Abstract
Light is one of the essential environmental factors in the production process of laying hens, which can directly affect their behavior, growth and development, and production performance. The spectral sensitivity of humans is different from that of poultry, and the perceived illuminance units of human and poultry are lux and clux, respectively. If the light management of laying hen production is carried out according to human perceived illuminance, the growth and development of laying hens during pullet rearing may be adversely affected due to the discomfort of the perceived illuminance. Preliminary research has found that blue-green LED light can improve the immune function of laying hens during the brooding and rearing periods. However, the differences of the effects caused by blue-green light on the immune performance and bone development of laying hens during pullet rearing are still unclear for the 2 spectral sensitivities. A total of 120 Jinghong layer chickens were raised from 1 d to 13 wk of age in one of three groups with a white LED light (light intensity unit lux, WL) group, a blue-green LED light (light intensity unit lux, HBGL) group, and blue-green LED light (light intensity unit clux, PBGL) group, and unlimited feed and water were provided during the whole experiment. At 7 and 13 wk of age, the immune performance, bone parameters, and related gene expression were investigated. The results showed that compared with the WL groups, HBGL and PBGL increased the immunoglobulin A (IgA) content at 13 wk of age and the IgM content at 7 wk of age (P < 0.05). The bone mineral density (BMD) at 7 and 13 wk of age and tibial strength (TS) at 13 wk of age of the pullets in the WL group were significantly higher than those in the HBGL and PBGL group (P < 0.05). Osteoclastogenesis inhibitory factor gene (OPG mRNA) expression was increased in the layer chickens at the age of 7 and 13 wk for the WL group (P < 0.05). Compared with the WL group and PBGL group, the melanopsin gene (OPN4 mRNA) transcription level of hypothalamus and pineal gland of the chickens under HBGL significantly increased at 7 and 13 wk of age (P < 0.05). In conclusion, blue-green LED light with two perceived illuminance (human and poultry) can increase the Ig content and the immune performance of layer chickens, and blue-green LED light (light intensity unit lux) can promote the expression of OPN4 gene in the hypothalamus and pineal gland. In addition, white LED light can enhance bone quality by increasing tibia OPG gene expression.
Key words: spectral sensitivity, layer chickens, blue-green LED light, immune performance, bone development
INTRODUCTION
Light is one of the essential environmental factors in the production process of laying hens, which affects their growth and development, behavior, physiology, metabolism, and production performance. The effect of light on laying hens is the result of the combined effects of light color, light intensity, photoperiod, and light source (Phillips and Piggins, 1992; Manser, 1996).
Light color is mainly caused by the distribution of light wavelengths (representing the distribution of each color). Research has demonstrated that chickens have color perception, and light color has different effects on chickens. Short-wavelength light (blue and green light) has a positive effect on the growth and immunity of chickens, quieting the chickens, reducing stress (Xie et al., 2008a,b; Baxter et al., 2014; Wei et al., 2020), and green light promotes weight gain of chickens. In addition, long-wavelength light (red light) can promote the sexual organs development, sex hormones secretion and sexual maturity, reduce feather pecking and cannibalism, improve egg production performance, and reduce egg quality and the fertilization rate (O'connor et al., 2011; Li et al., 2018; Shi et al., 2019; Hanlon et al., 2020). The total luminous power produced in the visual part of the spectrum is defined as light intensity, and light intensity has different effects on chickens. Previous studies have shown that high-illumination light will make the chickens irritable, frightened, and active, increasing fighting behavior, causing serious pecking, and causing a decline in eggshell quality, an increase in the number of deformed eggs, and an increase in mortality (Phillips and Piggins, 1992; Xie et al., 2008a,b; O'connor et al., 2011; Liu et al., 2017; Shi et al., 2019; Wei et al., 2020). Low illumination is helpful to keep chickens quiet, deposit fat, and enable faster weight gain (Renema and Robinson, 2001; Kristensen et al., 2007; Shi et al., 2019).
Light sources of different colors are considered to have different intensity, so it is difficult to distinguish the effects of these 2 light characteristics on laying hens (Prayitno et al., 1997). In addition, the perceived illuminance depends on the spectral power distribution of a light source and the poultry sensitivity to the particular wavelengths (Kristensen et al., 2007). Consequently, light sources may be perceived as different colors and illuminance by chickens than they are by humans. Compared to humans, poultry are more sensitive to light and can detect the flicker of low-frequency fluorescent lamps. The critical fusion frequency for poultry, which can detect the level of difference between continuous and intermittent lighting, is about 105 Hz (Nuboer et al., 1992a) to 120 Hz (Widowski and Duncan, 1996). Due to the difference in spectral sensitivity between humans and poultry and the different types of light sources used, the difference in the perceived illuminance between humans and poultry may reach 20% (Nuboer et al., 1992b; Prescott et al., 2003). And there are differences in the spectral sensitivity characteristic curve between humans and poultry (Lewis and Morris, 2000). However, there is little research on the effects of two perceived illuminance (human and poultry) on laying hens, which may cause an adverse effect on the growth and development of layers during pullet rearing.
Preliminary research has found that blue-green LED light can improve the immune function of laying hens during the brooding and rearing periods (Wei et al., 2020). Hence, we explored the effect of blue-green lights with 2 perceived illuminance (human and poultry) on the immune performance, bone development, and related gene expression of the layer chickens, with the hope of providing a theoretical basis for lighting applied during the brooding and early rearing periods of laying hens, which is conducive to further improving the light environment and the health and welfare of laying hens.
