Abstract
A pregnant mother undergoes significant changes in acid-base status as well as sodium and calcium metabolism to combat her physiological needs of pregnancy. Pregnant patients experience mild respiratory alkalosis due to the stimulation of the respiratory center by progesterone. This is associated with a corresponding increase in bicarbonate excretion by kidneys; as a result, the pH remains slightly high (7.40–7.45) but within the normal range. Pregnant women are predisposed to starvation ketosis as compared to nonpregnant states due to relative insulin resistance and increased production of the counter-regulatory hormone. Physiological mild hyponatremia occurs during pregnancy due to increased AVP secretion caused by resetting of osmoreceptors in the hypothalamus at a lower osmolality, but values below 130 mEq/L require a diagnostic workup and intervention. Gestational diabetes insipidus can occur due to increased production or decreased destruction of enzyme vasopressinase. Secretion of parathyroid hormone-related peptide by the placenta and breasts and two- to three-fold increased calcium and phosphate absorption in the maternal gut are the key changes in calcium metabolism during pregnancy. Though rare, both hypo- and hypercalcemia in pregnancy are associated with significant maternofetal morbidity and mortality.
How to cite this article
Ahmed A. Fetomaternal Acid–Base Balance and Electrolytes during Pregnancy. Indian J Crit Care Med 2021; 25(Suppl 3):S193–S199.
Keywords: Acid–base disturbance, Electrolytes in pregnancy, Hypercalcemia in pregnancy, Hypernatremia in pregnancy, Hypocalcemia in pregnancy, Hyponatremia in pregnancy
Introduction
Maternal physiology undergoes substantial acid–base and electrolyte changes during pregnancy which should be differentiated from pathological changes indicating the onset or progression of various disease processes. Among the acid–base alterations, respiratory alkalosis is the normal physiological response to pregnancy. Metabolic acidosis and alkalosis, as well as respiratory acidosis, when encountered, should be considered pathological, and further workup is warranted. Serum sodium and serum osmolality are mildly reduced with a rise in the circulating volume, but serum potassium, magnesium, ionic calcium, and phosphate levels do not undergo significant changes during pregnancy. Hyponatremia below 130 mEq/L and derangement of any other electrolytes from normal values require a diagnostic workup and intervention. The current article describes the physiological and pathological acid–base and electrolyte changes in pregnancy.
Fetomaternal Acid–Base Balance during Pregnancy
Respiratory Alkalosis in Pregnancy
Pregnant patients hyperventilate due to the stimulation of the respiratory center by the progesterone hormone.1 The increase in minute ventilation (product of tidal volume and respiratory rate) is brought about by an increase in tidal volume with the respiratory rate remaining normal. Renal response to this phenomenon is a compensatory increase in the excretion of bicarbonate (HCO3). As a result, the maternal pH remains in the normal range but slightly toward alkalosis (7.40–7.45), while PaCO2 is reduced to 27–32 mm Hg and HCO3 is reduced to 17–19 mmol/L in normal pregnancy.2 Variations in these values in different studies reflect changes at different altitudes. The clinical significance of this phenomenon is that a normal PaCO2 (35–40 mm Hg) during pregnancy may indicate an early phase of respiratory decompensation.
Metabolic Acidosis in Pregnancy
Like nonpregnant states, metabolic acidosis can occur in pregnancy due to a high anion gap or nongap metabolic acidosis due to different pathological conditions. High anion gap metabolic acidosis results from the production/addition of new acid in the body and is mainly classified as lactic acidosis, ketoacidosis, acidosis due to renal failure, and acidosis due to drugs or poisons. Normal gap metabolic acidosis results due to bicarbonate losses through the gut or kidneys with subsequent gain in chloride ions. Therefore, nongap metabolic acidosis is also called hyperchloremic metabolic acidosis. The pathophysiology of various types of metabolic acidosis remains the same as that in nonpregnant states. However, certain conditions require special attention.
