SUMMARY
The Northern House Mosquito Culex pipiens sensu stricto is one of the most important disease vector mosquitoes in temperate zones across the northern hemisphere, responsible for the emergence of West Nile Virus over the last two decades. It comprises two ecologically distinct forms. An aboveground form pipiens diapauses in winter and primarily bites birds, while a belowground form molestus thrives year round in subways, basements and other human-made, belowground habitats, bites mammals, and can even lay eggs without a blood meal. The two forms hybridize in some but not all places, leading to a complex ecological mosaic that complicates predictions of vectorial capacity. Moreover, the origin of the belowground molestus is contentious, with iconic populations from the London Underground subway system being held up by evolutionary biologists as a preeminent example of rapid, in situ, urban adaptation and speciation. We review the recent and historical literature on the origin and ecology of this important mosquito and its enigmatic forms. A synthesis of genetic and ecological studies spanning 100+ years clarifies a striking latitudinal gradient: behaviorally divergent and reproductively isolated forms in northern Europe gradually break down into what appear to be well-mixed, intermediate populations in North Africa. Moreover, a continuous narrative thread dating back to the original description of form molestus in Egypt in 1775 refutes the popular idea that belowground mosquitoes in London evolved in situ from their aboveground counterparts. These enigmatic mosquitoes are more likely derived from populations in the Middle East, where human-biting and other adaptations to human environments may have evolved on the timescale of millennia rather than centuries. We outline several areas for future work and discuss the implications of these patterns for public health and for our understanding of urban adaptation in the Anthropocene.
Introduction
In the winter of 1940 to 1941, the Second World War was raging and Londoners faced a sustained, eight-month, Nazi bombing campaign known as The Blitz1. During one particularly intense period from September to November, bombs were dropped for fifty-seven nights in a row. Many who had lost their homes or simply wanted a more secure place to sleep sought nightly refuge in the city’s extensive underground subway system. Tens of thousands of men, women, and children slept shoulder-to-shoulder on station platforms, escalators, and sometimes in the subway tunnels themselves1. The underground system provided life-saving protection from bombs, but the Londoners who took refuge there nevertheless found themselves attacked by a different enemy— mosquitoes. The London Underground had also become home to a belowground ecotype of the widespread, bird-biting mosquito Culex pipiens sensu stricto2. Unlike their aboveground cousins, these pesky biters were well equipped for life in man-made basements, subways, and cesspits; they were active year-round, could mate in confined spaces, often laid their first clutch of eggs without a blood meal, and voraciously attacked humans2.
The London Underground Mosquitoes, as they became known, captured the attention of the general public and scientific community alike. Evolutionary biologists hypothesized that they had evolved in situ within the one hundred year period since the construction of the London Underground and continue to cite these populations as an iconic example of rapid urban adaptation3–9. For vector biologists and public health researchers, on the other hand, the discovery merely helped confirm observations that had been trickling in since at least the 1920s of similar belowground populations in other European (and North American) cities10,11. The aboveground and belowground ecotypes were eventually named Cx. pipiens form pipiens and Cx. pipiens form molestus11–13, and hybridization between them may have contributed to the emergence of West Nile Virus in the United States and Europe over the past several decades (see Conclusions and Outlook). Today, Cx. pipiens represents the most intensively studied temperate vector across the Northern Hemisphere14,15, and while it is made up of two distinct ecotypes at colder northern latitudes in Europe, the dichotomy breaks down elsewhere—leading to a complex ecological mosaic and a rich, but often confusing, literature.
Here, we review what is known about the origin and ecology of this important mosquito and its major ecotypes, focusing in particular on their putative native range across the Western Palearctic — namely Europe, western Asia, North Africa, and the Middle East (Fig. 1). We synthesize the observations of early naturalists with more recent genetic and behavioral data to illustrate how above and belowground ecotypes in the north transition through highly variable yet still structured populations at middle latitudes to more homogenous intermediate populations in northwestern Africa. We also examine the widely cited theory that belowground mosquitoes in London and other urban areas evolved from their aboveground counterparts in situ over the last few hundred years— finding surprisingly little, if any, support for this hypothesis.
Figure 1. Cx. pipiens mosquitoes are found in temperate areas around the world.
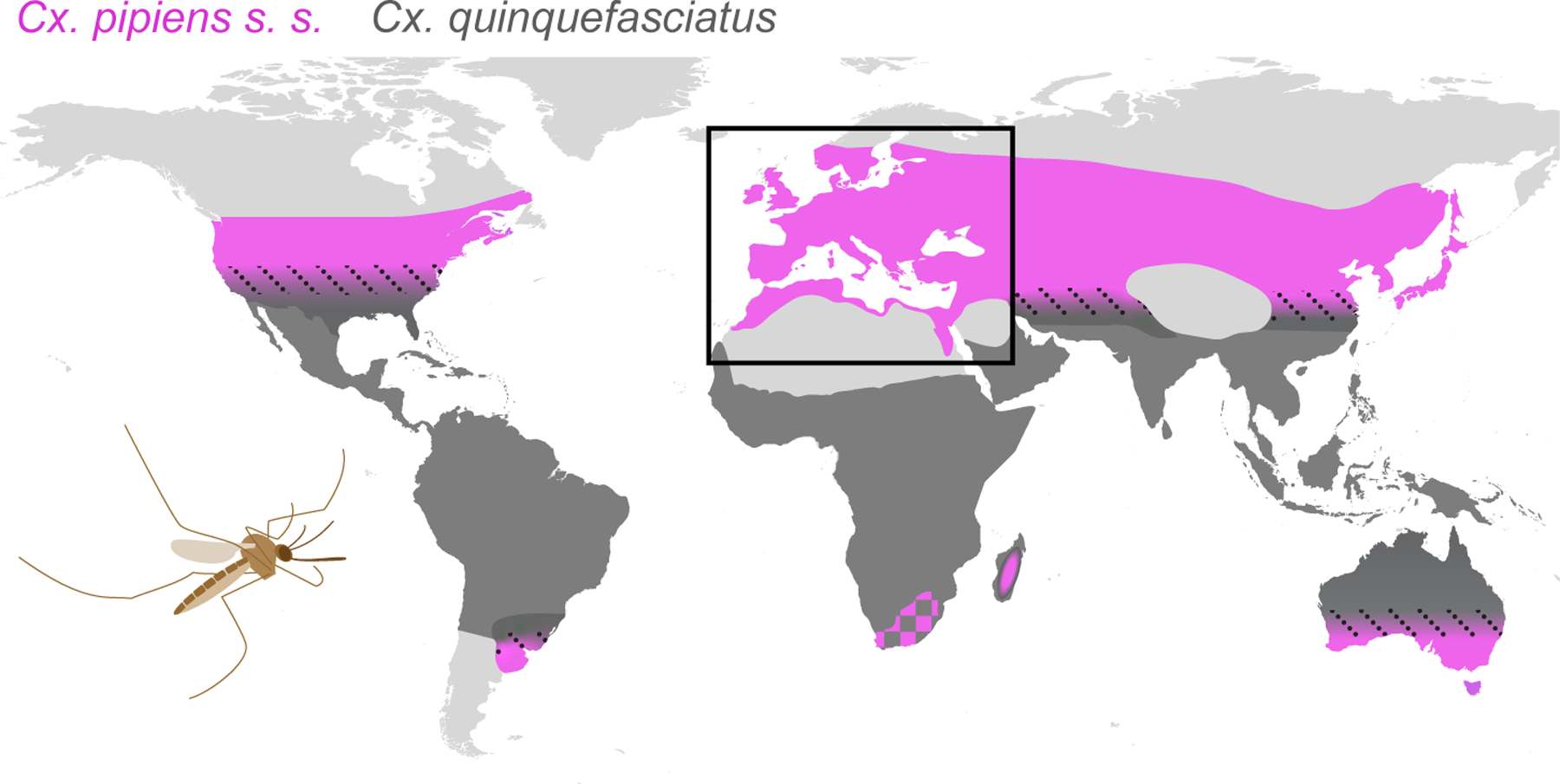
Shaded areas show the approximate global distributions of the vector mosquito Cx. pipiens sensu stricto (pink) and its tropical sibling Cx. quinquefasciatus (dark grey) (modified from 11,13,15). The two species hybridize where they come into contact (dotted black lines), except in South Africa (pink-grey checkers)97, where the local ‘Cx. pipiens’ do not interbreed with coexisting Cx. quinquefasciatus and may represent a third species55. Our review focuses on ecological diversity within Cx. pipiens across the Western Palearctic (black rectangle), where this diversity is highest and likely originated. Cx. pipiens populations in East Asia are recognized as a distinct subspecies (Cx. p. pallens), while those in the Americas and Australia are thought to have been established relatively recently15,82,84. Cx. pipiens may also occur sporadically at high elevations across tropical Africa14.
Culex pipiens comprises distinct above and belowground ecotypes in colder northern climates
The Northern House Mosquito Culex pipiens sensu stricto (hereafter Cx. pipiens) is one of the most common mosquitoes found breeding in human habitats throughout the temperate northern hemisphere (Fig. 1). In the higher latitudes of northern Europe, Asia, and North America, aboveground populations match the canonical description of Cx. pipiens form pipiens (hereafter pipiens) (Fig. 2). Like most mosquitoes, females require a blood meal to synthesize eggs, and males require open space to form mating swarms at dusk16. Females primarily bite birds14 and are therefore important vectors of pathogens that circulate within bird populations, including avian malaria and West Nile Virus17. Although larvae thrive in human sources of water, such as aboveground water tanks, irrigation ditches, and open sewers, they can also be found in natural environments14. Finally, shortening autumn days trigger a reproductive diapause, allowing mated females to survive the long, cold winter at latitudes as high as 60–65°N in Scandinavia and northern Russia14,18.
