Abstract
Hypertensive disorders of pregnancy can be classified as chronic hypertension (present before pregnancy), gestational hypertension (onset after 20 weeks of pregnancy), and preeclampsia (onset after 20 weeks of pregnancy, along with proteinuria and other organ dysfunction). Preeclampsia and related disorders are a major cause of maternal and fetal morbidity and mortality. Preeclampsia is believed to result from an angiogenic imbalance in the placenta circulation. Antenatal screening and early diagnosis may help improve outcomes. Severe preeclampsia is characterized by SBP ≥160 mm Hg, or DBP ≥110 mm Hg, thrombocytopenia (platelet count <100 × 109/L), abnormal liver function, serum creatinine >1.1 mg/dL, or a doubling of the serum creatinine concentration in the absence of other renal diseases, disseminated intravascular coagulation, pulmonary edema, new-onset headache, or visual disturbances. Severe preeclampsia or eclampsia (preeclampsia with seizures) needs ICU management and is the main cause of morbidity and mortality. Severe hypertension can also result in life-threatening intracranial hemorrhage. Blood pressure control, seizure prevention, and appropriate timing of delivery are the cornerstones of the management of preeclampsia. Besides intravenous antihypertensive drugs, intravenous magnesium sulfate is the drug of choice to prevent or treat seizures, when preparing for urgent delivery. At present, delivery remains the most effective treatment for preeclampsia, and organ dysfunction rapidly recovers after delivery. Novel therapeutic interventions are under development to reduce complications.
How to cite this article
Narkhede AM, Karnad DR. Preeclampsia and Related Problems. Indian J Crit Care Med 2021;25(Suppl 3):S261–S266.
Keywords: Cerebral venous sinus thrombosis, Disseminated intravascular coagulation, Hypertensive emergency, Maternal mortality, Obstetric critical care, Preeclampsia, Pregnancy
Introduction
Pregnancy is a physiological phenomenon with a high potential for severe pathologic states that can be life-threatening for both the mother and the fetus. Preeclampsia and related disorders are a common cause of fetal-maternal morbidity and mortality. Preeclampsia and related disorders complicate around 5–7% of pregnancies and contribute from 35 to 44.93% of obstetric intensive care unit (ICU) admissions.1–5 Worldwide, hypertensive disorders are the most common causes of maternal mortality, accounting for nearly 14% of maternal deaths.6,7 In low- to middle-income countries, many of these deaths could be attributed to lack of access to basic antenatal care and intensive care facilities.
Definitions and Diagnostic Criteria8
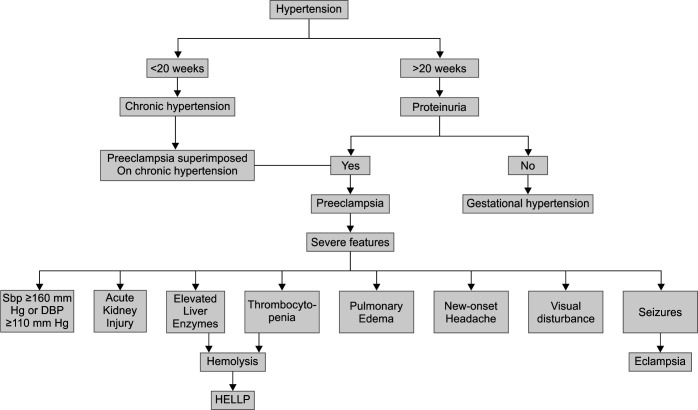
Gestational hypertension is defined as an SBP ≥140 mm Hg or DBP ≥90 mm Hg or both, on two occasions at least 4 hours apart after 20 weeks of gestation, in a woman with previously normal blood pressure, in the absence of other features of preeclampsia (Flowchart 1).
Flowchart 1.
Hypertensive disorders in pregnancy
Preeclampsia
-
Blood pressure
-
– Systolic blood pressure (SBP) of 140 mm Hg or more or diastolic blood pressure of 90 mm Hg or more on two occasions at least 4 hours apart after 20 weeks of gestation in a woman with previously normal blood pressure
Or
-
– SBP of 160 mm Hg or more or DBP of 110 mm Hg or more [severe hypertension can be confirmed within a short interval (minutes) to facilitate timely antihypertensive therapy].