MATERIALS AND METHODS
Experimental Pullet House and Experimental Animals
A total of 120 healthy and similar weight Jinghong layer chickens (Beijing Yukou Poultry Co., Ltd., Beijing, China) were raised from 1 d to 13 wk of age in one of 3 groups, the white LED light (light intensity unit lux, WL) group, the blue-green LED light (light intensity unit lux, HBGL) group, or the blue-green LED light (light intensity unit clux, PBGL) group. Each cage (length × width × height, 72 cm × 65 cm × 40 cm) was distributed at the 4 tiers of the stacked cage system, and the 3 different light treatments were randomly assigned in houses (Figure 1). The air temperature (T) and the relative humidity (RH) of the houses was maintained between 16°C and 36°C and between 40 and 60%, respectively, during the whole experiment, following the same environmental requirements for the birds at different ages. The hens were fed without restriction, and unlimited water was provided during the whole experiment period. All the layer chickens in this experiment were managed by trained staff with standing guidelines for the Jinghong laying hens.
Figure 1.
Oblique view of part of the cages and the experimental treatment arrangements.
Light Design
Since there is a difference in spectral sensitivity between humans and poultry, and the perceived illuminance depends on the spectral power distribution of a light source and the chicken sensitivity to the particular wavelengths, the lux (based on human spectral sensitivity) is not strictly appropriate for describing levels of illuminance in poultry houses (Philips Lighting, 1988b; Nuboer et al., 1992a; Lewis and Morris, 2000). The clux (described as the irradiance of the light source, measured at bird head height in 5 nm intervals, was obtained by integrating for per unit wavelength) can represent the illuminance perceived by the poultry (Prescott and Wathes, 1999; Kristensen et al., 2007).
Light intensities in the WL group and HBGL group were set according to daily production management, and the unit was lux. The value of light intensity in the PBGL group was the same as that in WL and HBGL, the unit of which was clux. The photoperiod and light intensity in this experiment are shown in Table 1.
Table 1.
Light program.
| Day(d)/week (wk) | Photoperiod (h) | Light intensity(lux) | Light intensity (clux) | Clux/lux |
|---|---|---|---|---|
| 1–3 d | 24 | 50 | 50 | 2.28 |
| 4–7 d | 22 | 50 | 50 | 2.28 |
| 2 wk | 20 | 30 | 30 | 2.13 |
| 3 wk | 18 | 15 | 15 | 1.99 |
| 4 wk | 16 | 8 | 8 | 1.92 |
| 5 wk | 14 | 8 | 8 | 1.92 |
| 6 wk | 12 | 8 | 8 | 1.92 |
| 7 wk | 10 | 8 | 8 | 1.92 |
| 8–13 wk | 9 | 8 | 8 | 1.92 |
The LED lamp used in the experiment was a strip tube (220 V/5 A, Wuxi Huazhaohong Optoelectronics Technology Co., Ltd., China), mounted vertically in front of the cage to improve light uniformity. The spectral characteristics involved in this study are shown in Figure 2. The average light intensity (lux) at the level of the chicken head in the middle of each chicken cage was measured with an illuminance meter (SRI-2000, Shangze Optoelectronics Co., Ltd., Taiwan, China), and based on spectral composition, the light intensity (lux) was converted to clux. A shading cloth was installed between adjacent individual cages to avoid unintended irradiation to the hens from the lamps in the other cages. In order to adequately block light, the connection section between feeder and drinker in the adjacent cages was sealed by opaque tape. The surface of the lamp was wiped with 75% alcohol regularly to avoid excessive dust affecting the light intensity.
Figure 2.
Spectral characteristics.
Immune Performance
At 7 wk of age (50 d) and 13 wk of age (92 d), 6 pullets (the different individuals) were randomly selected in each light treatment group, and blood from the right jugular vein was taken with a disposable collection needle. The collected samples were stored under conditions of −20°C before being delivered to Beijing Huaying Biotechnology Research Institute (Beijing, China) for tests the same day. The immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) levels were determined using the colorimetric method (Mindray BS-420 automatic biochemical analysis instrument, Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China).
Bone Development
At 7 wk of age (50 d) and 13 wk of age (92 d), 6 pullets (the different individuals) were randomly selected in each light treatment group, and their left leg and right leg tibia were immediately removed after euthanization. The skin and flesh of the leg tibia were removed with a medical scalpel, soaked, and wrapped in gauze with saline, the left tibia was tested for tibia traits and the right tibia was tested for weight and strength within 12 h. The tibia traits (bone mineral density [BMD], bone mineral content [BMC], and bone area [BA]) were detected by a dual-energy X-ray bone mineral density instrument (Lunar-iDXA, GE Healthcare, Madison, WI). The tibia weight was detected with a thousandth electronic balance (JM, Shanghai Lingke Industrial Development Co., Ltd., China) and the tibia strength was detected with a universal material testing machine (INSTRON3367, USA).
Total RNA Isolation and Real-Time Quantitative PCR
In the morning of the 7th wk (50 days old) and 13th wk (92 days old), 3 pullets (the different individuals) were randomly selected in each light treatment group. After euthanization, the pineal gland, hypothalamus, spleen, and tibia were separated under low illumination light conditions. The collected samples were stored under conditions of −80°C for total RNA extraction. Immunoglobulin G-γ (IgG-γ mRNA) abundance in the spleen, osteoclastogenesis inhibitory factor gene (OPG mRNA) abundance in the tibia, and melanopsin gene (OPN4 mRNA) abundance in the hypothalamus and pineal tissue were performed for fluorescence using real-time quantitative PCR analysis.
Statistical Analysis
All statistical analyses were performed using general linear models (GLE) parameterized with SPSS (IBM SPSS Statistics 23.0, Armonk, NY). The sample order and body weight were the random factor, whereas the sampling week and the light treatment were the fixed factors. The linear mixed model equation was as following:
where Yijkl is the trait we have investigated; µ is the model constant; LTi is the effect of light treatment (i = 1 to 3); SWj is the effect of sampling week (j = 7, 13); SOk is the effect of sample order (k = 1 to 6); BWl is the effect of body weight.
