Abstract
Campylobacteriosis is one of the most common types of bacterial gastroenteritis affecting humans, and poultry is considered a major source of the causative organism, Campylobacter spp. Broilers may arrive contaminated at slaughterhouses, and transport crates could be considered a potential source of contamination. Thus, cleaning and disinfection procedures are crucial to avoid cross-contamination among flocks. Despite its public health importance in Latin American countries, virulence factors of Campylobacter jejuni remain poorly studied in this region. Thus, this study aimed to: 1) determine the occurrence of contaminated crates at a poultry slaughterhouse, 2) compare the contamination before and after the cleaning and disinfection procedures, and 3) detect virulence-associated genes in C. jejuni strains by PCR. Campylobacter spp. were recovered from 8 of the 10 flocks evaluated, and C. jejuni was detected as the main species. There was no significant difference in the Campylobacter detection or quantification between crates at the reception platform and crates after the cleaning/disinfection processes. However, crates after 24 h of natural drying, presented a significant (P < 0.05) lower amount of Campylobacter cells than before the cleaning and disinfection processes. A negative relationship (R2 = 0.210, P = 0.045) between environmental conditions and Campylobacter quantification was found for transport crates after 24 h of natural drying. There was no significant difference (P > 0.05) in the detection of two C. jejuni virulence genes, flaA (encode a major flagellin protein) and cadF (encode an adhesion and fibronectin-binding protein), among various stages of the cleaning and disinfection processes. Our results demonstrate the high contamination levels of Campylobacter strains in broiler flocks and the potential involvement of poultry transport crates in transmitting these bacteria. This study also suggests that ineffective cleaning and disinfection procedures can increase Campylobacter contamination and facilitate the spread of bacteria in poultry establishments.
Key words: broiler, Campylobacter, cleaning and disinfection, drying, transport crate
INTRODUCTION
Campylobacter spp. is the agent of campylobacteriosis, one of the most common types of human bacterial gastroenteritis worldwide (CDC, 2021; EFSA and ECDC, 2021; Rodrigues et al., 2021; WHO, 2021). Poultry is considered a major reservoir of Campylobacter and a source of bacterial transmission to humans (Humphrey et al., 2014). Campylobacteriosis is usually associated with handling raw poultry, consuming raw or undercooked poultry meat, and cross-contamination of raw and cooked foods (Silva et al., 2011).
Broiler infection usually occurs on the farm, and during the transportation of live birds, stress could cause a disturbance of intestinal functions and may increase the spread of pathogens through feces (Keener et al., 2004; Hansson et al., 2005). As the crates are reused among flocks from different farms, they can be a potential reservoir for microorganisms, including Campylobacter spp., representing a risk of infection for susceptible broilers (Slader et al., 2002; Hansson et al., 2005; Hastings et al., 2011). Thus, cleaning and disinfection procedures are crucial to avoid cross-contamination among flocks. Previous studies have shown that Campylobacter contamination was retained or substantially increased when the cleaning and disinfection procedures were ineffective (Peyrat et al., 2008; Borges et al., 2020; Perdoncini et al., 2022).
Considering that Campylobacter may be transferred from animals to humans, it is important to know the genetic profile of circulating strains in broiler populations. However, despite its public health importance in Latin American countries, virulence factors and mechanisms of Campylobacter jejuni pathogenesis remain poorly studied (Levican et al., 2019). These virulence marks are associated with some of the mechanisms essential for bacterial survival and pathogenesis, including bacterial motility, adhesion, colonization/invasion of intestinal epithelial cells, production of toxins, and secreted proteins (Biswas et al., 2011; Bolton, 2015).
C. jejuni exhibits bipolar flagella, and its extracellular filament structural components are mainly composed of a major flagellin protein (FlaA; encoded by flaA), which is highly conserved among isolates (Guerry, 2007; Hermans et al., 2011). Campylobacter adhesion to fibronectin (CadF), encoded by cadF, is an adhesion and fibronectin-binding protein involved in the process of invasion, influencing the microfilament organization in host cells (Monteville et al., 2003; Bolton 2015).
The present study aimed to determine the occurrence of contaminated transport crates at slaughterhouses by qualitative and quantitative microbiological analysis, compare the contamination before and after the cleaning/disinfection procedures, and detect virulence-associated genes in C. jejuni strains.
MATERIALS AND METHODS
Experimental Design
Sample collection was performed via a cross-sectional observational study using convenience sampling. The samples were collected over a time period of nine months and were carried out in a slaughterhouse located in the Rio Grande do Sul state in southern Brazil. The slaughterhouse was under the authority of the Federal Inspection Service and had a slaughter capacity of 150,000 broilers per day.
A total of ten visits were conducted at the establishment, and at each visit, one flock was evaluated, totaling 10 flocks analyzed. For each flock, three crates were randomly sampled, totaling 30 crates at the end of sampling period. The crates were evaluated at three stages: reception platform after broiler removal, after cleaning and disinfection procedures in the transport crate sanitation room, and after 24 h of natural drying in a separated room.
