Abstract
Our understanding of the cellular composition and architecture of cancer has primarily advanced using 2D models and thin slice samples. This has granted spatial information on fundamental cancer biology and treatment response. However, tissues contain a variety of interconnected cells with different functional states and shapes, and this complex organization is impossible to capture in a single plane. Furthermore, tumours have been shown to be highly heterogenous, requiring large‐scale spatial analysis to reliably profile their cellular and structural composition. Volumetric imaging permits the visualization of intact biological samples, thereby revealing the spatio‐phenotypic and dynamic traits of cancer. This review focuses on new insights into cancer biology uniquely brought to light by 3D imaging and concomitant progress in cancer modelling and quantitative analysis. 3D imaging has the potential to generate broad knowledge advance from major mechanisms of tumour progression to new strategies for cancer treatment and patient diagnosis. We discuss the expected future contributions of the newest imaging trends towards these goals and the challenges faced for reaching their full application in cancer research.
Keywords: archival tissue, cancer biology, cancer (immuno)therapy, 3D imaging
Subject Categories: Cancer, Methods & Resources
As part of our cancer reviews series, Rios and colleagues revisit recent advances in spatiotemporal imaging microscopy and related experimental methods, highlighting their successful application to cancer biology.

Introduction
Visualization of tissues in three dimensions (3D) has increased our understanding of their structural organization and how this relates to organ development and function. Advances in tissue clearing (Richardson & Lichtman, 2015; Almagro et al, 2021) coupled with light sheet (Reynaud et al, 2015), confocal (Jonkman et al, 2020) and multiphoton (Andresen et al, 2009) microscopy technologies allow for the observation of complex structures within large intact tissue specimens and led to discoveries in many fields of interest, including embryonic development (Belle et al, 2017), brain connectivity and function (Kim et al, 2013; Renier et al, 2016; Murakami et al, 2018; Ueda et al, 2020), organ morphology (Hannezo et al, 2017; Li et al, 2017, 2019) and stem cell biology (Snippert et al, 2010; Rios et al, 2011, 2014; Scheele et al, 2017). In addition, super‐resolution imaging (Bates et al, 2007; Huang et al, 2008a, 2009) has expanded our ability to differentiate ultrastructures beyond the diffraction limit. A concomitant evolution in quantitative analysis and computational modelling has furthermore transformed fluorescent microscopy from a descriptive methodology into a powerful spatial and phenotypic (single‐cell) technology (Messal et al, 2019; Albanese et al, 2020; Stoltzfus et al, 2020; Zhao et al, 2020; van Ineveld et al, 2021). By providing 3D context of tissue architecture and cellular composition, and even subcellular organization of healthy and diseased tissue, these techniques can also uniquely unveil spatio‐dynamic traits of cancer. Moreover, an increased number of sample preparation methods (Box 1) designed for preserving endogenous fluorescence (Li et al, 2019; Rios et al, 2019; Messal et al, 2021), performing large sample immunolabelling (Renier et al, 2014; Ku et al, 2020) and moving towards multiplex imaging (Murray et al, 2015; Goltsev et al, 2018; Stoltzfus et al, 2020; preprint: Seo et al, 2021; van Ineveld et al, 2021; van Ineveld et al, in press), allowed adaptation of 3D imaging from application in fluorescently‐engineered animal models to studying biological processes in human samples. Together with advances in molecular and human tissue modelling techniques, this now provides important new arsenal for imaging modalities to explore human cancer biology (Rios & Clevers, 2018), covering key tumour features, such as competition and plasticity, also discussed elsewhere in this review series (Brabletz et al, 2021; Parker et al, 2021). 3D imaging‐driven knowledge of cancer biology includes insights into tumour architecture and its evolving heterogeneity (Oshimori et al, 2015; Tanaka et al, 2017; Messal et al, 2019; Rios et al, 2019), metastatic seeding (Kubota et al, 2017; Pan et al, 2019), interplay with the tumour microenvironment (TME) (Garofalo et al, 2015; Brown et al, 2018) and response to neoadjuvant treatments (Kubota et al, 2017; Kingston et al, 2019). Here, we review recent advances in 3D (live) imaging technology and novel understanding of tumour biology generated by it (Table 1; Figure 1). Furthermore, we discuss expected future contributions and the technological requirements that need to be met and challenges overcome to reach the full application of 3D imaging in the field of cancer research.
Table 1.
Milestone discoveries in cancer research using 3D imaging.
| Cancer discovery | Impact | Microscopy | Sample preparation | Study |
|---|---|---|---|---|
| Macroscale | ||||
| Tumour cell dissemination via lymph node blood vessels | New active process for metastasis at distant site |
Light sheet Multiphoton |
Fixed; CUBIC IVM |
Brown et al (2018); Pereira et al (2018) |
| Previously unrecognized micrometastases and degree of overlap with therapeutic antibody distribution | Metastatic burden assessment and treatment evaluation | Light sheet | Fixed, whole animal; CUBIC, DISCO | Kubota et al (2017); Pan et al (2019) |
| Epithelial mechanical forces that lead to aggressive forms of cancer | Biophysical prediction and potential targeting of tumour aggressiveness | Confocal | Fixed; FLASH | Messal et al (2019); Fiore et al (2020) |
| Archival | ||||
| Heterogeneity in lymphatic microvasculature and identification of vascular versus lymphatic invasion | Tumour staging and patient stratification | Light sheet | FFPE; DIPCO | Tanaka et al (2017); Tanaka et al (2018) |
| Her2‐enriched membrane protrusions in breast cancer biopsies | Biomarker ultrastructure that might relate to tumour aggressiveness | Super‐resolution; STORM | FFPE | Creech et al (2017) |
| High dimensionality | ||||
| Relation between tumour cells and fibroblasts in hypoxic tumour environment | Cancer cell functional profiling in relation to the TME | Spinning disc Confocal | Fixed; RNA multiplexing ExSeq | Alon et al (2021) |
| Developmental trajectory and heterogeneity mapping of Wilms tumour | Identification of new tumour cell subset | Multispectral confocal | Fixed; mLSR‐3D/FUnGI | van Ineveld et al (2021) |
| Time resolved | ||||
| Tumour plasticity and clonal evolution restriction in breast tumours | Clonal evolution of cancer | Multispectral confocal | Fixed; LSR‐3D/FUnGI | Rios et al (2019) |
| Stemness represents a reversible tumour cell state that can be gained or lost over time | Cancer stem cell plasticity in tumour progression | Multiphoton | IVM; mammary imaging windows | Zomer et al (2013) |
| Colorectal cancer metastasis linked to mutations that allow growth independence of niche signals | Driver mechanism of late‐stage cancer progression | Multiphoton | IVM; abdominal imaging windows | Fumagalli et al (2017) |
| Glioma cell functional interaction endows stemness and therapy resistance. Connections with neurons potentiates invasive growth | Importance of functional interconnections between tumour cells and environmental cells in tumour progression | Multiphoton; scanning electron microscopy | IVM; cranial imaging windows; optogenetics | Osswald et al (2015); Venkataramani et al (2019); Xie et al (2021) |
| Interplay with extracellular matrix regulates breast tumour dissemination and cooperation with macrophages supports intravasation | Role of the TME in tumour metastasis | Multiphoton | IVM; mammary imaging windows; skin‐fold windows | Harney et al (2015); Ilina et al (2020) |
| In vivo functional heterogeneity of CAR T cells and action radius of anti‐PD1 | Cancer immunotherapy mode of action and targets to improve efficacy | Confocal; multiphoton | IVM; skin‐fold windows | Arlauckas et al (2017); Cazaux et al (2019) |
| Organoids | ||||
| Highly heterogeneous and multi‐phasic Wilms tumour‐derived organoid cultures | Resemblance of human cancer organoid biobank to tumour tissue | Multispectral; confocal | Fixed; mLSR‐3D/FUnGI | Calandrini et al (2020) |
| Widespread chromosomal instability in human cancer, yet with heterogenous outcome for cell death | Functional heterogeneity shaping human tumour evolution | Confocal; spinning disc | Live | Bolhaqueiro et al (2019) |
| Tumour cells actively eliminate healthy cells by JNK‐dependent growth promotion | Mechanism of tumour cell competition in human cancer | Confocal; spinning disc | Live and fixed | Krotenberg Garcia et al (2021) |
| Widespread behavioural heterogeneity in engineered T cells targeting human cancer organoids | Opportunities to improve treatment efficacy by skewing towards T cell functional behaviour | Multispectral; confocal | Live | preprint: Dekkers et al (2021) |
Figure 1. Key 3D imaging technologies driving insight into cancer biology.
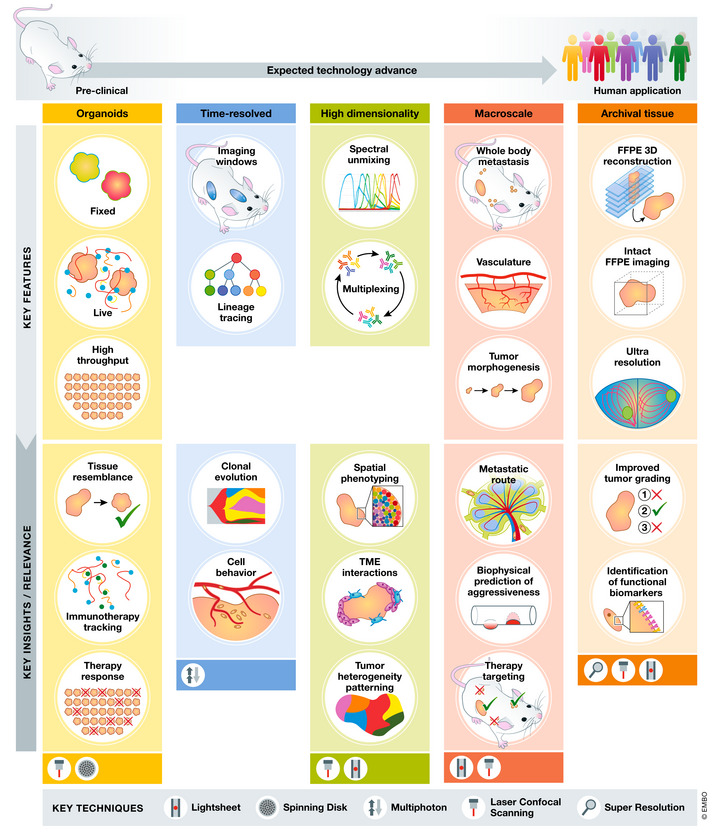
Technology advance in various areas of 3D imaging and key insight in cancer biology obtained by it.
Box 1. Sample preparation and acquisition methods for 3D imaging of cancer biology.
To interrogate cancer biology using 3D imaging, optical clearing to reach tissue transparency and fluorescent labelling of markers of interest form critical components of the workflow. To achieve this, various sample preparation pipelines have been developed, each with their own advantages related to the kind of tumour tissue that needs to be imaged and the type of fluorescence detected. Furthermore, the chosen microscope technology for acquisition will depend on these factors and needs to be balanced between the required resolution and imaging volume.
Optical clearing. A large body of clearing methods to homogenize the refractive index of tissues and thereby render them transparent have been developed and extensively reviewed elsewhere (Richardson & Lichtman, 2015; Ueda et al, 2020; Almagro et al, 2021). 3D imaging methods, like CUBIC (Susaki et al, 2014; Kubota et al, 2017) and various DISCO approaches (Erturk et al, 2012; Renier et al, 2014; Pan et al, 2016, 2019; Molbay et al, 2021) (Table 2), can achieve entire organ and even mouse whole‐body transparency and have been instrumental in cancer research for visualization and quantification of bodywide metastases (Kubota et al, 2017; Pan et al, 2019). Other clearing agents have been developed to offer more rapid and routine sample preparation pipelines that are furthermore better at conserving tissue morphology and reporter fluorescence, while being compatible with a wide range of antibody labels. FLASH (Table 2) implements an antigen retrieval step tailored to the tissue of interest that, importantly, includes preservation of epithelial markers (Messal et al, 2021). It has been applied to reveal the epithelial physical properties dictating the morphology of neoplasms in the pancreas (Messal et al, 2019), of which the epithelium could not effectively be stained with existing 3D imaging protocols that had been mostly developed for the brain (Messal et al, 2021) and the same was true for other abdominal organs. Ce3D (Table 2) was similarly developed to tackle issues of antibody‐based immunolabelling observed with alternative clearing methods, resulting in a protocol robustly compatible with extensive multiplexing (Li et al, 2017, 2019). It has subsequently been applied to interrogate the subset composition and spatial distribution of immune cells in the TME (Stoltzfus et al, 2020). Finally, the clearing agent FUnGI (Table 2) was developed to preserve mammary gland tissue integrity and allow for concurrent immunolabelling and detection of endogenous fluorescence to reveal the clonal evolution of breast cancer through lineage tracing combined with phenotypic profiling (Rios et al, 2019). Altogether, a wide range of clearing agents are available to interrogate cancer biology through 3D imaging, and the method of choice will depend on the scale and type of tissue to be imaged, as well as the fluorescent labelling strategy.
Fluorescent labelling. 3D visualization of markers of interest will depend on their robust fluorescence which can be achieved through specific labelling with fluorescent antibodies or dyes. This often requires a permeabilization step to enable penetration of antibodies and dyes deep and uniformly into the tissue (Almagro et al, 2021; Messal et al, 2021). In addition, markers of interest can be visualized through endogenous, reporter‐driven fluorescence. This requires clearing agents that optimally preserve such endogenous fluorescent expression (Li et al, 2019; Rios et al, 2019), or dedicated sample preparation techniques to boost the endogenous signal deep inside the tissue. vDISCO (Table 2), for instance, implements high‐pressure perfusion of nanobodies against fluorescent proteins to enhance their signal intensity by two orders of a magnitude (Cai et al, 2019; Pan et al, 2019). In the future, label‐free imaging (Rust et al, 2006; Gavgiotaki et al, 2020), for instance exploiting differential autofluorescence of distinct cell types (Rivenson et al, 2019), might become a valuable addition to increase the number of labels in 3D imaging approaches, or circumvent the need for fluorescent labelling all together.
Microscope technology. Light sheet technology captures large fields of view using low NA objectives. It thereby allows for rapid imaging of very large, even centimetre‐scale, specimens, such as entire mice and full human organs, yet at the expense of some resolution. Light sheet imaging has been widely applied to capture the macroscopic properties of cancer, including blood and lymph vasculature (Brown et al, 2018; Kastelein et al, 2020) and whole‐body tumour dissemination (Kubota et al, 2017; Pan et al, 2019). In contrast, confocal or two‐photon microscopes use high NA objectives to achieve higher resolution and magnification, yet at the sacrifice of some working depth and require lengthier imaging times, thereby more susceptible to photobleaching. When equipped with a multispectral detector, confocal/multiphoton imaging can be especially useful for multi‐marker imaging of cancer biology at high resolution, exploiting contemporary advances in unmixing of spectrally overlapping fluorophores (Seo et al, 2021; van Ineveld et al, 2021) to achieve high‐dimensional and high‐definition profiling of cancer.
3D anatomical mapping of cancer
Large‐scale 3D imaging has been instrumental for unravelling the structural landmarks of cancer in intact tumours and even organisms. In this section, we review the insights into tumour architecture and systemic cancer biology generated by 3D imaging in the context of tumour progression and treatment. We also highlight examples of how such technology can be exploited towards prediction of cancer aggressiveness and treatment targeting efficacy, when combined with advanced computational modelling.