And
-
-
Proteinuria
-
– Three hundred milligrams or more per 24-hour urine collection (or this amount extrapolated from a timed collection)
Or
-
– Protein/creatinine ratio of 0.3 mg/dL or more
Or
-
– Dipstick reading of 2+ (used only if other quantitative methods not available)
Or
In the absence of proteinuria, new-onset hypertension with the new onset of any of the following:
– Thrombocytopenia: platelet count less than 100 × 109/L
– Renal insufficiency: serum creatinine concentrations greater than 1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal diseases
– Impaired liver function: elevated blood concentrations of liver transaminases to twice normal concentration
– Pulmonary edema
– New-onset headache unresponsive to medication and not accounted for by alternative diagnoses
– Visual symptoms
-
Preeclampsia with Severe Features
SBP ≥160 mm Hg or DBP ≥110 mm Hg on two occasions at least 4 hours apart (unless antihypertensive therapy is initiated before this time)
Thrombocytopenia (platelet count less than 100 × 109/L)
Impaired liver function that is not accounted for by alternative diagnoses and as indicated by abnormally elevated blood concentrations of liver enzymes (to more than twice the upper limit normal concentrations), or by severe persistent right upper quadrant or epigastric pain unresponsive to medications
Renal insufficiency (serum creatinine concentration more than 1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal diseases)
Pulmonary edema
New-onset headache unresponsive to medication and not accounted for by alternative diagnoses
Visual disturbances
Eclampsia is defined by new-onset tonic-clonic, focal, or multifocal seizures in the absence of other causative conditions such as epilepsy, cerebral arterial ischemia, and infarction, intracranial hemorrhage, or drug use.
Hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome is one of the more severe forms of preeclampsia with a drop in hemoglobin with lactate dehydrogenase (LDH) elevated to 600 IU/L or more, aspartate aminotransferase, and alanine aminotransferase elevated more than twice the upper limit of normal, and the platelets count less than 100 × 109/L.8
Risk Factors for Preeclampsia
Risk factors for preeclampsia include nulliparity, multifetal gestations, preeclampsia in a previous pregnancy, chronic hypertension, pregestational diabetes, gestational diabetes, thrombophilia, systemic lupus erythematosus (SLE), prepregnancy body mass index (BMI) greater than 30, antiphospholipid antibody (APLA) syndrome, maternal age 35 years or older, kidney disease, assisted reproductive technology, and obstructive sleep apnea.8 However, it should be noted that most cases of preeclampsia occur in healthy nulliparous women with no obvious risk factors.
Pathogenesis of Preeclampsia
The pathogenesis of preeclampsia is best explained by the two-stage paradigm considered to involve two stages: (1) abnormal placentation and (2) development of maternal syndrome9
Abnormal placentation: Placental ischemia and hypoxia play an important role in the pathogenesis of preeclampsia.10 Acute fibrinoid necrosis of vessel wall and defective myometrial spiral artery remodeling are prevalent in histopathologic studies of preeclamptic placentae. Abnormalities in cytotrophoblast differentiation with defective placentation and failure to transform uterine spiral arteries are central to the pathogenesis of preeclampsia. Persistent elevated expression of hypoxia-inducible factor 1α (HIF1α) in preeclamptic placentae could indicate its pathogenic role in preeclampsia. Imbalance between prooxidative and antioxidative mechanisms mediated by the heme oxygenase pathway also leads to abnormal placentation. Immune mechanisms implicated in defective placentation include abnormalities in activation of decidual natural killer cells, allorecognition of paternal major histocompatibility complex HLA-C, predominance of T helper 1 cells and related cytokines, and abnormal activation of complement system.9