Sample Collection
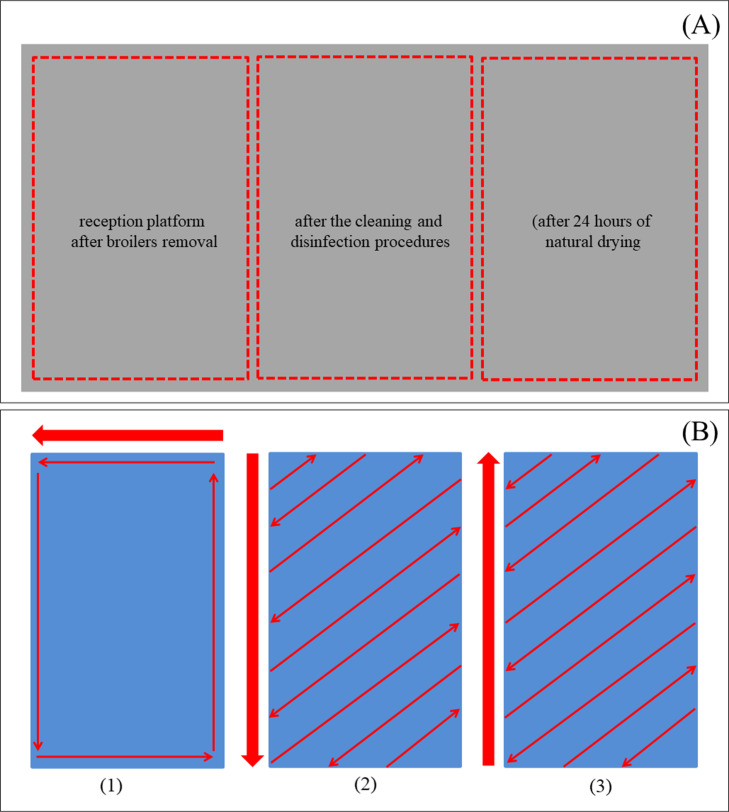
Crates selected for this study were made of high-density polyethylene, presented bottom surface with orifices, and all have the same size and weight (77 cm length × 57 cm width × 27 cm height, 5 kg weight). For sample collection, crates floor (bottom surface) was divided into three sections (25.6 cm × 19 cm each) using a paper mold, which was autoclaved at 121°C for 30 min prior to use and discarded after each sample collection. Crates were identified using a numbered security seal to identify the first quadrant to be collected: reception platform after broiler removal (left side of the compartment), immediately after the cleaning and disinfection procedures (center area of the compartment), and after 24 h of natural drying (right side of the compartment) (Figure 1A). Each section of the crate was swabbed throughout in the same direction using commercial sponge-sticks with neutralizing buffer (SSL10NB; 3MTM, Maplewood, MN) (Figure 1B). The sponges were packed in sterile bags with 50 mL of Brucella broth (Oxoid, Basingstoke, UK). After collection, all samples were transported to the laboratory in isothermal boxes containing ice. The samples were analyzed within 2 to 4 h of being transported from the slaughterhouse.
Figure 1.
Crate sampling. (A) Sections collected: platform after broiler removal (left side of the compartment), immediately after the cleaning and disinfection procedures (center area of the compartment), and after 24 h of natural drying (right side of the compartment). (B) Swab direction for all compartments: outer edges (1), top to bottom (2), bottom to top (3).
Cleaning and Disinfection Procedures of Transport Crates
The cleaning procedure of transport crates started immediately after removal of birds at the slaughterhouse and included the physical removal with water spray of fecal material, dirt, and debris. The cleaning and disinfection procedures were carried out in four stages within a spray tunnel: 1) water spray, 2) application of chlorinated alkaline detergent (Idrosan; AEB Group, Viseu, Portugal), 3) rinsing with water spray, and 4) application of 1% benzalkonium chloride-based disinfectant (Quatesan, AEB Group, Viseu, Portugal). After applying the disinfectant, the crates were not rinsed again. The compounds used did not change during the collection period. The water used in the tunnel was pumped at a pressure of 10 kg/cm2 with an average temperature of 45 to 50°C, pH 6.75 to 7.02, and chlorination at 0.3 to 2 ppm.
Temperature and Relative Humidity
The temperature and relative humidity inside the cleaning and disinfection rooms were measured during each visit using a digital thermometer (Thermo-Hygrometer AK28; Akso, São Leopoldo, Brazil). The thermometer was placed above the crates.
Qualitative Detection Method
Detection of Campylobacter spp. by conventional isolation was performed according to the methodology described by Sierra-Arguello et al. (2021). An aliquot (1 mL) of each sample was homogenized in Bolton broth (9 mL) (Oxoid) supplemented with antimicrobials (cefoperazone 20 mg/L, vancomycin 20 mg/L, trimethoprim 20 mg/L, and cycloheximide 50 mg/L; SR0183, Oxoid) and incubated under microaerophilic conditions using a gas tank with a mixture of 10% CO2, 5% O2, and 85% N2 for 48 h at 41.5°C. After incubation, 10 µL of the suspension was spread onto an acetate membrane with 0.65 µm pores (Sartorius, Gottingen, Germany) and placed on the surface of a modified charcoal cefoperazone deoxycholate agar (mCCDA) plate (Oxoid) with selective supplements (cefoperazone, 32 mg/L; amphotericin B, 10 mg/L; SR0155, Oxoid). The plate was incubated under microaerophilic conditions for 48 h at 41.5°C. Subsequently, one suspect colony (grayish, often with a metallic appearance, flat and moist with a tendency to spread) were seeded in blood agar (BA) plates (Oxoid) supplemented with 5% sterile defibrinated sheep blood (Laborclin, Curitiba, Brazil). Campylobacter spp. colonies were examined under a phase-contrast microscope (Olympus B201 optical microscope; Olympus Corporation, Tokyo, Japan) to monitor its typical movement and morphology (Fonseca et al., 2006). Colonies were characterized based on their ability to hydrolyze hippurate and indoxyl acetate, and catalase reaction (Public Health England, 2018). Isolates were stored in 15% glycerol at −80°C.
Quantification by Most Probable Number Method
The most probable number (MPN) method was used to quantify Campylobacter spp. in all crates, as described by Silva et al. (2017). Briefly, samples were homogenized for 20 s and an aliquot (1 mL) was transferred to 9 mL of Bolton broth supplemented with 2% novobiocin. Successive decimal dilutions were performed, in triplicate, in three tubes MPN series. Material was incubated under microaerophilic conditions for 48 h at 41.5°C. After incubation, samples were seeded on mCCDA agar with selective supplement and incubated under microaerophilic conditions for 48 h at 41.5°C. Colonies were confirmed based on their growth on BA supplemented with sterile defibrinated sheep blood, typical movement and morphology in phase-contrast microscopy, their ability to hydrolyze hippurate and indoxyl acetate, and catalase reaction. Counting was performed according to the protocol proposed by Blodgett (2006). Because of the lack of a normal distribution of the data, these values may not be compared by the calculation of simple means. Thus, the results were expressed as MPN/g and then transformed into log10 MPN/g to perform statistical analysis (Perdoncini et al., 2022). In addition, we used the formula described by Thomas Jr., (1942) to calculate the overall or pooled MPN for each stage of cleaning and disinfection processes:
where P is the number of positive tubes, N is the total quantity of sample in all negative tubes, and T is the total quantity of sample in all tubes.