Figure 2. Cx. pipiens comprises two ecotypes in colder, northern latitudes.
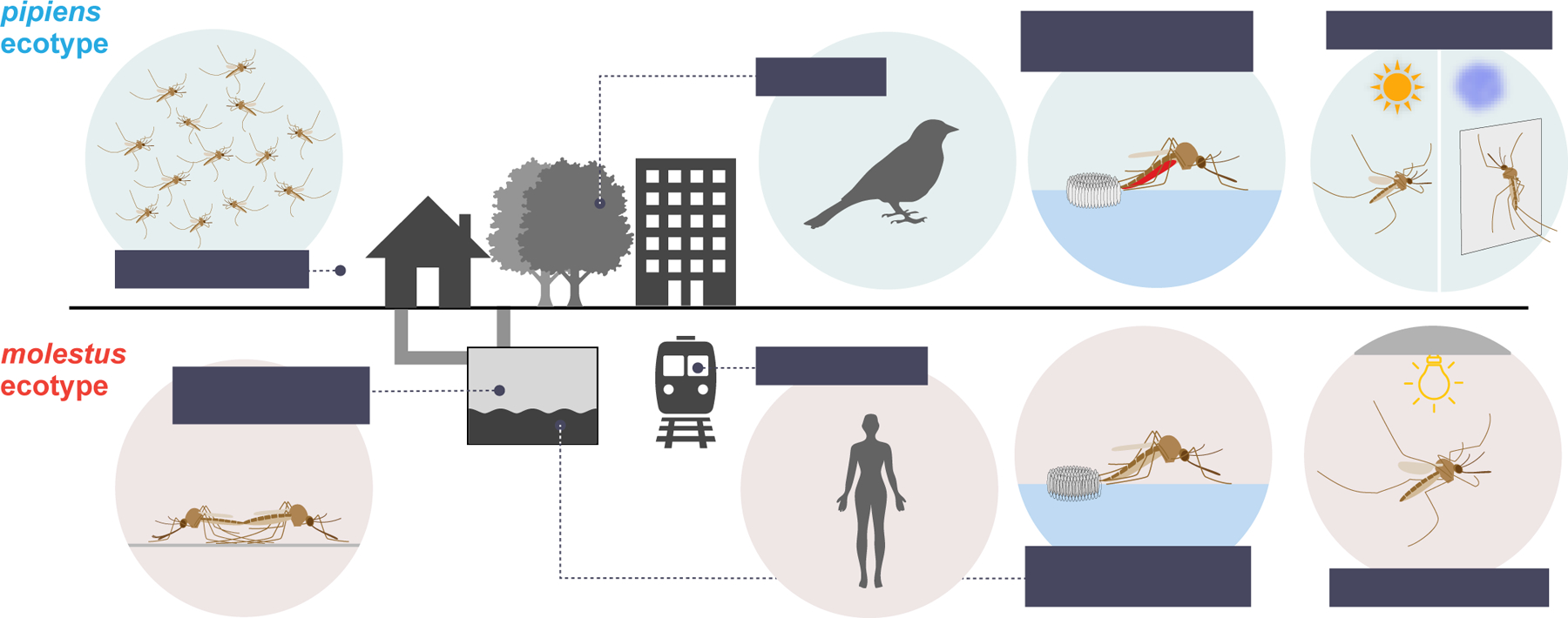
Form pipiens lives aboveground, while the form molestus is confined to man-made belowground habitats. The schematics summarize several striking behavioral and physiological differences (see references in main text).
Until the early 1900’s, all Cx. pipiens populations documented from northern Europe resembled those described above. Medical entomologists then began to make some puzzling observations—Cx. pipiens look-alikes breeding in cellars/cesspits and biting people. In 1923, P. G. Shute went to investigate a ‘mosquito nuisance’ affecting homes in the center of London and found dozens of Cx. pipiens females engorged with mammalian blood resting on the walls and ceilings of bedrooms2. He could not identify the breeding site. In 1929, E. Roubaud and P. de Boissezon independently published reports of Cx. pipiens breeding through the winter in a wine cellar near Paris19 and the boiler room of a medical dispensary in Toulouse20. Similar reports trickled in from other areas of France21, Germany22, Denmark23, the United States24, and of course the London Underground2.
The belowground mosquitoes were somewhat variable, but they clearly differed from their aboveground counterparts in four key behaviors25 (Fig. 2). First, they did not diapause in winter. Instead, they could be found breeding throughout the year in their buffered belowground habitat19. Second, they did not require open space for swarming and mating. Instead, they could mate in cages as small as 50 cubic centimeters26. Males were even observed to approach resting females27, which is unusual as mosquitoes typically initiate mating during flight16. Third, mated females were able to lay their first clutch of eggs without a bloodmeal. Roubaud called this ability ‘autogeny’19, and it allowed belowground populations to thrive without access to animal hosts. However, females were also willing to bite, and bloodmeals allowed them to lay additional, larger egg clutches. When belowground females did bite, they were not only attracted to birds but also voraciously attacked humans and other mammals. It was this last trait that earned them the name Cx. pipiens form molestus (hereafter molestus), originally coined for a human-biting form of Cx. pipiens in Egypt28.
Early authors noted subtle morphological differences between pipiens and molestus ecotypes (e.g. [10,29]), but they are considered morphologically indistinguishable today12. They are also capable of mating and producing fertile offspring in captivity10, begging the question of how they remain distinct in nature. Microhabitat segregation and selection against migrants are likely contributors. For example, long, cold winters confine the non-diapausing molestus to its buffered belowground environment30, while lack of bird hosts and open space for mating should prevent pipiens from successfully breeding belowground. As we will see, however, residual reproductive isolation appears to be present even when both are active aboveground, suggesting the presence of additional barriers.
The distinction between ecotypes breaks down further south
While populations of Cx. pipiens in northern Europe usually fit neatly into above and belowground ecotypes, it has long been clear that the situation is more complex further south. Closed belowground environments in southern Europe are still consistently occupied by mosquitoes that fit the canonical description of molestus31–33 (Fig. 2), but aboveground populations run the gamut from pipiens-like to molestus-like to many things in between. The earliest hints in the literature come from 19th century descriptions of new human-biting ‘species’ from Croatia34 and Italy35, which have since been subsumed under the Cx. pipiens umbrella13,29,36. These mosquitoes lived aboveground, but readily entered homes and were said to be far more bloodthirsty than the ‘common’ Cx. pipiens. Later, as belowground populations were being described in the north, southern populations characterized by all four key molestus behaviors were found breeding aboveground in rural areas of Greece and Malta27,29. Nevertheless, most populations from southern Europe were more pipiens-like or exhibited a mix of traits that put them somewhere in between the two northern extremes11,37,38. One strain from southern France, for example, was willing to bite humans and lacked diapause, but could lay only a few (if any) eggs without a blood meal and mated in modestly-sized, but not very small, cages38. Even further south in northwestern Africa, this type of intermediate behavior appeared to be the norm39–41.
The advent of genetic approaches such as allozymes and microsatellites corroborated the variability of Mediterranean populations31–33,42–47. Moreover, by allowing rapid assessment of many individuals, they confirmed that this variation is still partially structured by habitat. More or less pure molestus and pipiens can still often be found in canonical belowground and aboveground environments31–33,42. But gene flow is present, and many populations are genetically mixed—especially in intermediate habitats such as belowground sites with access to the open air and aboveground urban or suburban habitats31–33,42,48,49. A microsatellite study from 2004 included northern and southern European samples in the same analysis, providing some of the first holistic evidence that genetically ‘pure’ aboveground and belowground individuals prevail in the north (UK, Sweden, Germany, and northern France), while individuals with mixed ancestry are common in the south (southern France, Italy)43.
Importantly, Mediterranean populations don’t just vary from place to place or habitat to habitat. There is also widespread structure within populations. Multi-locus studies indicate that molestus-like and pipiens-like individuals can be caught at the same trapping sites with fewer than expected intermediates45,47. In Portugal, the molestus-like individuals were more common inside animal shelters and human dwellings than outdoors (another potential level of microhabitat segregation), but most samples included a mix45. The widespread detection of heterozygote deficits at single loci (e.g., [32,33]) also points to extensive structure within aboveground populations in southern Europe.
What reproductive barriers are responsible for lingering structure at lower latitudes where mild winters allow both ecotypes to thrive aboveground? A remarkable field study from 1981 points to mating behavior31. Italian researchers released tens of thousands of genetically identifiable aboveground ‘molestus’ from urban Rome in a rural area where pipiens-like mosquitoes were dominant. They then monitored the genetic make-up of mating swarms for months thereafter. Males swarming near the ground were almost always derived from the introduced molestus, while those captured two meters up near the foliage of trees were the local pipiens. Hybrids were rare, both in the swarms and at breeding sites, even three to four months after the initial release. The ability of molestus to mate in confined spaces (Fig. 2) may thus reflect a generalized change in swarming behavior that limits hybridization even in open air environments31,50–52.