The effects in the statistical model were tested simultaneously, and the effects were removed from the original model when they were not significant. When the effect was statistically different (P < 0.05), further analysis was needed. The independent sample t test (t test) was applied for post hoc group comparisons. The data are presented as the means ± standard error (SE).
RESULTS AND DISCUSSIONS
Effects of Blue-Green Lights With Two Perceived Illuminance (Human and Poultry) on the Immune Performance of Layer Chickens
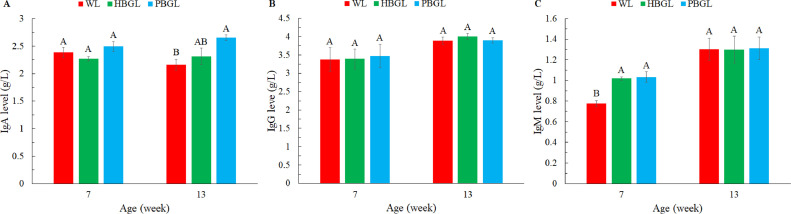
The influence of blue-green lights with 2 perceived illuminance (human and poultry) on the immune performance of layer chickens is shown in Figure 3. The immunoglobulin M (IgM) content was significantly affected by age (P < 0.01), and the immunoglobulin A (IgA) and immunoglobulin G (IgG) content were not significantly affected by age. The results showed that at 7 wk of age, the content of IgM in the blue-green LED light (light intensity unit lux, HBGL) group and blue-green LED light (light intensity unit clux, PBGL) group were significantly higher than the IgM content of layer chickens under white LED light (light intensity unit lux, WL) group (P < 0.05), and there was no significant difference in the content of IgA and IgG of the layer chickens in each group. At 13 wk of age, the IgA content of chickens in the PBGL group was significantly higher than that of chickens in WL group (P < 0.05), and there was no significant difference in the content of IgG and IgM of the chickens in each group.
Figure 3.
Effects of blue-green lights with two perceived illuminance (human and poultry) on the immune performance of layer chickens. (A) IgA content; (B) IgG content; (C): IgM content. WL: white LED light (lux), HBGL: blue-green LED light (lux), and PBGL: blue-green LED light (clux). Data are presented as the means ± SE. A, B: Within a column in different weeks, different capital letters indicate significant differences (P < 0.05). Light intensities in the WL and HBGL groups were set according to daily production management, and the unit was lux. The value of the light intensity in the PBGL group was the same as that in WL and HBGL, the unit of which was clux.
The results of this research are inconsistent with a previous study on the promotion of blue-green light on IgG content (Wei et al., 2020), which may be caused by different lighting environments. However, the total Ig content of the HBGL group and the PBGL group was higher than that of the WL group, which is consistent with the research results of Xie et al. (2008a), Zhang et al. (2014) and Hassan et al. (2013), that blue and green light can promote the immune performance of laying hens. This is because laying hens tend to be quiet under blue and green light, and have little stress response to the environment, which may lead to increased overall immunoglobulin concentration and enhanced immune performance. In addition, there are 2 ways of light acting on poultry: visual effects and nonvisual effects (Jácome et al., 2014). During the brooding and early rearing periods of laying hens, the effects of lighting on the physiology and welfare of layer chickens are mediated predominantly by vision. Moreover, research on the effects of different light color on laying hens have conflicting results (Pyrzak et al., 1987; Wei et al., 2020). The reason may be that the light intensity was not controlled in past studies of the light color on poultry behavior, physiology, and production, or the light intensity was measured in an energy unit or lux.
Serum immunoglobulin (IgA, IgG, and IgM) are important indicators of the functional status of the body's humoral immune system; their content can show the strength of the immune function, and they play an important role in immune regulation and defense against infection (Narat, 2003). IgA plays an important role in protecting the body's intestines, respiratory tract, and eyes against microbial infections (Lillehoj and Trout, 1996; Carsetti et al., 2010); IgG's main role is antibacterial, antiviral, and neutralizing toxins, and it plays an important role in anti-infection (Lillehoj and Trout, 1996; Narat, 2003); and IgM combines to dissolve pathogenic bacteria to achieve immune effects (Larsson et al., 1993; Ratcliffe, 2006). Blue-green light has a significant effect on the immune performance of the layer chickens, but whether it will continue to affect the immune performance of the laying hens remains to be explored.
Effects of Blue-Green Lights With Two Perceived Illuminance (Human and Poultry) on the Skeletal Development of Layer Chickens
The influence of blue-green lights with 2 perceived illuminance (human and poultry) on the bone parameters of the layer chicken is shown in Table 2. The tibial bone mineral density (BMD), bone mineral content (BMC), and bone area (BA) of laying hens increased as the pullets aged (P < 0.01). At 7 wk of age, the BMD of layer chickens in the white LED light (light intensity unit lux, WL) group was significantly higher than that of the blue-green LED light (light intensity unit lux, HBGL) group (P < 0.05), and there was no significant difference in the BMC and BA of the pullets in each group. At 13 wk of age, the BMD of the pullets in the WL group was significantly higher than that in the blue-green LED light (light intensity unit clux, PBGL) group (P < 0.05); there was no significant difference in the BMC of the pullets in each group, and the BA content of pullets in the PBGL group was significantly higher than in the WL group (P < 0.05).
Table 2.