DNA Extraction
The template DNA for PCR was extracted using a protocol adapted from Borsoi et al. (2009). Briefly, the bacterial culture (1 mL) was boiled at 95°C for 10 min. After centrifugation at 8,000 × g for 2 min, the supernatant was stored at −20°C and used as template DNA.
Multiplex-PCR Assay for Identification of Campylobacter spp
After bacterial isolation, Campylobacter spp. (C. jejuni and C. coli) were identified by multiplex PCR (Perdoncini et al., 2015). The primer sequences, amplicon size, and controls are described in Table 1. C. jejuni (ATCC 29428) and C. coli (ATCC 43478) were used as positive controls. Arcobacter spp. was used as a negative control.
Table 1.
Virulence-associated genes: target genes, primers, sequences, PCR conditions, amplicon size, gene function, and references.
| Target gene | Primers | Sequence (5ˊ→3ˊ) | PCR conditions | Amplicon size (bp) | Campylobacter species or gene function | References |
|---|---|---|---|---|---|---|
| 16S - rRNA | MD16S1 MD16S2 |
F: ATCTAATGGCTTAACCATTAAAC R: GGACGGTAACTAGTTTAGTATT |
35 cycles: 95°C – 30 s 59°C – 90 s 72°C – 60 s |
857 | Campylobacter genus | Denis et al., 1999; Linton et al., 2000 |
| mapA | MDmapA1 MDmapA2 |
F: CTATTTTATTTTTGAGTGCTTGTG R: GCTTTATTTGCCATTTGTTTTATTA |
589 | Campylobacter jejuni | Denis et al., 1999 | |
| ceuE | ceuE1 ceuE2 |
F: AATTGAAAATTGCTCCAACTATG R: TGATTTTATTATTTGTAGCAGCG |
462 | Campylobacter coli | Denis et al., 1999 | |
| flaA | flaAF flaAR |
F: GGATTTCGTATTAACACAAATGGTGC R: CTGTAGTAATCTTA AACATTTTG |
30 cycles: 94°C – 60 s 48°C – 60 s 72°C – 60 s |
1700 | encodes the major flagellin protein (FlaA) | http://campynet.vetinst.dk/Fla.htm |
| cadF | F2B R1B |
F:TGGAGGGTAATTTAGATATG R: CTAATACCTAAAGTTGAAAC |
30 cycles: 94°C – 60 s 54°C – 60 s 72°C – 60 s |
400 | encodes an adhesion and fibronectin-binding protein involved in the process of invasion | Konkel et al., 1999 |
PCR for the Detection of Virulence-Associated Genes (flaA and cadF)
The confirmed Campylobacter isolates were screened for the presence of two viluence-associated genes, flaA and cadF. The primers, PCR conditions, and amplicon size generated in this study are listed in Table 1. All PCR amplifications were performed in a reaction mixture (25 µL) consisting of 10X PCR Buffer (3 µL; 200 mM Tris–HCl [pH 8.4], 500 mM KCl; Invitrogen, Waltham, MA), Taq thermostable DNA polymerase (0.3 µL, 5 U/µL) (Invitrogen), MgCl2 (1.2 µL, 25 mM) (Invitrogen), dNTPs (2.5 µL, 2.5 mM) (Invitrogen), extracted template DNA (2 µL), and forward and reverse primers (0.5 µL each, 10 pmol/L) (Invitrogen). Sterile Milli-Q water was added in sufficient quantity to achieve a final volume of 25 µL. All reactions were performed in a thermal cycler (Swift MaxPro; Esco, Singapore), as described in Table 1. For visualization of the PCR products, 10 µL aliquots were subjected to electrophoresis in a 2% agarose gel in Tris-acetylated EDTA (TAE) buffer for 2 h at 100 V. The DNA bands stained with ethidium bromide were viewed under an ultraviolet transilluminator and photographed. The size of the PCR amplicons was compared to that of the 100 bp DNA ladder. C. jejuni (ATCC 33291) was used as a positive control.
Statistical Analysis
All statistical analyses were performed using SPSS Statistics software (version 22.0; IBM, Armonk, NY). Fisher's exact test was used to compare flock positivity by the qualitative detection method. One-way ANOVA, followed by Tukey's Honestly Significant Difference (HSD) test, was used to compare Campylobacter quantification by MPN (log10 MPN/g) at the three stages (Perdoncini et al., 2022). The t test was used to compare the average temperature and relative humidity of the crates. Simple and multiple linear regressions were performed to predict the influence of temperature and relative humidity on Campylobacter quantification. Statistical significance was set at P < 0.05, and Bonferroni correction was applied to adjust the confidence intervals for multiple hypothesis testing.
RESULTS
Qualitative Detection Method
Campylobacter spp. was recovered from 8 of 10 (80%) evaluated flocks. C. jejuni was detected in 6 (60%) flocks, whereas both C. jejuni and C. coli were simultaneously detected in 2 (20%) flocks. The results of the qualitative detection at each stage of cleaning and disinfection procedures are shown in Table 2. There were no significant differences (P > 0.05) in Campylobacter detection between the cleaning and disinfecting stages.
Table 2.