Another possible source of reproductive isolation between pipiens and molestus is cytoplasmic incompatibility caused by the bacterial endosymbiont Wolbachia pipientis53,54. Almost all Cx. pipiens individuals in nature are infected with Wolbachia (but see [55]), and when a male and female carry bacteria from incompatible genetic groups, mating can result in partial or complete embryonic lethality. There are at least four genetic groups of Wolbachia segregating in Cx. pipiens in Europe and North Africa56, though not yet any evidence that they separate mosquitoes with pipiens- and molestus-like ecology. The effect of Wolbachia variation on genetic structure in natural populations remains an active area of research.
In summary, the complex patterns of variation observed within Cx. pipiens across the Mediterranean basin likely reflect a variable mosaic of selection on behavior and physiology across urban-rural, indoor-outdoor, and aboveground-belowground environments, alongside the presence of significant (but incomplete) reproductive barriers.
Compilation of genetic studies highlights a striking latitudinal gradient
The studies described above point to a latitudinal gradient across Europe and North Africa; differentiation between above and belowground populations gradually decreases from north to south, presumably because the winters become less severe and non-diapausing molestus-like mosquitoes are more and more able to survive aboveground11,49. To better illustrate this pattern, we synthesized genetic data from 214 diverse Cx. pipiens populations scattered across the region (Fig. 3, Table S1, Supplemental Methods). Our synthesis is made possible by the widespread use of a simple, PCR assay based on sequence variation flanking a microsatellite locus called CQ11. CQ11 shows more or less fixed differences between canonical pipiens and molestus populations in Europe and North America57. As a single locus, it is not reliable for classifying individual mosquitoes in places where gene flow occurs. However, a flurry of recent studies has used it for the rapid and economical diagnosis of populations as pipiens-like, molestus-like, or something in between.
Figure 3. Genetic data illustrate a latitudinal gradient from distinct northern ecotypes to intermediate southern populations.
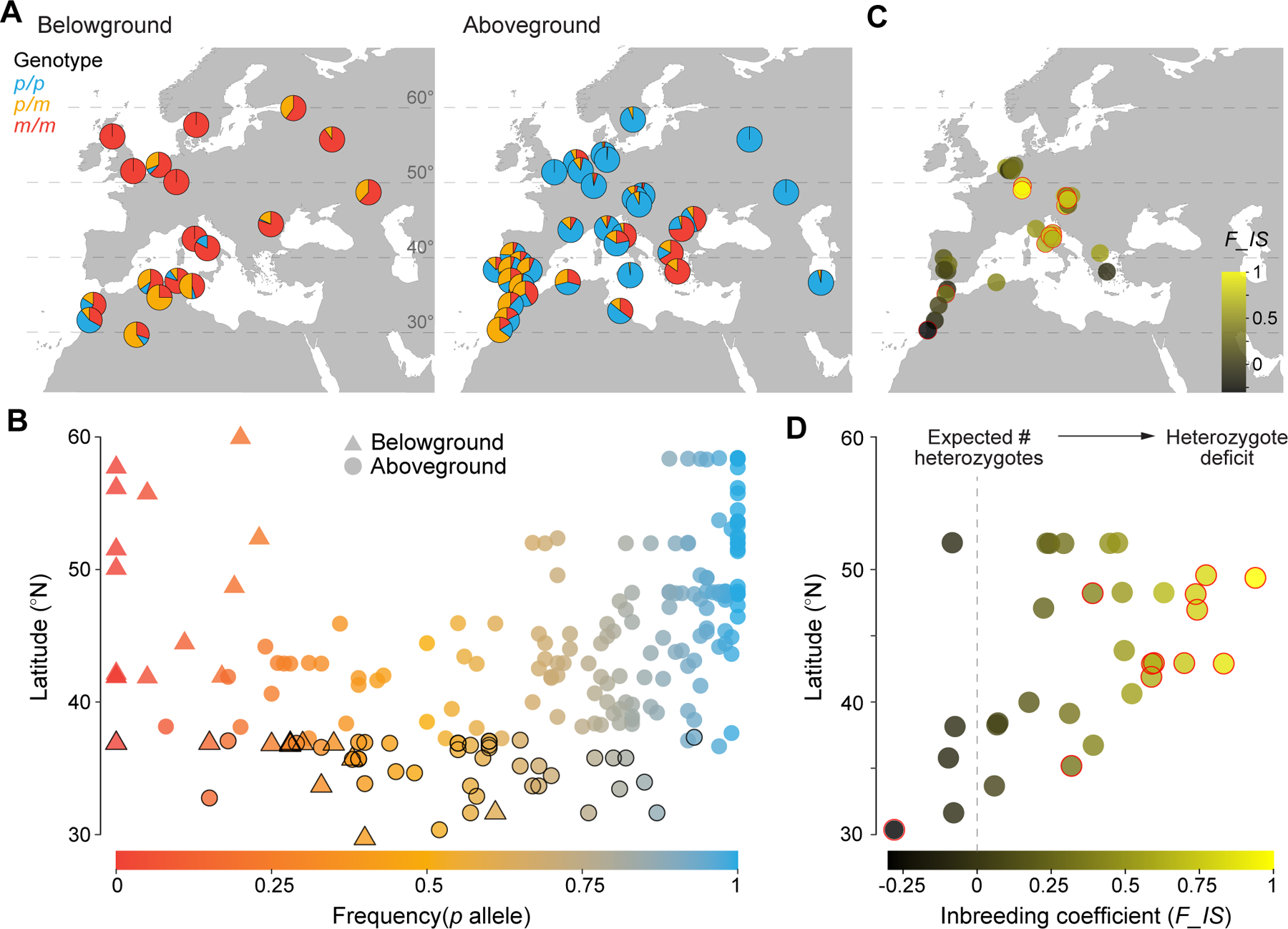
Compilation of published CQ11 genotype data for up to 214 diverse Cx. pipiens populations (see Table S1 and Supplemental Methods for details). A, Genotype frequencies for 18 belowground populations (left, N≥8 individuals each) and 35 geographically representative aboveground populations (right, N≥30 except two Russian populations N=7–12). p, pipiens allele; m, molestus allele. B, Allele frequencies for 214 populations (N≥5 individuals each). Symbols with black outline indicate North Africa. C-D, Inbreeding coefficient F_IS for 32 aboveground populations with N≥30 and minor allele frequency≥0.05. Red outlines mark significant departures from Hardy-Weinberg equilibrium, with positive and negative values indicating a heterozygote deficit and excess, respectively. Pies/dots in A and C are jittered from their exact locations for visibility. Taken together, these data suggest that genetically distinct aboveground and belowground ecotypes in northern Europe break down into highly variable, structured populations at middle latitudes and more reliably intermediate, well-mixed populations in North Africa. However, it is important to keep in mind that single-locus data may not be representative of genome-wide patterns.
Northern populations (>45°N) fall into two discrete classes, as expected, with most belowground and aboveground individuals being homozygous for the molestus and pipiens alleles, respectively (Fig. 3A and 3B). Further south, along the European coast of the Mediterranean (35–45°N), aboveground populations become extremely variable, showing nearly every possible configuration of genotype and allele frequencies (Fig. 3A and 3B). Importantly, many aboveground sites at middle latitudes harbored fewer heterozygotes than one would expect in a well mixed population at Hardy-Weinberg equilibrium (Fig. 3C and 3D). This can be seen in the uniformly positive (and often significant) inbreeding coefficients between 40°N and 50°N (Fig. 3D) and hints at the lingering reproductive isolation between aboveground pipiens- and molestus-like individuals noted in previous work.
At the lowest latitudes, along the North African coast of the Atlantic and Mediterranean, mosquitoes are more reliably intermediate (Fig. 3A and 3B). Few sampling localities yield the extreme allele frequencies characteristic of the two canonical ecotypes (Fig. 3B), and heterozygote frequencies are largely as expected for well mixed, unstructured populations (Fig. 3C and 3D). Even here, however, the molestus allele is slightly more frequent belowground (Fig. 3B).
Taken together, these patterns produce a V-shape in a plot of allele frequency by latitude, with below and aboveground populations lining up along the left and right sides of the ‘V’ (Fig. 3B). The frequency of the pipiens allele is negatively correlated with latitude belowground (N=26, Pearson’s r=−0.61, P=9.3*10−4), but positively correlated with latitude aboveground (N=188, Pearson’s r=0.56, P=2.2*10−16).
Continent-scale patterns of variation at the CQ11 locus are consistent with those seen in smaller multilocus datasets and mirror regional variation in behavior (previous section). It is nevertheless important to remember that selection acting on individual loci can render single-locus data unreliable for inferring population-scale phenomena. More work is also required to understand the connection between genotype and phenotype at the individual level. We know little about the genes underlying ecotype-specific behaviors (but see [58,59]), and while CQ11 genotypes roughly predict individual behavior in some mixed populations32,44, they are in no way prescriptive.
The contentious theory of in situ adaptation in the London Underground
As explained above, molestus populations from the London Underground became famous during WWII (Fig. 4). Early authors mused over a potential local origin (e.g., [2]), but they received little (if any) research attention over the subsequent fifty years. Then, in 1999, Byrne and Nicols published an influential allozyme study confirming that London Underground mosquitoes were reproductively isolated from their aboveground counterparts and hypothesizing that they evolved in situ3 (Fig. 4). They suggested that aboveground pipiens colonized the ‘Tube’ not long after it was constructed in the mid 1800s. Physically separated from their aboveground ancestors, the colonists then quickly evolved human biting, the ability to mate in confined spaces, and other key molestus behaviors as an adaptation to their concrete, subterranean home. Today, evolutionary biologists frequently hold up the London Underground Mosquito as a likely example of rapid urban adaptation4–9 (Fig. 4), and the popular science media treats the theory as fact60–65. What evidence is there to support these claims? And if unsupported, where, when, and why did molestus first evolve?
