Summary
Background
Following the proposed worldwide switch from trivalent oral poliovirus vaccine (tOPV) to bivalent types 1 and 3 OPV (bOPV) in 2016, inactivated poliovirus vaccine (IPV) will be the only source of protection against poliovirus type 2. With most countries opting for one dose of IPV in routine immunisation schedules during this transition because of cost and manufacturing constraints, optimisation of protection against all poliovirus types will be a priority of the global eradication programme. We assessed the immunogenicity and safety of a novel monovalent high-dose inactivated poliovirus type 2 vaccine (mIPV2HD) in infants.
Methods
This observer-blind, comparative, randomised controlled trial was done in a single centre in Panama. We enrolled healthy infants who had not received any previous vaccination against poliovirus. Infants were randomly assigned (1:1) by computer-generated randomisation sequence to receive a single dose of either mIPV2HD or standard trivalent IPV given concurrently with a third dose of bOPV at 14 weeks of age. At 18 weeks, all infants were challenged with one dose of monovalent type 2 OPV (mOPV2). Primary endpoints were seroconversion and median antibody titres to type 2 poliovirus 4 weeks after vaccination with mIPV2HD or IPV; and safety (as determined by the proportion and nature of serious adverse events and important medical events for 8 weeks after vaccination). The primary immunogenicity analyses included all participants for whom a post-vaccination blood sample was available. All randomised participants were included in the safety analyses. This trial is registered with ClinicalTrials.gov, number NCT02111135.
Findings
Between April 14 and May 9, 2014, 233 children were enrolled and randomly assigned to receive mIPV2HD (117 infants) or IPV (116 infants). 4 weeks after vaccination with mIPV2HD or IPV, seroconversion to poliovirus type 2 was recorded in 107 (93·0%, 95% CI 86·8–96·9) of 115 infants in the mIPV2HD group compared with 86 (74·8%, 65·8–82·4) of 115 infants in the IPV group (difference between groups 18·3%, 95% CI 5·0–31·1; p<0·0001), and median antibody titres against poliovirus type 2 were 181 (95% CI 72·0–362·0) in the mIPV2HD group and 36 (18·0–113·8) in the IPV group (difference between groups 98·8, 95% CI 60·7–136·9; p<0·0001). Serious adverse events were reported for six (5%) of 117 infants in the mIPV2HD group and seven (6%) of 116 infants in the IPV group during the 8-week period after vaccination; none were related to vaccination. No important medical events were reported.
Interpretation
Our findings lend support to the use of mIPV2HD as an option for stockpiling for outbreak response or primary protection in selected areas at risk for emergence of poliovirus type 2 during the next phase of the polio eradication plan.
Funding:
Bill & Melinda Gates Foundation.
Introduction
The worldwide eradication of polio is closer than ever, with the number of cases caused by wildtype poliovirus decreasing substantially in recent years.1 Currently, only two countries, Pakistan and Afghanistan, are regarded as endemic for polio, where transmission of wild poliovirus has never been interrupted.2 Also, of the three serotypes of wild poliovirus, only type 1 is currently circulating in these endemic countries. Wild poliovirus type 2 is deemed eradicated because the last naturally occurring case was seen in 1999. However, Sabin poliovirus type 2 accounts for roughly 97% of recent circulating vaccine-derived poliovirus outbreaks that typically occur in areas with low immunisation coverage and about 26–31% of cases of vaccine-associated paralytic poliomyelitis.3 No cases of wild poliovirus type 3 have been reported since November, 2012, the longest period ever for interruption of type 3 circulation.
With such historic progress being made in interrupting transmission of wild poliovirus, estimates suggest that the current burden of vaccine-related poliomyelitis is probably greater than that caused by wild poliovirus.1 The overall worldwide risk of vaccine-associated paralytic poliomyelitis is estimated to be 4·7 cases per million livebirths, which means an annual incidence of about 498 cases.3 Additionally, the mean number of reported cases of circulating vaccine-derived poliovirus has been 76 per year during the period between 2005 and 2013.1 By contrast, the total number of polio cases caused by wildtype strains was 416 in 2013 and 359 in 2014.4 Therefore, vaccination policies for the eradication programme need to ensure that adequate focus is given to the elimination of all types of polioviruses to achieve and sustain polio eradication in the long term.
With this epidemiological backdrop, the Polio Eradication and Endgame Strategic Plan 2013–2018 was developed by the Global Polio Eradication Initiative (GPEI) in 2013 with the aim of wiping out the last cases of polio from all causes by 2018.5 As a first step towards eliminating vaccine-related polio disease, the Endgame Plan recommends replacement of trivalent oral poliovirus vaccine (tOPV, which protects against types 1, 2, and 3), with bivalent oral poliovirus vaccine (bOPV, which protects against types 1 and 3), by April, 2016, preceded by the introduction of at least one dose of inactivated poliovirus vaccine (IPV) in routine immunisation programmes worldwide. From 2016 onwards, a mixed bOPV-IPV regimen in the Expanded Program on Immunization schedule is recommended, in which the one dose of IPV would be used with the primary intent to prime the population for immunity against poliovirus type 2. Additionally, this dose of IPV will boost immunity against types 1 and 3. The final step of the Endgame Plan would be to stop all OPV use after 2018–19 and to use only IPV for protection against polio.5,6 This strategy was endorsed by the Strategic Advisory Group of Experts on immunization (SAGE) in October, 2015.7
The current formulation of IPV with D-antigen (D-Ag) content of 40, 8, and 32 units for poliovirus types 1, 2, and 3, respectively, in IPV stand-alone or combination vaccines was established on the basis of a series of pivotal studies by Salk and his co-workers.8–12 Although the formulations containing 320-32-64 and 80-8-16 D-Ag units produced higher rates of seroconversion, the 40-8-32 D-Ag unit formulation was chosen because it induced sufficient immune response in infants after administration in full primary series of three or more doses and could be manufactured in adequate quantities.13 The immune response from IPV to poliovirus type 2 is low and might be related to its sensitivity to formalin inactivation.14 When given at or after 2 months of age, currently available IPV provides 32–77% seroconversion against poliovirus type 2.15–18 Achieving better protection against poliovirus type 2 from a single dose of inactivated vaccine could have substantial public health benefit, particularly during the period when tOPV will be replaced by bOPV worldwide, putting type 2 protection at some risk.