Maternal syndrome: The abnormal placenta secretes excessive antiangiogenic factors that cause maternal vascular inflammation and endothelial dysfunction. Imbalance between proangiogenic and antiangiogenic factors as evident from increased soluble fms-like tyrosine kinase-1 (sFlt1) and soluble endoglin along with decreased activity of placental growth factor (PlGF) leads to vascular injury, inflammation, and thrombosis and manifests as hypertension and organ dysfunctions. Hypertension could be mediated through agonist autoantibodies that bind to angiotensin II type 1 receptor. Other possible mechanisms causing hypertension include decreased levels of nitric oxide, increased activity of endothelin 1, and decreased placental expression of cystathionine γ-lyase with reduced hydrogen sulfide levels. The intense vasoconstriction along with capillary leak makes these patients prone to develop pulmonary edema. The exact mechanisms behind proteinuria in preeclampsia are not clear; however, findings of glomeruloendotheliosis and loss of endothelial glycocalyx have been implicated.9 Thrombocytopenia may result from increased platelet activation and consumption. Hemolysis as indicated by elevated LDH levels may be seen in patients with HELLP syndrome. Hematocrit may be normal despite hemolysis due to hemoconcentration.8 Elevated liver enzymes in severe preeclampsia are secondary to periportal necrosis. Placental ischemia can lead to fetal growth retardation, oligohydramnios, placental abruption, and increased risk of spontaneous preterm delivery.8 Convulsions develop as a consequence of cerebral vasospasm, hypertensive encephalopathy, breakdown of cerebral autoregulation, vasogenic and cytotoxic edema, cerebral microhemorrhages, and metabolic encephalopathy.11
Clinical Features
Most of the patients with preeclampsia are asymptomatic initially and diagnosed during routine antenatal visits. Onset is generally insidious with symptoms like edema which are often passed off as “normal” effects of pregnancy. Hypertension, edema, and proteinuria are defining features of preeclampsia. Preeclampsia should be differentiated from gestational hypertension of chronic hypertension; proteinuria and edema are absent in gestational hypertension and chronic hypertension. The other clinical features indicative of organ-system involvement are seen in severe preeclampsia. New-onset headache may be a sign of cerebral edema or posterior reversible encephalopathy syndrome (PRES). Visual symptoms include blurred vision, photopsia, scotomata, or blindness. Stroke due to intracranial hemorrhage is a serious complication and always associated with severe uncontrolled hypertension. Hyperreflexia and ankle clonus could be signs of neurological involvement, and seizures are the defining features for eclampsia. Pulmonary edema causing breathlessness occurs due to severe hypertension, left ventricular failure, or, rarely, capillary leak. Epigastric, upper abdominal, or retrosternal pain radiating to the right hypochondrium is also a feature of severe preeclampsia and occurs due to hepatic swelling or hemorrhage. Oliguria is common in severe preeclampsia due to intravascular volume contraction due to severe vasoconstriction, hypoproteinemia, and anasarca. Patients may present with features of antepartum or postpartum hemorrhage due to thrombocytopenia, disseminated intravascular coagulation, or abruptio placentae.
Laboratory features include proteinuria, raised serum creatinine, thrombocytopenia, microangiopathic hemolysis (elevated LDH, indirect bilirubin, and schistocytes in the peripheral blood smear), elevated hepatic transaminases, and elevated uric acid (sign of hypovolemia). Deranged prothrombin time may be seen due to liver dysfunction and is more common in acute fatty liver of pregnancy. Prolonged prothrombin time, partial thromboplastin time, fibrin degradation products, and reduced fibrinogen levels are characteristic of disseminated intravascular coagulation. Neuroimaging is done in patients with seizures, visual symptoms, or altered consciousness to look for PRES, intracranial hemorrhage, or cerebral venous sinus thrombosis.
Differential Diagnosis
Various other conditions may mimic features of preeclampsia with severe features. HELLP syndrome is closely mimicked by hematologic abnormalities like thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). Neurological features and renal involvement are more common in HUS and TTP. Acute fatty liver of pregnancy can mimic HELLP syndrome but has more severe hepatic dysfunction, prolonged prothrombin time, and a higher incidence of acute kidney injury. SLE may present with hypertension, proteinuria, nephritis, thrombocytopenia, and seizures.
Mimics of preeclampsia include:
Preexisting renal disease: hypertension and/or proteinuria before 20 weeks gestation favor a diagnosis of preexisting renal disease.
Renal artery stenosis: early-onset hypertension without proteinuria and neurofibromatosis favor this diagnosis.
Pheochromocytoma: diaphoresis, palpitations, blood pressure fluctuations, headache, chest pain, and postural hypotension favor pheochromocytoma.
Cushing's syndrome/primary hyperaldosteronism: hypokalemia, early-onset hypertension, proximal myopathy, thin skin, and osteoporosis favor these diagnoses.
