Abstract
Context
The implication of severe dental caries in children may have its effect on general health apart from affecting the orodental tissues. Many children reporting with severe caries have shown weighing less due to malnourishment resulting in anemia and modified somatic growth.
Aims
Study aimed to assess and compare anthropometric measurements, hemoglobin level, and salivary parameters among caries-free and severe early childhood caries (E-CCC) children.
Settings and design
For caries-free group [Group I] and S-ECC group [Group II] data were obtained from age-matched children with similar socioeconomic status.
Materials and methods
Children with severe caries and without caries from the age-group 3- 6 years participated in the study. Children were measured for height, weight, measurement of mid-upper arm circumference, and waist circumference. Hemoglobin level was recorded. The collected unstimulated saliva was assessed for flow rate, salivary pH, and its buffering capacity.
Statistical analysis used
Both descriptive and inferential statistical analyses were carried out using Windows software and SPSS (21). Tests of significance namely t-test and Chi-square test were used along with regression analysis.
Results
Caries experience showed no statistical difference for age and gender among the sample population. Significant difference was found for all anthropometric measurements. When comparison for hemoglobin was done for both groups I and II, significant difference was observed [p = 0.003].
Conclusion
Children with severe dental caries in the present study had low hemoglobin which, if persisted, can lead to anemia. Though the anthropometric parameters may appear normal in children diagnosed with severe early childhood caries, pediatric dentist should ensure the hemoglobin level test, as iron deficiency can affect growth and development of the child, if left undiagnosed.
How to cite this article
Deshpande AN, Sudani U, Wadhwa M, et al. Association of Anthropometric Measurements, Hemoglobin Level and Salivary Parameters among Caries-free and S-ECC Children. Int J Clin Pediatr Dent 2022;15(S-2):S164-S171.
Keywords: Body mass index, Buffering capacity, Early childhood caries, Iron deficiency, Salivary pH, Salivary flow, Waist circumference
Introduction
Early childhood caries though has got its mention in literature over a century, still it poses a challenge on developing and industrialized nation. In spite of increase in awareness in past decade, early childhood caries remains as the most prevalent childhood oral disease.1 Dental caries has been regarded as disease caused due to transmissible bacteria of mutans streptococci strain. They are dental hard tissue adherent and produce acid by breakdown of sugars in the food resulting in demineralization of enamel and dentin over a period of time. Severe early childhood caries has been associated with children up to the age of 6 years; showing signs of caries on smooth surfaces of maxillary anterior teeth. If the number of cavitated lesions or extracted or filled due to dental caries is more than or equal to the age of child, it is regarded as S-ECC.2
The implication of severe dental caries in children may have its effect on general health apart from affecting the orodental tissues. Many children reporting with severe caries have found to be weighing less due to malnourishment resulting in anemia and modified somatic growth.3 The probability of deficiency in vitamins and minerals is high in children with severe dental caries. Various researches are suggestive4,5 of weight less than normal in those children having severe caries; and recent evidence6,7 had similar observations as well showing deviation from normal anthropometric measurements for weight, height, body mass index [BMI], and circumference of arm and waist.
There are very few researches conducted to have and understanding of S-ECC with micronutrients. Study conducted in Canada reported the association of low iron levels in children with high caries index where 11% were found to be anemic and 6% showed iron deficiency.8 Biological process of dental caries may change due to several internal and external factors such as immature host defense, nature of saliva, ways of feeding, and style of brushing in early age. There are studies where it has been found that though fermentable carbohydrate was high in them, they still did not develop S-ECC. In recent years, endogenous factors, such as salivary characteristics and components, have been suggested as predisposing factors in children for development of ECC.9 Saliva is regarded as a key host-dependent factor to preserve the oral tissues integrity, which is mainly because of following characteristics: modifies oral milieu; acts as a buffer agent; maintains demineralization-remineralization balance, and has bacteriostatic action.1
The association of an anthropometric measurements, hemoglobin level, and salivary parameter and S-ECC in this age-group remains ambiguous. Data published about correlation between anthropometry and S-ECC is too limited and has only recently begun to be explored. Therefore, this study involving both cases and control is designed for further clarification regarding the association between S-ECC and anthropometric measurements, hemoglobin level and salivary parameters [salivary flow, pH, and buffer capacity]. In this study, anthropometric measurements and hemoglobin level are included to see if there is any difference in overall nutritional status of children without caries and with caries.
Materials and Methods
Study was conducted on 400 young children with and without caries who had reported to the department for orodental complaints or to the medical outpatient department within campus for vaccination and checkup. This study was initiated after approval of institutional ethics committee [SVIEC/ON/DENT/BNPG-13/D14232]. Written informed consent forms were obtained from parents to express their willingness for participation in study.
The S-ECC children with minimum one pulpally involved tooth and who were not taking medication since last 3 months were included in the study. To confirm the pulp involvement, intraoral periapical radiograph was used as investigation to confirm the pulp involvement. All children in this study had a contributory medical history and were considered by their physicians and dentists as not healthy were excluded from the study. The clinical examination of all children was entirely done by single investigator.