Starvation ketosis of pregnancy: Increased susceptibility of pregnant women to starvation ketosis was recognized as early as 1970. Pregnant women show an exaggerated response to fasting due to the metabolic milieu favoring insulin resistance with the background of increased counter-regulatory hormone-like placental glucagon and placental lactogen secretion. There is an increased breakdown of fatty acids leading to the production of ketone bodies and high anion gap metabolic acidosis. Frise et al. reported four cases of starvation ketosis during pregnancy, all of which were precipitated by a short duration of vomiting (within 24 hours) in the third trimester. All were managed with an emergency cesarean section with a good maternal outcome. The newborns had Apgar scores between 8 and 10 at 5 minutes, except for one baby who had an Apgar score of 5 at 5 minutes.3 There are case reports of successful management of starvation ketosis with dextrose infusion. Ekanem et al. reported electrolyte and acid–base changes during prolonged labor in a case–control study including 200 Nigerian women (95 = prolonged labor, 105 = normal labor). Around 92% of patients with prolonged labor had ketonuria as compared to 1.9% of patients with normal delivery.4
Lactic acidosis in pregnancy: Lactic acidosis (acidosis due to decreased tissue perfusion) is seen in all types of shock {hemorrhagic, distributive (mainly septic), cardiogenic, and obstructive} in pregnancy. It can be due to more than one etiology as it is not uncommon for septic shock patients to develop major bleed due to coagulopathy (hemorrhagic shock) and simultaneously compromised left ventricular contractility (cardiogenic shock) due to septic cardiomyopathy. Such patients frequently develop acute kidney injury and retention of renal acids, thus further adding to high anion gap metabolic acidosis. Treatment involves dealing with the primary pathology (with antimicrobials and source control) and hemodynamic support.
Metabolic Alkalosis
Causes of metabolic alkalosis in pregnant patients are similar to those in the nonpregnant population. Two mechanisms responsible for metabolic alkalosis are loss of chloride-rich fluid from the body either through the gut (e.g., persistent vomiting)/kidneys (e.g., diuretics) or factors causing increased aldosterone levels. Aldosterone causes increased sodium absorption and potassium and hydrogen ion excretion via kidneys. Former requires volume replacement with chloride-containing fluid, while potassium correction and addressing the cause of increased aldosterone level are needed for the latter.
Respiratory Acidosis
The residual effect of anesthetic drugs after general anesthesia is the most common cause of respiratory acidosis in pregnant women undergoing cesarean section. Other causes could be central nervous system depression following antiepileptic drugs for eclampsia, hypo/hypernatremia, stroke, cerebral venous thrombosis, respiratory muscle fatigue in patients with increased work of breathing, hypermagnesemia, etc.
Fetomaternal Electrolyte Changes during Pregnancy
Sodium Homeostasis in Pregnancy
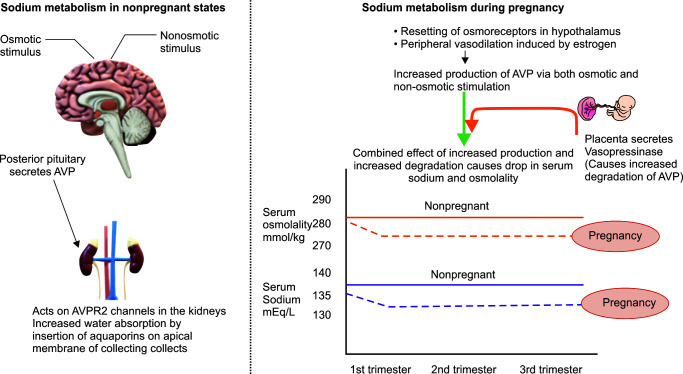
Sodium homeostasis shows significant changes during pregnancy, which are brought about by increased AVP (arginine–vasopressin) secretion from the posterior pituitary (Fig. 1).5 AVP mediates its action via AVP R1 and AVP R2 receptors located in different parts of the body. Osmoregulatory action of AVP is mediated via AVP R2 receptors located on the basolateral membrane of renal collecting ducts of cortical and medullary nephrons. Stimulation of these receptors causes the insertion of aquaporin-2 channels on the apical membrane, which causes water absorption in the collecting ducts.
Fig. 1.
