Abstract
Alcaligenes eutrophus (Ralstonia eutropha) NH9, isolated in Japan, utilizes 3-chlorobenzoate as its sole source of carbon and energy. Sequencing of the relevant region of plasmid pENH91 from strain NH9 revealed that the genes for the catabolic enzymes were homologous to the genes of the modified ortho-cleavage pathway. The genes from strain NH9 (cbnR-ABCD) showed the highest homology (89 to 100% identity at the nucleotide level) to the tcbR-CDEF genes on plasmid pP51 of the 1,2,4-trichlorobenzene-degrading bacterium Pseudomonas sp. strain P51, which was isolated in The Netherlands. The structure of the operon, including the lengths of open reading frames and intervening sequences, was completely conserved between the cbn and tcb genes. Most nucleotide substitutions were localized within and proximal to the cbnB (tcbD) gene. The difference in the chloroaromatics that the two strains could use as growth substrates seemed to be due to differences in enzymes that convert substrates to chlorocatechols. The restriction map of plasmid pENH91 was clearly different from that of pP51 except in the regions that contained the cbnR-ABCD and tcbR-CDEF genes, respectively, suggesting that the chlorocatechol gene clusters might have been transferred as units. Two homologous sequences, present as direct repeats in both flanking regions of the cbnR-ABCD genes on pENH91, were found to be identical insertion sequences (ISs), designated IS1600, which formed a composite transposon designated Tn5707. Although the tcbR-CDEF genes were not associated with similar ISs, a DNA fragment homologous to IS1600 was cloned from the chromosome of strain P51. The sequence of the fragment suggested that it might be a remnant of an IS. The two sequences, together with IS1326 and nmoT, formed a distinct cluster on a phylogenetic tree of the IS21 family. The diversity of the sources of these IS or IS-like elements suggests the prevalence of ISs of this type.
Contamination with recalcitrant chlorinated aromatic compounds is a very serious environmental problem (3). In the most common examples of bacterial aerobic degradation of chlorinated aromatics, the modified ortho-cleavage pathway plays a significant role, participating in the complete degradation of chlorocatechols that have been generated from various chlorinated aromatics through convergent pathways (61).
Three evolutionarily related clusters of genes of gram-negative bacteria that encode enzymes in the modified ortho-cleavage pathway have been well described (for reviews, see references 14, 26, 55, and 61). These are clcABD, tfdCDEF, and tcbCDEF. The clcABD genes are responsible for degradation of 3-chlorocatechol and were cloned from plasmid pAC27 of Pseudomonas putida, which is a 3-chlorobenzoate (3-CB)-degrading bacterium (9, 11, 22). The tfdCDEF genes are present on plasmid pJP4 and are responsible for the degradation of 3,5-dichlorocatechol, which is produced from 2,4-dichlorophenoxyacetate by the products of the tfdAB genes in Alcaligenes eutrophus (Ralstonia eutropha) JMP134 (17, 18, 46). The tcbCDEF genes are located on plasmid pP51 and are responsible for the degradation of 3,4,6-trichlorocatechol, generated from 1,2,4-trichlorobenzene by the products of the tcbAB genes in Pseudomonas sp. strain P51 (62, 64). These three gene clusters of gram-negative bacteria have apparently evolved from common ancestral chlorocatechol genes (62). Recently, the study of both the catechol and the chlorocatechol ortho-cleavage pathways of Rhodococcus opacus 1CP has shown that the chlorocatechol ortho-cleavage genes of this strain have evolved, independently of those of gram-negative bacteria, from the common origin of the catechol ortho-cleavage genes in all bacteria (19, 20).
The worldwide distribution of genes for chlorocatechol degradation has been suggested by the discovery of several isolates from different places (4, 6, 10–12, 17, 30, 37, 39, 58, 59, 64) and has recently been demonstrated more systematically by the studies of Fulthorpe et al. (23, 24, 33). However, the means of dissemination of the gene clusters is less clear: there have been only a few examples of identical or highly homologous plasmids which carried the modified ortho-pathway genes, indicating that they are transferred by plasmids (4, 10, 17). Although the similar operon-like structures of the gene clusters of the modified ortho-cleavage pathway suggest they might have spread as units on a transposable element, there has been only one documented example of a transposable element that carries the modified ortho-pathway genes (35).
In this paper, we report the structure of genes for chlorocatechol-degrading enzymes of A. eutrophus NH9, which was isolated in Japan. The nucleotide sequences of the cbnR-ABCD genes carried by a composite transposon structure are highly homologous to those of the tcbR-CDEF genes of strain P51, which was isolated in The Netherlands. This is the first report, to our knowledge, of nearly identical two-gene clusters of the modified ortho pathway which were disseminated by insertion sequences (ISs).
MATERIALS AND METHODS
Strains and plasmids.
Cosmid pPSA842 and Escherichia coli B378 (53) were used to construct a genomic library of plasmid pENH91 of A. eutrophus NH9. Plasmid pKT230 (5) was used for subcloning of the genes for catabolic enzymes. P. putida KT2440 (5) was used as the host strain for growth tests of complementation by subcloned fragments of DNA. The 3.3-kb SalI-SphI fragment containing DR2 was inserted into pUC18 to yield pEUDR2. The 4.7-kb EcoRI fragment containing DR1 was inserted into pBluescript KS(−) (Stratagene, La Jolla, Calif.) to yield pELDR1. Other bacterial strains and phages used in this study, and growth conditions, have been described elsewhere (45).
Media and culture conditions.
The ability of strain NH9 to grow on dichlorobenzene and on 1,2,4-trichlorobenzene was tested basically as described by van der Meer et al. (64). A preculture of NH9 was inoculated into liquid basal salts medium (45) supplemented with either 3.5 mmol of 1,2- or 1,4-dichlorobenzene/liter or 3.2 mmol of 1,2,4-trichlorobenzene/liter and was incubated at 30°C.
Cloning of genes for catabolism of 3-CB.
Plasmid DNA was isolated from strain NH9 as described previously (45). It was partially digested with Sau3A to generate fragments predominantly of 30 to 50 kb and subjected to centrifugation in a 10 to 40% sucrose density gradient. Fractions containing DNA fragments of 30 to 50 kb were pooled. This DNA was inserted into BamHI-digested broad-host-range cosmid pPSA842, packaged with a packaging extract (Gigapack Plus; Stratagene) by the procedure described by the manufacturer, and transduced into E. coli B378. The individual cosmid clones were mobilized into strain NH9D, a 3-CB− derivative of NH9 that had been cured of plasmid pENH91 (45), as described by Franklin (21). The transconjugants were then screened for growth on plates that contained 0.1% 3-CB and streptomycin (25 μg/ml).
Manipulation of DNA.