Anthropometric Measurements
On the 1st day of enrollment following parameters were recorded: weight, height, body mass, arm, and waist circumference [WC]. Standard technique for measuring various parameters was adopted and the equipment used was calibrated at regular intervals [Fig. 1] For height measuring tape and for weight electronic digital scale [Venus Digital LCD Weighing Scale, Ace Incorporation, Jaipur, India] were used. BMI for children was calculated with the help of BMI percentile calculator in metric version by filling age [years; months], sex, height [cm], and weight [kg] given by Centers for Disease Control and Prevention for child and teen.4 No stretch tape was used to measure the circumference of mid-upper arm circumference [MUAC] [Fig. 2]. The study population was obtained from age-matched children with same socioeconomic status [SES], which was calculated by Modified Kuppuswamy5 SES scale.
Fig. 1.
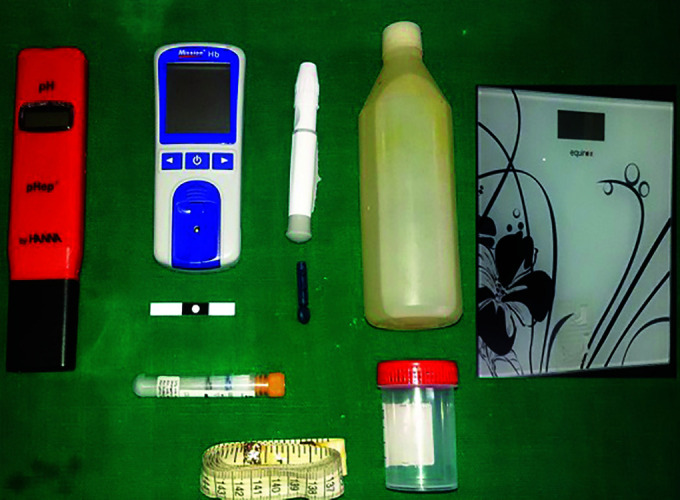
Photograph of armamentarium used in the study for measurements
Fig. 2.
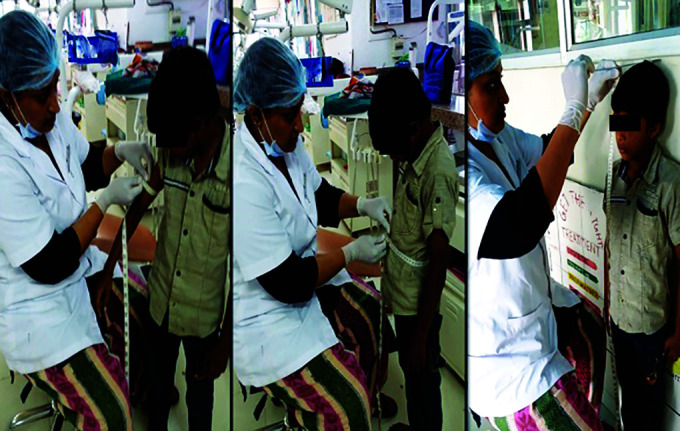
Photograph of anthropometric measurements taken for study
Clinical Examination
Each child was examined on an ordinary upright chair with the help of sterilized mouth mirror and explorer in an adequate natural light. Prior to examination, the child was asked to rinse the mouth thoroughly. The examination was done by one examiner only to eliminate error. It was carried out in the uniform manner, starting from the maxillary right quadrant and then in a clockwise direction.
Collection of Saliva
The parents were requested to perform normal oral hygiene procedures after breakfast on the day of saliva sampling [1 day following oral assessment]. The children were instructed not to have any food till the saliva sample was collected [1 hour 30 minutes after breakfast]. The unstimulated saliva was collected in small sterile cups under resting condition using the method described by Dawes.10,11 [In this method, plastic cylinders were used and the child was instructed to keep spitting for a duration of 5 minutes] [Fig. 3]. The rate of saliva flow was measured by weighing and checking the volume in grams per milliliter to compute it in mL/min.11 Salivary pH was determined by means of a pH meter [Hanna Instruments HI 98107 pH Tester, with +/-0.1 Accuracy], which measured the hydrogen ion concentration. The titration of the samples of saliva was done with 0.1 mol/L hydrochloric acid to evaluate the buffering capacity. Salivary buffering capacity [SBC] was categorized into high [> pH 5.5], medium [pH 5.5- 4.5], and low buffer function [< pH 4.5].
Fig.3.
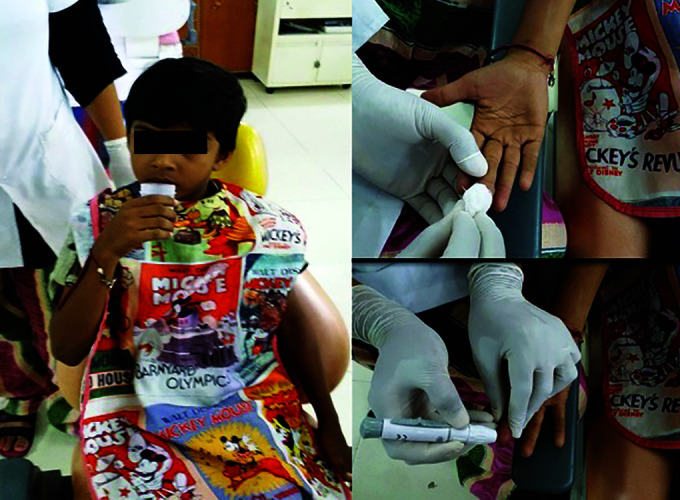
Photograph of saliva and blood collection
Hemoglobin Level
Blood samples were obtained on the day of study by examiner. Hemoglobin level was measured chair side with digital hemoglobin meter [Portable Hemoglobin Meter AM-4751 by ATICO Medical Pvt. Ltd., Ambala, India]. [Fig. 3]
Statistical Analysis
Both descriptive and inferential statistical analysis were carried out using Windows software and SPSS 21 [Statistical Package for the Social Sciences, IBM Corporation, USA]. Test of significance was done using t-test and Chi-square test along with regression analysis to evaluate association between anthropometric measurements, hemoglobin level, and salivary parameters among caries-free and S-ECC Children.