Campylobacter detection in 30 transport crates1: frequencies of positive crates by qualitative method, quantification by most probable number (MPN) method (log10 MNP/g), and overall or pooled MPN, according to the stage of cleaning and disinfection processes.
| Stage of cleaning and disinfection processes | Frequencies of Campylobacter detection % (n/N)2 |
Quantification of Campylobacter (log10 MPN/g)3 mean ± standard-deviation |
Overall or pooled MPN (MPN/g)4 |
|---|---|---|---|
| before the cleaning and disinfection processes | 63 (19/30)a | 0.81 ± 0.89a | 40.13 |
| after the cleaning and disinfection processes | 70 (21/30)a | 0.70 ± 0.77ab | 24.12 |
| after 24 hours of natural drying | 46 (14/30)a | 0.30 ± 0.57b | 5.79 |
Three transport crates of each flock, totaling 30 crates evaluated in this study.
Different letters in the same column represent significant differences among stages of cleaning and disinfection processes.
Fisher's exact test (adjusted P-value = 0.0169).
One-way ANOVA, followed by Tukey's Honestly Significant Difference (HSD) test (P < 0.05).
According to the formula described by Thomas (1942).
Quantification by Most Probable Number Method
The results of the quantitative detection according to the stages of cleaning and disinfection procedures are shown in Table 2. There was no significant difference (P > 0.05) in Campylobacter quantification between crates at the reception platform after broiler removal and crates after the cleaning/disinfection procedures. This is likely related to the significant variation within each group (0–1,100 MPN/g). However, after 24 h of natural drying, Campylobacter strains could not be detected or counted using the MPN method.
Temperature and Relative Humidity
Table 3 describes the temperatures and relative humidity obtained during the sample collection period, according to the cleaning and disinfection stages. Temperature and relative humidity remained similar (P > 0.05) before and after the cleaning and disinfection processes and during the collection after 24 h of natural drying.
Table 3.
Temperatures and relative humidity obtained1 over the sample collection period, according to the cleaning and disinfection stage: mean, standard-deviation, maximum, and minimum values.
| Parameters | During cleaning and disinfection process (before and after) |
At the collection after 24 h of natural drying |
||
|---|---|---|---|---|
| Temperature (°C) | Relative humidity (%) | Temperature (°C) | Relative humidity (%) | |
| Mean (SD) | 17 (5.43)a | 80 (10.38)a | 16.4 (5.97)a | 80 (9.09)a |
| Maximum | 25.2 | 90 | 24.3 | 90 |
| Minimum | 10.3 | 61 | 10 | 62 |
Different letters in the same line represent significant differences (P < 0.05) between temperatures OR relative humidity.
The temperature and relative humidity were measured inside the cleaning and disinfection rooms, and the thermometer was placed above the crates.
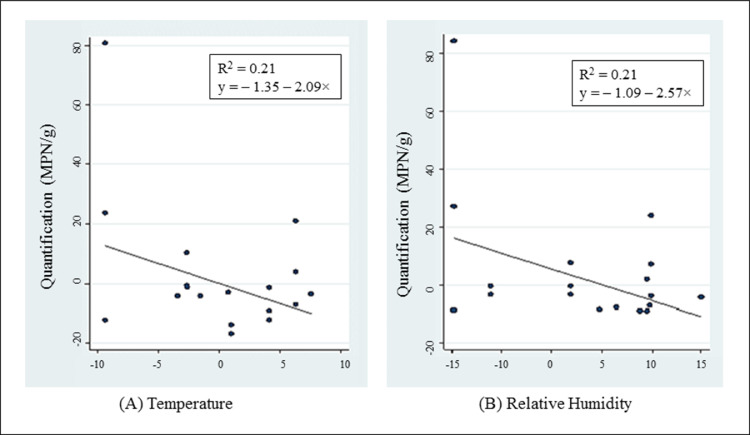
Simple linear regression was applied to predict the influence of temperature and relative humidity on the growth and survival of Campylobacter. MPN quantification in crates at the reception platform after broiler removal was not related to temperature (r2 = 0.090, P = 0.875) or relative humidity (r2 = 0.015, P = 0.513). Similarly, the levels of Campylobacter in crates after the cleaning and disinfection procedure were not associated with temperature (r2 = 0.037, P = 0.311) or relative humidity (r2 = 0.021, P = 0.442). Finally, no relationships were found in the quantification of crates after 24 h of natural drying with temperature (r2 = 0.017, P = 0.816) and relative humidity (r2 = 0.082, P = 0.124). No relationship was found between environmental conditions and Campylobacter quantification during the cleaning and disinfection procedures (before: R2 = 0.017, P = 0.497; after: R2 = 0.039,P = 0.485). However, a negative relationship (R2 = 0.210, P = 0.046) was found for crates after 24 h of natural drying, indicating that every unit increase in both temperature and relative humidity led to a decrease in the MPN/g values (Figure 2).
Figure 2.
Influence of the temperature and relative humidity on Campylobacter spp. contamination: multiple linear regression for crates after 24 h of natural drying.
Detection of Virulence-Associated Genes (flaA and cadF)
A total of 20 C. jejuni strains were identified in this study and were selected for detecting virulence-associated genes by PCR. The frequencies of flaA and cadF are listed in Table 4. There was no significant difference (P > 0.05) in the detection frequencies of the genes among various stages of cleaning and disinfection procedures.
Table 4.
Absolute and relative frequencies of virulence-associated genes, flaA and cadF, detected by PCR in Campylobacter jejuni strains (n = 20), according to the stage of cleaning and disinfection processes.
| Stage of cleaning and disinfection processes1 | Frequency of detection % (n/N) |
|
|---|---|---|
| flaA | cadF | |
| Before the cleaning and disinfection processes (n = 8) | 75 (6/8)a | 87.5 (7/8)a |
| After the cleaning and disinfection processes (n = 9) | 66.6 (6/9)a | 100 (9/9)a |
| After 24 h of natural drying (n = 3) | 100 (3/3)a | 100 (3/3)a |
| TOTAL (n = 20) | 75 (15/20) | 95 (19/20) |
Different letters in the same column represent significant difference (adjusted P-value = 0.0169) (Fisher's exact test).
Crates were swabbed using commercial sponge-sticks with neutralizing buffer (SSL10NB; 3MTM) and the sponges were packed in sterile bags with 50 mL of Brucella broth (Oxoid). The template DNA for PCR was extracted using a protocol adapted from Borsoi et al. (2009).
