Abstract
We describe a case of a 2-year-old girl with congenital megaureter presenting as intraabdominal cystic masses. The patient presented with a lump in abdomen that has been getting bigger since birth accompanied by pain. Ultrasonography that was taken when the patient was 2 years old showed a cystic mass with thick septation and pelvocaliectasis of the left kidney. One month after US, patient underwent 3D CT Scan which showed cystic masses in the upper to lower abdomen with no visualization of the normal structure of the left kidney and ureter. Non-contrast MRU that was taken 3 month after the CT Scan showed a thick-walled cystic mass resembling a tortuous tubular mass associated with the pelvocalyceal system without any distal obstruction. VCUG examination that was taken 2 weeks after the non-contrast MRU showed no reflux. This case reports can help clinicians to confirm persistent urinary tract dilatation, exclude the presence of VUR and differentiate primary megaureters from other causes of hydronephrosis including obstruction of the VUJ, posterior urethral valves, and ureterocele from radiological studies.
Keywords: Megaureter, Cystic intraabdominal mass, Pelvocaliectasis, Tortous ureter, Hydronephrosis
Introduction
Congenital abnormalities of the lower urinary tract are a significant cause of morbidity in infancy. Megaureter especially is one of the most common congenital abnormalities in infants, where the term “megaureter” is a descriptive term for the diameter of the ureteral lumen, which means the size of the ureter is enlarged [1,2]. Methods for imaging the urinary tract in children have developed significantly in recent years. Radiological examination has a significant impact on the diagnosis and management of megaureters, therefore, it is important that the indications, limitations, and complications associated with this procedure to be known by radiologists, urologists, and pediatricians who treat urinary tract diseases in children [3].
Case report
A 2-year-old girl presented with a lump in her stomach that has been getting bigger since birth, accompanied by pain, especially in the left side of the abdomen. This patient has had a physical examination at the pediatric clinic at Hasan Sadikin Hospital when the patient was 2-years-old, whereupon it was found that the abdomen is enlarged, especially on the left side. On the next day, the patient was then assessed with Abdominal Ultrasonography (US) and laboratory examinations in the form of routine blood tests. A cystic mass with septation was found in the abdomen, accompanied by pelvocalyectasis of the left kidney (Fig. 1). The patient was suspected of having a cystic mass in the lower abdomen with pelvocalyectasis of the left kidney.
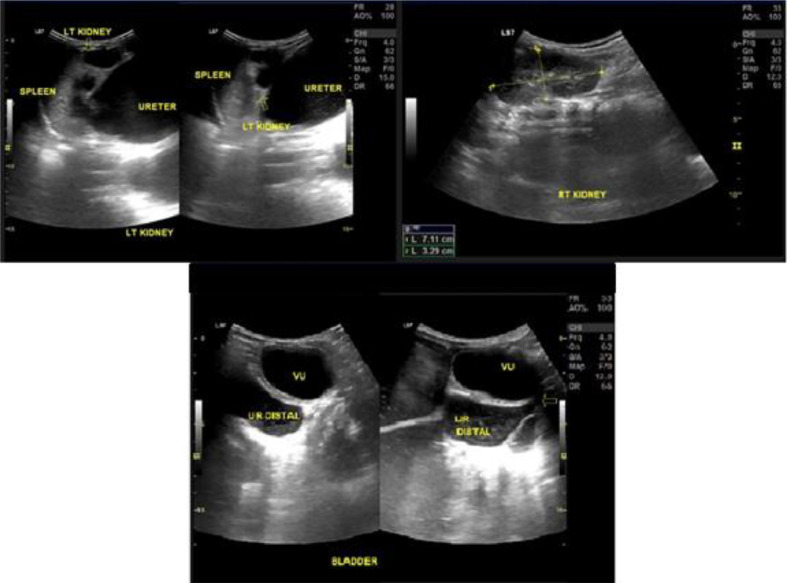
Fig. 1.
Ultrasonography examination showing pelvocaliectasis of the left kidney (yellow arrow) with proximal to distal ureteral dilatation that looks like an intra-abdominal cystic mass. (Color version of figure is available online.)
