Abstract
The placenta is a temporary, multifunctional organ composed of both maternal and fetal components. It maintains homeostasis to ensure the growth of the fetus and well-being of the mother. Abnormalities in placental development have been known to be responsible for several disorders of pregnancy. Conditions coincident with pregnancy can upset the homeostasis and result in critical illness, which can greatly impact placental function and in turn affect the fetus. Decreased blood flow, acidemia, hypercarbia, and hypoxia seen in critically ill pregnant mothers can result in fetal death. Understanding the physiological changes and functioning of the maternal–fetal–placental unit will aid in better management of critically ill mothers.
How to cite this article
Taggarsi DA, Krishna B. Placenta in the Critically Ill Mother. Indian J Crit Care Med 2021;25(Suppl 3):S200–S205.
Keywords: Critical care, Obstetric critical illness, Placenta, Pregnancy
Introduction
The placenta is the earliest organ to form during the course of embryonic development, and it is the key organ that has resulted in the success of viviparity among mammals. It has been referred to as “chimeric” as it comprises both maternal and fetal components.1 One of the earliest papers on comparative placentation described the mammalian placenta as “an apposition or fusion of the fetal membranes to the uterine mucosa for physiological exchange.”2
Recent studies have demonstrated that the placenta has many roles. It essentially performs the functions of all the diverse organ systems that are hitherto undeveloped in the fetus and is instrumental in fetal maturation. These include the bidirectional transfer of both nutrients and wastes, metabolic functions (like the liver), gas transfer (such as the lungs), barrier function to protect against some viruses, immune barrier against maternal immunological responses, regulation of early development of the embryo, and endocrine function.1,3,4 The placental endocrine function has a wide-ranging impact on maternal health by secretion of placental progesterone, lactogen, and estrogen. Recent research has revealed that it is also a source of serotonin that plays a role in the development of the fetal forebrain. Disturbances such as those seen in preterm neonates have been linked with childhood attention and anxiety disorders, as well as autism.3,4 The physiological changes occurring in pregnancy create a balance to ensure both maternal and fetal well-being.
The critically ill pregnant patient can develop hypoxia, hypotension, acidosis, and other physiological disturbances.5 The placenta plays a central role in a group of diseases that have been described as the “great obstetrical syndromes.”6 The fetoplacental unit is greatly affected by nonobstetric illnesses as well.
Normal Placental Anatomy and Hemodynamics
The human placenta has been described as hemochorial, i.e., vessels lined by endothelial cells containing the fetal blood, whereas the maternal blood is in spaces that are lined by subtypes of specialized trophoblastic cells.4,7 The placenta is formed from the differentiation of the trophectoderm into the trophoblastic cells, which give rise to syncytiotrophoblasts and cytotrophoblasts. These interact with the maternal tissues to form the placenta. The fetal end is known as the chorionic plate which gives rise to the villi. The villous space is divided into cotyledons and the villi are surrounded by maternal blood. Transport of nutrients and waste takes place across this interface either by active transport or passive diffusion.1,4,7 The cytotrophoblastic cells invade the maternal spiral arteries by a process known as “endothelial mimicry”3 and line them. There is extensive remodeling of the spiral arteries which occurs, including vascular smooth muscle loss and replacement of extracellular matrix of the vessel with fibrinoid substance. This prevents constriction and reduces arterial tone resulting in decreased peripheral vascular resistance.8 This results in the high-flow and low-resistance circulation characteristic of the uteroplacental unit.9 A recent study used magnetic resonance imaging (MRI) of placental flows to demonstrate that the velocity of blood reduces as it passes from the wall of the uterus to the placenta. This supports the theory that trophoblastic invasion into the spiral artery results in a reduction in the velocity of blood flow to the placenta.10 Uterine natural killer cells have been found to play an important role in mediating spiral artery transformation by secretion of chemokines and cytokines and also promoting trophoblastic invasion and arterial remodeling.11
Critical Illness in Pregnancy
During pregnancy, the body of the mother goes through many physiological changes. Critical illness results in derangements that can impact uteroplacental blood flow and transfer of oxygen and nutrients.
Critical illness in pregnancy can be related to the pregnancy itself (obstetric causes) or due to nonobstetric causes (Table 1). Defective placentation is one of the most common causes of obstetric critical illnesses. It results in preeclampsia, eclampsia, abruptio placentae, and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome.
