Abstract
The process by which bacterial cells build their intricate flagellar motility apparatuses has long fascinated scientists. Our understanding of this process comes mainly from studies of purified flagella from two species, Escherichia coli and Salmonella enterica. Here, we used electron cryo‐tomography (cryo‐ET) to image the assembly of the flagellar motor in situ in diverse Proteobacteria: Hylemonella gracilis, Helicobacter pylori, Campylobacter jejuni, Pseudomonas aeruginosa, Pseudomonas fluorescens, and Shewanella oneidensis. Our results reveal the in situ structures of flagellar intermediates, beginning with the earliest flagellar type III secretion system core complex (fT3SScc) and MS‐ring. In high‐torque motors of Beta‐, Gamma‐, and Epsilon‐proteobacteria, we discovered novel cytoplasmic rings that interact with the cytoplasmic torque ring formed by FliG. These rings, associated with the MS‐ring, assemble very early and persist until the stators are recruited into their periplasmic ring; in their absence the stator ring does not assemble. By imaging mutants in Helicobacter pylori, we found that the fT3SScc proteins FliO and FliQ are required for the assembly of these novel cytoplasmic rings. Our results show that rather than a simple accretion of components, flagellar motor assembly is a dynamic process in which accessory components interact transiently to assist in building the complex nanomachine.
Keywords: assembly, bacterial flagellar motor, cryo‐ET, high‐torque, tomography
Subject Categories: Microbiology, Virology & Host Pathogen Interaction
Cryo‐ET imaging of the assembly process of the flagellum in a diverse array of Proteobacteria reveals the presence of transient cytoplasmic rings required for the final assembly of high‐torque motors.

Introduction
Having undergone billions of years of optimization through natural selection, the bacterial flagellum represents a rich system to study how biological nanomachines are assembled at the macromolecular level. A prime example of a multi‐component complex that is built through a tightly regulated self‐assembly process (Macnab, 2003), the flagellum consists of a long extracellular filament driven by a cell envelope‐embedded motor at its base, connected through a flexible joint known as the hook (Fig EV1).
Figure EV1. Schematic of the Escherichia coli flagellar motor.
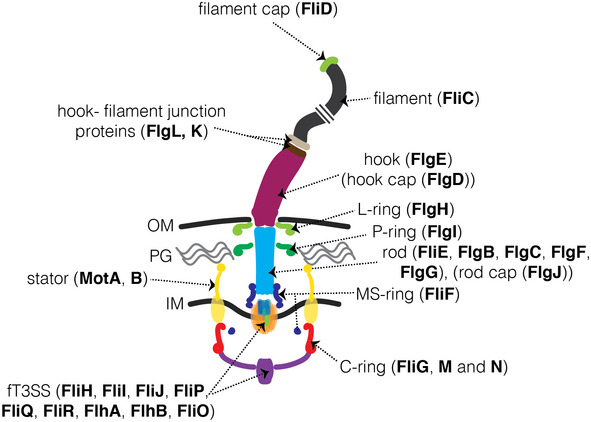
A schematic representation of the E. coli flagellum highlighting its major parts and their constituent proteins. The same color code is used in all main and supplemental figures, except Appendix Fig S3.
Our current understanding of how this structure assembles is based primarily on the popular model organisms Escherichia coli and Salmonella enterica (both Gammaproteobacteria). Flagellar biogenesis is believed to proceed in an inside‐out fashion (Fig 1), beginning with the assembly of a part of the flagellar type III secretion system (fT3SS) known as the core complex (fT3SScc), an integral inner membrane (IM) complex consisting of five proteins (FliP, FliQ, FliR, FlhB, and FlhA) (Abrusci et al, 2013). A sixth protein, FliO, is believed to be required for the assembly of the fT3SScc but does not form part of the complex (Fabiani et al, 2017; Fukumura et al, 2017). The assembly of the fT3SScc initiates with FliP, which forms a pentameric platform on which FliQ, FliR, and FlhB assemble to create a FliP5FliQ4FliR1FlhB1 subcomplex upon which a nonameric FlhA ring is built (Fabiani et al, 2017; Kuhlen et al, 2018; Minamino et al, 2019; Milne‐Davies et al, 2021). The C‐terminal domains of both FlhA and FlhB remain in the cytoplasm (Fig 1, top).
Figure 1. Current understanding of flagellar assembly based on the canonical systems of Escherichia coli and Salmonella enterica .
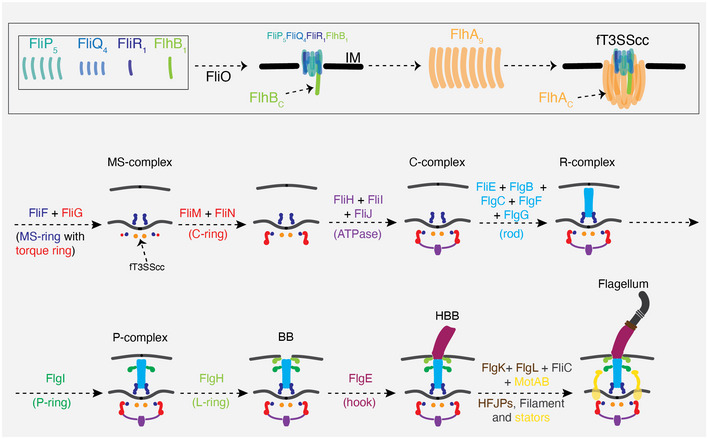
A schematic summary of our current understanding of how E. coli and S. enterica assemble the fT3SScc (top) and flagellum and the various flagellar complexes formed during this process. See also (Jones & Macnab, 1990; Kubori et al, 1992; Macnab, 2003; Li & Sourjik, 2011; Fabiani et al, 2017). The color code for the different parts of the flagellum is used in all subsequent schematics (except Appendix Fig S3), and labels are shown only in some schematics to avoid redundancy. HFJPs = hook‐filament junction proteins.
Upon formation of the fT3SScc, the MS‐ring (FliF) and C‐ring (FliG, FliM, and FliN) assemble, followed by the cytoplasmic fT3SS ATPase (FliH, FliI, and FliJ) inside the C‐ring. Biogenesis of the fT3SS promotes secretion of additional periplasmic and extracellular components across the IM including the flagellar rod (FliE, FlgB, FlgC, FlgF, and FlgG), hook (FlgE), hook‐filament junction proteins (FlgK and FlgL), filament capping protein (FliD), and filament subunits (FliC) (Macnab, 2003) (Fig 1, bottom). The periplasmic P‐ring (FlgI) and L‐ring (FlgH) which assemble around the rod are secreted through the IM via the Sec pathway (Macnab, 2003). Additional chaperones and capping proteins assist in the assembly process. While all known motors share this conserved core, various embellishments are found in different species, highlighting the continuous evolution of this nanomachine (Chen et al, 2011; Zhao et al, 2014; Qin et al, 2017; Chaban et al, 2018; Kaplan et al, 2019a).
The C‐ring, MS‐ring, rod, hook, and filament make up the rotor of the motor. Torque is generated by a ring of IM‐embedded ion channels known as stators which translate the flux of ions into rotation of the C‐ring (also called the torque ring) through interaction with FliG (Chang et al, 2020; Deme et al, 2020; Santiveri et al, 2020). While the majority of motors use H+‐ or Na+‐dependent stators, others can use different cations (Terahara et al, 2012; Ito & Takahashi, 2017). In addition, stators have also been discovered that can use either Na+ or H+ in a pH‐dependent manner, as well as motors with dual stator systems (Terahara et al, 2008; Thormann & Paulick, 2010). The motors of E. coli and S. enterica have dynamic stators that continually associate and disassociate from the rotor in response to the external environment (Lele et al, 2013). The motors of some other species generate higher torque with wider, more highly‐occupied stator rings stabilized by periplasmic scaffolds and chaperones that directly interact with the stators to maintain their integrity (Beeby et al, 2016; Kaplan et al, 2019a; Ribardo et al, 2019).
The assembly of the flagellum is believed to proceed sequentially, with the addition of each component building upon the previous one (Li & Sourjik, 2011). Additional mechanisms evolved to control the lengths of the different sections of the flagellum. For example, the periplasmic rod (driveshaft) is believed to grow until it hits the outer membrane (OM) (Cohen et al, 2017). FliK is thought to limit the length of the extracellular hook to ~ 50–55 nm (Erhardt et al, 2011; Kodera et al, 2015; Guse et al, 2020). Different models have been proposed for the assembly of the extracellular filament. First, it was proposed that the secreted subunits interact head‐to‐tail inside the channel of the flagellum and that the folding of subunits at the tip of the growing filament provides a pulling force on the head‐to‐tail chain (Evans et al, 2013). A more recent model suggests that filament assembly is instead governed by an injection‐diffusion mechanism in which the growth kinetics decrease quadratically, thus providing an explanation for why growth does not continue indefinitely (Chen et al, 2017; Hughes, 2017; Renault et al, 2017). For a recent review see (Renault et al, 2019).
