Plasmacytoid dendritic cells (pDC) have the unique ability to rapidly mount high‐level antiviral type I interferon (IFN‐I) responses during diverse virus infections. In COVID‐19 patients, reduced pDC numbers correlate with diminished IFN‐I serum levels and enhanced disease severity. However, the molecular mechanisms underlying SARS‐CoV‐2‐mediated pDC stimulation to induce cytokine responses are still largely unclear. In this issue of the EMBO Journal, van der Sluis and colleagues tackled this question by using an innovative hematopoietic stem and progenitor cells (HSPC)‐pDC system that allows gene editing and the detailed analysis of pDC sensing mechanisms.
Subject Categories: Immunology; Microbiology, Virology & Host Pathogen Interaction
While plasmacytoid dendritic cells (pDCs) do not support SARS‐CoV‐2 replication, they do sense the virus via TLR2 and TLR7 to induce divergent branches of cytokine responses.

In the late 50's, pDC were first described as a rare immune cell subset. They were re‐discovered four decades later as the natural type I interferon (IFN‐I) producing cell type in virus infection (reviewed in Reizis, 2019). For a long time, it was assumed that pDC primarily derive from a common dendritic cell progenitor. This concept was challenged by mouse studies suggesting that the majority of pDC derive from lymphoid progenitors. Very recently, it was shown that pDC and conventional DC share a common origin, thus reinitiating the debate about the origin of pDC (Feng et al, 2022). Approximately 0.1–0.5% of nucleated cells in the blood are pDC. Interestingly, infected cells activate pDC more vigorously than free virus to produce IFN‐I and they may even form interferogenic synapses (reviewed in Reizis, 2019). Many viruses such as hepatitis C virus (HCV) and human cytomegalovirus (HCMV) do not replicate in pDC, underscoring the need for pDC to interact with infected cells. In the case of HCV, we discovered that purified virus particles do not stimulate IFN‐I production in human pDC, whereas crude virus preparations and HCV infected cells do so (Grabski et al, 2015), indicating that mainly factors derived from infected cells trigger IFN‐I responses in pDC.
The detailed mechanistic analysis of pDC is hampered by their low abundance and their short life span under in vitro conditions (Fig 1A). Hence, such studies rely on inhibitors, which are often unspecific, or involve pDC from patients with rare genetic defects. Genetically modified mice can be used as a source of genetically manipulated pDC, but results from such experiments cannot easily be translated to conditions in humans, and also in mice pDC are scarce and difficult to target selectively. The ability to differentiate pDC from murine bone marrow cells has bolstered pDC research, but equivalent technologies could not be adapted in the human system. First attempts to differentiate human pDC from hematopoietic progenitors resulted in cells that showed low‐level IFN‐I responses upon treatment with synthetic Toll‐like receptor 9 (TLR9) agonists (Demoulin et al, 2012). Laustsen et al (2018) discovered that IFN‐I priming is needed to differentiate CD34+ HSPC‐derived pDC (HSPC‐pDC) in a manner that they can mount similarly profound IFN‐I responses as blood‐derived pDC. In contrast to blood‐derived pDC, HSPC‐pDC can be gene edited (Fig 1A). However, their cytokine responses other than IFN‐I still may deviate from that of blood‐derived pDC, highlighting the need to verify key observations in primary human cells (Laustsen et al, 2018).
Figure 1. Combining blood‐derived and differentiated human pDC for research.