With the aim of improving type 2 immunogenicity with a single dose, monovalent high-dose inactivated poliovirus type 2 vaccine (mIPV2HD) was formulated, containing 32 D-Ag units of poliovirus type 2, which is four times the content in IPV. The content of poliovirus type 2 in this monovalent formulation is equivalent to that in the previous experimental 320-32-64 unit trivalent formulation, which was not associated with any safety issues in initial studies.8–10,13 The advantages of such a high-dose type 2 formulation could be a higher proportion of infants protected from paralysis with a single dose, better kinetics of priming, or both. These characteristics would be important for a stockpile vaccine candidate in case of an outbreak of poliovirus type 2 from sources such as undetected circulation of vaccine-derived poliovirus type 2 after the switch from tOPV to bOPV, accidental (or intentional) release from a laboratory or vaccine manufacturer, or immunodeficiency-related vaccine-derived poliovirus excretion in a population with low coverage. Since bOPV has been shown to be more immunogenic than tOPV,19 the omission of poliovirus types 1 and 3 from IPV and focus on type 2 response could also allow the use of this vaccine in routine immunisation along with bOPV for a limited period of time in selected areas at high risk for type 2 emergence.
Although there were a priori no major safety concerns for this mIPV2HD vaccine candidate, the safety profile of this formulation was assessed in a phase 1 study in 80 healthy adults in Belgium (NCT01997632). No vaccine-related serious adverse events were reported up to 6 months after vaccination and no treatment-emergent clinically significant abnormal laboratory values were seen. The reactogenicity profile for mIPV2HD was similar to that of the control, commercially available IPV. We report the results of a phase 2 trial of this vaccine in a naive human population.
Methods
Study design and participants
This observer-blind, comparative, randomised, controlled, clinical trial was done between April 10, 2014, and Jan 30, 2015, in a single centre in Panama. Parents or legal guardians were advised about the trial during the late stage of pregnancy or at the first postnatal visit, when the physician assessed eligibility and obtained written informed consent.
Participants were healthy infants aged about 6 weeks (accepted range 5–8 weeks). Only one infant was enrolled per household. Infants were excluded if they had been previously vaccinated against poliovirus, had a confirmed or suspected immunodeficiency, a low birthweight (<2500 g), or had a known allergy to any component of the study vaccines. Infants were also excluded if a household member had received OPV within the previous 3 months or was scheduled to receive OPV during the study period.
Other vaccines were provided according to the national immunisation schedule of Panama to ensure that participants were fully protected (appendix). The study vaccines were thus given concomitantly (but in a different limb) with DTPw-HBV-Hib (diphtheria, tetanus, whole-cell-pertussis plus hepatitis B virus plus Haemophilus influenzae type b) or DTPw-Hib, followed by hepatitis B vaccine, pneumococcal conjugate vaccine, and oral rotavirus vaccine.
The study was approved by the ethical review board of the Hospital del Nino, Panama, and was done in accordance with the Declaration of Helsinki, the International Conference on Harmonisation guideline for Good Clinical Practice, and the codes and regulations of Panama regarding research on human participants. Oversight of the study was provided by an independent data safety and monitoring board.
Randomisation and masking
Eligible infants were randomly assigned (1:1) into the two study groups by computer-generated randomisation envelopes. Randomisation was done directly by the unmasked personnel administering the corresponding vaccine prescribed on the envelope assignation. The randomisation list remained concealed from the sponsor, investigators, and the data safety and monitoring board unless the board ruled otherwise. The study was open-label for the vaccine administrators, but the site staff responsible for safety follow-up (who were different from the vaccinating nurses) and parents or guardians of the study participants were not aware of the treatment group to which the child had been assigned. Laboratory personnel responsible for processing and analysing samples and all other assessments were blinded to group allocation.
Procedures
Infants first received two doses of bOPV (Sanofi Pasteur, Lyon, France) at 6 and 10 weeks of age. At 14 weeks, infants received one intramuscular dose of either mIPV2HD (0·5 mL; Bilthoven Biologicals, Bilthoven, Netherlands) containing 32 D-Ag units of inactivated type 2 poliovirus or IPV (0·5 mL; Sanofi Pasteur), which was given concurrently with a third dose of bOPV. At 18 weeks, all infants were challenged with one dose of mOPV2 (Polio Sabin Mono Two; GlaxoSmithKline, Rixensart, Belgium).
Neutralising antibody titres for poliovirus types 1, 2, and 3 were assessed at 6, 14, 15, 18, and 19 weeks of age. Serum samples were prepared immediately after collection of blood, stored at −20°C, and sent frozen to the US Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA) laboratory for analysis using the WHO standard microneutralisation assay, as described previously.18 Neutralisation titres were estimated by the Spearman-Kärber method20 and expressed as the reciprocal titre of the calculated 50% endpoint. Titres greater than 1448 (the highest dilution tested) were attributed the value 1448. Intestinal shedding of poliovirus type 2 was assessed in stool samples (5–10 g per sample) collected by WHO-approved protocols and kits once weekly during a period of 3 weeks after mOPV2 challenge; samples were transported frozen to the CDC laboratory for analysis by culture in accordance with the WHO protocol.21 Antibody titres against type 2 poliovirus were expressed as log10 50% cell culture infective dose (CCID50) per gram of stool.
Study staff recorded medical history and provided training and a diary card for parents to record safety data and medication use. In addition to filling out the cards, parents or guardians were asked at each of the site visits to provide information about any adverse event that occurred since their last visit. A toll-free telephone number was also provided for parents or guardians to call the study team if medical advice was required. Solicited local and general adverse events were recorded for 1 week after administration of study vaccines; unsolicited adverse events and concomitant drug treatment were recorded for 4 weeks post vaccination; and serious adverse events were recorded over the entire study period of 34 weeks. Important medical events, defined as medically significant events that did not meet the criteria for a serious adverse event, but that required medical or surgical consultation or intervention to prevent the event from becoming serious, were also recorded during the entire study period. Two blood samples were collected (before vaccination and 1 week after) for routine serum chemistry and haematological laboratory testing.
Outcomes
Primary immunogenicity outcomes were the proportion of infants with antibody seroconversion to poliovirus type 2 and median antibody titres against poliovirus type 2 at 18 weeks of age (ie, 4 weeks after vaccination with mIPV2HD or IPV). For infants who were seronegative (titre <8) at the time of vaccination, seroconversion was defined as achieving an antibody titre of at least eight after vaccination; for infants who were seropositive (titre ≥8), seroconversion was defined as a titre four times higher than the expected fall in maternal antibody concentrations based on the prevaccination titre (using a half-life of 24 days for maternal antibody). Secondary immunogenicity outcomes included the proportion of infants with seroconversion to poliovirus type 2 and median antibody titres 1 week after vaccination; quantitative index (percentage of infants who shed the virus, amount of virus shed, duration of shedding) of type 2 viral shedding during a 3-week period after mOPV2 challenge; and the proportion of infants with seroconversion and median antibody titres for poliovirus types 1 and 3 at 14 and 18 weeks of age.
The primary safety outcome was the proportion of infants with serious adverse events and important medical events during the 8-week period after vaccination with mIPV2HD or IPV. Secondary safety outcomes included the proportion of infants with serious adverse events and important medical events during the entire study; the frequency and severity of solicited local and systemic adverse events on the day of vaccination with mIPV2HD or IPV and the following 7 days; frequency of abnormal laboratory values on the day of vaccination with mIPV2HD or IPV and 1 week later; and frequency and severity of unsolicited adverse events from the day of vaccination with mIPV2HD or IPV and the following 28 days.