Mimics for HELLP syndrome include:
Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome: severe hemolysis, marked thrombocytopenia, milder elevation of liver enzymes, high LDH favor diagnosis of HUS/TTP. ADAMTS-13 activity is reduced in TTP.
Acute fatty liver of pregnancy: severe coagulopathy, the milder thrombocytopenia, hypoglycemia, and renal dysfunction favor this diagnosis.
Systemic lupus erythematosus: extrarenal manifestations disease, active urine sediment, decreased complement activity, and elevated dsDNA favors the diagnosis of SLE over HELLP syndrome.
Antiphospholipid antibody syndrome: widespread thrombosis with rapidly progressive multi-organ failure is suggestive of APLA.
Viral hepatitis: fever, markedly elevated liver enzymes and LDH, minimally elevated bilirubin, and coagulopathy in the absence of proteinuria or hypertension favor diagnosis of viral hepatitis.
Generally, any seizure presenting after 20 weeks of gestation is to be managed as eclampsia unless proven otherwise. Certain conditions with overlapping features include epilepsy, subarachnoid hemorrhage, hypertensive intracerebral bleed, cerebral venous sinus thrombosis, and cerebral malaria.
Predictive and Diagnostic Strategies for Preeclampsia
None of the predictive modalities like clinical factors, biomarkers, or imaging tools by themselves has a modest predictive ability. They have been utilized as parts of predictive algorithms with fair success. One such tool is the uterine artery Doppler flow-velocity measured at around 13 weeks of gestation. The levels of biomarkers like PlGF, sFLT1 and sENG, sFLT1:PlGF ratio, and PlGF:sENG ratio have good predictive performance in early-onset disease, severe disease with high negative predictive values. These angiogenic biomarkers can also be utilized to differentiate preeclampsia from diseases with overlapping features.9
Preventive Interventions
The role of aspirin in the prevention of preeclampsia is most studied and various international guidelines recommend the use of low-dose aspirin 75–150 mg in pregnant women at risk of developing preeclampsia.12 Analysis of multiple trials has shown that low-dose aspirin 75–150 mg initiated before 16 weeks of gestation is effective in reducing preeclampsia13 (Table 1).
Table 1.
Aspirin for prevention of preeclampsia8
| Risk factors | Preventive strategy | |
|---|---|---|
| High risk | History of preeclampsia | Low-dose aspirin If ≥1, high-risk factors are present |
| Chronic renal disease | ||
| Type 1 or type 2 diabetes | ||
| Chronic hypertension | ||
| Autoimmune disease (SLE, APLA syndrome) | ||
| Multifetal gestation | ||
| moderate-risk | Nulliparity | Low-dose aspirin If ≥2 moderate-risk factors are present |
| Obesity (BMI >30) | ||
| Family history of preeclampsia (mother, sister) | ||
| Age ≥35 years | ||
| Personal history (>10 years pregnancy interval, previous adverse pregnancy outcome) | ||
| Low socioeconomic status |
Oral calcium supplementation (1 g/day) may help prevent preeclampsia especially among women with low dietary calcium intake. Various other agents with mixed results or under evaluation for prevention of preeclampsia include metformin, low molecular weight heparin, arginine, pravastatin, and vitamin supplementation.12
Management
A systematic approach is essential for the management of critically ill obstetric patients with preeclampsia and related disorders. A multidisciplinary team approach, including various specialties like obstetrics, intensive care medicine, anesthesiology, and neonatology is required to formulate a treatment plan. A systematic five-step approach is recommended to improve outcomes:14
Step 1. To differentiate between obstetric and medical disorders with overlapping features and initiate any specific therapy for the same;
Step 2. To identify various organ dysfunctions and support failing organ systems;
Step 3. To assess maternal and fetal risk from continuing pregnancy and to decide whether delivery/termination of pregnancy will improve outcome;
Step 4. To choose the time and mode of delivery, if necessary, like vaginal or operative; and
Step 5. To optimize organ functions to ensure safe delivery.
Early and appropriate diagnosis is of utmost importance. Critically ill obstetric patients may have overlapping clinical features. Certain specific features may help clinch a diagnosis and initiate specific treatments if available. This has been discussed in brief in the section on differential diagnosis.