Table 1.
List of causes of critical illness in pregnancy5
| Conditions directly related to pregnancy | Preexisting conditions aggravated by pregnancy | Conditions with more susceptibility during pregnancy |
|---|---|---|
| Abnormal placentation Preeclampsia Eclampsia Placental abruption HELLP syndrome Placenta praevia |
Cardiovascular diseases Congenital heart disease Pulmonary hypertension Valvular heart diseases Coarctation of aorta Systemic hypertension |
Infections Falciparum malaria Hepatitis E Varicella pneumonia Listeriosis Urinary tract infection |
| Infectious Chorioamnionitis Septic abortion Puerperal sepsis |
Respiratory Cystic fibrosis Obstructive sleep apnea Bronchial asthma |
Hematologic Deep vein thrombosis DIC HUS/TTP |
| Postpartum Postpartum hemorrhage Retained products of conception |
Renal Chronic kidney disease Glomerulonephritis |
Respiratory Pulmonary thromboembolism Aspiration |
| Cardiac Peripartum cardiomyopathy |
Endocrine Prolactinomas |
Endocrine Sheehan syndrome |
| Hepatic Acute fatty liver of pregnancy |
Hepatic Cirrhosis Budd–Chiari syndrome |
|
| Endocrine Gestational diabetes mellitus |
Connective tissue diseases Scleroderma Systemic lupus erythematosus |
|
| Miscellaneous Amniotic fluid embolism |
Neurologic Epilepsy Intracranial tumors |
|
| Hematological Sickle cell disease |
HELLP syndrome, hemolysis, elevated liver enzymes, and low platelets; DIC, disseminated intravascular coagulation; HUS, hemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura
Certain preexisting conditions such as congenital heart disease, connective tissue diseases, diabetes, liver diseases like cirrhosis, and neurological diseases like epilepsy may worsen during the pregnancy.5 Such illnesses disturb the physiological balance and adversely affect the mother and fetus.
Placental Role in the “Great Obstetric Syndromes”
The role of spiral arterial remodeling is one of the theories to explain the pathophysiology of preeclampsia. Abnormal remodeling of the spiral artery was first associated with the development of preeclampsia in 1964.12 It has since been implicated to cause preterm labor, intrauterine growth restriction, preterm premature rupture of membranes, abruptio placentae, and late spontaneous abortion—collectively known as the “great obstetric syndromes.”6 Defective placentation occurs in the paracentral part where features like thrombosis of the spiral arteries and placental infarcts have been identified, whereas normal physiological changes are present in the center of the placental bed.6 These physiological changes usually extend deep into the myometrium, involving the radial arteries.12 In preeclampsia, the myometrial segment fails to undergo physiological changes.13
It is also characterized by acute atherosis within the vessels and is similar to pathological changes seen secondary to hypertension. This has been corroborated by MRI evaluation of placental flows in preeclampsia, which showed greater velocity of flow through the intervillous space and increased resistance to flow, unlike what is seen in normal pregnancy.10 This defective remodeling results in intrauterine hypoxia secondary to vasospasm and reduced placental flows. It results in increased oxidative stress and abnormal systemic inflammatory response in the mother. This can result in fetal hypoxia, fetal acidosis, and growth retardation. It forms the basis of the “two-stage theory of preeclampsia.” The first stage is the abnormal placentation and the second stage is immune activation.14 Maternal endothelial dysfunction has been attributed to be the reason behind the clinical features of preeclampsia.15 It has been proposed that this is due to the production of free radicals which result in increased circulating inflammatory mediators, like soluble vascular endothelial growth factor (VEGF) receptor (sFLT1), and soluble E-selectin (cause endothelial cell activation) and leukocyte activation with increased Th1 cytokine; all of which result in features like hypertension, kidney injury, and proteinuria and liver injury.15,16
While this theory has been challenged by some, it has been largely accepted as the underlying reason for preeclampsia. Large number of studies have validated the theory that angiogenic factor imbalance results in pathophysiological changes seen in preeclamptic patients.15 This led to the use of antioxidants (vitamin C and vitamin E) and low-dose aspirin as a means of reducing oxidative stress in the placental unit.17,18 However, larger trials have uncovered uncertainties around benefits of aspirin19,20 or vitamin C20 especially in the later stages of gestation.