Statistical analysis
For sample size calculations, the proportion of infants with seroconversion to poliovirus type 2 after one dose of mIPV2HD was considered to be 45%. With a sample size of 108 infants, the power of the study was 90% to declare superiority of mIPV2HD over IPV if seroconversion was 23% higher in the mIPV2HD group than in the IPV group. This sample size also provided 90% power to declare superiority of mIPV2HD over IPV if the difference in median neutralisation titres against type 2 poliovirus was greater than nine; it also provided a 95% probability of detecting any safety signal with a true frequency of about one in 40 participants, using the rule of three. Assuming that 10% of the participants would not be evaluable, 120 infants were recruited in each group. The actual retention was adequate for power calculations for primary outcomes.
For the primary objective related to immunogenicity, we used the 95% Fisher exact CI for the difference in binomial proportions to assess the superiority of the proportion of type 2 seroconversion. Superiority was to be concluded if the 95% CI did not include the null value. We also compared the proportion of infants with seroconversion between groups with Fisher’s exact test. A Hodges-Lehmann estimate, together with the 95% distribution-free CI (ie, Moses confidence limit) and a Mann-Whitney-Wilcoxon test, was used to determine whether the difference between the type 2 median neutralisation titres in the two groups was statistically significant.
Intestinal immunity to poliovirus type 2 was assessed with the quantitative shedding index,18 which was based on the average log10-transformed values of virus concentration in stool samples collected at three different timepoints at weekly intervals. Titres of virus shed at each timepoint and the shedding index were compared between groups using the Mann-Whitney-Wilcoxon test.
The primary immunogenicity analyses included all participants for whom a post-vaccination blood sample was available. All randomised participants were included in the analyses of safety and reactogenicity. Analyses were done with SAS version 9.2. This trial is registered with ClinicalTrials.gov, number NCT02111135.
Role of the funding source
One of the authors of this report (ASB) is an employee of the study sponsor, and was involved in the study design, data interpretation, and writing of the report. All authors had full access to all the data from the study; the corresponding author had final responsibility for the decision to submit for publication.
Results
Between April 14 and May 9, 2014, 233 infants were enrolled and randomly assigned to receive mIPV2HD (n=117) or IPV (n=116; figure). All 233 infants received the allocated polio vaccines at 6 weeks (bOPV), 10 weeks (bOPV), and 14 weeks (bOPV and mIPV2HD or IPV) of age and 230 (99%) infants received the mOPV2 challenge 4 weeks later at 18 weeks of age. Overall, 201 (87%) randomised infants completed the study.
Figure. Trial profile.
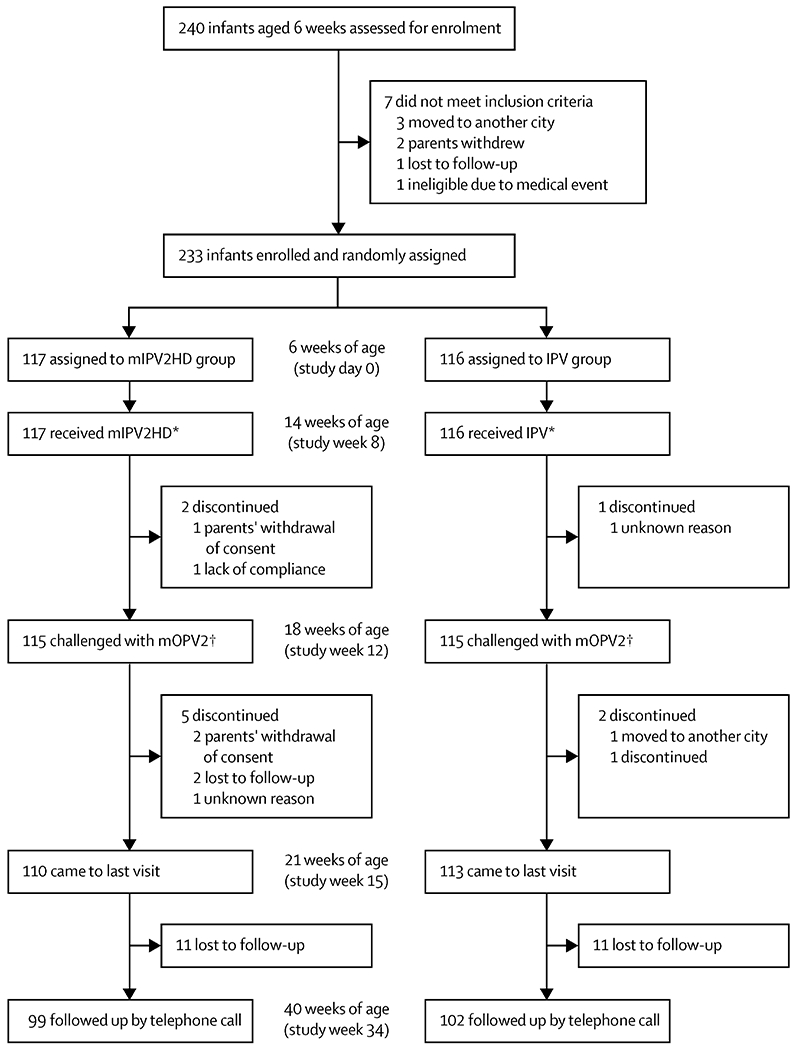
Polio vaccines were administered at 6 weeks (bOPV), 10 weeks (bOPV), and 14 weeks of age (bOPV and mIPV2HD or IPV). Infants received the mOPV2 challenge at 18 weeks of age. mIPV2HD=monovalent inactivated poliovirus vaccine, type 2, high dose. IPV=trivalent inactivated poliovirus vaccine. mOPV2=monovalent oral poliovirus vaccine, type 2. *All randomised participants were included in the analyses of safety and reactogenicity. †The primary immunogenicity analyses included all participants for whom a post-vaccination blood sample was available.
The mean age of the infants at enrolment was 6·1 weeks (SD 0·9) and most were Hispanic (90%). The two groups were well-balanced with respect to sex, breastfeeding, and day-care attendance (table 1).
Table 1:
Participant characteristics
| mIPV2HD group (n=117) | IPV group (n=116) | |
|---|---|---|
| Age (weeks) | 6·1 (0·84) | 6·1 (0·91) |
|
| ||
| Sex | ||
| Male | 58 (50%) | 63 (54%) |
| Female | 59 (50%) | 53 (46%) |
|
| ||
| Race | ||
| White | 2 (2%) | 1 (1%) |
| Black or African-American | 5 (4%) | 10 (9%) |
| Asian | 0 | 0 |
| Hispanic | 107 (91%) | 102 (88%) |
| Other | 3 (3%) | 3 (3%) |
|
| ||
| Breastfed | ||
| Yes | 114 (97%) | 112 (97%) |
| No | 3 (3%) | 4 (3%) |
|
| ||
| Attending a day-care centre | ||
| Yes | 1 (1%) | 0 |
| No | 116 (99%) | 116 (100%) |
Data are mean (SD) or n (%). mIPV2HD=monovalent inactivated poliovirus vaccine, type 2, high dose. IPV=trivalent inactivated poliovirus vaccine.