Subcloning and sequencing of DNA fragments and hybridization were performed as described elsewhere (51) unless otherwise stated. Conjugation was performed as described by Franklin (21). Southern hybridization experiments for cloning the 3.7-kb SalI fragment from strain P51 were performed with a digoxigenin labeling kit (Boehringer Mannheim, Mannheim, Germany) according to the protocol from the manufacturer. Sequences were determined by the dideoxy chain-termination method (52) with automated sequencers (373A [Perkin-Elmer–Applied Biosystems Inc., Foster City, Calif.], ALFred [Pharmacia, Uppsala, Sweden], and DSQ-1000L [Shimadzu, Kyoto, Japan]), with the dye-primer or dye-terminator kits supplied by the respective manufacturers.
Nucleotide sequence accession numbers.
The nucleotide sequence of the 6,959-bp SacI-KpnI region containing the cbnR-ABCD genes and the deduced amino acid sequences have been deposited in the DDBJ, GenBank, and EMBL databases under accession no. AB019032. The nucleotide sequence containing the 1,300-bp region from strain P51, homologous to part of IS1600, has been deposited under accession no. AB019033. The nucleotide sequence of orfL is included in the sequence that contains IS1600 (DR2) (from the SphI site to outside of the KpnI site of pENH91 [Fig. 1b]) that was deposited previously (45) and that has been updated with correction of the sequence of DR2 (accession no. D64144).
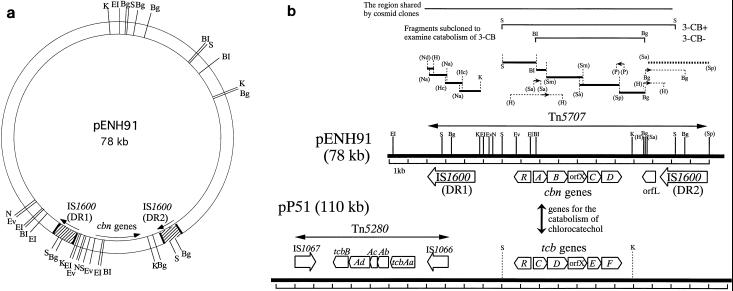
FIG. 1.
(a) Restriction map of pENH91. BI, BamHI; Bg, BglII; EI, EcoRI; Ev, EcoRV; K, KpnI; N, NheI; S, SacI. (b) Schematic representation of regions containing degradative genes and insertion sequences on plasmids pENH91 and pP51. The open arrows for the cbn genes, orfL, and the tcb genes show the locations and the directions of transcription of the ORFs. The orientation of the open arrows of IS1600, IS1066, and IS1067 are in agreement with the direction of transcription of the ORFs within the ISs. The strategies for subcloning and sequencing the catabolic region on plasmid pENH91 are shown above the linear map. Fragments shared by cosmid clones or subcloned to examine the 3-CB phenotype are shown by thin solid lines at the top of the figure. The thick solid lines above the map of pENH91 indicate DNA fragments that were sequenced in both directions by using nested sets of deletions or subcloned restriction fragments. The sequence indicated by the thick dotted line (a 3.3-kb SalI-SphI fragment) was reported previously (45) but was corrected in this study. The thin dotted lines with small arrows indicate subcloned fragments used to sequence the boundary sites between the sequenced fragments described above. The small arrows indicate the lengths and directions of the sequences determined (5′ to 3′). Restriction sites are abbreviated as follows, in addition to those defined in panel a: H, HindIII; Hc, HincII; Na, NaeI; Nd, NdeI; P, PstI; Sa, SalI; Sm, SmaI; and Sp, SphI. The restriction sites in parentheses are those determined only for subcloning of related fragments; thus, other sites recognized by such enzymes within the linear map were not determined before sequencing. The map of pP51 is based on material in references 62, 63, 65, and 66. Only the SacI and KpnI sites described in the text are shown for pP51.
RESULTS
Cloning of genes for catabolic enzymes.
The genes for degradation of 3-CB by A. eutrophus NH9 are carried on plasmid pENH91 (45). A library of genes in pENH91 was constructed in E. coli B378 by use of the broad-host-range cosmid pPSA842. The individual cosmid clones were mobilized from E. coli into A. eutrophus NH9D, a 3-CB− derivative that had been cured of pENH91 (45), and transconjugants were selected on minimal agar plates that contained 3-CB and streptomycin. Among about 200 clones examined, 8 had the 3-CB+ phenotype. A comparison of the restriction maps of the inserts of the positive clones showed that they all included a common 13-kb region (Fig. 1b). A physical map of pENH91 was constructed by further restriction analysis (Fig. 1a). For subcloning, a 9.2-kb SacI fragment (Fig. 1b) from this 13-kb region was inserted into the broad-host-range vector pKT230 to yield pEKC1, which was mobilized into A. eutrophus NH9D (3-CB−) and P. putida KT2440. Cells of both strains harboring pEKC1 grew on 3-CB-supplemented mineral salts agar plates. A 5.8-kb BamHI-BglII fragment from within the 9.2-kb SacI fragment did not confer the 3-CB+ phenotype on either strain. These results showed that the genes for catabolism of 3-CB were located within the 9.2-kb SacI fragment.
Determination of sequences of genes for degradative enzymes.
Sequencing analysis of the 9.2-kb SacI fragment revealed seven long open reading frames (ORFs) (Fig. 1b). Six of the ORFs formed a cluster and exhibited strong homology to ORFs in the following clusters of chlorocatechol-degradative genes (in order of relatedness): (i) the tcbR-CDXEF genes on plasmid pP51 in Pseudomonas sp. strain P51 (62, 63); (ii) the clcR-ABXDE genes on plasmids pAC27 in P. putida AC866 and pWR1 in Pseudomonas sp. strain B13 (13, 22, 32); and (iii) the tfdR and tfdCDEF genes on plasmid pJP4 in A. eutrophus JMP134 (40, 46) (“X” denotes the third ORF in the cluster of degradative genes tcbCDEF and clcABDE; the functions of these genes are unknown). In particular, the extent of homology between the six ORFs of NH9 and ORFs of the tcbR-CDXEF genes of Pseudomonas sp. strain P51 was very great (Table 1).
TABLE 1.