DISCUSSION
Although Brazil is among the largest producers and exporters of poultry worldwide, data on Campylobacter infections in Brazilian flocks are still insufficient (Gomes et al., 2018). In addition, there is currently no internal monitoring program adopted by the Brazilian poultry companies, which most likely explains the lack of national data (Borges et al., 2020). However, recent studies have shown that Campylobacter contamination is high among broiler flocks in this country (Würfel et al., 2019; Borges et al., 2020; Pozza et al., 2020; Perdoncini et al., 2022). Thus, control procedures during the slaughtering process are essential to avoid carcass contamination.
Previous studies have shown that most flocks arrive to the slaughterhouse with Campylobacter. Positive cloacal swabs at the reception of slaughterhouses show that broilers were contaminated on the farm (Borges et al., 2020; Sierra-Arguello et al., 2021; Perdoncini et al., 2022). Since the probability of a broiler to become colonized increases during rearing, it is estimated that 60–80% of the flocks may be Campylobacter-positive at slaughter age (Hermans et al. 2012). This is in agreement with our findings, since 80% (8/10) of the flocks tested positive for this pathogen in the present study. This high occurrence of Campylobacter in broiler transport crates may reflect a high prevalence of this microorganism among flocks during the pre-slaughter period. However, this hypothesis requires further evaluation.
As expected, C. jejuni was the most frequently detected species and is the main reported member of thermotolerant Campylobacter in chicken meat (Perdoncini et al., 2015; Skarp et al., 2016; Sinulingga et al., 2020; Perdoncini et al., 2022). In addition, the persistence of this pathogen in slaughterhouses has been reported worldwide (Melo et al., 2019). C. coli, also detected in this study, is the second most reported Campylobacter species that cause human infections (Gomes et al., 2018). However, these infections are usually sporadic and seasonal in high-income countries (Igwaran and Okoh, 2019).
A high level of contamination in the cloacal swabs suggested the likelihood of contaminated feces being deposited in transport crates, thereby increasing the risk of contamination. Our results indicate that 63% of the crates were Campylobacter-positive prior to the cleaning and disinfection processes. Contaminated crates, which are reused among flocks from different farms, can be a potential reservoir for Campylobacter spp. and represent a risk of infection for susceptible broilers (Slader et al., 2002; Berrang et al. 2003; Hastings et al., 2011). In addition, the ineffective cleaning and disinfection processes can contribute to increased levels of chicken meat contamination. According to the local animal health authorities, the insufficient cleaning of transport crates in one establishment was responsible to the spread of Campylobacter strains among farms in Sweden. The high levels of flocks contamination contributed to the occurrence of large outbreaks widespread in all regions of the country in 2 periods: 2016/2017 and 2020. In 2016/2017, more than 5,000 campylobacteriosis cases were reported by the authorities (Food Safety News 2020, Moazzami et al., 2021).
Thus, cleaning and disinfection processes are crucial to avoid cross-contamination among flocks. However, quantitative results from the MPN method have shown that values obtained before (0.81 log10 MPN/g) and after (0.70 log10 MPN/g) cleaning/disinfection processes present a non significant reduction in the number of total bacteria. In addition, the total number of positive crates increased from 19 to 21. This suggested an increase in the number of contaminated crates, even if the number of bacteria present in these crates remained similar. Similar results were observed by Perdoncini et al. (2022), who also described that crates from one flock that tested negative before cleaning and disinfection were positive after the process.
Several factors can explain the ineffectiveness of the cleaning and disinfection processes. The characteristics of crate surfaces, such as cracks, crevices, texture, and hydrophobic tendency, may also affect the ability of these processes to reduce Campylobacter contamination. Furthermore, long-term use of these crates induces damage to crate surfaces, creating more harborage sites for the accumulation of contaminated material (Berrang and Northcutt, 2005; Allen et al., 2008; Moazzami et al., 2021).
The process of transporting and slaughtering broilers is often hastened so that the crates can be reused on the same day to transport birds from different farms. Thus, these crates are potential vehicles for the transmission of Campylobacter (Dzieciolowski et al., 2022). However, previous studies have reported that drying transport crates for an extended period (24 h or 48 h) could effectively reduce the microbial load to an undetectable level (Berrang and Northcutt, 2005). In this study, the qualitative method detected Campylobacter in 46% of the crates, with no significant reduction compared to the contamination in the crates before and immediately after the cleaning and disinfection process. However, the quantitative method detect a significant Campylobacter reduction in these crates, when compared to the crates before the cleaning and disinfection procedures. This difference between the 2 techniques may be related to the limit of detection of the quantitative method, which is 3 MPN/mL for the three-tube MPN series (Perdoncini et al., 2022). This result indicates that natural drying crates favors the reduction of contamination. Recently, Dzieciolowski et al. (2022) have shown that using a chemical disinfectant in combination with hot air drying (dehumidifier) was the most effective treatment for significantly reducing bacterial contamination.
In addition to the characteristics of crate surfaces and slaughterhouses, the cleaning and disinfection processes themselves are important to guarantee a decrease in Campylobacter contamination. The type of treatment (such as manual wash, spray, dipping), the active compound, exposure time, sanitizer concentration, temperature, relative humidity, and pH may affect the process efficiency (Berrang and Northcutt, 2005; Moazzami et al., 2021). Thus, we also evaluated the influence of relative humidity and temperature on the bacterial load in the cleaning room. The average relative humidity and temperature before and after cleaning/disinfection processes were similar to those observed after 24 h of natural drying. Our results demonstrated that, when analyzed individually, these two factors did not influence bacterial contamination during the cleaning and disinfection processes. However, when these factors were analyzed together, they negatively affected the bacterial count after 24 h of drying, but not before and after the cleaning/disinfection processes. Both relative humidity and temperature have a major influence on the growth of Campylobacter spp. (Smith et al., 2016). Previous studies have shown that dry conditions at relatively warm temperatures cause stress in Campylobacter (Berrang et al., 2004; Dzieciolowski et al., 2022). These results are interesting because Campylobacter persists throughout slaughter, surviving at the temperatures associated with the different processing steps, drying conditions, and oxygen levels (Slader et al., 2002).