Changes in sodium metabolism during pregnancy. AVP, arginine–vasopressin; AVPR2, arginine–vasopressin receptor 2
In nonpregnant states, AVP is secreted from the posterior pituitary in response to osmotic and nonosmotic stimuli. During pregnancy, its secretion is increased via both mechanisms. The osmotic mechanism triggering AVP secretion includes resetting of osmoreceptors at a lower threshold in the hypothalamus, i.e., 275–285 mOsmol/kg instead of 285–295 mOsmol/kg in nonpregnant states. Besides this, the human chorionic gonadotropin hormone causes increased stimulation of the thirst center, leading to increased water intake by pregnant women. Among the nonosmotic mechanisms, arterial vasodilation–mediated neurohormonal and sympathetic system activation plays an important role in increasing AVP secretion. During delivery, a further increase in AVP secretion is brought about by pain and sympathetic stimulation.
As a result of the above-described mechanisms, serum sodium is generally decreased by 5 mmol/L, and serum osmolality is reduced by 10 mOsmol/kg during normal pregnancy. These changes start appearing early in the first trimester and reach a plateau by 8–10 weeks of gestation.
Interestingly, there is also increased AVP breakdown during pregnancy due to secretion of enzyme vasopressinase by the placental trophoblast. The balance between increased production and increased degradation of AVP is such that serum sodium remains around 130–135 mmol/L during pregnancy. Values below 130 mmol/L require a search for pathological causes.
Disorders of Sodium Homeostasis during Pregnancy
-
Hyponatremia in pregnancy: Pregnant patients are prone to develop pathological hyponatremia (serum sodium <130 mmol/L) due to various reasons like poor intake associated with nausea and vomiting in the first trimester, pain-mediated increased stimulation of AVP secretion in the third trimester, and the use of excessive hypotonic saline and oxytocin during delivery. Oxytocin bears structural similarity with vasopressin and can cause fluid retention by action on AVP R2 channels in renal collecting ducts.
One must note that pregnancy can unmask previous subclinical hyponatremia due to other causes not related to pregnancy. These patients should be approached with the standard protocol of managing hyponatremia as in nonpregnant states, a detailed description of which is beyond the scope of this review. Care must be taken while managing patients with euvolemic hypotonic hyponatremia (syndrome of inappropriate antidiuresis [SIAD]). In nonpregnant states, management of euvolemic hyponatremia requires fluid restriction, which can be relatively harmful in pregnancy due to the risk of oligohydramnios and dehydration.6
Therefore, pregnant patients who are known cases of euvolemic hyponatremia (SIAD) should have a water intake of around 1.5 L/day along with ultrasound-guided monitoring of amniotic fluid index and subsequent fluid intake titration.
Acute hyponatremia below 120 mEq/L requires urgent correction by infusion of hypertonic saline, irrespective of etiology due to the risk of seizures. The rise in sodium level should not exceed 8–10 mEq/L in 24 hours. One must anticipate neonatal hyponatremia in babies delivered to such patients.
-
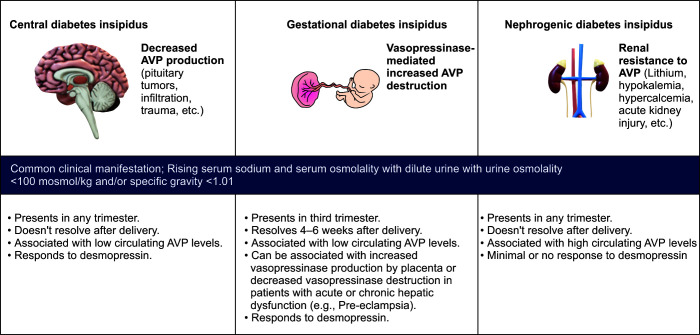
Hypernatremia in pregnancy: Besides iatrogenic causes (e.g., hypertonic saline, sodium bicarbonate infusion), pregnant women can experience hypernatremia due to pregnancy-specific causes (gestational diabetes insipidus) as well as due to the unmasking of previous subclinical pathology unrelated to pregnancy (central or nephrogenic diabetes insipidus).7
Diabetes insipidus is a condition that arises due to decreased production (central diabetes insipidus), increased destruction (gestational diabetes insipidus), or decreased action of AVP hormone on renal collecting ducts (renal diabetes insipidus). Irrespective of the cause, clinical features of diabetes insipidus remain the same, i.e., production of dilute urine with urine osmolality <100 mOsmol/kg and/or specific gravity <1.01 with rising serum sodium and serum osmolality (Fig. 2).