Placental Role in Acute Fatty Liver of Pregnancy
Acute fatty liver of pregnancy has been attributed to an increase in circulating fatty acids in the mother and oxidative stress.21 One of the prevailing theories is that this occurs due to a fetal deficiency of mitochondrial long-chain acyl-coenzyme A dehydrogenase (LCHAD). However, it has been observed that in a majority of cases of acute fatty liver of pregnancy, fatty acid oxidation disorders could not be demonstrated in the fetus.22
It has been proposed that defective mitochondrial function in the placenta could lead to oxidative stress and free radical formation. This could further result in fatty acid deposition in the placenta and subsequent rise in serum fatty acids leading to acute fatty liver of pregnancy.23
Placenta: Immunity and Infections
Pregnancy was initially considered to be an immunosuppressed state.24,25 In 1953, Medawar proposed the immunological paradox where he compared the fetus to a semi-allogeneic graft that could survive for 9 months without immunosuppression. He proposed that this was because of three main reasons: (i) placental barrier between mother and fetus, (ii) immature fetal antigens that failed to elicit an immune response in the mother, and (iii) pregnancy induced a state of immunological inertness. Most of these theories have been disproved.26
Subsequent investigators proposed that throughout pregnancy, there is a balance between Th1 and Th2 responses mediated by estrogen and progesterone level fluctuations. It was widely believed that it resulted in a predominantly anti-inflammatory or Th2 cytokine state.24 Any insults such as infections that affect this were thought to be the perpetrators of disease states in the pregnant mother as well as fetal development.22,27
Another proposition has been that pregnancy consists of three separate immunological phases. The first trimester is a predominantly proinflammatory phase resulting in symptoms such as nausea. The second trimester is an anti-inflammatory state and allows for optimal growth of the fetus. The third trimester has been described as a proinflammatory phase which finally culminates in expulsion of the products of conception.28
Placenta and Infections
The placenta is believed to be capable of interacting with various pathogens as it displays tropism to some. It has also been described as an active immunological site capable of generating immune responses to infections.24 In early stages, placental vascular development is dependent on a balance between various proangiogenic as well as antiangiogenic factors. Infections like malaria and human immunodeficiency virus (HIV) can cause inflammation and immunological responses, which throw these factors off balance. This can translate to inadequate placental vascular development and adverse fetal outcomes.25 Viremia is essential for the virus to reach the fetoplacental unit. A number of developmental abnormalities in the fetus have been associated with congenital infections such as Toxoplasmosis, Other—syphilis, varicella zoster, parvovirus B19, Rubella, Cytomegalovirus, and Herpes simplex (TORCH). However, even in the absence of placental transfer, maternal infections can impact fetal well-being. Activation of maternal immune responses by certain viral infections can result in developmental abnormalities in the fetal heart, brain, and lung.27,28 This may also produce an inflammatory response in the placental unit, which may have long-lasting effects on fetal immunity including the ability to respond to vaccines and the development of allergic tendencies. It can also trigger abortion or preterm labor.29,30 This has also been described as the fetal inflammatory response syndrome31 (FIRS) where the neonate is found to have high levels of cytokines [interleukin (IL)-6, IL-1, IL-8, and tumor necrosis factor (TNF)-alpha] in the absence of identifiable infections. These have been known to impact neuronal development and have long-term effects on the developing fetal brain.25,28
Viral infections can also cause direct placental damage and impact fetal well-being. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been associated with villous stromal–vascular karyorrhexis, villous infarction, perivillous deposition of fibrin, and large intervillous thrombi, which have resulted in fetal demise.32
What Happens to the Placenta in Critical Illness?
In critical illness, both obstetric and nonobstetric, the mother and fetus are subjected to stressors such as immunological activation, hypoxia, acidosis, organ hypoperfusion, and systemic inflammation. In subsequent sections, we will discuss the impact of each of these on the utero-feto placental unit.
Placenta in Shock States in the Critically Ill
In shock states, there is decreased organ perfusion that results in decreased blood flow to the placenta. Compensatory maternal responses are not protective to the fetus as they focus on maintenance on perfusion to vital organs of the mother.33 Alpha-adrenergic receptors present in the placental vascular bed respond to sympathetic stimulation which results in constriction and further reduction in flows.