Results
Data of 400 participants were analyzed that included 200 Caries Free [Group-I] and 200 S-ECC [Group II]. Demographic data for both groups were recorded: for gender distribution were 98 [24.5] in caries free and 114 [28.5] in S-ECC were male, 102 [25.5] in caries free and 86 [21.5] in S-ECC group in female. SES was also recorded on the basis of upper, upper middle, middle, and upper lower category.
Weight, BMI, MUAC, and WC showed [Table 1] highly significant association between dental caries status.
Table 1.
Anthropometric measurements of caries-free and S-ECC group
| Variables | Group | N | Mean ± SD | 95% Confidence Interval | t- test p-value |
|---|---|---|---|---|---|
| Height | Caries free | 200 | 104.18 ± 10.671 cm | -7.229-3.370 | 0.000 HS |
| S-ECC | 200 | 109.48 ± 8.877 cm | |||
| Weight | Caries free | 200 | 15.10 ± 2.886 Kg | -1.545-0.054 | 0.000 HS |
| S-ECC | 200 | 15.90 ± 4.520 Kg | |||
| BMI | Caries free | 200 | 14.093 ± 2.022 Kg/m2 | -0.221-0.286 | 0.000 HS |
| S-ECC | 200 | 13.305 ± 3.504 Kg/m2 | |||
| Mid-upper arm circumference | Caries free | 200 | 14.869 ± 1.2598 cm | 0.392-0.949 | 0.001 HS |
| S-ECC | 200 | 14.198 ± 1.564 cm | |||
| Waist circumference | Caries free | 200 | 49.980 ± 2.453 cm | 2.850-4.029 | 0.000 HS |
| S-ECC | 200 | 46.540 ± 3.456 cm |
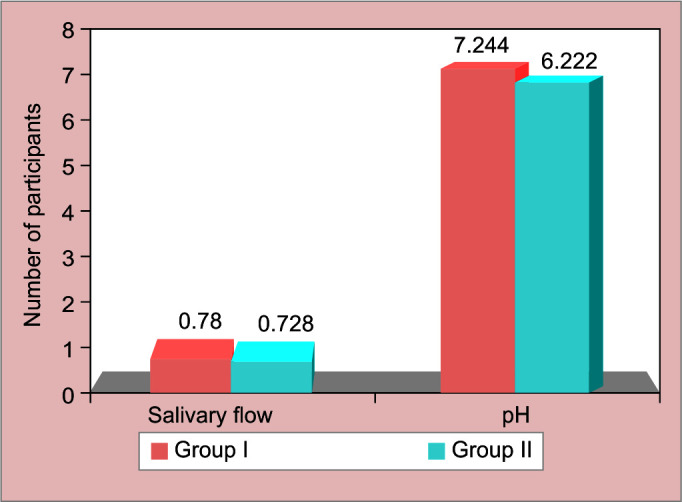
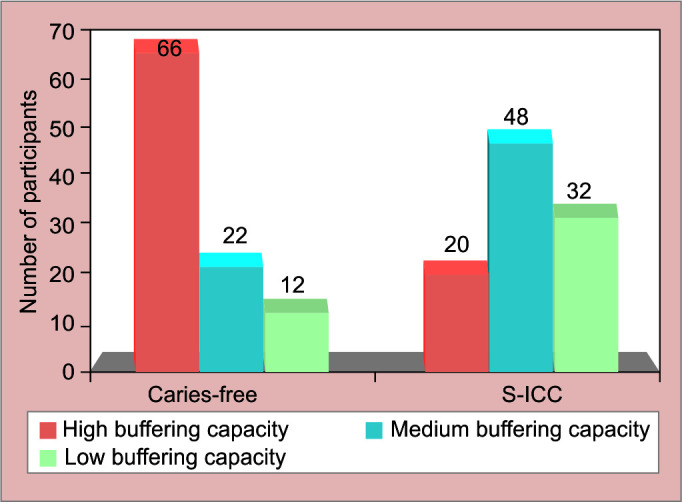
Hemoglobin level was lower in carious children then caries free [Table 2, [Fig. 4]. Statistical significant [p = 0.003 highly significant] difference was observed between both the groups. Comparison of salivary parameters for participants in both the groups depicted shows mean and standard deviation of salivary flow rate [SFR] and pH for both study groups [Fig. 5, Table 3]. Comparison between two groups for buffering capacity of saliva showed high statistically significant difference. Chi-square test of association is used to analyze the association between presence of phenomenon and the group [Table 2, Fig. 6].
Table 2.