Homology between cbn and tcb genes
| Corresponding genes | G+C content (%) | Length
|
Homology (%)
|
||
|---|---|---|---|---|---|
| Nucleotides (bp) | Amino acids | Nucleotide level | Amino acid level | ||
| cbnR | 66.1 | 885 | 294 | 99.9 | 99.7 |
| tcbR | 66.2 | 885 | 294 | ||
| cbnA | 65.0 | 756 | 251 | 98.0 | 95.6 |
| tcbC | 65.2 | 756 | 251 | ||
| cbnB | 63.3 | 1,113 | 370 | 88.9 | 97.0 |
| tcbD | 63.7 | 1,113 | 370 | ||
| orfXcbn | 64.3 | 1,011 | 336 | 97.9 | 97.0 |
| orfXtcb | 63.3 | 1,011 | 336 | ||
| cbnC | 61.0 | 717 | 238 | 99.9 | 99.6 |
| tcbE | 61.0 | 717 | 238 | ||
| cbnD | 62.9 | 1,059 | 352 | 100 | 100 |
| tcbF | 62.9 | 1,059 | 352 | ||
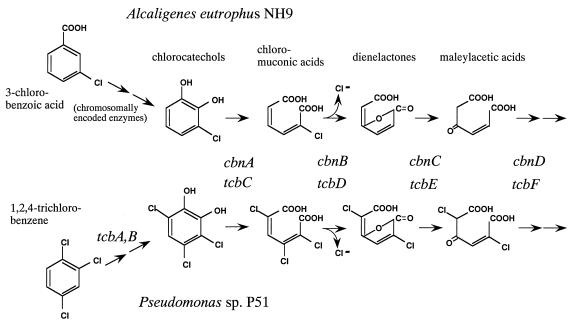
From the high homology to known chlorocatechol-degradative genes and by analogy to the pathways formed by the products of these gene clusters (62), it was apparent that the sequenced degradative genes of strain NH9 encoded enzymes of the modified ortho-cleavage pathway (Fig. 2). Since strain NH9D grew on benzoate, strain NH9 was assumed to harbor genes for benzoate 1,2-dioxygenase and 1,2-dihydro-1,2-dihydroxybenzoate dehydrogenase, which might convert (chloro)benzoate into (chloro)catechol, either on its chromosome (29) or on the additional plasmid pENH92 (45).
FIG. 2.
Pathways for degradation of 3-chlorobenzoic acid by A. eutrophus NH9 and for degradation of 1,2,4-trichlorobenzene by Pseudomonas sp. strain P51. The pathway for degradation of 1,2,4-trichlorobenzene by Pseudomonas sp. strain P51 was first described by van der Meer et al. (64).
Since the enzymes encoded by the chlorocatechol-degradative genes of NH9 were responsible for degradation of 3-CB, the genes were designated cbnR-ABCD, with cbnA, -B, and -C encoding chlorocatechol 1,2-dioxygenase, chloromuconate cycloisomerase, and dienelactone hydrolase, respectively (62, 64). Recent studies have suggested that cbnD, corresponding to tcbF, might encode maleylacetate reductase (31, 32, 56). cbnR was presumed to be a regulatory gene that belonged to the lysR family (54, 63). orfX, between cbnB and cbnC, corresponded to the third ORF in the tcbCDEF and clcABDE gene clusters, whose products have unknown functions. The strongly conserved amino acid sequences encoded by orfX in cbnABXCD and in tcbCDXEF suggest that the products of these ORFs might play a role that is indispensable for the function of the gene cluster in some as-yet-unknown fashion.
The extent of the homology of each gene in the cbnR-ABXCD cluster to the corresponding gene in the clcR-ABXDE cluster (13, 22, 32) ranged from 59 to 72% at the nucleotide level and from 51 to 76% at the amino acid level. Homology to the tfdR and tfdCDEF genes of strain JMP134 (40, 46) was 58 to 66% at the nucleotide level and 52 to 67% at the amino acid level. The degradative genes cbnA, cbnB, and cbnR are considered to be evolutionarily related to the functionally similar genes in the catechol ortho-cleavage pathway, namely, catA, catB, and catR (55). The homology between the cbnR-AB genes and the corresponding cat genes of P. putida PRS2000 (28) and Pseudomonas sp. strain RB1 (2, 50) was 51 to 57% at the nucleotide level and 31 to 45% at the amino acid level.
Comparison of the cbn and tcb gene clusters.
The regions containing the chlorocatechol-degradative genes of the two plasmids, namely, the 6,959-bp SacI-KpnI regions of pENH91 and pP51, were compared (Fig. 1b and 3 and Table 1). All of the corresponding ORFs were the same length (Table 1). Hence, all the differences between the coding regions of the cbnR-ABCD and tcbR-CDEF genes were substitutions. The cbnA and cbnB genes overlapped by 4 bp. One nucleotide was present between cbnB and the third ORF in the cbn degradative operon. The intergenic region between the third ORF of cbn and cbnC consisted of 21 bp. cbnC overlapped with cbnD by 4 bp. All of these structural features of the cbn genes were the same as those of the corresponding regions of the tcb genes. The nucleotide sequence of the promoter region between cbnR and cbnA (150 bp) was identical to that of the region between tcbR and tcbC.
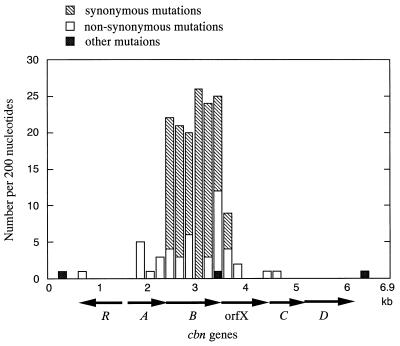
FIG. 3.
Numbers of nucleotide substitutions and other mutations in the region of the cbn gene cluster in comparison to the region of the tcb gene cluster. The numbers per 200 nucleotides are shown, counted from the SacI site in the 6,959-bp region that contains the cbn gene cluster. The corresponding regions of nucleotides in the two clusters are as follows: cbn 1 to 287 and tcb 1 to 287, cbn 289 to 6578 and tcb 288 to 6577, and cbn 6579 to 6959 and tcb 6579 to 6959.
Nucleotide substitutions between cbnA and tcbC resulted in a slight decrease in the percentage of homology between the deduced amino acid sequences (Table 1). Eleven of 13 nucleotide substitutions caused nonsynonymous substitutions at the amino acid level. However, the four amino acids that are supposed to coordinate the ferric ion at the active site (Tyr-130, Tyr-164, His-188, and His-198) were conserved in CbnA, as were another 28 amino acids that are conserved among catechol 1,2-dioxygenases (43). Although homology at the nucleotide level was lowest among the cycloisomerase genes cbnB and tcbD (Table 1), the majority of nucleotide substitutions between cbnB and tcbD were synonymous substitutions (in 102 of 113 codons) and reflected the high frequency of nucleotide substitutions at the third base in the codon (107 of 124 nucleotides [Fig. 3b]). Consequently, the homology at the amino acid level between CbnB and TcbD remained high, in contrast to the case of CbnA and TcbC (Table 1). There was one nucleotide substitution between cbnR and tcbR and between cbnC and tcbE, resulting in one amino acid substitution in each pair. The nucleotide sequence of cbnD was identical to that of tcbF. All of the putative ribosome-binding sites for each of the cbn genes were located at the same respective positions, and all had the same sequences as those for the tcb genes (62, 63).
The nucleotide sequences of the flanking regions of the two gene clusters within the SacI-KpnI fragments were nearly identical: the 637-bp nucleotide sequence of the downstream flanking region of cbnR was identical to that of tcbR except that one nucleotide was missing from the latter. The 618-bp nucleotide sequence of the downstream flanking region of cbnD was identical to that of tcbF except for the insertion of one nucleotide in the latter. These results suggested that the highly homologous regions of the two plasmids extended beyond both the SacI site and the KpnI site (Fig. 1b).