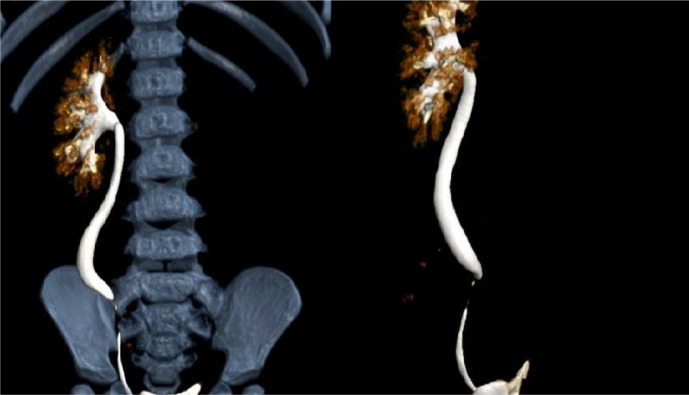
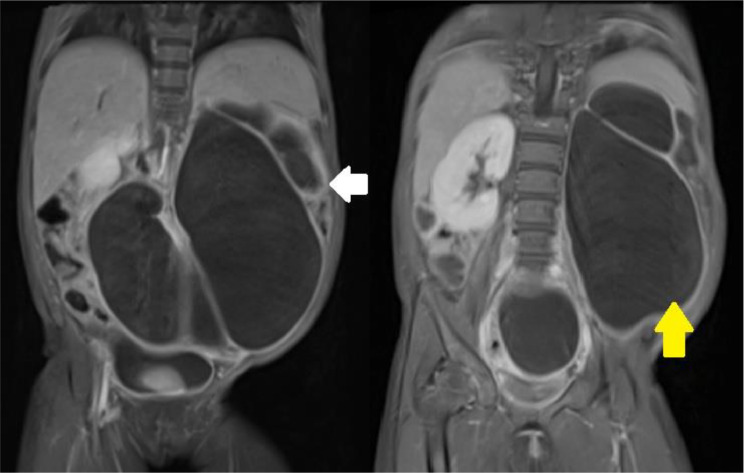
The patient then underwent other radiological examinations to confirm the cystic appearance on US examination and to look for the cause of the hydronephrosis of the left kidney. One month after US, patient underwent CT urography examination that shows multiple cystic masses in the upper to lower abdomen with pelvocalyectasis of the left kidney (Fig. 2). From the CT Scan 3D Urography, the normal structure of the kidney and left ureter was not visualized (Fig. 3). On the T2WI MRI sequence that was performed 3 months after CT Scan, the non-contrast urography showed a thick-walled cystic mass resembling a tortuous tubular structure connected with the pelvicalyceal system without any distal obstruction (Fig. 3). To confirm the cause, patient underwent VCUG examination 2 weeks after non-contrast MRU, but no reflux was found (Fig. 4).
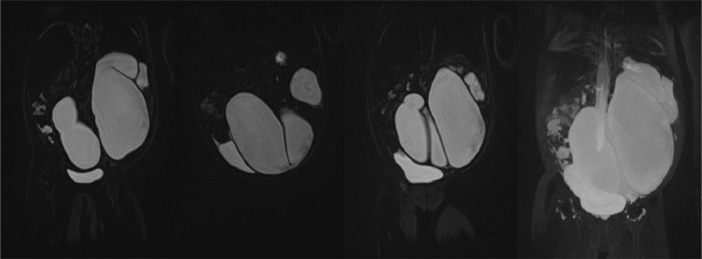
Fig. 5.
MR Coronal section 3D non-contrast urography shows a tortuous tubular structure occupying almost the entire abdominal area, especially on the left where there is a dilated ureter (megauereter).
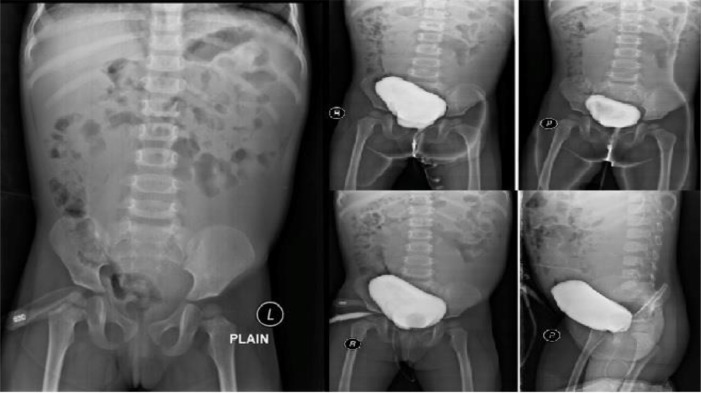
Fig. 6.