Among the pregnancy-specific causes of hypernatremia, gestational diabetes insipidus requires special consideration. Though rare (2–6 cases per 1,00,000 pregnancies), gestational diabetes insipidus can lead to serious maternofetal implications like severe oligohydramnios, hypernatremia, and altered sensorium. Pathogenesis involves increased secretion of placental vasopressinase, which causes rapid degradation of AVP hormone and subsequent production of dilute urine, hypernatremia, and dehydration. Patients with increased placental mass (e.g., twin pregnancy) and pregnancy conceived via in vitro fertilization have been shown to be associated with increased vasopressinase production. High levels of vasopressinase can also occur due to abnormalities of liver function, as seen in preeclampsia, HELLP, or chronic liver disease due to hampered vasopressinase degradation in the liver. Patients with placental abruption can develop diabetes insipidus due to the sudden release of a large amount of vasopressinase. Gestational diabetes insipidus typically resolves in 4–6 weeks after delivery with a gradual degradation of circulating vasopressinase.
Fig. 2.
Causes of diabetes insipidus in pregnancy. AVP, arginine–vasopressin
Treatment consists of a synthetic analog of AVP hormone named desmopressin (DDAVP). Desmopressin is resistant to placental vasopressinase and can be safely used in pregnancy as it has not been found to have vasopressor activity or increased incidence of preeclampsia. There is a theoretical risk of desmopressin causing increased uterine contractility due to its similarity to oxytocin, but its intranasal use has 75 times less oxytocic action as compared to oxytocin.
Magnesium Homeostasis in Pregnancy
Magnesium is required for more than 300 enzymatic reactions in the body. It plays a crucial role in regulating the vasomotor tone of the blood vessels, and its deficiency has been linked with hypertension in nonpregnant states. Serum magnesium levels remain unchanged or marginally reduced in pregnancy due to hemodilution. Magnesium homeostasis has not been well understood in pregnancy as it is an intracellular ion and serum magnesium level is an unreliable marker of magnesium deficiency.8,9 Red cell magnesium is a more accurate measure of magnesium status but is not readily available. The gold standard for measuring magnesium deficiency is using magnesium loading and measuring its urinary excretion. Daily magnesium requirement is 300–360 mg in healthy women, and it is increased further by another 40 – 50 mg in pregnancy. Magnesium homeostasis is regulated via absorption in the small intestine and excretion through the kidneys.
Magnesium has been postulated to play a protective role in preterm labor by antagonizing the calcium-mediated uterine contractions, but robust data in this field are lacking. It plays a therapeutic role in patients with preeclampsia via inhibition of angiotensin-II- and endothelin-I-mediated vasoconstriction and immunomodulatory action. Its infusion in preeclamptic women has been found to be associated with a 50% reduction in progression to eclampsia and maternal deaths. Besides this, magnesium supplementation improves glucose tolerance in gestational diabetes mellitus, but the results of most studies have the limitation of using serum magnesium level rather than red cell magnesium for identifying patients with magnesium deficiency.9
Disorders of Magnesium Homeostasis during Pregnancy
-
Hypomagnesemia in pregnancy: Poor nutritional intake is the most common cause of hypomagnesemia in pregnancy. Other causes include chronic diarrhea, diabetes mellitus, drugs (loop diuretics, thiazides, aminoglycosides, amphotericin B, calcineurin inhibitors, and previous cisplatin use), and genetic disorders. Magnesium replacement can be done via the oral or intravenous route. Its deficiency can coexist with other electrolyte abnormalities in various syndromes like hypokalemia, hypomagnesemia, hypophosphatemia, and hypocalcemia in refeeding syndrome or hypokalemia hypomagnesemia with hypercalcemia in Gittelman's syndrome.10
Hypomagnesemia causes increased PTH secretion like hypocalcemia, but very profound hypomagnesemia interferes with the release of PTH hormone, which can lead to hypocalcemia with hypomagnesemia.