Identification of DR1 and DR2 as identical ISs.
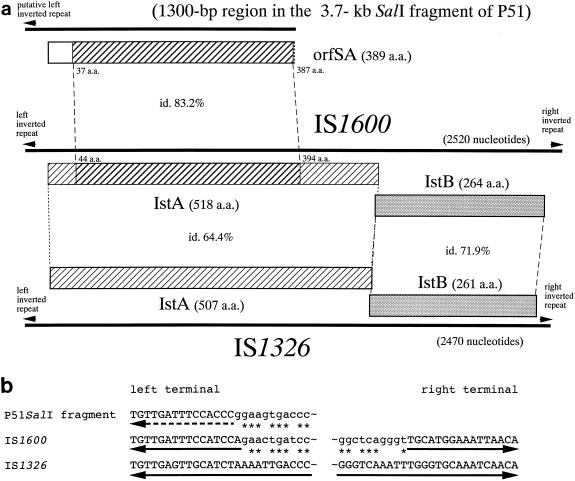
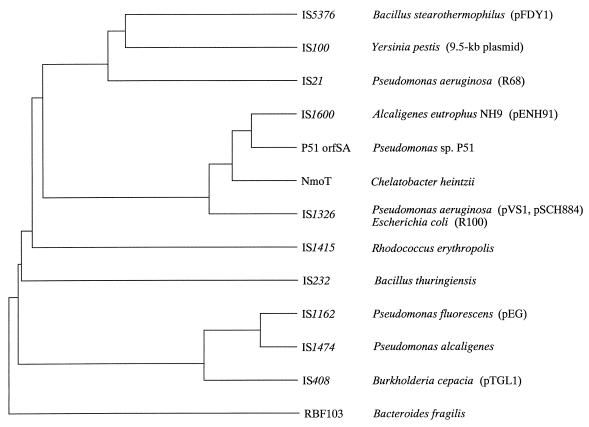
Two directly oriented homologous elements, DR1 and DR2, were found at the ends of the catabolic gene region on pENH91 (45). We found that the identification in our previous report (45) of one nucleotide, C, at position 850 from the left inverted repeat of DR2 was a sequencing error, and we eliminated it from the sequence of DR2. With this correction, the nucleotide sequences of the two ORFs, ORFA1 and ORFA2 (45), which were homologous to the first and the second half of the IstA gene of IS21 (48), respectively, were found to form one ORF that corresponded to the entire length of the IstA gene. The amino acid sequence deduced from the revised nucleotide sequence showed the highest homology to that of IstA of IS1326 (7) among the ISs of the IS21 family (Fig. 4a). Since DR2 was found to have the perfect structure of an IS of the IS21 group, it was designated IS1600, and the two genes were designated istA and istB (Fig. 4a). Interestingly, although the terminal inverted repeats of IS1326 (26 bp) were considerably longer than those of IS1600 (16 bp), some nucleotides of IS1600 proximal to the inverted repeats matched the nucleotides within the inverted repeats of IS1326 (Fig. 4b).
FIG. 4.
(a) Schematic representation of the ORFs in related IS or IS-like elements. The hatched areas indicate the regions with homology at the amino acid level. Percentages of identical amino acids (id.) are shown. (b) Terminal regions of the elements shown in panel a. The solid arrows indicate the inverted repeats and their orientations (7, 45). The dotted arrow below the nucleotide sequence of the SalI fragment from P51 indicates a putative left inverted repeat that was delineated on the basis of similarity to the sequence of IS1600. The asterisks indicate identical nucleotides in the juxtaposed sequences that are not included in the inverted repeats of IS1600 and the SalI fragment from P51.
A 4.7-kb EcoRI fragment containing DR1 was cloned from plasmid pENH91 (Fig. 1b). Sequencing analysis revealed that DR1 was identical to DR2.
An additional ORF in the region between cbnD and DR2.
In a 2-kb region between cbnD and DR2, we found an ORF of considerable length that exhibited some similarity to known proteins at the amino acid level (Fig. 1b) and designated it orfL. The deduced amino acid sequence of orfL (240 amino acids [aa]) was homologous to those of many bacterial polypeptides that are known to be components of membrane-bound transport systems for amino acids. In particular, LivF of E. coli (1) and BraG of Pseudomonas aeruginosa PAO (27) were 42 and 39% homologous, respectively, to orfL at the amino acid level. orfL seemed to be complete in length in comparison with LivF (237 aa) and BraG (233 aa). Both LivF and BraG are located at the downstream ends (in the direction of transcription) in their respective gene clusters. Because of the location and apparent direction of transcription of orfL in the region bracketed by the two ISs, it appeared that orfL might have been separated from the rest of the genes in the cluster by an excision event involving IS1600 (DR2).
Cloning and sequencing of a DNA fragment from strain P51 with homology to IS1600.
We investigated whether the tcbR-CDEF gene cluster was also associated with an IS1600 (or IS1600-like) sequence on plasmid pP51. Total DNA was extracted from cells of strain P51 that had been grown on Luria-Bertani medium (51) and subjected to Southern hybridization experiments. The presence of plasmid pP51 DNA in the total DNA was confirmed with a 3.5-kb BamHI-PstI fragment containing a part of the cbn gene cluster as the probe. We then used the same membrane and a 2.3-kb HindIII-SphI fragment containing a part of IS1600 (DR2) as the probe. A hybridizing band was observed, and this fragment was cloned from the total DNA of strain P51 into pUC19 as a 3.7-kb SalI fragment. In a subsequent hybridization experiment, using the cloned 3.7-kb SalI fragment as the probe, the SalI-digested total DNA from strain P51 (which retained the tcb genes) and from derivative strains of P51 that had been cured spontaneously of the plasmid P51 after successive cultures on Luria-Bertani liquid medium gave the same pattern of signals. Therefore, it appeared that the cloned 3.7-kb SalI fragment resided on the chromosome of strain P51.
Sequence of a ca. 2-kb region of the cloned 3.7-kb SalI fragment revealed that the region homologous to IS1600 extended for 1,300 bp, with nucleotide homology of 81%. The 1,300-bp region was flanked by nonhomologous marginal regions of ca. 0.2 and 0.5 kb on each side. Thus, the homologous region had not been truncated by the cloning procedure with the SalI sites. The homologous 1,300-bp region started with a 15-bp sequence that resembled the left inverted repeat of IS1600 (Fig. 4b). It was followed by a 115-bp intervening region and then by an ORF (designated orfSA; 1,170 bp) which showed the highest homology with a part of IstA of IS1600 (83% at the amino acid level [Fig. 4a and 5]) in the database. In addition to the fact that the 1,300-bp region seemed to lack the 3′ portion of istA, it was obviously not followed by istB and an inverted repeat at the other end. Thus, this fragment seemed to be a remnant of an IS.
