple dimensions of platypnea- orthodeoxia syndrome: a review. Respir. Med. 2017;129:313–318. doi: 10.1016/j.rmed.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Rossier P.H., Buhlmann A., Wiesinger K. Springer Berlin Gottingen Heildelberg; 1958. Physiologie und Pathophysiologie der Atmung. [Google Scholar]
- 4.Pristipino C., Sievert H., D'Ascenzo Fa, Mas J.L., Meier B., Scacciatella P., et al. European position paper on the management of patients with patent foramen ovale. Part II - decompression sickness, migraine, arterial deoxygenation syndromes and select high-risk clinical conditions. Eur. Heart J. 2021;42:1545–1553. doi: 10.1093/eurheartj/ehaa1070. SPECIAL ARTICLE doi:10.1093/eurheartj/ehaa 1070. [DOI] [PubMed] [Google Scholar]
- 5.Godart F., Rey C., Prat A., Vincentelli A., Chmaït A. Atrial right-to-left shunting causing severe hypoxaemia despite normal right-sided pressures. Report of 11 consecutive cases corrected by percutaneous closure. Eur. Heart J. 2000;21(6):483–489. doi: 10.1053/euhj.1999.1944. Mar. [DOI] [PubMed] [Google Scholar]
- 6.Hagen P.T., Scholz D.G., Edwards W.D. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin. Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 7.Bancal C. Patent foramen ovale and hypoxaemia with or without elevated right heart pressures. Rev. Mal. Respir. 2011;28(8):967–977. doi: 10.1016/j.rmr.2011.07.001. Oct. [DOI] [PubMed] [Google Scholar]
- 8.Layou M.E., Aboulhosn J.A., Tobis J.M. Potential role of Patent Foramen Ovale in exacerbating hypoxemia in chronic pulmonary disease. Tex. Heart Inst. J. 2017;44(3):189–197. doi: 10.14503/THIJ-16-6027. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soliman A., Shanoudy H., Liu J. Increased prevalence of patent foramen ovale in patients with severe chronic obstructive pulmonary disease. J. Am. Soc. Echocardiogr. 1999;12:99–105. doi: 10.1378/chest.113.1.91. [DOI] [PubMed] [Google Scholar]
- 10.Boerrigter B.G., Boonstra A., Westerhof N., Postmus P.E., Vonk-Noordegraaf A. Cardiac shunt in COPD as a cause of severe hypoxaemia: probably not so uncommon after all. Eur. Respir. J. 2011;37 4:960–962. doi: 10.1183/09031936.00058410. [DOI] [PubMed] [Google Scholar]
- 11.Hacievliyagil S.S., Gunen H., Kosar F.M., Sahin I., Kilic T. Prevalence and clinical significance of a patent foramen ovale in patients with chronic obstructive pulmonary disease. Respir. Med. 2006;100(5):903–910. doi: 10.1016/j.rmed.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh Z.F., Kelly J.L., Shrikrishna D., de Villa M., Mullen M.J., Hopkinson N.S. Patent foramen ovale is not associated with hypoxemia in severe chronic obstructive pulmonary disease and does not impair exercise performance. Am. J. Respir. Crit. Care Med. 2014;189 5:540–547. doi: 10.14503/THIJ-16-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilic H., Kevser Gülsoy G. Patent foramen ovale among patients with mild chronic obstructive pulmonary disease and unexplained hypoxia. Echocardiography. 2010;27(6):687–690. doi: 10.1111/j.1540-8175.2009.01105.x. Jul. [DOI] [PubMed] [Google Scholar]
- 14.Martolini D., Tanner R., Davey C., Patel M.S., Elia D., Purcell H. Significance of patent foramen ovale in patients with GOLD Stage II chronic obstructive pulmonary disease. J. COPD F. 2014;12:185–192. doi: 10.15326/jcopdf.1.2.2013.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riemer A.T.R.K. Intrapulmonary arteriovenous anastomoses. Physiological, pathophysiological, or both? Ann. Am. Thoracic Soc. 2007;10(Issue 5):504–508. doi: 10.1513/AnnalsATS.201308-265ED. [DOI] [PubMed] [Google Scholar]
- 16.Roisin R.R. Hepatopulmonary syndrome - a liver-induced lung vascular disorder. N.Engl. J. Med. 2008;29(22):2378–2387. doi: 10.1056/NEJMra0707185. 358. PMID:18509123, May. [DOI] [PubMed] [Google Scholar]
- 17.Messe S.R., Murat Tuzcu E., Catha G., Ring J.C. Percutaneous device closure of patent foramen ovale for secondary stroke prevention. A call for completion of randomized clinical trials: a science advisory from the American heart association/American stroke association and the American college of cardiology Foundation.The American academy of neurology affirms the value of this science advisory. Circulation. 2009;119:2743–2747. doi: 10.1016/j.jacc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Fenster B.E. Effectiveness of percutaneous closure of patent foramen ovale for hypoxemia. Am. J. Cardiol. 2013;112(8):1258–1262. doi: 10.1155/2020/1513409. Oct 15. [DOI] [PubMed] [Google Scholar]
- 19.El Tahlawi M., Jop B., Bonello B., Dragulescu A., Rouault F., Habib G., et al. Should we close hypoxaemic patent foramen ovale and interatrial shunts on a systematic basis? Arch. Cardiovasc. Dis. 2009 Nov;102(11):755–759. doi: 10.1016/j.acvd.2009.09.009. Epub 2009 Nov 14. [DOI] [PubMed] [Google Scholar]