To reduce Campylobacter contamination on transport crates, researchers have investigated the efficacy of numerous physical, chemical, and biological methods for reducing the bacterial load. Consequently, new methods (such as heating, irradiation, and ultraviolet light) have been developed to act synergistically with temperature, relative humidity, and disinfectants to reduce contamination (Tam et al., 2006; Peyrat et al., 2008; Berrang et al., 2011; Moazzami et al., 2021; Dzieciolowski et al., 2022).
Studies during the last decade have demonstrated the increased pathogenic potential of C. jejuni isolates from Brazilian poultry (Melo et al., 2019). Thus, we also evaluated the pathogenic potential of C. jejuni strains based on the detection of two virulence markers; high overall frequencies of flaA (75%) and cadF (95%) were detected. The results were similar among the three sample collection stages. These high frequencies were expected, as previous studies have reported similar results (Wieczorek and Osek, 2008; Mizel and Bates, 2010; Rizal et al., 2010; Melo et al., 2019; Sierra-Arguello et al., 2021). The high occurrence of flaA, which codes for the flagellar protein FlaA, demonstrates its importance to the survival and viability of Campylobacter strains under varying environmental and stress-inducing conditions (Le et al., 2012). cadF is one of the markers that determine the adherence of Campylobacter spp.; it is required for maximal attachment and invasion of mammalian cells by binding to fibronectin (Monteville et al., 2003). CadF protein may play a role in gut colonization during human infection and may also be an important mediator of material and information transfer between the cells and their environment (Shoaf-Sweeney et al., 2008).
Our results demonstrate the high contamination of Campylobacter strains in broiler flocks and the potential involvement of poultry transport crates in the transmission of Campylobacter. However, it is important to emphasize that a larger number of establishments in a shorter sample collection period should be analyzed in the future since biosecurity measures may vary among them. This study suggests that ineffective cleaning and disinfection procedures maintain bacterial count at similar levels, and, thus, may facilitate the spread of bacteria in poultry establishments. Drying crates is essential to reduce Campylobacter contamination. It can be also controlled by improving the existing approaches, such as including a microbiological contamination control in transportation crates after the cleaning and disinfection procedures. Other preventive strategies could include the use of biodegradable crates or disposable crate liners to avoid cross-contamination between different flocks of birds and drying the cleaned crates before reusing them.
ACKNOWLEDGMENTS
This research was supported by the Brazilian National Council for Scientific and Technological Development (CNPq) through the concession of a scholarship to Rafaela Bom Morgan. The authors also wish to acknowledge CNPq for its financial support through the concession of research Grants Numbers 303086/2013-0 and 476092/2013-2
DISCLOSURES
The authors have no conflicts of interest to report.
REFERENCES
- Allen V.M., Burton C.H., Wilkinson D.J., Whyte R.T., Harris J.A., Howell M., Tinker D.B. Evaluation of the performance of different cleaning treatments in reducing microbial contamination of poultry transport crates. Br. Poult. Sci. 2008;49:233–240. doi: 10.1080/00071660802094206. [DOI] [PubMed] [Google Scholar]
- Berrang M.E., Northcutt J.K., Fletcher D.L., Cox N.A. Role of dump cage fecal contamination in the transfer of Campylobacter to carcasses of previously negative broilers. J. App. Poult. Res. 2003;12:190–195. [Google Scholar]
- Berrang M.E., Northcutt J.K., Cason J.A. Recovery of Campylobacter from broiler feces during extended storage of transport cages. Poult. Sci. 2004;83:1213–1217. doi: 10.1093/ps/83.7.1213. [DOI] [PubMed] [Google Scholar]
- Berrang M.E., Northcutt J.K. Water spray and immersion in chemical sanitizer to lower bacterial numbers on broiler transport coop flooring. J. Appl. Poult. Res. 2005;14:315–321. doi: 10.1093/ps/84.11.1797. [DOI] [PubMed] [Google Scholar]
- Berrang M.E., Hofacre C.L., Meinersmann R.J. Forced hot air to dry feces and kill bacteria on transport cage flooring. J. Appl. Poult. Res. 2011;20:567–572. [Google Scholar]
- Biswas D., Townsend H.G., Potter A., Allan B.J. Genes coding for virulence determinants of Campylobacter jejuni in human clinical and cattle isolates from Alberta, Canada, and their potential role in colonization of poultry. Int. Microbiol. 2011;14:25–32. doi: 10.2436/20.1501.01.132. [DOI] [PubMed] [Google Scholar]
- Blodgett, R. 2006. BAM Appendix 2: most probable number from serial dilutions. Appendix 2 – Most probable number from serial dilutions. US Food and Drug Administration (FDA). Accessed Feb. 2019. https://www.fda.gov/food/laboratory-methods-food/bam-appendix-2-most-probable-number-serial-dilutions
- Bolton D.J. Campylobacter virulence and survival factors. Food Microbiol. 2015;48:99–108. doi: 10.1016/j.fm.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Borges K.A., Cisco I.C., Furian T.Q., Tedesco D.C., Rodrigues L.B., Nascimento V.P., Santos L.R. Detection and quantification of Campylobacter spp. in Brazilian poultry processing plants. J. Infect. Dev. Ctries. 2020;14:109–113. doi: 10.3855/jidc.11973. [DOI] [PubMed] [Google Scholar]