Comparison of saliva buffering capacity (SBC) for caries-free and S-ECC group
| Group 1 Caries free N (%) | Group 2 S-ECC N (%) | Total N (%) | Chi-square test p-value | ||
|---|---|---|---|---|---|
| Buffering capacity | High [above pH 5.5] | 132 [66.0] | 40 [20.0] | 172 [43.0] | 0.000 HS |
| Medium [from pH 5.5 to 4.5] | 44 [22.0] | 96 [48.0] | 140 [35.0] | ||
| Low [below pH 4.5] | 24 [12.0] | 64 [32.0] | 88 [22.0] | ||
| Total | 200 [100] | 200 [100] | 400 [100] | ||
Fig. 4.
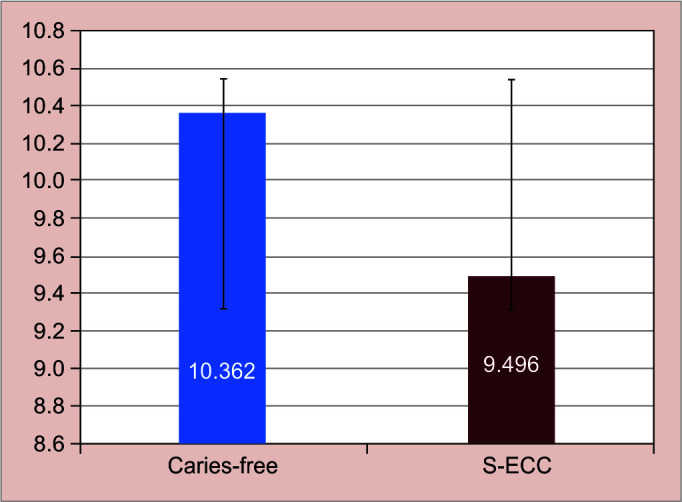
Graphical presentation of hemoglobin level
Fig. 5.
Graphical presentation of comparison of salivary flow rate and pH
Table 3.
Comparison of salivary flow rate (SFR), salivary pH, and hemoglobin level for caries-free and S-ECC group
| Variables | Group | N | Mean ± SD | 95% confidence interval | Chi-square test p-value |
|---|---|---|---|---|---|
| Salivary flow | Caries free | 200 | 0.780 ± 0.067 mL/min | 0.022-0.080 | 0.000 HS |
| S-ECC | 200 | 0.728 ± 0.198 mL/min | |||
| pH | Caries free | 200 | 7.2440 ± 0.643 | 0.194-0.447 | 0.006 HS |
| S-ECC | 200 | 6.9228 ± 0.656 | |||
| Hb | Caries free | 200 | 10.362 ± 1.689 | 1.172-0.559 | 0.003 S |
| S-ECC | 200 | 9.496±1.421 |
Fig. 6.
Graphical presentation of buffering capacity of saliva in both groups
Multiple linear was applied for Hb as dependent variable where R square indicates that the predictability of the model is 60.6% and adjusted Rsq is 69.5%. As the adjusted Rsq was very large, ANOVA was applied further and it was significant as the p value is <0.05 indicating that the variables have a role in predicting the Hb Level among the patients. As shown in Table 4, DEFT, DEFS salivary flow, and BMI were significant. Stating from above table, the regression equation for predicting Hb level among the study participants is as follows: Hb = 8.224-0.123 [DEFT] + 0.009 [DEF]) + 0.213 [salivary flow] + 0.102 [BMI].
Table 4.
Multiple linear regression analysis for predicting Hb level among caries-free and S-ECC children
| Model | unstandardized coefficients | standardized coefficients | t | Sig. | ||
|---|---|---|---|---|---|---|
| B | Std. error | Beta | ||||
| 1 | (Constant) | 8.224 | 1.165 | 7.060 | 0.000 | |
| DEFT | -0.123 | 0.082 | -0.258 | -1.490 | 0.037 | |
| DEFS | 0.009 | 0.053 | 0.029 | 0.170 | 0.045 | |
| Salivary flow | 0.213 | 0.559 | 0.020 | 0.382 | 0.003 | |
| pH | 0.068 | 0.119 | 0.028 | 0.572 | 0.568 | |
| BMI | 0.102 | 0.028 | 0.181 | 3.575 | 0.000 | |
a. Dependent Variable: Hb
Multiple linear was applied for BMI as dependent variable where R square indicates that the predictability of the model is 61.9% 61.9%. and adjusted Rsq is 71%]. As the adjusted Rsq was very large, ANOVA was applied further and it was significant as the p value is <0.05 indicating that the variables have a role in predicting the BMI level among the patients. As shown in Table 5, DEFT, DEFS, salivary flow, and pH were significant. Stating from above table, the regression equation for predicting BMI level among the study participants is as follows: BM I = 21.891-34.104 [DEFT] -1.224 [DEFS] -3.598 [salivary flow] -0.678 [pH].
Table 5.
