Highlights
-
•
Inorganic element based nanoformulations were prominent in the delivery drug leads.
-
•
Polymer and lipid based nanoformulations are emerging as novel formulations.
-
•
Majority of investigations on nanoherbal formulations were on in vitro models.
-
•
Proper glycemic control was an important property in nanoherbalformulations.
Keywords: herbal remedies, inorganic nanoformulations, lipid-based nanoformulations, polymeric nanoformulations, type 2 diabetes mellitus
Abstract
Background
Herbal remedies are used to manage type 2 diabetes mellitus (type 2 DM) as the sole treatment or as a complementary therapy. Limitations of herbal remedies, such as poor stability and limited absorption, impede their development as therapeutic agents, which could be overcome by nanoformulations.
Objectives
This review attempts to summarize the studies reported between 2009 and 2020 in the development of medicinal plant-based nanoformulations for the management of type 2 DM, discuss formulation methods, mechanisms of action, and identify gaps in the literature to conduct future research on nanoparticle-based herbal treatment options targeting type 2 DM.
Methods
To retrieve articles published between January 2009 and December 2020, the electronic databases PubMed, Science Direct, and Google Scholar were searched with the keywords nanoparticle, plant, and diabetes in the entire text. Peer-reviewed research articles on herbal nanoformulations published in English-language based on in vitro and/or in vivo models of type 2 DM and/or its complications were included. The literature search and selection of titles/abstracts were carried out independently by 2 authors. The list of full-text articles was selected considering inclusion and exclusion criteria, with the agreement of all the authors.
Results
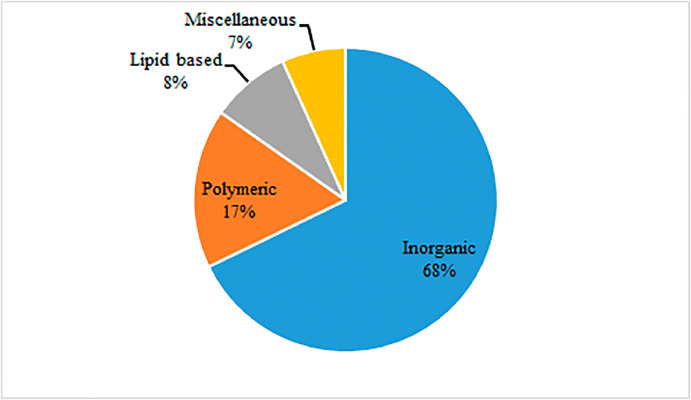
Among the reported studies, 68% of the studies were on inorganic herbal nanoformulations, whereas 17% and 8% were of polymer-based and lipid-based herbal nanoformulations, respectively. Some of the important biological properties of nanoformulations included improvement in glycemic control and insulin levels, inhibition of the formation of advanced glycation end products, and regeneration of pancreatic β cells. The aforementioned properties were observed by screening nanoformulations using in vitro cellular and noncellular models, as well as in vivo animal models of type 2 DM studied for acute or subacute durations. Only 2 clinical trials with patients with diabetes were reported, indicating the need for further research on medicinal plant-based nanoformulations as a therapeutic option for the management of type 2 DM.
Conclusions
Medicinal plant extracts and isolated compounds have been nanoformulated using various methods. The properties of the nanoformulations were found superior to those of the corresponding herbal extracts and isolated compounds. At both the preclinical and clinical levels, there are a number of poorly explored research areas in the development and bioactivity assessment of herbal nanoformulations. (Curr Ther Res Clin Exp. 2022; 83:XXX–XXX) © 2022 Elsevier HS Journals, Inc.
Introduction
The epidemic of type 2 diabetes mellitus (DM) is a major global health burden. Global estimates revealed that 463 million adults had type 2 DM in 2019.1 Approximately 90% of diabetes cases are type 2, with the highest proportion being reported in low- and middle-income countries.1 The prevalence of DM among the Sri Lankan adult population is reported to be 8.7%.1 DM is responsible for 9% of all deaths in Sri Lanka.2
Type 2 DM is characterized by insulin deficiency or insulin resistance or a combination of both.3 External factors (eg, high-calorie foods, lack of exercise, and obesity) and increased insulin requirements imposed by insulin resistance, glucotoxicity, and elevated free fatty acids may all contribute to β-cell deterioration in type 2 DM.4 Although the underlying mechanism is uncertain, hyperglycemia, and elevated free fatty acids are reported as factors promoting inflammation and oxidative stress in type 2 DM.5
Metformin is the first-line treatment for most patients with type 2 DM in allopathic medicine. Other oral hypoglycemic agents include sulfonylureas (eg, glibenclamide), glucagon-like peptide-1 agonists (eg, exenatide), sodium-glucose cotransporter-2 inhibitors (eg, dapagliflozin), dipeptidyl peptidase-4 inhibitors (eg, sitagliptin), thiazolidinediones (eg, pioglitazone), α-glucosidase inhibitors (eg, acarbose), and in some cases parenteral insulin is also used for the management of type 2 DM.6 Although a number of allopathic medicines have been developed and are in use for the treatment of type 2 DM, they are primarily aimed at glycemic control. None of these medications has been proven to provide a complete cure. Even with careful use, some of these medications may cause undesirable side effects such as gastrointestinal discomfort, hypoglycemia, and lactic acidosis. As a result, herbal medicines with few side effects have attracted the interest of researchers working on therapeutic options for type 2 DM.7
Plant extracts, in particular, have been shown to be capable of targeting the underlying pathophysiology of diseases and to have multiple mechanisms of action due to the synergistic effects produced by combinations of phytochemicals present. The multitargeting potentials of herbal medicines are beneficial in the treatment of multifactorial chronic diseases such as type 2 DM.8,9
Lack of standardization, poor stability, unpleasant taste, and reduced absorption leading to low bioavailability have been identified as major limitations in the formulation of medicines directly from medicinal plants.10 Recent advances in nanotechnology are useful in delivering plant-derived bioactive compounds, overcoming the aforementioned limitations.11,12 There are few reviews published on recent investigations on herbal nanoformulations for the management of type 2 DM; however, there is lack of emphasis on different nanoformulation design methods. This review attempts to summarize the studies reported between 2009 and 2020 in the development of medicinal plant-based nanoformulations for the treatment of type 2 DM, discuss formulation design, mechanisms of action, and to identify the needs and gaps in literature as an aid to conduct future research on nanoparticle-based herbal treatment options targeting type 2 DM.
Methods
The online electronic databases PubMed, Science Direct, and Google Scholar were searched with the keywords nanoparticle, plant, and diabetes in the text. Data were collected from January 2009 to December 2020 from peer-reviewed research articles in English-language academic journals. The references of the retrieved articles were also screened for relevant articles. Studies on herbal nanoformulations using in vitro and/or in vivo models of type 2 DM and/or its complications, as well as clinical trials based on efficacy testing of nanoformulations in patients with DM, were selected. In vitro test models often provide useful information about the mechanism(s) of action(s) of novel formulations, which would be useful for future research. In vitro models are frequently employed as preliminary screening tools to investigate the anti-DM activity and toxicity of new nanoformulations. Taking this into consideration, more emphasis was placed in this review on in vitro studies. Studies on conventional herbal formulations and nanoformulations of commercial oral hypoglycemic agents were excluded. The literature search and selection of titles/abstracts were carried out independently by 2 authors. The list of full-text articles was selected considering inclusion and exclusion criteria, with the agreement of all the authors. As a result, 110 hits were obtained from the PubMed database. A total of 950 hits were obtained in the Science Direct database under the category of research articles, and 17,500 hits were obtained through Google Scholar. The extensive amount of literature from the latter 2 databases was then sorted by relevance in descending order, and the first 100 hits from each database, namely Science Direct and Google Scholar, were selected to reduce grey literature and retrieve the most relevant articles. Accordingly, a total of 310 articles were collected from all 3 databases and after removing 15 articles that were found as duplicates, 295 articles were selected for further screening. After carefully reviewing the titles and/or abstracts of that remaining 295 articles, 50 were excluded as being reviews, 186 were excluded for being irrelevant to the scope of the current review, and 2 full-text articles were excluded for not being from peer-reviewed journals. The remaining 57 full-text articles were selected for the present review. Another 7 full-text articles were obtained by searching the references of each of the 57 full-text articles, with the key word nano in the title of the references and excluding irrelevant references. For the review, a total of 64 full-text articles were examined (see the Supplemental Figure in the online version at doi:XXXXX). The study design is shown in Figure 1.
