Abstract
Myomodulation (MyoMo) using hyaluronic acid (HA) fillers can be considered as a novel aesthetic treatment for gummy smile (GS). However, literature is still lacking information about this procedure. For this reason, the aim of the present case report was to describe a technique for MyoMo with HA as an efficient alternative for the management of GS. A 36-year-old male patient attended to our clinic complaining about excessive gingival exposure when smiling. After a clinical and digital assessment, poor bone support was observed in the premaxilla area, resulting in an increasing contraction of the levator labii superioris alaeque nasi and levator anguli oris. A multilayer approach with HA filling was performed in order to impair muscle movement and limit excessive lip elevation. As a result, a reduction on the elevation of the upper lip while smiling was achieved, and an improvement on GS was achieved. Thus, it can be proposed that Myomo with HA acid may be an effective treatment for GS, with lasting and satisfactory results. However, high-quality studies are required in order to compare patient's satisfaction with this treatment and any other possible alternative.
Keywords: Hyaluronic acid, Smile, Gingival exposure, Gummy smile, Cosmetics
Graphical abstract

1. Introduction
Facial aesthetics is associated with a pleasant smile, which involves a perfect balance among teeth, gum, and lips. An excessive gingival display is usually seeing as a facial displeasure for many patients, affecting their self-stem and social behavior. This condition, also known as gummy smile (GS), or gingival smile, is defined as a gingival exposure of more than 2 mm when smiling, and it frequently leads patients to seek professional help with dentists.1
GS present multiple etiologies that must be identified for its correct management. It is known that the act of smiling is performed by different facial muscles, that working in harmony contribute to a normal and graceful appearance in facial expression. However, the balance among these muscles may be disrupted due to structural deficiencies in youth or to bone and/or soft tissue loss with aging. When GS is caused by hypermobility of the upper lip due to muscular overactivity, minimally invasive procedures like botulinum toxin applications and, more recently, myomodulation (MyoMo) using hyaluronic acid (HA) are therapeutic options to be considered.1, 2, 3
MyoMo using HA is a novel therapeutic approach for GS. In general terms, the presence of the filler impairs muscle movement, preventing its complete contraction and limiting excessive lip elevation.4 Moreover, HA presents a high reversibility potential by using hyaluronidase, an enzyme that allows the product to degrade when vascular events or unaesthetic results appear.5 Despite the vast literature about facial harmonization using HA, there is almost any information about MyoMo using HA for GS, probably due to its novelty among clinicians.1 Thus, hereby we aimed to describe a technique about the use of HA for Myomo to improve GS.
2. Case report
A healthy 36-year-old male patient attended to our private practice in São Paulo, Brazil, complaining about excessive gingival display when smiling (Fig. 1). A social embarrassment during photo shoots or while smiling, making him to hide his smile behind the hands, was related by the patient. A comprehensive clinical evaluation revealed that the patient was systemically and dentally healthy.
Fig. 1.
Initial photographic assessment of the patient. Different angles from the A) right side, B) frontal view, and C) left side show an excessive gingival exposure.
Facial analysis was made by two experienced clinicians (MG and VR) using a photographic and digital assessment (Fig. 2). To assess the gingival display, first the height of the right central incisor was determined (9.3 mm). Then, using the height measurement as a reference, the gingival display was calculated considering the distance between the lowest margin of the upper lip and superior to the midportion of the gingival margin of the maxillary anterior teeth (Fig. 2). The excessive gingival display of each tooth in relation to the upper lip is described in Table 1. After clinical evaluation, it was also observed a deficient support in the premaxilla area, resulting in an increased contraction of the levator labii superioris alaeque nasi (LLSAN) and levator anguli oris (LAO) muscles. After explaining the case to the patient, we proposed the use of MyoMo with HA to impair muscular movement and reduce gingival exposure.
Fig. 2.
Digital assessment of the A) teeth size and a B) closer look to the height measurement of the right central incisor (9.3 mm). C) The height of the central right incisor was used as a guide to measure the D) excessive gingival display related to all anterior teeth. Excessive gingival display was assessed considering the distance from the lowest margin of the upper lip and superior to the midportion of the gingival margin of the maxillary anterior teeth. T1 = right central incisor. G = gingival display measurement of 1 – left canine; 2 – left lateral incisor; 3 – left central incisor; 4 - right central incisor; 5 – right lateral incisor; 6 – right canine.
Table 1.