At baseline, 139 (60%) of infants had detectable maternal antibodies against poliovirus type 2 (71 [62%] in the mIPV2HD group; 68 [59%] in the IPV group). This number fell to 83 (35%) at the time of vaccination with mIPV2HD or IPV at 14 weeks of age (44 [38%] in the mIPV2HD group; 39 [33%] in the IPV group). 4 weeks after vaccination (at 18 weeks of age), seroconversion to poliovirus type 2 was recorded in 107 (93·0%; 95% CI 86·8–96·9) of 115 infants in the mIPV2HD group and 86 (74·8%; 65·8–82·4) of 115 infants in the IPV group (difference between groups 18·3% [95% CI 5·0–31·1]; p<0·0001; table 2). The median neutralising antibody titre against type 2 poliovirus 4 weeks after vaccination (18 weeks of age) was 181 (95% CI 72·0–362·0) in the mIPV2HD group and 36 (18·0–113·8) in the IPV group (difference between groups 98·8 [95% CI 60·7–136·9]; p<0·0001).
Table 2:
Seroprotection, seroconversion, and median neutralising antibody titres for poliovirus type 2, by age
| mIPV2HD group |
IPV group |
Difference (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| n/N | Outcome* | n/N | Outcome* | |||
| 6 weeks | ||||||
| Seroprotection | 71/114 | 62·3% (52·7–71·2) | 68/115 | 59·1% (49·6–68·2) | .. | .. |
|
| ||||||
| 14 weeks | ||||||
| Seroprotection | 44/115 | 38·3% (29·4–47·8) | 39/115 | 33·9% (25·3–43·3) | .. | .. |
|
| ||||||
| 15 weeks | ||||||
| Seroprotection | 114/115 | 99·1% (95·3–100·0) | 108/114 | 94·7% (88·9–98·0) | .. | .. |
| Seroconversion | 107/115 | 93·0% (86·8–96·9) | 87/114 | 76·3% (67·4–83·8) | 16·7 (3·7 to 29·1) | <0·0001 |
| Reciprocal titre | .. | 288 (90·5–910·2) | .. | 45 (18·0–144·0) | .. | .. |
|
| ||||||
| 18 weeks | ||||||
| Seroprotection | 113/115 | 98·3% (93·9–99·8) | 106/114 | 93·0% (86·6–96·9) | .. | .. |
| Seroconversion | 107/115 | 93·0% (86·8–96·9) | 86/115 | 74·8% (65·8–82·4) | 18·3 (5·0 to 31·1) | <0·0001 |
| Reciprocal titre | .. | 181 (72·0–362·0) | .. | 36 (18·0–113·8) | 98·8 (60·7 to 136·9) | <0·0001 |
|
| ||||||
| 19 weeks | ||||||
| Seroprotection | 111/112 | 99·1% (95·1–100·0) | 113/114 | 99·1% (95·2–100·0) | .. | .. |
| Seroconversion | 110/112 | 98·2% (93·7–99·8) | 104/114 | 91·2% (84·5–95·7) | 7·0 (−6·4 to 19·9) | 0·0338† |
| Seroconversion‡ | 1/2 | 50·0% (1·3–98·7) | 7/8 | 87·5% (47·3–99·7) | .. | .. |
| Reciprocal titre | .. | 362 (144·0–910·2) | .. | 181 (56·9–455·1) | .. | .. |
Seroprotection defined as neutralising titre of at least 8. Seroconversion assessed with respect to status at 14 weeks of age. For infants who were seronegative (titre <8), seroconversion was defined as achieving an antibody titre of at least 8; for infants who were seropositive (titre ≥8), seroconversion was defined as a titre four times higher than the expected fall in maternal antibody concentrations based on the prevaccination titre. Proportion of infants with seroconversion compared between groups using Fisher’s exact test. Median neutralising antibody titres compared between groups using the Mann-Whitney-Wilcoxon test. mIPV2HD=monovalent inactivated poliovirus vaccine, type 2, high dose. IPV=trivalent inactivated poliovirus vaccine. n=number of infants seroprotected or seroconverted. N=total number of infants at each timepoint.
Data for seroprotection and seroconversion are % (95% CI); data for reciprocal titre are median (IQR).
Exploratory analysis.
Seroconversion in infants seronegative at 18 weeks of age. Seroconversion was defined as achieving an antibody titre of at least 8.
A significant difference between groups in the proportion of infants with seroconversion to poliovirus type 2 was seen as early as 1 week after vaccination with mIPV2HD or IPV (93·0% [95% CI 86·8–96·9] in the mIPV2HD group vs 76·3% [67·4–83·8] in the IPV group; p<0·0001; table 2). Median antibody titres against type 2 poliovirus were also higher in the mIPV2HD group than in the IPV group at this time (288 [95% CI 90·5–910·2] vs 45 [18·0–114·0]). 1 week after the mOPV2 challenge, seroconversion to poliovirus type 2 reached 98·2% (95% CI 93·7–99·8) in the mIPV2HD group compared with 91·2% (84·5–95·7) in the IPV group, indicating priming for type 2 from the single dose of inactivated vaccines. One of the two infants who remained seronegative after vaccination with mIPV2HD and seven of eight infants who remained seronegative after vaccination with IPV seroconverted within a week of challenge.
Following mOPV2 challenge, 80 (84%) of 95 infants in the mIPV2HD group and 76 (84%) of 91 infants in the IPV group shed type 2 poliovirus at any timepoint (table 3). The shedding index was 3·95 log10 CCID50 in the mIPV2HD group and 4·07 log10 CCID50 in the IPV group; the distribution of shedding indices in the two groups is shown as reverse cumulative distribution curves in the appendix. Virus titres and the proportion of infants shedding virus in each group were highest 1 week after challenge and diminished thereafter; there were no significant differences between study groups.