Light sheet technology for studying blood and lymphatic vasculature
The blood and lymphatic vascular systems not only represent structural landmarks of the microenvironment within malignant tissues, but also a gateway for whole‐body tumour dissemination. They thereby hold important implications for cancer targeting and late metastatic progression. Volumetric imaging allows to fully appreciate the complex organization of these networks in 3D and has provided key information on how these structures in the direct tumour microenvironment (Lin et al, 2016) are actively remodelled by the tumour (Liu et al, 2013; Shen et al, 2019). 3D imaging of blood vasculature has also helped to assess the efficacy of therapy delivery (Lee et al, 2019a), as well as micro‐vascular damage as a side effect of cancer treatment (Craver et al, 2016). Light sheet technology, which can capture large field of views using low numerical aperture (NA) objectives, yet in detriment to some resolution, has been demonstrated especially suited to study such macroscopic structures within tissue (Box 1). Combined with advances in optical clearing, it has been instrumental in revealing the full vasculature network of organs, such as the murine brain (Todorov et al, 2020). Moreover, it has been applied to entire tumours, uniquely revealing their chaotic and immature angioarchitecture and identifying tumour areas with poorly‐perfused microvasculature (Dobosz et al, 2014; Kastelein et al, 2020). Next to providing a cause to hypoxia, a well‐recognized tumour feature that often leads to an invasive phenotype of cancer, these findings thereby also explain varying responses to systemic treatment that have been linked to perfusion constraints (Mendler et al, 2016; Viallard et al, 2020). As such, modulation of key signalling molecules involved in vascular remodelling, such as VEGF and BMP9, is of therapeutic interest to normalize the tumour vasculature, and 3D imaging has been used to visualize the outcome of such approaches. This indeed revealed a decrease in hypoxia and increased perfusion (Viallard et al, 2020), beneficial for drug delivery.
An additional layer of complexity can be reached by also taking into account the lymphatic system. Here, light sheet 3D imaging uniquely revealed that metastasizing cells can exit the lymph node by invading local lymph node blood vessels, instead of using efferent lymphatic vessels (Brown et al, 2018), a finding also confirmed through intravital imaging (Pereira et al, 2018) (Table 1), discussed later on in this review. This key observation received a lot of attention, as it shed new light on an ongoing debate whether lymph node metastasis could be an active route for tumour cells to disperse to distant sites. It might also have implications for treatment decisions, as few tumour cells located adjacent to or within blood vessels are expected to be more predictive of poor prognosis, compared to potentially larger tumour cell deposits distant from blood vessels (2018; Dart, 2018; Tjan‐Heijnen & Viale, 2018). For large‐scale 3D imaging, deep and homogeneous penetration of antibodies even in difficult to access anatomical structures, such as bone tissue, is essential (Box 1) (Tainaka et al, 2018). As an example, decalcified vertebral segments immunolabelled using the iDISCO+ method (see Table 2 for glossary of 3D imaging and data analysis tools) provided a major step forward in mapping the 3D architecture and function of vertebral lymphatic vessels (Jacob et al, 2019), of interest for cancer research, as the vertebral column represents a common site for metastasis. Thus, 3D imaging of blood and lymph vasculature has generated critical insights into large‐scale cancer architecture of therapeutic relevance for improving drug delivery and predicting metastatic routes.
Table 2.
Glossary of 3D imaging methodologies and data tools.
| Abbreviation | Name | Reference |
|---|---|---|
| BEHAV3D | Behavioral‐phenotypic characterization of dynamic immune‐organoid 3D imaging | preprint: Dekkers et al (2021) |
| Ce3D | Clearing enhanced 3D | Li et al (2017, 2019) |
| CODEX | CO‐Detection by indEXing | Goltsev et al (2018) |
| CUBIC | Clear, Unobstructed Brain Imaging Cocktails and Computational analysis | Susaki et al (2014) |
| CytoMAP | histo‐Cytometric Multidimensional Analysis Pipeline | Stoltzfus et al (2020) |
| DeepMACT | Deep learning‐enabled Metastasis Analysis in Cleared Tissues | Pan et al (2019) |
| DIPCO | Diagnosing Immunolabeled Paraffin‐embedded Cleared Organs | Tanaka et al (2017, 2018) |
| 3DISCO | 3D Imaging of Solvent‐Cleared Organs | Erturk et al (2012) |
| iDISCO+ | immunolabeling‐enabled three‐Dimensional Imaging of Solvent‐Cleared Organs | Renier et al (2016) |
| uDISCO | Ultimate DISCO | Pan et al (2016) |
| vDISCO | nanobody VhH boosted DISCO | Pan et al (2019) |
| DVEX | Drosophila Virtual Expression eXplorer | Karaiskos et al (2017) |
| ExSeq | Expansion Sequencing | Alon et al (2021) |
| FLASH | Fast Lightmicroscopic analysis of Antibody‐Stained wHole organs | Messal et al (2019) |
| FTIR | Fourier‐Transform InfraRed spectroscopy | Rivenson et al (2019) |
| FUnGI | Fructose, Urea and Glycerol for Imaging | Rios et al (2019) |
| Geo‐seq | Geographical position sequencing | Chen et al (2017) |
| LSR‐3D | Large‐scale Single‐cell Resolution 3D | Rios et al (2019) |
| LvSEM | Low‐vacuum Scanning Electron Microscopy | Jones (2012) |
| mLSR‐3D | multispectral Large‐scale Single‐cell Resolution 3D | van Ineveld et al (2021) |
| Opal | multiplexed IHC protocol Opal | Nolan et al (2017); Viratham Pulsawatdi et al (2020) |
| PICASSO | blind unmixing technique | Seo et al (2021) |
| SHG | Second Harmonic Generation | Ren et al (2017) |
| SMART 3D | Spatial filtering‐based background removal and Multi‐chAnnel forest classifiers‐based 3D ReconsTruction | Guldner et al (2016) |
| STAPL‐3D | SegmenTation Analysis by ParaLlelization of 3D datasets | van Ineveld et al (2021) |
| STARmap | Spatially‐resolved Transcript Amplicon Readout mapping | Wang et al (2018) |
| STORM | Stochastic Optical Reconstruction Microscopy | Rust et al (2006) |
| SWITCH | System‐Wide control of Interaction Time and kinetics of CHemicals | Murray et al (2015) |
| THG | Third Harmonic Generation | Gavgiotaki et al (2020) |
| Thick PS‐LvSEM | Thick Paraffin Sections ‐ Low‐vacuum Scanning Electron Microscopy | Sawaguchi et al (2018) |
| Tomo‐seq | Tomos (slice) sequencing | Junker et al (2014) |
Whole‐body 3D imaging to assess metastatic burden
3D imaging plays an essential role in studying late manifestation of cancer, by enabling quantification of metastasis in cleared secondary organs. Moreover, as further discussed below, the highly potent clearing techniques CUBIC (Susaki et al, 2014) and derivates of 3DISCO (Erturk et al, 2012) (Table 2) have allowed for pan‐scale imaging to study systemic cancer biology, including distant dissemination, and explore therapeutic avenues (Table 1). Together with upgraded light sheet microscope systems and advanced fluorescent labelling pipelines, they allow for the detection of cells in microscopic quantities, even single metastasized tumour cells (Pan et al, 2019), while scanning massive volumes to image entire mice and full human organs (Box 1) (Kubota et al, 2017; Cai et al, 2019; Zhao et al, 2020). Kubota et al (2017) used CUBIC to quantify metastasis at the whole mouse body level and assess their response to chemotherapy (Kubota et al, 2017). Panoramic imaging further improved with the development of uDISCO (Pan et al, 2016), followed by next‐generation DISCO, vDISCO (Pan et al, 2019) (Table 2). The unprecedented scale of whole‐body imaging poses significant challenges for data handling and analysis, thereby deep learning‐based quantification forms an indispensable part of this technology advance. The DeepMACT analysis pipeline (Table 2; Box 2) (Pan et al, 2019) allows mapping of individual metastasis in whole‐animal cancer models and quantifying spatial differences in their response to immunotherapy. This uniquely revealed a previously unrecognized micro‐metastasis pattern at numerous sites across the body, as well as their degree of overlap with the distribution of a monoclonal antibody therapy, indicative of targeting (Pan et al, 2019). These examples also highlight how 3D imaging methods initially developed for other areas of interest, for example, brain function, are subsequently applied in cancer research, leading to new biological insight in cancer (Figure 2). Building from this seminal work, whole‐body distribution mapping of novel targeted or cellular therapies is a promising avenue that could uniquely be addressed by these 3D panoramic imaging technologies.
Figure 2. Timeline illustrating the developmental cycle between (imaging) technology advance and new knowledge.
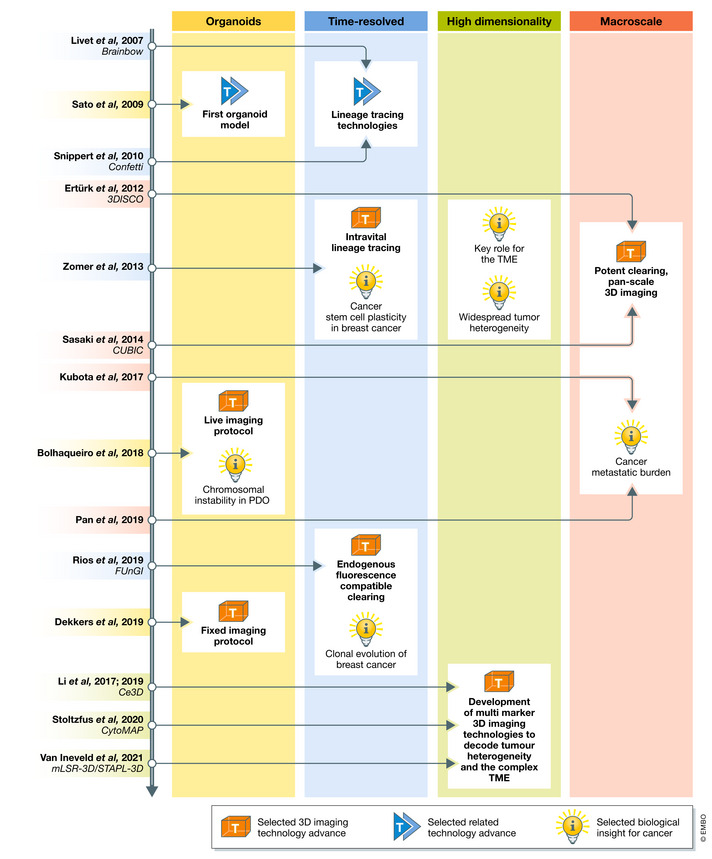
Timeline of selected advances in 3D imaging (T, orange box) or related technologies (T, blue arrows) and biological insights (I, lightbulb) for the main areas of 3D imaging, to illustrate the developmental cycle between technology and knowledge advance.
Box 2. Artificial intelligence to analyse complex 3D imaging data.
With both scale and dimensionality of 3D imaging data rapidly increasing, automated analysis pipelines become imperative for extracting the large amount of spatial and phenotypic single‐cell information. Artificial intelligence (AI)‐based data analysis depends on high‐quality imaging data to train algorithms to automatically detect single objects, such as cells and regions, in 3D imaging data and perform quantitative analysis. Several recent 3D image analysis pipelines that have been applied for cancer research implement either deep learning or machine learning to achieve rapid, accurate, automated and unbiased analysis.
DeepMACT (Table 2) is a deep learning‐based pipeline that employs a U‐Net‐like convolutional neural network exploiting 2D maximum‐intensity projections with high signal‐to‐noise ratio to rapidly and unbiasedly segment and quantify tumour metastases in whole mouse 3D imaging data (Pan et al, 2019). As such, DeepMACT has been instrumental in mapping the metastatic landscape of tumours, including thousands of micrometastases and their targeting by therapeutic antibodies.
CytoMAP (Table 2) employs machine learning‐based data clustering to group cells into local neighbourhoods based on their cell type and position to explore the architectural organization of cells within tissues (Stoltzfus et al, 2020). It can thereby resolve the spatial relationships of cells, for instance the interrelations between immune and tumour cells in the TME.
STAPL‐3D (Table 2) implements deep learning algorithms: 3D‐UNET for membrane prediction and Stardist for nuclear prediction, to accurately and timely segment millions of cells in large 3D imaging datasets (van Ineveld et al, 2021). The resulting high‐dimensional dataset allows for omics‐like analysis, such as unbiased cell population clustering, revealing novel tumour cell subsets. STAPL‐3D, furthermore, offers a co‐affine registration method to obtain large volumes of high‐quality 3D imaging data for training the deep learning algorithms.
Confocal and two‐photon microscopy resolves refined tumour growth patterns
As opposed to light sheet technology, confocal or two‐photon microscopes offer more mainstream systems for large‐scale 3D imaging with higher resolution, yet at the sacrifice of some working depth (Box 1). Therefore, recent efforts have been directed at obtaining easy‐to‐use protocols for routine 3D imaging of intact tumours with clearing methodologies that preserve epitope integrity, limit distortion of tissue and allow for rapid sample preparation (Box 1) (Li et al, 2017, 2019; Rios et al, 2019; Stoltzfus et al, 2020; Messal et al, 2021). Typically combined with confocal and multiphoton imaging using high NA objectives, such methodologies paved the way for studying tissue disruption during cancer morphogenesis at high resolution. Indeed, growth patterns of lesions in murine cancer models have been suggested to be a shared consequence of both genetic alterations and the geometry of normal epithelium. Using the FLASH protocol (Table 2) with subsequent morphometrics analysis and mathematical modelling, Messal et al (2019) showed that physical constraints such as epithelial curvature can not only dictate morphology, but also predict tumour cell infiltration in the surrounding parenchyma in cleared pancreatic neoplastic tissues (Messal et al, 2019). Similarly, in skin cancer, different forms of the disease have been shown to be the consequence of biophysical and biological constraint differences, with decrease in basement membrane stiffness promoting invasive squamous cell carcinoma (Fiore et al, 2020) (Table 1). Such spatial analyses are not restricted to primary tumour, but also relevant for metastasis. Multiphoton microscopy combined with an integrative analysis pipeline, SMART 3D (Table 2), revealed the spatial landscape of experimental brain metastasis derived from breast cancer cells and identified, for instance, proliferative differences and specific metastasis‐associated astrogliosis (Guldner et al, 2016). These examples illustrate the power of confocal/multiphoton 3D imaging together with computational modelling to not only quantify global spatial changes occurring during cancer progression, but also predict biophysical forces that lead to aggressive forms of cancer.
Together, this body of work demonstrates the value of 3D imaging to unravel the macroscopic landscape of cancer across the full trajectory from early stage progression to end stage metastasis, as well as during treatment response. Importantly, these 3D imaging results have allowed to start drawing connections between cancer architecture and clinical outcome, such as tumour aggressiveness based on biophysical properties of the surrounding tissue (Table 1; Figure 1). To fully explore the predictive power of 3D imaging and start using it as a diagnostic tool, 3D imaging of archival samples stored in patient biobanks together with relevant clinical data will be the next step forward, as discussed in the following section.
3D visualization of archival tissue
For 3D imaging to translate into a clinical application that can facilitate patient diagnosis and treatment stratification, compatibility with pathological formalin‐fixed paraffin‐embedded (FFPE) tissue is key. FFPE samples are current practice for diagnosis and therefore an extremely well‐documented and widely available resource that is for now not fully exploited for its clinical and research potential by routine 2D sectional haematoxylin‐eosin (H&E) imaging. Indeed, standard histological practices only allow for the observation of a few slices of material, which ends up being a fraction of the entire sample, thereby severely limiting in information when analysing spatially heterogeneous tissue, like tumour tissue. This deficiency is underscored by current 2D methods for histological grading of primary tumours leading to inconsistency and misdiagnosis (Kruskal et al, 1997; Brown et al, 2010; Catalona et al, 2017; Epstein, 2018; Ahdoot et al, 2020). Although challenging to obtain high‐quality volumetric data from FFPE‐embedded samples compared to freshly fixed tissue, tailored protocols and pipelines could greatly improve accuracy of diagnosis and reduce the observed variability of cancer staging, thereby offering a powerful quantitative and unbiased imaging tool for treatment guidance. Furthermore, the vast amount of existing FFPE samples would open up a unique source of 3D information for preclinical research. This information stored within biobanks containing billions of FFPE tissue blocks together with relevant clinical information, including diagnosis and patient outcome, would allow for correlative and predictive analyses (Baker, 2012; Kokkat et al, 2013). Many laboratories have begun to explore 3D histopathology of FFPE primary patient material for tumour grading (van Royen et al, 2016) or to assign novel treatment options, such as immunotherapy (Si et al, 2019). In this section, we discuss the two major trends in 3D histopathological imaging: 3D reconstruction of 2D sections and 3D imaging of intact FFPE samples. Moreover, super‐resolution imaging of such samples might provide additional insight by revealing malignant ultrastructures linked to disease progression and clinical outcome.