Our current understanding of flagellar assembly comes mainly from biochemistry and negative‐staining electron microscopy of purified motors, and lower‐resolution light microscopy of cells (Jones & Macnab, 1990; Kubori et al, 1992; Li & Sourjik, 2011). Higher‐resolution electron cryo‐tomography (cryo‐ET) of native motors inside intact cells captured late assembly stages in Borrelia burgdorferi (Zhao et al, 2013) and non‐canonical Gammaproteobacteria (Kaplan et al, 2019b), but images of the flagellar assembly process from its earliest stages (the fT3SScc and the MS‐ring) inside the cell are still lacking. Here, we investigated how flagellar motors are built in situ using cryo‐ET. We identified early flagellar intermediates that precede the formation of the C‐ring and rod and discovered that in the absence of the torque ring protein, FliG, the MS‐ring does not assemble. Moreover, we identified novel cytoplasmic rings that surround the FliG torque ring during assembly. These ring(s) are built before the C‐ring is fully assembled and persist until the stator ring assembles, but are not found in the fully assembled flagellum. By imaging mutants of candidate genes, we discovered that the fT3SScc proteins FliO and FliQ are required for the formation of the novel cytoplasmic rings.
Results
We identified flagellar motor assembly intermediates in electron cryotomograms of several Proteobacteria species: Hylemonella gracilis, Helicobacter pylori, Campylobacter jejuni, Shewanella oneidensis, Pseudomonas aeruginosa, and Pseudomonas fluorescens (Table EV1). These species have various flagellar arrangements: monotrichous, amphitrichous, and lophotrichous (see Table EV1). For convenience, we refer to flagellar intermediates by the names indicated in Fig 1. In some cases, established terms are available, such as the fT3SScc, basal body (BB), and hook‐basal‐body (HBB) complex. For the others, we named them according to either the last component to join the complex or its dominant structural feature. Thus, we have the MS‐complex, the C‐complex, the R‐complex, and the P‐complex. Table 1 summarizes the number of examples we identified for each of these complexes in each species. Note that schematics and interpretation of complexes where not enough examples were found to produce a subtomogram average are tentative. In addition to collecting new cryotomograms for this study, we also mined the Jensen lab tomography database (Ding et al, 2015; Ortega et al, 2019), which contains more than 40,000 cryotomograms of ~ 90 bacterial species collected in the course of various projects.
Table 1.
Summary of the number of examples of each flagellar intermediate identified in each species investigated in this study.
| Species | No. of tomograms | MS‐complex | C‐complex | R‐complex | P‐complex | Basal body | HBB | Motor without collar | Flagellum |
|---|---|---|---|---|---|---|---|---|---|
| Helicobacter pylori | 46 | 11 | 3 | – | – | 9 | 1 | – | 34 |
| Helicobacter pylori fliP* | 76 | 64 | 15 | – | – | – | – | – | – |
| H. pylori ∆flgS fliP* | 84 | 21 | 2 | – | – | – | – | – | – |
| Helicobacter pylori ∆fliM fliP* | 265 | 121 | – | – | – | – | – | – | – |
| Helicobacter pylori ∆fliG fliP* | 47 | – | – | – | – | – | – | – | – |
| Helicobacter pylori ∆fliO fliP* | 267 | 47 | 15 | – | – | – | – | – | – |
| Helicobacter pylori ∆fliQ fliP* | 220 | 94 | 3 | – | – | – | – | – | – |
| Helicobacter pylori ∆fliF fliP* | 455 | – | – | – | – | – | – | – | – |
| Shewanella oneidensis ∆flgH | 59 | – | – | – | 13 | – | – | – | – |
| Campylobacter jejuni ∆flhBC | 19 | 4 | 15 | – | – | – | – | – | – |
| Campylobacter jejuni ∆flhAC | 54 | 8 | 30 | – | – | – | – | – | – |
| Pseudomonas fluorescens | 32 | – | – | 2 | – | 2 | – | – | 4 |
| Hylemonella gracilis | 76 | 5 | 1 | 1 | 35 | 1 | 7 | 8 | 86 |
Novel transient cytoplasmic rings surround the FliG ring during the assembly of high‐torque motors in Beta‐ and Epsilonproteobacteria
In Hylemonella gracilis, a Betaproteobacterium whose thin cells yield high‐quality cryo‐ET images, we identified eight classes of flagellar motor intermediates corresponding to the MS‐complex, the C‐complex, the R‐complex, the P‐complex, BB, HBB, otherwise full flagella lacking the periplasmic collar characteristic of this species (see (Chen et al, 2011)), and full flagella with the periplasmic collar (Fig 2 and Movies EV1 and EV2). For many of these classes, we were able to collect enough examples for subtomogram averaging to reveal greater detail (Fig 2A and D, and F–H and Appendix Fig S1). We observed a novel ~55‐nm‐wide ring located in the cytoplasm 12 nm below the IM (green arrows in Fig 2A–F). This ring was present at the earliest stage of assembly we observed, the MS‐complex, and persisted through assembly of the hook. The ring was absent only in the two final complexes where the filament was assembled, either without the periplasmic collar surrounding the P‐ring (Fig 2G) or with the collar present and the stators assembled (the fully assembled flagellum) (Fig 2H).
Figure 2. The flagellar assembly pathway of the Betaproteobacterium Hylemonella gracilis .
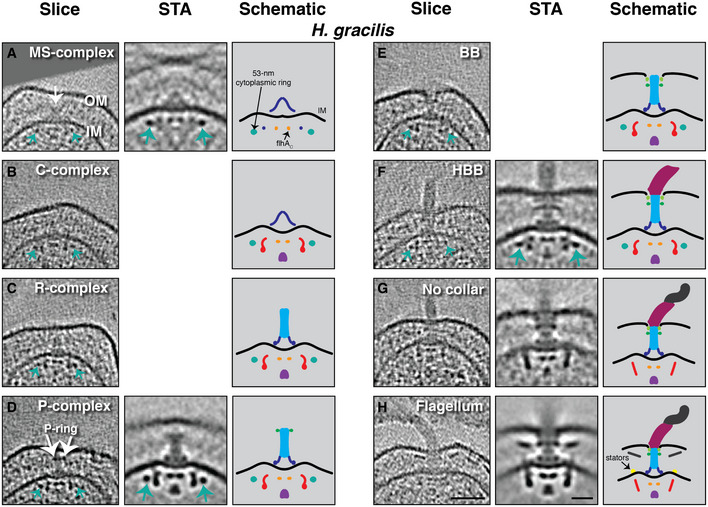
-
A–HSlices through electron cryotomograms of H. gracilis cells (left panels), central slices through subtomogram averages (STA, middle panels), and schematic representations (right panels) representing the various assembly stages observed. White arrow in (A) points to the MS‐complex; green arrows point to the novel 55‐nm‐wide cytoplasmic ring. Scale bars in (H) are 50 nm for the cryotomogram slice and 20 nm for the STA and apply to all panels. Empty panels represent stages where not enough particles were found to produce a STA. OM, outer membrane; IM, inner membrane.
The position of the P‐ring in P‐complexes was variable (Fig EV2), which explains why the P‐ring was not as well‐resolved in the subtomogram average of this complex (Fig 2D). We noted that the OM of H. gracilis frequently undulates, with the distance between the OM and IM varying significantly around the cell (Fig EV3). Both S. enterica and E. coli tether their OM to the PG with a protein known as Braun’s lipoprotein (Lpp) (Miller & Salama, 2018). The H. gracilis genome lacks an Lpp homolog, raising the question of how this bacterium controls its periplasmic space and whether this is related to the variable location of the P‐ring. It has recently been shown that some β‐barrel proteins can perform the same function as Lpp in some Gram‐negative bacteria (Godessart et al, 2020; Sandoz et al, 2020).
Figure EV2. The variable position of the P‐ring in Hylemonella gracilis P‐complexes.
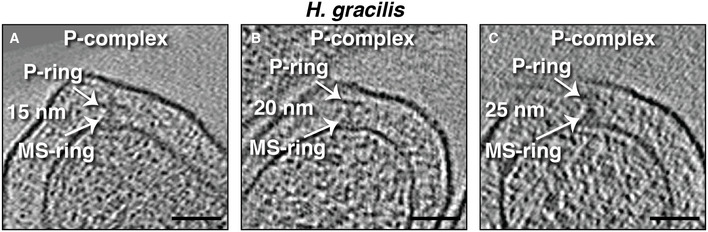
-
A–CSlices through electron cryotomograms of H. gracilis showing P‐complexes. The variable distance between the MS‐ and P‐rings is indicated (15, 20 and 25 nm, respectively). Scale bars are 50 nm.
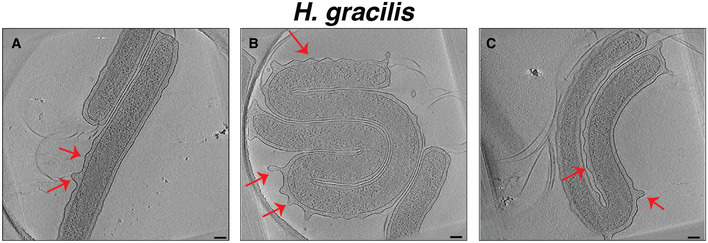
Figure EV3. The undulating outer membrane of Hylemonella gracilis .
-
A–CSlices through electron cryotomograms of H. gracilis highlighting undulating OM (indicated by red arrows). Scale bars are 100 nm.