Various types of shock result in tissue hypoperfusion, resulting in increased anaerobic metabolism and rising lactate levels. The placenta serves as a lactate buffer and shields the fetus against increased maternal lactate levels. The placenta plays an active role by ensuring that lactate flux to the maternal circulation is higher than lactate flux to the fetus. On the contrary, it is hypothesized that it can enhance its own metabolic activity to supply lactate to the fetus when levels are low.34 In the absence of this buffer, or if it gets overwhelmed, there can be progressive fetal acidosis and can lead to fetal demise.
Placenta, Acidosis, and Hypoxia
In the first trimester of gestation, the spiral arteries are plugged with the invading trophoblastic cells and there is no maternal blood in the villous spaces. This ensures that the placental and fetal development occurs in an environment with low oxygen tension. Placental nutrition is provided by increased endometrial secretions.3 This is done because the developing fetus is at high risk of damage, which may be mediated by reactive oxygen species in the presence of higher oxygen tension and absence of protective antioxidant substances. Blood enters the intervillous space only by 12–14 weeks of gestation.35
A fetus normally has a very low PaO2 (35 mm Hg) with a PaCO2 of 42 mm Hg. The high oxygen content of blood is maintained by the fetal hemoglobin that shifts the oxygen hemoglobin dissociation curve to the left. This is an essential physiological adaptation to maximize oxygen delivery to the fetus.
An important regulator of fetoplacental vascular tone has been proposed to be hypoxia itself; however, its impact has been poorly studied. In the event of acute hypoxia, it has been observed that there is excess sympathetic activation in the fetus.36 By way of alpha-adrenergic stimulation, there is preferential diversion of blood to the heart, brain, and placenta in the fetus. Concomitantly, there is decreased supply to other organs like the lungs and the gut where there is vasoconstriction mediated by alpha-adrenergic stimulation.37 There are also compensatory mechanisms observed in the fetus during periods of acute hypoxia. In animal models, it has been seen that there is a 60% decrease in oxygen consumption by the fetus during hypoxic episodes by a shift toward anaerobic metabolism. These mechanisms allow the fetus to tolerate acute hypoxia for about 30 minutes. It is accompanied by fetal bradycardia, increased fetal blood pressure, and subsequently fetal acidosis.38 Fetal acidosis can further hamper oxygen transport across the placenta by shifting the oxygen hemoglobin dissociation curve to the right.
Analogous to the pulmonary circulation, experimental studies have also demonstrated the presence of fetoplacental vasoconstriction during periods of hypoxia also known as hypoxic fetoplacental vasoconstriction (HFPV). This was first described in 1987.39 This response was observed irrespective of pCO2 and pH levels. The response appears to be mediated via hypoxic inhibition of Kv (voltage-gated potassium channels) channels, which results in a change in the membrane potential. This results in opening of calcium channels and subsequent contraction of vascular smooth muscle and vasoconstriction.40 It is hypothesized that this helps to optimize fetal oxygenation by diverting fetal blood to well-oxygenated zones of the placenta. The role of nitric oxide (NO) in HFPV is still controversial. In one study, it was observed that while the baseline vascular tone was influenced by NO, it played no role in HFPV.40 In another study, the HFPV was abolished by NO.41 The presence of HFPV has been challenged by certain investigators42 and requires further investigation.
It has been demonstrated that vascular tone in the term human placenta is influenced to an extent by extracellular pH. Acidemia causes relaxation of the chorionic plate arteries over a range of pH values between 6.4 and 7.4.43 This is consistent with the findings that chronic hypoxia results in increased uterine artery blood flow which consequently maintains total oxygen delivery when oxygen tension is reduced.37 This response appears to be blunted in preeclampsia and may contribute to increased fetal stress during periods of acidosis.
Maternal pH and pCO2 are important factors that maintain oxygen transfer across the placenta. Normally, maternal hyperventilation ensures a state of respiratory alkalosis which results in transfer of CO2 from fetal-to-maternal circulation. This results in a fall in maternal pH and rise in fetal pH (double Bohr effect), which ensures saturation of fetal hemoglobin with oxygen (Fig. 1).35
Fig. 1.