Effects of blue-green lights with two perceived illuminance (human and poultry) on the bone parameters of layer chickens.
| Age (Wk) | Treatment | Bone mineral density (g/cm2) | Bone mineral content (g) | Bone mineral area (cm2) |
|---|---|---|---|---|
| 7 | WL | 0.153 ± 0.002a | 0.830 ± 0.030a | 5.53 ± 0.09a |
| HBGL | 0.146 ± 0.002b | 0.746 ± 0.069a | 5.16 ± 0.34a | |
| PBGL | 0.151 ± 0.001ab | 0.810 ± 0.020a | 5.54 ± 0.07a | |
| 13 | WL | 0.177 ± 0.001a | 1.336 ± 0.048a | 7.52 ± 0.11b |
| HBGL | 0.174 ± 0.002ab | 1.360 ± 0.063a | 7.73 ± 0.11ab | |
| PBGL | 0.172 ± 0.001b | 1.366 ± 0.018a | 8.05 ± 0.06a |
WL: white LED light (lux), HBGL: blue-green LED light (lux), and PBGL: blue-green LED light (clux). Data are presented as the means ± SE.
Within a column at the same age, different lowercase letters indicate significant differences (P < 0.05). Light intensities in the WL and HBGL groups were set according to daily production management, and the unit was lux. The value of the light intensity in the PBGL group was the same as that in WL and HBGL, the unit of which was clux.
BMD is a biophysical parameter with experimental and clinical significance; it is an important indicator of bone quality and can reflect bone development (Riczu et al., 2004; Amoroso et al., 2013), and it is positively correlated with bone strength (Mccoy et al., 1996). The research results show that white LED light can improve bone mineral density, probably because white light belongs to full-wavelength light, and the long-wavelength light part contains higher energy, which can directly pass through the skull of the layer and act on the hypothalamus, synthesizing estradiol (E2) that is essential for stimulating the switch from structural bone to medullary bone deposition of calcium in bones, such as the tibia (Baxter et al., 2014; Bédécarrats, 2015; Bain et al., 2016; Takeshima et al., 2019). In this study, blue-green LED light (light intensity unit lux) did not increase bone parameters, which is inconsistent with previous results of high illumination light increasing chicken activity and enhancing bone quality (Renema and Robinson, 2001; Li et al., 2018), which may be due to the chicken ages, breed, and different light sources.
Figure 4 shows the effect of blue-green lights with two perceived illuminance (human and poultry) on the weight and strength of the tibia of the layer chickens. The weight and strength of the tibia of the layer chickens was significantly affected by age (P < 0.01). At 7 and 13 wk of age, there was no significant difference in the tibia weight of the pullets in each group. At 7 wk of age, there was no significant difference in the tibia strength of the pullets in each group. At 13 wk of age, the tibia strength of pullets in the WL group was significantly higher than the tibia strength of pullets in the HBGL and PBGL groups (P < 0.05).
Figure 4.
Effects of blue-green lights with two perceived illuminance (human and poultry) on tibia weight and strength of layer chickens. (A) Tibia weight; (B) Tibia strength. WL: white LED light (lux), HBGL: blue-green LED light (lux), and PBGL: blue-green LED light (clux). Data are presented as the means ± SE. A, B: Within a column in different weeks, different capital letters indicate significant differences (P < 0.05). Light intensities in the WL and HBGL groups were set according to daily production management, and the unit was lux. The value of the light intensity in the PBGL group was the same as that in WL and HBGL, the unit of which was clux.
The results of this research are inconsistent with previous observations that high-illuminance light stimulated the activity of laying hens and increased bone strength (Pang et al., 1974; Newberry et al., 1988). There may be a threshold between the light color and the light intensity. Only when this range is exceeded, the laying hens will make corresponding physiological and behavioral responses to the light (Prayitno et al., 1997). Studies have shown that the direct cause of osteoporosis in laying hens is low activity in caged laying hens rather than malnutrition (Rennie et al., 1997; Bishop et al., 2000). Therefore, it is far from enough to prevent osteoporosis by simply supplementing feed nutrition. Light environment regulation is one of the effective methods to increase the activity of laying hens, which can achieve the effect of preventing osteoporosis. Previously results demonstrated that the bone development of layers during pullet rearing determined subsequent production performance and mortality (Fleming et al., 2003; Hester et al., 2011). Thus, by advancing early bone growth of chicks through light stimulation will have long-term health benefits in reducing osteoporosis in laying hens at the end of the laying period.
Effects of Blue-Green Lights With Two Perceived Illuminance (Human and Poultry) on the Expression of Related Genes in Layer Chickens
The effect of blue-green lights with 2 perceived illuminance (human and poultry) on the expression of immunoglobulin G-γ (IgG-γ) in the spleen of layer chickens is shown in Figure 5. The IgG-γ mRNA expression level was significantly affected by the chickens’ age (P < 0.01). In terms of gene expression, at 7 wk of age, there was no significant difference in the spleen IgG-γ mRNA expression of each group of pullets. At 13 wk of age, the expression level of IgG-γ mRNA in the spleen of pullets in the white LED light (light intensity unit lux, WL) group was significantly higher than that in the blue-green LED light (light intensity unit lux, HBGL) group (P < 0.01), and the expression level of IgG-γ mRNA in the spleen of pullets in the blue-green LED light (light intensity unit clux, PBGL) group was significantly higher than that in the HBGL group (P < 0.01).
Figure 5.
Effect of blue-green lights with two perceived illuminance (human and poultry) on the spleen IgG-γ mRNA express of layer chickens. WL: white LED light (lux), HBGL: blue-green LED light (lux), and PBGL: blue-green LED light (clux). Data are presented as the means ± SE. A, B: Within a column in different weeks, different capital letters indicate significant differences (P < 0.05). Light intensities in the WL and HBGL groups were set according to daily production management, and the unit was lux. The value of the light intensity in the PBGL group was the same as that in WL and HBGL, the unit of which was clux.