We next examined other high‐torque motors, in the Epsilonproteobacteria Helicobacter pylori and Campylobacter jejuni (Fig EV4). In cryotomograms of H. pylori, in addition to fully assembled flagella, we identified the MS‐complex, the C‐complex, the BB, and the HBB (Fig 3A–D). Interestingly, the MS‐complex contained two cytoplasmic rings (one electron‐dense and one weaker) surrounding the FlhAC densities close to the IM, as well as two additional larger cytoplasmic rings with diameters of 58 nm and 75 nm, located 17 nm below the IM (Fig 3A, dark and light green rings). These lower rings were not present at the corresponding position in fully assembled motors (Qin et al, 2017) (Fig EV4), or in the BB complex. They were also not readily apparent in cryotomograms of the C‐complex, although we did not find enough examples of the complex for subtomogram averaging to definitively confirm this. In fully assembled H. pylori flagella, a periplasmic structure called the cage surrounds the rod and the MS‐ring and stabilizes the stator ring (Qin et al, 2017). In individual particles, we observed a density that may correspond to the lower part of this cage in the C‐complex, suggesting that it is assembled before the rest of the basal body (Fig 3B and Movie EV3).
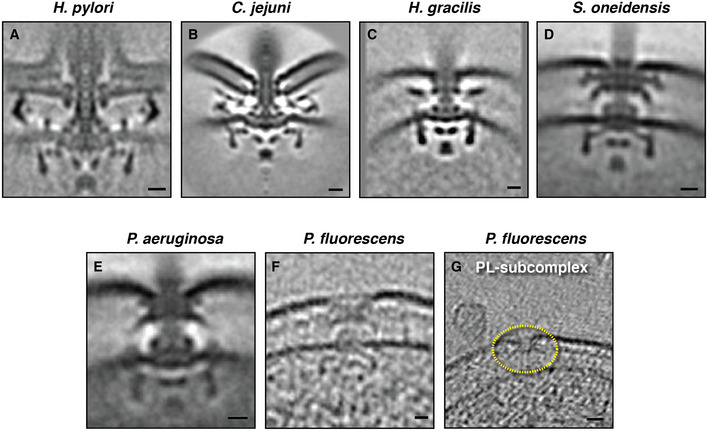
Figure EV4. The flagellar motors of the species investigated in this study.
-
A–ECentral slices through the STAs of fully assembled high‐torque motors in species in which we identified novel cytoplasmic rings interacting with the FliG ring during assembly. The H. pylori motor is from (Qin et al, 2017), C. jejuni motor from (Beeby et al, 2016), S. oneidensis and P. aeruginosa motors from (Kaplan et al, 2019a).
-
F, GSlices through electron cryotomograms of P. fluorescens highlighting the presence of a basal body (F) and a disassembly PL‐subcomplex (G, yellow dotted circle), showing the similarity of the motor of this species to that of P. aeruginosa.
Data information: Scale bars (A–F) 10 nm, (G) 20 nm.
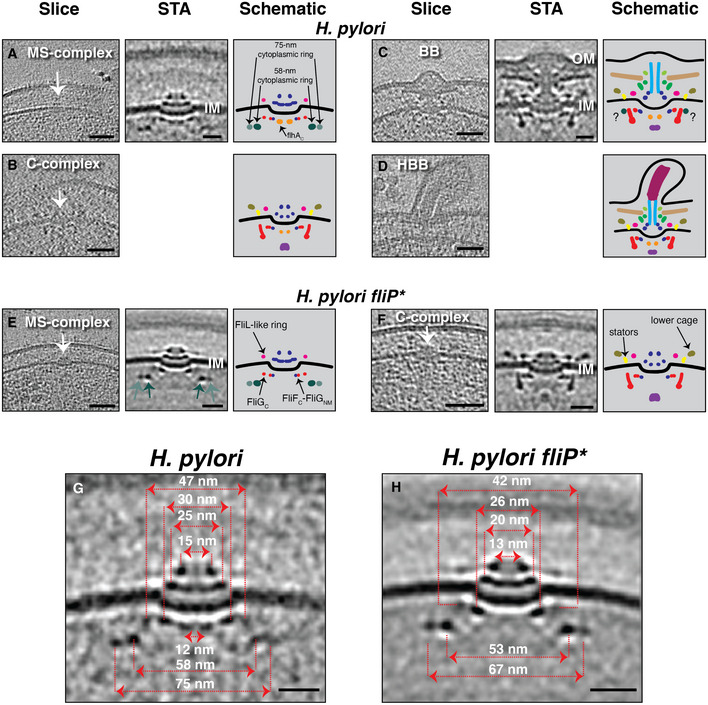
Figure 3. Flagellar assembly intermediates in Helicobacter pylori .
-
A–DSlices through electron cryotomograms of H. pylori (left panels) indicating the presence of various flagellar intermediates. Middle panels show central slices through STAs. Right panels show schematic representations. Question marks in the schematic in (C) indicate that it is unclear whether the densities surrounding the upper part of the C‐ring are the same as the novel cytoplasmic rings seen in the MS‐complex. Empty panels indicate that there were not enough examples to produce a STA.
-
E, FCryotomogram slices, STAs, and schematics of H. pylori fliP* cells showing the MS‐complex (E) and the C‐complex (F). The two novel cytoplasmic rings are highlighted with light and dark green arrows in the STA panel. White arrows in (A‐F) point to the particles.
-
G, HCentral slices through the STAs of the MS‐complexes of the motile H. pylori (G) and H. pylori fliP* mutant (H) with the diameters of their various constituent rings indicated.
Data information: Scale bars are 50 nm for cryotomogram slices and 20 nm for STAs.
In a non‐motile strain of H. pylori which does not produce FliP due to a naturally occurring point mutation (Chang et al, 2018) (henceforth referred to as fliP*), we identified many MS‐ and C‐complexes (Fig 3E and F and Appendix Fig S2). Subtomogram averaging revealed that these complexes are similar to those in motile cells (Fig 3A–D) but lack the FlhAC densities (Fig 3E and F). A ring surrounding the MS‐ring, previously putatively attributed to FliL in the fully assembled motor (Qin et al, 2017), was seen associated with the MS‐complex (Fig 3E). In addition, the entire MS‐complex in the fliP* strain was narrower than in motile cells (Fig 3G and H). For example, the two lower cytoplasmic rings in the fliP* strain had diameters of 53 and 67 nm, respectively, compared to diameters of 58 and 75 nm in motile cells. A subtomogram average of the C‐complex in the fliP* strain confirmed that the lower cage and the stator ring are assembled at this stage and that the novel cytoplasmic rings are no longer present, or at least not in the same location they occupy in the MS‐complex (Fig 3F).
The improved quality of the subtomogram average of the MS‐complex in the fliP* strain allowed us to tentatively assign densities to FliF and FliG (Fig EV5) and compare our structure to available high‐resolution structures of the purified MS‐ring (Johnson et al, 2020; Kawamoto et al, 2021). Manually fitting the high‐resolution structure of the purified MS‐ring from S. enterica (PDB 6SCN) (Johnson et al, 2020) into the average allowed us to provisionally assign densities to different periplasmic domains including the drive‐shaft‐housing ring (the Beta collar), the C‐ring template (the ring building motif 3 (RBM3) ring), and the RBM2+RBM1 rings. Additionally, fitting the crystal structure of FliG (which is known to interact with FliF) from Aquifex aeolicus (PDB 3HJL) (Lee et al, 2010) suggested that the dense ring directly surrounding the (here absent) FlhAC densities probably contains the C terminus of FliF (FliFC) and the N‐ and middle domains of FliG (FliGMN), while the peripheral ring contains the C terminus of FliG (FliGC) (Fig EV5).
Figure EV5. Tentative component assignment to various MS‐complex rings in Helicobacter pylori .
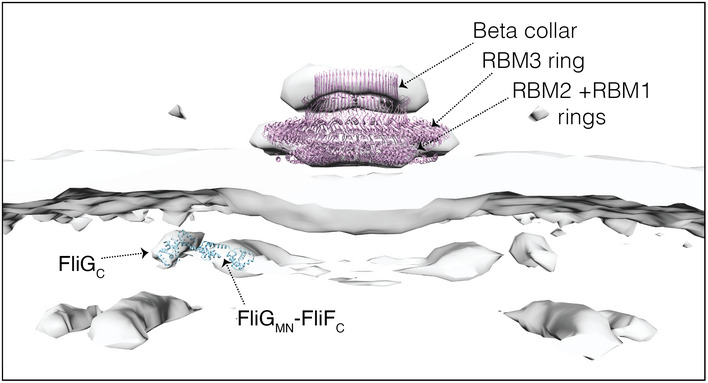
Surface rendering of the MS‐complex of H. pylori fliP* with the crystal structures of Aquifex aeolicus FliG (PDB 3HJL) and S. enterica MS‐ring (PDB 6SCN) docked inside it. RBM, ring‐building motif.