3D reconstruction of serial 2D sections
3D reconstruction of serial 2D sections allows the exploration of full FFPE tissue blocks. The advantages of this approach include full compatibility with current tissue staining protocols and a vast number of antibodies validated for immunohistochemistry. Such superimposing of stained FFPE tissue sections, for example, has been applied to reveal location‐dependent PD‐L1 expression in colon cancer (Korehisa et al, 2018), a specific invasion pattern of lung adenocarcinoma (Yagi et al, 2020), and a “branching tree”‐like structure of prostate cancer (Tolkach et al, 2018). This structural insight would have been impossible to retrieve from a single 2D section. Quantitative analysis can be performed on these in silico 3D reconstructions as well, for instance, quantifying invasive tumour progression (Booth et al, 2015). Such quantified spatial information could aid pathologists in making more refined classification through more detailed sub‐staging of patients (Jansen et al, 2019). However, 3D rendering from serial sectioning is tremendously laborious and time consuming, as for large volumes thousands of slides must be processed and imaged. Automated staining machines and slide scanners can potentially relieve some of this time burden. Still, artefacts can arise, due to tissue deformations associated with sectioning.
3D imaging of intact FFPE tissue
To overcome limitations of serial 2D sectioning, protocols for 3D imaging of intact FFPE specimens are emerging that rely on similar principles of optical clearing, as described for freshly fixed tissue, and are compatible with immunolabelling and confocal or light sheet microscopy (Rios et al, 2016; van Royen et al, 2016; Nojima et al, 2017; Chen et al, 2019; Yoshizawa et al, 2020; van Ineveld et al, in press). A big advantage of this approach from a pathological point of view is that the imaged tissue remains intact and can be re‐embedded for later use, enabling the implementation of 3D imaging into conventional pathological practice. A prime example of this approach is the comprehensive overview of prostate cancer growth patterns revealed by 3D imaging of archival FFPE samples. In 3D, as opposed to 2D, two architecturally different subgroups could be observed (Verhoef et al, 2019). Since therapeutic decisions are based on tumour growth patterns (Gleason score), this highlights the clinical importance of such 3D imaging. Clinical application of 3D archival tissue imaging was further demonstrated by DIPCO, an iDISCO‐based protocol for FFPE tissue (Table 2), shown to have better accuracy compared to 2D histological analysis for diagnosis and stratification of patient prognosis (Tanaka et al, 2017, 2018). In addition, vessel radius, impossible to reliably quantify in 2D, could stratify prognosis for ovarian cancer patients, and vascular or lymphatic invasive cancer was more efficiently detected in 3D (Table 1). More recently, this protocol was updated to include in situ hybridization for RNA detection, further improving the molecular phenotyping capabilities of the technique (Tanaka et al, 2020). It was applied to assess small populations of cancer stem cells expressing PROM1 and LGR5 in 3D, allowing the identification of neoplastic cells positioning themselves preferentially parallel to muscular blood vessels in pancreatic cancer (Noe et al, 2018).
Super‐resolution to differentiate malignant ultrastructure beyond the diffraction limit
For investigation of ultrastructures beyond the roughly 200 nm diffraction limit (Weiss, 2000), conventional light microscopy is not sufficient. This limitation can be circumvented by super‐resolution techniques, such as LvSEM (Jones, 2012) or STORM (Rust et al, 2006) (Table 2) that are capable of achieving below 1 nm or 20 nm resolution, respectively. The application of electron microscopy to thick paraffin sections, termed Thick PS‐LvSEM (Table 2), allows for the investigation of FFPE material sections of up to 30 μm in thickness (Sawaguchi et al, 2018). Applied on FFPE renal biopsies, this technique revealed various glomerular lesions, with spike formation on the basement membrane clearly identified in membranous nephropathy samples (Miyazaki et al, 2012), illustrating its potential for 3D investigation of complex tissue structures at the electron microscopic level. However, while offering very high resolution, this technology comes at a cost of limited (30 μm) section thickness. STORM achieves 3D super‐resolution imaging through highly accurate localization of single fluorescent molecules (Huang et al, 2008b). Applied to clinical FFPE sections of cancerous tissue, it revealed ultrastructures resolvable to a depth of 800 nm (Creech et al, 2017). It can thereby be used to study ultrastructure and functional markers of various cancer types, including breast. In this regard, Her2 receptor, an important biomarker for breast cancer diagnosis, could be accurately visualized on membrane protrusions in FFPE breast cancer biopsies. Previously shown to be implicated in persistent localization and signalling of Her2 in breast cancer cells in vitro (Jeong et al, 2016), this demonstrates that similar Her2‐enriched protrusions appear to exist in vivo and may play a functional role in tumour biology (Table 1) (Creech et al, 2017). Thus, although for now limited to very thin sections, super‐resolution imaging may offer additional molecular insights into disease mechanisms and diagnosis by uncovering ultrastructure information from FFPE samples. Together with 3D imaging technology advance for archival samples, this can create a very complete picture of tumour characteristics associated with poor prognosis and treatment response.
This improved discriminative potential of 3D archival tissue imaging will further expand with the number of molecules, and thereby cell types and ultrastructures, that can be visualized. Advances in label‐free imaging approaches, including THG, SHG and FTIR spectroscopy (Ren et al, 2017; Rivenson et al, 2019; Gavgiotaki et al, 2020) (Table 2), can help achieve this goal and have already been applied to 2D tissue sections to discriminate morphology differences between malignant and healthy tissue (Gavgiotaki et al, 2020). These approaches might offer easy‐to‐implement alternatives to antibody labelling of thick FFPE tissues. Other exciting avenues in multi‐marker 3D imaging, discussed in the next section, could add to this purpose as well and provide powerful technologies to decipher the complex composition of tumours and identify the many cell types present in the millions of archival tissues available around the world. Overall, FFPE material will continue to be the golden standard for processing and efficiently storing clinical samples. After overcoming barriers in cost and technical feasibility, 3D imaging has the potential to become more embedded in the clinical workflow to improve diagnostic accuracy and validate new biomarkers for diagnostic purposes and to monitor treatment response.
Multi‐marker imaging to resolve tumour heterogeneity and interaction with the microenvironment
Advancing multi‐marker 3D imaging is the next step forward for unravelling tumour heterogeneity and interaction with the complex TME (Figure 2). Especially the immune compartment that has been increasingly recognized for its role in tumour progression and is thereby an important focus of ongoing therapy development. Indeed, multiple research groups have applied 3D imaging to spatially map diverse immune cell subsets (Cuccarese et al, 2017; Kim et al, 2018; Lee et al, 2019b), as well as the expression of immune regulatory molecules (Lee et al, 2019a). This showcases the clinically relevant resolving capacity of 3D microscopy that can be further expanded to capture the entire cellular heterogeneity of cancer by overcoming the traditional limit of 4‐5 markers acquired at a time. Therefore, recent technology advance has focused on increasing the number of markers that can be visualized in a single specimen through multiplexing and multispectral imaging approaches discussed in this section.
Multiplexed 3D imaging to elucidate tumour microenvironment relationships
Multiplexing represents a relatively straightforward method for achieving multi‐marker imaging, by implementing sequential rounds of protein or RNA labelling and acquisition. For RNA detection, DVEX (Karaiskos et al, 2017), Tomo‐seq (Junker et al, 2014) and Geo‐seq (Chen et al, 2017) (Table 2) pioneered so‐called spatial transcriptomics with some degree of 3D location retention, but the field has truly advanced into 3D by STARmap (Wang et al, 2018) (Table 2), enabling in situ sequencing of hydrogel transformed tissues at millimetre scale. ExSeq (Alon et al, 2021) (Table 2) improves this approach by enlarging the tissue sample, resulting in better spatial precision and improving RNA accessibility. Applied to a core biopsy from breast cancer metastasis, it elucidated the microenvironmental relationship between cells in hypoxic environments, showing that tumour cells overexpress HIF1A when in close proximity to HSPG2 positive fibroblasts (Table 1). However, for large specimens, the multiplexing experimental procedure can become incredibly time consuming and complex, as sample co‐registration is required to integrate data from each sequential imaging round in 3D. STARmap, for example, can visualize 1,020 genes in 5 days on thin sections, but this number was drastically reduced to 28 in thick tissue, while almost doubling experimental duration (Wang et al, 2018). Compared to RNA visualization, multiplexed protein detection has proven even more difficult and has mostly been performed on thin sections. The SWITCH protocol (Table 2) achieved 3D multiplex immunolabelling of more than 10 proteins on human brain slices, demonstrating its potential to resolve multiple cell subpopulations, as well as their spatial orientation in relation to cortical layers and blood vessels (Murray et al, 2015). However, due to the limited spectrum of species‐specific secondary antibodies that can be used for detection, harsh sample preparations, including multiple staining, stripping and washing rounds, are required to remove primary and secondary antibodies at every cycle, which can lead to sample degradation. In recent years, novel multiplexing technologies based on DNA‐barcoding antibodies, including CODEX (Goltsev et al, 2018) (Table 2), implement a single step primary antibody staining procedure, followed by sequential imaging cycles for deep‐phenotyping, comparable to flow or mass cytometry. For now only used on 2D sections, this could offer an exciting future avenue for multiplexed 3D imaging.
Multispectral 3D imaging to decode tumour heterogeneity and map the TME
Alternate approaches to increase the number of markers visualized rely on multispectral imaging of spectrally resolvable fluorophores (Valm et al, 2017; Coutu et al, 2018; Li et al, 2019). CytoMAP, implementing histo‐cytometry‐based 8‐10 colour imaging (Gerner et al, 2012) (Table 2), was used in 2D to characterize the immune TME compartment of murine colorectal tumours, while its 3D potential was shown on 500 μm thick lymph node slices when combined with Ce3D clearing (Table 2) (Li et al, 2019; Stoltzfus et al, 2020). CytoMAP analysis of colorectal tumours elucidated the spatial relationships between immune cell subsets in the TME (Box 2), for instance, revealing mutual exclusion of tumour‐associated macrophages (TAMs) and T cells, in line with an inhibitory role of TAMs on T cell tumour infiltration (Petty et al, 2019; Fang et al, 2021). These findings underscore the impact of understanding the spatial organization of cells within the tumour architecture for inferring their functional relationships. However, the multi‐marker imaging strategy of CytoMAP depends on post‐acquisition compensation of spectral overlap, requiring single‐stained control samples and increasing acquisition time. This not only complicates and lengthens the imaging process, but also leads to reduced efficiency of light collection and sometimes photobleaching, due to repetitive illumination. To overcome these limitations, single‐acquisition can be performed using a multispectral detector/camera (Box 1). The Opal method (Table 2) covalently deposits spectrally distinct Opal fluorophores onto antigens of interest and thereby allows recording of 8 markers in a single acquisition. Although it has paved the way for applying multispectral imaging to decode the TME immune composition (Nolan et al, 2017; Viratham Pulsawatdi et al, 2020), it has so far only been performed in 2D. To enable multispectral 3D imaging with a high number of markers, we recently developed mLSR‐3D (Table 2), which implements “on the fly” linear unmixing to remove the need for post‐acquisition processing. As such, 8 spectrally‐resolved, yet highly overlapping, fluorophores can be separated during single‐scan acquisition, enabling fast and light‐efficient high‐dimensional imaging. Combined with a deep learning‐based segmentation pipeline, STAPL‐3D (Table 2), hundreds of molecular and spatial features could be extracted from millions of cells in 3D imaging datasets, for the first time, allowing omics‐like analysis of imaging data (Box 2). The power of this 3D imaging and computational advance was demonstrated by the identification of novel and rare tumour cell subsets in paediatric kidney Wilms Tumour (van Ineveld et al, 2021). This showcases the resolving capacity of multispectral 3D imaging with inclusion of 8 molecular markers, a number that is expected to continue to increase as technology advances. A recent preprint, for instance, describes PICASSO (preprint: Seo et al, 2021) (Table 2), a technique capable of unmixing a number of fluorophores equal to the number of input images and thereby already vastly exceeding the current best practice of 8‐colour linear unmixing. Fifteen‐colour 3D compatible imaging was demonstrated, and in theory over 27 fluorophores could be imaged and unmixed with the right technical setup and materials, indicating the order of magnitude in terms of fluorescent labels that large‐scale imaging is heading towards.
With both multiplexed and multispectral imaging still rapidly evolving, we see a clear application for high‐dimensional 3D imaging in probing the heterogeneity of both the tumour itself, as well as its cellular environment (Figure 2). In addition, as discussed above, multi‐marker imaging has the important potential of adding molecular classification to histological scoring of FFPE samples, thereby identifying novel biomarkers to refine diagnosis or guide treatment decisions.
Dynamic facets of cancer biology revealed through lineage tracing and intravital imaging
The above‐described 3D imaging approaches have generated considerable advances in our understanding of cancer biology, by resolving large‐scale tissue structures and whole‐body metastatic patterns, as well as by profiling in great detail the cellular heterogeneity of tumours and their microenvironment. However, they do not take into account the critical dimension of time, thus providing only a snapshot of these processes. Lineage tracing tools can serve as a cellular clock recording the developmental history of tumour cells. Moreover, intravital microscopy (IVM) allows to visualize cancer biology in a living animal over time and can thereby generate important insight into evolving heterogeneity and the dynamic behaviour of tumour cells, especially in relation to their TME, which is also subject to continuous change. In this section, we discuss these advances in understanding spatio‐dynamic aspects of cancer biology brought by multi‐coloured lineage tracing and IVM (Figure 2).
Tumour intraclonal plasticity revealed by 3D multi‐coloured lineage tracing
Tumours are inherently heterogeneous and constantly evolving to not only expand, but also adapt to selective pressure inflicted by their TME, as well as treatments. Additional levels of complexity, added by, for instance, genetic background and cell‐of‐origin, make tumour evolution towards later disease stages and treatment resistance arduous to predict. Several multi‐coloured lineage tracing approaches (Livet et al, 2007; Snippert et al, 2010; Kretzschmar & Watt, 2012; Yum et al, 2021) have paved the way for better understanding these complex processes, by addressing the clonal evolution of tumours in vivo (Figure 2). Fluorescently‐engineered animal or xenograft models, carrying inducible reporters for temporal and/or spatial restricted expression of fluorescent tags, such as confetti, allow tracking of either cell populations or individual cell fates throughout the progression of cancer (Lamprecht et al, 2017; Yanai et al, 2017; Rios et al, 2019; Tang et al, 2019; Tiede et al, 2021; Yum et al, 2021). Combined with an optical clearing agent that preserves reporter expressed endogenous fluorescence (Box 1), it allowed for studying clonal diversity within intact tumours. For instance, LSR‐3D imaging (Table 2) of confetti lineage tracing breast cancer models revealed profound clonal restriction during tumour progression, mechanisms also observed in other cancers (Vermeulen et al, 2013; Snippert et al, 2014; Brown et al, 2017; Ying et al, 2018; Bruens et al, 2020) and potentially resulting from active removal of aberrant cells to protect healthy tissue (Ohoka et al, 2015; Kon et al, 2017). Integration with clonal RNA sequencing and immunolabelling revealed the molecular heterogeneity of these predominant stem‐ or progenitor cell‐derived clones, with cellular phenotypes varying along a spectrum of reversible states in between epithelial and mesenchymal fate. Altogether, this indicates inherent tumour plasticity and illustrates that many clonal evolution routes can lead to effective cancer progression (Rios et al, 2019) (Table 1).