Figure 4. The theory of in situ evolution in the London Underground Mosquito rose to prominence despite contradictory evidence in the broader scientific literature.
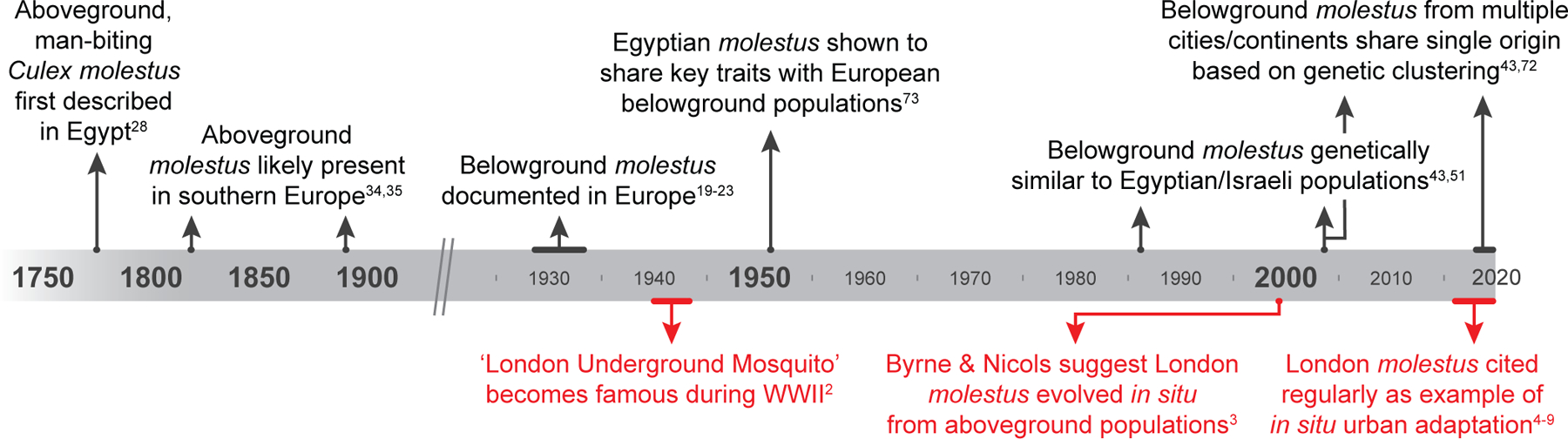
Black lettering describes research on diverse European and Middle Eastern populations. Red lettering describes research and popular attention paid to London Underground mosquitoes. The notoriety of London populations generated a popular narrative of rapid, in situ, urban adaptation that is at odds with genetic and ecological data linking them to previously described taxa in both Europe and the Middle East.
Byrne and Nicols analyzed genetic variation at twenty allozyme loci in London populations. Belowground mosquitoes from eight Tube stations were relatively homogenous and more closely related to each other than to nearby aboveground mosquitoes. This is the main result of the paper, supporting the conclusion that London Underground populations are reproductively isolated and share a single origin. The authors then go on to suggest that they evolved in situ based on the fact that all but one of the specific allozyme alleles common belowground could also be readily found aboveground in London. While this pattern is certainly consistent with in situ evolution, it is also consistent with the idea that molestus migrated to London from another part of the species’ range. Fixed genetic differences can take thousands to millions of years to accumulate between diverging taxa, even when geographically distant, and may be restricted to narrow parts of the genome directly involved in key traits66. This is especially true when selection acts on pre-existing variants67 and/or gene flow is ongoing68. Moreover, as explained above, molestus is not confined to London, and subsequent genomic analyses show that belowground mosquitoes from locations as distant as the UK, Spain, Portugal, Germany, Belarus, Kyrgyzstan, and the USA form a single genetic cluster43,69–71 (but see [72]). Most molestus populations are thus likely to share a common origin, and there is no particular reason to believe that this origin is the London Underground.
If not London, then where did molestus first evolve? A persistent narrative thread in the literature dating back over 200 years points to Egypt and surrounding areas (Fig. 4). We have so far avoided mention of Egyptian populations because they have received little recent attention. However, the name molestus originated in Egypt’s Nile delta, when, in 1775, the Swedish explorer and Linnaeus-disciple Peter Forskål described Cx. molestus—a new ‘species’ morphologically similar to Cx. pipiens that “bothers sleepers at night” and is “difficult to avoid unless with well-closed curtains”12,28. The name was later somewhat controversially adopted for northern belowground populations based solely on their shared human-biting behavior10,38,40. However, subsequent work confirms that Egyptian mosquitoes not only tend to bite humans and other mammals (Fig. 5A), but also mate in confined spaces, remain active year-round, and show at least low levels of autogeny73. Decades-old allozyme data51 and more recent microsatellite work43 also document a genetic link between molestus in Europe and aboveground populations in Egypt, Israel, and Jordan. Byrne and Nicols were aware of these data, and although they favored in situ evolution, they carefully presented a Middle Eastern origin as an alternative in the introduction of their paper. Surprisingly, however, this possibility was overlooked in subsequent evolutionary reviews and syntheses4–9.
Figure 5. Cx. pipiens populations from the Middle East are mammal-biting, consistent with their recent and possibly ancient role in disease transmission.
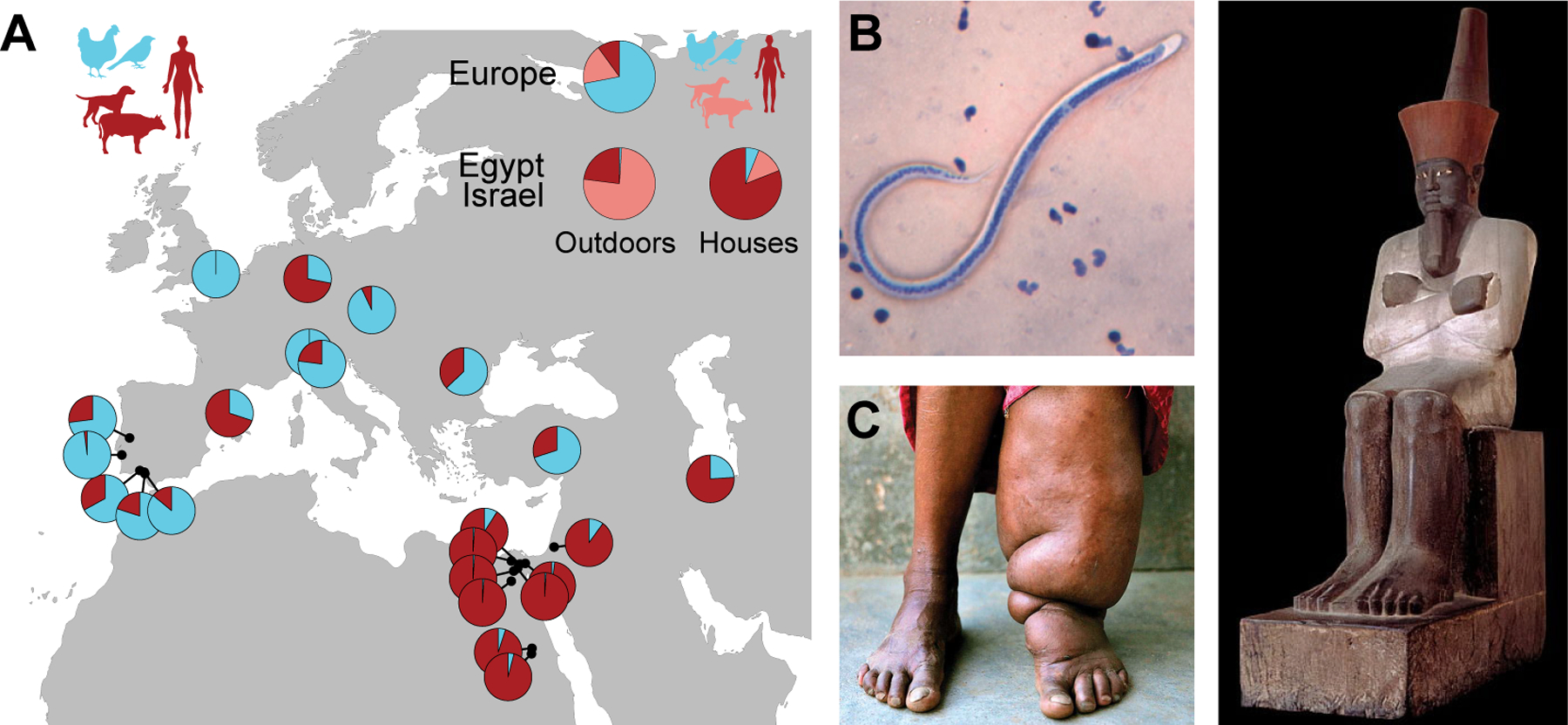
A, Published host-choice data for 27 aboveground Cx. pipiens populations (Table S2, see Supplemental Methods). Pies placed on the map show fraction of bloodmeals taken from birds (light blue) or mammals (maroon) in nature. Inset summarizes data for outdoor and indoor house collection sites separately, with mammal meals further divided into human and non-human fractions. Mosquitoes from aboveground populations in Egypt and Israel consistently bite mammals, including humans. B, Wuchereria bancrofti is a human-specific filarial worm that was prevalent in Egypt and transmitted by Cx. pipiens during the 20th c76. Photo shows blood smear with ~0.3 mm long larva. C, W. bancrofti infections can cause swelling in the legs known as elephantiasis. D, A statue of the pharaoh Mentuhotep II with an enlarged left leg is one of several ancient Egyptian artifacts that suggest W. bancrofti infections have been prevalent in the Middle East for millennia77. Images in (B-D) from www.cdc.gov/dpdx/lymphaticfilariasis/index.html (B), www.dnaindia.com/india/report-an-elephantine-problem-haunts-bihar-jharkhand-uttar-pradesh-2741585 (C), and www.meisterdrucke.uk/fine-art-prints/Egyptian-11th-Dynasty/603893/Statue-of-Nebhepetre-Mentuhotep-II,-from-the-Funerary-Temple-of-Mentuhotep-II,-Deir-el-Bahri,-Middle-Kingdom-(painted-sandstone).html (D).