No reflux found on VCUG examination.
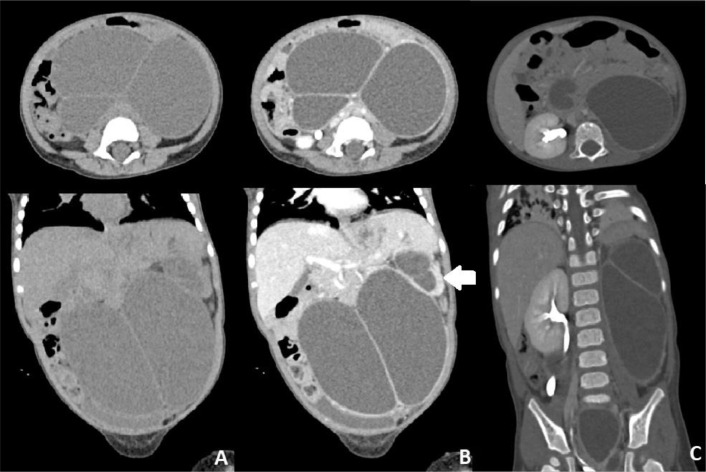
Fig. 2.
Abdominal Axial and coronal CT scan showing multiple hypodense lesions with thick septa over most of the abdominal area with pelvocalyectasis of the left kidney.
Fig. 3.
3D CT Urology showing the left kidney is not visualized.
Fig. 4.
MR Urography on coronal T2WI showing hydronephrosis of the left kidney (white arrow) with a tortuous tubular hypointense appearance that appears to be associated with the pelvicalyceal system of the left kidney without associated distal obstruction (yellow arrow). (Color version of figure is available online.)
Based on several radiological examinations, it can be concluded that the multiple cystic masses that occupy almost the entire abdominal area are dilated and tortuous ureters without obstruction. In addition, it is also important to examine whether or not the patient has reflux from the lower urinary tract to the upper urinary tract that causes hydronephrosis of the left kidney. For this purpose, an examination using fluoroscopy is carried out, namely Voiding Cystoureterography (VCUG).
The patient was operated at the age of 4 years old, and at the time of the operation a cystoscopy was performed and followed by a nephroureterectomy. From the results of cystoscopy, the right ureteral opening was normal and there was no left ureteral opening. Nephroureterectomy showed that the left ureter was enlarged tortuously with the smallest diameter of more or less 1 cm and the largest diameter is more or less 5 cm. After surgery, the patient urine was examined with a urine culture in the laboratory and the results were no bacteria found.
Discussion
One of the most frequently detected congenital abnormalities of the urinary tract in children is ureteral dilatation or 'mega'-ureter. Megaureter is a general term denoting the presence of an enlarged ureter with or without concomitant dilatation of the upper collecting system. There are many synonyms for this condition, including hydroureter and megaureter, but all represent a larger-than-normal diameter ureter with or without associated dilatation of the renal pelvis. This term implies a congenital abnormality, and because the neonate's ureter contains a large number of elastic fibers, it can become very wide. Normal ureters in children rarely exceed 5 mm in diameter. In practice, ureters with a diameter of 7 mm or more should be considered megaureters. Congenital ureteral dilatation can be caused by vesicoureteral reflux, obstructive disease, high urine flow from the unconcentrated kidney, and abnormalities of ureteral muscle development. Bacterial toxins from infection in the system can increase the degree of megaureter and can even dilate normal ureters with toxic paralysis of muscle cells [2], [3], [4], [5], [6].