Intravital imaging combined with lineage tracing uncovered the dynamic role of cancer stem cells (CSCs) in cancer progression (Huels et al, 2018; Tang et al, 2019; Fumagalli et al, 2020). CSCs can self‐renew to sustain tumour growth (Batlle & Clevers, 2017; Lenos et al, 2018), and longitudinal IVM combined with confetti lineage tracing revealed that only a minor population of CSCs is able to generate long‐lived clones (Zomer et al, 2013), in line with LSR‐3D results discussed above. In addition, dynamic analysis of clonal evolution uncovered CSCs that displayed a delayed onset of growth, or sudden regression (Zomer et al, 2013), indicating that stemness represents a reversible cellular state that can be gained or lost by cancer cells over time (Table 1). IVM has also showed that plasticity of CSCs plays a role in metastasis (Fumagalli et al, 2020; van Rheenen & Scheele, 2021). In addition, live recording of epithelial‐to‐mesenchymal transition (EMT) and mesenchymal‐to‐epithelial transition (MET) in mammary tumours showed that a small population of mesenchymal cells is able to disseminate to distant sites and reacquire an epithelial state to colonize secondary organs (Beerling et al, 2016; Harper et al, 2016). Such state changes have also been shown for tumour cells escaping drug‐induced cell death (Biehs et al, 2018), suggesting that epithelial/mesenchymal cell state plasticity might be a common way for cancer cells to progress into late stages of the disease, or to resist treatment. IVM has also allowed to track human CSC dynamics, by xeno‐grafting human cancer organoids engineered with a confetti‐construct (Fumagalli et al, 2017). This showed that metastatic capability is directly linked to specific mutations that allow growth independence of certain niche signals, thereby identifying key driver mechanisms of colorectal cancer progression (Fumagalli et al, 2017). Thus, 3D (IVM) imaging combined with lineage tracing provides a powerful tool to study the clonal dynamics of CSCs and has elucidated their (plastic) cellular states contributing to cancer initiation, progression and treatment resistance.
Intravital microscopy offers a unique view into the ever‐evolving behaviour of tumour cells
IVM not only generated insight into CSC biology and tumour cell plasticity described above, but also enabled studying tumour cell behaviour (Alieva et al, 2019; Ilina et al, 2020), including response to treatment and inter‐cellular communication strategies (Osswald et al, 2015; Zomer et al, 2015; Lagerweij et al, 2017; Venkataramani et al, 2019; Xie et al, 2021), as well as their dynamic metabolic (Kondo et al, 2021) and pathway activation states (Hirata et al, 2015), through implementation of fluorescent biosensors. Importantly, it allows to analyse these critical cellular features within the tumour’s natural environment (Alieva et al, 2017; Conway et al, 2018), thereby taking into account critical interplay with TME immune‐ (Marangoni et al, 2013; Harney et al, 2015; Arlauckas et al, 2017; Park et al, 2019) and tissue cell types (Venkataramani et al, 2019), as well as extracellular matrix components (Hirata et al, 2015; Ilina et al, 2020). IVM, for instance, has shown that glioma tumour cells are integrated into a functional communication network via direct gap junction‐mediated contact, which endows these cells with stemness properties (Xie et al, 2021) and resistance to radiotherapy (Osswald et al, 2015). In addition, glioma cells have been shown to connect to presynaptic neurons in their environment, which enables them to “feed” on neuronal activity, potentiating their invasive and growth properties (Venkataramani et al, 2019) (Table 1). In this study, IVM was combined with optogenetics to show that in vivo stimulation of neurons induced synchronized calcium activity patterns in glioma cells that correlated with tumour cell migration episodes. Due to its unique dynamic readout, providing a longitudinal outlook is an important leverage of IVM that can be used to study tumour evolution over long periods of time, or response to treatment in a living animal. This also allows to capture TME components that are not necessarily present at a particular timepoint, but instead arrive from distant sites (i.e., circulating tumour or immune cells) (Osswald et al, 2015; Zomer et al, 2015; Alieva et al, 2017; Fumagalli et al, 2020). As such, IVM has brought a deep insight into tumour cell dissemination and metastasis—some of the biggest challenges in the current treatment of solid tumours—and how these processes relate to the TME. Plasticity of cadherin expression by tumour cells in interplay with extracellular matrix confinement, for instance, was shown to regulate collective and single tumour cell dissemination (Ilina et al, 2020). Moreover, cooperation with environmental cells has been shown to facilitate local vascular permeability and tumour intravasation (Harney et al, 2015) (Table 1). Real‐time IVM of intravenously injected high–molecular‐weight compounds was performed to mark sites of vascular permeability, which showed that breast cancer carcinoma cells migrate together with Tie2hi macrophages towards blood vessels, where intravasation co‐occurs with transient vascular permeability (Harney et al, 2015).
Exploiting IVM for understanding and improving immune‐mediated cancer control
IVM can also be highly instrumental for increasing our understanding of immune‐mediated cancer control, largely exploited for therapy development (Boulch et al, 2019), for instance, by uncovering fundamental principles of immune activation. A cornerstone example of the power of IVM in this context, is the discovery of a three‐way communication scenario that lies at the base of CD4+ and CD8+ T cell activation by dendritic cells (Eickhoff et al, 2015; Hor et al, 2015). It was shown that both T cell subsets were first independently activated by different dendritic cells at separate anatomical locations, followed by a second activation step in which both T cells interact with the same dendritic cell, essential for CD4 help delivery and full activation of the CD8 T cell. Without the unique readout in both space and time offered by IVM, this sophisticated, sequential and location‐dependent immune activation mechanism could not have been uncovered. Most recently, IVM has demonstrated its potential for studying the dynamic targeting mechanisms of (engineered) T cell immunotherapy (Marangoni et al, 2013; Arlauckas et al, 2017; Cazaux et al, 2019; Murty et al, 2020) (Table 1). Clear successes for haematological malignancies have sparked efforts to translate these approaches to solid tumours, but efficacy so far has been limited (Chen & Mellman, 2017). IVM can shed important light on some of the key capacities therapeutic cells need to have in order to effectively treat solid tumours, including proper homing to and infiltration of the tumour and potent (serial) killing behaviour (Weigelin et al, 2021). In addition, it has allowed to trace the activity of checkpoint inhibitors in vivo (Arlauckas et al, 2017), thereby revealing that binding dynamics could be prolonged through blockade of Fcγ receptors before checkpoint inhibitor administration (Table 1).
Recent and expected IVM technology advance
To overcome some of the challenges associated with IVM, such as tissue access and tagging of multiple cell types, various contemporary technological advances have been directed that can now be applied towards a better understanding of cancer. In vivo imaging relies on the visual access to tissues of interest via skin flaps or imaging windows, which limits the organs or locations that can be studied with this approach. Next to, amongst others, cranial, dorsal skin fold, mammary and abdominal imaging windows (Huang et al, 2021), the development of a permanent lung window (Entenberg et al, 2018) has allowed researchers to investigate tumour cell arrival and fate in this organ. This presents a huge asset for cancer research, since the lung represents a common metastatic niche for various types of cancer. In addition, developments in intravital endoscopy have granted visual access to deep brain regions, thereby allowing investigation of tumour and vasculature progression of previously inaccessible deep lying brain tumours (Barretto et al, 2011).
Next to these advances in tissue accessibility, increasing the number of cell types that can be distinguished is a major trend in IVM in order to get a better picture of the complex and dynamic TME and its role in tumour progression (Ricard & Debarbieux, 2014; Dawson et al, 2020, 2021). Application of label‐free imaging approaches in IVM (You et al, 2018; preprint: Bakker et al, 2020), such as SHG and THG (Table 2) discussed above, holds promise for less perturbative imaging. This will enable the simultaneous acquisition of spatiotemporal dynamics and morphological and molecular data from distinct cell types, as well as their subcellular features and environmental components. Along these efforts, a recent approach has demonstrated the synchronized detection of autofluorescence and harmonic generation signals from tumour cells, to identify tumour cells, tumour‐associated vesicles, macrophages, endothelial cells, red blood cells, leukocytes and the redox states of these different cell types (You et al, 2018). This revealed an increased redox ratio in migrating leukocytes, compared to leukocytes clustering in the tissue, indicative of heightened metabolic activity.
In conclusion, dynamic 3D imaging offers an unprecedented view of the evolving processes underlying tumour progression and therapy mechanisms of action, including tumour cell behaviour and TME interplay revealed by IVM, as well as clonal restriction assessed through fluorescent lineage tracing. Most recent advances in IVM have enabled imaging access to key organs of interest, as well as increased the number of markers that can be visualized for complex tumour and tissue characterization. Despite these advances, the major drawback of IVM remains its throughput capacity. Therefore, to study and develop personalized treatments for cancer, other imaging technologies and accompanying imaging‐accessible models are required.
Visualize the organoid revolution for personalized investigation of human cancer
3D in vitro models span the gap between 2D cell cultures and whole‐animal systems, while offering throughput potential. Tumour organoids in particular have revolutionized cancer research, by providing an easily accessible and versatile model that faithfully captures characteristics and patient treatment response of tumour tissue (Tuveson & Clevers, 2019). With cancer model systems moving into 3D, volumetric imaging technologies present an exciting method to characterize these models and fully exploit their biological potential (Figure 2). In this section, we discuss recent developments in 3D imaging technologies specifically dedicated to organoids and how they have advanced cancer research.
Fixed 3D imaging of organoids to characterize modelled tumour organization
More complex organization and faithful modelling of tissue architecture, as exemplified by the first stem cell‐derived murine small intestinal organoids (Sato et al, 2009), is regarded a key strength of organoids compared to 2D culture systems. To capture this fine‐grained cellular organization, dedicated sample preparation methods have been developed that allow preservation and multi‐coloured visualization of intact organoids at high resolution (Dekkers et al, 2019; van Ineveld et al, 2020) (Figure 2). Such fixed organoid 3D imaging has been widely applied to organoids of various species and tissue origin (Hu et al, 2018; Schutgens et al, 2019; Dekkers et al, 2020; Post et al, 2020), as well as disease states. This has demonstrated their suitability to capture morphological changes upon pathology, such as the formation of syncytia in human airway organoids infected with respiratory syncytial virus (RSV) (Sachs et al, 2019). In addition, 3D imaging can be used to assess the structural resemblance of organoids to their original tissue, for instance the comparable spatial organization of luminal and basal cells in murine (Jamieson et al, 2017) and human breast organoids (Dekkers et al, 2020) as seen in vivo (Rios et al, 2014, 2019), a feature that cannot be fully appreciated in 2D. A demonstration of the power of 3D organoid imaging for cancer modelling was provided by Calandrini and colleagues, who demonstrated that human Wilms tumour‐derived organoid cultures were highly heterogeneous and multi‐phasic, containing organoids of both epithelial and blastemal‐like origin, similar to Wilms tumour tissue (Calandrini et al, 2020) (Table 1). Furthermore, these organoids structurally form a complex network that also contains stromal cells. This showcases the value of fixed 3D imaging to characterize the complex morphologies of organoid cancer models. Moreover, we expect 3D imaging to be highly useful in ongoing efforts to increase the complexity of tumour biology modelled by organoids, through addition of critical TME components, such as immune cells (Rios & Clevers, 2018; Yuki et al, 2020). Here, the multi‐marker potential of fixed 3D imaging described above will be highly suited to map the different cell types present. In addition, live 3D imaging can be used to study human cancer processes in these models, as further discussed in the next session.
Live 3D imaging of organoids for understanding tumour progression and treatment response
Unlike 2D cultures, the complex organoid architecture, when combined with live 3D imaging, provides an excellent platform to study often subtle changes in morphometric interactions and signalling dynamics in response to carcinogenesis and drug treatment. Live 3D imaging has been applied in patient‐derived organoids to show widespread chromosomal instability in human colorectal cancer, yet the outcome of mitotic errors varied between organoids and was not consistently followed by cell death (Bolhaqueiro et al, 2019) (Table 1; Figure 2). This showcases the value of live 3D organoid imaging to reveal factors that shape human tumour evolution and heterogeneity. Recently, Krotenberg Garcia et al (2021) applied live‐cell imaging combined with fixed 3D imaging to study competition between murine intestinal wild‐type and tumour cells within the same organoid and showed that cancerous organoid cells actively eliminated wild‐type cells through JNK‐dependent mechanisms boosting their own growth (Krotenberg Garcia et al, 2021) (Table 1). Thus, organoid live‐cell imaging has the potential to study cell competition and holds promise for studying tumour clonal competition, discussed above, in an in vitro human setting. Considering that organoids can be propagated in culture long‐term, while maintaining features of their original tissue (Drost & Clevers, 2018; Bleijs et al, 2019), they also offer an important opportunity to study drug responses in a patient‐specific manner. In this regard, live 3D imaging can be applied to study responsiveness to molecular targeted therapy, exemplified by a recent study analysing the response of colorectal cancer organoids to tankyrase inhibitors (Badder et al, 2020). In addition, Ponsioen et al (2021) developed a fluorescence resonance energy transfer (FRET) sensor to assess drug responses in colorectal cancer organoids in real‐time, thereby providing a mechanistic explanation for the efficacy of combination therapies with EGFR inhibitors observed in patients (Ponsioen et al, 2021).
Next to molecular and conventional therapies, we envision great potential for live 3D imaging in organoid co‐cultures used for cellular immunotherapy development and screening (Cattaneo et al, 2020). Not only to monitor treatment response over time, as previously done to evaluate CAR T cell targeting efficacy against human colon cancer organoids (Schnalzger et al, 2019), but also with the important potential to visualize and dynamically analyse the therapeutic cells alongside tumour cells. This creates a critical opportunity to define immune cell functional properties based on their single‐cell dynamics captured into a behavioural landscape (Crainiciuc et al, 2022). In this light, we recently developed BEHAV3D (Table 2), a large content dynamic 3D imaging‐transcriptomic platform for the in‐depth characterization of organoid‐immunotherapy co‐cultures (preprint: Dekkers et al, 2021). When applied to various solid tumour organoid biobanks and T cell therapy products, both single and population organoid death dynamics could be extracted, as well as the behavioural and transcriptomic profile of individual therapeutic T cells. This strikingly revealed the molecular signature of a sub‐population of serial killer T cells, a desired asset for solid tumour targeting and furthermore hinted at combinatory drug strategies to improve targeting (Table 1). Together, this illustrates how live‐cell 3D imaging can be used as a tool to study in depth the effects of cancer therapies in human tumour organoid models and guide the optimization of (immune)therapy products.
Advances in high‐throughput organoid imaging offer opportunities for personalized medicine
A key strength of 3D organoid imaging for personalized treatment approaches lies in its high‐throughput potential. Advanced imaging and analysis techniques allow for the screening of hundreds of thousands of organoids on a subcellular, single‐cell and single organoid levels to assess phenotypic identity and therapy response. An elegant demonstration of the power of large‐scale organoid imaging was provided by Lukonin et al (2020), who imaged and analysed over 400K healthy intestinal organoids exposed to a library of compounds with known targets (Lukonin et al, 2020). This led to the identification of novel regulators of cell‐fate decisions that could be exploited to improve intestinal regeneration. Similarly, very recently a novel high‐content screening platform has been described that allows non‐invasive live 3D imaging of various organoid cultures using light sheet microscopy (preprint: Beghin et al, 2021). Such advances in high‐throughput (live) organoid imaging hold great promise for personalized medicine applications by not only assessing organoid viability, but also morphology and cellular composition, which can offer additional mechanistic insights beyond what standard viability assays can provide.
Altogether, applications of both fixed and live 3D organoid imaging can be envisioned across all stages of cancer research, from basic characterization of organoid models and carcinogenesis processes to translational applications, such as drug responses in patient‐derived organoids. This might lead to drug repurposing, as well as the discovery of new agents and combination strategies that effectively tackle tumour heterogeneity adequately modelled by patient‐derived organoids (Sasaki & Clevers, 2018).