- Borsoi A., Santin E., Santos L.R., Salle C.T.P., Moraes H.L.S., Nascimento V.P. Inoculation of newly hatched broiler chicks with two Brazilian isolates of Salmonella Heidelberg strains with different virulence gene profiles, antimicrobial resistance, and pulsed field gel electrophoresis patterns to intestinal changes evaluation. Poult. Sci. 2009;88:750–758. doi: 10.3382/ps.2008-00466. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2021. Salmonella and food. Accessed Apr. 2021. https://www.cdc.gov/foodsafety/communication/salmonella-food.html
- Denis M., Soumet C., Rivoal K., Ermel G., Blivet D., Salvat G., Colin P. Development of a m-PCR assay for simultaneous identification of Campylobacter jejuni and C. coli. Lett. Appl. Microbiol. 1999;29:406–410. doi: 10.1046/j.1472-765x.1999.00658.x. [DOI] [PubMed] [Google Scholar]
- Dzieciolowski T., Boqvist S., Rydén J., Hansson I. Cleaning and disinfection of transport crates for poultry – comparison of four treatments at slaughter plant. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA), and European Centre for Disease Prevention (ECDC) The European Union One Health 2019 zoonoses report. EFSA J. 2021;19:1–286. doi: 10.2903/j.efsa.2021.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca B.B., Soncini R.A., Gimarães A.R., Rossi D.A. Campylobacter spp. in eggs from cloacal swab positive breeder hens. Braz. J. Microbiol. 2006;37:573–575. [Google Scholar]
- Food Safety News. 2020. Sweden declares end to Campylobacter outbreak. Accessed Mar. 2022. https://www.foodsafetynews.com/2020/12/sweden-declares-end-to-campylobacter-outbreak-100s-were-sickened/
- Gomes C.N., Passaglia J., Vilela F.P., Silva F.M.H.S.P., Duque S.S., Falcão J.P. High survival rates of Campylobacter coli under different stress conditions suggest that more rigorous food control measures might be needed in Brazil. Food Microbiol. 2018;73:327–333. doi: 10.1016/j.fm.2018.02.014. [DOI] [PubMed] [Google Scholar]
- Guerry P. Campylobacter flagella: not just for motility. Trends Microbiol. 2007;15:456–461. doi: 10.1016/j.tim.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Hansson I., Ederoth M., Andersson L., Vagsholm I., Engvall E.O. Transmission of Campylobacter spp. to chickens during transport to slaughter. J Appl. Microbiol. 2005;99:1149–1157. doi: 10.1111/j.1365-2672.2005.02689.x. [DOI] [PubMed] [Google Scholar]
- Hastings R., Colles F.M., McCarthy N.D., Maiden M.C.J., Sheppard S.K. Campylobacter genotypes form poultry transportation crates indicate a source of contamination and transmission. J Appl. Microbiol. 2011;110:266–267. doi: 10.1111/j.1365-2672.2010.04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D., Van Deun K., Martel A., Van Immerseel F., Messens W., Heyndrickx M., Haesebrouck F., Pasmans F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011;42:82. doi: 10.1186/1297-9716-42-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D., Pasmans F., Messens W., Martel A., Van Immerseel F., Rasschaert G., Heyndrickx M., Van Deun K., Haesebrouck F. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis. 2012;12:89–98. doi: 10.1089/vbz.2011.0676. [DOI] [PubMed] [Google Scholar]
- Humphrey S., Chaloner G., Kemmett K., Davidson N., Williams N., Kipar A., Humphrey T., Wigley P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. MBio. 2014;5:1–7. doi: 10.1128/mBio.01364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igwaran A., Okoh A.I. Human campylobacteriosis: a public health concern of global importance. Heliyon. 2019;5:e02814. doi: 10.1016/j.heliyon.2019.e02814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keener K.M., Bashor M.P, Curtis P.A., Sheldon B.W., Kathariou S. Comprehensive review of Campylobacter and poultry processing. Compr. Rev. Food Sci. Food Saf. 2004;3:105–116. doi: 10.1111/j.1541-4337.2004.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Konkel M.A, Kim B.J., Rivera-Amill V., Garvis S.G. Identification of proteins required for the internalization of Campylobacter jejuni into cultured mammalian cells. Adv. Exp. Med. Biol. 1999;473:215–224. doi: 10.1007/978-1-4615-4143-1_22. [DOI] [PubMed] [Google Scholar]
- Le M.T., Porcelli I., Weight C.M., Gaskin D.J.H., Carding S.R., van Vliet A.H.M. Acid–shock of Campylobacter jejuni induces flagellar gene expression and host cell invasion. Eur. J. Microbiol. Immunol. 2012;2:12–19. doi: 10.1556/EuJMI.2.2012.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levican A., Ramos-Tapia I., Briceño I., Guerra F., Mena B., Varela C., Porte L. Genomic analysis of chilean strains of Campylobacter jejuni from human faeces. Biomed. Res. Int. 2019;8:1–12. doi: 10.1155/2019/1902732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton D., Gilbert M., Hitchen P.G., Dell A., Morris H.R., Wakarchuk W.W., Gregson N.A., Wren B.D. Phase variation of a beta-1,3 galactosyltransferase involved in generation of the ganglioside GM1-like lipo-oligosaccharide of Campylobacter jejuni. Mol. Microbiol. 2000;37:501–514. doi: 10.1046/j.1365-2958.2000.02020.x. , In this issue. [DOI] [PubMed] [Google Scholar]
- Melo R.T., Grazziotin A.L., Valadares Júnior E.C., Prado R.R., P.Mendonça E., Monteiro G.P., Peres P.A.B.M., Rossi D.A. Evolution of Campylobacter jejuni of poultry origin in Brazil. Food Microbiol. 2019;82:489–496. doi: 10.1016/j.fm.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Mizel S.B., Bates J.T. Flagellin as an adjuvant: cellular mechanisms and potential. J. Immunol. 2010;185:5677–5682. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazzami M., Fernström L.L., Hansson I. Reducing Campylobacter jejuni, Enterobacteriaceae and total aerobic bacteria on transport crates for chickens by irradiation with 265-nm ultraviolet light (UV–C LED) Food Control. 2021;119 doi: 10.4315/JFP-20-395. [DOI] [PubMed] [Google Scholar]