The presence of molestus in Egypt and surrounding areas in recent times doesn’t necessarily mean that it originated there, but several additional lines of evidence make this idea plausible. First, unlike the situation in Europe, molestus appears to be the only form present in Egypt and Israel. There is no evidence for cryptic genetic structure51, and aboveground mosquitoes are just as likely to behave like molestus as those found breeding belowground74. An Egyptian origin thus alleviates the mental acrobatics required to imagine initial divergence in sympatry. Second, epidemiological data suggest that molestus has been present in Egypt for millennia. Wuchereria bancrofti is a filarial worm that only infects humans and causes lymphatic filariasis75 (Fig. 5B). Although recently eliminated from Egypt, it was recognized as a major public health problem throughout the 1900s, with Cx. pipiens mosquitoes serving as the primary vector76. Most people with lymphatic filariasis have no symptoms, but some develop severe swelling in the legs or other extremities known as elephantiasis (Fig. 5C). Interestingly, ancient Egyptian papyrus and pharaonic sculptures indicate that this condition, and thus most likely mammal-biting Cx. pipiens, were common in Egypt as long ago as 2000 B.C.77 (Fig. 5D; but note the possibility that Anopheline mosquitoes78 contributed to transmission in ancient times).
Finally, the long history of agriculture and dense human civilization in Egypt and surrounding areas provide a novel ‘human’ niche in which one can easily imagine key molestus behaviors (Fig. 2) first emerging. Abundant humans and domestic animals may have favored mammal-biting, and the presence of these hosts in houses and animal shelters at night (when Cx. pipiens is most active) may have favored the ability to enter and mate in confined spaces. Breeding year-round is likely ancestral within the species79. The only molestus trait that cannot be easily linked to ancient aboveground habitats is autogeny. Indeed, only a minority of molestus females from contemporary Egyptian and Israeli populations can lay eggs without a bloodmeal73,74. Ancient irrigation ditches and cesspits may have provided the kind of nutrient-rich larval habitat permissive for initial emergence of this behavior, but autogenous egg clutches are smaller than those laid after a bloodmeal, and the trait is likely under strong selection only when hosts are scarce33,80. We therefore hypothesize that autogeny was further selected in modern, belowground habitats. Many authors have shown that the trait responds quickly to the hard selection imposed by lack of vertebrate hosts, as long as at least a few females in the starting population can lay eggs without a bloodmeal40.
Ultimately, hypotheses surrounding the origin of molestus must be tested with better genomic data—ideally whole genome sequences from a large sample of individuals, including representatives from Egypt and other ecologically and geographically diverse populations. How old is molestus? Where and in what context did it likely first emerge? If molestus originated thousands of years ago in the Middle East, when did it spread north and come into contact with pipiens in southern Europe? When and from where did it colonize modern belowground habitats? Humans are clearly capable of spreading molestus from place to place in modern times81–84, but might at least some molestus populations be independently derived, e.g. via the reassortment of standing genetic variation present in admixed aboveground populations49? The London Underground Mosquito almost certainly did not evolve de novo from local aboveground populations within the last 200 years, but there are still many interesting questions to answer surrounding its ultimate origin and spread.
Conclusions and outlook
For better or for worse, Culex pipiens mosquitoes have been capturing the attention of scientists and the general public for centuries. They are the most common mosquitoes in human habitats across the temperate northern hemisphere and display a level of ecogenetic variation that alternately inspires and frustrates even the most astute entomologists, vector biologists, and public health officials. We have reviewed what is known about this variation across the western Palearctic, clarifying a latitudinal gradient in ecology, microhabitat segregation, and gene flow (Fig. 6). In northern Europe and Asia, the species comprises two divergent ecotypes, with pipiens aboveground and molestus in man-made belowground environments. Harsh winters and strong divergent selection likely limit gene flow and allow the two to maintain a remarkable suite of divergent behaviors (Fig. 2). As one moves south into the Mediterranean basin, gene flow increases. There are still two genetic clusters corresponding to pipiens- and molestus-like individuals, but they are less extreme and less restricted to their respective above and belowground habitats. Aboveground mosquitoes are particularly diverse and sometimes display a mix of traits that makes them difficult to categorize. Finally, gene flow is high at low latitudes, with little to no difference between microhabitats in North Africa. Populations in the wetter, western parts of North Africa are intermediate, but those in Egypt and surrounding areas are genetically and behaviorally similar to northern molestus. Indeed, several lines of evidence suggest that molestus first evolved in association with early agricultural societies in the Middle East, before migrating north and eventually colonizing northern belowground habitats like the London Underground during modern times (Fig. 4, 5).
Figure 6. Summary of ecogenetic variation within Cx. pipiens across the western Palearctic.
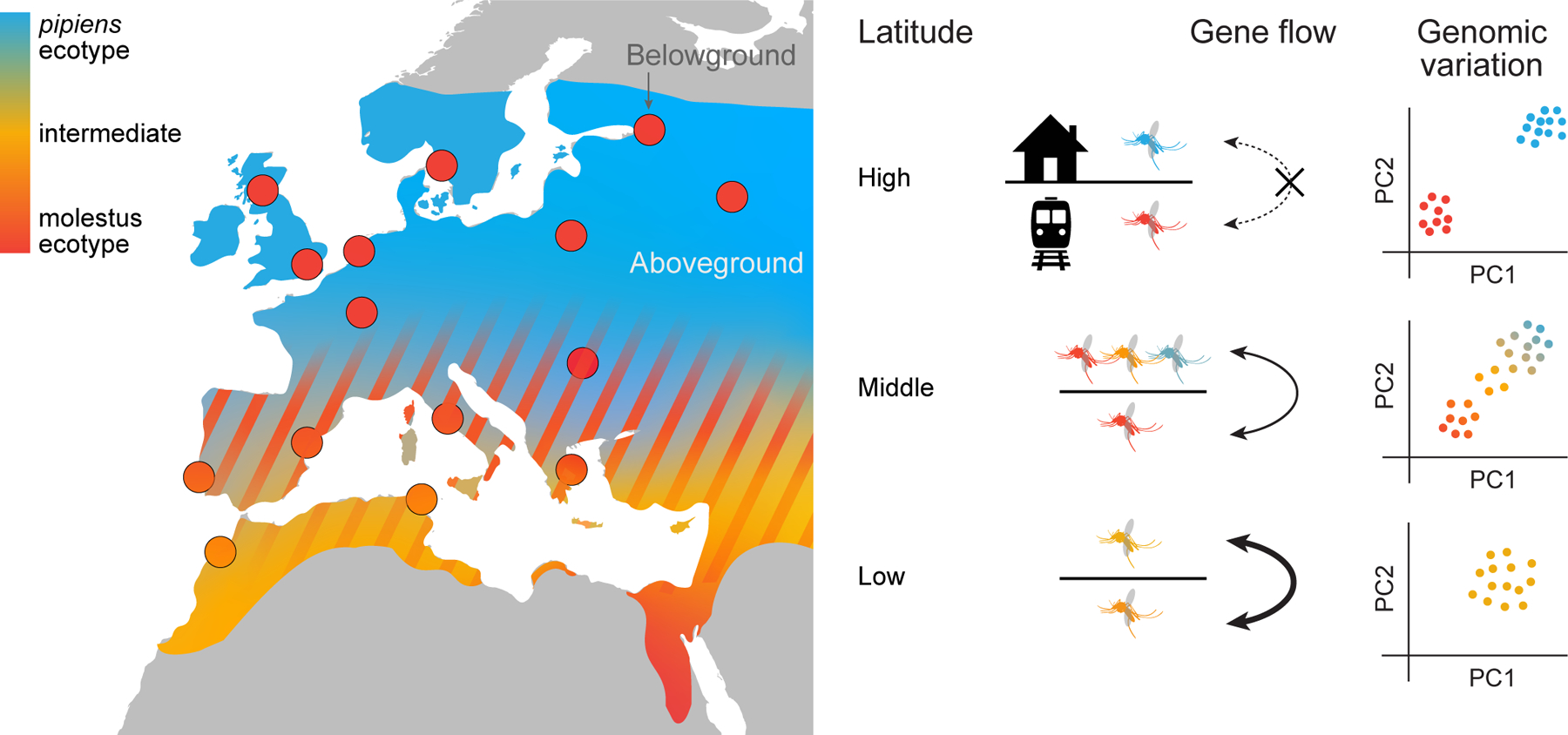
The available data suggest a latitudinal model in which aboveground populations (represented by background map color) and belowground populations (represented by circles) are reproductively isolated in the north, where they correspond to the canonical pipiens (blue) and molestus (red) ecotypes. Gene flow increases as one moves south, generating a complex ecological mosaic with variable aboveground populations at middle latitudes (hatching), and a genetically and ecologically intermediate form in the wetter, western parts of North Africa (orange). Schematics at right show the types of mosquitoes typically found above and below ground in each latitudinal band and expected patterns of genomic variation (hypothetical principal components analyses of multilocus data). Belowground molestus populations from the north (red circles) are behaviorally and genetically similar to Egyptian populations (solid red background color). molestus may have originally evolved thousands of years ago in association with early Egyptian or other Middle Eastern agricultural societies, before moving north and eventually colonizing urban belowground habitats during modern times.