Conclusion and future outlook
The bright future of 3D imaging for cancer research
Due to advances in sample preparation protocols, microscope technology and computational approaches (Box 1 and 2), we can now achieve incredible cellular detail in large tissue volumes, demonstrating an invaluable asset of 3D imaging for cancer research (Table 1). Various 3D imaging approaches have now been applied across a wide range of cancer subtypes (Table 3). This has already generated critical insight into key aspects of cancer biology, such as tumour cell clonal evolution and metastatic behaviour in concert with the TME, as well as biophysical tissue constraints related to tumour progression (Figure 1). While many of these 3D imaging techniques have been primarily designed for fresh tissue, application on preserved clinical specimens is increasing, thereby providing access to the most abundant source of patient material stored in biobanks around the world, together with relevant clinical data (Baker, 2012; Kokkat et al, 2013). With accompanying advances in artificial intelligence (Box 2) and data handling, this offers a unique opportunity for 3D imaging to correlate tumoural heterogeneity in 3D space with clinical outcome, such as patient survival and treatment response. Because of their high‐throughput nature and strong resemblance to in vivo tumour tissue, human organoids offer powerful experimental models to functionally test the identified correlations in a patient‐specific manner. Together, this might in the near future transform 3D imaging from a mostly lab applied methodology to a potential diagnostic tool guiding patient stratification and treatment decisions. Application of 3D imaging in in vivo preclinical models, furthermore, has the potential to find cues for predicting cancer aggressiveness, as well as expected treatment response, for instance through whole‐body mapping of metastases and overlaying it with therapy distribution. The latter can be an important and urgently needed step towards preventing overtreatment of cancer, by identifying metastasis sites excluded from certain therapy distribution and thereby unlikely to benefit from such treatments.
Table 3.
Overview of applied 3D imaging methods per cancer subtype.
| Cancer | Experimental model | Imaging method | Study |
|---|---|---|---|
| Bladder | Patient material, FFPE or fresh | 3D‐reconstructed 2D tissue sections | Jansen et al (2019) |
| Light sheet | Tanaka et al (2017); Tanaka et al (2018); Tanaka et al (2020) | ||
| Brain | In vivo models | Confocal | Lagerweij et al (2017) |
| Intravital | Barretto et al (2011); Ricard and Debarbieux (2014); Osswald et al (2015); Lagerweij et al (2017); Alieva et al (2017); Alieva et al (2019); Venkataramani et al (2019); Murty et al (2020); Xie et al (2021) | ||
| Breast | Patient material, FFPE or fresh | Confocal | Chen et al (2019) |
| Light sheet | Tanaka et al (2020) | ||
| Super‐resolution | Creech et al (2017) | ||
| 3D‐reconstructed 2D tissue sections | Booth et al (2015) | ||
| In vivo models | Intravital | Zomer et al (2013); Harney et al (2015); Zomer et al (2015); Beerling et al (2016); Harper et al (2016); Entenberg et al (2018); You et al (2018); Ilina et al (2020); Kondo et al (2021) | |
| Light sheet | Dobosz et al (2014) | ||
| Confocal | Tiede et al (2021) | ||
| Spectral confocal | Rios et al (2019) | ||
| Organoids | Light sheet | Tanaka et al (2020) | |
| Live 3D imaging | preprint: Dekkers et al (2021) | ||
| Spectral confocal | Dekkers et al (2020) | ||
| Colon / intestine | Patient material, FFPE or fresh | Light sheet | Tanaka et al (2017); Tanaka et al (2020) |
| Confocal | Liu et al (2013) | ||
| 3D‐reconstructed 2D tissue sections | Korehisa et al (2018) | ||
| Organoids | Light sheet | Tanaka et al (2020) | |
| Live 3D imaging | Bolhaqueiro et al (2019); Schnalzger et al (2019); Badder et al (2020); Ponsioen et al (2021) | ||
| Fixed and live 3D imaging | Krotenberg Garcia et al (2021) | ||
| In vivo models | Intravital | Marangoni et al (2013); Arlauckas et al (2017); Fumagalli et al (2017); Fumagalli et al (2020) | |
| Head and neck | Patient material, FFPE or fresh | Light sheet | Tanaka et al (2017); Si et al (2019) |
| Organoids | Live 3D imaging | preprint: Dekkers et al (2021) | |
| Kidney | Patient material, FFPE or fresh | Light sheet | Tanaka et al (2020) |
| Multispectral confocal | van Ineveld et al (2021) | ||
| Organoids | Spectral confocal | Schutgens et al (2019); Calandrini et al (2020) | |
| Liver | Patient material, FFPE or fresh | Light sheet, confocal | Yoshizawa et al (2020) |
| Lung | Patient material, FFPE or fresh | Light sheet | Tanaka et al (2017); Tanaka et al (2020) |
| 3D‐reconstructed 2D tissue sections | Yagi et al (2020) | ||
| In vivo models | Light sheet | Viallard et al (2020) | |
| Lymphoma | Patient material, FFPE or fresh | Confocal | Nojima et al (2017) |
| In vivo models | Intravital | Cazaux et al (2019) | |
| Ovarian | Patient material, FFPE or fresh | Light sheet | Tanaka et al (2017); Tanaka et al (2020); Kastelein et al (2020) |
| Pancreatic | Patient material, FFPE or fresh | Light sheet | Tanaka et al (2017); Noe et al (2018) |
| Confocal | Lin et al (2016) | ||
| In vivo models | Confocal | Messal et al (2021) | |
| Confocal | Lin et al (2016); Shen et al (2019) | ||
| Intravital | Conway et al (2018) | ||
| Prostate | Patient material, FFPE or fresh | 3D‐reconstructed 2D tissue sections | Tolkach et al (2018) |
| Confocal | Van Royen et al (2016); Verhoef et al (2019) | ||
| Skin | Patient material, FFPE or fresh | Light sheet | Tanaka et al (2020) |
| In vivo models | Confocal | Fiore et al (2020) | |
| Intravital | Zomer et al (2015); Hirata et al (2015); Park et al (2019) | ||
| Soft tissue sarcoma | In vivo models | Intravital | Tang et al (2019) |
| Thyroid | Patient material, FFPE or fresh | Light sheet | Tanaka et al (2020) |
Increasing the dimensionality of 3D datasets
Imaging technologies are constantly under development to meet the diverse needs of researchers and clinicians, and efforts have been especially put towards increasing the markers and processes that can be visualized in 3D specimens. Genetically encoded reporters for live 3D imaging have been used to extend the application of live imaging to visualization of key processes in cancer biology (Hirata et al, 2015; Kondo et al, 2021; Ponsioen et al, 2021). Development of novel reporters (Mahlandt et al, 2021), multiplexing approaches that combine several reporters (Regot et al, 2014; Chavez‐Abiega et al, 2022) and improved genome editing technologies (Bollen et al, 2022) can be expected to further expand fluorescent reporter applications and increase the richness of information that can be extracted from live 3D imaging data.
Current high‐dimensional profiling already allows for omics‐like analysis of 3D imaging datasets, and the power of this advancement was demonstrated by the identification of unique tumour cell subsets based on combined molecular marker expression and spatial data (van Ineveld et al, 2021) (Table 1), clearly positioning 3D imaging as the next step forward in single‐cell technology development. Through advances in label‐free imaging, artificial intelligence, multiplexing and linear unmixing (Box 1), the number of labels per single sample is expected to continue to rise steeply in the upcoming years. Therefore, we can imagine the field of microscopy to catch‐up with single‐cell sequencing, by drastically increasing its multi‐dimensional capability. Compared to still mainstream single‐cell RNA sequencing approaches, this will offer the critical advantage of also providing spatial context and taking into account cell morphologies. In addition, it will prevent cell loss associated with tissue dissociation that can be especially detrimental for the detection of rare cell subsets, or when disproportionally affecting certain cell populations (Denisenko et al, 2020).
Next to increasing the number of markers imaged, efforts are put towards bioinformatic solutions for integrating multi‐omics (imaging, RNAseq, DNAseq, ATACseq, etc.) datasets, even further enhancing the dimensionality of information retrieved from a single biological specimen (Rodriques et al, 2019; Cang & Nie, 2020; Liu et al, 2020; Mantri et al, 2021; Payne et al, 2021; Zanfardino et al, 2021). These future directions will greatly aid in understanding the complex cellular heterogeneity of tumours and their microenvironment, as well as the heterogenous composition and mode of action of cellular cancer therapies. The latter application exemplified by the integration of dynamic 3D imaging with single‐cell transcriptomics to unveil the underlying molecular mechanisms of solid tumour targeting by cellular immunotherapy (preprint: Dekkers et al, 2021). In addition, with progress in genetic lineage tracing tools (Baron & van Oudenaarden, 2019), we can expect that in the near future a combination of 3D imaging and transcriptomics will further expand the work performed on fluorescent lineage tracing of tumour cells. This could generate a complete spatial and molecular picture of cancer clonal evolution with important implications for understanding and tackling late disease progression and treatment resistance. Technology advance might also include crossovers between different imaging modalities. An example of cancer biology that can be uncovered by such an integrative approach results from combining 3D confocal fluorescence imaging and atomic force microscopy (Staunton et al, 2016). By quantifying the force response of metastatic breast adenocarcinoma cells invading collagen hydrogels, it was found that invading cells stiffen through actomyosin contractility, which might be a key and potentially druggable process in the metastasizing potential of cancer cells. This thereby demonstrates that key insights of potential therapeutic importance can also be uncovered by integration of multiple imaging strategies.
A sharp increment in the dimensionality of data generated from large, intact samples comes at the expense of increasingly large and complex data files. Therefore, parallel efforts should be put towards solutions for data storage and processing (Horl et al, 2019), as well as deep learning‐ (LeCun et al, 2015; Litjens et al, 2017; Belthangady & Royer, 2019; Falk et al, 2019; Moen et al, 2019; Isensee et al, 2021) or machine learning (Berg et al, 2019)‐enabled automated analysis pipelines (Box 2). In addition, it should be safeguarded that the obtained 3D imaging technology advance will be available to the scientific community at large and adheres to FAIR data principles (Wilkinson et al, 2016), which will require the publication of detailed protocols, solutions and guidelines for data sharing, ensuring reproducibility (Miura & Norrelykke, 2021), as well as adequate training to operate the most advanced microscope systems. This will require the joint effort of the imaging community as a whole, together with input and collective technology development from cross‐over disciplines, such as single‐cell transcriptomics. Sharing of 3D imaging data to a similar extend as currently achieved for sequencing data will be important. The BioImage Archive, a central archival system at EMBL‐EBI (Hartley et al, 2022), now provides infrastructure for archiving and accessing existing imaging data. Although already a highly commendable resource, supporting patient‐identifiable data in the future could further advance its application in cancer research.
Thus, by further increasing the phenotyping power of imaging through enhancing its multi‐dimensional capability and implementing correlative multi‐omics strategies, we envision enhanced tools for the simultaneous recording of molecular, spatial and dynamic cellular features, beyond what current single‐cell technologies can offer. This technology advance can fill a broad research need across scientific disciplines and, importantly, be used to better exploit the spatial, dynamic and morphological features of cancer for drug discovery and development.
Disclosure and competing interests statement
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to thank members of the Rios group for helpful discussions. ACR received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement no 804412). This work was financially supported by the Princess Máxima Center for Pediatric Oncology and a St. Baldrick’s Foundation (SBF) Robert J. Arceci International Innovation award.
The EMBO Journal (2022) 41: e109675.
This article is part of the Cancer Reviews series.