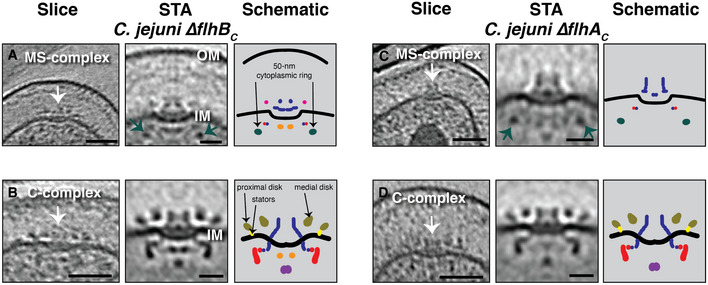
We also examined cryotomograms of another Epsilonproteobacterium, Campylobacter jejuni (see (Abrusci et al, 2013; Beeby et al, 2016)). In wild‐type C. jejuni cells, we found only fully assembled flagella. In mutants lacking the C‐terminal domain of either FlhA (ΔflhAC ) or FlhB (ΔflhBC ), however, which do not assemble full flagella, we identified MS‐ and C‐complexes (Fig 4). In the ΔflhBc strain, the MS‐complex comprised the MS‐ring, putative FliG densities, the fT3SScc (as indicated by the presence of the cytoplasmic FlhAC densities), and, notably, a ~ 50‐nm‐wide cytoplasmic ring located ~ 17 nm below the IM (as in H. pylori) (Fig 4A). The same 50‐nm ring was also seen in ΔflhAC MS‐complexes, which of course lack the cytoplasmic FlhAC densities (Fig 4C). Neither mutant retained the 50 nm cytoplasmic ring in the C‐complex (Fig 4B and D). Fully assembled flagella of C. jejuni have two concentric periplasmic discs which assist the assembly of the stator ring (Beeby et al, 2016). Similar to the lower portion of the cage in H. pylori, we observed these discs in the C‐complex of both ΔflhBc and ΔflhAC cells (Fig 4B and D).
Figure 4. Flagellar intermediates in Campylobacter jejuni mutants.
-
A–DSlices through electron cryotomograms (left panels), central slices through STAs (middle panels), and schematic representations (right panels), of C. jejuni ΔflhBC and ΔflhAC illustrating different flagellar intermediates (highlighted by white arrows). Green arrows in (A) and (C) point to the ~ 50‐nm cytoplasmic ring associated with the MS‐complex. Scale bars are 50 nm for cryotomogram slices, 20 nm for STAs.
To summarize, we found unexpected cytoplasmic rings encircling the FliG ring in high‐torque motors of Betaproteobacteria (H. gracilis) and Epsilonproteobacteria (H. pylori and C. jejuni). These rings assemble very early, associating with the MS‐complex before the C‐ring is built and then disassemble (or at least do not stay in their original positions) upon addition of the stators and their stabilizing periplasmic scaffolds (assembly stages corresponding to the C‐complex in Epsilonproteobacteria and the full flagellum in H. gracilis).
The novel rings are dependent on components of the flagellar type III secretion system
In an attempt to identify the protein(s) forming these unexpected cytoplasmic rings, we produced and imaged various mutants in the H. pylori fliP* background. We chose this strain because: (i) H. pylori has multiple polar flagella (up to six per cell); hence, there is a higher chance of identifying flagellar intermediates compared to species with a single flagellum at each pole like C. jejuni; (ii) the process of generating mutants is better‐established in H. pylori than in some other species like H. gracilis; and (iii) since fliP* cells do not proceed to build full flagella, early flagellar assembly stages (like the MS‐complex where the novel rings are clearest) are abundant. We generated the following mutants in H. pylori fliP*: ΔflgS, ΔfliM, ΔfliG, ΔfliF, ΔfliO, and ΔfliQ. Despite multiple attempts, we were unable to produce a ΔfliR mutant.
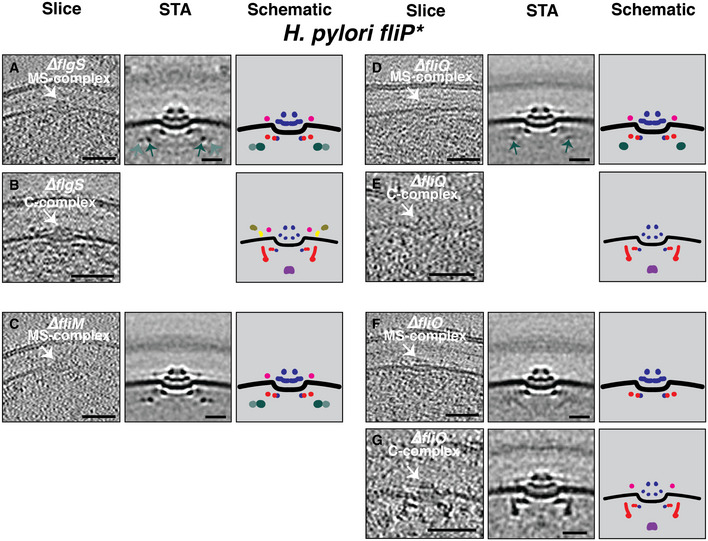
In contrast to Gammaproteobacteria in which all of the basal body and hook genes belong to the same transcriptional class (Chilcott & Hughes, 2000), in Epsilonproteobacteria genes encoding the basal body are expressed from a hierarchy of two classes, regulated by the cytoplasmic proteins FlgS and FlgR (Gilbreath et al, 2011; Lertsethtakarn et al, 2011) (Appendix Fig S3). FlgS, a sensor kinase that is auto‐phosphorylated upon assembly of the fT3SS and the MS‐ and C‐rings (Joslin & Hendrixson, 2009; Lertsethtakarn et al, 2011) (Appendix Fig S3), is required for phosphorylation of FlgR and activation of σ54 and σ28, resulting in expression of class II and class III genes, respectively (Lertsethtakarn et al, 2011). We hypothesized that at least one of the novel cytoplasmic rings might contain FlgS physically interacting with the MS‐complex. However, we saw no difference in the MS‐complexes present in the ∆flgS fliP* strain compared to fliP* (Fig 5A), nor in two individual examples of the C‐complex (Fig 5B and Movie EV4). Also, in Epsilonproteobacteria, the C‐ring protein FliM is known to assemble after and interact with, FliG, followed by FliY and then FliN (Henderson et al, 2020). We therefore imaged cells lacking FliM. Again, we saw no difference between the cytoplasmic rings in MS‐complexes of ∆fliM fliP* and fliP* strains (Fig 5C and Appendix Fig S4). As expected, no C‐complexes were observed in the ΔfliM strain. We also imaged ∆fliG fliP* and ∆fliF fliP* strains, but detected no MS‐ or C‐complexes in these two strains despite collecting 47 cryotomograms of ∆fliG fliP*, and 455 cryotomograms of ∆fliF fliP*.
Figure 5. The effect of various mutants on the MS‐ and C‐complexes of Helicobacter pylori fliP* .
-
A–GSlices through electron cryotomograms (left panels), central slices of subtomogram averages (middle panels), and schematic representations (right panels) of the MS‐ and C‐complexes (white arrows on the slices) of different mutants in H. pylori fliP*. Light and dark green arrows in (A and D) point to the ~ 53‐nm and ~ 67‐nm cytoplasmic rings. Scale bars for cryotomogram slices are 50 nm, and 20 nm for STAs. Empty panels indicate that there were not enough examples to produce a STA.
In addition to these proteins, which we reasoned were likely candidates for the novel cytoplasmic rings, we examined the effect of the two other early‐assembling fT3SScc proteins, fliQ and fliO, on these early flagellar assembly complexes. FliQ is a transmembrane protein and is one of the first fT3SScc components to assemble as part of the FliP5FliQ4FliR1 subcomplex (Minamino et al, 2019). In ∆fliQ fliP* cells, the MS‐complex contained the periplasmic putative FliL ring and the 53‐nm cytoplasmic ring, but lacked the 67‐nm ring (Fig 5D and Appendix Fig S5). Putative C‐complexes in ∆fliQ fliP* cells additionally lacked the lower part of the cage and the stators normally present at this stage (Fig 5E and Movie EV5), although not enough examples were identified to produce a subtomogram average (see Table 1). FliO is required for assembly of the fT3SScc, but is not thought to form part of the complex (Fabiani et al, 2017; Fukumura et al, 2017). Interestingly, in ∆fliO fliP* cells, both the 53‐nm and 67‐nm cytoplasmic rings were missing in the MS‐complex (Fig 5F), and the C‐complex again lacked the lower cage and the stators (Fig 5G). Nonetheless, some faint, angled densities were present in the MS‐complex in this mutant, in the location where the novel rings would otherwise appear (Fig 5F, middle panel).
These results show that without either the 67‐nm ring (∆fliQ fliP*) or both cytoplasmic rings (∆fliO fliP*), the stator ring and associated stabilizing structures (lower cage) do not assemble.