The spleen is the largest peripheral lymphoid organ in chickens, and it is mainly involved in humoral and cellular immunity (Zhang et al., 2019). IgG-γ mRNA expressing cells can be observed in spleen sections (Zheng et al., 2000). However, there was no significant difference in the immunoglobulin G of the pullets in this paper, which may be because the expression of spleen IgG-γ mRNA is more involved in the body's cellular immunity (Lewis et al., 2019; Song et al., 2021).
Figure 6 shows the expression of the osteoprotegerin gene (OPG mRNA) in the tibia of each group of pullets at different weeks. The tibia OPG mRNA expression level was not significantly affected by chicken age. The results showed that at 7 and 13 wk of age, the expression level of the OPG gene in the tibia of the WL group was significantly higher than that of the HBGL and PBGL groups (P < 0.05).
Figure 6.
Effect of blue-green lights with two perceived illuminance (human and poultry) on tibia OPG mRNA express of layer chickens. WL: white LED light (lux), HBGL: blue-green LED light (lux), and PBGL: blue-green LED light (clux). Data are presented as the means ± SE. A, B: Within a column in different weeks, different capital letters indicate significant differences (P < 0.05). Light intensities in the WL and HBGL groups were set according to daily production management, and the unit was lux. The value of the light intensity in the PBGL group was the same as that in WL and HBGL, the unit of which was clux.
The effect of WL on the tibia OPG gene expression level may be caused by different spectral composition. WL contains a higher proportion of long-wavelength light, which can activate the hypothalamo-pituitary-gonadal (HPG) axis (Prayitno et al., 1997; Bédécarrats, 2015; Bain et al., 2016), that indirectly influence inducing osteoclast differentiation and activation, and regulating OPG gene expression (Kong et al., 1999; Takeshima et al., 2019). Osteoprotegerin, also known as an osteoclast inhibitor, is mainly involved in the regulation of bone cells and bone resorption in the body, and OPG gene expression is positively correlated with bone density and bone mass (Benoit, 1964; Boyce and Xing, 2008). In this study, the OPG gene expression, bone mineral density, and bone strength of the pullets in the WL group increased significantly. It therefore follows that WL may promote the skeletal development of pullets by promoting the expression of the OPG gene in the tibia.
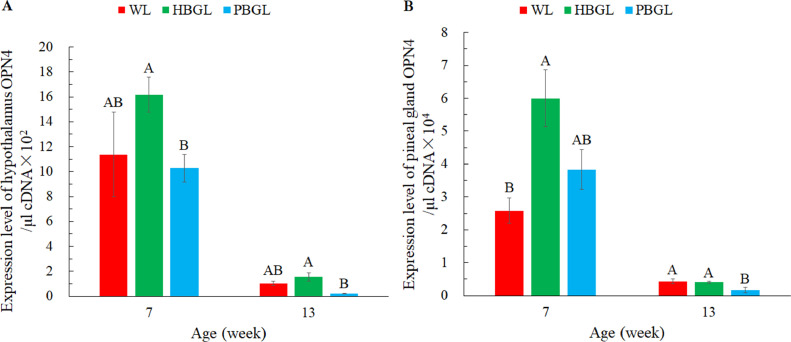
Figure 7 shows the effect of blue-green lights with 2 perceived illuminance (human and poultry) on the expression of the melanopsin (OPN4) gene in the hypothalamus and pineal gland of layer chickens. The expression level of the OPN4 gene in the hypothalamus and pineal gland of layer chickens were significantly affected by age (P < 0.01). According to the results of fluorescence real-time quantitative PCR, at 7 wk of age, the expression of OPN4 mRNA in the pineal gland of the HBGL group was significantly higher than that of the PBGL group, and the expression of OPN4 mRNA in the hypothalamus of the HBGL group was significantly higher than that of the WL group (P < 0.05). At 13 wk of age, the OPN4 mRNA expression of the pineal gland of the HBGL group was significantly higher than that of the PBGL group, and the hypothalamic OPN4 mRNA expression of the WL group and HBGL group were significantly higher than that of the PBGL group (P < 0.05).
Figure 7.
Effect of blue-green lights with two perceived illuminance (human and poultry) on the hypothalamus and pineal gland OPN4 mRNA express of layer chickens. (A) Hypothalamus OPN4 mRNA; (B) Pineal gland OPN4 mRNA. WL: white LED light (lux), HBGL: blue-green LED light (lux), and PBGL: blue-green LED light (clux). Data are presented as the means ± SE. A, B: Within a column in different weeks, different capital letters indicate significant differences (P < 0.05). Light intensities in the WL and HBGL groups were set according to daily production management, and the unit was lux. The value of the light intensity in the PBGL group was the same as that in WL and HBGL, the unit of which was clux.
The results of this paper are consistent with the previous research that chicken melanopsin cOPN4-1S and cOPN4-2L have blue-sensitive photopigments (Torii et al., 2007). However, the expression of OPN4 mRNA in the pineal gland and hypothalamus of the PBGL group did not increase significantly, possibly because the perceived light intensity did not reach the threshold for stimulating the pineal gland and hypothalamus (Prayitno et al., 1997). In poultry, there are 2 photoreceptors, called retinal photoreceptors on the retina (in the eye) and extra-retinal photoreceptors (in the pineal gland and the hypothalamus) (Kumar et al., 2004), that will coordinate light spectrum responses (Hanlon et al., 2020). Furthermore, an absorption spectrum with OPN4 was between 410 and 480 nm, and the maximum spectral absorption predicted reaching deep brain perception was 492 nm (Foster and Follett, 1985; Hanlon et al., 2020). Thus, blue light (lux) can't affect OPN4 expression by directly stimulating the deep brain. But after poultry are exposed to light, firstly, through the mediation of melanopsin, then, integrating the incoming information of cone cells and rod cells, the light response process of the nonvisual imaging system is initiated in the body to produce different physiological responses (Bailey and Cassone, 2005; Torii et al., 2007). However, the interaction and specific underlying mechanisms of light on retinal and extracretinal receptors in chickens have yet to be determined.