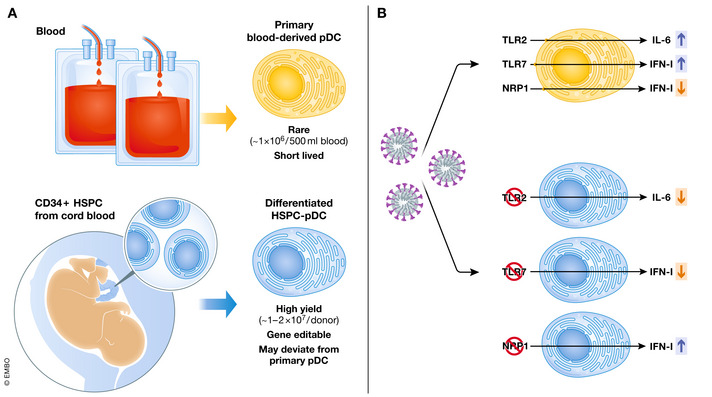
(A) Strengths and limitations of using primary blood‐derived pDC or pDC differentiated from CD34+ HSPC (HSPC‐pDC) from cord blood for in vitro experiments. Blood‐derived pDC are ex vivo isolated primary cells and thus show functional traits that resemble those of pDC in vivo. However, they can only be isolated in small numbers and have a short life span, making it almost impossible to genetically modify them. In contrast, HSPC‐derived pDC can be obtained in higher quantities and gene modification by CRISPR/Cas9 editing or other systems is possible. However, as they are differentiated cells, certain functions of HSPC‐pDC may deviate from their in vivo counterparts. Therefore, critical results should be validated by the analysis of primary cells, if possible. (B) Schematic depiction of pDC stimulation by SARS‐CoV‐2. Upon exposure to SARS‐CoV‐2, pDC mount TLR2‐dependent IL‐6 and TLR7‐dependent IFN‐I responses. Binding of SARS‐CoV‐2 to neuropilin‐1 (NRP1) expressed by pDC diminishes their IFN‐I responses.
IFN‐I responses are critical in COVID‐19, as indicated by 15–20% of severely diseased patients showing inborn errors of IFN‐I immunity or autoantibodies against IFN‐I (Zhang et al, 2022). In early stages of COVID‐19, reduced IFN‐I and enhanced TNFα and IL‐6 responses are associated with increased disease severity, whereas diminished IFN‐I production is correlated with decreased function and numbers of peripheral blood pDC (reviewed in Greene & Zuniga, 2021). In later disease stages, excessive IFN‐I responses or treatment with exogenous IFN‐I can be detrimental (Greene & Zuniga, 2021). In line with this, also van der Sluis et al (2022) found reduced numbers of blood pDC in hospitalized COVID‐19 patients and the extent of pDC reduction correlated with disease severity and with high flow oxygen supplementation. Onodi et al (2021) showed that upon in vitro exposure to SARS‐CoV‐2, pDC produce high amounts of IFN‐I, but they do not express ACE2 and do not support productive infection. Onodi et al (2021) further proposed that in pDC endosomal TLRs sense SARS‐CoV‐2, which was confirmed by Asano et al (2021) who showed that pDC from TLR7‐deficient patients do not mount IFN‐I responses upon SARS‐CoV‐2 exposure, whereas they still express IL‐6 and CXCL‐10.
In the accompanying study, van der Sluis et al (2022) found that similar to blood pDC, HSPC‐pDC produce IFN‐I and IFN‐III upon SARS‐CoV‐2 exposure and that they do not support virus replication. CRISPR/Cas9‐mediated gene editing confirmed that the TLR7/MyD88 axis is a key sensor for IFN‐I induction. In accordance with Asano et al (2021), IL‐6 expression was not affected by TLR7 deletion. Instead, van der Sluis et al (2022) uncovered that IL‐6 production was dependent on TLR2 and TLR2/6 sensing of the SARS‐CoV‐2 envelope protein. Consistently, in blood‐derived pDC, the SARS‐CoV‐2 envelope protein induced IL‐6, but not IFN‐I expression (Fig 1B). So far, TLR2‐dependent responses of pDC were rarely studied, and TLR2 was more discussed in the context of bacterial pDC stimulation, for example, as a sensor of components from commensal bacteria that render pDC tolerogenic (reviewed in Reizis, 2019 and Greene & Zuniga, 2021).