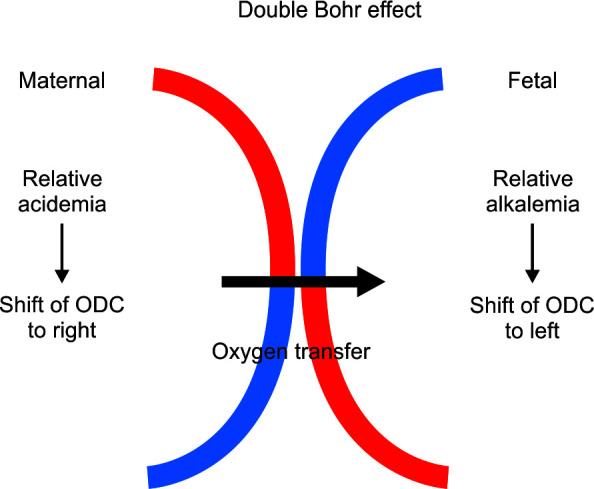
Representation of double Bohr effect in placenta. At tissue level in the placenta, CO2 diffuses from fetus to mother and produces relative acidemia at the maternal side. There is a rise in pH on the fetal side. This facilitates greater diffusion of oxygen from mother to fetus. ODC, oxygen dissociation curve; CO2, carbon dioxide
In the presence of maternal hypercapnia, there is impaired CO2 diffusion from fetus to the mother resulting in fetal acidemia and decreased affinity of hemoglobin to oxygen. This can further compound fetal hypoxia (Fig. 2).44 Thus, targeting a lower pH while ventilating the pregnant mother may aid in oxygen transfer to the fetus.
Fig. 2.

Effects of maternal hypercarbia on fetal oxygenation and pH. ODC, oxygen dissociation curve; CO2, carbon dioxide
Anemia and Placental Oxygen Transfer
Anemia has been demonstrated to have a large impact on oxygen transfer across the placenta. It has been demonstrated that uterine oxygen delivery is predominantly impacted by blood flow, oxygen saturation, and hemoglobin concentration.45 As physiological anemia of pregnancy already reduces the hematocrit, a further drop in hemoglobin may significantly affect fetal oxygen levels.
Effect of Vasoactive Drugs on Uteroplacental Flow
Cardiogenic shock has been observed in critically ill pregnant women. Some of the commonly used agents in treatment include dopamine, dobutamine, and milrinone. The impact of vasopressors and inotropes on uteroplacental flows has been evaluated largely by conducting animal studies.46 Dopamine at higher doses results in reduction in uterine blood flows and leading to a progressive decrease in fetal arterial pH and rise in fetal arterial pCO2. Milrinone was found to produce an increase in uterine blood flow.47 Dobutamine was found to result in a relatively unchanged mean arterial pressure (MAP) but reduced uterine blood flow. The effect, however, was less than that of dopamine.46 It was observed in another study that the fall occurred after 60 minutes, probably due to effects of 3-O methyldobutamine (a metabolite of dobutamine). This reduction, however, did not result in decreased fetal oxygen concentration. In fact, fetal oxygenation improved with dobutamine. This was explained by the increase in maternal hemoglobin as a result of splenic contraction caused by dobutamine.48
Vasopressor agents used in septic shock also reduce blood flow to the uterus by causing vasoconstriction. Uterine blood flow is reduced in response to use of epinephrine, norepinephrine as well as dopamine.49 Phenylephrine and ephedrine have been found to preserve placental blood flow compared to the rest of the vasopressors, especially during spinal anesthesia. Among the two, studies showed that ephedrine caused more fetal acidosis by resulting in increased fetal oxygen consumption, increased fetal CO2 production, and increased lactate.50
Effect of Extracorporeal Circulation on Placenta
Extracorporeal circulation membrane oxygenation and cardiopulmonary bypass result in significant variations in uterine blood flow and oxygen delivery. Extracorporeal circulation results in hemodilution, systemic inflammation, leukocyte activation, alteration in coagulation, hypotension, and hypothermia.51 All of these factors can affect oxygen delivery to the fetus, especially hemodilution (as explained above). Uterine contractions occurring during extracorporeal circulation have been associated with fetal death.51
Placental Transfer of Drugs
The placenta acts like a selective barrier and there is transfer of drugs which can occur to the fetus. While due attention must be given to this, essential treatment still must be given to the pregnant mother irrespective of teratogenic concerns after advising them about risks involved. Teratogenic effects of commonly used drugs like antibiotics are highest in the first trimester.34 A Cochrane review recently concluded that antibiotic use in the second and third trimesters did not significantly increase risk of congenital abnormalities. However, they concluded that they had insufficient evidence to do a comprehensive evaluation of effects on the fetus.52
Pregnancy results in a number of hemodynamic changes such as hemodilution, increase in circulating blood volume, decreased gastric motility, and increase in glomerular filtration rate. This can impact usual drug pharmacokinetics and must be kept in mind while deciding dosage. Therapeutic drug level monitoring can be employed when possible.53
Conclusion
The placenta is one of the most unique organs that has developed during the course of evolution. Its diversity of function is what results in successful viviparity. The placenta has an unquestionable role in both maternal and fetal health and can cause serious effects on both. We are still trying to understand the complexity of its workings. Critical illness poses an additional challenge. Due consideration to the anatomical and physiological nature of the materno-fetoplacental relationship will result in better outcomes for both the mother and fetus.