Megaureter is a common diagnosis in children referred to a pediatric urologist for urological evaluation, representing 23% of children with urinary tract obstruction. This disorder is more common in boys than girls, and is more common on the left side. It can be bilateral in 25% of cases, and the contralateral kidney is absent or dysplastic in 10%-15% of cases. There is no clear pattern of genetic inheritance, although some cases appear to run in families [1,7].
Megaureters can be primary or secondary, with or without reflux, with or without obstruction, or without reflux nor obstruction. Primary megaureter is a term that covers all cases of megaureter due to idiopathic congenital changes in the vesicoureteral junction. There are 3 main categories of primary megaureters: obstructive primary megaureters, reflux primary megaureters, and non-obstructive non-reflux primary megaureters. Secondary megaureter occurs as a result of several disorders involving the bladder or urethra (eg, urethral valve, neuropathic bladder dysfunction, urethral stricture, ureteroceles, and acquired obstruction) [3,6,8].
Methods for imaging the urinary tract in children have developed significantly in recent years. Computed tomography scan, MR imaging, and echo-enhanced cystosonography and the more traditional radiological examinations, including nuclear imaging, US, VCUG, and intravenous urography (IVU) are also used as modalities of inspection. Imaging of the urinary tract has a significant impact on the diagnosis and management of patients with megaureter. Therefore, it is important that the indications, limitations and complications associated with this procedure are known to radiologists, urologists, and pediatricians who treat urinary tract diseases in children [3].
Primary obstructive megaureter is associated with adynamics of the distal ureteral segment with dilatation of its proximal portion and is a common cause of obstructive uropathy in children and is considered a functional adynamic secondary obstruction of the distal ureteral segment. This may be associated with increased collagen levels, leading to fibrosis and obstruction, or atrophy of the inner longitudinal muscles in the distal segment of the ureter, leading to impaired transmission of peristalsis. It could also be due to hypertrophy of the outer compressive circular muscle, also causing obstruction. This is analogous to achalasia of the esophagus or Hirschsprung's colon due to a lack of ganglion cells in the wall of the ureter but the exact cause has not been proven. Primary megaureter with reflux is caused by abnormalities that occur in the area of the vesicoureteral junction that can inhibit normal antireflux mechanisms. This can be caused by a short vertical portion of the intramural segment, congenital paraureteric diverticulum, ureterocele with or without duplication of the collecting system and others. Primary megaureter without reflux and without obstruction is considered the most common cause of primary megaureter in neonates, and although the vesicoureteral junction is found to be normal without reflux and obstruction, the ureter may be enlarged. The cause is still unknown until now [8,9].
The radiological features of the 3 types of megaureters have the same characteristics, namely enlarged ureters with a diameter of > 7 mm which are sometimes very conspicuous. In all radiological modalities capable of visualizing the ureter (CT, US, MRI, IVU), the megaureter appears as a tubular structure posterior to the bladder [2,3,10].
Primary obstructive megaureter is a form of megaureter with characteristic clinical, radiological, and ultrastructural features in which the ureteral drainage system is inadequate. The important criteria in this type are ureteral dilatation and the absence of bladder dysfunction or urethral obstruction. In mild forms, the ureter appears fully distended in the pelvic region and is more tapered or narrowed in normal caliber. The shape of the calyces system is usually concave, sometimes convex, and sometimes shows atrophy of the renal cortex. In more severe degrees of obstruction, the ureters appear more dilated proximally but rarely tortuously as in reflux megaureters. The presence of a discrepancy between a dilated ureter and an empty bladder represents an unfilled segment of obstruction and is visualized by postvoiding technique on a pyelogram. Ultrasonography examination showed hydronephrosis due to active peristaltic waves. CT and MR urogram findings of the primary obstructive megaureter include dilatation of the proximal ureter associated with a dilated collecting system and gradual smooth descent of the urinary tract. However, functional imaging with dynamic renography using 99 mTc-mercapto acetyltriglycine is often necessary to properly diagnose the condition and triage the patient to appropriate therapy [9,10].
In primary reflux megaureters, vesicoureteral reflux can be seen on fluoroscopy. The essential defect that occurs in this type is due to a deficiency of the ureterotrigonal unit. The distal ureter is weakened with inadequate fixation of the ureter to the bladder, with absence of connections in the submucosa. This abnormality can cause pathological changes and displacement of the ureteral orifice. This type can also be caused by the presence of a paraureteral saccule or diverticulum that can interfere with the vesicoureteral junction valve mechanism [10].