Multiple linear regression analysis for predicting body mass index (BMI) among caries-free and S-ECC children
| Model | Unstandardized coefficients | Standardized coefficients | t | Sig. | ||
|---|---|---|---|---|---|---|
| B | Std. error | Beta | ||||
| 1 | (Constant) | 21.891 | 1.741 | 12.575 | 0.000 | |
| DEFT | -34.104 | 0.142 | -0.709 | -4.226 | 0.000 | |
| DEFS | -1.224 | 0.093 | 0.450 | 2.650 | 0.008 | |
| Salivary flow | 81.470 | 427.053 | -0.186 | -3.703 | 0.000 | |
| pH | 9.368 | 91.308 | -0.156 | -3.261 | 0.001 | |
a. Dependent Variable: BMI
Discussion
Dental caries has effect on overall health of an individual. S-ECC children had severe dental condition, most of the children did not visit a dentist until serious decay was observed or they suffered a toothache.
Demographic Data
The study population was obtained from age-matched children with similar SES for both noncarious and severe caries children. Both groups were compared in terms of their age [p = 0.105]. Most of the participants from both the groups belonged to upper middle class. No statistical difference was found when SES was compared.
Anthropometry
Previous research done by Tanner JM et al. in 199611 had relied solely on body weight to assess nutritional health. The rationale for this study was that the clinical assessment of nutritional status in children with S-ECC to be affirmative required numerous tests to determine malnutrition. Various measurements of anthropometry such as height, weight arm, and WC can be suggestive of nutritional status. Vital nutrients are important for child's growth, which can be elicited by various blood tests, which are diagnosed as iron deficiency or pernicious anemia.
On the contrary, BMI was only found to be valid for initial screening for children with the risk of obesity or being overweight. Additional anthropometric assessment was used for having clarity pertaining to somatic nutritional effect in children with severe caries: (1) MUAC and (2) WC measurement. If low measurements are observed for an individual, it indicates insufficient caloric intake. In this study, the MUAC was found to be in acceptable range for both groups of children suggestive of adequate protein intake in both groups [> 13.5 cm] suggestive of adequate protein intake in both the groups.12 On statistical analysis for comparing MUAC, significant statistical difference was found between the groups where the mean value of S-ECC [14.198 ± 1.564 cm] group was lower than that of caries free [14.869 ± 1.2598 cm] group, which indicates that severe ECC group had less muscle mass compared to noncarious group. Though no significant p value was found four in MUAC in both groups, children were above the acceptable range of malnutrition.12
Compared to previous study,11 this study included more subjects and methodology was also modified. Firstly, age and gender-specific BMI were used as a weight descriptor. Secondly, S-ECC children with one pulpal involvement teeth were included in the study. In various anthropometric measurements, all showed highly significant difference between both the groups. As the MUAC was above 13.5, it is suggestive that children with shorter height in S-ECC group may have chances of catching up their height, after dental rehabilitation, and appropriate diet. Weight was considered separately, children with S-ECC were shorter than controls. Though p value was not significant for weight of children. The mid-arm and WC measurements showed acceptable range of muscle mass/muscle protein storage in study population, but had lower values than their age-matched controls.
Hemoglobin Level
The nutritional value is best tested by various specific blood investigations and Hb level though not a specific indicator of nutritional status. It is considered to be acceptable, as it requires less blood withdrawal and the procedure can be carried out chair side. In present study, the mean value for S-ECC and Caries-free group was 9.496 ± 1.432 gm% and 10.362 ± 1.689 gm%, respectively. These results suggest that iron levels in children's diet was insufficient to sustain required level of hemoglobin. Looker et al.13 in his population-based study reported 6% prevalence of iron deficiency. The studies’ most notable finding was that the hemoglobin levels of substantial proportion of young children were unsatisfactory meeting the definition of anemia as stated by Mother Child Malnutrition organization have regarded children with Hb < 11.0 g/dL as anemic and Hb < 7.0 g/dL as severe anemia.14
There is negligible research comparing relationship between dietary iron levels and dental caries. By comparing hemoglobin levels in children of both the groups, present study was able to investigate this association. Although high significant difference was found pertaining to iron concentration, both the groups had low Hb levels. This is largely similar with findings by other investigator that identified 65% of study group children having Hb value of less than 12 g/dL.15
It was noticed that low hemoglobin levels [mean 9.4 gm%] were prevalent in children with severe caries as compared to their counterparts [mean 10.362 gm%]. When the WHO's anemia subgroups were applied, 29.5% of our study population was considered to be anemic.
In recent times, Shaoul R, et al. in 2012 described a compatible strong link to be present between widespread caries in young children and low hemoglobin levels. The levels of hemoglobin in children with rampant caries were substantially lower than in control group. Study by Shaoul R et al.15 moreover the current study shows that there is an association between hemoglobin levels and S-ECC [high statistical significant difference p = 0.007].
There are numerous reasonable clarifications as to why the iron levels of a child are related with the occurrence of S-ECC. A plausible reason for low hemoglobin level frequently seen in young children could be due to inflammatory oral tissue response, which is associated with pulp involvement or dentoalveolar abscess, a sequelae of dental caries. Inflammation observed with progressive ECC initiates a cascade of events that finally result in the generation of cytokines, which may further impede erythropoiesis and lower blood hemoglobin levels, decreased hemoglobin levels are prevalent in many chronic illnesses and made it to “anemia of chronic disease” in severe conditions.13 S-ECC could be one of the manifestations of such conditions.