Footnotes
Source of support: Nil
Conflict of interest: None
Orcid
Dipali Anand Taggarsi https://orcid.org/0000-0001-8168-179X
Bhuvana Krishna https://orcid.org/0000-0002-0003-6797
References
- 1.Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120(4):1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mossman HW. Comparative morphogenesis of the fetal membranes and accessory uterine structures. Contrib Embryol Carneg Instn. 1937;26:129–246. doi: 10.1016/0143-4004(91)90504-9. [DOI] [PubMed] [Google Scholar]
- 3.Maltepe E, Fisher SJ. Placenta: the forgotten organ. Annu Rev Cell Dev Biol. 2015;31:523–552. doi: 10.1146/annurev-cellbio-100814-125620. [DOI] [PubMed] [Google Scholar]
- 4.Soares MJ, Varberg KM, Iqbal K. Hemochorial placentation: development, function, and adaptations. Biol Reprod. 2018;99(1):196–211. doi: 10.1093/biolre/ioy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guntupalli KK, Hall N, Karnad DR, Bandi V, Belfort M. Critical illness in pregnancy: part I: an approach to a pregnant patient in the ICU and common obstetric disorders. Chest. 2015;148(4):1093–1104. doi: 10.1378/chest.14-1998. [DOI] [PubMed] [Google Scholar]
- 6.Brosens I, Puttemans P, Benagiano G. Placental bed research: I. The placental bed: from spiral arteries remodeling to the great obstetrical syndromes. Am J Obstet Gynecol. 2019;221(5):437–456. doi: 10.1016/j.ajog.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 7.Huppertz B. The anatomy of the normal placenta. J Clin Pathol. 2008;61(12):1296–1302. doi: 10.1136/jcp.2008.055277. [DOI] [PubMed] [Google Scholar]
- 8.Aplin JD, Myers JE, Timms K, Westwood M. Tracking placental development in health and disease. Nat Rev Endocrinol. 2020;16(9):479–494. doi: 10.1038/s41574-020-0372-6. [DOI] [PubMed] [Google Scholar]
- 9.Hu XQ, Zhang L. Hypoxia and mitochondrial dysfunction in pregnancy complications. Antioxidants. 2021;10(3):405. doi: 10.3390/antiox10030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellschaft NS, Hutchinson G, Shah S, Jones NW, Bradley C, Leach L, et al. The haemodynamics of the human placenta in utero. PLoS Biol. 2020;18(5):e3000676. doi: 10.1371/journal.pbio.3000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris LK, Benagiano M, D'elios MM, Brosens I, Benagiano G. Placental bed research: II. Functional and immunological investigations of the placental bed. Am J Obstet Gynecol. 2019;221(5):457–469. doi: 10.1016/j.ajog.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Brosens I. A study of the spiral arteries of the decidua basalis in normotensive and hypertensive pregnancies. BJOG. 1964;71(2):222–230. doi: 10.1111/j.1471-0528.1964.tb04270.x. [DOI] [PubMed] [Google Scholar]
- 13.De Wolf F, Robertson WB, Brosens I. The ultrastructure of acute atherosis in hypertensive pregnancy. Am J Obstet Gynecol. 1975;123(2):164–174. doi: 10.1016/0002-9378(75)90522-0. [DOI] [PubMed] [Google Scholar]
- 14.Redman CW. 10 Immunological aspects of pre-eclampsia. Bailliere's Clin Obstet Gynaecol. 1992;6(3):601–615. doi: 10.1016/s0950-3552(05)80012-4. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed A, Ramma W. Unravelling the theories of pre‐eclampsia: are the protective pathways the new paradigm? Br J Pharmacol. 2015;172(6):1574–1586. doi: 10.1111/bph.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. 2015;213(4):S115–S122. doi: 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallenburg HC, Makovitz JW, Dekker GA, Rotmans P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet. 1986;327(8471):1–3. doi: 10.1016/s0140-6736(86)91891-x. [DOI] [PubMed] [Google Scholar]