Patients with preeclampsia commonly present with accelerated hypertension to the ICU. These patients have contracted intravascular volume due to a capillary leak which makes blood pressure control without compromising organ perfusion and fetoplacental perfusion a challenging task. Overzealous blood pressure control is to be avoided as it can compromise organ perfusion, worsen renal function, and decrease fetoplacental perfusion. Vasodilator antihypertensives are the logical choice in this condition. However, contracted volume with vasodilation may decrease cardiac output by decreasing venous return and preload. These can be prevented by appropriate plasma volume expansion. However, overzealous volume expansion may precipitate pulmonary edema. Hemodynamic monitoring may help fine-tune and individualize the fluid-vasodilator balance in patients with severe preeclampsia.15
The objectives of antihypertensive therapy include the prevention of organ dysfunction like pulmonary edema, myocardial ischemia, renal injury, seizures/stroke, and placental abruption. Gradual reduction in blood pressure to 140–160 mm Hg systolic and 90–100 mm Hg diastolic is optimal. Abrupt drop in blood pressure may compromise uteroplacental perfusion. Antihypertensive agents used in the treatment of acute severe hypertension include intravenous (IV) labetalol, IV hydralazine, oral nifedipine, and IV nicardipine. Intravenous labetalol is the first-line drug. Oral nifedipine may be used in resource-limited setting16 (Table 2).
Table 2.
Antihypertensive drugs for severe hypertension
| Drug | Dose | Comment |
|---|---|---|
| Labetalol | 10–20 mg iv, followed by 20–80 mg every 10–30 minutes (maximum dose 300 mg) or continuous infusion at 1–2 mg/minute | First-line agent The onset of action is 1–2 minutes Avoid bronchial asthma, decompensated heart failure, heart block, bradycardia |
| Hydralazine | 5 mg iv, followed by 5–10 mg every 20–40 minutes (maximum dose 20 mg) or continuous infusion 0.5–10 mg/hour | Onset 10–20 minutes Can cause maternal hypotension, headache |
| Nifedipine | 10–20 mg po, repeated in 30 minutes if required, then 10–20 mg every 3–6 hours. Maximum daily dose 180 mg | Onset 5–10 minutes Reflex tachycardia, headaches Used commonly in resource-limited settings |
| Nicardipine | Continuous infusion 3 mg/hour; increase by 0.5 mg/hour every 20 minutes to a maximum of 15 mg/hour | Onset 10–20 minutes Tachycardia, headaches |
Once the acute severe hypertension is controlled, the patient should be started on oral antihypertensive agents if expectant management is planned. Oral labetalol 100–600 mg per dose, 3–4 times a day (maximum 2400 mg/day) followed by modified release oral nifedipine 30–60 mg per dose 1–2 times a day (maximum 120 mg/day) or oral methyldopa 250–100 mg per dose 3–4 times a day (maximum 300 mg/day) are used.
Magnesium sulfate is recommended for treatment and prophylaxis of eclampsia. The Magpie Trial showed a 58% reduction in the incidence of seizures in preeclampsia patients with severe features with the use of magnesium sulfate prophylaxis.17 Magnesium sulfate is the drug of choice for the treatment of seizures in eclampsia. The intravenous magnesium sulfate regimen includes a loading dose of 4–6 g over 15 minutes followed by an infusion of 1–2 g/hour. Magnesium levels should be monitored every 4–6 hours, especially in patients with renal dysfunction and dose titrated accordingly. Patients should be monitored clinically for a decrease in urine output, loss of patellar reflexes, respiratory depression, or cardiac effects (Table 3). Decreased urine output may warrant more frequent monitoring, and adverse effects need stopping of magnesium infusion and may need administration of calcium gluconate. Intramuscular magnesium sulfate may be considered in resource-limited setting-loading dose of 5 g intramuscular in each buttock followed by 5 g intramuscular in alternate buttock every 4 hours. Magnesium sulfate should be continued for 24 hours after delivery or last seizure.8 Brain imaging preferably magnetic resonance imaging should be performed in patients with eclampsia to rule out intracranial hemorrhage, posterior reversible encephalopathy syndrome (PRES), or cerebral venous sinus thrombosis.
Table 3.