Initial and final measurements of gingival display for each tooth.
| TOOTH | INITIAL | FINAL |
|---|---|---|
| G1 | 2.4 mm | 0 |
| G1 | 3.1 mm | 0 |
| G3 | 2.0 mm | 0 |
| G4 | 2.0 mm | 0 |
| G5 | 3.3 mm | 0 |
| G6 | 2.2 mm | 0 |
*G1 – left canine; G2 – left lateral incisor; G3 – left central incisor; G4 - right central incisor; G5 – right lateral incisor; G6 – right canine.
The technique used for MyoMo was the injection of HA as a multilayer approach, where a supraperiosteal and a subcutaneous injection of HA was performed (Fig. 3). First, a NASHA® technology hyaluronic acid filler (Restylane Lyft® with Lidocaine; Galderma, Uppsala, Sweden) was selected for this the supraperiostial injection. Formulated with 20 mg/mL hyaluronic acid and 1% crosslinking, with homogeneous particles, firm gel (G prime – 549) and less flexible (cohesivity – 411) with high tissue projection capability (particle size – 750–1000 μm) and focal integration.6 A 0.2 ml bolus of the HA filler was deeply injected (supraperiosteal), bilaterally, at the piriformis fossa using a 29G x ½” needle (Terumo®) that is included in the product. A 10 s careful aspiration was performed always before injection by retrieving the syringe plunger at least 0.2 ml. After confirming no blood aspiration, which may reveal an injection on a blood vessel, a HA retroinjection among the subcutaneous layer of the skin was performed (Fig. 3). The procedure was performed carefully, maintaining the angulation of the syringe in accordance with a perpendicular vector to the bone, in order to form a pillar, bellow the LLSAN muscle.
Fig. 3.
A) Myomodulation planning and B) schematic representation of the technique. White spots indicate a supraperiosteal hyaluronic acid filler bolus (0.2 ml of Restylane Lyft®) injected underneath the levator labii superioris alaeque nasi muscle (shown in red), and yellow lines indicate the subcutaneous hyaluronic acid retroinjections (0.2 ml Restylane®). Blue spot shows the injection point for botulinum toxin in the depressor septi nasi muscle.
Then, in the nasolabial fold of each side, a 0.2 ml of HA (Restylane®, Galderma) was retroinjected in the subcutaneous layer using a 22 G x 50 mm cannula (Prodeep®, Alur.). Different from the HA injected as a bolus, this one is formulated with 20 mg/mL hyaluronic acid and 1% crosslinking, with homogeneous particles (particle size – 350–450 μm), firm gel (G prime – 565) and less flexible (cohesivity – 349) and a higher quantity of particles for each 1 ml. These properties give the product more flexibility, which is necessary for this region since it is more active.7 The injection was realized at a constant pressure to allow a uniform tissue accommodation of the product (Fig. 3). As a complement, the patient received an injection of 2 units of botulinum toxin (abobotulinumtoxinA, Dysport®, Ipsen) in the depressor septi nasi (DSN) muscle, in order to extend the duration of MyoMo by regulating muscle force.
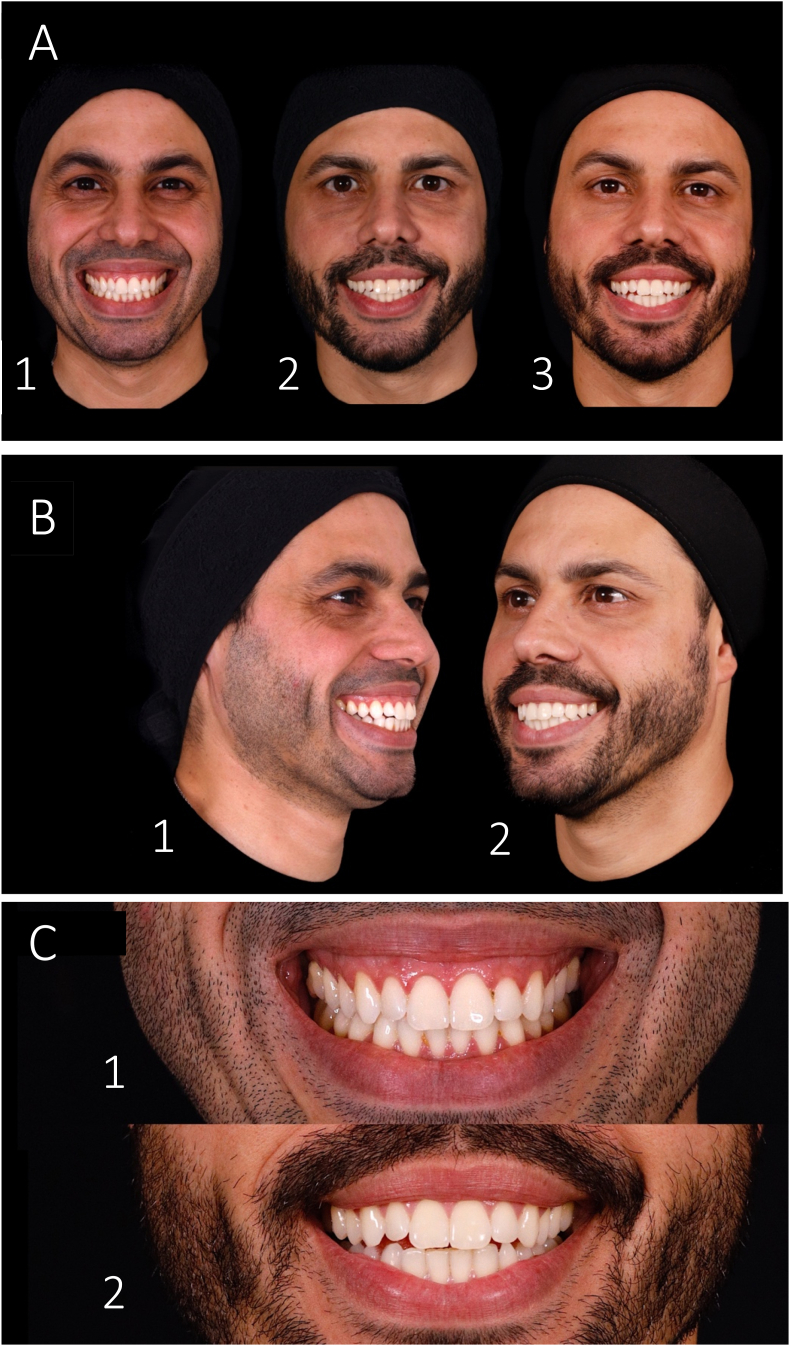