The novel rings induce a conformational change in the FliG torque ring
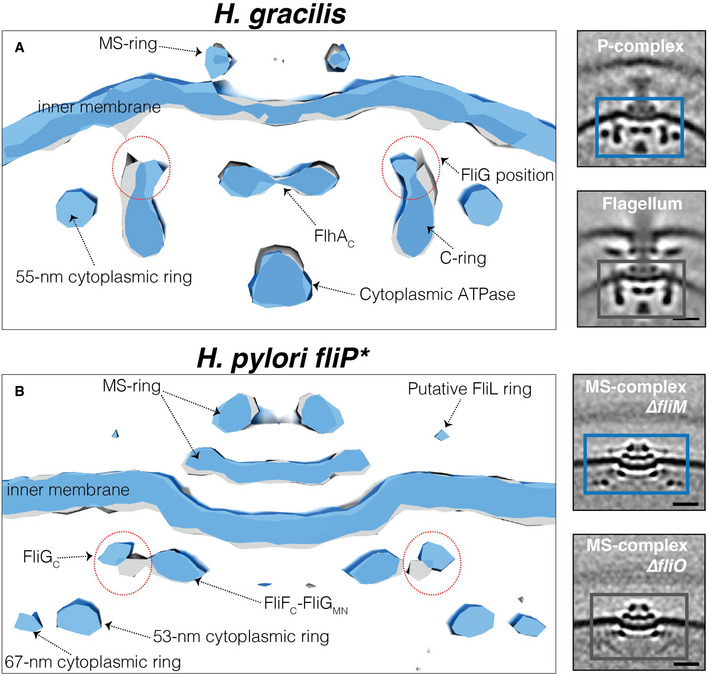
The fact that the novel cytoplasmic rings are assembled before the full C‐ring (in the MS‐complex) and are present in flagellar intermediates lacking the cytoplasmic part of the flhAC protein (in fliP* H. pylori and ΔflhAC C. jejuni cells) suggests that they interact with FliGC, which is the only other part of the MS‐complex accessible to the cytoplasm. To investigate this further, we compared our best subtomogram averages (containing the most particles) of assembly intermediates with and without the novel rings. We first compared the average of the P‐complex (with the 55‐nm ring) to that of the fully assembled flagellum of H. gracilis and observed that the upper part of the C‐ring (occupied by FliG) was pushed inward in the presence of the cytoplasmic ring (Fig 6A). We then compared the MS‐complexes of ∆fliM fliP* (with 53‐nm and 67‐nm rings present) and ∆fliO fliP* (both rings absent) H. pylori and found that the FliGC density was pushed upward (closer to the IM) and outward in the presence of the rings (Fig 6B). These results indicate that the novel cytoplasmic rings interact with and alter the position of, FliGC.
Figure 6. The novel cytoplasmic rings affect the position of the torque ring protein FliG.
-
A, BSurface renderings of the motor of the fully assembled flagellum (grey) and the P‐complex (blue) of H. gracilis (A), and the MS‐complex of ΔfliM ΔfliP* (blue) and ΔfliO ΔfliP* in H. pylori (grey) (B). Boxed areas in the STAs shown on the right represent the surface‐rendered areas shown on the left. Red dotted circles in the left panels highlight the different orientations of the FliG protein in these structures. Scale bar is 20 nm.
The novel rings are also present in Gammaproteobacteria motors with a stabilized stator ring
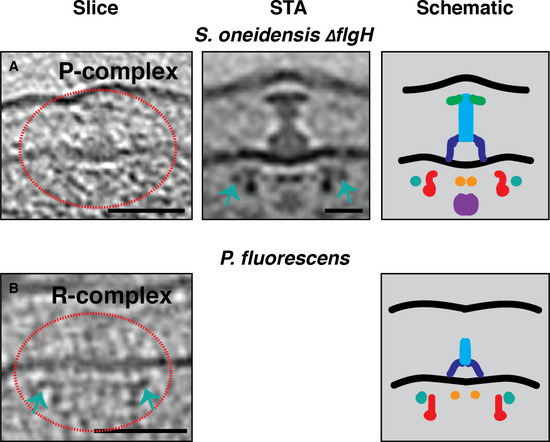
We next investigated whether the novel cytoplasmic rings are present in flagellar motors of other classes of bacteria with a stable stator ring. We first examined the motor of Shewanella oneidensis, which as in Vibrio species, is characterized by periplasmic structures (the H‐ and T‐rings) that stabilize the stator ring (Kaplan et al, 2019a) (Fig EV4D). In cryotomograms of wild‐type cells, we observed abundant fully assembled flagella, but few intermediates. This could be due either to the growth conditions used or to the fact that HBB genes are clustered in a single transcriptional class, perhaps rendering their assembly faster than in Epsilonproteobacteria motors. To arrest the process, we imaged S. oneidensis ΔflgH which lacks the L‐ring protein FlgH and halts assembly at the P‐complex. In electron cryotomograms of this strain, we observed that the P‐complex showed a clear ~ 55‐nm cytoplasmic ring surrounding the upper part of the C‐ring occupied by FliG (Fig 7A).
Figure 7. The novel cytoplasmic rings are associated with the assembly of Gammaproteobacteria motors with a stabilized stator ring.
-
A, BSlices through electron cryotomograms (left panels), central slices through STAs (middle panels), and schematic representations (right panels) of S. oneidensis ΔflgH and P. fluorescens illustrating the presence of the ~ 55‐nm cytoplasmic rings during the assembly of these motors (green arrows). Red dotted circles highlight the complexes in the cryotomograms slices. Scale bars are 50 nm for cryotomogram slices, 20 nm for the STA.
We next explored Pseudomonas spp. While our cryotomograms of wild‐type P. aeruginosa contained predominantly fully assembled flagella, previous work from another group reported the structure of the C‐complex (Zhu et al, 2019). Examining that structure, we noticed an ~ 56–57‐nm cytoplasmic ring surrounding the FliG densities in the C‐ring (Appendix Fig S6). No comment was made about this ring in the publication. In cryotomograms from the Jensen lab database of Pseudomonas fluorescens, which has a motor similar to that of P. aeruginosa (Fig EV4E–G), we also found a ~ 55‐nm cytoplasmic ring in the R‐complex (Fig 7B).
Together, these results indicate that Gammaproteobacteria motors with a stabilized stator ring also contain an ~ 55‐nm cytoplasmic ring surrounding the FliG ring during their assembly, suggesting a common regulatory mechanism for high‐torque motors that contain periplasmic scaffolds stabilizing their stator rings. We summarize our findings from Beta‐, Gamma‐, and Epsilonproteobacteria in Fig 8.
Figure 8. Model of how high‐torque motors are built based on in situ cryo‐ET data.
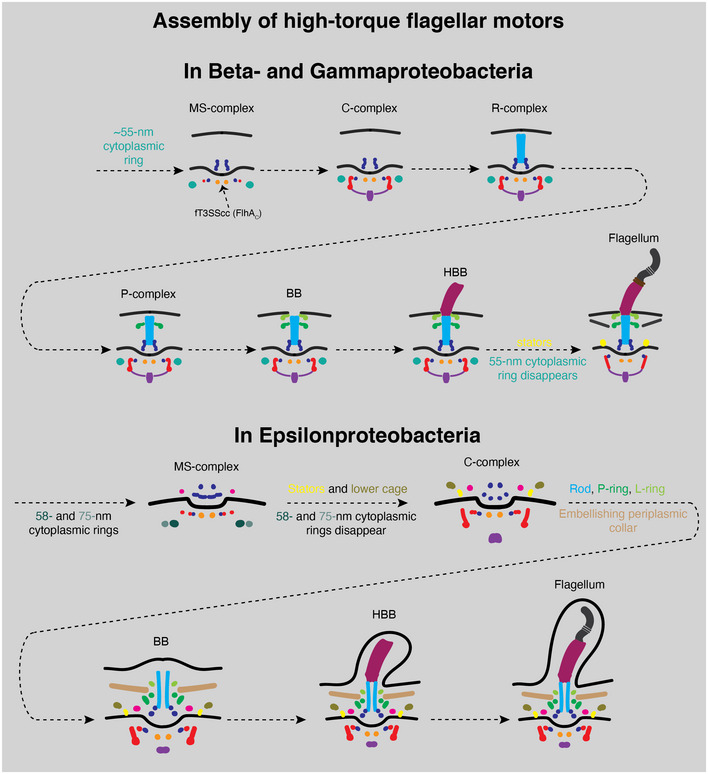
A schematic summary of our cryo‐ET imaging results illustrating how the high‐torque motors of Beta‐, Gamma‐, (top) and Epsilonproteobacteria (bottom) are assembled.
Discussion
Here, we imaged flagellar assembly in situ using cryo‐ET in diverse bacterial species with high‐torque motors (i.e., species that use periplasmic scaffolds to stabilize the stator ring). We discovered cytoplasmic rings encircling the FliG torque ring that persists until the stators and their stabilizing structures are assembled. These novel rings likely interact with FliG, as its conformation is changed in their presence. We identified these cytoplasmic rings in H. gracilis, H. pylori, C. jejuni, P. aeruginosa, P. fluorescens, and S. oneidensis, representing Beta‐, Gamma‐, and Epsilonproteobacteria. By imaging various mutants in the H. pylori fliP* background, we showed that the rings (at least in this species) were dependent on FliO and FliQ, with the outermost ring disappearing in a ∆fliQ fliP* mutant and both rings disappearing in a ∆fliO fliP* mutant. As both FliO and FliQ are transmembrane proteins, we postulate that these fT3SScc proteins are involved in regulating other proteins that form (or are required to form) the rings, rather than forming the rings directly. Previous biochemical studies have indicated a regulatory role for FliO in H. pylori, where it is required for the optimal expression of other flagellar genes (Tsang & Hoover, 2014). However, direct physical involvement of these proteins in the formation of the novel cytoplasmic rings cannot be totally excluded.