Figure 1.
Study design.
Results
Based on the type of nanomaterial used in the formulation, review of 64 full-text articles resulted in the identification of 3 major categories of herbal nanoformulations targeting type 2 DM. These include polymer-based nanoformulations, inorganic nanoformulations, and lipid-based nanoformulations.
The biological activities of nanoformulations were screened using in vitro cellular and noncellular models, as well as in vivo models. The rat epithelial cell line LC-540 induced with hydrogen peroxide, mouse-derived pancreatic β-cells, insulin-resistance model synthesized using the human hepatoma cell line HepG2, and the rat insulinoma cell line INS-1 have all been used as cellular models to evaluate the anti-DM activities and toxicity of nanoformulations. Commonly used noncellular in vitro tests include α-amylase, α-glucosidase, and DPP-4 inhibitory assays. In vivo studies were mostly conducted on rat/mouse models induced with type 2 DM. Only 2 clinical trials on herbal nanoformulations against type 2 DM were identified, and the details are reported in the section on Clinical Studies on Herbal Nanoformulations against Type 2 DM.
Polymer-based nanoformulations
Polymer-based nanoparticles with diameters ranging from 10 to 1000 nm are ideal for delivering plant-derived natural products.13 These nanoparticles are synthesized using biodegradable and biocompatible polymers and can be used to control the release and delivery of bioactive compounds. Natural polymers (eg, alginate and chitosan) as well as synthetic polymers (eg, polyvinyl alcohol, poly[L-lactic acid], polyethylene glycol [PEG], and poly [lactic-co-glycolic acid]), are widely used for the synthesis of polymer based nanoparticles.13
Polymer-based nanoformulations can be either nanocapsules or nanospheres depending on the structural differences of the polymer used (Figure 2).
Figure 2.
Basic structure of (A) nanocapsules and (B) nanospheres.
Nanocapsules contain a lipid core surrounded by a polymeric membrane. The bioactive compounds can either be adsorbed to the polymeric membrane or dissolved in the lipid core. Nanospheres are only composed of a polymeric structure in which bioactive compounds are retained or adsorbed.14
Natural Polymer-Based Nanoformulations
Chitosan-based nanoformulations were found to be the most abundant polymer-based nanoformulations studied for the management of type 2 DM. The ionotropic gelation method, in which the chitosan solution (positively charged) is dissolved in acetic acid or any polyanionic solution (negatively charged) with or without a stabilizing agent, is the most commonly used method for the synthesis of chitosan nanoparticles.15 Ethanol (70%) extract of the whole plant of Echinacea purpurea (L) Moench (Family: Asteraceae) was nanoencapsulated using a mixture of chitosan and silica. The nanoparticles were synthesized by mixing the chitosan solution and the silicate solution (10:1) and then adding plant extract to that mixture (11:1). According to this study, nanoparticle showed an mean (SD) size of 218 (42) nm, with an encapsulation efficiency of 66.9%.16 The antioxidant activity and toxicity of this nanoformulation were investigated in vitro using LC-540 cells. Treatment of hydrogen peroxide-induced LC-540 cells with nanoformulation showed a concentration-dependent reduction (highest at 25 µg/mL) in cellular nitric oxide production, thus reducing oxidative stress. The toxicity of the nanoformulation was determined using 3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyl tetrazolium bromide (MTT) assay. Cell viability increased significantly to 70% when treated with nanoformulation at a concentration of 25 µg/mL.16 This nanoformulation was further investigated for its in vivo anti-DM activity in Sprague-Dawley rats induced with type 2 DM, and the details are summarized in Table 1.
Table 1.
Plant extracts encapsulated nanoformulations investigated for antidiabetic activity and toxicity in vivo using animal models.
| Plant | Plant extract | Plant family | Nanoformulation | Properties of nanoparticles(size, shape, zeta potential) | Animal model | in vivo bioactivities/toxicities | Reference |
|---|---|---|---|---|---|---|---|
| Cassia fistula L | Aqueous extract of stem bark | Leguminosae | Gold NPs | 55.2–98.4 nm, rectangular and triangular | Wistar rats with type 2 DM induced by intraperitoneal injection of STZ (60 mg/kg) | ↓Fasting blood glucose, HbA1c, renal markers (serum urea, creatinine, uric acid) Improvement in lipid profile |
36 |
| Chamaecostus cuspidatus (Nees & Mart) CD Specht & DW Stev | Aqueous extract of leaves | Costaceae | Gold NPs | 50 nm, spherical | Wistar rats with type 2 DM induced by intraperitoneal injection of nicotinamide (110 mg/kg) followed by STZ (45 mg/kg) | ↓Plasma glucose, TC | 37 |
| Cinnamomum cassia (L) J Presl | Aqueous extract of whole plant | Lauraceae | Silver NPs | NM | Sprague-Dawley rats with type 2 DM induced by intraperitoneal injection of nicotinamide (100 mg/kg) followed by STZ (60 mg/kg) | ↓Serum urea, creatinine, fasting blood glucose, glutathione, MDA, ALT (EC 2.6.1.2), AST (EC 2.6.1.1) Regeneration of glomerular network and ↓interstitial space with infiltrations indicating beneficial in diabetes-induced kidney damage |
38 |
| Citrullus colocynthis (L) Schrad, Momordica balsamina L, and Momordica dioica Roxb ex Willd | Methanolic extract of fruits | Cucurbitaceae | Nanoliposome | 450 nm, −22.7 mV | Swiss albino rats with type 2 DM induced by intraperitoneal administration of nicotinamide (120 mg/kg) followed by STZ (60 mg/kg) | ↓Fasting blood glucose | 39 |
| Couroupita guianensis Aubl | Aqueous extract of leaves | Lecythidaceae | Gold NPs | 47 nm, spherical, −36.3 mV | Wistar rats with type 2 DM induced by intraperitoneal injection of STZ (60 mg/kg) | ↓Blood glucose, serum creatinine, urea, bilirubin, AST, ALT ↑Lipid peroxidation, SOD (EC 1.15.1.1), glutathione peroxidase, and CAT (EC 1.11.1.6) Improvement of lipid profile ↓Histological injury in the hepatic, renal, and pancreatic tissues |
40 |
| Echinacea purpurea (L) Moench | 70% v/v ethanol extract of whole plant | Asteraceae | Chitosan NPs | 218 (42) nm* | Sprague-Dawley rats with type 2 DM induced by feeding HFD (40% calories from fat) followed by STZ (33 mg/kg) intraperitoneal injection | ↓Blood glucose, insulin resistance, plasma FGF-21 resistance, reactive oxygen species level Improvement of testis tissue structure, sperm quality and DNA integrity indicating beneficial against diabetes induced male infertility |