While gross ecological patterns are beginning to come into focus, many questions remain unanswered, and the increasing threat of West Nile Virus and other mosquito-borne pathogens makes continuing research efforts essential85,86. West Nile Virus is a bird virus for which humans are dead-end hosts87. Spillover therefore requires mosquitoes that are willing to bite both birds and mammals. Research in US cities suggests that hybridization between pipiens and molestus produces such bridge vectors and may drive West Nile Virus epidemics in major urban areas43,88,89. This idea has motivated much of the recent CQ11 genotyping work, and we hope that our synthesis of these data (Fig. 3, 6) will help public health officials across the western Palearctic better predict the potential for West Nile Virus transmission in their region. One should keep in mind, however, that CQ11 genotypes are only loose correlates of behavior and middle latitude populations are remarkably heterogeneous. Some of this heterogeneity is linked to local variation in urbanicity32,47,48 (but see [90]), but it remains difficult to predict exactly where or in what situations one will find pipiens, molestus, both, or something in between. Progress will likely require grappling with genome-wide patterns of genetic variation and standardizing the collection of behavioral data across studies and countries. Integrating behavioral and genomic data with knowledge of human population density, land use, and microclimate for a large sample may then clarify the key environmental factors that shape ecological diversity.
Understanding the environmental determinants of Cx. pipiens diversity is important because it can help us predict changes under future climate and land use regimes91. It is widely accepted that the geographic distributions of many animals and plants, including mosquitoes, will shift as the planet warms92,93. The latitudinal nature of the patterns reviewed here (Fig. 6) suggests that the dynamics and ecology of preexisting Cx. pipiens populations will also change. Warmer winters may expand the latitudinal zone within which molestus can survive aboveground, increasing hybridization between ecotypes in northern areas where they currently remain distinct. Such changes would be invisible at the morphological level, but nevertheless have potential consequences for disease transmission.
Although beyond the scope of the current review, the genetics and ecology of Cx. pipiens mosquitoes outside the western Palearctic region is also complex, in part due to interactions with related species13,72,79. Notably, both ecotypes of Cx. pipiens hybridize extensively with their tropical sibling Cx. quinquefasciatus when they come into contact in Asia, Australia, and the Americas (Fig. 1). Cx. quinquefasciatus females require a bloodmeal to lay eggs, but are reminiscent of molestus in other ways, including in their willingness to bite humans and mate in captivity. This raises the possibility that gene flow or ancestral variation may have contributed to the evolution of molestus behavior. The only place where the temperate and tropical sibling species are fully reproductively isolated is in southern Africa, where local Cx. pipiens populations may actually represent a third, possibly ancestral species55. Understanding the evolutionary history (and future) of pipiens and molestus ecotypes will likely require consideration of this broader evolutionary context.
Finally, our review of the origin of London molestus has interesting implications for understanding urban plant and animal communities. The London Underground Mosquito is frequently held up as an iconic example consistent with rapid urban adaptation. It is not exactly clear how this idea grew such long legs, but the weight of the evidence instead suggests that molestus’ success in modern cities is largely the result of ancestral traits that first evolved in a different time and place—on the timescale of millennia rather than centuries. Whole genome sequences from geographically diverse populations will be critical for testing this hypothesis and putting dates on key evolutionary events94. Interestingly, Aedes aegypti is another vector mosquito that likely first arose in association with newly sedentary human societies in the Holocene, but is now taking advantage of the modern urban niche95. The extent to which ancestral traits (versus newly evolved adaptations) underlie the success of contemporary urban taxa is an important outstanding question in urban ecology and evolution8,96. We expect future work on molestus to continue to inform this relatively new field and to provide a captivating conduit for communicating these ideas to the general public.
Supplementary Material
Acknowledgements.
We thank Dina Fonseca, Megan Fritz, and Matthew Aardema for discussion and/or comments on the manuscript. We also thank Ali Bouattour, Bruno Gomes, Chantal Vogels, Daniel Bravo-Barriga, Dina Fonseca, Hans-Peter Fuehrer, Marco Di Luca, and Stefanie Becker for providing raw CQ11 data underlying previous work. Y.H. is supported by a Masason Foundation Fellowship, a Honjo International Fellowship, and a Centennial Fellowship. C.S.M.’s laboratory is also supported by a Pew Scholar Award, a Searle Scholar Award, a Klingenstein-Simons Fellowship, a Rosalind Franklin New Investigator Award, and the New York Stem Cell Foundation. C.S.M. is a New York Stem Cell Foundation – Robertson Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests. The authors declare no competing interests.
REFERENCES
- 1.Gardiner J (2011). The Blitz: The British Under Attack (HarperPress) https://www.harpercollins.com/products/the-blitz-the-british-under-attack-juliet-gardiner
- 2.Shute PG (1951). Culex molestus. Trans. R. Entomol. Soc. Lond 102, 380–382. [Google Scholar]
- 3.Byrne K, and Nichols RA (1999). Culex pipiens in London Underground tunnels: differentiation between surface and subterranean populations. Heredity 82, 7–15. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MTJ, and Munshi-South J (2017). Evolution of life in urban environments. Science 358, eaam8327. [DOI] [PubMed] [Google Scholar]
- 5.Thompson KA, Rieseberg LH, and Schluter D (2018). Speciation and the City. Trends Ecol. Evol 33, 815–826. [DOI] [PubMed] [Google Scholar]
- 6.Reznick DN, Losos J, and Travis J (2019). From low to high gear: there has been a paradigm shift in our understanding of evolution. Ecol. Lett 22, 233–244. [DOI] [PubMed] [Google Scholar]
- 7.Otto SP (2018). Adaptation, speciation and extinction in the Anthropocene. Proc. R. Soc. B Biol. Sci 285, 20182047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivkin LR, Santangelo JS, Alberti M, Aronson MFJ, de Keyzer CW, Diamond SE, Fortin M-J, Frazee LJ, Gorton AJ, Hendry AP, et al. (2019). A roadmap for urban evolutionary ecology. Evol. Appl 12, 384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendry AP, Gotanda KM, and Svensson EI (2017). Human influences on evolution, and the ecological and societal consequences. Philos. Trans. R. Soc. Lond. B Biol. Sci 372, 20160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jobling B (1938). On two subspecies of Culex pipiens L. (Diptera). Trans. R. Entomol. Soc. Lond 87, 193–216. [Google Scholar]
- 11.Mattingly PF (1951). The Culex pipiens complex. Trans. R. Entomol. Soc. Lond 102, 331–382. [Google Scholar]
- 12.Harbach RE, Harrison BA, and Gad AM (1984). Culex (Culex) molestus Forskal (Diptera: Culicidae): neotype designation, description, variation, and taxonomic status. Proc. Entomol. Soc. Wash 86, 521–542. [Google Scholar]
- 13.Harbach RE (2012). Culex pipiens: species versus species complex – taxonomic history and perspective. J. Am. Mosq. Control Assoc 28, 10–23. [DOI] [PubMed] [Google Scholar]
- 14.Vinogradova EB (2000). Culex Pipiens Pipiens Mosquitoes: Taxonomy, Distribution, Ecology, Physiology, Genetics, Applied Importance and Control (Pensoft Publishers) https://www.nhbs.com/culex-pipiens-pipiens-mosquitoes-taxonomy-distribution-ecology-physiology-genetics-applied-importance-and-control-book
- 15.Farajollahi A, Fonseca DM, Kramer LD, and Marm Kilpatrick A (2011). “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol 11, 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clements AN (1999). The Biology of Mosquitoes Volume 2: Sensory Reception and Behaviour (CABI publishing). https://www.cabi.org/bookshop/book/9780851993133/ [Google Scholar]
- 17.European Centre for Disease Prevention and Control (2020). Culex pipiens - Factsheet for experts https://www.ecdc.europa.eu/en/all-topics-z/disease-vectors/facts/mosquito-factsheets/culex-pipiens-factsheet-experts.