Clinical effects of serum magnesium level
| Serum magnesium level | |||
|---|---|---|---|
| Clinical effect | mmol/L | mEq/L | mg/dL |
| Therapeutic | 2–3.5 | 4–7 | 5–9 |
| Loss of patellar reflex | >3.5 | >7 | >9 |
| Respiratory paralysis | >5 | >10 | >12 |
| Cardiac effects | >12.5 | >25 | >30 |
Delivery versus Expectant Management
For patients with preeclampsia requiring intensive care, prompt delivery, usually by cesarean section, rapidly reverses all the life-threatening manifestations of organ failure. Expectant management may be appropriate for gestational hypertension or preeclampsia without severe features in the preterm period and would favor better fetal outcomes. The risks of late preterm expectant management include severe preeclampsia, eclampsia, HELLP syndrome, and placental abruption are to be weighed against the risks of preterm delivery like neonatal complications of prematurity including neonatal respiratory complications and the need for prolonged neonatal intensive care. Nevertheless, in all critically ill obstetric patients, saving the pregnant mother's life takes precedence over improving fetal outcomes in terms of morbidity and mortality.14 The HYPITAT Trial showed that the induction of labor after completing 36 weeks of gestation significantly reduced new-onset severe preeclampsia, HELLP syndrome, pulmonary edema, or placental abruption as a composite outcome, without increasing neonatal complications, when compared to expectant management beyond 36 weeks of gestation.18 The HYPITAT II trial showed that a similar approach between 34 and 37 weeks of gestation worsened neonatal respiratory complications.19
Before 34 weeks of gestation, expectant management is intended to provide neonatal benefit at the expense of maternal risk. This approach needs intense in-hospital monitoring and delivery is recommended with any deterioration in maternal and fetal parameters. If time permits, two doses of intravenous corticosteroids should be administered 48–72 hours before delivery for fetal lung maturity.
During expectant management any time in pregnancy, immediate delivery is needed whenever there is deterioration in maternal or fetal condition. Predictive systems like sFlt-1/PlGF ratio and fullPIERS may help in identifying patients at risk of adverse outcomes early by up to 48 hours which is a clinically useful period that allows steroid administration and planning for delivery.20
The various organ system dysfunctions should be managed supportively. Pulmonary edema should be managed with oxygen supplementation or ventilation. Airway management in preeclampsia patients is difficult due to both anatomical and physiological challenges. Coagulation disturbances due to diffuse intravascular coagulation or thrombocytopenia should also be treated with appropriate blood products. Acute kidney dysfunction should be managed with judicious fluid therapy and hemodynamic monitoring.
Certain complications of preeclampsia or preeclampsia itself may present in the postpartum period. The incidence of postpartum eclampsia ranges from 17 to 37%.21 Around 30% of HELLP syndrome cases occur in the postpartum period.22 Appearance of these serious complications in the postpartum period warrants monitoring of preeclampsia patients in the postpartum period for features of severe preeclampsia.
Novel therapeutic strategies for preeclampsia under investigation include injection of recombinant VEGF121 or PlGF, inhibition of sFLT1 by small molecules or small interfering RNA, and selective depletion of sFLT1 with antibodies or apheresis; none of these is recommended in clinical practice as yet.9
Conclusion
Preeclampsia and related disorders are a major cause of maternal and fetal morbidity and mortality. Angiogenic imbalance is the major hallmark of preeclampsia. Screening and early diagnosis may help improve outcomes. Blood pressure control, seizure prevention, and appropriate timing of delivery are the cornerstones of the management of preeclampsia. At present, delivery remains the most effective treatment for preeclampsia. Novel therapeutic interventions are under development to reduce complications.