16 |
| Elettaria cardamomum (L) Maton | 1,8-cineole-rich supercritical carbon dioxide extract of seeds | Zingiberaceae | PEGylated nanoliposomal formulation | NM | Wistar albino rats with type 2 DM | ↓Fasting blood glucose ↑Insulin sensitivity Improvement of lipid profile |
41 |
| Eysenhardtia polystachy (A Gray) S Watson | 50% v/v methanol extract of bark | Fabaceae | Silver NPs | 10–12 nm, spherical, −32.25 mV | Zebra fish with DM induced by immersion of fish in 111 mmol/L glucose | ↓Blood glucose, serum TC and TG ↑Insulin secretion Protection of β-cells from oxidative stress |
29 |
| Gymnema sylvestre (Retz) Schult | Gymnemic acids rich fraction extracted from leaves | Asclepiadaceae | Silver NPs | 21.5 nm, spherical | Wistar rats with type 2 DM induced by STZ (40 mg/kg) intraperitoneal injection | ↓Blood glucose ↑Insulin Improvement of lipid profile |
42 |
| Hibiscus subdariffa Rottb | Aqueous extract of leaves | Malvaceae | ZnO NPs | 16–60 nm, spherical | Swiss albino mice with type 2 DM induced by intraperitoneal injection of STZ (100 mg/kg) followed by feeding high sucrose- HFD | ↓Blood glucose, IL-4, IL-10 ↑mRNA expression and inflammatory markers (TNF-α, IL-1β, IL-6) Down-regulated gene expressions of GLUT-2 and glucokinase |
43 |
| Lawsonia inermis L | Aqueous extract of leaves | Lythraceae | Silver NPs and cerium oxide NPs | 50 nm, spherical | Healthy Swiss albino mice Wistar rats with type 2 DM induced by intraperitoneal injection of STZ (40 mg/kg) |
Nontoxic ↓Blood glucose ↑Insulin Improvement of lipid profile Regeneration of pancreatic islets |
44 |
| Momordica charantia L | Aqueous extract of fruits | Cucurbitaceae | ZnO, cerium oxide and silver NPs | 22.5–55.8 nm, spherical | Healthy Swiss albino mice Wistar rats with type 2 DM induced by intraperitoneal injection of STZ (40 mg/kg) |
Nontoxic ↓Fasting blood glucose ↑Insulin β-cell regeneration |
45 |
| Morus indica L | Aqueous extract of leaves | Moraceae | ZnO NPs | 6–12 nm | Wistar rats with type 2 DM induced with STZ (40 mg/kg) intraperitoneal injection | ↓Liver and renal enzymes Regeneration of liver cells and reappearance of damaged glomeruli and tubules indicated beneficial in diabetic nephropathy |
34 |
| Morus spp | Aqueous extract of leaves | Moraceae | Silver NPs | 35 nm, −15 mV | Albino rats with DM induced by intraperitoneal injection of STZ (60 mg/kg) | ↓Fasting blood glucose Amelioration of retinal damage indicating beneficial in diabetes retinopathy |
46, 47 |
| Musa paradisiaca L | Aqueous extract of stem | Musaceae | Silver NPs | 30–60 nm, spherical | Sprague-Dawley rats with type 2 DM induced with nicotinamide (110 mg/kg) followed by STZ (60 mg/kg) intraperitoneal injection | ↓Blood glucose, HbA1c ↑Insulin, glycogen |
48 |
| Plicosepalus acaciae (Zucc) Wiens & Polhill and Plicosepalus curviflorus (Benth ex Oliv) Tiegh | Methanolic extract of whole plant | Loranthaceae | SLN (Compritol 888 ATO as solid lipid and sodium dodecyl sulfate as surfactant) |
Size: 22–70 nm, spherical | Wistar rats with type 2 DM induced by HFD (58% calories as fat) followed by ip injection of STZ (35 mg/kg) | ↓Blood glucose, HbA1c, insulin resistance, MDA ↑Pancreatic GSH, SOD, CAT |
49 |
| Pouteria sapota (Jacq) HE Moore & Stearn | Aqueous extract of leaves | Sapotaceae | Silver NPs | 20–110 nm | Wistar albino rats with type 2 DM induced by intraperitoneal injection of STZ (50 mg/kg) | ↓Blood glucose, liver function markers (AST, ALT, ALP (EC 3.I.3.1)), renal markers (serum creatinine, serum urea) ↑Insulin, SOD, CAT activities |
32 |
| Psoralea corylifolia L | Aqueous extract of seeds | Fabaceae | Silver NPs | 18 nm, circular | Healthy albino mice | Nontoxic | 50 |
| Punica granatum L | Methanolic extract of fruit peel | Punicaceae | Gold NPs | 20 nm, spherical | BALB/c mice with diabetes nephropathy induced by intraperitoneal injection of STZ (200 mg/kg) | Inhibit protein glycation Induce scavenging of reactive oxygen species ↓Inflammation in nephritic tissues, renal fibrosis |
51 |
| Sambucus nigra L | Aqueous extract of fruit | Adoxaceae | Gold NPs | 21.3 nm, spherical, −22.6 mV | Wistar rats with type 2 DM induced by intramuscular injection of STZ (30 mg/kg) | ↓MDA, GSH to GSSG ratio in muscle, liver, and blood, COX-2, ProMMP-2 | 52 |
| Smilax glabra Roxb | Aqueous extract of rhizomes | Smilacaceae | Gold NPs | 50−90 nm, hollow | Wistar rats with type 2 DM induced by HFD-STZ | ↓Body weight, glucose levels, inflammatory markers such as leptin Improvement in lipid profile |
53 |
| Stevia rebaudiana (Bertoni) Bertoni | 70% v/v methanol extract of leaves | Asteraceae | Chitosan NPs | < 73.27 nm, spherical | Healthy Wistar rats Wistar rats with type 2 DM induced by intraperitoneal injection of STZ (55 mg/kg) |
Non toxic ↓Fasting blood glucose, HbA1c ↑SOD, CAT, GSH Regeneration of pancreatic islet cells |
54 |
| Syzygium cumini (L) Skeels | Aqueous extract of seeds | Myrtaceae | Poly-ƹ-caprolactone NPs | NM | Wistar rats induced with type 2 DM by ip. injection of STZ (60 mg/kg) followed by infected with Candida albicans by intraperitoneal injection of C albicans suspension (105 CFU/mL) | ↓Serum glucose, cholesterol, and creatinine ↓Protein oxidation, lipid peroxidation indicating potential to treat DM-related fungal infections |
55 |
| Zingiber officinale Roscoe | Ethanolic extract of rhizome | Zingiberaceae | Silver NPs | 123.8 (0.16) nm*, spherical, –20.2 mV | Wistar albino rats with type 2 DM induced by intraperitoneal injection of STZ (150 mg/kg) | ↓Blood glucose | 56 |
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CAT = catalase; CFU = colony forming unit; COX-2 = cyclooxygenase-2; DM = diabetes mellitus; DNA = deoxyribonucleic acid; FGF-21 = fibroblast growth factor-21; GLUT-2 = glucose transporter-2; GSH = reduced glutathione; GSSG = oxidized glutathione; HbA1c = glycated hemoglobin; HFD = high fat diet; IL = interleukin; MDA = malondialdehyde; mRNA = messenger ribonucleic acid; ProMMP; NM = not mentioned; NPs = nanoparticles; PEG = polyethylene glycol; ProMMP-2 = promatrix metalloproteinase-2; SOD = superoxide dismutase; SLN = solid lipid nanoparticles; STZ = streptozotocin; TC = total cholesterol; TG = triglycerides; TNF-α = tumor necrosis factor α.
Value is presented as mean (SD).