Moreover, van der Sluis et al (2022) showed that antibody binding to the pDC marker neuropilin‐1 (NRP1, BDCA‐4, CD304), which together with ACE2 is a cellular (co‐)receptor for SARS‐CoV‐2, reduced TLR7 agonist‐induced IFN‐I expression in HSPC‐ and blood‐derived pDC. Neuropilin‐1‐deficient HSPC‐pDC showed increased IFN‐I responses upon SARS‐CoV‐2 exposure, but IL‐6 expression stayed normal. These data support the concept that SARS‐CoV‐2 binding to neuropilin‐1 represents a viral escape strategy to evade pDC‐derived IFN‐I.
In summary, van der Sluis et al (2022) combined studies with genetically engineered HSPC‐pDC and primary blood‐derived pDC to show that SARS‐CoV‐2 triggers pDC via TLR7 and TLR2 and thus induces divergent branches of cytokine responses. This observation points toward new options to selectively modulate the profile of pDC‐derived cytokine responses. Earlier studies showed that pDC interact with infected cells in their surroundings to efficiently mount IFN‐I responses. Thus, it will be interesting to address whether pDC that encounter SARS‐CoV‐2 infected cells also deploy TLR2 and TLR7 for their activation. Furthermore, future studies will be needed to address the role of neuropilin‐1‐mediated modulation of IFN‐I responses by pDC in more detail, not only in the context of SARS‐CoV‐2, but also for other viruses.
The EMBO Journal (2022) 41: e111208.
See also: R M van der Sluis et al (May 2022)
References
- Asano T, Boisson B, Onodi F, Matuozzo D, Moncada‐Velez M, Maglorius Renkilaraj MRL, Zhang P, Meertens L, Bolze A, Materna M et al (2021) X‐linked recessive TLR7 deficiency in ~1% of men under 60 years old with life‐threatening COVID‐19. Sci Immunol 6: eabl4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoulin S, Roncarati P, Delvenne P, Hubert P (2012) Production of large numbers of plasmacytoid dendritic cells with functional activities from CD34(+) hematopoietic progenitor cells: use of interleukin‐3. Exp Hematol 40: 268–278 [DOI] [PubMed] [Google Scholar]
- Feng J, Pucella JN, Jang G, Alcántara‐Hernández M, Upadhaya S, Adams NM, Khodadadi‐Jamayran A, Lau CM, Stoeckius M, Hao S et al (2022) Clonal lineage tracing reveals shared origin of conventional and plasmacytoid dendritic cells. Immunity 55: 405–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabski E, Wappler I, Pfaender S, Steinmann E, Haid S, Dzionek A, Pietschmann T, Kalinke U (2015) Efficient virus assembly, but not infectivity, determines the magnitude of hepatitis C virus‐induced interferon alpha responses of plasmacytoid dendritic cells. J Virol 89: 3200–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene TT, Zuniga EI (2021) Type I interferon induction and exhaustion during viral infection: plasmacytoid dendritic cells and emerging COVID‐19 findings. Viruses 13: 1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laustsen A, Bak RO, Krapp C, Kjær L, Egedahl JH, Petersen CC, Pillai S, Tang HQ, Uldbjerg N, Porteus M et al (2018) Interferon priming is essential for human CD34+ cell‐derived plasmacytoid dendritic cell maturation and function. Nat Commun 9: 3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodi F, Bonnet‐Madin L, Meertens L, Karpf L, Poirot J, Zhang SY, Picard C, Puel A, Jouanguy E, Zhang Q et al (2021) SARS‐CoV‐2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4. J Exp Med 218: e20201387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B (2019) Plasmacytoid dendritic cells: development, regulation, and function. Immunity 50: 37–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis RM, Cham LB, Gris‐Oliver A, Gammelgaard KR, Pedersen JG, Idorn M, Ahmadov U, Hernandez SS, Cémalovic E, Godsk SH et al (2022) TLR2 and TLR7 mediate distinct immunopathological and antiviral plasmacytoid dendritic cell responses to SARS‐CoV‐2 infection. EMBO J e109622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Bastard P, Karbuz A, Gervais A, Tayoun AA, Aiuti A, Belot A, Bolze A, Gaudet A, Bondarenko A et al (2022) Human genetic and immunological determinants of critical COVID‐19 pneumonia. Nature 603: 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]