Table 3:
Quantitative viral shedding for poliovirus type 2 after mOPV2 challenge
| mIPV2HD group | IPV group | p value | |
|---|---|---|---|
| 19 weeks (1 week after challenge) | |||
|
| |||
| n/N | 88/108 | 83/106 | .. |
| % (95% CI) | 81·5% (72·9–88·3) | 78·3% (69·2–85·7) | 0·6109* |
| Median (log) CCID50 (IQR) | 4·86 (2·84–6·20) | 4·45 (2·75–6·22) | 0·4740* |
|
| |||
| 20 weeks (2 weeks after challenge) | |||
|
| |||
| n/N | 67/108 | 64/105 | .. |
| % (95% CI) | 62·0% (52·2–71·2) | 60·9% (50·9–70·3) | 0·8888* |
| Median (log) CCID50 (IQR) | 2·83 (2·75–4·83) | 3·06 (2·75–4·72) | 0·8173* |
|
| |||
| 21 weeks (3 weeks after challenge) | |||
|
| |||
| n/N | 46/101 | 46/100 | .. |
| % (95% CI) | 45·5% (35.6–55.8) | 46·0% (36·0–56·3) | 1·0000* |
| Median (log) CCID50 (IQR) | 2·75 (2·75–3·47) | 2·75 (2·75–4·47) | 0·5365* |
|
| |||
| Infants shedding virus at any timepoint | |||
|
| |||
| n/N | 80/95 | 76/91 | .. |
| % (95% CI) | 84·2% (75·3–90·9) | 83·5% (74·3–90·5) | 1·0000 |
|
| |||
| Shedding indext † | |||
|
| |||
| Median (log) CCID50 (IQR) | 3·95 (3·18–4·85) | 4·07 (3·21–4·84) | 0·6429 |
The proportions of infants shedding virus were compared between groups using Fisher’s exact test Viral titres were compared between groups using the Mann-Whitney-Wilcoxon test. mOPV2=monovalent oral poliovirus vaccine, type 2. mIPV2HD=monovalent inactivated poliovirus vaccine, type 2, high dose. IPV=trivalent inactivated poliovirus vaccine. n=number of infants shedding virus. N=total number of infants at each timepoint. CCID50=50% cell culture infective dose.
Exploratory analysis.
Computed as the average of log10-transformed values of viral titres measured in stool collected on 7, 14, and 21 days after mOPV2 challenge.
At 6 weeks of age, the proportions of infants with neutralising antibodies against poliovirus type 1 were 62% and 73% in the mIPV2HD and IPV groups, respectively, and the proportions of infants with neutralising antibodies against poliovirus type 3 were 36% and 30%, respectively (table 4). At 14 weeks of age (ie, 4 weeks after the second dose of bOPV), seroconversion to poliovirus type 1 was 92% in the mIPV2HD group and 87% in the IPV group; seroconversion to poliovirus type 3 was 97% in the mIPV2HD group and 97% in the IPV group. At this timepoint, at least 99% of infants had protective antibody levels against poliovirus types 1 and 3, and median antibody titres were 1448. A third dose of bOPV together with mIPV2HD (no type 1 or 3 polio antigen) or IPV led to all infants being seroprotected with no further increase in antibody titres.
Table 4:
Seroprotection, seroconversion, and median neutralising antibody titres for poliovirus type 1 and type 3, by age
| mIPV2HD group (n=115) |
IPV group (n=115) |
|||
|---|---|---|---|---|
| n/N | Outcome* | n/N | Outcome* | |
| Type 1 | ||||
|
| ||||
| 6 weeks | ||||
| Seroprotection | 71/114 | 62·3% (52·7–71·2) | 84/115 | 73·0% (64·0–80·9) |
| Reciprocal titre | .. | 11·31 (5·66–36·00) | .. | 18·00 (7·11–90·51) |
| 14 weeks | ||||
| Seroprotection | 114/115 | 99·1% (95·3–100·0) | 114/115 | 99·1% (95·3–100·0) |
| Seroconversion | 106/115 | 92·1% (85·5–96·3) | 100/115 | 87·0% (79·4–92·5) |
| Reciprocal titre | .. | 1448 (1448–1448) | .. | 1448 (1448–1448) |
| 18 weeks | ||||
| Seroprotection | 115/115 | 100·0% (96·8–100·0) | 115/115 | 100·0% (96·8–100·0) |
| Seroconversion | 110/115 | 95·6% (90·1–98·6) | 105/115 | 91·3% (84·6–95·8) |
| Reciprocal titre | .. | 1448 (1448–1448) | .. | 1448 (1448–1448) |
|
| ||||
| Type 3 | ||||
|
| ||||
| 6 weeks | ||||
| Seroprotection | 41/114 | 36·0% (27·2–45·5) | 35/115 | 30·4% (22·2–39·7) |
| Reciprocal titre | .. | 5·66 (5·66–14·22) | .. | 5·66 (5·66–11·31) |
| 14 weeks | ||||
| Seroprotection | 115/115 | 100·0% (96·8–100·0) | 114/115 | 99–1% (95·3–100·0) |
| Seroconversion | 111/115 | 96·5% (91·3–99·0) | 112/115 | 97·4% (92·6–99·5) |
| Reciprocal titre | .. | 1448 (1152·06–1448) | .. | 1448 (910·17–1448) |
| 18 weeks | ||||
| Seroprotection | 115/115 | 100·0% (96·8–100·0) | 115/115 | 100·0% (96·8–100·0) |
| Seroconversion | 113/115 | 98·2% (93·8–99·8) | 113/115 | 98·3% (93·9–99·8) |
| Reciprocal titre | .. | 1448 (1448–1448) | .. | 1448 (1448–1448) |
Data for seroprotection and seroconversion are % (95% CI); data for reciprocal titre are median (IQR). Seroprotection defined as neutralising titre of at least 8. Seroconversion assessed with respect to status at 6 weeks of age. For infants who were seronegative (titre <8), seroconversion was defined as achieving an antibody titre of at least 8; for infants who were seropositive (titre ≥8), seroconversion was defined as a titre four times higher than the expected fall in maternal antibody concentrations based on the prevaccination titre. mIPV2HD=monovalent inactivated poliovirus vaccine, type 2, high dose. IPV=trivalent inactivated poliovirus vaccine.
n=114 at 6 weeks
During the course of the study, eight (7%) of 117 infants in the mIPV2HD group and 15 (13%) of 116 infants in the IPV group had serious adverse events; of these, six (5%) infants and seven (6%) infants, respectively, had serious adverse events during the 8-week period after vaccination with mIPV2HD or IPV (table 5). None of the serious adverse events were judged to be related to vaccination. No important medical events were reported during this 8-week period. No clinically relevant differences were apparent in the proportion, nature, or severity of solicited injection site reactions and systemic events between the two vaccine groups. The proportion of infants who had other, unsolicited events during the 28-day period after mIPV2HD or IPV vaccination was also similar in the two groups. The causal relation between the polio vaccines and the systemic reactogenicity cannot be determined with certainty, because other Expanded Program on Immunization vaccines were given at the same visit. Nearly all laboratory values that were out of the normal range before and after vaccination were deemed as without clinical relevance; no change in laboratory parameter was attributed to the study vaccines.