FIG. 5.
Phylogenetic tree of members of the IS21 family based on the alignment of IstA or IstA-like proteins. The alignment was performed with ClustalW software and adjusted manually to incorporate the results reported by Haas et al. (25). The tree was constructed with the Genetyx software program (Software Development Co., Tokyo, Japan) by the unweighted-pair group method with mathematical averages. Accession numbers (and references), except those for IS1600 and P51 orfSA, are as follows (from top to bottom): X67861 (68), Z32853 (47), X14793 (48), L49438 (69), U38187 (7), AF002247 (42), M38370 (41), X79443 (57), U67315 (70), L09108 (8), and U05888 (49).
DISCUSSION
In this study, we analyzed the region of plasmid pENH91 that contained the chlorocatechol-degradative genes of A. eutrophus NH9. The chlorocatechol-degradative genes of NH9 (cbnR-ABCD) were found to be highly homologous to those of Pseudomonas sp. strain P51 (tcbR-CDEF) (62, 63). The lengths of the corresponding ORFs, the overlaps of ORFs, and the intervening sequences between ORFs were the same in the two gene clusters. Highly conserved nucleotide sequences and the identical overall structures of the two gene clusters indicated that the horizontal transfer and the divergence at the nucleotide level of the two clusters had occurred relatively recently in the evolutionary history of the clusters of genes in the modified ortho pathway (55).
The frequency of nucleotide divergence between the regions that contained cbnB and tcbD was significantly higher than that between the other corresponding regions of the two clusters (Table 1). This point is of particular interest, since the genes for chloromuconate cycloisomerase are generally more conserved than the other three genes in the cluster of modified ortho-pathway genes (62). If selective pressure on the genes caused such divergence, several features should be noted here. Even though the two clusters were located on plasmids, the G+C content of each gene in the two clusters, which ranged from 61 to 66% in either cluster (Table 1), cannot be regarded as being significantly different from the chromosomal G+C content of A. eutrophus (66.3 to 66.8%) (15) or of species of Pseudomonas (e.g., P. putida, 59.6 to 63.4% [38]). Furthermore, the G+C contents of cbnB (63.3%) and tcbD (63.7%) are similar and are in the middle range of those of all the genes in the respective clusters. Therefore, the nucleotide divergence between cbnB and tcbD cannot be attributed to “GC pressure” from the host. Alternatively, one possible explanation for the rapid divergence of cbnB and tcbD is that some pattern of biased codon usage forced the bacteria in which these genes were located to replace nucleotides in an effort to adapt to their genetic backgrounds (for example, the pool of transfer RNAs). However, we found no significant differences in codon usage patterns between cbnB and tcbD or between cbnB (tcbD) and the other genes in the clusters. At present, it is unknown whether any selective pressure forced the rapid nucleotide divergence of cbnB and tcbD.
The fact that numerous nucleotide substitutions were not limited to the cbnB (tcbD) gene but spanned a region of ca. 1.3 kb that contained cbnB (tcbD) (Fig. 3) suggests that this divergence might have been caused by physical conditions in this region of DNA and not by the genetic nature of cbnB (tcbD). The rate of substitutions is high throughout cbnB (tcbD). Substitutions in the flanking genes tended to be localized in regions proximal to cbnB (tcbD): most of the nucleotide substitutions (18 of 21) between orfXs (next to cbnB or tcbD) were located within 138 bp of the 5′ portions of orfXs (1,011 bp), and 5 of 14 nucleotide substitutions between cbnA and tcbC were located within 24 bp of the 3′ regions of the genes (756 bp). In the other corresponding parts of the two 6,959-bp SacI-KpnI fragments, the rate of mutation, including substitutions, insertions, and deletions, was one in several hundred base pairs, which might reflect the basal rate of spontaneous mutation. Some unidentified local structure of the DNA in the region containing cbnB (tcbD) might have made this region more vulnerable to substitutions during replication. The high homology maintained at the amino acid level might reflect some constraint for the function of chloromuconate cycloisomerases.
There was a difference between the chloroaromatic compounds that the two strains, NH9 and P51, utilized as substrates for growth. Strain P51 grew on either 1,2- or 1,4-dichlorobenzene, and it also grew on 1,2,4-trichlorobenzene (64). Strain NH9 was tested for growth on these compounds, but it failed to grow in liquid medium in the presence of these chlorobenzenes. On the other hand, strain P51 did not grow on 3-CB (64). This discrepancy in growth substrates might be explained by the difference in “upper-pathway” enzymes available to the two strains. Strain NH9 probably synthesizes enzymes that convert 3-CB to 3-chlorocatechol but not the enzymes that convert chlorobenzenes to any compounds that can be further metabolized. Strain P51 has been reported to synthesize the enzymes that convert chlorobenzenes to the corresponding chlorocatechols (64, 66), but it does not seem to synthesize enzymes that convert chlorobenzoates to chlorocatechols. The recruitment of the homologous chlorocatechol-degradative gene clusters by the two strains, which resulted in the difference in chloroaromatics utilizable as growth substrates, illustrates the economy with which bacteria adapt to xenobiotic compounds.
In contrast to reports on well-described catabolic transposons, such as the toluene transposons on TOL plasmids and the catabolic genes mobilized by IS1071 (44) (for reviews, see references 16, 60, and 67), there has been only one documented example of a transposable element that carries genes for the modified ortho pathway (35). The IS ISJP4 copy A and its incomplete copy C captured the genes tfdS-R-DIICIIEIIFIIBIIK to form a composite transposon on plasmid pJP4 in A. eutrophus JMP134 (35, 36). With regard to their characteristics as transposable elements, there are some differences between Tn5707 and the composite transposon formed by ISJP4. IS1600 of Tn5707 belongs to the IS21 family, while ISJP4 belongs to the IS5 group of the IS4 family (35). The ISJP4 transposon was apparently transposed to pJP4 as a composite transposon, as indicated by the presence of target site duplication at both ends (35). By contrast, the absence of such duplication at both ends of Tn5707 suggests that Tn5707 may not transpose as such a composite unit. Instead, this feature suggests mobilization of the chromosome that resulted from plasmid integration and subsequent excision mediated by IS1600 (45, 67). The existence of orfL in Tn5707 suggests that the origin of the region carried by Tn5707 might have been a bacterial chromosome, since genes for amino acid transport systems have been found on them (1, 27).
The catabolic genes carried by the ISJP4 transposon are different from those carried by Tn5707 in the following ways. Although the tfdDIICIIEIIFII genes are most homologous to the tcbCDEF genes, the homology between the corresponding genes ranged from 58 to 70% at the nucleotide level and from 27 to 65% at the amino acid level. The ISJP4 transposon contains duplicated regulatory genes, tfdR and tfdS (34, 71), as well as additional genes, namely, tfdBII, which might encode chlorophenol monooxygenase, and tfdK, which encodes an active transporter of 2,4-dichlorophenoxyacetate (36, 46).