References
- (2018) Tumor cells metastasize from lymph nodes. Cancer Discov 8: OF10 [DOI] [PubMed] [Google Scholar]
- Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, Bloom J, Gurram S, Siddiqui M, Pinsky P et al (2020) MRI‐targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med 382: 917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese A, Swaney JM, Yun DH, Evans NB, Antonucci JM, Velasco S, Sohn CH, Arlotta P, Gehrke L, Chung K (2020) Multiscale 3D phenotyping of human cerebral organoids. Sci Rep 10: 21487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alieva M, Leidgens V, Riemenschneider MJ, Klein CA, Hau P, van Rheenen J (2019) Intravital imaging of glioma border morphology reveals distinctive cellular dynamics and contribution to tumor cell invasion. Sci Rep 9: 2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alieva M, Margarido AS, Wieles T, Abels ER, Colak B, Boquetale C, Jan Noordmans H, Snijders TJ, Broekman ML, van Rheenen J (2017) Preventing inflammation inhibits biopsy‐mediated changes in tumor cell behavior. Sci Rep 7: 7529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almagro J, Messal HA, Zaw Thin M, van Rheenen J, Behrens A (2021) Tissue clearing to examine tumour complexity in three dimensions. Nat Rev Cancer 21: 718–730 [DOI] [PubMed] [Google Scholar]
- Alon S, Goodwin DR, Sinha A, Wassie AT, Chen F, Daugharthy ER, Bando Y, Kajita A, Xue AG, Marrett K et al (2021) Expansion sequencing: Spatially precise in situ transcriptomics in intact biological systems. Science 371: eaax2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen V, Alexander S, Heupel WM, Hirschberg M, Hoffman RM, Friedl P (2009) Infrared multiphoton microscopy: subcellular‐resolved deep tissue imaging. Curr Opin Biotechnol 20: 54–62 [DOI] [PubMed] [Google Scholar]
- Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM et al (2017) In vivo imaging reveals a tumor‐associated macrophage‐mediated resistance pathway in anti‐PD‐1 therapy. Sci Transl Med 9: eaal3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badder LM, Hollins AJ, Herpers B, Yan K, Ewan KB, Thomas M, Shone JR, Badder DA, Naven M, Ashelford KE et al (2020) 3D imaging of colorectal cancer organoids identifies responses to Tankyrase inhibitors. PLoS One 15: e0235319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M (2012) Biorepositories: building better biobanks. Nature 486: 141–146 [DOI] [PubMed] [Google Scholar]
- Bakker GJ, Weischer S, Heidelin J, Andresen V, Beutler M, Friedl P (2020) Intravital deep‐tumor and 4‐photon microscopy. bioRxiv 10.1101/2020.09.29.312827 [PREPRINT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron CS, van Oudenaarden A (2019) Unravelling cellular relationships during development and regeneration using genetic lineage tracing. Nat Rev Mol Cell Biol 20: 753–765 [DOI] [PubMed] [Google Scholar]
- Barretto RP, Ko TH, Jung JC, Wang TJ, Capps G, Waters AC, Ziv Y, Attardo A, Recht L, Schnitzer MJ (2011) Time‐lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy. Nat Med 17: 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates M, Huang B, Dempsey GT, Zhuang X (2007) Multicolor super‐resolution imaging with photo‐switchable fluorescent probes. Science 317: 1749–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Clevers H (2017) Cancer stem cells revisited. Nat Med 23: 1124–1134 [DOI] [PubMed] [Google Scholar]
- Beerling E, Seinstra D, de Wit E, Kester L, van der Velden D, Maynard C, Schäfer R, van Diest P, Voest E, van Oudenaarden A et al (2016) Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis‐enhancing stem cell capacity. Cell Rep 14: 2281–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghin A, Grenci G, Rajendiran H, Delaire T, Raffi SBM, Blanc D, Mets R, Ong HT, Acharya V, Sahini G et al (2021) High content 3D imaging method for quantitative characterization of organoid development and phenotype. bioRxiv 10.1101/2021.03.26.437121 [PREPRINT] [DOI] [Google Scholar]
- Belle M, Godefroy D, Couly G, Malone SA, Collier F, Giacobini P, Chedotal A (2017) Tridimensional visualization and analysis of early human development. Cell 169: 161–173.e12 [DOI] [PubMed] [Google Scholar]
- Belthangady C, Royer LA (2019) Applications, promises, and pitfalls of deep learning for fluorescence image reconstruction. Nat Methods 16: 1215–1225 [DOI] [PubMed] [Google Scholar]
- Berg S, Kutra D, Kroeger T, Straehle CN, Kausler BX, Haubold C, Schiegg M, Ales J, Beier T, Rudy M et al (2019) ilastik: interactive machine learning for (bio)image analysis. Nat Methods 16: 1226–1232 [DOI] [PubMed] [Google Scholar]
- Biehs B, Dijkgraaf GJP, Piskol R, Alicke B, Boumahdi S, Peale F, Gould SE, de Sauvage FJ (2018) A cell identity switch allows residual BCC to survive Hedgehog pathway inhibition. Nature 562: 429–433 [DOI] [PubMed] [Google Scholar]
- Bleijs M, van de Wetering M, Clevers H, Drost J (2019) Xenograft and organoid model systems in cancer research. EMBO J 38: e101654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhaqueiro ACF, Ponsioen B, Bakker B, Klaasen SJ, Kucukkose E, van Jaarsveld RH, Vivié J, Verlaan‐Klink I, Hami N, Spierings DCJ et al (2019) Ongoing chromosomal instability and karyotype evolution in human colorectal cancer organoids. Nat Genet 51: 824–834 [DOI] [PubMed] [Google Scholar]
- Bollen Y, Hageman JH, van Leenen P, Derks LLM, Ponsioen B, Buissant des Amorie JR, Verlaan‐Klink I, van den Bos M, Terstappen LWMM, van Boxtel R et al (2022) Efficient and error‐free fluorescent gene tagging in human organoids without double‐strand DNA cleavage. PLoS Biol 20: e3001527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth ME, Treanor D, Roberts N, Magee DR, Speirs V, Hanby AM (2015) Three‐dimensional reconstruction of ductal carcinoma in situ with virtual slides. Histopathology 66: 966–973 [DOI] [PubMed] [Google Scholar]
- Boulch M, Grandjean CL, Cazaux M, Bousso P (2019) Tumor Immunosurveillance and Immunotherapies: a fresh look from intravital imaging. Trends Immunol 40: 1022–1034 [DOI] [PubMed] [Google Scholar]
- Brabletz S, Schuhwerk H, Brabletz T, Stemmler MP (2021) Dynamic EMT: a multi‐tool for tumor progression. EMBO J 40: e108647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Hunt KK, Shen J, Huo L, Babiera GV, Ross MI, Meric‐Bernstam F, Feig BW, Kuerer HM, Boughey JC et al (2010) Histologic changes associated with false‐negative sentinel lymph nodes after preoperative chemotherapy in patients with confirmed lymph node‐positive breast cancer before treatment. Cancer 116: 2878–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Assen FP, Leithner A, Abe J, Schachner H, Asfour G, Bago‐Horvath Z, Stein JV, Uhrin P, Sixt M et al (2018) Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 359: 1408–1411 [DOI] [PubMed] [Google Scholar]
- Brown S, Pineda CM, Xin T, Boucher J, Suozzi KC, Park S, Matte‐Martone C, Gonzalez DG, Rytlewski J, Beronja S et al (2017) Correction of aberrant growth preserves tissue homeostasis. Nature 548: 334–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruens L, Ellenbroek SIJ, Suijkerbuijk SJE, Azkanaz M, Hale AJ, Toonen P, Flanagan DJ, Sansom OJ, Snippert HJ, van Rheenen J (2020) Calorie restriction increases the number of competing stem cells and decreases mutation retention in the intestine. Cell Rep 32: 107937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Förstera B, Zhao S, Bhatia HS, Parra‐Damas A, Mrowka L, Theodorou D et al (2019) Panoptic imaging of transparent mice reveals whole‐body neuronal projections and skull‐meninges connections. Nat Neurosci 22: 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandrini C, Schutgens F, Oka R, Margaritis T, Candelli T, Mathijsen L, Ammerlaan C, van Ineveld RL, Derakhshan S, de Haan S et al (2020) An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat Commun 11: 1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang Z, Nie Q (2020) Inferring spatial and signaling relationships between cells from single cell transcriptomic data. Nat Commun 11: 2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, DeKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL et al (2017) Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol 197: S200–S207 [DOI] [PubMed] [Google Scholar]
- Cattaneo CM, Dijkstra KK, Fanchi LF, Kelderman S, Kaing S, van Rooij N, van den Brink S, Schumacher TN, Voest EE (2020) Tumor organoid‐T‐cell coculture systems. Nat Protoc 15: 15–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazaux M, Grandjean CL, Lemaitre F, Garcia Z, Beck RJ, Milo I, Postat J, Beltman JB, Cheadle EJ, Bousso P (2019) Single‐cell imaging of CAR T cell activity in vivo reveals extensive functional and anatomical heterogeneity. J Exp Med 216: 1038–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez‐Abiega S, Gronloh MLB, Gadella TWJ, Bruggeman FJ, Goedhart J (2022) Single cell imaging of ERK and Akt activation dynamics and heterogeneity induced by G protein‐coupled receptors. J Cell Sci 135: jcs259685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer‐immune set point. Nature 541: 321–330 [DOI] [PubMed] [Google Scholar]
- Chen J, Suo S, Tam PP, Han JJ, Peng G, Jing N (2017) Spatial transcriptomic analysis of cryosectioned tissue samples with Geo‐seq. Nat Protoc 12: 566–580 [DOI] [PubMed] [Google Scholar]
- Chen Y, Shen Q, White SL, Gokmen‐Polar Y, Badve S, Goodman LJ (2019) Three‐dimensional imaging and quantitative analysis in CLARITY processed breast cancer tissues. Sci Rep 9: 5624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JRW, Warren SC, Herrmann D, Murphy KJ, Cazet AS, Vennin C, Shearer RF, Killen MJ, Magenau A, Mélénec P et al (2018) Intravital imaging to monitor therapeutic response in moving hypoxic regions resistant to PI3K pathway targeting in pancreatic cancer. Cell Rep 23: 3312–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu DL, Kokkaliaris KD, Kunz L, Schroeder T (2018) Multicolor quantitative confocal imaging cytometry. Nat Methods 15: 39–46 [DOI] [PubMed] [Google Scholar]
- Crainiciuc G, Palomino‐Segura M, Molina‐Moreno M, Sicilia J, Aragones DG, Li JLY, Madurga R, Adrover JM, Aroca‐Crevillén A, Martin‐Salamanca S et al (2022) Behavioural immune landscapes of inflammation. Nature 601: 415–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craver BM, Acharya MM, Allen BD, Benke SN, Hultgren NW, Baulch JE, Limoli CL (2016) 3D surface analysis of hippocampal microvasculature in the irradiated brain. Environ Mol Mutagen 57: 341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creech MK, Wang J, Nan X, Gibbs SL (2017) Superresolution imaging of clinical formalin fixed paraffin embedded breast cancer with single molecule localization microscopy. Sci Rep 7: 40766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuccarese MF, Dubach JM, Pfirschke C, Engblom C, Garris C, Miller MA, Pittet MJ, Weissleder R (2017) Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat Commun 8: 14293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart A (2018) Take a left here. Nat Rev Cancer 18: 337 [DOI] [PubMed] [Google Scholar]
- Dawson CA, Mueller SN, Lindeman GJ, Rios AC, Visvader JE (2021) Intravital microscopy of dynamic single‐cell behavior in mouse mammary tissue. Nat Protoc 16: 1907–1935 [DOI] [PubMed] [Google Scholar]
- Dawson CA, Pal B, Vaillant F, Gandolfo LC, Liu Z, Bleriot C, Ginhoux F, Smyth GK, Lindeman GJ, Mueller SN et al (2020) Tissue‐resident ductal macrophages survey the mammary epithelium and facilitate tissue remodelling. Nat Cell Biol 22: 546–558 [DOI] [PubMed] [Google Scholar]
- Dekkers JF, Alieva M, Wellens LM, Ariese HCR, Jamieson PR, Vonk AM, Amatngalim GD, Hu H, Oost KC, Snippert HJG et al (2019) High‐resolution 3D imaging of fixed and cleared organoids. Nat Protoc 14: 1756–1771 [DOI] [PubMed] [Google Scholar]
- Dekkers JF, Alieva M, Cleven A, Keramati F, Brazda P, Rebel HG, Wezenaar AKL, Puschhof J, Buchholz M, Barrera Román M et al (2021) Behavioral‐transcriptomic landscape of engineered T cells targeting human cancer organoids. bioRxiv 10.1101/2021.05.05.442764 [PREPRINT] [DOI] [Google Scholar]
- Dekkers JF, Whittle JR, Vaillant F, Chen H‐R, Dawson C, Liu K, Geurts MH, Herold MJ, Clevers H, Lindeman GJ et al (2020) Modeling breast cancer using CRISPR‐Cas9‐mediated engineering of human breast organoids. J Natl Cancer Inst 112: 540–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisenko E, Guo BB, Jones M, Hou R, de Kock L, Lassmann T, Poppe D, Clément O, Simmons RK, Lister R et al (2020) Systematic assessment of tissue dissociation and storage biases in single‐cell and single‐nucleus RNA‐seq workflows. Genome Biol 21: 130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobosz M, Ntziachristos V, Scheuer W, Strobel S (2014) Multispectral fluorescence ultramicroscopy: three‐dimensional visualization and automatic quantification of tumor morphology, drug penetration, and antiangiogenic treatment response. Neoplasia 16: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost J, Clevers H (2018) Organoids in cancer research. Nat Rev Cancer 18: 407–418 [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H, Garbi N, Kaisho T, Germain RN, Kastenmuller W (2015) Robust anti‐viral immunity requires multiple distinct T cell‐dendritic cell interactions. Cell 162: 1322–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entenberg D, Voiculescu S, Guo P, Borriello L, Wang Y, Karagiannis GS, Jones J, Baccay F, Oktay M, Condeelis J (2018) A permanent window for the murine lung enables high‐resolution imaging of cancer metastasis. Nat Methods 15: 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JI (2018) Prostate cancer grading: a decade after the 2005 modified system. Mod Pathol 31: S47–S63 [DOI] [PubMed] [Google Scholar]
- Erturk A, Becker K, Jahrling N, Mauch CP, Hojer CD, Egen JG, Hellal F, Bradke F, Sheng M, Dodt HU (2012) Three‐dimensional imaging of solvent‐cleared organs using 3DISCO. Nat Protoc 7: 1983–1995 [DOI] [PubMed] [Google Scholar]
- Falk T, Mai D, Bensch R, Çiçek Ö, Abdulkadir A, Marrakchi Y, Böhm A, Deubner J, Jäckel Z, Seiwald K et al (2019) U‐Net: deep learning for cell counting, detection, and morphometry. Nat Methods 16: 67–70 [DOI] [PubMed] [Google Scholar]
- Fang W, Zhou T, Shi HE, Yao M, Zhang D, Qian H, Zeng Q, Wang Y, Jin F, Chai C et al (2021) Progranulin induces immune escape in breast cancer via up‐regulating PD‐L1 expression on tumor‐associated macrophages (TAMs) and promoting CD8(+) T cell exclusion. J Exp Clin Cancer Res 40: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore VF, Krajnc M, Quiroz FG, Levorse J, Pasolli HA, Shvartsman SY, Fuchs E (2020) Mechanics of a multilayer epithelium instruct tumour architecture and function. Nature 585: 433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli A, Drost J, Suijkerbuijk SJE, van Boxtel R, de Ligt J, Offerhaus GJ, Begthel H, Beerling E, Tan EH, Sansom OJ et al (2017) Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc Natl Acad Sci U S A 114: E2357–E2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli A, Oost KC, Kester L, Morgner J, Bornes L, Bruens L, Spaargaren L, Azkanaz M, Schelfhorst T, Beerling E et al (2020) Plasticity of Lgr5‐negative cancer cells drives metastasis in colorectal cancer. Cell Stem Cell 26: 569–578.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo S, D’Alessandro G, Chece G, Brau F, Maggi L, Rosa A, Porzia A, Mainiero F, Esposito V, Lauro C et al (2015) Enriched environment reduces glioma growth through immune and non‐immune mechanisms in mice. Nat Commun 6: 6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavgiotaki E, Filippidis G, Tsafas V, Bovasianos S, Kenanakis G, Georgoulias V, Tzardi M, Agelaki S, Athanassakis I (2020) Third Harmonic Generation microscopy distinguishes malignant cell grade in human breast tissue biopsies. Sci Rep 10: 11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN (2012) Histo‐cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37: 364–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltsev Y, Samusik N, Kennedy‐Darling J, Bhate S, Hale M, Vazquez G, Black S, Nolan GP (2018) Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell 174: 968–981.e915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldner IH, Yang L, Cowdrick KR, Wang Q, Alvarez Barrios WV, Zellmer VR, Zhang Y, Host M, Liu F, Chen DZ et al (2016) An integrative platform for three‐dimensional quantitative analysis of spatially heterogeneous metastasis landscapes. Sci Rep 6: 24201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannezo E, Scheele C, Moad M, Drogo N, Heer R, Sampogna RV, van Rheenen J, Simons BD (2017) A unifying theory of branching morphogenesis. Cell 171: 242–255.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, Condeelis JS (2015) Real‐time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage‐derived VEGFA. Cancer Discov 5: 932–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, Avivar‐Valderas A, Nagi C, Girnius N, Davis RJ et al (2016) Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature 540: 588–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley M, Kleywegt GJ, Patwardhan A, Sarkans U, Swedlow JR, Brazma A (2022) The BioImage Archive ‐ building a home for life‐sciences microscopy data. J Mol Biol 167505 10.1016/j.jmb.2022.167505 [DOI] [PubMed] [Google Scholar]