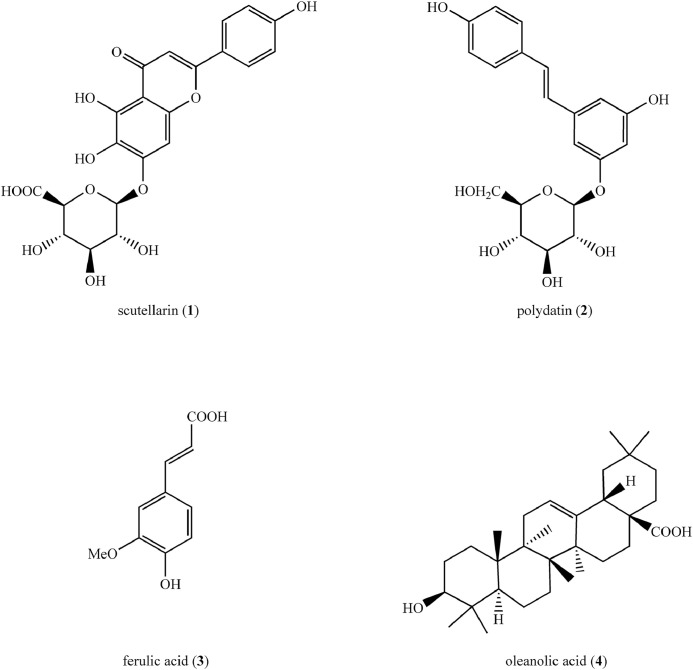
Scutellarin (1) (Figure 3), a flavone isolated from Erigeron breviscapus (Vaniot) Hand-Mazz (Family: Asteraceae) was encapsulated into deoxycholic acid/chitosan/vitamin B-12 nanoparticles.17 Nanoparticles were synthesized using deoxycholic acid-modified chitosan (to improve water solubility) and vitamin B-12 (to target the small intestine). The scutellarin-loaded nanoparticles were spherical, measuring 150 to 250 nm in diameter, with a mean (SD) zeta potential of −16.5 (3.1) mV. In vitro intracellular uptake of scutellarin and scutellarin-loaded nanoparticles were evaluated against Caco-2 monolayer of the human epithelial cell line. In the cell count kit-8 assay, scutellarin-loaded nanoparticles showed a 5-fold increase in intracellular uptake when compared with scutellerin at 250 µg/mL, as well as low-toxicity to Caco-2 cells (ie, cell viability of 85.02% [4.34%]).17 This nanoformulation was further tested in vivo using healthy Sprague-Dawley rats to assess its pharmacokinetics. In brief, the plasma concentration of scutellarin was estimated up to 24 hours upon oral administration of a single dose of (40 mg/kg) free scutellarin and scutellarin encapsulated nanoparticles. A significant (P < 0.05) increase in the total area under the curve of scuttelarin was observed upon administration of nanoparticles (22.096 [2.064] ng/h/mL) compared with that of free scutellarin (6.419 [1.051] ng/h/mL) indicating improvement of bioavailability of scutellarin due to nanoencapsulation. Furthermore, the in vivo bioactivity of nanoparticles was investigated using Sprague-Dawley rats with type 2 DM induced by feeding a high-fat diet (30% calories from fat) followed by streptozotocin (33 mg/kg) intraperitoneal injection. A significant (P < 0.05) reduction of retinal resistivity index, increase in blood flow velocity, and improvement in retinal architecture were observed in diabetic rats treated with nanoparticles indicating positive effects against type 2 DM-induced retinopathy. In addition, the in vivo acute toxicity studies conducted using zebrafish embryo showed that the nanoparticles are nontoxic up to a concentration of 250 µg/mL.17 The results of in vivo studies conducted using Sprague-Dawley rats and zebrafish embryo are summarized in Table 2.
Figure 3.
Structures of compounds (1–4) isolated from medicinal plants used in synthesizing natural polymer-based nanoformulations as potential therapeutics for the treatment of type 2 diabetes mellitus.
Table 2.
Nanoformulations encapsulated with phytochemical compounds investigated for antidiabetic activity and toxicity in vivo using animal models.
| Phytochemical compound | Nanoformulation | Properties of nanoparticles(size, shape, zeta potential) | Animal model | in vivo bioactivities/toxicities | Reference |
|---|---|---|---|---|---|
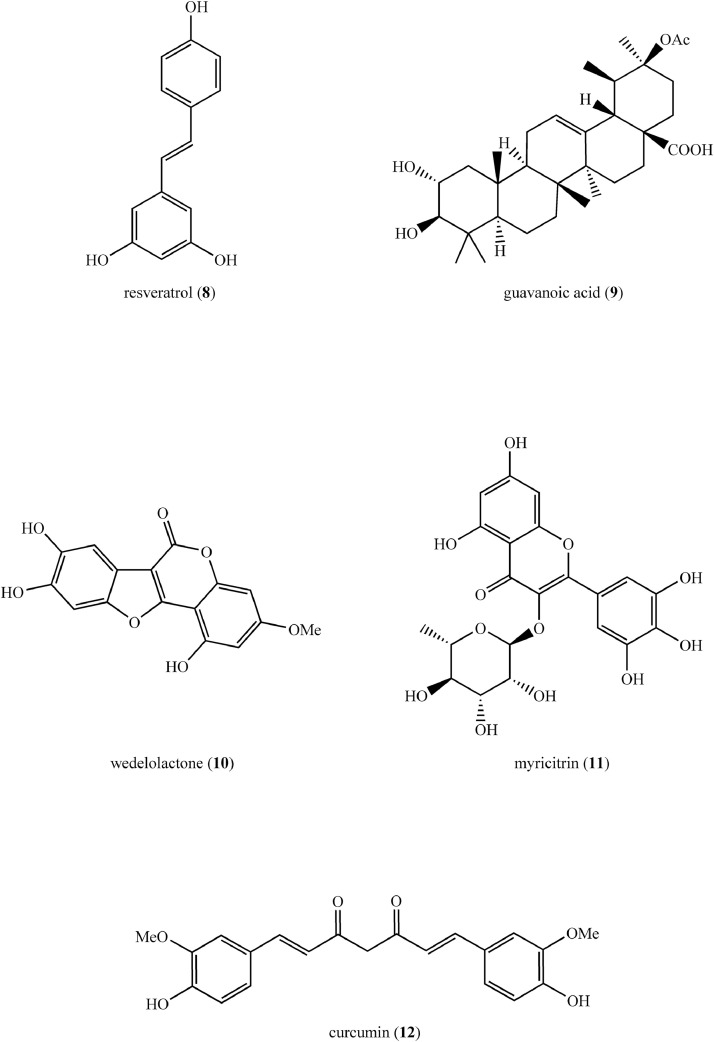
| Curcumin (12) (Figure 5) | Curcumin in sodium bicarbonate buffer | 200 nm | Albino rats with DM induced by intraperitoneal injection of STZ (60 mg/kg) | ↓TG, myocardial enzymes [CK-MB (EC 2.7.3.2), LDH (EC 1.1.1.27), AST]. Improvement of myocardial cell morphology Regulation of oxidative markers in heart tissue indicating beneficial in treating diabetes cardiomyopathy |
57 |
| Ferulic acid (3) (Figure 3) | Chitosan-tripolyphosphate NPs | 119.5 (3.9) nm†, −14.2 (0.5) mV† | Healthy Wistar albino rats Wistar albino rats with type 2 DM induced by intraperitoneal injection of STZ (50 mg/kg) |
Non toxic Delayed release ↑Bioavailability ↓Fasting blood glucose Improved lipid profile |
58 |
| Myricitrin (11) (Figure 5) | NLC | 76.1 nm, −5.51 mV | NMRI mice with type 2 DM induced by intraperitoneal nicotinamide (120 mg/kg) | ↓Plasma glucose ↑Plasma insulin, muscle glut-4 gene expression, CAT, pancreatic islet diameter |
80 |
| Oleanolic acid (4) (Figure 3) | Polygalacturonic acid NPs | 200 nm, −44.7 mV | Wistar rats with type 2 DM induced by HF and high sucrose diet followed by ip injection of STZ (35 mg/kg) | ↓Fasting blood glucose ↑Intestinal absorption Improvement in lipid profile |
20 |
| Polydatin (2) (Figure 3) | Chitosan NPs | 144.25 (3.37) nm†, spherical, +17.9 (3.99) mV† | Wistar albino rats with type 2 DM induced by intraperitoneal injection of nicotinamide (110 mg/kg) followed by STZ (50 mg/kg) | ↓Fasting serum glucose, glycogen ↑Fasting serum insulin |
19 |
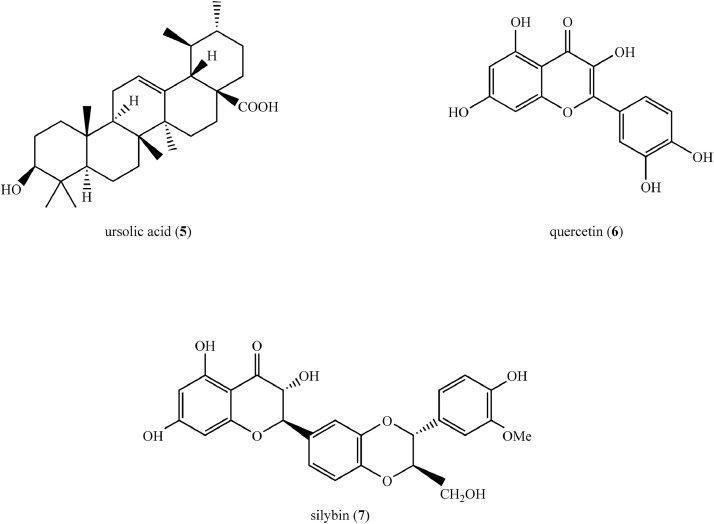
| Quercetin (6) (Figure 4) | PEG-b-(PELG-g-PZLL) NPs | 32 nm | Healthy Sprague Dawley rats Sprague Dawley rats with type 2 DM induced by HFD (40% calories from fat) followed by intraperitoneal injection of STZ (35 mg/kg) |
Improved circulation time Sustained release ↓Fasting blood glucose, MDA, blood urea nitrogen, serum creatinine and urine protein, ICAM-1 expression ↑SOD Improvement of lipid profile ↓Renal histopathological abnormalities indicating beneficial in treating Diabetes nephropathy |
21 |
| Resveratrol (8) (Figure 5) | Gold NPs | 10 nm, spherical | Wistar rats with type 2 DM induced by STZ (60 mg/kg) intravenous injection | ↓Permeability of blood–retinal barrier, retinal vessels, VEGF-1, retinal mRNA expressions of VEGF-1, TNF-α, MCP-1, ICAM-1, IL-6, IL-1β, phosphorylation of NF-κB p65, ERK1/2 ↑Retinal expression of renal PEDF indicating beneficial in treating diabetes retinopathy |
59 |
| Scutellarin (1) (Figure 3) | Chitosan-deoxycholic acid-vitamin B12 NPs | 150 to 250 nm, spherical, −16.5 (3.1) mV† | Zebra fish embryo Healthy Sprague–Dawley rats Sprague-Dawley rats with type 2 DM induced by feeding HFD (30% calories from fat) followed by STZ (33 mg/kg) intraperitoneal injection |
Non toxic ↑Bioavailability Down-regulation in the central retinal artery resistivity index and the expression of angiogenesis proteins of retinas indicating the potential for treating diabetic retinopathy |
17 |
| Silybin (7) (Figure 4) | Chitosan-PLGA NPs | 184.6 nm, spherical, +21 mV | Wistar rats with type 2 DM induced by intraperitoneal injection of STZ (50 mg/kg) | ↓Blood glucose, TC, TG, fructosamine, HbA1c, liver function parameters (ALT, AST, ALP), MDA ↑Insulin, liver glycogen, SOD, CAT activities β-cell regeneration in pancreas |
22 |