An important observation is that these cytoplasmic rings persisted until the stators and their stabilizing periplasmic scaffolds assembled. For example, while C. jejuni and H. pylori lacked the novel cytoplasmic rings in the C‐complex (where the stators and the stabilizing lower cage are already assembled), the rings persisted through later stages in H. gracilis and S. oneidensis, where the periplasmic scaffolds that stabilize the stators are also assembled later. Moreover, the ∆fliQ fliP* and ∆fliO fliP* H. pylori mutants, which did not assemble the novel cytoplasmic rings, were also unable to assemble the stators and the lower cage. Nonetheless, the rest of the C‐ring proteins could still assemble in these mutants, indicating that the interaction between FliG and the subsequently added C‐ring protein FliM (which interacts with a different part of FliG than the stators (Brown, 2002)) is not affected by the presence/absence of the novel cytoplasmic rings. Taken together, these observations suggest that the interaction between FliG and the surrounding cytoplasmic rings is required for the assembly of the stator ring.
It is possible that these rings lock FliG in a certain conformation that stabilizes the stators before other periplasmic scaffolds are built in high‐torque motors. Previous studies on the motor of S. enterica, which does not have a fixed stator ring but rather a variable number of stators depending on the external environment, did not report these rings (Kubori et al, 1992). This could be either because the novel rings are found only in high‐torque motors with a stable stator ring and absent in E. coli and S. enterica, or because they were lost in purification of assembly intermediates in S. enterica.
It was recently shown that the chemotaxis response regulator CheY interacts with the C‐ring of fully assembled flagella, forming an associated ring ~ 50 nm in diameter that locks the switch complex in clockwise direction by interacting with the C‐ring protein FliM (Carroll et al, 2020; Chang et al, 2020; Rossmann et al, 2020). While superficially similar, the novel rings we discovered assemble before FliM and even in its absence (in ∆fliM fliP* H. pylori), indicating that they are of a different nature.
Recently, Johnson et al (2021), Yamaguchi et al (2021), and Tan et al (2021) reported the molecular structures of the flagellar P‐ and L‐rings from S. enterica. Johnson and colleagues observed an outer ring formed by a protein called YecR surrounding the L‐ring in their structure, which they hypothesized helps to remodel the lipid bilayer around the L‐ring protein, thereby facilitating its assembly in the OM. However, such a ring was not present in the structures solved by Yamaguchi et al and Tan and colleagues. Interestingly, while Johnson et al purified their particles from a mutant that halts flagellar assembly at the BB stage (lacking the hook protein), Yamaguchi et al and Tan et al purified their particles from a mutant that proceeds to make the HBB (lacking the filament protein). Our discovery here that transient components are stably present only at certain assembly stages of the flagellar motor and disappear at later stages might explain this difference. It is plausible that the YecR ring is similarly a transient component that helps the assembly of the L‐ring in S. enterica and disassembles after the hook is built. Alternatively, of course, differences in the purification protocols used in these studies might have affected the stability of the YecR ring.
The evolutionary history of macromolecular complexes, including the bacterial flagellum, is reflected in their assembly pathways (Liu & Ochman, 2007; Ghosal et al, 2019; Beeby et al, 2020). In our cryotomograms, FliG was always associated with the MS‐ring and we failed to identify any MS‐related complexes in ΔfliG fliP* mutants, suggesting that FliG is essential for the assembly of the MS‐ring, in agreement with previous studies (Li & Sourjik, 2011; Terashima et al, 2020). On the other hand, we found that the MS‐complex and the novel cytoplasmic rings can still assemble in mutants without a fully assembled fT3SScc, simply adopting a smaller diameter. This ability of the MS‐ring to assemble independently of a fully assembled fT3SScc in these mutants raises the question of how FliF finds and assembles around the fT3SScc in wild‐type cells. Recently, it has been suggested that the absence of the fT3SScc might result in rings with lower symmetry in the closely related injectisome (Butan et al, 2019), and this notion has been used to explain the ability of FliF to form rings with different symmetries in vitro (Johnson et al, 2020). Similarly, we found that all cytoplasmic and periplasmic components of the C‐complex, including the cytoplasmic ATPase and the stators with their stabilizing scaffolds, can assemble in the absence of a functional fT3SScc in H. pylori. The independent modular assembly of these components might be a vestige of different evolutionary pasts of these proteins before they combined to produce a new hybrid complex performing a novel motility function.
Materials and Methods
Strains and growth conditions
Pseudomonas fluorescens were grown in K10 medium as detailed in (Boyd et al, 2014). H gracilis were grown as described in (Briegel et al, 2009; Chen et al, 2011; Kaplan et al, 2020) Wild‐type C. jejuni and mutants were grown as described in (Abrusci et al, 2013; Beeby et al, 2016). S. oneidensis ΔflgH were prepared as described in (Kaplan et al, 2019b) and grown aerobically in LB culture at 30°C with shaking at 175 rpm to OD600 2.4–2.8.
A motile revertant H. pylori 26695 isolate was selected by serial passage in Brucella broth supplemented with 10% heat‐inactivated fetal bovine serum at 37°C, 5% CO2 for 4 days until cultures reached an OD600 ~ 0.4. Non‐motile H. pylori fliP* mutants were propagated on TSAII blood agar plates (BD Biosciences) at 37°C, 5% CO2 for either 24 or 48 h prior to collection with a sterile cotton swab for grid preparation.
Helicobacter pylori mutants (ΔfliM fliP*, ΔfliO fliP*, ΔflgS fliP*, ΔfliG fliP*, ΔfliQ fliP*, ΔfliF fliP*) were grown directly from glycerol stocks on sheep blood agar at 37°C with 5% CO2 for 48 h. Then, the cells were either collected from the plate using a cotton swab and dissolved in PBS and spun down and plunge‐frozen directly or the cells were spread on a new plate and allowed to grow for 24 h under the same conditions before plunge‐freezing. No difference could be discerned between the two samples by cryo‐ET.
Helicobacter pylori mutagenesis
Flagellar mutants were generated in the non‐motile H. pylori 26695 background as previously described (Shaffer et al, 2011). Briefly, constructs were generated to replace the coding region of the gene of interest with an in‐frame, non‐polar kanamycin resistance cassette. The target gene and approximately 500 base pairs (bp) upstream and downstream of flanking regions were amplified and cloned into pGEM T‐Easy (Promega). This construct was used as a template for inverse PCR to remove the majority of the target gene coding region and to introduce incompatible restriction sites for directional cloning. A kanamycin resistance cassette driven by a promoter transcribed in the same direction as the endogenous operon was cloned into the ligated inverse PCR plasmid. H. pylori 26695 was transformed via natural competence, and single colonies resistant to kanamycin (12.5 μg/ml) were selected. PCR was used to verify that the kanamycin resistance cassette had inserted into the target locus in the same orientation as operon transcription.
Cryo‐ET sample preparation and imaging
R2/2 carbon‐coated 200 mesh copper Quantifoil grids (Quantifoil Micro Tools) were glow‐discharged for 60 s. Then, cells were mixed with BSA‐treated 10‐ or 20‐nm gold solution. 4 μl of this mixture was applied on the grids in a Vitrobot chamber (FEI). Subsequently, the extra fluid was blotted off using a Whatman filter paper in the Vitrobot chamber with 100% humidity and the grids were plunge‐frozen in a liquid ethane/propane mixture. Cells were then imaged using either an FEI Polara 300 keV field emission gun electron microscope (FEI company, Hillsboro, OR, USA) equipped with a Gatan image filter and K2 Summit direct electron detector in counting mode (Gatan, Pleasanton, CA, USA), or a Titan Krios 300 keV field emission gun transmission electron microscope (Thermo Fisher Scientific) equipped with a Gatan imaging filter and a K2 Summit direct detector in counting mode (Gatan). Data were collected using either the UCSF Tomography software (Zheng et al, 2007) or SerialEM (Mastronarde, 2005), with each tilt series ranging from −60° to 60° in 1°, 2° or 3° increments, and an underfocus of ~ 5–10 μm.
The majority of H. pylori mutants were imaged using the fast‐incremental single exposure (FISE) method (Chreifi et al, 2019; Eisenstein et al, 2019) with SerialEM software (Mastronarde, 2005), using a dose‐symmetric tilt scheme adapted from Hagen et al (2017), a tilt range of −60° to +60°, 3° tilt increment, target defocus of −6 µm, pixel size of 4.49 Å, frame rate of 0.05 s/frame, and a total dose of 130 e−/Å2. FISE tilt‐series were gain normalized and motion‐corrected using IMOD’s alignframes (Kremer et al, 1996).
Cumulative electron doses used for each individual tilt series are shown in Table EV1.
Image processing and subtomogram averaging
Three‐dimensional reconstructions of tilt‐series were performed either automatically through the RAPTOR pipeline used in the Jensen lab (Ding et al, 2015) or with the IMOD software package (Kremer et al, 1996). The PEET program (Nicastro, 2006) was used to produce sub‐tomogram averages with 2‐fold symmetrization along the particle Y‐axis. Surface renderings were visualized in Chimera (Pettersen et al, 2004). The number of particles that were averaged is listed in the table below:
Author contributions
Grant J Jensen: Conceptualization; Resources; Formal analysis; Funding acquisition; Writing—review and editing. Mohammed Kaplan: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Writing—original draft; Writing—review and editing. Catherine M Oikonomou: Formal analysis; Writing—review and editing. Cecily R Wood: Data curation; Methodology; Writing—review and editing. Georges Chreifi: Data curation; Writing—review and editing. Poorna Subramanian: Data curation; Writing—review and editing. Davi R Ortega: Data curation; Writing—review & editing. Yi‐Wei Chang: Data curation; Writing—review and editing. Morgan Beeby: Data curation; Writing—review and editing. Carrie Shaffer: Data curation; Funding acquisition; Methodology; Writing—review and editing.