- Hirata E, Girotti MR, Viros A, Hooper S, Spencer‐Dene B, Matsuda M, Larkin J, Marais R, Sahai E (2015) Intravital imaging reveals how BRAF inhibition generates drug‐tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell 27: 574–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hor JL, Whitney PG, Zaid A, Brooks AG, Heath WR, Mueller SN (2015) Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T cell activation to localized viral infection. Immunity 43: 554–565 [DOI] [PubMed] [Google Scholar]
- Hörl D, Rojas Rusak F, Preusser F, Tillberg P, Randel N, Chhetri RK, Cardona A, Keller PJ, Harz H, Leonhardt H et al (2019) BigStitcher: reconstructing high‐resolution image datasets of cleared and expanded samples. Nat Methods 16: 870–874 [DOI] [PubMed] [Google Scholar]
- Hu H, Gehart H, Artegiani B, LÖpez‐Iglesias C, Dekkers F, Basak O, van Es J, Chuva de Sousa Lopes SM, Begthel H, Korving J et al (2018) Long‐term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 175: 1591–1606.e19 [DOI] [PubMed] [Google Scholar]
- Huang B, Bates M, Zhuang X (2009) Super‐resolution fluorescence microscopy. Annu Rev Biochem 78: 993–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Jones SA, Brandenburg B, Zhuang X (2008a) Whole‐cell 3D STORM reveals interactions between cellular structures with nanometer‐scale resolution. Nat Methods 5: 1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Wang W, Bates M, Zhuang X (2008b) Three‐dimensional super‐resolution imaging by stochastic optical reconstruction microscopy. Science 319: 810–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Garrett A, Bose S, Blocker S, Rios AC, Clevers H, Shen X (2021) The frontier of live tissue imaging across space and time. Cell Stem Cell 28: 603–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huels DJ, Bruens L, Hodder MC, Cammareri P, Campbell AD, Ridgway RA, Gay DM, Solar‐Abboud M, Faller WJ, Nixon C et al (2018) Wnt ligands influence tumour initiation by controlling the number of intestinal stem cells. Nat Commun 9: 1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilina O, Gritsenko PG, Syga S, Lippoldt J, La Porta CAM, Chepizhko O, Grosser S, Vullings M, Bakker G‐J, Starruß J et al (2020) Cell‐cell adhesion and 3D matrix confinement determine jamming transitions in breast cancer invasion. Nat Cell Biol 22: 1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ineveld RL, Ariese HCR, Wehrens EJ, Dekkers JF, Rios AC (2020) Single‐cell resolution three‐dimensional imaging of intact organoids. J Vis Exp 10.3791/60709 [DOI] [PubMed] [Google Scholar]
- van Ineveld RL, Collot R, Barrera Roman M, Pagliaro A, Bessler N, Ariese HCR, Kleinnijenhuis M, Kool M, Alieva M, Chuva de Sousa Lopes SM et al (in press) Multispectral confocal 3D imaging of intact healthy and tumor tissue using mLSR‐3D. Nat Protoc [DOI] [PubMed] [Google Scholar]
- van Ineveld RL, Kleinnijenhuis M, Alieva M, de Blank S, Barrera Roman M, van Vliet EJ, Martínez Mir C, Johnson HR, Bos FL, Heukers R et al (2021) Revealing the spatio‐phenotypic patterning of cells in healthy and tumor tissues with mLSR‐3D and STAPL‐3D. Nat Biotechnol 39: 1239–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee F, Jaeger PF, Kohl SAA, Petersen J, Maier‐Hein KH (2021) nnU‐Net: a self‐configuring method for deep learning‐based biomedical image segmentation. Nat Methods 18: 203–211 [DOI] [PubMed] [Google Scholar]
- Jacob L, Boisserand LSB, Geraldo LHM, de Brito Neto J, Mathivet T, Antila S, Barka B, Xu Y, Thomas J‐M, Pestel J et al (2019) Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun 10: 4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson PR, Dekkers JF, Rios AC, Fu NY, Lindeman GJ, Visvader JE (2017) Derivation of a robust mouse mammary organoid system for studying tissue dynamics. Development 144: 1065–1071 [DOI] [PubMed] [Google Scholar]
- Jansen I, Lucas M, Savci‐Heijink CD, Meijer SL, Liem E, de Boer OJ, van Leeuwen TG, Marquering HA, de Bruin DM (2019) Three‐dimensional histopathological reconstruction of bladder tumours. Diagn Pathol 14: 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, VanHouten JN, Dann P, Kim W, Sullivan C, Yu H, Liotta L, Espina V, Stern DF, Friedman PA et al (2016) PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2‐mediated breast cancer. Proc Natl Acad Sci U S A 113: E282–E290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CG (2012) Scanning electron microscopy: preparation and imaging for SEM. Methods Mol Biol 915: 1–20 [DOI] [PubMed] [Google Scholar]
- Jonkman J, Brown CM, Wright GD, Anderson KI, North AJ (2020) Tutorial: guidance for quantitative confocal microscopy. Nat Protoc 15: 1585–1611 [DOI] [PubMed] [Google Scholar]
- Junker JP, Noel ES, Guryev V, Peterson KA, Shah G, Huisken J, McMahon AP, Berezikov E, Bakkers J, van Oudenaarden A (2014) Genome‐wide RNA tomography in the zebrafish embryo. Cell 159: 662–675 [DOI] [PubMed] [Google Scholar]
- Karaiskos N, Wahle P, Alles J, Boltengagen A, Ayoub S, Kipar C, Kocks C, Rajewsky N, Zinzen RP (2017) The Drosophila embryo at single‐cell transcriptome resolution. Science 358: 194–199 [DOI] [PubMed] [Google Scholar]
- Kastelein AW, Vos LMC, van Baal JOAM, Koning JJ, Hira VVV, Nieuwland R, van Driel WJ, Uz Z, van Gulik TM, van Rheenen J et al (2020) Poor perfusion of the microvasculature in peritoneal metastases of ovarian cancer. Clin Exp Metastasis 37: 293–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Li R, Ng TSC, Courties G, Rodell CB, Prytyskach M, Kohler RH, Pittet MJ, Nahrendorf M, Weissleder R et al (2018) Quantitative imaging of tumor‐associated macrophages and their response to therapy using (64)Cu‐labeled macrin. ACS Nano 12: 12015–12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Chung K, Deisseroth K (2013) Light microscopy mapping of connections in the intact brain. Trends Cogn Sci 17: 596–599 [DOI] [PubMed] [Google Scholar]
- Kingston BR, Syed AM, Ngai J, Sindhwani S, Chan WCW (2019) Assessing micrometastases as a target for nanoparticles using 3D microscopy and machine learning. Proc Natl Acad Sci U S A 116: 14937–14946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkat TJ, Patel MS, McGarvey D, LiVolsi VA, Baloch ZW (2013) Archived formalin‐fixed paraffin‐embedded (FFPE) blocks: a valuable underexploited resource for extraction of DNA, RNA, and protein. Biopreserv Biobank 11: 101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon S, Ishibashi K, Katoh H, Kitamoto S, Shirai T, Tanaka S, Kajita M, Ishikawa S, Yamauchi H, Yako Y et al (2017) Cell competition with normal epithelial cells promotes apical extrusion of transformed cells through metabolic changes. Nat Cell Biol 19: 530–541 [DOI] [PubMed] [Google Scholar]
- Kondo H, Ratcliffe CDH, Hooper S, Ellis J, MacRae JI, Hennequart M, Dunsby CW, Anderson KI, Sahai E (2021) Single‐cell resolved imaging reveals intra‐tumor heterogeneity in glycolysis, transitions between metabolic states, and their regulatory mechanisms. Cell Rep 34: 108750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korehisa S, Ikeda T, Okano S, Saeki H, Oki E, Oda Y, Hashizume M, Maehara Y (2018) A novel histological examination with dynamic three‐dimensional reconstruction from multiple immunohistochemically stained sections of a PD‐L1‐positive colon cancer. Histopathology 72: 697–703 [DOI] [PubMed] [Google Scholar]
- Kretzschmar K, Watt FM (2012) Lineage tracing. Cell 148: 33–45 [DOI] [PubMed] [Google Scholar]
- Krotenberg Garcia A, Fumagalli A, Le HQ, Jackstadt R, Lannagan TRM, Sansom OJ, van Rheenen J, Suijkerbuijk SJE (2021) Active elimination of intestinal cells drives oncogenic growth in organoids. Cell Rep 36: 109307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal JB, Kane RA, Sentovich SM, Longmaid HE (1997) Pitfalls and sources of error in staging rectal cancer with endorectal us. Radiographics 17: 609–626 [DOI] [PubMed] [Google Scholar]
- Ku T, Guan W, Evans NB, Sohn CH, Albanese A, Kim JG, Frosch MP, Chung K (2020) Elasticizing tissues for reversible shape transformation and accelerated molecular labeling. Nat Methods 17: 609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota SI, Takahashi K, Nishida J, Morishita Y, Ehata S, Tainaka K, Miyazono K, Ueda HR (2017) Whole‐body profiling of cancer metastasis with single‐cell resolution. Cell Rep 20: 236–250 [DOI] [PubMed] [Google Scholar]
- Lagerweij T, Dusoswa SA, Negrean A, Hendrikx EML, de Vries HE, Kole J, Garcia‐Vallejo JJ, Mansvelder HD, Vandertop WP, Noske DP et al (2017) Optical clearing and fluorescence deep‐tissue imaging for 3D quantitative analysis of the brain tumor microenvironment. Angiogenesis 20: 533–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht S, Schmidt EM, Blaj C, Hermeking H, Jung A, Kirchner T, Horst D (2017) Multicolor lineage tracing reveals clonal architecture and dynamics in colon cancer. Nat Commun 8: 1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521: 436–444 [DOI] [PubMed] [Google Scholar]
- Lee SS, Bindokas VP, Kron SJ (2019a) Multiplex three‐dimensional mapping of macromolecular drug distribution in the tumor microenvironment. Mol Cancer Ther 18: 213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Bindokas VP, Lingen MW, Kron SJ (2019b) Nondestructive, multiplex three‐dimensional mapping of immune infiltrates in core needle biopsy. Lab Invest 99: 1400–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenos KJ, Miedema DM, Lodestijn SC, Nijman LE, van den Bosch T, Romero Ros X, Lourenço FC, Lecca MC, van der Heijden M, van Neerven SM et al (2018) Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer. Nat Cell Biol 20: 1193–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Germain RN, Gerner MY (2017) Multiplex, quantitative cellular analysis in large tissue volumes with clearing‐enhanced 3D microscopy (Ce3D). Proc Natl Acad Sci U S A 114: E7321–E7330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Germain RN, Gerner MY (2019) High‐dimensional cell‐level analysis of tissues with Ce3D multiplex volume imaging. Nat Protoc 14: 1708–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Peng SJ, Shen CN, Pasricha PJ, Tang SC (2016) PanIN‐associated pericyte, glial, and islet remodeling in mice revealed by 3D pancreatic duct lesion histology. Am J Physiol Gastrointest Liver Physiol 311: G412–G422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, van der Laak J, van Ginneken B, Sanchez CI (2017) A survey on deep learning in medical image analysis. Med Image Anal 42: 60–88 [DOI] [PubMed] [Google Scholar]
- Liu YA, Pan ST, Hou YC, Shen MY, Peng SJ, Tang SC, Chung YC (2013) 3‐D visualization and quantitation of microvessels in transparent human colorectal carcinoma [corrected]. PLoS One 8: e81857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang M, Deng Y, Su G, Enninful A, Guo CC, Tebaldi T, Zhang DI, Kim D, Bai Z et al (2020) High‐spatial‐resolution multi‐omics sequencing via deterministic barcoding in tissue. Cell 183: 1665–1681.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW (2007) Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450: 56–62 [DOI] [PubMed] [Google Scholar]
- Lukonin I, Serra D, Challet Meylan L, Volkmann K, Baaten J, Zhao R, Meeusen S, Colman K, Maurer F, Stadler MB et al (2020) Phenotypic landscape of intestinal organoid regeneration. Nature 586: 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlandt EK, Arts JJG, van der Meer WJ, van der Linden FH, Tol S, van Buul JD, Gadella TWJ, Goedhart J (2021) Visualizing endogenous Rho activity with an improved localization‐based, genetically encoded biosensor. J Cell Sci 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantri M, Scuderi GJ, Abedini‐Nassab R, Wang MFZ, McKellar D, Shi H, Grodner B, Butcher JT, De Vlaminck I (2021) Spatiotemporal single‐cell RNA sequencing of developing chicken hearts identifies interplay between cellular differentiation and morphogenesis. Nat Commun 12: 1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni F, Murooka TT, Manzo T, Kim EY, Carrizosa E, Elpek NM, Mempel TR (2013) The transcription factor NFAT exhibits signal memory during serial T cell interactions with antigen‐presenting cells. Immunity 38: 237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendler CT, Feuchtinger A, Heid I, Aichler M, D’Alessandria C, Pirsig S, Blechert B, Wester H‐J, Braren R, Walch A et al (2016) Tumor uptake of anti‐CD20 fabs depends on tumor perfusion. J Nucl Med 57: 1971–1977 [DOI] [PubMed] [Google Scholar]
- Messal HA, Almagro J, Zaw Thin M, Tedeschi A, Ciccarelli A, Blackie L, Anderson KI, Miguel‐Aliaga I, van Rheenen J, Behrens A (2021) Antigen retrieval and clearing for whole‐organ immunofluorescence by FLASH. Nat Protoc 16: 239–262 [DOI] [PubMed] [Google Scholar]
- Messal HA, Alt S, Ferreira RMM, Gribben C, Wang VM, Cotoi CG, Salbreux G, Behrens A (2019) Tissue curvature and apicobasal mechanical tension imbalance instruct cancer morphogenesis. Nature 566: 126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Norrelykke SF (2021) Reproducible image handling and analysis. EMBO J 40: e105889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki H, Uozaki H, Tojo A, Hirashima S, Inaga S, Sakuma K, Morishita Y, Fukayama M (2012) Application of low‐vacuum scanning electron microscopy for renal biopsy specimens. Pathol Res Pract 208: 503–509 [DOI] [PubMed] [Google Scholar]
- Moen E, Bannon D, Kudo T, Graf W, Covert M, Van Valen D (2019) Deep learning for cellular image analysis. Nat Methods 16: 1233–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molbay M, Kolabas ZI, Todorov MI, Ohn TL, Erturk A (2021) A guidebook for DISCO tissue clearing. Mol Syst Biol 17: e9807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami TC, Mano T, Saikawa S, Horiguchi SA, Shigeta D, Baba K, Sekiya H, Shimizu Y, Tanaka KF, Kiyonari H et al (2018) A three‐dimensional single‐cell‐resolution whole‐brain atlas using CUBIC‐X expansion microscopy and tissue clearing. Nat Neurosci 21: 625–637 [DOI] [PubMed] [Google Scholar]
- Murray E, Cho J, Goodwin D, Ku T, Swaney J, Kim S‐Y, Choi H, Park Y‐G, Park J‐Y, Hubbert A et al (2015) Simple, scalable proteomic imaging for high‐dimensional profiling of intact systems. Cell 163: 1500–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty S, Haile ST, Beinat C, Aalipour A, Alam IS, Murty T, Shaffer TM, Patel CB, Graves EE, Mackall CL et al (2020) Intravital imaging reveals synergistic effect of CAR T‐cells and radiation therapy in a preclinical immunocompetent glioblastoma model. Oncoimmunology 9: 1757360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noë M, Rezaee N, Asrani K, Skaro M, Groot VP, Wu P‐H, Olson MT, Hong S‐M, Kim SJ, Weiss MJ et al (2018) Immunolabeling of cleared human pancreata provides insights into three‐dimensional pancreatic anatomy and pathology. Am J Pathol 188: 1530–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima S, Susaki EA, Yoshida K, Takemoto H, Tsujimura N, Iijima S, Takachi KO, Nakahara Y, Tahara S, Ohshima K et al (2017) CUBIC pathology: three‐dimensional imaging for pathological diagnosis. Sci Rep 7: 9269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan E, Savas P, Policheni AN, Darcy PK, Vaillant F, Mintoff CP, Dushyanthen S, Mansour M, Pang JB, Fox SB et al (2017) Combined immune checkpoint blockade as a therapeutic strategy for BRCA1‐mutated breast cancer. Sci Transl Med 9: eaal4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka A, Kajita M, Ikenouchi J, Yako Y, Kitamoto S, Kon S, Ikegawa M, Shimada T, Ishikawa S, Fujita Y (2015) EPLIN is a crucial regulator for extrusion of RasV12‐transformed cells. J Cell Sci 128: 781–789 [DOI] [PubMed] [Google Scholar]
- Oshimori N, Oristian D, Fuchs E (2015) TGF‐beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell 160: 963–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M et al (2015) Brain tumour cells interconnect to a functional and resistant network. Nature 528: 93–98 [DOI] [PubMed] [Google Scholar]
- Pan C, Cai R, Quacquarelli FP, Ghasemigharagoz A, Lourbopoulos A, Matryba P, Plesnila N, Dichgans M, Hellal F, Erturk A (2016) Shrinkage‐mediated imaging of entire organs and organisms using uDISCO. Nat Methods 13: 859–867 [DOI] [PubMed] [Google Scholar]