| Ursolic acid (5) (Figure 4) | Polyvinyl alcohol NPs | 246.4 nm, −31.2 (5.17) mV† | Wistar albino rats with type 2 DM induced by intraperitoneal injection of STZ (60 mg/kg) | ↑Blood glucose Improvement of lipid profile Antioxidant effects |
60 |
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CAT = catalase; CK-MB = creatine kinase-MB; DM = diabetes mellitus; ERK1/2 = extracellular signal-regulated kinase; HbA1c = glycated haemoglobin; HFD = high fat diet; ICAM-1 = intercellular adhesion molecule-1; IL = interleukin; LDH = lactate dehydrogenase; MCP-1 = monocyte chemotactic proteins-1; MDA = malondialdehyde; mRNA, Messenger ribonucleic acid; NF-κB p65 = nuclear factor κB p65; NLC = nanostructured lipid carriers; NPs = nanoparticles; PEDF = pigment epithelium-derived factor; PEG-b-(PELG-g-PZLL) = polyethylene glycol-b-[poly(ethylenediamine L-glutamate)-g-poly(ε-benzyoxycarbonyl-L-lysine)]; PLGA = poly (lactic-co-glycolic acid); SOD = superoxide dismutase; STZ = streptozotocin; TC = total cholesterol; TG = triglycerides; TNF-α =tumor necrosis factor-α; VEGF-1 =vascular endothelial growth factor.
*BOLDFACE NUMBER IN PARENS EXPLANATION.
Value is presented as mean (SD).
Withanolides, a group of natural C-28 steroids extracted from aqueous extracts of berries of Withania coagulans (Stock) Dunal (Family: Solanaceae) was nanoformulated by entrapping in chitosan nanoparticles followed by coating with starch. Chitosan nanoparticles were synthesized by electrospraying of a chitosan solution directly into a solution consisting sodium tripolyphosphate (cross-linking agent) and polysorbate 80. These nanoparticles were mixed in a 1:1 ratio with a starch solution containing sodium trimetaphosphate (a cross-linking agent) to form a starch coat. The aqueous extract was added to the nanoparticle mixture before electrospraying to encapsulate withanolides. When compared with uncoated nanoparticles, enteric starch coating delayed the release of extract in the stomach by 2.5 times (ie, release of extract from uncoated nanoparticles was 38%, whereas the release of starch coated nanoparticles was 16%, within 2 hours). The bioactivity of the encapsulated extract was then tested in vitro in mouse-derived pancreatic β-cells and found to be nontoxic to cells while also promoting insulin secretion at a concentration of 1 µmol/L.18
Polydatin (2) (Figure 3), a glycoside isolated from the roots and rhizomes of Polygonum cuspidatum Siebold & Zucc (Family: Polygonaceae) was nanoformulated using chitosan by a modified ionotropic gelation method. Spherical particles with an average size of 144.25 (3.37) nm, a zeta potential of +17.9 (3.99) mV, and entrapment efficiency of 96.74% (0.39%) were obtained. Because of the high zeta potential, the nanoparticles were coated with polysorbate 80 to prevent aggregation. The in vitro release profile demonstrated a sustained release pattern, with the percentage of release remaining below 20% after 12 hours at pH 6.8. In Vero cell lines (kidney epithelial cells isolated from the African green monkey Cercopithecus aethiops), the nanoformulation was found to be nontoxic and showed around 95% cell viability in the range 10 to100 µg/mL.19 This nanoformulation was then screened in vivo for anti-DM activity in Wistar albino rats with type 2 DM, and the results are summarized in Table 2.
Polygalacturonic acid, a natural polysaccharide, was used in the nanoencapsulation of oleanolic acid (4) (Figure 3), a natural triterpenoid, extracted from Ligustrum lucidum W.T. Aiton (Family: Oleaceae). Nanoparticles with an average diameter of 200 nm, a zeta potential of − 44.7 mV, and encapsulation efficiency of 76.59% (4%) were obtained. The MTT assay revealed that the nanoformulation was nontoxic in the range 10 to 150 µg/mL. The nanoformulation was then screened in an insulin resistance model prepared using HepG2 cells exposed to high concentrations (10–7 mol/L) of insulin treatment. An endocytosis assay confirmed the uptake of nanoparticles by insulin-resistant cells. The degraded morphology of insulin-resistant cells returned to normal after treatment with the nanoformulation. Furthermore, the expression of tyrosine phosphatase 1B decreased significantly, whereas the expression of protein kinase B and insulin receptor substrate-1 increased significantly. Changes in the expression of the above insulin-resistant related proteins suggested that the insulin-resistant reversal effect induced by the nanoformulation is mediated by promoting the insulin receptor substrate-1/phosphatidylinositol-3 kinase/protein kinase B signaling pathway while inhibiting the activity of the tyrosine phosphatase 1B enzyme simultaneously to increase glucose uptake.20 This nanoformulation was tested for anti-DM activity in vivo using Wistar rats with type 2 DM, and the results are summarized in Table 2.
Synthetic polymer-based nanoformulations
Quercetin (6) (Figure 4), a plant flavonoid, was encapsulated in PEG-b-(poly[ethylenediamine L-glutamate][PELG]-g-poly[ε- benzyoxycarbonyl-L-lysine] [PZLL]), a linear-brush copolymer. This copolymer was synthesized using a series of chemical reactions. In summary, ring-opening polymerization of γ-benzyl L-glutamate-N-carboxyanhydride with PEG amine as the macroinitiator, led to the formation of PEG-b-poly(γ-benzyl L-glutamate). Aminolysis of PEG-b-poly(γ-benzyl L-glutamate) with ethanediamine yielded PEG-b-PELG. PEG-b-PELG was used as the macroinitiator to initiate the ring-opening polymerization of ε-benzyoxycarbonyl-L-lysine-N-carboxyanhydride to synthesize PEG-b-(PELG-g-PZLL). PEG and PELG were linear in this copolymer, with PELG acting as a brush backbone and PZLL acting as brush side chains. The encapsulation efficiency of quercetin was 53.2%. The average diameter of the PEG-b-(PELG-g-PZLL) copolymer increased from 9 to 32 nm upon encapsulation of quercetin. In vitro release studies revealed a biphasic release profile of encapsulated quercetin, with an initial fast release followed by a sustained and steady release phase. PEG-b-(PELG-g-PZLL) at 200 µg/mL on HeLa cells showed low cell toxicity (ie, cell viability >75%).21 The pharmacokinetics and anti-DM properties of this nanoformulation were assessed in vivo using Sprague-Dawley rats, and the results are summarized in Table 2.