In mothers, it can present as lethargy and weakness. Profound deficiency presents as tetany, cardiac arrhythmias, and an increased tendency for renal stone formation.
It can lead to impaired placental development and fetal growth.
Hypermagnesemia in pregnancy: Hypermagnesemia in pregnancy mostly results from iatrogenic overdosing in patients with compromised renal function, as normal kidneys are efficient in excreting an extra magnesium load. Clinical symptoms correlate with plasma concentration. Plasma concentration of 1.8–3.0 mmol/L is therapeutic in the management of preeclampsia, while values above 3.5 mmol/L are associated with the loss of patellar reflex. Respiratory paralysis is seen with plasma concentration of 5–6.5 mmol/L, followed by conduction abnormalities at a concentration >7.5 mmol/L and cardiac arrest at a concentration >12.5 mmol/L. Magnesium infusions in preeclamptic mothers can lead to neonatal hypermagnesemia, which is mostly asymptomatic, but extremely high levels can present as hypotonia and central nervous system depression in newborns.
Calcium and Phosphate Homeostasis in Pregnancy
Serum ionic calcium and serum phosphate levels remain unchanged during pregnancy. There is a decrease in serum total calcium due to a decrease in serum albumin and the bound fraction of calcium, brought about by volume expansion in pregnancy. Daily calcium allowance in nonpregnant healthy adults is 1000 mg/day, which is increased to 1200 mg/day during pregnancy.
In nonpregnant states, the calcium metabolism is beautifully controlled by three hormones (parathyroid hormone, calcitonin, and 1,25 dihydroxycholecalciferol, i.e., the active form of vitamin D3) and three organs, namely bones, kidneys, and small intestine, summarized below.11
Our body's calcium (99%) is stored in the bones in the form of hydroxyapatite. The remaining 1% is distributed extracellularly (plasma and interstitium) as well as intracellularly. The plasma calcium exists in two forms, i.e., 50% free ionic calcium, which is metabolically available for various chemical reactions and bound form 40% of which is bound to albumin while 10% is bound to complex anions like citrate and bicarbonate. Under normal conditions, PTH is secreted by parathyroid glands and is the key regulator of ionic calcium levels in the human body. The PTH acts on osteoclasts and causes the release of calcium and phosphate from the bones, thus maintaining normal levels in the plasma. It also acts on kidneys to cause decreased calcium and increased phosphate excretion.
The calcitonin hormone is secreted by the parafollicular C cells located in the thyroid glands and acts by decreasing the osteoclastic activity in the bones and limiting calcium reabsorption in the kidneys.
-
Vitamin D3 (cholecalciferol) is converted into an active form before it can act as a hormone. It undergoes hydroxylation in the liver to form 25(OH)D3, which further undergoes hydroxylation in the kidneys to form 1,25(OH)2D3, the active form of vitamin D3. Hydroxylation in the kidneys is brought about by the enzyme 1-alpha hydroxylase. The active form of vitamin D3 causes increased intestinal and renal absorption of calcium and phosphate. PTH plays a role in stimulating the conversion of vitamin D3 into its active form 1,25(OH)2D3 in the kidneys.
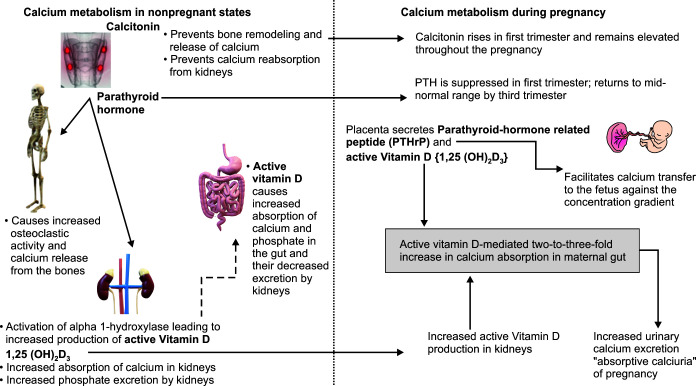
In pregnant states, calcium metabolism undergoes significant changes to promote fetal growth and skeletal development (Fig. 3).