In addition to the differences in inherent characteristics between Tn5707 and the ISJP4 composite transposon, our present results illustrate the role of the IS elements in the recent dissemination of genes in the modified ortho pathway by the strong homology between the two clusters, cbnR-ABCD and tcbR-CDEF. On the phylogenetic tree of IstAs of the IS21 family, IstA of IS1600 formed a distinct cluster together with orfSA from strain P51, IstA of IS1326, and NmoT (Fig. 5). The branching point of the IstA of IS1326 and the other three elements suggests that these four elements diverged relatively recently in the evolution of the members of IS21 family. IS1326 was found in integrons in antibiotic-resistant clinical isolates (7). NmoT is a putative transposase that corresponds to IstAs and was found proximal to nitrilotriacetate-degradative genes in Chelatobacter heintzii (69). The various origins of the four elements indicate the recent wide distribution of the related IS (-like) elements among bacteria, which in turn raises the possibility that these IS(-like) elements might have been involved in recent genetic rearrangements of various kinds.
ACKNOWLEDGMENTS
We are grateful to Jan Roelof van der Meer for providing strain P51. We also thank Toshiko Kajiwara for assistance with the experiments and Sally M. McFall for helpful comments on the manuscript.
This work was supported by the Program for the Promotion of Basic Research Activities for Innovative BioSciences and by the Ministry of Agriculture, Forestry, and Fisheries of Japan.
REFERENCES
- 1.Adams M D, Wagner L M, Graddis T J, Landick R, Antonucci T K, Gibson A L, Oxender D L. Nucleotide sequence and genetic characterization reveal six essential genes for the LIV-I and LS transport systems of Escherichia coli. J Biol Chem. 1990;265:11436–11443. [PubMed] [Google Scholar]
- 2.Aldrich T L, Frantz B, Gill J F, Kilbane J J, Chakrabarty A M. Cloning and complete nucleotide sequence determination of the catB gene encoding cis,cis-muconate lactonizing enzyme. Gene. 1987;52:185–195. doi: 10.1016/0378-1119(87)90045-x. [DOI] [PubMed] [Google Scholar]
- 3.Alexander M. Biodegradation of chemicals of environmental concern. Science. 1981;211:132–138. doi: 10.1126/science.7444456. [DOI] [PubMed] [Google Scholar]
- 4.Amy P S, Schulke J W, Frazier L M, Seidler R J. Characterization of aquatic bacteria and cloning of genes specifying partial degradation of 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1985;49:1237–1245. doi: 10.1128/aem.49.5.1237-1245.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagdasarian M, Lurz R, Rückert B, Franklin F C H, Bagdasarian M M, Frey J, Timmis K N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 6.Bhat M A, Tsuda M, Horiike K, Nozaki M, Vaidyanathan C S, Nakazawa T. Identification and characterization of a new plasmid carrying genes for degradation of 2,4-dichlorophenoxyacetate from Pseudomonas cepacia CSV90. Appl Environ Microbiol. 1994;60:307–312. doi: 10.1128/aem.60.1.307-312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown H J, Stokes H W, Hall R M. The integrons In0, In2, and In5 are defective transposon derivatives. J Bacteriol. 1996;178:4429–4437. doi: 10.1128/jb.178.15.4429-4437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrne A M, Lessie T G. Characteristics of IS401, a new member of the IS3 family implicated in plasmid rearrangements in Pseudomonas cepacia. Plasmid. 1994;31:138–147. doi: 10.1006/plas.1994.1015. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee D K, Chakrabarty A M. Genetic rearrangements in plasmids specifying total degradation of chlorinated benzoic acids. Mol Gen Genet. 1982;188:279–285. doi: 10.1007/BF00332688. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee D K, Chakrabarty A M. Genetic homology between independently isolated chlorobenzoate-degradative plasmids. J Bacteriol. 1983;153:532–534. doi: 10.1128/jb.153.1.532-534.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee D K, Kellogg S T, Hamada S, Chakrabarty A M. Plasmid specifying total degradation of 3-chlorobenzoate by a modified ortho pathway. J Bacteriol. 1981;146:639–646. doi: 10.1128/jb.146.2.639-646.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhry G R, Huang G H. Isolation and characterization of a new plasmid from a Flavobacterium sp. which carries the genes for degradation of 2,4-dichlorophenoxyacetate. J Bacteriol. 1988;170:3897–3902. doi: 10.1128/jb.170.9.3897-3902.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coco W M, Rothmel R K, Henikoff S, Chakrabarty A M. Nucleotide sequence and initial functional characterization of the clcR gene encoding a LysR family activator of the clcABD operon in Pseudomonas putida. J Bacteriol. 1993;175:417–427. doi: 10.1128/jb.175.2.417-427.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daubaras D L, Chakrabarty A M. The environment, microbes and bioremediation: microbial activities modulated by the environment. Biodegradation. 1992;3:125–135. [Google Scholar]
- 15.Davis D H, Doudoroff M, Stanier R Y. Proposal to reject the genus Hydrogenomonas: taxonomic implications. Int J Syst Bacteriol. 1969;19:375–390. [Google Scholar]
- 16.Di Gioia D, Peel M, Fava F, Wyndham R C. Structures of homologous composite transposons carrying cbaABC genes from Europe and North America. Appl Environ Microbiol. 1998;64:1940–1946. doi: 10.1128/aem.64.5.1940-1946.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Don R H, Pemberton J M. Properties of six pesticide degradation plasmids isolated from Alcaligenes paradoxus and Alcaligenes eutrophus. J Bacteriol. 1981;145:681–686. doi: 10.1128/jb.145.2.681-686.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Don R H, Weightman A J, Knackmuss H-J, Timmis K N. Transposon mutagenesis and cloning analysis of the pathways for degradation of 2,4-dichlorophenoxyacetic acid and 3-chlorobenzoate in Alcaligenes eutrophus JMP134(pJP4) J Bacteriol. 1985;161:85–90. doi: 10.1128/jb.161.1.85-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eulberg D, Golovleva L A, Schlömann M. Characterization of catechol catabolic genes from Rhodococcus opacus 1CP. J Bacteriol. 1997;179:370–381. doi: 10.1128/jb.179.2.370-381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eulberg D, Kourbatova E M, Golovleva L A, Schlömann M. Evolutionary relationship between chlorocatechol catabolic enzymes from Rhodococcus opacus 1CP and their counterparts in proteobacteria: sequence divergence and functional convergence. J Bacteriol. 1998;180:1082–1094. doi: 10.1128/jb.180.5.1082-1094.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franklin F C H. Broad host range cloning vectors for gram negative bacteria. In: Glover D M, editor. DNA cloning. I. Oxford, England: IRL Press; 1985. pp. 165–184. [Google Scholar]