Figure 4.
Structures of compounds (5–7) isolated from medicinal plants used to synthesize synthetic polymer-based nanoformulations as potential therapeutics for the treatment of type 2 diabetes mellitus.
Silybin (7) (Figure 4), a natural flavonoid, was encapsulated using chitosan and poly (lactic-co-glycolic acid) (PLGA) using a solvent diffusion technique. Briefly, silybin and PLGA were dissolved in acetone and the resulting solution was added to a pluronic F-127 (a nonionic surfactant) solution. Followed by evaporation of acetone, a chitosan solution was added to the silybin encapsulated PLGA nanoparticles to produce the final chitosan-embedded silybin encapsulated PLGA nanoparticles via polyelectrolyte deposition of chitosan. Spherical nanoparticles with an average particle size of 184.6 nm, a zeta potential of +21 mV, and encapsulation efficiency of 92.11% were obtained. The in vitro release of silybin from nanoparticles was investigated using dialysis method and the pattern of release was biphasic.22 The anti-DM activity of this nanoformulation was subsequently assessed in vivo using Wistar rats with type 2 DM and the results are presented in Table 2.
Inorganic nanoformulations
Plant based natural products particularly crude extracts and fractions can be delivered using inorganic nanoparticles which are highly stable, hydrophilic, and biocompatible. They allow controlled drug release while also protecting against gastric acid and enzyme degradation. Inorganic nanoparticles such as gold, silver, and zinc oxide stabilized with surfactants and entrapped with hydrophilic bioactive compounds are used in the form of aqueous colloid solutions.23
Gold nanoparticles synthesized using a methanol extract of the whole plant of Eclipta alba (L) Hassk (Family: Asteraceae) were investigated using the RIN-5F pancreatic β cell line treated with STZ (10 mM) for 24 hours. The nanoparticles were synthesized by mixing an aqueous solution of plant extract with a solution of auric chloride. The resulting nanoparticles were spherical in shape, with a diameter of 26 nm and zeta potential of −12 mV. The MTT assay revealed that when STZ-induced RIN-5F cells were treated with nanoparticles at doses of 15, 30, and 60 µg/mL for 24 hours, their viability increased, indicating the effectiveness of nanoparticles against STZ induced cytotoxicity. The 2,2,1-diphenyl-1-picrylhydrazyl (DPPH) assay was used to measure the superoxide radical scavenging activity and the hydroxyl radical scavenging activity of nanoparticles, and gold nanoparticles exhibited a concentration-dependent increase in antioxidant activity. Apoptotic staining followed by flow cytometry of treated cells revealed gold nanoparticles’ antiapoptotic potential. The antiapoptotic pathway was evaluated by Western blot analysis using specific primary antibodies against Bcl-2-associated X protein, B-cell lymphoma-2, nuclear factor-κB, and β-actin. Down-regulation of Bcl-2, up-regulation of Bax, and modulation of nuclear factor-κB was observed as the anti-apoptotic role of nanoparticles.24
An aqueous ethanol (50% v/v) extract of the aerial parts of Leucosidea sericea Eckl & Zeyh (LSTE) (Family: Rosaceae) and its procyanidin fractions of dimers and trimers designated as F1 and F2, with F2 being the most polar fraction, were used to synthesize gold nanoparticles. Nanoparticles with diameters of 6, 24, and 21 nm and zeta potentials of −33.59, −32.5, and −29.1 mV were obtained for LSTE, F1, and F2, respectively. F1-coated nanoparticles showed the highest α-amylase inhibitory activity with an half maximal inhibitory concentration of 1.88 µg/mL. F2 nanoparticles showed the strongest α-glucosidase activity at 4.5 µg/mL. F1 and F2 nanoparticles also showed the highest antioxidant activity in ferric reducing antioxidant power assay and 2′-azino-bis-3-ethylbenzotiazolin-6-sulfonic acid assay.25
Guavanoic acid (9) (Figure 5), a triterpenoid isolated from the leaves of Psidium guajava L (Family: Myrtaceae) was formulated as gold nanoparticles. They showed in vitro anti-DM activities in terms of protein tyrosine phosphatase 1B, α-amylase, and α-glucosidase enzyme inhibition. In addition, guavanoic acid-functionalized gold nanoparticles exhibited anti-DM potential by improving insulin-dependent glucose uptake in L6 rat skeletal muscle cells. Cytotoxicity studies revealed that at 265 (0.01) µg/mL, these nanoparticles inhibited the growth of cells by 50%. Lactate dehydrogenase enzyme release assay was performed in differentiated L6 myoblasts treated at 1 to 100 µg/mL gold nanoparticles, and cell viability of 75% was observed at 100 µg/m.26
Figure 5.
Structures of compounds (8–12) isolated from medicinal plants used to synthesize inorganic, lipid based, and miscellaneous nanoformulations as potential therapeutics for the treatment of type 2 diabetes mellitus.
Gold nanoparticles were synthesized from aqueous extracts of Terminalia bellirica Roxb (Family: Combretaceae) fruits and seeds using various methods, with microwave-assisted synthesis being the most efficient. The nanoparticles were spherical, hexagonal, and triangular in shape, with the smallest diameter being 5.1 nm. In vitro anti-DM activity of gold nanoparticles was investigated by an α-amylase inhibitory assay using Aspergillus oryzae derived α-amylase and porcine pancreatic α-amylase. At 150 µmol/L, gold nanoparticles showed 46% and 42% inhibition of plant and animal amylases, respectively.27
The anti-DM efficacy of gold nanoformulation of wedelolactone (10) (Figure 5), a coumarin isolated from Wedelia calendulacea Rich (Family: Asteraceae), and Eclipta prostrata (L) L (Family: Asteraceae) was investigated using RIN-5F cell lines (rat islet cell line). The average particle size was 102.7 nm, with a zeta potential of −10.1 mV. Insulin secretion was significantly improved in glucose-stressed pancreatic cells treated with the nanoformulation at 10, 20, 40, and 80 µmol/L, both in low (5 mmol/L) and high glucose medium (25 mmol/L). A diabetogenic agent di (2-ethylhexyl) phthalate plasticizer, was used to model oxidative stress in pancreatic cells. The treatment of di (2-ethylhexyl) phthalate plasticizer-exposed cells with nanoparticles (10, 20, 40, and 80 µmol/L) for 24 hours improved cell viability, increased secretion of insulin, decreased levels of reactive oxygen species, and restored antioxidant enzymes, indicating its ability to reduce oxidative stress, which is beneficial in the treatment of type 2 DM.28
Silver nanoparticles were synthesized using 50% methanol extract of dried bark of Eysenhardtia polystachy (A Gray) S Watson (Family: Fabaceae). The majority of the nanoparticles were spherical, with a diameter of 10 to 12 nm and zeta potential of −32.25 mV, and they remained physically stable for 6 months. Toxicity assessment using RAW 264.7 (murine macrophage cell line) showed more than 90% viability after 24 hours of exposure to the nanoparticles at 50 to 560 µg/mL. Pretreatment of hydrogen peroxide-treated RAW 264.7 cells with nanoparticles at 560 µg/mL increased the cell viability by 96%, indicating its antioxidant property. Pretreatment of INS-1 cells with nanoparticles before hydrogen peroxide exposure confirmed the protective effects of nanoparticles against oxidative stress for β-cell damage.29 This nanoformulation was tested for anti-DM activity in vivo using zebrafish, and the results are summarized in Table 1.