The key change in the maternal physiology that facilitates calcium delivery to the fetus is the increased intestinal calcium and phosphorus absorption in the mother's gut mediated by a two- to three-fold rise in the levels of 1,25(OH)2D3.12 This rise in the level of active vitamin D3 is mediated due to a multifold increase in the production of the enzyme 1-alpha hydroxylase in maternal kidneys. Another source of active vitamin D3 during gestation is the placenta. The increased calcium absorption by the gut under the influence of active vitamin D3 leads to increased urinary calcium excretion by kidneys which is also called absorptive calciuria. Therefore, pregnancy is associated with an increased tendency for stone formation.
The PTH remains suppressed to a low normal range in the first trimester and rises to the mid-normal range in the third trimester. This facilitates the accrual of calcium by maternal bones during the initial phase of the pregnancy, only to be used by the fetus in the later stages when the calcium requirement increases in the fetal skeleton. The PTH does not cross the placenta.
The calcitonin level rises in the first trimester and remains elevated throughout the pregnancy. It is also secreted by the breasts and placenta during pregnancy and prevents calcium release and bone remodeling in the maternal skeleton. Like the PTH hormone, calcitonin does not cross the placenta.
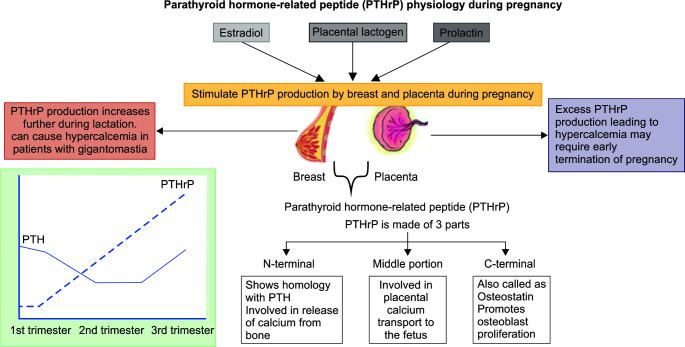
Parathyroid hormone-related peptide (PTHrP) is a unique hormone that remains undetected in nonpregnant states but plays a key role in calcium metabolism during pregnancy and lactation.13 It is secreted from the placenta and breast tissue under the influence of estradiol, placental lactogen, and prolactin. Its level rises steadily throughout the pregnancy and peaks in the third trimester. PTHrP exerts its action via binding to the PTH receptors but disassociates from the receptors in a different manner. During pregnancy, the fetal calcium level is higher than maternal calcium, and calcium transfer requires active movement against the concentration gradient, which is mediated via the PTHrP hormone (Fig. 4).
Fig. 3.
Changes in calcium metabolism during pregnancy
Fig. 4.
Parathyroid hormone (PTH) and parathyroid hormone-related peptide (PTHrP)
Disorders of Calcium Homeostasis in Pregnancy
-
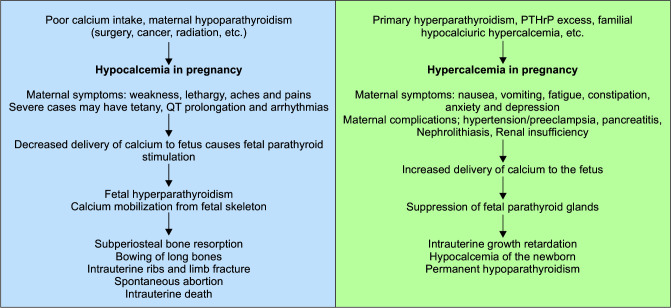
Hypocalcemia in pregnancy: The most common cause of hypocalcemia in pregnancy is poor oral calcium intake and vitamin D3 deficiency. Kumar et al. reported hypocalcemia in 66.4% of women in a study done on 545 healthy pregnant Indian women.14 All cases of hypocalcemia were due to low socioeconomic status-related poor intake. Besides the above-mentioned causes, hypocalcemia can occur due to hypoparathyroidism related to various causes like neck surgery for thyroid/laryngeal cancer, radiation exposure, granulomatous infiltration of parathyroid glands (sarcoidosis and tuberculosis), or drug exposure (L-asparaginase-induced parathyroid necrosis).12
Maternal hypocalcemia leads to decreased placental transfer of calcium and subsequent fetal parathyroid stimulation. This leads to fetal hyperparathyroidism and calcium mobilization from the fetal skeleton to maintain fetal calcium levels. As a result, multiple fetal bone deformities ensue, like subperiosteal bone resorption, intrauterine fracture of ribs and limbs, bowing of long bones, and low birth weight. Maternal calcium and vitamin D supplementation form the mainstay of the therapy (Fig. 5).