Melanopsin is a photoreceptor pigment protein expressed by autonomous photoreceptor ganglion cells, and it is expressed in poultry retina, hypothalamus, pineal gland, and suprachiasmatic nucleus (Chaurasia et al., 2005). Among them, the vertebrate melanopsin encoding genes are OPN4-1 (OPN4x or OPN4a) and OPN4-2 (OPN4m or OPN4b), and only OPN4-2 is expressed in mammalian retina, while poultry retina expresses OPN4-1 and OPN4-2 (Kumar et al., 2007). And the transcription of OPN4-1 and OPN4-2 genes in the pineal gland of chickens not only showed significant circadian rhythm (Holthues et al., 2004), but also showed tissue difference (Tomonari et al., 2007). In addition, the expression of melanopsin is also related to the synthesis of melatonin (MEL) (Lucas, 1999), and the secretion of MEL affects the body's biological rhythm and immune function (Holthues and Vollrath, 2004; Xie et al., 2008a,b). Therefore, blue-green LED light (light intensity unit lux, HBGL) may increase the content of melanopsin by promoting the expression of the OPN4 gene expression in the hypothalamus and pineal gland, and cooperate with melatonin to positively regulate the physiological metabolism and growth and development of the pullets. In addition, the results of this study can also provide a theoretical basis for how the blue-green light affects the immune function of laying hens.
CONCLUSIONS
Blue-green LED light (light intensity unit lux and clux) can increase the immunoglobulin content of layer chickens (0–13 wk old), white LED light (light intensity unit lux) can increase the bone mineral density and bone strength of the tibia by promoting the expression of tibial osteoclastogenesis inhibitory factor (OPG) mRNA, and blue-green LED light (light intensity unit lux) can increase the expression of melanopsin (OPN4) mRNA in the hypothalamus and pineal gland. In summary, blue-green LED light with two perceived illuminance (light intensity unit lux and clux) can improve the immune function of layer pullets, but blue-green LED light (light intensity unit lux) can promote the expression of OPN4 gene in the hypothalamus and pineal gland. In addition, white LED light (light intensity unit lux) increases the expression of OPG genes in the tibia to elevate bone quality. This result can provide a theoretical basis for the selection of suitable light for the brooding and early rearing periods of laying hens.
ACKNOWLEDGMENTS
The animal study proposal was approved by The Laboratory Animal Ethical Committee of China Agricultural University. This study was funded by the National Key R&D Program of China (2017YFB0404000). Thanks are extended to our colleagues at the Shangzhuang experimental station and the Department of Agricultural Structure and Environmental Engineering, College of Water Resources & Civil Engineering, China Agricultural University, Beijing, China.
REFERENCES
- Amoroso L., Baraldi A., Barreiro F., Pacheco M., Alva J., Soares N., Pacheco L., Melaré M. Bone densitometry and calcium serum levels in chickens treated with filtered or unfiltered water. Rev. Bras. Ciênc. Avíc. 2013;15:379–384. [Google Scholar]
- Bailey M.J., Cassone V.M. Melanopsin expression in the chick retina and pineal gland. Mol. Brain. Res. 2005;134:345–348. doi: 10.1016/j.molbrainres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Bain M.M., Nys Y., Dunn I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016;57:330–338. doi: 10.1080/00071668.2016.1161727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M., Joseph N., Osborne V.R., Bédécarrats G.Y. Red light is necessary to activate the reproductive axis in chickens independently of the retina of the eye. Poult. Sci. 2014;93:1289–1297. doi: 10.3382/ps.2013-03799. [DOI] [PubMed] [Google Scholar]
- Bédécarrats G.Y. Control of the reproductive axis: Balancing act between stimulatory and inhibitory inputs. Poult. Sci. 2015;94:810–815. doi: 10.3382/ps/peu042. [DOI] [PubMed] [Google Scholar]
- Benoit J. The role of the eye and of hypothalamus in the photostimulation of gonads in the duck. Ann. N. Y. Acad. Sci. 1964;117:204–215. doi: 10.1111/j.1749-6632.1964.tb48175.x. [DOI] [PubMed] [Google Scholar]
- Bishop S.C., Fleming R.H., McCormack H.A., Flock D.K., Whitehead C.C. Inheritance of bone characteristics affecting osteoporosis in laying hens. Br. Poult. Sci. 2000;41:33–40. doi: 10.1080/00071660086376. [DOI] [PubMed] [Google Scholar]
- Boyce B.F., Xing L.P. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsetti R., Rosado M.M., Wardmann H. Peripheral development of B cells in mouse and man. Immunol. Rev. 2010;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- Chaurasia S.S., Rollag M.D., Jiang G., Hayes W.P., Haque R., Natesan A., Iuvone P.M., Provencio I. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J. Neurochem. 2005;92:158–170. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- Fleming R.H., Mccormack H.A., Mcteir L., Whitehead C.C. Effects of dietary particulate limestone, vitamin K3 and fluoride and photostimulation on skeletal morphology and osteoporosis in laying hens. Br. Poult. Sci. 2003;44:683–689. doi: 10.1080/00071660310001643688. [DOI] [PubMed] [Google Scholar]