Silver nanoparticles synthesized using a methanolic extract of dried seeds of Syzygium cumini (Family: Myrtaceae) were investigated in embryonic H9C2 cells derived from the heart of glucose-stressed rats to assess its beneficial effects in diabetic cardiomyopathy. Nanoparticles with a diameter ranging from 40 to 100 nm and a zeta potential of −19.6 mV were obtained. Nanoparticles showed in vitro antioxidative potential of 66.87% (0.7%) and 86.07% (0.92%) DPPH and 2′-azinobis-3-ethylbenzotiazolin-6-sulfonic acid assays, respectively. The MTT cell toxicity assay was used to select a safe dose of nanoparticles (20 µg/mL) for glucose-stressed cardiac cell lines. As an indicator of inducing oxidative stress in cardiac cells, the results showed decreased cell size and lipid peroxide formation with deformities of cell nuclei. All of the changes suggested that nanoformulation could be used to treat diabetic cardiomyopathy.30
Aqueous extract of bulbs of Allium cepa L (Family: Liliaceae) coated silver nanoparticles were spherical in shape with a size ranging from 49 to 73 nm, and they were uniformly distributed. In vitro anti-DM activities, in terms of α-amylase inhibition and α-glucosidase inhibition, were evaluated. The results showed that α-amylase inhibitory potential of nanoparticles was significantly (P < 0.05) higher than that of acarbose, whereas α-glucosidase inhibitory activity of nanoparticles was not significantly (P > 0.05) varied from that of acarbose at all the tested concentrations. In the DPPH assay, the nanoparticles demonstrated dose dependent antioxidant properties. The MTT assay was used to determine the cytotoxicity of the synthesized silver nanoparticles against the mouse adipocyte 3T3 L1 cell line. A 65% cell viability was observed at the highest tested concentration (100 µg/mL).31
Silver nanoparticles synthesized from aqueous extract of dried leaves of Pouteria sapota (Jacq) H.E. Moore & Stearn (Family: Sapotaceae) were within 20 to 110 nm size range. The nanoformulations showed significant in vitro antidiabetic activity compared with the free extract in terms of inhibition of nonenzymatic glycation of hemoglobin (–62.09% [0.64%]), inhibition of α-amylase (52% [0.4%]), and glucose uptake by yeast cells by 67.28% (0.38%) and 65.85% (0.96%) at concentrations of 5 mmol/L and 10 mmol/L, respectively.32 This formulation was further investigated in vivo using Wistar rats for its anti-DM activity and the findings are summarized in Table 1.
Aqueous extracts of dried leaves of Areca catechu L (Family: Arecaceae) were nanoformulated using zinc oxide by a solution combustion synthesis method, in which a mixture of Zn(NO3)2•6H2O and plant extract were kept in a preheated muffle furnace maintained at 400°C. The resulting nanoparticles were crystalline in nature, with an average crystallite size of 29 nm. Spherical agglomeration was observed in the particles. The in vitro anti-DM activity of the nanoparticles was investigated using a glucose uptake by yeast cells assay (Saccharomyces cerevisiae). Percentage glucose uptake was concentration-dependent, where glucose uptake was highest (66.81%) at the highest concentration (250 µg/mL) of nanoparticles.33
Zinc oxide nanoparticles synthesized from the aqueous leaf extract of Morus indica L (Family: Moraceae) were approximately 6 to 12 nm in size. In vitro studies showed inhibition of the production of advanced glycation end products, thus reducing the formation of glycated hemoglobin, inhibiting protein glycation, and inhibiting red blood cell autoxidation. Zinc oxide nanoparticles were synthesized by mixing aqueous extracts of dried leaves (20 mL) with zinc nitrate hexahydrate (2 g). Molecular docking studies revealed that masking of lysine and arginine residues in proteins is among the possible mechanisms underlying the potent antiglycation activity of these nanoparticles.34 This formulation was further investigated in vivo using Wistar rats for its antidiabetic activity, and the results are summarized in Table 1.
A novel polysaccharide (ie, RTFP-3) composed mainly of arabinose, galactose, fructose, glucose, mannose, and xylose isolated from the fruit of Rosa roxburghii Tract (Family: Rosaceae) was nanoformulated using selenium. RTFP-3–coated selenium nanoparticles at a concentration of 2 mg/mL had an average diameter of 104.5 nm and zeta potential of −40.4 mV. These were physically stable for 30 days. Pretreatment of hydrogen peroxide-induced INS-1 cells with nanoformulation at a dose of 2 µg/mL resulted in approximately 89.34% cell viability, reversed hydrogen peroxide-induced caspase-8, caspase-9, and caspase-3 activation, and significantly decreased the production of reactive oxygen species. Furthermore, it inhibited hydrogen peroxide-induced mitochondrial damage and increased insulin secretion, indicating that pancreatic β-cells were protected from oxidative stress, which is beneficial in the treatment of type 2 DM.35
In addition to the previously mentioned nanoformulations, several inorganic nanoformulations synthesized using plant extracts have been studied for their anti-DM activities, but the studies have been limited to in vitro noncellular studies. These nanoformulations had greater anti-diabetic effects than the respective free extracts. Table 3 summarizes these findings.
Table 3.
Inorganic nanoformulations prepared using plant extracts and investigated in vitro for antidiabetic activity.
| Plant | Plant part used | Plant family | Nanoformulation | Properties of nanoparticles(size, shape, zeta potential) | In vitro antidiabetic activity | Reference |
|---|---|---|---|---|---|---|
| Ananas comosus L Merr | Peels of fruit | Bromeliaceae | Silver NPs | NM | Inhibition of α-glucosidase (EC 3.2.1.3), Antioxidant activity |
61 |
| Andrographis paniculata (Burm f) Wall ex Nees | Leaves | Acanthaceae | Zinc oxide NPs | 96–115 nm, spherical and hexagonal | Inhibition of α-amylase (EC 3.2.1.1), α-glucosidase Antioxidant activity Anti-inflammatory activity |
62 |
| Argyreia nervosa (Burm f) Bojer | Leaves | Convolvulaceae | Silver NPs | 15 nm, spherical | Inhibition of α-amylase, α-glucosidase Antioxidant activity |
63 |
|
Azadirachta indicia A Juss, Hibiscus rosa-sinensis L, Murraya koenigii L Spreng, Moringa oleifera Lam, and Tamarindus indica L |
Leaves |
Meliaceae, Malvaceae, Rutaceae, Moringaceae, Fabaceae |
Zinc oxide NPs | 25–32 nm, spherical | Inhibition of α-amylase, α-glucosidase Antioxidant activity *Highest activity was observed from nanoparticles of Tamarindus indica |
64 |
| Bauhinia variegata L | Flower | Fabaceae | Silver NPs | 5–15 nm, spherical | Inhibition of α-amylase Antioxidant activity |
65 |
| Calophyllum tomentosum Wight | Leaves | Calophyllaceae | Silver NPs | 24 nm, spherical | Inhibition of α- amylase, α- glucosidase, dipeptidyl peptidase-4 | 66 |
| Colpomenia sinuosa (Mertens Ex Roth) Derbés & Solier | Whole plant | Scytosiphonaceae | Silver NPs | NM | Inhibition of α-amylase, α-glucosidase | 67 |
| Costus igneus N E Br | Leaves | Costaceae | Zinc oxide NPs | 26.55 nm, hexagonal | Inhibition of α-amylase, α-glucosidase Antioxidant activity |
68 |
| *Costus pictus D Don | Leaves | Costaceae | Silver NPs | NM | Inhibition of HbA1c formation, α-amylase | 69 |
| Dioscorea bulbifera L | Tubers | Dioscoreaceae | Copper NPs | 12 –16 nm, spherical | Inhibition of α-amylase, α-glucosidase Antioxidant activity |
70 |
|
Gnidia glauca (Fresen) Gilg and Plumbago zeylanica L |
Leaves, flowers and stem Leaves |
Thymelaeaceae Plumbaginaceae |
Copper NPs | Nanoparticles of leaf extract of Gnidia glauca: 70–93 nm, spherical Nanoparticles of flower extract of Gnidia glauca: 5 nm, spherical Nanoparticles of Plumbago zeylanica: 1–5 nm, spherical |
Highest inhibition of α-amylase by NPs of Gnidia glauca leaf extract and flower extract Highest inhibition of α-glucosidase by NPs of Gnidia glauca stem extract and leaf extract |
71 |
| Lonicera japonica Thunb | Leaves | Caprifoliaceae | Silver NPs | 53 nm, spherical and hexagonal, –35.6 mV | Inhibition of α-amylase, α-glucosidase Antioxidant activity |
72 |
| Millettia pinnata L Panigrahi | Flower | Fabaceae | Copper NPs | 13 to 35 nm, polygons, square, spherical and hexagonal | Inhibition of α-amylase, α-glucosidase, HbA1c formation Antioxidant activity |
73 |
| Ocimum basilicum L, Ocimum sanctum L | Leaves | Lamiaceae | Silver NPs | 3–25 nm, spherical | Inhibition of α-amylase, α-glucosidase |
74 |
| Pisum sativum L | Outer peel of fruit | Fabaceae | Silver NPs | 10–25 nm | Inhibition of α-glucosidase | 75 |
| Psoralea corylifolia L | Seeds | Fabaceae | Silver NPs | 18.0 nm, circular | Inhibition of phosphatase -1B | 50 |
| Pterocarpus santalinus L f | Heartwood | Fabaceae | Zinc oxide NPs | 20 nm | Inhibition of α-amylase, α-glucosidase |
76 |
| Tephrosia tinctoria Pers | Stem | Fabaceae | Silver NPs | 73 nm, spherical | Inhibition of α-amylase, α-glucosidase ↑Cellular uptake of glucose as observed by glucose uptake in RBC assay |
77 |
HbA1c = glycated hemoglobin; NM = not mentioned; NPs = nanoparticles; RBC = red blood cells.