Furthermore, the pain experienced by children with S-ECC is correspondingly documented by altered eating habits.8 This may set a stage for nutritional deficiencies such as low levels of iron.16 Lastly, alterations in adequacy of nutrition in young children may be influenced by home finance. A family's capacity to acquire healthy food may be limited due to financial constraints. The social status has been linked to the increased occurrence of anemia.17
Whereas, in present study, most of the children were from upper middle class and associated with anemia and the reason associated for altered eating habits rather than insufficient funds. Children with S-ECC showed low hemoglobin level, which demonstrates substantial influence on health.
The potential to detect early indicators of low iron levels in young children with widespread caries may help patients to obtain required treatments even before long-term consequences of iron deficiency manifests.
Salivary Parameters
At least three risk factors are associated with caries development: (1) micro-organisms; (2) substrate/oral environment; and (3) host/teeth. One of the authors18 had found that weight and height of the children may influence SFR. The overall secretion rate in this study was estimated to be between 0.3 and 1.5 mL/min, with a mean flow rate of 0.78 ± 0.485 mL/min. In this study, SFR was more in caries-free children than in children with S-ECC. Various researchers reported similar findings, stating that the caries-free [DMFT/dmft = 0] group had higher mean values than the caries-active group.18–20
This unstimulated flow is what is secreted by the salivary glands majority of the time and is essential for providing the protection functions to the teeth against dental caries.21 In general, the lesser the flow rate of saliva, the poorer the cleansing action on tooth surfaces, hence the greater the microbial attacks and greater the risk of dental caries.22,23 In their investigation, Kaur et al.24 reported that 90% of the caries-free groups had SFRs of > 0.7 mL/min; similarly Seekinabi and Hiremath25 reported 100% of their caries-free individuals had an SFR of > 0.7 mL/min. Whereas, few studies stated that there is no statistical difference between caries-free and caries-active children for SFR as found in present study.26–28
Salivary pH
Caries-free Group had a significantly higher mean salivary pH value [7.2440 ± 0.643] than that of S-ECC Group. According to Prabhakar et al.,19 pH in caries affected children was ranging from 6.20 to 7.90 with no statistical significance. In caries-affected children, pH of saliva was marginally lower as compared to non-carious children. Research by Zhou et al.29 had contrary findings where pH was highly significant in early childhood caries group. As the mineral loss from enamel begins once the pH goes below 5.5, the levels found in this study are suggestive that etched ions would be insufficient to yield in mineral loss from the tooth's inorganic composition.
Study by Cunha-Cruz et al. in 201328 stated that 60% higher mean increment of dental caries was reported when comparing individuals with resting pH of saliva of ≤6 to those with 6.4. The finding demonstrates that low salivary pH was not related to increased caries index, whereas the mean of all scores was acidic for resting saliva as compared to stimulated saliva with mean stimulated SFR was 1.4 mL/ min.
Swerdlove in 194230 and Malekipour et al. in 200831 found no statistical association between the occurrence of carious lesions and the pH of normal resting saliva. Similarly, Lamberts et al.32 found no significant correlation between increased salivary pH and caries incidence in both caries-free and caries-active participants in 1983.32
Salivary Buffering Capacity
The majority of study participants [43%] had a buffer capacity of >5.5 pH, whereas 35% had a pH of 4.5 to 5.5. The mean SBC was significantly different between the study groups [p 0.001]. The SBC of the caries-free group was substantially greater than that of the S-ECC group. Low SBC inhibits the formation of cariogenic microorganisms in the salivary glands, which may contribute to high-caries scores.
The results obtained are consistent with those reported by Prabhakar et al. in 2009.19 However, the findings of their research were insignificant. Caries-affected children's SBC was only marginally lower than that of caries-free children. In a research published by Malekipour et al. in 2008,31 patients with caries-active and caries-free individuals had pH values of 6.67 ± 0.03 and 6.76 ± 0.03, respectively, though the difference was statistically insignificant. Another analysis revealed that saliva from children with severe caries had a stronger buffer potential compared to caries-free.23,33 Nasiru et al.20 found a similar decline in the mean SFR and SBC when the DMFT/dmft score increased [p > 0.05]. SBC pH > 6.0 was found in 77.6% of research participants, with a larger frequency [78.9%] in the caries-free group. The caries-free participants had a slightly larger mean redox potential, but it was not statistically significant.
Similarly, Bagherian and Gholanireza1 evaluated salivary variables in children with and without early childhood caries and found that buffer potential of saliva in children with severe caries was higher [8.03 ± 0.91], but not significant when compared to those with no caries [7.43 ±0.82].
On contradictory, research done by Sullivan et al.33 and Tukiakumala et al.34 revealed no significant relationship between salivary pH and flow rate of saliva for mean caries score. Similar findings were found by Tulunoglu OS,35 wherein no correlation was observed between pH level and caries activity.
According to the literature,1,23,31,33 salivary buffering capacity rather than saliva flow rate appears to have a major role in the prevention of dental caries. Low buffer potential of saliva can be attributed to several salivary parameters such as fluoride level, immunoglobulin, and bacterial load in caries-active group.36
Limitations
Since the study was cross-sectional, it was difficult to discern actual cause and effect. Identifying cavity-free individuals from similar regions and background to participate was equally challenging. Despite all odds, the sample size provided adequate statistical power to determine whether or not there were any relationships.
Considering the study population, a systematic random sampling was not employed in the current study as children with severe caries are difficult to detect and access. Because the data had to be categorized into age, gender, and percentile categories in order to compare with reference values, the analysis was constrained. As a result, subcategories were too narrow for statistical testing of groups or means to be accurate.