Hypercalcemia in pregnancy: Hypercalcemia in pregnancy is a rare disorder with limited literature in this field. But physicians should remain aware of its pathophysiology as it presents with nonspecific symptoms which are difficult to diagnose clinically and cause significant fetomaternal morbidity/mortality. In mothers, hypercalcemia presents as nausea, vomiting, generalized aches, and pains with or without anxiety and depression.15 There are case reports of hypercalcemia presenting as hyperemesis gravidarum in pregnancy. It can lead to maternal complications like hypertension, preeclampsia, pancreatitis, nephrolithiasis, and renal insufficiency. Maternal hypercalcemia causes suppression of fetal parathyroid glands and can present as hypocalcemia of newborns or permanent hypoparathyroidism. It can also lead to intrauterine growth retardation and intrauterine fetal death. Various case reports and case series describing causes have shown primary hyperparathyroidism as the most common cause of hypercalcemia in pregnancy. There are also case reports of hypercalcemia caused by excessive PTHrP secretion by placenta or uterine fibromas or mammary glands, which resolve with the termination of pregnancy or fibroma removal (Fig. 5).16
Fig. 5.
Clinical manifestations of hypo- and hypercalcemia in pregnancy
Lactation increases PTHrP secretion, so patients with primary hyperparathyroidism can experience a rise in calcium levels after delivery, and these patients should be advised against breastfeeding.
Treatment of hypercalcemia in pregnancy includes hyperhydration and calcitonin. Parathyroidectomy can be considered in second trimester in patients with primary hyperparathyroidism. Some authors have used cinacalcet, a calcium mimetic agent, to suppress PTH secretion, but its safety is unknown in pregnancy.
Potassium Homeostasis in Pregnancy
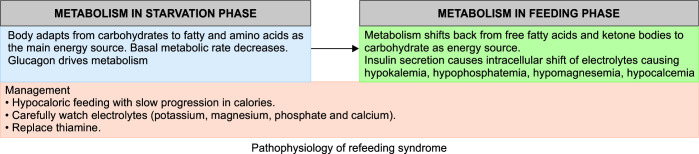
Potassium levels do not change significantly during pregnancy. Causes and management of hypo- and hyperkalemia in pregnancy are the same as those in nonpregnant states. Recommended daily potassium intake is 90–120 mmol/day (3510–4000 mg/day) for young adult and pregnant women but increased to 4400 mg/day for lactating mothers. Special consideration should be given to electrolyte correction and thiamine replacement while managing patients with hyperemesis gravidarum as there is a risk of refeeding syndrome. This syndrome is seen in patients who are fed after a phase of starvation/poor nutritional intake and is characterized by hypokalemia, hypophosphatemia, hypomagnesemia, and hypocalcemia (Fig. 6). Management includes a gradual increase in calorie intake with simultaneous electrolyte and thiamine replacement.17
Fig. 6.
Mechanism of refeeding syndrome
Conclusion
Fetomaternal acid–base and electrolyte changes are essential determinants of pregnancy outcomes. Many of these changes may present with nonspecific clinical symptoms like lethargy, fatigue, or irritability. It is essential to differentiate between physiological changes from those occurring due to the pathological processes for appropriate patient management.