In addition to the CRediT author contributions listed above, the contributions in detail are:
MK and GJJ designed research. MK, CRW, PS, YWC, MB, and CLS prepared samples. MK, GC, PS, YWC, and MB collected data. MK, CMO, DRO, CLS, and GJJ analyzed data. MK wrote the manuscript and all authors edited it.
Disclosure and competing interests statement
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Table EV1
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Movie EV5
Acknowledgements
This project was funded by the NIH (grant R01 AI127401 to G.J.J and NIH P20 GM130456 to C.L.S.) and a Baxter postdoctoral fellowship from Caltech to M.K. Cryo‐ET work was performed in the Beckman Institute Resource Center for Transmission Electron Microscopy at the California Institute of Technology. We are grateful to Prof. Marc Erhardt (Humboldt‐Universität zu Berlin) for critically reading an initial version of this work.
The EMBO Journal (2022) 41: e109523.
Data availability
The following subtomogram averages have been deposited in the Electron Microscopy Data Bank (https://www.ebi.ac.uk/emdb/) under the following accession codes: fully assembled flagellar motor of H. gracilis (EMD‐25702; https://www.ebi.ac.uk/emdb/EMD‐25702), the MS‐complex of H. pylori ΔfliM fliP* (EMD‐25703; http://www.ebi.ac.uk/pdbe/entry/EMD‐25703), the MS‐complex of H. pylori fliP* (EMD‐25704; http://www.ebi.ac.uk/pdbe/entry/EMD‐25704), the MS‐complex of H. pylori ΔfliQ fliP*, (EMD‐25705; http://www.ebi.ac.uk/pdbe/entry/EMD‐25705).
References
- Abrusci P, Vergara‐Irigaray M, Johnson S, Beeby MD, Hendrixson DR, Roversi P, Friede ME, Deane JE, Jensen GJ, Tang CM et al (2013) Architecture of the major component of the type III secretion system export apparatus. Nat Struct Mol Biol 20: 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeby M, Ferreira JL, Tripp P, Albers S‐V, Mitchell DR (2020) Propulsive nanomachines: the convergent evolution of archaella, flagella and cilia. FEMS Microbiol Rev 44: 253–304 [DOI] [PubMed] [Google Scholar]
- Beeby M, Ribardo DA, Brennan CA, Ruby EG, Jensen GJ, Hendrixson DR (2016) Diverse high‐torque bacterial flagellar motors assemble wider stator rings using a conserved protein scaffold. Proc Natl Acad Sci U S A 113: E1917–E1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd CD, Smith TJ, El‐Kirat‐Chatel S, Newell PD, Dufrene YF, O’Toole GA (2014) Structural features of the pseudomonas fluorescens biofilm adhesin LapA required for LapG‐dependent cleavage, biofilm formation, and cell surface localization. J Bacteriol 196: 2775–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel A, Ortega DR, Tocheva EI, Wuichet K, Li Z, Chen S, Müller A, Iancu CV, Murphy GE, Dobro MJ et al (2009) Universal architecture of bacterial chemoreceptor arrays. Proc Natl Acad Sci U S A 106: 17181–17186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PN (2002) Crystal structure of the middle and C‐terminal domains of the flagellar rotor protein FliG. The EMBO Journal 21: 3225–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butan C, Lara‐Tejero M, Li W, Liu J, Galán JE (2019) High‐resolution view of the type III secretion export apparatus in situ reveals membrane remodeling and a secretion pathway. Proc Natl Acad Sci U S A 116: 24786–24795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BL, Nishikino T, Guo W, Zhu S, Kojima S, Homma M, Liu J (2020) The flagellar motor of Vibrio alginolyticus undergoes major structural remodeling during rotational switching. eLife 9: e61446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban B, Coleman I, Beeby M (2018) Evolution of higher torque in Campylobacter‐type bacterial flagellar motors. Sci Rep 8: 97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y‐W, Shaffer CL, Rettberg LA, Ghosal D, Jensen GJ (2018) In vivo structures of the Helicobacter pylori cag Type IV secretion system. Cell Rep 23: 673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Zhang K, Carroll BL, Zhao X, Charon NW, Norris SJ, Motaleb MA, Li C, Liu J (2020) Molecular mechanism for rotational switching of the bacterial flagellar motor. Nat Struct Mol Biol 27: 1041–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Zhao Z, Yang J, Peng K, Baker MA, Bai F, Lo C‐J (2017) Length‐dependent flagellar growth of Vibrio alginolyticus revealed by real time fluorescent imaging. eLife 6: e22140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Beeby M, Murphy GE, Leadbetter JR, Hendrixson DR, Briegel A, Li Z, Shi J, Tocheva EI, Müller A et al (2011) Structural diversity of bacterial flagellar motors: Structural diversity of bacterial flagellar motors. EMBO J 30: 2972–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcott GS, Hughes KT (2000) Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar typhimurium and Escherichia coli. Microbiol Mol Biol Rev 64: 694–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chreifi G, Chen S, Metskas LA, Kaplan M, Jensen GJ (2019) Rapid tilt‐series acquisition for electron cryotomography. J Struct Biol 205: 163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EJ, Ferreira JL, Ladinsky MS, Beeby M, Hughes KT (2017) Nanoscale‐length control of the flagellar driveshaft requires hitting the tethered outer membrane. Science 356: 197–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deme JC, Johnson S, Vickery O, Aron A, Monkhouse H, Griffiths T, James RH, Berks BC, Coulton JW, Stansfeld PJ et al (2020) Structures of the stator complex that drives rotation of the bacterial flagellum. Nat Microbiol 5: 1553–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HJ, Oikonomou CM, Jensen GJ (2015) The caltech tomography database and automatic processing pipeline. J Struct Biol 192: 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein F, Danev R, Pilhofer M (2019) Improved applicability and robustness of fast cryo‐electron tomography data acquisition. J Struct Biol 208: 107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt M, Singer HM, Wee DH, Keener JP, Hughes KT (2011) An infrequent molecular ruler controls flagellar hook length in Salmonella enterica: Infrequent molecular ruler controls flagellar hook length in S. enterica . EMBO J 30: 2948–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LDB, Poulter S, Terentjev EM, Hughes C, Fraser GM (2013) A chain mechanism for flagellum growth. Nature 504: 287–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani FD, Renault TT, Peters B, Dietsche T, Gálvez EJC, Guse A, Freier K, Charpentier E, Strowig T, Franz‐Wachtel M et al (2017) A flagellum‐specific chaperone facilitates assembly of the core type III export apparatus of the bacterial flagellum. PLoS Biol 15: e2002267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura T, Makino F, Dietsche T, Kinoshita M, Kato T, Wagner S, Namba K, Imada K, Minamino T (2017) Assembly and stoichiometry of the core structure of the bacterial flagellar type III export gate complex. PLoS Biol 15: e2002281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D, Jeong KC, Chang Y‐W, Gyore J, Teng L, Gardner A, Vogel JP, Jensen GJ (2019) Molecular architecture, polar targeting and biogenesis of the Legionella Dot/Icm T4SS. Nat Microbiol 4: 1173–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbreath JJ, Cody WL, Merrell DS, Hendrixson DR (2011) Change Is Good: variations in common biological mechanisms in the epsilonproteobacterial genera Campylobacter and Helicobacter . Microbiol Mol Biol Rev 75: 84–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godessart P, Lannoy A, Dieu M, Van der Verren SE, Soumillion P, Collet J‐F, Remaut H, Renard P, De Bolle X (2020) β‐Barrels covalently link peptidoglycan and the outer membrane in the α‐proteobacterium Brucella abortus. Nat Microbiol 6, 27–33 [DOI] [PubMed] [Google Scholar]
- Guse A, Rohde M, Erhardt M (2020). Controlling minimal and maximal hook‐length of the bacterial flagellum (Microbiology). bioRxiv 10.1101/2020.03.25.007062 [PREPRINT] [DOI] [Google Scholar]
- Hagen WJH, Wan W, Briggs JAG (2017) Implementation of a cryo‐electron tomography tilt‐scheme optimized for high resolution subtomogram averaging. J Struct Biol 197: 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LD, Matthews‐Palmer TRS, Gulbronson CJ, Ribardo DA, Beeby M, Hendrixson DR (2020) Diversification of Campylobacter jejuni flagellar C‐ring composition impacts its structure and function in motility, flagellar assembly, and cellular processes. MBio 11, e02286‐19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KT (2017) Flagellum length control: how long is long enough? Curr Biol 27: R413–R415 [DOI] [PubMed] [Google Scholar]
- Ito M, Takahashi Y (2017) Nonconventional cation‐coupled flagellar motors derived from the alkaliphilic Bacillus and Paenibacillus species. Extremophiles 21: 3–14 [DOI] [PubMed] [Google Scholar]