- 18.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145–1154. doi: 10.1016/S0140-6736(06)68433-X. Vitamins in Pre-eclampsia (VIP) Trial Consortium. [DOI] [PubMed] [Google Scholar]
- 19.Villa PM, Kajantie E, Räikkönen K, Pesonen AK, Hämäläinen E, Vainio M, et al. Aspirin in the prevention of pre‐eclampsia in high‐risk women: a randomised placebo‐controlled PREDO Trial and a meta‐analysis of randomised trials. BJOG. 2013;120(1):64–74. doi: 10.1111/j.1471-0528.2012.03493.x. [DOI] [PubMed] [Google Scholar]
- 20.Rossi AC, Mullin PM. Prevention of pre-eclampsia with low-dose aspirin or vitamins C and E in women at high or low risk: a systematic review with meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2011;158(1):9–16. doi: 10.1016/j.ejogrb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Naoum EE, Leffert LR, Chitilian HV, Gray KJ, Bateman BT. Acute fatty liver of pregnancy: pathophysiology, anesthetic implications, and obstetrical management. Anesthesiology. 2019;130(3):446–461. doi: 10.1097/ALN.0000000000002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182–193. doi: 10.1016/j.clinre.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan SK, Thangaraj KR, Eapen CE, Ramachandran A, Mukhopadhya A, Mathai M, et al. Liver injury in acute fatty liver of pregnancy: possible link to placental mitochondrial dysfunction and oxidative stress. Hepatology. 2010;51(1):191–200. doi: 10.1002/hep.23245. [DOI] [PubMed] [Google Scholar]
- 24.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370(23):2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weckman AM, Ngai M, Wright J, McDonald CR, Kain KC. The impact of infection in pregnancy on placental vascular development and adverse birth outcomes. Front Microbiol. 2019;10:1924. doi: 10.3389/fmicb.2019.01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rendell V, Bath NM, Brennan TV. Medawar's paradox and immune mechanisms of fetomaternal tolerance. OBM Transplant. 2020;4(1):26. doi: 10.21926/obm.transplant.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63(6):425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardenas I, Aldo P, Koga K, Means R, Lang S, Mor G. Subclinical viral infection in pregnancy leads to inflammatory process at the placenta with non-lethal fetal damage: S-12. Am J Reprod Immunol. 2009;61(6):397. doi: 10.4049/jimmunol.1000289. [DOI] [Google Scholar]
- 30.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65(suppl_3):S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 31.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179(1):194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 32.Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154(1):23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoyama K, Seaward PG, Lapinsky SE. Fetal outcome in the critically ill pregnant woman. Crit Care. 2014;18(3):1–7. doi: 10.1186/cc13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barta E. Lactate transport at the uteroplacental unit – a theoretical study. bioRxiv. 2020 doi: 10.1101/2020.10.23.351841. [DOI] [Google Scholar]
- 35.Carter AM. Placental gas exchange and the oxygen supply to the fetus. Compr Physiol. 2011;5(3):1381–1403. doi: 10.1002/cphy.c140073. [DOI] [PubMed] [Google Scholar]
- 36.Omo-Aghoja L. Maternal and fetal acid-base chemistry: a major determinant of perinatal outcome. Ann Med Health Sci Res. 2014;4(1):8–17. doi: 10.4103/2141-9248.126602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parer JT. Effects of hypoxia on the mother and fetus with emphasis on maternal air transport. Am J Obstet Gynecol. 1982;142(8):957–961. doi: 10.1016/0002-9378(82)90774-8. [DOI] [PubMed] [Google Scholar]