- 22.Frantz B, Chakrabarty A M. Organization and nucleotide sequence determination of a gene cluster involved in 3-chlorocatechol degradation. Proc Natl Acad Sci USA. 1987;84:4460–4464. doi: 10.1073/pnas.84.13.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulthorpe R R, McGowan C, Maltseva O V, Holben W E, Tiedje J M. 2,4-Dichlorophenoxyacetic acid-degrading bacteria contain mosaics of catabolic genes. Appl Environ Microbiol. 1995;61:3274–3281. doi: 10.1128/aem.61.9.3274-3281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulthorpe R R, Rhodes A N, Tiedje J M. High levels of endemicity of 3-chlorobenzoate-degrading soil bacteria. Appl Environ Microbiol. 1998;64:1620–1627. doi: 10.1128/aem.64.5.1620-1627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas D, Berger B, Schmid S, Seitz T, Reimmann C. Insertion sequence IS21: related insertion sequence elements, transpositional mechanisms, and application to linker insertion mutagenesis. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular biology of pseudomonads. Washington, D.C: American Society for Microbiology; 1996. pp. 238–249. [Google Scholar]
- 26.Harwood C S, Parales R E. The β-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol. 1996;50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 27.Hoshino T, Kose K. Cloning, nucleotide sequences, and identification of products of the Pseudomonas aeruginosa PAO bra genes, which encode the high-affinity branched-chain amino acid transport system. J Bacteriol. 1990;172:5531–5539. doi: 10.1128/jb.172.10.5531-5539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houghton J E, Brown T M, Appel A J, Hughes E J, Ornston L N. Discontinuities in the evolution of Pseudomonas putida cat genes. J Bacteriol. 1995;177:401–402. doi: 10.1128/jb.177.2.401-412.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson B F, Stanier R Y. Dissimilation of aromatic compounds by Alcaligenes eutrophus. J Bacteriol. 1971;107:468–475. doi: 10.1128/jb.107.2.468-475.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ka J O, Holben W E, Tiedje J M. Genetic and phenotypic diversity of 2,4-dichlorophenoxyacetic acid (2,4-D)-degrading bacteria isolated from 2,4-D-treated field soils. Appl Environ Microbiol. 1994;60:1106–1115. doi: 10.1128/aem.60.4.1106-1115.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasberg T, Daubaras D L, Chakrabarty A M, Kinzelt D, Reineke W. Evidence that operons tcb, tfd, and clc encode maleylacetate reductase, the fourth enzyme of the modified ortho pathway. J Bacteriol. 1995;177:3885–3889. doi: 10.1128/jb.177.13.3885-3889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasberg T, Seibert V, Schlömann M, Reineke W. Cloning, characterization, and sequence analysis of the clcE gene encoding the maleylacetate reductase of Pseudomonas sp. strain B13. J Bacteriol. 1997;179:3801–3803. doi: 10.1128/jb.179.11.3801-3803.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leander M, Vallaeys T, Fulthorpe R. Amplification of putative chlorocatechol dioxygenase gene fragments from α- and β-proteobacteria. Can J Microbiol. 1998;44:482–486. doi: 10.1139/cjm-44-5-482. [DOI] [PubMed] [Google Scholar]
- 34.Leveau J H J, van der Meer J R. The tfdR gene product can successfully take over the role of the insertion element-inactivated TfdT protein as a transcriptional activator of the tfdCDEF gene cluster, which encodes chlorocatechol degradation in Ralstonia eutropha JMP134(pJP4) J Bacteriol. 1996;178:6824–6832. doi: 10.1128/jb.178.23.6824-6832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leveau J H J, van der Meer J R. Genetic characterization of insertion sequence ISJP4 on plasmid pJP4 from Ralstonia eutropha JMP134. Gene. 1997;202:103–114. doi: 10.1016/s0378-1119(97)00460-5. [DOI] [PubMed] [Google Scholar]
- 36.Leveau J H J, Zehnder A J B, van der Meer J R. The tfdK gene product facilitates uptake of 2,4-dichlorophenoxyacetate by Ralstonia eutropha JMP134(pJP4) J Bacteriol. 1998;180:2237–2243. doi: 10.1128/jb.180.8.2237-2243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mäe A A, Marits R O, Ausmees N R, Kôiv V M, Heinaru A L. Characterization of a new 2,4-dichlorophenoxyacetic acid degrading plasmid pEST4011: physical map and localization of catabolic genes. J Gen Microbiol. 1993;139:3165–3170. [Google Scholar]
- 38.Mandel M. Deoxyribonucleic acid base composition in the genus Pseudomonas. J Gen Microbiol. 1966;43:273–292. doi: 10.1099/00221287-43-2-273. [DOI] [PubMed] [Google Scholar]
- 39.Matheson V G, Forney L J, Suwa Y, Nakatsu C H, Sexstone A J, Holben W E. Evidence for acquisition in nature of a chromosomal 2,4-dichlorophenoxyacetic acid/α-ketoglutarate dioxygenase gene by different Burkholderia spp. Appl Environ Microbiol. 1996;62:2457–2463. doi: 10.1128/aem.62.7.2457-2463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matrubutham U, Harker A R. Analysis of duplicated gene sequences associated with tfdR and tfdS in Alcaligenes eutrophus JMP134. J Bacteriol. 1994;176:2348–2353. doi: 10.1128/jb.176.8.2348-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menou G, Mahillon J, Lecadet M M, Lereclus D. Structural and genetic organization of IS232, a new insertion sequence of Bacillus thuringiensis. J Bacteriol. 1990;172:6689–6696. doi: 10.1128/jb.172.12.6689-6696.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagy I, Schoofs G, Vanderleyden J, De Mot R. Transposition of the IS21-related element IS1415 in Rhodococcus erythropolis. J Bacteriol. 1997;179:4635–4638. doi: 10.1128/jb.179.14.4635-4638.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakai C, Uyeyama H, Kagamiyama H, Nakazawa T, Inouye S, Kishi F, Nakazawa A, Nozaki M. Cloning, DNA sequencing, and amino acid sequencing of catechol 1,2-dioxygenases (pyrocatechase) from Pseudomonas putida mt-2 and Pseudomonas arvilla C-1. Arch Biochem Biophys. 1995;321:353–362. doi: 10.1006/abbi.1995.1405. [DOI] [PubMed] [Google Scholar]