- Foster R.G., Follett B.K. The involvement of a rhodopsin-like photopigment in the photoperiodic response of the Japanese quail. J. Comp. Physiol. A. 1985;157:519–528. [Google Scholar]
- Hanlon C., Ramachandran R., Zuidhof M.J., Bédécarrats G.Y. Should I lay or should I grow: photoperiodic versus metabolic cues in chickens. Front. Physiol. 2020;11:707. doi: 10.3389/fphys.2020.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M.R., Sultana S., Choe H.S., Ryu K.S. Effect of monochromatic and combined light colour on performance, blood parameters, ovarian morphology and reproductive hormones in laying hens. Ital. J. Anim. Sci. 2013;12:359–364. [Google Scholar]
- Hester P.Y., Wilson D.A., Settar P., Arango J.A., O'Sullivan N.P. Effect of lighting programs during the pullet phase on skeletal integrity of egg-laying strains of chickens. Poult. Sci. 2011;90:1645–1651. doi: 10.3382/ps.2011-01411. [DOI] [PubMed] [Google Scholar]
- Holthues H., Engel L., Spessert R., Vollrath L. Circadian gene expression patterns of melanopsin and pinopsin in the chick pineal gland. Biochem. Bioph. Res. Co. 2004;326:160–165. doi: 10.1016/j.bbrc.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Holthues H., Vollrath L. The phototransduction cascade in the isolated chick pineal gland revisited. Brain. Res. 2004;999:175–180. doi: 10.1016/j.brainres.2003.11.059. [DOI] [PubMed] [Google Scholar]
- Jácome I.M.T.D., Rossi L.A., Borille R. Influence of artificial lighting on the performance and egg quality of commercial layers: a review. Braz. J. Poult. Sci. 2014;16:337–344. [Google Scholar]
- Kong Y.Y., Yoshida H., Sarosi I., Tan H.L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A.J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C.R., Lacey D.L., Mak T.W., Boyle W.J., Penninge J.M. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Kristensen H.H., Prescott N.B., Perry G.C., Ladewiga J., Ersbøllb A.K., Overvada K.C., Wathesc C.M. The behavior of broiler chickens in different light sources and illuminances. Appl. Anim. Behav. Sci. 2007;103:75–89. [Google Scholar]
- Kumar S., Nayak T.Jegla, Panda S. Role of a novel photopigment, melanopsin, in behavioral adaptation to light. Cell. Mol. Life. Sci. 2007;64:144–154. doi: 10.1007/s00018-006-5581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Singh B.P., Rani S. The bird clock: a complex, multioscillatory and highly diversified system. Biol. Rhythm Res. 2004;35:121–144. [Google Scholar]
- Larsson A., Balow R.M., Lindahl T.L., Forsberg P.O. Chicken antibodies: taking advantage of evolution- a review. Poult. Sci. 1993;72:1807–1812. doi: 10.3382/ps.0721807. [DOI] [PubMed] [Google Scholar]
- Lewis P.D., Morris T.R. Poultry and coloured light. World Poult. Sci. J. 2000;56:189–207. [Google Scholar]
- Lewis S.M., Williams A., Eisenbarth S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019;4:1–12. doi: 10.1126/sciimmunol.aau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.M., Li B.M., Zhao Y., Shi Z.X., Liu Y., Zheng W.C. Layer pullet preferences for light colors of light-emitting diodes. Animal. 2018;13:1245–1251. doi: 10.1017/S1751731118002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H.S., Trout J.M. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 1996;9:349–360. doi: 10.1128/cmr.9.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Xin H.W., Sekhon J., Wang T. Effect of fluorescent vs. poultry-specific light-emitting diode lights on production performance and egg quality of W-36 laying hens. Poult. Sci. 2017;97:834–844. doi: 10.3382/ps/pex371. [DOI] [PubMed] [Google Scholar]
- Lucas R.J. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- Manser C.E. Effects of lighting on the welfare of domestic poultry: a review. Anim. Welfare. 1996;5:341–360. [Google Scholar]
- Mccoy M.A., Reilly G.A.C., Kilpatrick D.J. Density and breaking strength of bones of mortalities among caged layers. Res. Vet. Sci. 1996;60:185–186. doi: 10.1016/s0034-5288(96)90017-x. [DOI] [PubMed] [Google Scholar]
- Narat M. Production of antibodies in chickens. Food. Technol. Biotechnol. 2003;41:259–267. [Google Scholar]
- Newberry R.C., Hunt J.R., Gardiner E.E. Influence of light intensity on behavior and performance of broiler chickens. Poult. Sci. 1988;67:1020–1025. doi: 10.3382/ps.0671020. [DOI] [PubMed] [Google Scholar]
- Nuboer J.F.W., Coemans M.A.J.M., Vos J.J. Artificial lighting in poultry houses: do hens perceivethe modulation of fluorescent lamps as flicker? Br. Poult. Sci. 1992;33:123–133. doi: 10.1080/00071669208417449. [DOI] [PubMed] [Google Scholar]
- Nuboer J.F.W., Coemans M.A.J.M., Vos J.J. Artificial lighting in poultry houses: are photometric units appropriate for describing illumination intensities? Br. Poult. Sci. 1992;33:135–140. doi: 10.1080/00071669208417449. [DOI] [PubMed] [Google Scholar]
- O'connor E., Parker M., Davey E., Grist H., Owen R., Szladovits B., Abeyesinghe S. Effect of low light and high noise on behavioural activity, physiological indicators of stress and production in laying hens. Br. Poult. Sci. 2011;52:666–674. doi: 10.1080/00071668.2011.639342. [DOI] [PubMed] [Google Scholar]