- 38.Parer JT. The effect of acute maternal hypoxia on fetal oxygenation and the umbilical circulation in the sheep. Eur J Obstet Gynecol Reprod Biol. 1980;10(2):125–136. doi: 10.1016/0028-2243(80)90090-8. [DOI] [PubMed] [Google Scholar]
- 39.Howard RB, Hosokawa T, Maguire MH. Hypoxia-induced fetoplacental vasoconstriction in perfused human placental cotyledons. Am J Obstet Gynecol. 1987;157(5):1261–1266. doi: 10.1016/s0002-9378(87)80307-1. [DOI] [PubMed] [Google Scholar]
- 40.Hampl V, Bíbová J, Stranák Z, Wu X, Michelakis ED, Hashimoto K, et al. Hypoxic fetoplacental vasoconstriction in humans is mediated by potassium channel inhibition. Am J Physiol Heart Circ Physiol. 2002;283(6):H2440–H2449. doi: 10.1152/ajpheart.01033.2001. [DOI] [PubMed] [Google Scholar]
- 41.Byrne BM, Howard RB, Morrow RJ, Whiteley KJ, Adamson SL. Role of the L-arginine nitric oxide pathway in hypoxic fetoplacental vasoconstriction. Placenta. 1997;18(8):627–634. doi: 10.1016/s0143-4004(97)90003-5. [DOI] [PubMed] [Google Scholar]
- 42.Wareing M. Oxygen sensitivity, potassium channels, and regulation of placental vascular tone. Microcirculation. 2014;21(1):58–66. doi: 10.1111/micc.12069. [DOI] [PubMed] [Google Scholar]
- 43.Ali TY, Broughton Pipkin F, Khan RN. The effect of pH and ion channel modulators on human placental arteries. PLoS One. 2014;9(12):e114405. doi: 10.1371/journal.pone.0114405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeLuca L, Holzman I, Gibbs K. Newborn ventilatory response to maternal chronic hypercapnia. J Perinatol. 2012;32(10):804–806. doi: 10.1038/jp.2011.164. [DOI] [PubMed] [Google Scholar]
- 45.Wilkening RB, Meschia GI. Fetal oxygen uptake, oxygenation, and acid-base balance as a function of uterine blood flow. Am J Physiol. 1983;244(6):H749–H755. doi: 10.1152/ajpheart.1983.244.6.H749. [DOI] [PubMed] [Google Scholar]
- 46.Fishburne JI, Meis PJ, Urban RB, Greiss FC, Wheeler AS, James FM, et al. Vascular and uterine responses to dobutamine and dopamine in the gravid ewe. Am J Obstet Gynecol. 1980;137(8):944–952. doi: 10.1016/s0002-9378(16)32836-8. [DOI] [PubMed] [Google Scholar]
- 47.Santos AC, Baumann AL, Wlody D, Pedersen H, Morishima HO, Finster M. The maternal and fetal effects of milrinone and dopamine in normotensive pregnant ewes. Am J Obstet Gynecol. 1992;166(1):257–262. doi: 10.1016/0002-9378(92)91869-C. [DOI] [PubMed] [Google Scholar]
- 48.Hu H, Pasca I. Management of complex cardiac issues in the pregnant patient. Crit Care Clin. 2016;32(1):97–107. doi: 10.1016/j.ccc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 49.James FM. Greiss FC Jr, Kemp RA. An evaluation of vasopressor therapy for maternal hypotension during spinal anesthesia. Anesthesiology. 1970;33(1):25–34. doi: 10.1097/00000542-197007000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Kee WD, Lee A, Khaw KS, Ng FF, Karmakar MK, Gin T. A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: the effects on fetal acid-base status and hemodynamic control. Anesth Analg. 2008;107(4):1295–1302. doi: 10.1213/ane.0b013e31818065bc. [DOI] [PubMed] [Google Scholar]
- 51.Kapoor MC. Cardiopulmonary bypass in pregnancy. Ann Card Anaesth. 2014;17(1):33. doi: 10.4103/0971-9784.124133. [DOI] [PubMed] [Google Scholar]
- 52.Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P, Ota E. Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity. Cochrane Database Syst Rev. 2015;1:CD002250. doi: 10.1002/14651858.CD002250.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Bookstaver PB, Bland CM, Griffin B, Stover KR, Eiland LS, McLaughlin M. A review of antibiotic use in pregnancy. Pharmacotherapy. 2015;35(11):1052–1062. doi: 10.1002/phar.1649. [DOI] [PubMed] [Google Scholar]