Fig. 4 shows the initial and final photographic protocol of the patient at different angles. A complete correction of the GS was achieved, and a reduction of the gingival display considering the distance from the lowest margin of the upper lip and the superior midportion of the gingival margin of the maxillary anterior teeth was reduced to zero, as shown in Table 1. A total satisfaction by the patient regarding the results of the treatment was obtained, and no adverse effects were reported during the treatment or in the subsequent days and follow-ups. Monthly follow-ups were realized until 6 months, with the patient still satisfied with the result.
Fig. 4.
Final photographic assessment of the patient. A) Patient's evolution during Myomodulation treatment: 1 – Before; 2–15 days after; and 3–60 days after myomodulation. B) Lateral view of the 1 – initial and 2 – result. C) Frontal view of the 1 – initial and 2 – final smile and gingival display of the patient.
3. Discussion
Commonly, HA fillers are used for static wrinkles, folds, and to restructure and volumize localized facial areas of lost volume.4,8,9 Recently, some authors have shown that, in addition to neurotoxins, HA-fillers can play an important role in limiting muscle movement and restoring muscle imbalance due to structural deficiencies with or without lost volume.4 Functional muscle groups contribute to facial movement and appearance, so structural congenital deficiencies or changes in muscle-action during aging can reflect in both of them.1,10
Treatment of GS must be carefully planned according to the primary cause. When the excessive gingival display is secondary to hyperactivity of the LLSAN muscle, the injection of botulinum toxin laterally to the alar cartilage wing is an effective and safe therapeutic approach. However, this technique may lead to an excessive limitation of the movement2 and requires regular reapplications every three to six months.1,3 On the other hand, MyoMo using HA fillers is presented as a safe and predictable procedure, that leads to an immediate outcome with minor movement impairment and long-lasting results.1
In the present case report, we planned MyoMo with HA using a technique previously described in literature.1,4 Diaspro et al. (2018), described a technique where patients were injected with 0.2–0.3 ml of HA into the paranasal area, at the most cranial portion of the nasolabial fold, about 3 mm from nasal wing.1 According to the author, the boluses were positioned on the bone surface to gently compress the lateral fibers of the LLSAN without invading it. An immediate improvement that lasted from 6 to 8 months was achieved. Additionally, in 2018, Maio described a technique where a 0.7 ml single bolus of HA was positioned with a 25 G cannula in the nasal spine area, at bone level.4 This technique corrected the excessive gingival display and repositioned the nose, creating a mechanical obstacle between the DSN and the elevators of the superior lip muscles.