Conclusion
The clinical significance of this study is that S-ECC should be considered an early signal of low hemoglobin levels. Pediatricians and pediatric dentist should consider S-ECC as a key marker for insufficient nutrition that can further lead to anemia in young children. Practitioners should therefore take this into account and focus on preventing and intervening S-ECC for wellbeing of the child. The results of this study recommend that S-ECC patients should have a complete blood count, meticulous measurement of height and weight, and a food intake evaluation, all of which should be conducted by a pedodontist, pediatrician, or clinical dietician.
Future Scope
Studies with population stratification based on age, gender, and percentile groups can give better insight to malnutrition and its correlation to severe caries. The randomized controlled study design would enable actual cause and effect to be determined.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Bagherian A, Asadikaram G. Comparison of some salivary characteristics between children with and without early childhood caries. Indian J. Dent. Res. 2012;23(05):628–632. doi: 10.4103/0970-9290.107380. [DOI] [PubMed] [Google Scholar]
- 2.Policy on Early Childhood Caries (ECC): ClassificationsConsequencesand Preventive Strategies. https://pubmed.ncbi.nlm.nih.gov/27931420/ Pediatr Dent. 2016;38(6):52–54. 27931420 Oct; . [PubMed] [Google Scholar]
- 3.Sheller B, Shervin S, Davidson BW. Body mass index of children with severe early childhood caries. https://pubmed.ncbi.nlm.nih.gov/19552226/ Pediatr Dent. 2009;3(03):216–221. [PubMed] [Google Scholar]
- 4.Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion. BMI Percentile Calculator for Child and Teen. U.S. Department of Health & Human Services. 2019. https://www.cdc.gov/healthyweight/bmi/calculator.html https://www.cdc.gov/healthyweight/bmi/calculator.html [online] Available from. . [Last accessed November, 2021]
- 5.Saleem SM. Modified Kuppuswamy scale updated for year 2018. https://www.researchgate.net/publication/323846030_MODIFIED_KUPPUSWAMY_SCALE_UPDATED_FOR_YEAR_2018 Indian J Res. 2018;7(03):6–7. [Google Scholar]
- 6.Köksal E, Tekçiçek M, Yalçin SS, et al. Association between anthropometric measurements and dental caries in Turkish school children. Cent Eur J Public Health. 2011;19(03):147–151. doi: 10.211/cejph.3648. [DOI] [PubMed] [Google Scholar]
- 7.Mishu MP, Tsakos G, Heilmann A, et al. Dental caries and anthropometric measures in a sample of 5- to 9-year-old children in Dhaka, Bangladesh. Community Dent Oral Epidemiol. 2018;46(05):449–456. doi: 10.1111/cdoe.12412. [DOI] [PubMed] [Google Scholar]
- 8.Schroth R J, Levi J, Kliewer E, et al. Association between iron status, iron deficiency anaemia, and severe early childhood caries: a case-control study. BMC Pediatr. 2013;13:22. doi: 10.1186/1471-2431-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong MCM, Schwarz E, Lo ECM. Patterns of dental caries severity in Chinese kindergarten children. Community Dent Oral Epid. 1997;25:343–347. doi: 10.1111/j.1600-0528.1997.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 10.Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance and the sensation of dry mouth in man. J Dent Res. 1987;66:648–653. doi: 10.1177/00220345870660S107. [DOI] [PubMed] [Google Scholar]
- 11.Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children. Arch Dis Child. 1996;41:613–635. doi: 10.1136/adc.41.220.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Early Detection and Referral of Children with Malnutrition. The Mother and Child Health and Education Trust A U.S. 501(c)(3) non profit organization. 2019. https://motherchildnutrition.org/early-malnutrition-detection/detection-referral-children-with-acute-malnutrition/interpretation-of-muac-indicators.html https://motherchildnutrition.org/early-malnutrition-detection/detection-referral-children-with-acute-malnutrition/interpretation-of-muac-indicators.html [online] Available from. . [Last accessed November, 2021]
- 13.Looker AC, Dallman PR, Carroll MD, et al. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 14.Kapur D, Agarwal KN, Sharma S. Detecting iron deficiency anemia among children (9-36 months of age) by implementing a screening program in an urban slum. https://pubmed.ncbi.nlm.nih.gov/12147896/ Indian Pediatr. 2002;39(07):671–676. [PubMed] [Google Scholar]
- 15.Shaoul R, Gaitini L, Kharouba J, et al. The association of childhood iron deficiency anaemia with severe dental caries. Acta Paediatr. 2012;101(02):e76–e79. doi: 10.4103/2231-0762.97697. [DOI] [PubMed] [Google Scholar]
- 16.Sinha N, Deshmukh PR, Garg . Epidemiological correlates of nutritional anemia among children (6-35 months) in rural Wardha, Central India. BS. Indian J Med Sci. 2008;62:45–54. doi: 10.4103/0019-5359.39366. [DOI] [PubMed] [Google Scholar]