Table 5:
Adverse events after vaccination with mIPV2HD or IPV
| mIPV2HD group (n=117) |
IPV group (n=116) |
Difference between groups (mIPV2HD-IPV), % (95% CI) | |||
|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | ||
| Any solicited adverse event within 1 week after mIPV2HD or IPV vaccination | 107 | 91·5% (84·8–95·8) | 112 | 96·6% (91·4–99·1) | −5·1% (−17·8 to 7·7) |
| Solicited injection site adverse events | 55 | 47·0% (37·7–56·5) | 55 | 47·4% (38·1–56·9) | −0·4% (−13·4 to 12·7) |
| Pain | 18 | 15·4% (9·4–23·2) | 13 | 11·2% (6·1–18·4) | 4·2% (−8·5 to 17·0) |
| Redness | 45 | 38·5% (29·6–47·9) | 47 | 40·5% (31·5–50·0) | −2·0% (−14·6 to 11·1) |
| Swelling | 0 | 0(0·0–3·1) | 3 | 2·6% (0·5–7·4) | −2·6% (−15·3 to 10·2) |
| Induration | 3 | 2·6% (0·5–7·3) | 5 | 4·3% (1·4–9·8) | −1·7% (−14·5 to 11·1) |
| Grade 3 solicited injection site adverse event | 0 | 0(0·0–3·1) | 1 | 0·9% (0·0–4·7) | −0·9% (−13·6 to 11·9) |
|
| |||||
| Any unsolicited adverse event within 4 weeks after mIPV2HD or IPV vaccination | 53 | 45·3% (36·1–54·8) | 58 | 50·0% (40·6–59·4) | −4·7% (−17·6 to 8·4) |
| Related to vaccine | 1 | 0·9% (0·0–4·7) | 0 | 0(0·0–3·1) | 0·9% (−11·9 to 3·6) |
|
| |||||
| Any serious adverse event during the 34-week study period | 8 | 6·8% (3·0–13·0) | 15 | 12·9% (7·4–20·4) | −6·1% (−18·7 to 6·8) |
| Serious adverse event within 8 weeks after mIPV2HD or IPV vaccination | 6 | 5·1% (1·9–10·8) | 7 | 6·0% (2·5–12·0) | −0·9% (−13·6 to 11·9) |
|
| |||||
| Any important medical event during the 34-week study period | 1 | 0·9% (0·0–4·7) | 1 | 0·9% (0·0–4·7) | 0 (−12·8 to 12·8) |
| Any important medical event within 8 weeks after mIPV2HD or IPV vaccination | 0 | 0 (0·0–3·1) | 0 | 0(0·0–4·7) | 0 (NA) |
The following vaccines were given concomitantly with polio vaccines: with bivalent types 1 and 3 oral poliovirus vaccine (bOPV) dose 1 at 6 weeks of age: diphtheria, tetanus, whole-cell pertussis, plus hepatitis B plus Haemophilus influenzae type b (DTPw-HepB-Hib) vaccine (or DTPw-Hib vaccine), pneumococcal vaccine, and rotavirus vaccine (infants who received DTPw-Hib vaccine at day 0 were vaccinated with hepatitis B vaccine at 10 weeks of age with bOPV dose 2); with mIPV2HD or IPV at 14 weeks of age: bOPV (dose 3) and rotavirus vaccine with mOPV2 challenge at 18 weeks of age, DTPw-HepB-Hib vaccine, and pneumococcal vaccine. A third dose of DTPw-HepB-Hib vaccine was given at 24 weeks of age; influenza vaccine was given at 24 and 28 weeks of age. mIPV2HD=monovalent inactivated poliovirus vaccine, type 2, high dose. IPV=trivalent inactivated poliovirus vaccine. NA=not applicable.
Discussion
This study is the first to report safety, reactogenicity, and immunogenicity of a novel mIPV2HD formulation. It is also an important addition to the clinical evidence base on new polio vaccination schedules with bOPV and IPV that will soon be adopted worldwide in accordance with the Endgame Strategic Plan.5,22 Our study shows that one dose of mIPV2HD induces a superior humoral immune response against poliovirus type 2 compared with IPV when given at 14 weeks in the Expanded Program on Immunization schedule along with the third dose of bOPV, as measured by differences in seroconversion and in median antibody titres. Furthermore, the kinetics of the immune response to poliovirus type 2 after the two vaccines was different, with a significantly higher proportion of infants with seroconversion in the mIPV2HD group than the IPV group as early as 1 week after vaccination.
The superior humoral immune response induced by one dose of mIPV2HD did not translate into improved intestinal immunity compared with standard IPV. After the challenge dose of mOPV2, the percentage of infants who shed virus, the quantity of virus shed, and the duration of shedding did not significantly differ between the two groups. This finding is consistent with earlier studies showing that IPV provides poor primary intestinal immunogenicity.17,23,24
Our results also show that two doses of bOPV for poliovirus types 1 and 3 induced excellent humoral immunogenicity in the population and environment studied. A third dose of bOPV along with IPV in the control group added only marginally to the high response from the first two doses. This finding will be important for the worldwide switch from tOPV to bOPV, which is scheduled for April, 2016, and preceded by the introduction of at least one dose of IPV. The promising data for priming for poliovirus type 2 from one dose of inactivated vaccine (IPV or mIPV2HD) in such schedules suggest potential for rapid immune response to a type 2 exposure in the future. These data also suggest that, for poliovirus types 1 and 3, bOPV produces very high proportions of seroconversion to types 1 and 3 when given in an Expanded Program on Immunization series and thus the lack of protection to these serotypes in the mIPV2HD candidate is not of major concern, particularly in settings similar to where this study was performed.
The safety profile of mIPV2HD was similar to that of IPV when given concomitantly in a paediatric vaccination schedule and there were no differences in safety events between the two groups. All serious adverse events and important medical events reported during the study were events that are routinely seen in children younger than 1 year of age and all were deemed unrelated to vaccination. There were no clinically relevant differences in injection site events between the two vaccines.
This study had limitations. There was no “OPV only” or “no polio vaccine” control group. Thus, although intestinal immunogenicity did not differ between groups, the effects of mIPV2HD on viral shedding compared with no vaccine or OPV administration could not be assessed. Also, the study was done in Panama where tOPV was still being used for routine immunisation, although no national or regional OPV immunisation campaigns were ongoing during the study period; thus, some background exposure of study participants to Sabin type 2 polioviruses could not be ruled out. However, with the measures taken to minimise this effect, and also the fact that any such effect would have affected both study groups, there is no reason to believe that this factor had any substantial effect on the findings and interpretations. Lastly, we studied the effect of a single dose of mIPV2HD; inclusion of booster dose(s) in the study design would have allowed an assessment of potential additional effects on intestinal mucosal immunity via activation of memory B cells. Future studies will also need to investigate a potential effect of mIPV2HD on concomitantly administered antigens.