- Monteville M.R., Yoon J.E., Konkel M.E. Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni requires the CadF outer-membrane protein and microfilament reorganization. Microbiol. 2003;149:153–165. doi: 10.1099/mic.0.25820-0. [DOI] [PubMed] [Google Scholar]
- Perdoncini G., Sierra-Arguello Y.M., Lima L.M., Trindade M.M., Gomes M.J.P., Santos L.R., Schmidt V., Nascimento V.P. Occurence of Campylobacter jejuni and C. coli on broiler carcasses after chilling in southern Brazil. Pesq.Vet. Bras. 2015;35:349–352. [Google Scholar]
- Perdoncini G., Sierra Arguello Y.M., Lima L.M., Furian T.Q., Borges K.A., Rodrigues L.B., Santos L.R., Borsoi A., Isolan L.W., Gomes M.J.P., Salle C.T.P., Moraes H.L.S., Nascimento V.P. Detection and quantification of Campylobacter in poultry slaughterhouses using conventional microbiological technique, most probable number, and real-time PCR. Foodborne Pathog. Dis. 2022;19:143–150. doi: 10.1089/fpd.2021.0071. [DOI] [PubMed] [Google Scholar]
- Peyrat M.B., Soumet C., Maris P., Sanders P. Phenotypes and genotypes of Campylobacter strains isolated after cleaning and disinfection in poultry slaughterhouses. Vet. Microbiol. 2008;128:313–326. doi: 10.1016/j.vetmic.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Pozza J.S., Voss-Rech D., Lopes L.S., Vaz C.S.L. A baseline survey of thermotolerant Campylobacter in retail chicken in southern Brazil. Poult. Sci. 2020;99:2690–2695. doi: 10.1016/j.psj.2019.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health England (PHE). 2018. UK Standards for Microbiology Investigations: Identification of Campylobacter species. Accessed Feb. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/685065/ID_23i3.1.pdf
- Rizal A., Kumar A., Vidyarthi A.S. Prevalence of pathogenic genes in Campylobacter jejuni isolated from poultry and human. Int. J. Food Saf. 2010;12:29–34. [Google Scholar]
- Rodrigues C.S., Armendaris P.M., de Sá C.V.G.C., Haddad J.P.A., de Melo C.B. Prevalence of Campylobacter spp. in chicken carcasses in slaughterhouses from south of Brazil. Curr. Microbiol. 2021;78:2242–2250. doi: 10.1007/s00284-021-02478-w. [DOI] [PubMed] [Google Scholar]
- Shoaf-Sweeney K.D., L.Larson C., Tang X., Konkel M.E. Identification of Campylobacter jejuni proteins recognized by maternal antibodies of chickens. Appl. Environ. Microbiol. 2008;74:6867–6875. doi: 10.1128/AEM.01097-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Arguello Y.M., Perdoncini G., Rodrigues L.B., Santos L L.R., Borges K.A., Furian T T.Q., Salle C.T.P., Moraes H.L.S., Gomes M.J.P., Nascimento V.P. Identification of pathogenic genes in Campylobacter jejuni isolated from broiler carcasses and broiler slaughterhouses. Sci. Rep. 2021;11:4588. doi: 10.1038/s41598-021-84149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva N., Junqueira V.C.A., Silveira N.F.A., Taniwaki M.H., Gomes R.A.R., Okazaki M.M. 5th rev. ed. Blucher; São Paulo, Brazil: 2017. Manual de Métodos de Análise Microbiológica de Alimentos e Água. [Google Scholar]
- Silva J., Leite D., Fernandes M., Mena C., Gibbs P.A., Teixeira P. Campylobacter spp. as a foodborne pathogen: a review. Front. Microbiol. 2011;2:1–12. doi: 10.3389/fmicb.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinulingga T.S., Aziz S.A., Bitrus A.A., Zunita Z., Abu J. Occurrence of Campylobacter species from broiler chickens and chicken meat in Malaysia. Trop. Anim. Health. Prod. 2020;52:151–157. doi: 10.1007/s11250-019-01995-y. [DOI] [PubMed] [Google Scholar]
- Skarp C.P.A., Hänninen M.L., Rautelin H.I.K. Campylobacteriosis: the role of poultry meat. Clin. Microbiol. Infect. 2016;22:103–109. doi: 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Slader J., Domingue G., Jorgensen F., McAlpine K., Owen R.J., Bolton F.J., Humphrey T.J. Impact of transport crate reuse and of catching and processing on Campylobacter and Salmonella contamination of broiler chickens. Appl. Environ. Microbiol. 2002;68:713–719. doi: 10.1128/AEM.68.2.713-719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Meade J., Gibbons J., McGill K., Bolton D., Whyte P. The impact of environmental conditions on Campylobacter jejuni survival in broiler faeces and litter. Infect. Ecol. Epidemiol. 2016;6:31685. doi: 10.3402/iee.v6.31685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam C.C., Rodrigues L.C., O'Brien S.J., Hajat S. Temperature dependence of reported Campylobacter infection in England, 1989–1999. Epidemiol. Infect. 2006;134:119–125. doi: 10.1017/S0950268805004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Jr H.A. Bacterial densities from fermentation tube tests. J. - Am. Water Works Assoc. 1942;34:572–576. , In this issue. [Google Scholar]
- Wieczorek K., Osek J. Identification of virulence genes in Campylobacter jejuni and C. coli isolates by PCR. Bull Vet. Inst. Pulawy. 2008;52:211–216. [Google Scholar]
- World Health Organization (WHO). 2021. Food safety. Accessed Apr. 2021. https://www.who.int/news-room/fact-sheets/detail/food-safety
- Würfel S.F.R., Silva W.P., G.Oliveira M., Kleinubing N.R., Lopes G.V., Gandra E.A., Dellagostin O.A. Genetic diversity of Campylobacter jejuni and Campylobacter coli isolated from poultry meat products sold on the retail market in Southern Brazil. Poult. Sci. 2019;98:932–939. doi: 10.3382/ps/pey365. [DOI] [PubMed] [Google Scholar]