- 17.Fretham SJ, Carlson ES, Georgieff MK. The role of iron in learning and memory. Adv Nutr. 2011;2:112–121. doi: 10.3945/an.110.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavanagh DA, O’Mullane DM, Smeeton N. Variation of salivary flow rate in adolescents. Arch Oral Biol. 1998;43:347–352. doi: 10.1016/s0003-9969(98)00020-x. [DOI] [PubMed] [Google Scholar]
- 19.Prabhakar AR, Dodawad R, Raju OS,. Evaluation of flow rate, pH, buffering capacity, calcium, total proteins and total antioxidant capacity levels of saliva in caries free and caries active children: An in vivo study. Int J Clin Pediatr Dent. 2009;2:9–12. doi: 10.5005/jp-journals-10005-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasiru, W O, Taiwo, J O, Ibiyemi O. Salivary flow rate, buffering capacity and dental caries among 6-12 year old schoolchildren, age and gender in Nigeria: a comparative study. IOSR J Dent Med Sci. 2019;18(01):72–79. doi: 10.9790/0853-1801017279. [DOI] [Google Scholar]
- 21.Edgar WM, Highman SM, Manning RH. Saliva stimulation and caries prevention. Adv Dent Res. 1994;8(02):239–245. doi: 10.1177/08959374940080021701. [DOI] [PubMed] [Google Scholar]
- 22.Lenander-Lumikari M, Loimaranta V. Saliva and Dental Caries. Adv Dent Res. 2000;14:40–47. doi: 10.1177/08959374000140010601. [DOI] [PubMed] [Google Scholar]
- 23.Diaz de Guillory C, Schoolfield JD, Johnson D, et al. Co-relationships between glandular salivary flow rates and dental caries. Gerodontology. 2014;31(03):210–219. doi: 10.1111/ger.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur A, Kwatra K S, Kamboj P. Evaluation of non microbial salivary caries activity parameters and salivary biochemical indicators in predicting dental caries. J Indian Soc Pedod Prev Dent. 2013;30(03):212–217. doi: 10.4103/0970-4388.105013. [DOI] [PubMed] [Google Scholar]
- 25.Sakeenabi B, Hiremath SS. Dental Caries experience and salivary streptococcus mutans, lactobacilli scores, salivary flow rate and salivary buffer capacity among 6 years old Indian school children. J Clin Exp Dent. 2011;3(05):e412–e417. doi: 10.4103/2231-0762.97697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birkhed D, Heintze U. In: Human Saliva: Clinical Chemistry and Microbiology, Tenouvo J, editor. Boca Raton, FL: CRC Press; 1989. Salivary secretion rate, buffer capacity and pH. pp. 25–73. (Ed). Volume 1. [Google Scholar]
- 27.Russell JI, MacFarlane TW, Aitchison TC, et al. Caries prevalence and microbiological and salivary caries activity tests in Scottish adolescents. Community Dent Oral Epidemiol. 1990;18:120–125. doi: 10.1111/j.1600-0528.1990.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 28.Cunha-Cruz J, Scott J, Rothen M, et al. Northwest practice-based research collaborative in evidence-based dentistry. Salivary characteristics and dental caries: evidence from general dental practices. J Am Dent Assoc. 2013;144(05):e31–e40. doi: 10.14219/jada.archive.2013.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q, Bai J, Qin M. Relationship between cariogenic microbe, salivary buffer capacity and early childhood caries. Chinese J Stomatol. 2007;42:581, 58–4. 18215361 [PubMed] [Google Scholar]
- 30.Swerdlove CK. Relation between the incidence of dental caries and the pH of normal resting saliva. J Dent Res. 1942;21:73–81. doi: 10.1177/00220345420210011101. [DOI] [Google Scholar]
- 31.Malekipour MR, Messripour M, Shirani F. Buffering capacityof saliva in patients with active dental caries. Asian J Biochem. 2008;3:280–283. doi: 10.3923/ajb.2008.280.283. [DOI] [Google Scholar]
- 32.Lamberts BL, Pederson ED, Shklair IL. Salivary pH-rise activities in caries-free and caries-active naval recruits. Arch Oral Biol. 1983;28(07):605–608. doi: 10.1016/0003-9969(83)90008-0. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan A. Correlation between caries incidence and secretion rate/buffer capacity of stimulated whole saliva in 5-7-year-old children matched for lactobacillus count and gingival state. https://pubmed.ncbi.nlm.nih.gov/2255991/ Sweden Dent J. 1990;14(03):131, 13–5. [PubMed] [Google Scholar]
- 34.Tukia-kulmala H, Tenovuo J. Intra- and inter-individual variation in salivary flow rate, buffer effect, Lactobacilli, and mutans streptococci among 11 to 12-year old schoolchildren. Acta Odontol Scand. 1993;51(01):31–37. doi: 10.3109/00016359309041145. [DOI] [PubMed] [Google Scholar]
- 35.Tulunoglu O, Demirtas S, Tulunoglu I. Total antioxidant levels of saliva in children related to caries, age and gender. Int J Paediatr Dent. 2006;16(03):186–191. doi: 10.1111/j.1365-263X.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 36.Pandey P, Reddy NV, Rao VA, et al. Estimation of salivary flow rate, pH, buffer capacity, calcium, total protein content and total antioxidant capacity in relation to dental caries severity, age and gender. Contemp Clin Dent. 2015;6(Suppl 1):S65–S71. doi: 10.4103/0976-237X.152943. [DOI] [PMC free article] [PubMed] [Google Scholar]