- 18.Hesson JC, Ostman O, Schäfer M, and Lundström JO (2011). Geographic distribution and relative abundance of the sibling vector species Culex torrentium and Culex pipiens in Sweden. Vector Borne Zoonotic Dis. 11, 1383–1389. [DOI] [PubMed] [Google Scholar]
- 19.Roubaud E (1929). Autogenous cycle of winter generations of Culex pipiens L. C. R. Acad. Sci 188, 735–738. [Google Scholar]
- 20.Boissezon P. de (1929). Notes on the reproduction of Culex pipiens L. in winter. Bull. Soc. Pathol. Exot 22, 549–553. [Google Scholar]
- 21.Legendre J (1931). Le moustique cavernicole ou l’adaptation de “Culex pipiens” a l’urbanisme moderne. Bull. Acad. Med 106, 86–89. [Google Scholar]
- 22.Hecht O (1932). Experimentelle beiträge zur biologie der stechmücken II. Z. Angew. Entomol 19, 579–607. [Google Scholar]
- 23.Wesenberg-Lund C (1920). Contributions to the biology of the Danish Culicidae (AF Høst & søn) https://www.worldcat.org/title/contributions-to-the-biology-of-the-danish-culicidae/oclc/14802295
- 24.Huff CG (1929). Ovulation requirements of Culex pipiens Linn. Biol. Bull 56, 347–350. [Google Scholar]
- 25.Knight KL (1951). A review of the Culex pipiens complex in the Mediterranean subregion (Diptera, Culicidae). Trans. R. Entomol. Soc. Lond 102, 354–364. [Google Scholar]
- 26.Callot J (1947). Étude sur quelques souches de Culex pipiens (sensu lato) et sur leurs hybrides. Ann. Parasitol. Hum. Comp 22, 380–393. [Google Scholar]
- 27.Tate P, and Vincent M (1936). The biology of autogenous and anautogenous races of Culex pipiens L. (Diptera: Culicidae). Parasitology 28, 115–145. [Google Scholar]
- 28.Forskål P (1775). Descriptiones Animalium, Avium, Amphibiorum, Piscium, Insectorum, Vermium: Quae In Itinere Orientali Observavit (Molleri, Hauniae) https://www.worldcat.org/title/descriptiones-animalium-avium-amphibiorum-piscium-insectorum-vermium-quae-in-itinere-orientali-observavit-petrus-forskal/oclc/63636078
- 29.Marshall JF, and Staley J (1937). Some notes regarding the morphological and biological differentiation of Culex pipiens Linnaeus and Culex molestus Forskål (Diptera, Culicidae). Proc. R. Entomol. Soc 12, 17–26. [Google Scholar]
- 30.Vinogradova EB (1961). Concerning the biological isolation of the subspecies of Culex pipiens L. (Diptera, Culicidae). Entomol. Obozr 40, 63–75. [Google Scholar]
- 31.Urbanelli S, Cianchi R, Petrarca V, Sabatinelli M, Coluzzi M, and Bullini L (1981). Adattamento all’ambiente urbano nella zanzara Culex pipiens (Diptera, Culicidae). In Ecologia Atti I Congressi nazionali S It. E., Moroni A, Ravera O and Anelli A, ed. (Zara), pp. 305–316. [Google Scholar]
- 32.Di Luca M, Toma L, Boccolini D, Severini F, La Rosa G, Minelli G, Bongiorno G, Montarsi F, Arnoldi D, Capelli G, et al. (2016). Ecological distribution and CQ11 genetic structure of Culex pipiens complex (Diptera: Culicidae) in Italy. PLoS One 11, e0146476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chevillon C, Eritja R, Pasteur N, and Raymond M (1995). Commensalism, adaptation and gene flow: mosquitoes of the Culex pipiens complex in different habitats. Genet. Res 66, 147–157. [DOI] [PubMed] [Google Scholar]
- 34.Germar EF (1817). Reise Nach Dalmatien Und in Das Gebiet Von Ragusa (F. A. Brockhaus) https://books.google.com/books/about/Reise_Nach_Dalmatien_und_in_Das_Gebiet_V.html?id=RYzzwAEACAAJ
- 35.Ficalbi E (1890). Notizie preventive sulle zanzare Italiane. Boll. Soc. Entomol. Ital. 21, 124–131. [Google Scholar]
- 36.Edwards FW (1921). A revision of the mosquitos of the Palaearctic region. Bull. Entomol. Res 12, 263–351. [Google Scholar]
- 37.Callot J, and Van Ty D (1943). Sur quelques souches françaises de Culex pipiens. Bull. Soc. Pathol. Exot. Filiales 36, 229–232. [Google Scholar]
- 38.Roubaud E, and Ghelelovitch S (1956). Observations sur le moustique anthropophile méditerranéen du groupe pipiens, Culex berbericus Roub. C. R. Acad. Sci. Paris 242, 2900–2903. [Google Scholar]
- 39.Roubaud E (1939). Le pouvoir autogène chez le biotype nord-africain du moustique commun Culex pipiens (L.). Bull. Soc. Pathol. Exot 36, 172–175. [Google Scholar]
- 40.Rioux JA, and Pech JM (1959). Le biotype autogene de Culex pipiens L. ne doit pasetre nomme Culex molestus Forskal (Diptera Culicidae). Cahiers Des Nat 15, 115–117. [Google Scholar]
- 41.Rioux JA, Juminer B, Kchouk M, and Croset H (1965). Présence du caractère autogène chez Culex pipiens pipiens L. dans un biotope épigé de l’Ile de Djerba. Arch. Inst. Pasteur Tunis 42, 1–8. [Google Scholar]
- 42.Petrarca V, Cianchi R, Sabatinelli G, Bianchi Bullini AP, Coluzzi M & Bullini L (1976). Alcuni dati sul differenziamento morfologico, ecologico, etologico e genetico di popolazioni urbane e rurali di Culex pipiens nel Lazio. Boll. Zool 43, 394–395. [Google Scholar]
- 43.Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, Fleischer RC, and Wilkerson RC (2004). Emerging vectors in the Culex pipiens complex. Science 303, 1535–1538. [DOI] [PubMed] [Google Scholar]
- 44.Gomes B, Sousa CA, Novo MT, Freitas FB, Alves R, Côrte-Real AR, Salgueiro P, Donnelly MJ, Almeida APG, and Pinto J (2009). Asymmetric introgression between sympatric molestus and pipiens forms of Culex pipiens (Diptera: Culicidae) in the Comporta region, Portugal. BMC Evol. Biol 9, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomes B, Sousa CA, Vicente JL, Pinho L, Calderón I, Arez E, Almeida AP, Donnelly MJ, and Pinto J (2013). Feeding patterns of molestus and pipiens forms of Culex pipiens (Diptera: Culicidae) in a region of high hybridization. Parasit. Vectors 6, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomes B, Kioulos E, Papa A, Almeida APG, Vontas J, and Pinto J (2013). Distribution and hybridization of Culex pipiens forms in Greece during the West Nile virus outbreak of 2010. Infect. Genet. Evol 16, 218–225. [DOI] [PubMed] [Google Scholar]
- 47.Osório HC, Zé-Zé L, Amaro F, Nunes A, and Alves MJ (2014). Sympatric occurrence of Culex pipiens (Diptera, Culicidae) biotypes pipiens, molestus and their hybrids in Portugal, Western Europe: feeding patterns and habitat determinants. Med. Vet. Entomol 28, 103–109. [DOI] [PubMed] [Google Scholar]
- 48.Martínez-de la Puente J, Ferraguti M, Ruiz S, Roiz D, Soriguer RC, and Figuerola J (2016). Culex pipiens forms and urbanization: effects on blood feeding sources and transmission of avian Plasmodium. Malar. J 15, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chevillon C, Rivet Y, Raymond M, Rousset F, and Pasteur N (1998). Migration/selection balance and ecotypic differentiation in the mosquito Culex pipiens. Mol. Ecol 7, 197–208. [Google Scholar]
- 50.Spielman A (1964). Studies on autogeny in Culex pipiens populations in nature: I. Reproductive isolation between autogenous and anautogenous populations. Am. J. Hyg 80, 175–183. [DOI] [PubMed] [Google Scholar]
- 51.Villani F, Urbanelli S, Gad A, Nudelman S, and Bullini L (1986). Electrophoretic variation of Culex pipiens from Egypt and Israel. Biol. J. Linn. Soc. Lond 29, 49–62. [Google Scholar]
- 52.Bullini L, and Coluzzi M (1980). Ethological mechanisms of reproductive isolation in Culex pipiens and Aedes mariae complexes (Diptera, Culicidae). Monit. Zool. Ital 14, 99–100. [Google Scholar]
- 53.Laven H (1959). Speciation by cytoplasmic isolation in the Culex pipiens-complex. Cold Spring Harb. Symp. Quant. Biol 24, 166–173. [DOI] [PubMed] [Google Scholar]
- 54.Yen JH, and Barr AR (1971). New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 232, 657–658. [DOI] [PubMed] [Google Scholar]
- 55.Dumas E, Atyame CM, Malcolm CA, Le Goff G, Unal S, Makoundou P, Pasteur N, Weill M, and Duron O (2016). Molecular data reveal a cryptic species within the Culex pipiens mosquito complex. Insect Mol. Biol 25, 800–809. [DOI] [PubMed] [Google Scholar]
- 56.Dumas E, Atyame CM, Milesi P, Fonseca DM, Shaikevich EV, Unal S, Makoundou P, Weill M, and Duron O (2013). Population structure of Wolbachia and cytoplasmic introgression in a complex of mosquito species. BMC Evol. Biol 13, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bahnck CM, and Fonseca DM (2006). Rapid assay to identify the two genetic forms of Culex (Culex) pipiens L. (Diptera: Culicidae) and hybrid populations. Am. J. Trop. Med. Hyg 75, 251–255. [PubMed] [Google Scholar]
- 58.Mori A, Romero-Severson J, and Severson DW (2007). Genetic basis for reproductive diapause is correlated with life history traits within the Culex pipiens complex. Insect Mol. Biol 16, 515–524. [DOI] [PubMed] [Google Scholar]
- 59.Hickner PV, Mori A, Rund SSC, Sheppard AD, Cunningham JM, Chadee DD, Duffield GE, and Severson DW (2019). QTL determining diel flight activity in male Culex pipiens mosquitoes. J. Hered 110, 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reznick DN (2011). The Origin Then and Now: An Interpretive Guide to the Origin of Species (Princeton University Press; ). https://press.princeton.edu/books/paperback/9780691152578/the-origin-then-and-now [Google Scholar]
- 61.Nye B (2014). Chapter 18: Mosquito in the Tube. In Undeniable: Evolution and the Science of Creation (Macmillan; ), pp. 137–144. https://us.macmillan.com/books/9781250074225/undeniable [Google Scholar]
- 62.Bull JW (2016). Humans Artificially Drive Evolution of New Species. EurekAlert! https://www.eurekalert.org/pub_releases/2016-06/nhmo-had062716.php.