- Pan C, Schoppe O, Parra‐Damas A, Cai R, Todorov MI, Gondi G, von Neubeck B, Böğürcü‐Seidel N, Seidel S, Sleiman K et al (2019) Deep learning reveals cancer metastasis and therapeutic antibody targeting in the entire body. Cell 179: 1661–1676.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M, McBain N, Wagner T, Edwards J, McConville R et al (2019) Tissue‐resident memory CD8(+) T cells promote melanoma‐immune equilibrium in skin. Nature 565: 366–371 [DOI] [PubMed] [Google Scholar]
- Parker TM, Gupta K, Palma AM, Yekelchyk M, Fisher PB, Grossman SR, Won KJ, Madan E, Moreno E, Gogna R (2021) Cell competition in intratumoral and tumor microenvironment interactions. EMBO J 40: e107271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AC, Chiang ZD, Reginato PL, Mangiameli SM, Murray EM, Yao C‐C, Markoulaki S, Earl AS, Labade AS, Jaenisch R et al (2021) In situ genome sequencing resolves DNA sequence and structure in intact biological samples. Science 371: eaay3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira ER, Kedrin D, Seano G, Gautier O, Meijer EFJ, Jones D, Chin S‐M, Kitahara S, Bouta EM, Chang J et al (2018) Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 359: 1403–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty AJ, Li A, Wang X, Dai R, Heyman B, Hsu D, Huang X, Yang Y (2019) Hedgehog signaling promotes tumor‐associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest 129: 5151–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsioen B, Post JB, Buissant des Amorie JR, Laskaris D, van Ineveld RL, Kersten S, Bertotti A, Sassi F, Sipieter F, Cappe B et al (2021) Quantifying single‐cell ERK dynamics in colorectal cancer organoids reveals EGFR as an amplifier of oncogenic MAPK pathway signalling. Nat Cell Biol 23: 377–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post Y, Puschhof J, Beumer J, Kerkkamp HM, de Bakker MAG, Slagboom J, de Barbanson B, Wevers NR, Spijkers XM, Olivier T et al (2020) Snake venom gland organoids. Cell 180: 233–247.e21 [DOI] [PubMed] [Google Scholar]
- Regot S, Hughey JJ, Bajar BT, Carrasco S, Covert MW (2014) High‐sensitivity measurements of multiple kinase activities in live single cells. Cell 157: 1724–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Choi H, Chung K, Bouma BE (2017) Label‐free volumetric optical imaging of intact murine brains. Sci Rep 7: 46306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, Autry AE, Kadiri L, Umadevi Venkataraju K, Zhou YU et al (2016) Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165: 1789–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier‐Lavigne M (2014) iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159: 896–910 [DOI] [PubMed] [Google Scholar]
- Reynaud EG, Peychl J, Huisken J, Tomancak P (2015) Guide to light‐sheet microscopy for adventurous biologists. Nat Methods 12: 30–34 [DOI] [PubMed] [Google Scholar]
- van Rheenen J, Scheele C (2021) Intravital microscopy to illuminate cell state plasticity during metastasis. Curr Opin Cell Biol 72: 28–35 [DOI] [PubMed] [Google Scholar]
- Ricard C, Debarbieux FC (2014) Six‐color intravital two‐photon imaging of brain tumors and their dynamic microenvironment. Front Cell Neurosci 8: 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DS, Lichtman JW (2015) Clarifying tissue clearing. Cell 162: 246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios AC, Capaldo BD, Vaillant F, Pal B, van Ineveld R, Dawson CA, Chen Y, Nolan E, Fu NY, 3DTCLSM Group et al (2019) Intraclonal plasticity in mammary tumors revealed through large‐scale single‐cell resolution 3D imaging. Cancer Cell 35: 618–632.e616 [DOI] [PubMed] [Google Scholar]
- Rios AC, Clevers H (2018) Imaging organoids: a bright future ahead. Nat Methods 15: 24–26 [DOI] [PubMed] [Google Scholar]
- Rios AC, Fu NY, Lindeman GJ, Visvader JE (2014) In situ identification of bipotent stem cells in the mammary gland. Nature 506: 322–327 [DOI] [PubMed] [Google Scholar]
- Rios AC, Fu NY, Jamieson PR, Pal B, Whitehead L, Nicholas KR, Lindeman GJ, Visvader JE (2016) Essential role for a novel population of binucleated mammary epithelial cells in lactation. Nat Commun 7: 11400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios AC, Serralbo O, Salgado D, Marcelle C (2011) Neural crest regulates myogenesis through the transient activation of NOTCH. Nature 473: 532–535 [DOI] [PubMed] [Google Scholar]
- Rivenson Y, Wang H, Wei Z, de Haan K, Zhang Y, Wu Y, Günaydın H, Zuckerman JE, Chong T, Sisk AE et al (2019) Virtual histological staining of unlabelled tissue‐autofluorescence images via deep learning. Nat Biomed Eng 3: 466–477 [DOI] [PubMed] [Google Scholar]
- Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, Welch J, Chen LM, Chen F, Macosko EZ (2019) Slide‐seq: a scalable technology for measuring genome‐wide expression at high spatial resolution. Science 363: 1463–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Royen ME, Verhoef EI, Kweldam CF, van Cappellen WA, Kremers GJ, Houtsmuller AB, van Leenders GJ (2016) Three‐dimensional microscopic analysis of clinical prostate specimens. Histopathology 69: 985–992 [DOI] [PubMed] [Google Scholar]
- Rust MJ, Bates M, Zhuang X (2006) Sub‐diffraction‐limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Methods 3: 793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N, Papaspyropoulos A, Zomer‐van Ommen DD, Heo I, Böttinger L, Klay D, Weeber F, Huelsz‐Prince G, Iakobachvili N, Amatngalim GD et al (2019) Long‐term expanding human airway organoids for disease modeling. EMBO J 38: e100300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Clevers H (2018) Studying cellular heterogeneity and drug sensitivity in colorectal cancer using organoid technology. Curr Opin Genet Dev 52: 117–122 [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ et al (2009) Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- Sawaguchi A, Kamimura T, Yamashita A, Takahashi N, Ichikawa K, Aoyama F, Asada Y (2018) Informative three‐dimensional survey of cell/tissue architectures in thick paraffin sections by simple low‐vacuum scanning electron microscopy. Sci Rep 8: 7479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele CL, Hannezo E, Muraro MJ, Zomer A, Langedijk NS, van Oudenaarden A, Simons BD, van Rheenen J (2017) Identity and dynamics of mammary stem cells during branching morphogenesis. Nature 542: 313–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnalzger TE, de Groot MH, Zhang C, Mosa MH, Michels BE, Roder J, Darvishi T, Wels WS, Farin HF (2019) 3D model for CAR‐mediated cytotoxicity using patient‐derived colorectal cancer organoids. EMBO J 38: e100928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutgens F, Rookmaaker MB, Margaritis T, Rios A, Ammerlaan C, Jansen J, Gijzen L, Vormann M, Vonk A, Viveen M et al (2019) Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol 37: 303–313 [DOI] [PubMed] [Google Scholar]
- Seo J, Sim Y, Kim J, Kim H, Cho I, Yoon Y, Chang J (2021) PICASSO: ultra‐multiplexed fluorescence imaging of biomolecules through single‐round imaging and blind source unmixing. bioRxiv 10.1101/2021.01.27.428247 [PREPRINT] [DOI] [Google Scholar]
- Shen C‐N, Goh K‐S, Huang C‐R, Chiang T‐C, Lee C‐Y, Jeng Y‐M, Peng S‐J, Chien H‐J, Chung M‐H, Chou Y‐H et al (2019) Lymphatic vessel remodeling and invasion in pancreatic cancer progression. EBioMedicine 47: 98–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si YU, Merz SF, Jansen P, Wang B, Bruderek K, Altenhoff P, Mattheis S, Lang S, Gunzer M, Klode J et al (2019) Multidimensional imaging provides evidence for down‐regulation of T cell effector function by MDSC in human cancer tissue. Sci Immunol 4: eaaw9159 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon‐Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD et al (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Schepers AG, van Es JH, Simons BD, Clevers H (2014) Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep 15: 62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton JR, Doss BL, Lindsay S, Ros R (2016) Correlating confocal microscopy and atomic force indentation reveals metastatic cancer cells stiffen during invasion into collagen I matrices. Sci Rep 6: 19686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus CR, Filipek J, Gern BH, Olin BE, Leal JM, Wu Y, Lyons‐Cohen MR, Huang JY, Paz‐Stoltzfus CL, Plumlee CR et al (2020) CytoMAP: a spatial analysis toolbox reveals features of myeloid cell organization in lymphoid tissues. Cell Rep 31: 107523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaki E, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe T, Yokoyama C, Onoe H, Eguchi M, Yamaguchi S et al (2014) Whole‐brain imaging with single‐cell resolution using chemical cocktails and computational analysis. Cell 157: 726–739 [DOI] [PubMed] [Google Scholar]
- Tainaka K, Murakami TC, Susaki EA, Shimizu C, Saito R, Takahashi K, Hayashi‐Takagi A, Sekiya H, Arima Y, Nojima S et al (2018) Chemical landscape for tissue clearing based on hydrophilic reagents. Cell Rep 24: 2196–2210.e9 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Kaczynska D, Kanatani S, Sahlgren C, Mitura P, Stepulak A, Miyakawa A, Wiklund P, Uhlen P (2018) Mapping of the three‐dimensional lymphatic microvasculature in bladder tumours using light‐sheet microscopy. Br J Cancer 118: 995–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Kanatani S, Tomer R, Sahlgren C, Kronqvist P, Kaczynska D, Louhivuori L, Kis L, Lindh C, Mitura P et al (2017) Whole‐tissue biopsy phenotyping of three‐dimensional tumours reveals patterns of cancer heterogeneity. Nat Biomed Eng 1: 796–806 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Kanatani S, Kaczynska D, Fukumoto K, Louhivuori L, Mizutani T, Kopper O, Kronqvist P, Robertson S, Lindh C et al (2020) Three‐dimensional single‐cell imaging for the analysis of RNA and protein expression in intact tumour biopsies. Nat Biomed Eng 4: 875–888 [DOI] [PubMed] [Google Scholar]
- Tang YJ, Huang J, Tsushima H, Ban GI, Zhang H, Oristian KM, Puviindran V, Williams N, Ding X, Ou J et al (2019) Tracing tumor evolution in sarcoma reveals clonal origin of advanced metastasis. Cell Rep 28: 2837–2850.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiede S, Kalathur RKR, Lüönd F, von Allmen L, Szczerba BM, Hess M, Vlajnic T, Müller B, Canales Murillo J, Aceto N et al (2021) Multi‐color clonal tracking reveals intra‐stage proliferative heterogeneity during mammary tumor progression. Oncogene 40: 12–27 [DOI] [PubMed] [Google Scholar]
- Tjan‐Heijnen V, Viale G (2018) The lymph node and the metastasis. N Engl J Med 378: 2045–2046 [DOI] [PubMed] [Google Scholar]
- Todorov MI, Paetzold JC, Schoppe O, Tetteh G, Shit S, Efremov V, Todorov‐Völgyi K, Düring M, Dichgans M, Piraud M et al (2020) Machine learning analysis of whole mouse brain vasculature. Nat Methods 17: 442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkach Y, Thomann S, Kristiansen G (2018) Three‐dimensional reconstruction of prostate cancer architecture with serial immunohistochemical sections: hallmarks of tumour growth, tumour compartmentalisation, and implications for grading and heterogeneity. Histopathology 72: 1051–1059 [DOI] [PubMed] [Google Scholar]
- Tuveson D, Clevers H (2019) Cancer modeling meets human organoid technology. Science 364: 952–955 [DOI] [PubMed] [Google Scholar]
- Ueda HR, Erturk A, Chung K, Gradinaru V, Chedotal A, Tomancak P, Keller PJ (2020) Tissue clearing and its applications in neuroscience. Nat Rev Neurosci 21: 61–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, Cohen AR, Davidson MW, Betzig E, Lippincott‐Schwartz J (2017) Applying systems‐level spectral imaging and analysis to reveal the organelle interactome. Nature 546: 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataramani V, Tanev DI, Strahle C, Studier‐Fischer A, Fankhauser L, Kessler T, Körber C, Kardorff M, Ratliff M, Xie R et al (2019) Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573: 532–538 [DOI] [PubMed] [Google Scholar]
- Verhoef EI, van Cappellen WA, Slotman JA, Kremers GJ, Ewing‐Graham PC, Houtsmuller AB, van Royen ME, van Leenders G (2019) Three‐dimensional analysis reveals two major architectural subgroups of prostate cancer growth patterns. Mod Pathol 32: 1032–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, Buczacki S, Kemp R, Tavare S, Winton DJ (2013) Defining stem cell dynamics in models of intestinal tumor initiation. Science 342: 995–998 [DOI] [PubMed] [Google Scholar]
- Viallard C, Audiger C, Popovic N, Akla N, Lanthier K, Legault‐Navarrete I, Melichar H, Costantino S, Lesage S, Larrivee B (2020) BMP9 signaling promotes the normalization of tumor blood vessels. Oncogene 39: 2996–3014 [DOI] [PubMed] [Google Scholar]
- Viratham Pulsawatdi A, Craig SG, Bingham V, McCombe K, Humphries MP, Senevirathne S, Richman SD, Quirke P, Campo L, Domingo E et al (2020) A robust multiplex immunofluorescence and digital pathology workflow for the characterisation of the tumour immune microenvironment. Mol Oncol 14: 2384–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Allen WE, Wright MA, Sylwestrak EL, Samusik N, Vesuna S, Evans K, Liu C, Ramakrishnan C, Liu J et al (2018) Three‐dimensional intact‐tissue sequencing of single‐cell transcriptional states. Science 361: eaat5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelin B, den Boer AT, Wagena E, Broen K, Dolstra H, de Boer RJ, Figdor CG, Textor J, Friedl P (2021) Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity. Nat Commun 12: 5217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S (2000) Shattering the diffraction limit of light: a revolution in fluorescence microscopy? Proc Natl Acad Sci U S A 97: 8747–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten J‐W, da Silva Santos LB, Bourne PE et al (2016) The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 3: 160018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Kessler T, Grosch J, Hai L, Venkataramani V, Huang L, Hoffmann DC, Solecki G, Ratliff M, Schlesner M et al (2021) Tumor cell network integration in glioma represents a stemness feature. Neuro Oncol 23: 757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Aly RG, Tabata K, Barlas A, Rekhtman N, Eguchi T, Montecalvo J, Hameed M, Manova‐Todorova K, Adusumilli PS et al (2020) Three‐dimensional histologic, immunohistochemical, and multiplex immunofluorescence analyses of dynamic vessel co‐option of spread through air spaces in lung adenocarcinoma. J Thorac Oncol 15: 589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai H, Atsumi N, Tanaka T, Nakamura N, Komai Y, Omachi T, Tanaka K, Ishigaki K, Saiga K, Ohsugi H et al (2017) Intestinal cancer stem cells marked by Bmi1 or Lgr5 expression contribute to tumor propagation via clonal expansion. Sci Rep 7: 41838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Sandoval M, Beronja S (2018) Oncogenic activation of PI3K induces progenitor cell differentiation to suppress epidermal growth. Nat Cell Biol 20: 1256–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Hong SM, Jung D, Noe M, Kiemen A, Wu PH, Wirtz D, Hruban RH, Wood LD, Oshima K (2020) Three‐dimensional analysis of extrahepatic cholangiocarcinoma and tumor budding. J Pathol 251: 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S, Tu H, Chaney EJ, Sun YI, Zhao Y, Bower AJ, Liu Y‐Z, Marjanovic M, Sinha S, Pu Y et al (2018) Intravital imaging by simultaneous label‐free autofluorescence‐multiharmonic microscopy. Nat Commun 9: 2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K, Cheng N, Nakano M, Kuo CJ (2020) Organoid models of tumor immunology. Trends Immunol 41: 652–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yum MK, Han S, Fink J, Wu S‐H, Dabrowska C, Trendafilova T, Mustata R, Chatzeli L, Azzarelli R, Pshenichnaya I et al (2021) Tracing oncogene‐driven remodelling of the intestinal stem cell niche. Nature 594: 442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanfardino M, Castaldo R, Pane K, Affinito O, Aiello M, Salvatore M, Franzese M (2021) MuSA: a graphical user interface for multi‐OMICs data integration in radiogenomic studies. Sci Rep 11: 1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Todorov MI, Cai R, ‐Maskari RAI, Steinke H, Kemter E, Mai H, Rong Z, Warmer M, Stanic K et al (2020) Cellular and molecular probing of intact human organs. Cell 180: 796–812.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer A, Ellenbroek SI, Ritsma L, Beerling E, Vrisekoop N, Van Rheenen J (2013) Intravital imaging of cancer stem cell plasticity in mammary tumors. Stem Cells 31: 602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SIJ et al (2015) In vivo imaging reveals extracellular vesicle‐mediated phenocopying of metastatic behavior. Cell 161: 1046–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]