Footnotes
Source of support: Nil
Conflict of interest: None
Orcid
Amit M Narkhede https://orcid.org/0000-0001-8589-8347
Dilip R Karnad https://orcid.org/0000-0001-9935-5028
References
- 1.Karnad DR, Guntupalli KK. Critical illness and pregnancy: review of a global problem. Crit Care Clin. 2004;20(4):555–576. doi: 10.1016/j.ccc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Munnur U, Karnad DR, Bandi VDP, Lapsia V, Suresh MS, Ramshesh P, et al. Critically ill obstetric patients in an American and an Indian public hospital: comparison of case-mix, organ dysfunction, intensive care requirements, and outcomes. Intensive Care Med. 2005;31(8):1087–1094. doi: 10.1007/s00134-005-2710-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karnad DR, Lapsia V, Krishnan A, Salvi VS. Prognostic factors in obstetric patients admitted to an Indian intensive care unit. Crit Care Med. 2004;32(6):1294–1299. doi: 10.1097/01.ccm.0000128549.72276.00. [DOI] [PubMed] [Google Scholar]
- 4.Mamatha K. A study on obstetric admissions to HDU/ICU in a tertiary care centre. Indian J Obstet Gynecol. 2019;7(2):177–181. doi: 10.21088/ijog.2321.1636.7219.9. [DOI] [Google Scholar]
- 5.Gupta H, Gandotra N, Mahajan R. Profile of obstetric patients in intensive care unit: a retrospective study from a tertiary care center in North India. Indian J Crit Care Med. 2021;25(4):388–391. doi: 10.5005/jp-journals-10071-23775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Say L, Chou D, Gemmill A, Tunçalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health. 2014;2(6):e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 7.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, et al. Global, regional, and national levels and causes of maternal mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):980–1004. doi: 10.1016/S0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin Number 222: gestational hypertension and preeclampsia. Obstet Gynecol. 2020;135(6):e237–e260. doi: 10.1097/AOG.0000000000003892. [DOI] [PubMed] [Google Scholar]
- 9.Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15(5):275–289. doi: 10.1038/s41581-019-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341(8858):1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 11.Karnad DR, Guntupalli KK. Neurologic disorders in pregnancy. Crit Care Med. 2005;33(10):S362–S371. doi: 10.1097/01.ccm.0000182790.35728.f7. [DOI] [PubMed] [Google Scholar]
- 12.Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398(10297):341–354. doi: 10.1016/S0140-6736(20)32335-7. [DOI] [PubMed] [Google Scholar]
- 13.Rolnik DL, Nicolaides KH, Poon LC. Prevention of preeclampsia with aspirin. Am J Obstet Gynecol. 2020;S0002-9378(20):30873–5. doi: 10.1016/j.ajog.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 14.Guntupalli KK, Hall N, Karnad DR, Bandi V, Belfort M. Critical illness in pregnancy: part I: an approach to a pregnant patient in the ICU and common obstetric disorders. Chest. 2015;148(4):1093–1104. doi: 10.1378/chest.14-1998. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin K, Scholten RR, Kingdom JC, Floras JS, Parker JD. Should maternal hemodynamics guide antihypertensive therapy in preeclampsia? Hypertension. 2018;71(4):550–556. doi: 10.1161/HYPERTENSIONAHA.117.10606. [DOI] [PubMed] [Google Scholar]
- 16.Shekhar S, Gupta N, Kirubakaran R, Pareek P. Oral nifedipine versus intravenous labetalol for severe hypertension during pregnancy: a systematic review and meta‐analysis. BJOG. 2016;123(1):40–47. doi: 10.1111/1471-0528.13463. [DOI] [PubMed] [Google Scholar]
- 17.Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–1890. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 18.Koopmans CM, Bijlenga D, Groen H, Vijgen SM, Aarnoudse JG, Bekedam DJ, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979–988. doi: 10.1016/S0140-6736(09)60736-4. [DOI] [PubMed] [Google Scholar]
- 19.Broekhuijsen K, van Baaren GJ, Van Pampus MG, Ganzevoort W, Sikkema JM, Woiski MD, et al. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet. 2015;385(9986):2492–2501. doi: 10.1016/S0140-6736(14)61998-X. [DOI] [PubMed] [Google Scholar]
- 20.Mirkovic L, Tulic I, Stankovic S, Soldatovic I. Prediction of adverse maternal outcomes of early severe preeclampsia. Pregnancy Hypertension. 2020;22:144–150. doi: 10.1016/j.preghy.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Sabiri B, Moussalit A, Salmi S, El Youssoufi S, Miguil M. Post-partum eclampsia: epidemiology and prognosis. J Gynecol Obstet Biol Reprod. 2007;36(3):276–280. doi: 10.1016/j.jgyn.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Rath W, Faridi A, Dudenhausen JW. HELLP syndrome. J Perinat Med. 2000;28(4):249–260. doi: 10.1515/JPM.2000.033. [DOI] [PubMed] [Google Scholar]