Acknowledgment
Author would like to thank Dr Suhail Sarwar Siddiqui, who contributed to editing the manuscript.
Footnotes
Source of support: Nil
Conflict of interest: None
Orcid
Armin Ahmed https://orcid.org/0000-0002-1626-7418
References
- 1.Hankins GD, Clark SL, Harvey CJ, Uckan EM, Cotton D, Van Hook JW. Third-trimester arterial blood gas and acid base values in normal pregnancy at moderate altitude. Obstet Gynecol. 1996;88(3):347–350. doi: 10.1016/0029-7844(96)00210-4. [DOI] [PubMed] [Google Scholar]
- 2.LoMauro A, Aliverti A. Respiratory physiology of pregnancy: physiology masterclass. Breathe (Sheff) 2015;11(4):297–301. doi: 10.1183/20734735.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frise CJ, Mackillop L, Joash K, Williamson C. Starvation ketoacidosis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2013;167(1):1–7. doi: 10.1016/j.ejogrb.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Ekanem EI, Umoiyoho A, Inyang-Otu A. Study of electrolyte changes in patients with prolonged labour in ikot ekpene, a rural community in niger delta region of Nigeria. ISRN Obstet Gynecol. 2012;2012:430265. doi: 10.5402/2012/430265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belzile M, Pouliot A, Cumyn A, Côté AM. Renal physiology and fluid and electrolyte disorders in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2019;57:1–14. doi: 10.1016/j.bpobgyn.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Pazhayattil GS, Rastegar A, Brewster UC. Approach to the diagnosis and treatment of hyponatremia in pregnancy. Am J Kidney Dis. 2015;65(4):623–627. doi: 10.1053/j.ajkd.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Ananthakrishnan S. Gestational diabetes insipidus: diagnosis and management. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101384. doi: 10.1016/j.beem.2020.101384. [DOI] [PubMed] [Google Scholar]
- 8.Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. 2012;5(Suppl. 1):i3–i14. doi: 10.1093/ndtplus/sfr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalton LM, Ní Fhloinn DM, Gaydadzhieva GT, Mazurkiewicz OM, Leeson H, Wright CP. Magnesium in pregnancy. Nutr Rev. 2016;74(9):549–557. doi: 10.1093/nutrit/nuw018. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy FP, Magee CN, Plant WD, Kenny LC. Gitelman's syndrome in pregnancy: case report and review of the literature. Nephrol Dial Transplant. 2010;25(4):1338–1340. doi: 10.1093/ndt/gfp688. [DOI] [PubMed] [Google Scholar]
- 11.Ali DS, Dandurand K, Khan AA. Hypoparathyroidism in pregnancy and lactation: current approach to diagnosis and management. J Clin Med. 2021;10(7):1378. doi: 10.3390/jcm10071378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almaghamsi A, Almalki MH, Buhary BM. Hypocalcemia in pregnancy: a clinical review update. Oman Med J. 2018;33(6):453–462. doi: 10.5001/omj.2018.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai NK, Martinez D. Physiological roles of parathyroid hormone-related protein. Acta Biomed. 2019;90(4):510–516. doi: 10.23750/abm.v90i4.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A, Agarwal K, Devi SG, Gupta RK, Batra S. Hypocalcemia in pregnant women. Biol Trace Elem Res. 2010;136(1):26–32. doi: 10.1007/s12011-009-8523-6. [DOI] [PubMed] [Google Scholar]
- 15.Rey E, Jacob CE, Koolian M, Morin F. Hypercalcemia in pregnancy – a multifaceted challenge: case reports and literature review. Clin Case Rep. 2016;4(10):1001–1008. doi: 10.1002/ccr3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appelman-Dijkstra NM, Ertl DA, Carola Zillikens M, Rjenmark L, Winter EM. Hypercalcemia during pregnancy: management and outcomes for mother and child. Endocrine. 2021;71(3):604–610. doi: 10.1007/s12020-021-02615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336(7659):1495–1498. doi: 10.1136/bmj.a301. [DOI] [PMC free article] [PubMed] [Google Scholar]