- Johnson S, Fong YH, Deme JC, Furlong EJ, Kuhlen L, Lea SM (2020) Symmetry mismatch in the MS‐ring of the bacterial flagellar rotor explains the structural coordination of secretion and rotation. Nat Microbiol 5: 966–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Furlong EJ, Deme JC, Nord AL, Caesar JJE, Chevance FFV, Berry RM, Hughes KT, Lea SM (2021) Molecular structure of the intact bacterial flagellar basal body. Nat Microbiol 6: 712–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CJ, Macnab RM (1990) Flagellar assembly in Salmonella typhimurium: analysis with temperature‐sensitive mutants. J Bacteriol 172: 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslin SN, Hendrixson DR (2009) Activation of the Campylobacter jejuni FlgSR two‐component system is linked to the Flagellar export apparatus. J Bacteriol 191: 2656–2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M, Ghosal D, Subramanian P, Oikonomou CM, Kjaer A, Pirbadian S, Ortega DR, Briegel A, El‐Naggar MY, Jensen GJ (2019a) The presence and absence of periplasmic rings in bacterial flagellar motors correlates with stator type. eLife 8: e43487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M, Subramanian P, Ghosal D, Oikonomou CM, Pirbadian S, Starwalt‐Lee R, Mageswaran SK, Ortega DR, Gralnick JA, El‐Naggar MY et al (2019b). In situ imaging of the bacterial flagellar motor disassembly and assembly processes. EMBO J 38: e100957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M, Sweredoski MJ, Rodrigues JPGLM, Tocheva EI, Chang Y‐W, Ortega DR, Beeby M, Jensen GJ (2020) Bacterial flagellar motor PL‐ring disassembly subcomplexes are widespread and ancient. Proc Natl Acad Sci U S A 117: 8941–8947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto A, Miyata T, Makino F, Kinoshita M, Minamino T, Imada K, Kato T, Namba K (2021) Native flagellar MS ring is formed by 34 subunits with 23‐fold and 11‐fold subsymmetries. Nat Commun 12: 4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodera N, Uchida K, Ando T, Aizawa S‐I (2015) Two‐ball structure of the flagellar hook‐length control protein FliK as revealed by high‐speed atomic force microscopy. J Mol Biol 427: 406–414 [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR (1996) Computer visualization of three‐dimensional image data using IMOD. J Struct Biol 116: 71–76 [DOI] [PubMed] [Google Scholar]
- Kubori T, Shimamoto N, Yamaguchi S, Namba K, Aizawa S‐I (1992) Morphological pathway of flagellar assembly in Salmonella typhimurium. J Mol Biol 226: 433–446 [DOI] [PubMed] [Google Scholar]
- Kuhlen L, Abrusci P, Johnson S, Gault J, Deme J, Caesar J, Dietsche T, Mebrhatu MT, Ganief T, Macek B et al (2018) Structure of the core of the type III secretion system export apparatus. Nat Struct Mol Biol 25: 583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LK, Ginsburg MA, Crovace C, Donohoe M, Stock D (2010) Structure of the torque ring of the flagellar motor and the molecular basis for rotational switching. Nature 466: 996–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele PP, Hosu BG, Berg HC (2013) Dynamics of mechanosensing in the bacterial flagellar motor. Proc Natl Acad Sci U S A 110: 11839–11844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertsethtakarn P, Ottemann KM, Hendrixson DR (2011) Motility and Chemotaxis in Campylobacter and Helicobacter . Annu Rev Microbiol 65: 389–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Sourjik V (2011) Assembly and stability of flagellar motor in Escherichia coli: Flagellar motor assembly. Mol Microbiol 80: 886–899 [DOI] [PubMed] [Google Scholar]
- Liu R, Ochman H (2007) Stepwise formation of the bacterial flagellar system. Proc Natl Acad Sci U S A 104: 7116–7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab RM (2003) How bacteria assemble flagella. Annu Rev Microbiol 57: 77–100 [DOI] [PubMed] [Google Scholar]
- Mastronarde DN (2005) Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 152: 36–51 [DOI] [PubMed] [Google Scholar]
- Miller SI, Salama NR (2018) The gram‐negative bacterial periplasm: size matters. PLoS Biol 16: e2004935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne‐Davies B, Wimmi S, Diepold A (2021) Adaptivity and dynamics in type III secretion systems. Mol Microbiol 115: 395–411 [DOI] [PubMed] [Google Scholar]
- Minamino T, Kawamoto A, Kinoshita M, Namba K (2020) Molecular organization and assembly of the export apparatus of Flagellar Type III secretion systems. Curr Top Microbiol Immunol 427: 91–107 [DOI] [PubMed] [Google Scholar]
- Nicastro D, Schwartz C, Pierson J, Gaudette R, Porter ME, McIntosh JR (2006) The molecular architecture of axonemes revealed by cryoelectron tomography. Science 313: 944–948 [DOI] [PubMed] [Google Scholar]
- Ortega DR, Oikonomou CM, Ding HJ, Rees‐Lee P, Alexandria , Jensen GJ (2019) ETDB‐Caltech: a blockchain‐based distributed public database for electron tomography. PLoS One 14: e0215531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
- Qin Z, Lin W, Zhu S, Franco AT, Liu J (2017) Imaging the motility and chemotaxis machineries in Helicobacter pylori by cryo‐electron tomography. J Bacteriol 199: e00695‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault TT, Abraham AO, Bergmiller T, Paradis G, Rainville S, Charpentier E, Guet CC, Tu Y, Namba K, Keener JP et al (2017) Bacterial flagella grow through an injection‐diffusion mechanism. eLife 6: e23136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault TT, Guse A, Erhardt M (2019). Export mechanisms and energy transduction in type‐III secretion machines. Curr Top Microbiol Immunol 427: 143–159 [DOI] [PubMed] [Google Scholar]
- Ribardo DA, Kelley BR, Johnson JG, Hendrixson DR (2019) A chaperone for the stator units of a bacterial flagellum. MBio 10: e01732‐19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann FM, Hug I, Sangermani M, Jenal U, Beeby M (2020) In situ structure of the Caulobacter crescentus flagellar motor and visualization of binding of a CheY‐homolog. Mol Microbiol 114: 443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz KM, Moore RA, Beare PA, Patel AV, Smith RE, Bern M, Hwang H, Cooper CJ, Priola SA, Parks JM et al (2021) β‐Barrel proteins tether the outer membrane in many Gram‐negative bacteria. Nat Microbiol 61: 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiveri M, Roa‐Eguiara A, Kühne C, Wadhwa N, Hu H, Berg HC, Erhardt M, Taylor NMI (2020) Structure and function of stator units of the bacterial flagellar motor. Cell 183: 244–257.e16 [DOI] [PubMed] [Google Scholar]
- Shaffer CL, Gaddy JA, Loh JT, Johnson EM, Hill S, Hennig EE, McClain MS, McDonald WH, Cover TL (2011) Helicobacter pylori exploits a unique repertoire of type IV secretion system components for pilus assembly at the bacteria‐host cell interface. PLoS Pathog 7: e1002237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Zhang X, Wang X, Xu C, Chang S, Wu H, Wang T, Liang H, Gao H, Zhou Y et al (2021) Structural basis of assembly and torque transmission of the bacterial flagellar motor. Cell 184: 2665–2679.e19 [DOI] [PubMed] [Google Scholar]
- Terahara N, Krulwich TA, Ito M (2008) Mutations alter the sodium versus proton use of a Bacillus clausii flagellar motor and confer dual ion use on Bacillus subtilis motors. Proc Natl Acad Sci U S A 105: 14359–14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terahara N, Sano M, Ito M (2012) A bacillus flagellar motor that can use both Na+ and K+ as a coupling ion is converted by a single mutation to use only Na+. PLoS One 7: e46248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima H, Hirano K, Inoue Y, Tokano T, Kawamoto A, Kato T, Yamaguchi E, Namba K, Uchihashi T, Kojima S et al (2020) Assembly mechanism of a supramolecular MS‐ring complex to initiate bacterial flagellar biogenesis in Vibrio species. J Bacteriol 202: e00236‐20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormann KM, Paulick A (2010) Tuning the flagellar motor. Microbiology 156: 1275–1283 [DOI] [PubMed] [Google Scholar]
- Tsang J, Hoover TR (2014) Requirement of the flagellar protein export apparatus component FliO for optimal expression of flagellar genes in Helicobacter pylori . J Bacteriol 196: 2709–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Makino F, Miyata T, Minamino T, Kato T, Namba K (2021) Structure of the molecular bushing of the bacterial flagellar motor. Nat Commun 12: 4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Norris SJ, Liu J (2014) Molecular architecture of the bacterial flagellar motor in cells. Biochemistry 53: 4323–4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhang K, Boquoi T, Hu B, Motaleb MA, Miller KA, James ME, Charon NW, Manson MD, Norris SJ et al (2013) Cryoelectron tomography reveals the sequential assembly of bacterial flagella in Borrelia burgdorferi. Proc Natl Acad Sci U S A 110: 14390–14395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SQ, Keszthelyi B, Branlund E, Lyle JM, Braunfeld MB, Sedat JW, Agard DA (2007) UCSF tomography: an integrated software suite for real‐time electron microscopic tomographic data collection, alignment, and reconstruction. J Struct Biol 157: 138–147 [DOI] [PubMed] [Google Scholar]
- Zhu S, Schniederberend M, Zhitnitsky D, Jain R, Galán JE, Kazmierczak BI, Liu J (2019) In situ structures of polar and lateral flagella revealed by cryo‐electron tomography. J Bacteriol 201: e00117‐19 [DOI] [PMC free article] [PubMed] [Google Scholar]