- Pang S.F., Ralph C.L., Reilly D.P. Melatonin in the chicken brain: its origin, diurnal variation, and regional distribution. Gen. Comp. Endocr. 1974;22:499–506. doi: 10.1016/0016-6480(74)90026-4. [DOI] [PubMed] [Google Scholar]
- Philips Lighting . Pages 3–26 in Vision. volume 4. Philips Lighting B. V.; The Netherlands: 1988. Correspondence course lighting application. [Google Scholar]
- Phillips C.J.C., Piggins D. CAB International; Oxford, UK: 1992. Pages 49–65 in Environmental Factors Influencing the Production and Welfare of Farm Animals: Photoperiod. [Google Scholar]
- Prayitno D., Phillips C., Stokes D. The effects of color and intensity of light on behavior and leg disorders in broiler chickens. Poult. Sci. 1997;76:1674–1681. doi: 10.1093/ps/76.12.1674. [DOI] [PubMed] [Google Scholar]
- Prescott N.B., Wathes C.M. Spectral sensitivity of the domestic fowl (Gallus g. domesticus) Br. Poult. Sci. 1999;40:332–339. doi: 10.1080/00071669987412. [DOI] [PubMed] [Google Scholar]
- Prescott N.B., Wathes C.M., Jarvis J.R. Light, vision and the welfare of poultry. Anim. Welfare. 2003;12:269–288. [Google Scholar]
- Pyrzak R., Snapir N., Goodman G., Perek M. The effect of light wavelength on the production and quality of eggs of the domestic hen. Theriogenology. 1987;28:947–960. [Google Scholar]
- Ratcliffe M.J.H. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev. Comp. Immunol. 2006;30:101–118. doi: 10.1016/j.dci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Renema R.A., Robinson F.E. Effects of light intensity from photostimulation in four strains of commercial egg layers: 1. ovarian morphology and carcass parameters. Poult. Sci. 2001;80:1112–1120. doi: 10.1093/ps/80.8.1112. [DOI] [PubMed] [Google Scholar]
- Rennie J.S., Fleming R.H., McCormack H.A., McCorquodale C.C., Whitehead C.C. Studies on effects of nutritional factors on bone structure and osteoporosis in laying hens. Br. Poult. Sci. 1997;38:417–424. doi: 10.1080/00071669708418012. [DOI] [PubMed] [Google Scholar]
- Riczu C.M., Saunders-Blades J.L., Yngvesson A.K., Robinson F.E., Korver D.R. End-of-Cycle bone quality in White- and Brown-Egg laying hens. Poult. Sci. 2004;83:375–383. doi: 10.1093/ps/83.3.375. [DOI] [PubMed] [Google Scholar]
- Shi H.P., Li B.M., Tong Q., Zheng W.C., Zeng D., Feng G.B. Effects of LED light color and intensity on feather pecking and fear responses of layer breeders in natural mating colony cages. Animals. 2019;9:1–15. doi: 10.3390/ani9100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B.C., Tang D.Z., Yan S.J., Fan H., Li G., Shahid M.S., Mahmood T., Guo Y.M. Effects of age on immune function in broiler chickens. J. Anim. Sci. Biotech. 2021;12:1–12. doi: 10.1186/s40104-021-00559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima K., Hanlon C., Sparling B., Bédécarrats G.Y. Spectrum lighting during pullet rearing and its impact on subsequent production performance in layers. J. Appl. Poult. Res. 2019;28:1262–1278. [Google Scholar]
- Tomonari S., Takagi A., Noji S., Ohuchi H. Expression pattern of the melanopsin-like (cOpn4m) and VA opsin-like genes in the developing chicken retina and neural tissues. Gene. Expr. Patterns. 2007;7:746–753. doi: 10.1016/j.modgep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Torii M., Kojima D., Okano T., Nakamuraa A., Terakitab A., Shichidab Y., Wadac A., Fukadaa Y. Two isoforms of chicken melanopsins show blue light sensitivity. FEBS. Lett. 2007;581:5327–5331. doi: 10.1016/j.febslet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Wei Y.X., Zheng W.C., Li B.M., Tong Q., Shi H.P. Effects of a two-phase mixed color lighting program using LED lights on layer chickens during brooding and rearing periods. Poult. Sci. 2020;99:4695–4703. doi: 10.1016/j.psj.2020.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widowski T.M., Duncan I.J.H. Laying hens do not have a preference for high-frequency versus low-frequency compact fluorescent light sources. Can. J. Anim. Sci. 1996;76:177–181. [Google Scholar]
- Xie D., Wang Z.X., Dong Y.L., Cao J., Wang J.F., Chen J.L., Chen Y.X. Effects of monochromatic light on immune response of broilers. Poult. Sci. 2008;87:1535–1539. doi: 10.3382/ps.2007-00317. [DOI] [PubMed] [Google Scholar]
- Xie D., Wang Z., Cao J., Dong Y., Chen Y. Effects of monochromatic light on proliferation response of splencyte in broilers. Anatom. Histol. Embryol. 2008;37:332–337. doi: 10.1111/j.1439-0264.2008.00849.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Sun X.J., Wang T.Z., Chen B., Huang Y.F., Chen H., Chen Q.S. The postembryonic development of the immunological barrier in the chicken spleens. J. Immunol. Res. 2019;2019:1–11. doi: 10.1155/2019/6279360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.Q., Cao J., Wang Z.X., Dong Y.L., Chen Y.X. Effect of a combination of green and blue monochromatic light on broiler immune response. J. Photoch. Photobio. B. 2014;138:118–123. doi: 10.1016/j.jphotobiol.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Zheng W.M., Izaki J., Furusawa S., Yoshimura Y. Localization of immunoglobulin G γ-Chain mRNA-expressing cells in the oviduct of laying and diethylstilbestrol-treated immature hens. Gen. Comp. Endocr. 2000;120:345–352. doi: 10.1006/gcen.2000.7573. [DOI] [PubMed] [Google Scholar]