- 44.Nakatsu C, Ng J, Singh R, Straus N, Wyndham R C. Chlorobenzoate catabolic transposon Tn5271 is a composite class I element with flanking class II insertion sequences. Proc Natl Acad Sci USA. 1991;88:8312–8316. doi: 10.1073/pnas.88.19.8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa N, Miyashita K. Recombination of a 3-chlorobenzoate catabolic plasmid from Alcaligenes eutrophus NH9 mediated by direct repeat elements. Appl Environ Microbiol. 1995;61:3788–3795. doi: 10.1128/aem.61.11.3788-3795.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perkins E J, Gordon M P, Caceres O, Lurquin P F. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J Bacteriol. 1990;172:2351–2359. doi: 10.1128/jb.172.5.2351-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Podladchikova O N, Dikhanov G G, Rakin A V, Heesemann J. Nucleotide sequence and structural organization of Yersinia pestis insertion sequence IS100. FEMS Microbiol Lett. 1994;121:269–274. doi: 10.1111/j.1574-6968.1994.tb07111.x. [DOI] [PubMed] [Google Scholar]
- 48.Reimmann C, Moore R, Little S, Savioz A, Willetts N S, Haas D. Genetic structure, function and regulation of the transposable element IS21. Mol Gen Genet. 1989;215:416–424. doi: 10.1007/BF00427038. [DOI] [PubMed] [Google Scholar]
- 49.Rogers M B, Bennett T K, Payne C M, Smith C J. Insertional activation of cepA leads to high-level β-lactamase expression in Bacteroides fragilis clinical isolates. J Bacteriol. 1994;176:4376–4384. doi: 10.1128/jb.176.14.4376-4384.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothmel R K, Aldrich T L, Houghton J E, Coco W M, Ornston L N, Chakrabarty A M. Nucleotide sequencing and characterization of Pseudomonas putida catR: a positive regulator of the catBC operon is a member of the LysR family. J Bacteriol. 1990;172:922–931. doi: 10.1128/jb.172.2.922-931.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 52.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savard P, Peloquin L, Sylvestre M. Cloning of Pseudomonas sp. strain CBS3 genes specifying dehalogenation of 4-chlorobenzoate. J Bacteriol. 1986;168:81–85. doi: 10.1128/jb.168.1.81-85.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schell M A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- 55.Schlömann M. Evolution of chlorocatechol catabolic pathways. Biodegradation. 1994;5:301–320. doi: 10.1007/BF00696467. [DOI] [PubMed] [Google Scholar]
- 56.Seibert V, Stadler-Fritzsche K, Schlömann M. Purification and characterization of maleylacetate reductase from Alcaligenes eutrophus JMP134(pJP4) J Bacteriol. 1993;175:6745–6754. doi: 10.1128/jb.175.21.6745-6754.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solinas F, Marconi A M, Ruzzi M, Zennaro E. Characterization and sequence of a novel insertion sequence, IS1162, from Pseudomonas fluorescens. Gene. 1995;155:77–82. doi: 10.1016/0378-1119(94)00922-f. [DOI] [PubMed] [Google Scholar]
- 58.Suwa Y, Wright A D, Fukumori F, Nummy K A, Hausinger R P, Holben W E, Forney L J. Characterization of a chromosomally encoded 2,4-dichlorophenoxyacetic acid/α-ketoglutarate dioxygenase from Burkholderia sp. strain RASC. Appl Environ Microbiol. 1996;62:2464–2469. doi: 10.1128/aem.62.7.2464-2469.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Top E M, Holben W E, Forney L J. Characterization of diverse 2,4-dichlorophenoxyacetic acid-degradative plasmids isolated from soil by complementation. Appl Environ Microbiol. 1995;61:1691–1698. doi: 10.1128/aem.61.5.1691-1698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuda M. Catabolic transposons in pseudomonads. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular biology of pseudomonads. Washington, D.C: American Society for Microbiology; 1996. pp. 219–228. [Google Scholar]
- 61.van der Meer J R, de Vos W M, Harayama S, Zehnder A J B. Molecular mechanisms of genetic adaptation to xenobiotic compounds. Microbiol Rev. 1992;56:677–694. doi: 10.1128/mr.56.4.677-694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Meer J R, Eggen R I L, Zehnder A J B, de Vos W M. Sequence analysis of the Pseudomonas sp. strain P51 tcb gene cluster, which encodes metabolism of chlorinated catechols: evidence for specialization of catechol 1,2-dioxygenases for chlorinated substrates. J Bacteriol. 1991;173:2425–2434. doi: 10.1128/jb.173.8.2425-2434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Meer J R, Frijters A C J, Leveau J H J, Eggen R I L, Zehnder A J B, de Vos W M. Characterization of the Pseudomonas sp. strain P51 gene tcbR, a LysR-type transcriptional activator of the tcbCDEF chlorocatechol oxidative operon, and analysis of the regulatory region. J Bacteriol. 1991;173:3700–3708. doi: 10.1128/jb.173.12.3700-3708.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Meer J R, van Neerven A R W, de Vries E J, de Vos W M, Zehnder A J B. Cloning and characterization of plasmid-encoded genes for the degradation of 1,2-dichloro-, 1,4-dichloro-, and 1,2,4-trichlorobenzene of Pseudomonas sp. strain P51. J Bacteriol. 1991;173:6–15. doi: 10.1128/jb.173.1.6-15.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Meer J R, Zehnder A J B, de Vos W M. Identification of a novel composite transposable element, Tn5280, carrying chlorobenzene dioxygenase genes of Pseudomonas sp. strain P51. J Bacteriol. 1991;173:7077–7083. doi: 10.1128/jb.173.22.7077-7083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Werlen C, Kohler H P E, van der Meer J R. The broad substrate chlorobenzene dioxygenase and cis-chlorobenzene dihydrodiol dehydrogenase of Pseudomonas sp. strain P51 are linked evolutionarily to the enzymes for benzene and toluene degradation. J Biol Chem. 1996;271:4009–4016. doi: 10.1074/jbc.271.8.4009. [DOI] [PubMed] [Google Scholar]
- 67.Wyndham R C, Cashore A E, Nakatsu C H, Peel M C. Catabolic transposons. Biodegradation. 1994;5:323–342. doi: 10.1007/BF00696468. [DOI] [PubMed] [Google Scholar]
- 68.Xu K, He Z Q, Mao Y M, Sheng R Q, Sheng Z J. On two transposable elements from Bacillus stearothermophilus. Plasmid. 1993;29:1–9. doi: 10.1006/plas.1993.1001. [DOI] [PubMed] [Google Scholar]
- 69.Xu Y, Mortimer M W, Fisher T S, Kahn M L, Brockman F J, Xun L. Cloning, sequencing, and analysis of a gene cluster from Chelatobacter heintzii ATCC 29600 encoding nitrilotriacetate monooxygenase and NADH:flavin mononucleotide oxidoreductase. J Bacteriol. 1997;179:1112–1116. doi: 10.1128/jb.179.4.1112-1116.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeo C C, Poh C L. Characterization of IS1474, an insertion sequence of the IS21 family isolated from Pseudomonas alcaligenes NCIB 9867. FEMS Microbiol Lett. 1997;149:257–263. doi: 10.1111/j.1574-6968.1997.tb10338.x. [DOI] [PubMed] [Google Scholar]
- 71.You I-S, Ghosal D. Genetic and molecular analysis of a regulatory region of the herbicide 2,4-dichlorophenoxyacetate catabolic plasmid pJP4. Mol Microbiol. 1995;16:321–331. doi: 10.1111/j.1365-2958.1995.tb02304.x. [DOI] [PubMed] [Google Scholar]