In conclusion, one dose of mIPV2HD was well tolerated in infants and induced a superior humoral immune response, both in terms of magnitude as well as kinetics of the response, compared with a single dose of currently available IPV. On the basis of the promising humoral immunogenicity and safety data from this study, mIPV2HD could be considered an important addition to the options of the polio endgame plan. Also, unlike the live attenuated mOPV2, mIPV2HD does not carry the rare but serious risk of generating new vaccine-derived polioviruses or vaccine-associated paralytic poliomyelitis. Nevertheless, the effect of mIPV2HD on primary intestinal immunogenicity was similar to current IPV. Among other factors, the effect of IPV on intestinal immunity depends on previous exposure to OPV. Two studies from India have shown that one dose of IPV given to OPV-vaccinated children significantly boosted intestinal mucosal immunity compared with no vaccine and this boost was higher than that achieved with an additional dose of OPV.25,26 Previous studies and reviews have also shown that when IPV was given to children who did not have previous OPV exposure, it had a marginal effect in reducing duration and median titre of viral shed, but the overall impact on viral shedding was substantially lower than that induced by OPV.1,18,23
Considering all of these factors, mIPV2HD could have an important role in individual protection and prevention of cases of paralysis by closing any humoral immunity gap and would also be effective in boosting intestinal immunity for OPV-vaccinated children. But with limited effects on inducing intestinal immunity in naive children, mIPV2HD is unlikely to be the only intervention for large-scale outbreak control of poliovirus type 2. This new formulation could indeed be stockpiled for concomitant use in outbreak response for control and prevention of poliovirus type 2 outbreaks following the transition from tOPV to bOPV. In situations where the risk of generating new vaccine-derived polioviruses with the use of mOPV2 is deemed high in an outbreak response scenario (eg, in areas of very poor immunisation coverage), mIPV2HD could also be an alternative to mOPV2 because it does not have any risk of generation of vaccine-associated paralytic poliomyelitis or vaccine-derived poliovirus. This situation might also apply to areas surrounding the epicentre of an outbreak. Enhanced environmental surveillance to rapidly detect any silent virus circulation after such use of inactivated vaccines in these areas would add value to the overall response strategy.
In view of the supply and cost constraints of IPV,5,27 mIPV2HD could be an alternative to IPV for primary protection against poliovirus type 2 during the period after OPV2 withdrawal and before all OPV cessation. However, such benefits have to be balanced with issues such as absence of any type 1 and 3 protection compared with IPV, need for an accelerated pathway for licensure for use in routine immunisation, and the challenges of multiple short-term changes in immunisation programmes before moving to use of IPV only by 2019. Although the safety and immunogenicity data of this candidate vaccine look promising, additional information such as immunogenicity in different age groups and with multiple doses, concomitant use with mOPV2 for outbreak control, and eventually alternative needle-free administration methods for easier use in outbreak response settings would further support informed policy decisions for polio vaccination for the endgame and beyond.
Supplementary Material
Research in context.
Evidence before this study
In 2012, to phase out the use of live type 2 poliovirus vaccine, WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) recommended that trivalent oral polio vaccine (tOPV) be replaced with bivalent oral polio vaccine (bOPV) containing only poliovirus types 1 and 3 in all countries by 2016. This change is to be preceded by the introduction of at least one dose of conventional trivalent inactivated poliovirus vaccine (IPV) in routine immunisation programmes to provide immunity to poliovirus type 2. However, a dose of inactivated poliovirus type 2 higher than the standard dose (8 D-Ag units) may be needed to ensure adequate immunity. With the aim of improving type 2 immunogenicity with a single dose, a new monovalent high-dose inactivated poliovirus vaccine (mIPV2HD), which contains four times the standard dose of inactivated poliovirus type 2 (32 D-Ag units), was formulated. Higher D-Ag content of IPV was investigated by Salk and co-workers in a series of dose-ranging studies several decades ago. We searched PubMed for papers published between Jan 1, 1955, and Feb 28, 2013, with the terms “IPV”, “high-dose”, “poliovirus”, and/or “type 2” and identified several published reports of clinical trials investigating high-dose IPV formulated from inactivated Sabin strains of poliovirus. However, we are not aware of any other published study in which a monovalent high-dose inactivated vaccine was compared with trivalent IPV in a mixed bOPV-IPV schedule, and where mOPV2 was used to assess intestinal immunity in such schedules.
Added value of this study
To our knowledge, this study is the first to report data for safety and humoral and intestinal immunogenicity of mIPV2HD formulation in a naive infant population using a mixed bOPV-IPV schedule. The humoral immune response to poliovirus type 2 with mIPV2HD was superior to that of IPV and the intestinal immunity to poliovirus type 2 was similar in both groups. These results provide evidence that mIPV2HD can be safely used in infants and show that a combined bOPV-mIPV2HD schedule would adequately protect against all three poliovirus types.
Implications of all the available evidence
With the upcoming worldwide switch from tOPV to bOPV, the only protection against poliovirus type 2 will come from IPV. In view of the supply and cost constraints of IPV, higher immunogenicity against poliovirus type 2 from a single dose of a monovalent high-dose formulation could be of advantage in settings at risk of emergence of this serotype. Our study showed an excellent safety and immunogenicity profile of mIPV2HD compared with currently available IPV and therefore is an important addition to the clinical evidence base for vaccine options for the polio endgame.
Acknowledgments
The study was funded by the Bill & Melinda Gates Foundation. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the US Centers for Disease Control and Prevention (CDC) and other contributing agencies. The use of trade names is for identification purposes only and does not constitute an endorsement by the CDC or the US Government. Important contributors were Ana Cecilia Villarreal who was the site coordinator for the study; Steve Self, Bhavesh R Borate, and Chris Gast of the Fred Hutchinson Cancer Research Center, who provided input into study design and data analysis, respectively; Chris Wilson of the Bill & Melinda Gates Foundation who provided input into study design; and Mohamed Amakrane, Marie-Cécile Bozonnat, and Anne Hepburn of 4Clinics, who performed the statistical analysis and assisted in the preparation of the study report and manuscript. We thank Victor Sales and Johnny Escobar of VaxTrials for their support in the study conduct; Mark Pallansch, CDC, for his expert advice during the development of the study design; María Luisa Ávila, Maria Teresa Valenzuela, Claudio Strunchiner, Luiza Helena Falleiros, and Carolina Danovaro for study supervision within the data safety monitoring board; Gerrit Van Roekel and Kim Bush of the Bill & Melinda Gates Foundation for coordinating vaccine supply; Jay Wenger and John Modlin of the Bill & Melinda Gates Foundation who provided input into study design and data analysis; and the laboratory staff at the CDC (Deborah Moore, Yiting Zhang, Sharla McDonald, Larin McDuffie, William Hendley, Patricia Mitchell, Mario Nicolas, Demetrius Mathis, Brittani Brown, and Jessica Wielgus) for performing poliovirus titrations and seroneutralisation assays. We also thank Bilthoven Biologicals, GlaxoSmithKline, and Sanofi Pasteur for kindly donating vaccines for the study. We are grateful to the Panama site members Juan Carlos Batista, Luis Casal, Evelyn Castillo, Rita Tello, María Guadalupe de Fletcher, John Solano, Onix Saldaña, and Stephany Álvarez for their participation.