The combination of reflux and obstruction in megaureter was first reported by Weiss and Lytton. In some cases, muscle cells in the intravesica and juxtavesica of the distal ureter are lackluster, so that the ureter is unable to drain urine adequately, resulting in obstruction. On VCUG examination, delayed reflux contrast clearance or a sharper cutoff distally may be seen. Severe hydronephrosis with renal atrophy may be seen on IVP examination which does not appear to be proportional to the degree of reflux that occurs during VCUG examination [10].
Megaureters without reflux and without obstruction show ureteral dilatation including those with ureteral dilatation during urosepsis. The toxins from the bacteria paralyze the muscles and make the ureters temporarily atonic. Good recovery after administration of antibacterial therapy is highly expected to occur. However, chronic bacteriuria can cause permanent damage to the ureteral muscles. Kidney polyuria causes loss of its ability and concentration resulting in uropathy and ureteral dilatation [10].
In primary megaureters without reflux, there is usually no or little hydronephrosis. Although rare, congenital megaureter can coexist with congenital megacalyces which makes the assessment of hydronephrosis more difficult [9].
Secondary megaureter occurs due to secondary processes due to abnormalities in the bladder and urethra. Abnormalities often occur in the pelvicourethral valves (PUV), which occur during the development of the ureteral bud and metanephric blastemal [10].
Patient consent
Informed consent for publication has been obtained.
Conclusion
Congenital megaureter is 1 of the most common causes of uropathy in children, so radiological examination is needed to support the diagnosis of megaureter. Radiologic modalities are needed to determine the cause, type, and can help determine the management of megaureter. Ultrasonography is the initial modality that can be used, followed by CT or MR urography and VCUG to determine the cause.
Footnotes
Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hodges SJ, Werle D, McLorie G, Atala A. Megaureter. The Scientific World Journal. 2010;10:603–612. doi: 10.1100/tsw.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier P-H, Augdal TA, Avni FE, Bacchetta J, Beetz R, Bjerre AK, et al. Standardization of pediatric uroradiological terms: a multidisciplinary European glossary. Pediatr Radiol. 2018;48(2):291–303. doi: 10.1007/s00247-017-4006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrocal T, López-Pereira P, Arjonilla A, Gutiérrez J. Anomalies of the distal ureter, bladder, and urethra in children: embryologic, radiologic, and pathologic features. Radiographics. 2002;22(5):1139–1164. doi: 10.1148/radiographics.22.5.g02se101139. [DOI] [PubMed] [Google Scholar]
- 4.Farrugia M-K, Hitchcock R, Radford A, Burki T, Robb A, Murphy F. British Association of Paediatric Urologists consensus statement on the management of the primary obstructive megaureter. J Pediatr Urol. 2014;10(1):26–33. doi: 10.1016/j.jpurol.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Teklali Y, Robert Y, Boillot B, Overs C, Piolat C, Rabattu PY. Endoscopic management of primary obstructive megaureter in pediatrics. J Pediatr Urol. 2018;14(5):382–387. doi: 10.1016/j.jpurol.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Mark Adams, WHHI C. In: Pediatric Surgery. 2. United States of America: Elseviers Saunders. Coran AG, editor. 2012. Megaureter and Prune-Belly Syndrome; pp. 1498–1505. editor. [Google Scholar]
- 7.Ibrahimi A, Ziani I. Primary obstructive megaureter. The Pan African Med J. 2020;37(296) doi: 10.11604/pamj.2020.37.296.26867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh DN, Bhatti W. Primary obstructive megaureter with ureteric calculi in a child. CHRISMED J of Health and Res. 2016;3(2):134. [Google Scholar]
- 9.Gaillard F. WY. Congenital Megaureter: radiopaedia.org; 2008 [updated 30 December 2021. Available at: https://radiopaedia.org/articles/congenital-megaureter.
- 10.Hanna MK. Megaureter. Urological Surgery in Neonates and Young Infants. 1: W. B Saunders Company. 1988:160–203. Volume 1. [Google Scholar]