- 63.Blakemore E (2016). The London Underground Has Its Own Mosquito Subspecies. Smithsonian Magazine https://www.smithsonianmag.com/smart-news/london-underground-has-its-own-mosquito-subspecies-180958566/.
- 64.Silver K (2016). The Unique Mosquito that Lives in the London Underground. BBC Earth https://www.bbc.com/earth/story/20160323-the-unique-mosquito-that-lives-in-the-london-underground.
- 65.Schilthuizen M (2019). Darwin Comes to Town: How the Urban Jungle Drives Evolution (Picador; ). https://us.macmillan.com/books/9781250127822 [Google Scholar]
- 66.Turner TL, Hahn MW, and Nuzhdin SV (2005). Genomic islands of speciation in Anopheles gambiae. PLoS Biol 3, e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barrett RDH, and Schluter D (2008). Adaptation from standing genetic variation. Trends Ecol. Evol 23, 38–44. [DOI] [PubMed] [Google Scholar]
- 68.Yeaman S, and Whitlock MC (2011). The genetic architecture of adaptation under migration-selection balance. Evolution 65, 1897–1911. [DOI] [PubMed] [Google Scholar]
- 69.Gomes B, Wilding CS, Weetman D, Sousa CA, Novo MT, Savage HM, Almeida APG, Pinto J, and Donnelly MJ (2015). Limited genomic divergence between intraspecific forms of Culex pipiens under different ecological pressures. BMC Evol. Biol 15, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yurchenko AA, Masri RA, Khrabrova NV, Sibataev AK, Fritz ML, and Sharakhova MV (2020). Genomic differentiation and intercontinental population structure of mosquito vectors Culex pipiens pipiens and Culex pipiens molestus. Sci. Rep 10, 7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weitzel T, Collado A, Jöst A, Pietsch K, Storch V, and Becker N (2009). Genetic differentiation of populations within the Culex pipiens complex and phylogeny of related species. J. Am. Mosq. Control Assoc 25, 6–17. [DOI] [PubMed] [Google Scholar]
- 72.Aardema ML, vonHoldt BM, Fritz ML, and Davis SR (2020). Global evaluation of taxonomic relationships and admixture within the Culex pipiens complex of mosquitoes. Parasit. Vectors 13, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knight KL, and Abdel Malek AA (1951). A morphological and biological study of Culex pipiens in the Cairo area of Egypt (Diptera-Culicidae). Bulletin de la Société Fouad 1er d’entomologie 35, 175–185. [Google Scholar]
- 74.Nudelman S, Galun R, Kitron U, and Spielman A (1988). Physiological characteristics of Culex pipiens populations in the Middle East. Med. Vet. Entomol 2, 161–169. [DOI] [PubMed] [Google Scholar]
- 75.Centers for Disease Control and Prevention (2019). Lymphatic Filariasis https://www.cdc.gov/dpdx/lymphaticfilariasis/index.html.
- 76.Ramzy RMR, Kamal HA, Hassan MA, and Haggag AA (2019). Elimination of lymphatic filariasis as a public health problem from the Arab Republic of Egypt. Acta Trop 199, 105121. [DOI] [PubMed] [Google Scholar]
- 77.Gordon CA, Jones MK, and McManus DP (2018). The history of Bancroftian Lymphatic Filariasis in Australasia and Oceania: is there a threat of re-occurrence in mainland Australia? Trop. Med. Int. Health 3, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manguin S, Bangs MJ, Pothikasikorn J, and Chareonviriyaphap T (2010). Review on global co-transmission of human Plasmodium species and Wuchereria bancrofti by Anopheles mosquitoes. Infect. Genet. Evol 10, 159–177. [DOI] [PubMed] [Google Scholar]
- 79.Aardema ML, Olatunji SK, and Fonseca DM (2021). The enigmatic Culex pipiens (Diptera: Culicidae) species complex: phylogenetic challenges and opportunities from a notoriously tricky mosquito group. Ann. Entomol. Soc. Am Advance Article, saab038. [Google Scholar]
- 80.Tsuji N, Okazawa T, and Yamamura N (1990). Autogenous and anautogenous mosquitoes: a mathematical analysis of reproductive strategies. J. Med. Entomol 27, 446–453. [DOI] [PubMed] [Google Scholar]
- 81.Markovich NY, and Zarechnaya SN (1992). Materials on the distribution of Culex pipiens over the territory of the USSR. Med. Parazitol 1, 5–9. [PubMed] [Google Scholar]
- 82.Mitchell CJ, Darsie RF Jr, and Monath TP (1984). Occurrence of autogenous Culex pipiens Linnaeus, 1758, (Diptera: Culicidae) in Argentina and notes on distribution of the complex. Mosq. Syst 16, 308–316. [Google Scholar]
- 83.Mogi M (2012). The forms of the Culex pipiens complex in East Asia, with ecological thoughts on their origin and interrelation. J. Am. Mosq. Control Assoc 28, 28–52. [DOI] [PubMed] [Google Scholar]
- 84.Kassim NFA, Webb CE, Wang Q, and Russell RC (2013). Australian distribution, genetic status and seasonal abundance of the exotic mosquito Culex molestus (Forskal) (Diptera: Culicidae). Aust. J. Entomol 52, 185–198. [Google Scholar]
- 85.Barrett ADT (2018). West Nile in Europe: an increasing public health problem. J. Travel Med 25, tay096. [DOI] [PubMed] [Google Scholar]
- 86.Ronca SE, Ruff JC, and Murray KO (2021). A 20-year historical review of West Nile virus since its initial emergence in North America: Has West Nile virus become a neglected tropical disease? PLoS Negl. Trop. Dis 15, e0009190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention (2020). West Nile Virus https://www.cdc.gov/westnile/index.html.
- 88.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P, and Fonseca DM (2007). Genetic influences on mosquito feeding behavior and the emergence of zoonotic pathogens. Am. J. Trop. Med. Hyg 77, 667–671. [PubMed] [Google Scholar]
- 89.Huang S, Hamer GL, Molaei G, Walker ED, Goldberg TL, Kitron UD, and Andreadis TG (2009). Genetic variation associated with mammalian feeding in Culex pipiens from a West Nile virus epidemic region in Chicago, Illinois. Vector Borne Zoonotic Dis 9, 637–642. [DOI] [PubMed] [Google Scholar]
- 90.Vogels CBF, Möhlmann TWR, Melsen D, Favia G, Wennergren U, and Koenraadt CJM (2016). Latitudinal diversity of Culex pipiens biotypes and hybrids in farm, Peri-Urban, and wetland habitats in Europe. PLoS One 11, e0166959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Couper LI, Farner JE, Caldwell JM, Childs ML, Harris MJ, Kirk DG, Nova N, Shocket M, Skinner EB, Uricchio LH, et al. (2021). How will mosquitoes adapt to climate warming? Elife 10, e69630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hickling R, Roy DB, Hill JK, Fox R, and Thomas CD (2006). The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol 12, 450–455. [Google Scholar]
- 93.Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, Barker CM, Moore CG, Carvalho RG, Coelho GE, Van Bortel W, et al. (2015). The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 4, e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beichman AC, Huerta-Sanchez E, and Lohmueller KE (2018). Using genomic data to infer historic population dynamics of nonmodel organisms. Annu. Rev. Ecol. Evol. Syst 49, 433–456. [Google Scholar]
- 95.Rose NH, Sylla M, Badolo A, Lutomiah J, Ayala D, Aribodor OB, Ibe N, Akorli J, Otoo S, Mutebi J-P, et al. (2020). Climate and urbanization drive mosquito preference for humans. Curr. Biol 30, 3570–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lambert MR, Brans KI, Des Roches S, Donihue CM, and Diamond SE (2021). Adaptive evolution in cities: progress and misconceptions. Trends Ecol. Evol 36, 239–257. [DOI] [PubMed] [Google Scholar]
- 97.Jupp PG (1978). Culex (Culex) pipiens pipiens Linnaeus and Culex (Culex) pipiens quinquefasciatus Say in South Africa: morphological and reproductive evidence in favour of their status as two species. Mosquito Systematics 10, 461–473. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