Footnotes
Declaration of interests
NM and XS-L report personal fees from VaxTrials, during the conduct of the study. All other authors declare no competing interests.
Contributor Information
Xavier Sáez-Llorens, Hospital del Niño, Ciudad de Panama, Panama.
Ralf Clemens, Global Research in Infectious Diseases, Rio de Janeiro, Brazil.
Geert Leroux-Roels, Center for Vaccinology (CEVAC), Ghent, Belgium.
José Jimeno, VaxTrials, Ciudad de Panama, Panama.
Sue Ann Costa Clemens, Instituto de Pós Graduação Carlos Chagas, Rio de Janeiro, Brazil.
William C Weldon, Centers for Disease Control and Prevention, Atlanta, GA, USA.
M Steven Oberste, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Natanael Molina, VaxTrials, Ciudad de Panama, Panama.
Ananda S Bandyopadhyay, Bill & Melinda Gates Foundation, Seattle, WA, USA.
References
- 1.Bandyopadhyay AS, Garon J, Seib K, et al. Polio vaccination: past, present and future. Future Microbiol 2015; 10: 791–808. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global Polio Eradication Initiative. Polio this week. Geneva, Switzerland: World Health Organization; 2015. http://www.polioeradication.org/Dataandmonitoring/Poliothisweek.aspx (accessed Nov 23, 2015). [Google Scholar]
- 3.Platt LR, Estívariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis 2014; 210 (suppl 1): S380–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polio Global Eradication Initiative. Polio eradication & endgame; midterm review July 2015. http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/GPEI-MTR_July2015.pdf (accessed Nov 23, 2015).
- 5.Global Polio Eradication Initiative. Polio Eradication & Endgame Strategic Plan 2013–2018. http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/PEESP_EN_US.pdf (accessed Nov 23, 2015).
- 6.WHO. Polio vaccines: WHO position paper, January 2014. Wkly Epidemiol Rec 2014; 89 73–92. [PubMed] [Google Scholar]
- 7.Global Polio Eradication Initiative. SAGE confirms global polio vaccine switch date as April 2016. Oct 26, 2015. http://www.polioeradication.org/mediaroom/newsstories/SAGE-confirms-global-polio-vaccine-switch-date-as-April-2016/tabid/526/news/1307/Default.aspx?popUp=true (accessed Nov 23, 2015).
- 8.Salk J, Cohen H, Fillastre C, et al. Killed poliovirus antigen titration in humans. Dev Biol Stand 1977; 41: 119–32. [PubMed] [Google Scholar]
- 9.Salk J, Van Wezel AL, Stoeckel P, et al. Theoretical and practical considerations in the application of killed poliovirus vaccine for the control of paralytic poliomyelitis. Dev Biol Stand 1980; 47: 181–98. [PubMed] [Google Scholar]
- 10.Salk J, Stoeckel P, van Wezel AL, et al. Antigen content of inactivated poliovirus vaccine for use in a one- or two-dose regimen. Ann Clin Res 1982; 14: 204–12. [PubMed] [Google Scholar]
- 11.Vidor E, Mescheivitz C, Plotkin S. Fifteen years of experience with Vero-produced enhanced potency inactivated poliovirus vaccine. Ped Infect Dis J 1997; 16: 312–22. [DOI] [PubMed] [Google Scholar]
- 12.Swartz TA, Handsher R, Stoeckel P, et al. Immunologic memory induced at birth by immunization with inactivated polio vaccine in a reduced schedule. Eur J Epidemiol 1989; 5: 143–45. [DOI] [PubMed] [Google Scholar]
- 13.WHO. WHO Expert Committee on Biological Standardization. World Health Organ Tech Rep Ser 1982; 673: 1–180. [PubMed] [Google Scholar]
- 14.Ferguson M, Wood DJ, Minor PD. Antigenic structure of poliovirus in inactivated vaccines. J Gen Virol 1993; 74: 685–90. [DOI] [PubMed] [Google Scholar]
- 15.Simasathien S, Migasena S, Beuvery C, et al. Comparison of enhanced potency inactivated poliovirus vaccine (eIPV) versus standard oral poliovirus vaccine (OPV) in Thai infants. Scand J Infect Dis 1994; 26: 731–38. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed AJ, AlAwaidy S, Bawikar S, et al. Fractional doses of inactivated poliovirus vaccine in Oman. N Engl J Med 2010; 362: 2351–59. [DOI] [PubMed] [Google Scholar]
- 17.Resik S, Tejeda A, Sutter RW, et al. Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med 2013; 368: 416–24. [DOI] [PubMed] [Google Scholar]
- 18.O’Ryan M, Bandyopadhyay AS, Villena R, et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study. Lancet Infect Dis 2015; 15: 1273–82. [DOI] [PubMed] [Google Scholar]
- 19.Sutter RW, John TJ, Jain H, et al. Immunogenicity of a bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet 2010; 376: 1682–88. [DOI] [PubMed] [Google Scholar]
- 20.Karber G Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch Exp Pathol Pharmakol 1931; 162: 480–83. [Google Scholar]
- 21.WHO. Polio laboratory manual, 4th edition. WHO/IVB/04.10. Geneva: World Health Organization, 2004. http://apps.who.int/iris/bitstream/10665/68762/1/WHO_IVB_04.10.pdf (accessed Nov 23, 2015). [Google Scholar]
- 22.Sutter RW, Bahl S, Deshpande JM, et al. Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label, randomised controlled trial. Lancet 2015; published online Sep 17 10.1016/S0140-6736(15)00237-8. [DOI] [PubMed] [Google Scholar]
- 23.The Cuba IPV Study Collaborative Group. Randomized, placebo-controlled trial of inactivated poliovirus vaccine in Cuba. N Engl J Med 2007; 356: 1536–4. [DOI] [PubMed] [Google Scholar]
- 24.Hird TR, Grassly NC. Systematic review of mucosal immunity inducted by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus shedding. PLoS Pathog 2012; 8: e1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John J, Giri S, Karthikeyan AS, et al. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: an open-label, randomised controlled trial. Lancet 2014; 384: 1505–12. [DOI] [PubMed] [Google Scholar]
- 26.Jafari H, Deshpande JM, Sutter RW, et al. Polio eradication. Efficacy of inactivated poliovirus vaccine in India. Science 2014; 345: 922–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Global Polio Eradication Initiative (GPEI). Availability and price of inactivated polio vaccine. http://www.polioeradication.org/tabid/488/iid/354/Default.aspx (accessed Nov 23, 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