Based on previous data, hereby we performed a bilateral supraperiosteal HA bolus-injection at the piriform fossa. Then, a retroinjection along nasolabial fold was performed to achieve the planned outcome, in a safe and efficient approach for the GS MyoMo. The HA bolus acts beneath the LLSAN compressing and stretching its fibers. The same dynamics is achieved by retroinjections in the subcutaneous, limiting the muscular movement and preventing the total contraction. As a result, a reduction on the elevation of the upper lip during smile is achieved. Resulting in a satisfactory effect to improve GS.
A proper selection of the product is mandatory to achieve good results. Each product possesses particular rheological and physicochemical characteristics, that have to be well known by the clinician to better address the final effect. The multiple layer approach demands different properties of the filler products, what justifies the multiple choices in these cases. According to this, to achieve the projection demanded to limit the muscle movement, the chosen product was the Restylane Lift® for the supraperiosteal boluses, since its higher elastic modulus G' and its bigger particles can tolerate the dynamics forces at the injection site, and to guarantee the compressive action. In the subcutaneous retroinjections, the chosen product was the Restylane®, with a high G' but smaller particles, which guarantees a more natural look when injected in more superficial layer.
4. Conclusion
Myomo using HA was an efficient approach to correct dynamic excessive gingival display (GS) in this patient. Due to the properties of HA, MyoMo using HA may have longer lasting and satisfactory results than neurotoxins. However, high-quality studies are required in order to compare patient's satisfaction with this treatment and any other possible alternative.
References
- 1.Diaspro A., Cavallini M., Patrizia P., Sito G. Gummy smile treatment: proposal for a novel corrective technique and a review of the literature. Aesthetic Surg J. 2018;38(12) doi: 10.1093/asj/sjy174. 1330–8. [DOI] [PubMed] [Google Scholar]
- 2.Suber J.S., Dinh T.P., Prince M.D., Smith P.D. OnabotulinumtoxinA for the treatment of a "gummy smile.". Aesthetic Surg J. 2014;34(3) doi: 10.1177/1090820X14527603. 432–7. [DOI] [PubMed] [Google Scholar]
- 3.Nasr M.W., Jabbour S.F., Sidaoui J.A., Haber R.N., Kechichian E.G. Botulinum toxin for the treatment of excessive gingival display: a systematic review. Aesthetic Surg J. 2015;36(1) doi: 10.1093/asj/sjv082. 82–8. [DOI] [PubMed] [Google Scholar]
- 4.de Maio M. Myomodulation with injectable fillers: an innovative approach to addressing facial muscle movement. Aesthetic Plast Surg. 2018;42(3):798–814. doi: 10.1007/s00266-018-1116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vieira M.G., Machado-Filho D.A., Alcantara A.R., Mendonça A., Kim J.H., Gonzalez Cortes A.R. Clinical management of nasal skin necrosis caused by hyaluronic acid filler. J Craniofac Surg. 2021;32(2) doi: 10.1097/SCS.0000000000006847. http://www.ncbi.nlm.nih.gov/pubmed/33705046 [Internet] e120–2. Available from: [DOI] [PubMed] [Google Scholar]
- 6.Edsman K., Nord L.I., Å Öhrlund, Lärkner H., Kenne A.H. Gel properties of hyaluronic acid dermal fillers. Dermatol Surg. 2012 Jul;38(7):1170–1179. doi: 10.1111/j.1524-4725.2012.02472.x. https://journals.lww.com/00042728-201207020-00009 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 7.Falcone S.J., Berg R.A. Crosslinked hyaluronic acid dermal fillers: a comparison of rheological properties. J Biomed Mater Res. 2008 Oct;87(1):264–271. doi: 10.1002/jbm.a.31675. http://www.ncbi.nlm.nih.gov/pubmed/18200557 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 8.Carruthers J., Carruthers A., Tezel A., Kraemer J., Craik L. Volumizing with a 20-mg/mL smooth, highly cohesive, viscous hyaluronic acid filler and its role in facial rejuvenation therapy. Dermatol Surg. 2010;36(SUPPL. 3):1886–1892. doi: 10.1111/j.1524-4725.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 9.Callan P., Goodman G.J., Carlisle I., et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Invest Dermatol. 2013;6:81–89. doi: 10.2147/CCID.S40581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teston A.P., Nardino D., Pivato L. Envelhecimento cutâneo: teoria dos radicais livres e tratamentos visando a prevenção e o rejuvenescimento. Uningá Rev. 2010;1:71–84. http://revista.uninga.br/index.php/uningareviews/article/view/451 [Internet] Available from: [Google Scholar]