Methanolic extract of plant material was used in nanoformulation. For all other plants, aqueous extract was used in nanoformulation.
Lipid-based nanoformulations
Solid lipid nanoparticles and nanostructured lipid carriers
Solid lipid nanoparticles (SLNs) range in size from 50 to 1000 nm and are synthesized from lipids that remain in the solid state at room and body temperatures. Natural bioactive compounds are commonly delivered using SLNs. An SLN is a spherical structure consisting of a solid lipid core surrounded by a phospholipid monolayer. Lipids (eg, fatty acids, mono, di- or triglycerides), and mixtures of glycerides or waxes are used in the synthesizing of solid lipid cores. A biocompatible surfactant (eg, polysorbate 80) is used to stabilize the formulation. Typically, when designing a formulation, the drug is dispersed in a solid lipid core of SLNs, and the SLNs are then dispersed in an aqueous medium to form an SLN suspension. Controlled drug release, drug targeting, protection of incorporated compounds against chemical degradation, avoidance of organic solvents, and incorporation of lipophilic and hydrophilic drugs are just a few of the benefits of SLNs.78 This nanoformulation method may result in insufficient drug loading. SLNs can be modified to overcome this limitation by incorporating both solid and liquid lipids into the lipid core. This modified version of SLNs is known as nanostructured lipid carriers (NLC) (Figure 6).79
Figure 6.
Basic structure of (A) solid lipid nanoparticles and (B) nanostructured lipid carriers.
Myricitrin (11) (Figure 5), a botanical flavonol glycoside, isolated from plants (ie, Myrica rubra Lour [Family: Myrtaceae], Eugenia uniflora L [Family: Myrtaceae], and Manilkara zapota [L] P. Royen [Family: Sapotaceae]) was delivered by incorporation of NLCs. For this purpose, NLCs were synthesized by a cold homogenization method using Compritol 888 ATO (Gattefosse, Saint-Priest, France) (a hydrophobic mixture of mono-, di-, and tri-behenate of glycerol) as the solid lipid and oleic acid as the liquid lipid. A mixture of polysorbate 80 and Span 20 (1:1) was used as the surfactant. The congelation was achieved by adding propylene glycol. The resulting mean particle size and zeta potential of the spherical NLC were 76.1 nm and −5.51 mV, respectively. In this study, the encapsulation efficiency was reported to be 56.2% and the in vitro release kinetics showed a controlled release of 50.34% myricitrin within 1 to 24 hours. The NLC preparations showed a significant (P < 0.05) increase in the total antioxidant capacity compared with metformin in the hyperglycemic (maintained in a medium containing 100 mM D-glucose) skeletal myoblast cell line. The antioxidant capacity of NLCs in mouse pancreatic tissue was comparable to metformin. The glycogen content in hyperglycemic skeletal myoblast cell lines also increased after NLC treatment. The cytotoxicity of the formulation at 10 µM was assessed using MTT assay and found to be nontoxic.80 This formulation was further investigated in vivo using NMRI (Naval Medical Research Institute) mice with type 2 DM for its anti-DM activity, and the results are summarized in Table 2.
SLNs synthesized using oat-derived peptides isolated from seeds of Avena sativa L (Family: Poaceae) were investigated for their dipeptidyl peptidase-4 (EC 3.4.14.5) inhibitory activity. The preparation included the use of glyceryl monostearate as a solid lipid and poloxamer 188 (polyethylene–polypropylene glycol) as a surfactant. The encapsulation efficiencies of the low molecular weight and high molecular weight peptide fractions observed were 75.6% and 69.8%, respectively. Zeta potentials were −20.9 mV and −19.1 mV, and particle sizes were 63.9 nm and 270.9 nm, for low molecular weight and high molecular weight peptide nanoparticles, respectively. The nanoparticles had spherical shapes and smooth surfaces. Accelerated stability assays at 4°C revealed that the nanoparticles remained stable for at least 1 month. A low cumulative release rate (<7.87%) within 2 hours in the gastric fluid was observed in vitro, indicating that the nanoparticles could remain stable in this acidic environment, preventing the polypeptide from leaking out. Dipeptidyl peptidase-4 inhibitory activity was higher in SLNs than in free peptide.81
Nanoliposomes
A nanoliposome is a vesicle formed by a phospholipid bilayer, usually stabilized with sterols. Phosphatidylcholine (PC), also known as lecithin, is a common phospholipid used in synthesizing nanoliposomes, where cholesterol is used to stabilize the liposomal membranes (Figure 7). PC derived from egg yolk and soybean is commonly used for nanoliposome production. However, commercially available PC derived from egg yolk and soybean products contains PC as well as other phospholipids such as phosphatidylethanolamine, phosphatidylserine, and phosphatidic acid in various compositions.82 Liposomes are biocompatible and biodegradable structures that are used in herbal drug delivery. They offer benefits such as increased drug solubility and bioavailability, increased in vitro and in vivo stability, and cell-specific targeting.83
Figure 7.
Basic structure of a nanoliposome.
Nanoliposomes were synthesized using Lipoid (Lipoid Kosmetik, Sennweidstrasse 44, Steinhausen 6312, Switzerland) S45 (soybean lecithin with 45% PC) to encapsulate a polyherbal mixture consisting of Citrullus colocynthis (L) Schrad. (Family: Cucurbitaceae), Momordica balsamina L (Family: Cucurbitaceae), and Momordica dioica Roxb. ex Willd (Family: Cucurbitaceae) (1:1:1) derived methanolic extracts. The average particle size of a vesicle was 450 nm, with an encapsulation efficiency of 92.1% (5.1%). In-vitro drug release studies showed that 92% of the formulation was released in 12 hours. The zeta potential of the vesicles was –22.7 mV and the formulation was stable for 2 months (physically and chemically) under accelerated stability testing conditions of 4 (1) °C and 40 (2) °C.39 This formulation was further investigated in vivo using Swiss albino rats for its anti-DM activity, and the results are summarized in Table 1. Furthermore, using animal models, several nanoformulations have been investigated for their bioactivities. Tables 1 and 2 summarize these results.
Clinical studies on herbal nanoformulations against type 2 DM
Although many herbal nanoformulations with enhanced biological activities against type 2 DM have been developed, only 2 have been clinically tested for their therapeutic effects.
Nanocurcumin is 1 such marketed curcumin (12) (Figure 5) (a polyphenol isolated from Curcuma longa L [Family: Zingiberaceae]) nanoformulation synthesized by a proprietary method using polysorbate 80. The anti-DM activity of nanocurcumin was studied in a randomized, placebo-controlled double-blind clinical trial in patients with DM. The study group that received 80 mg nanocurcumin capsules for 8 weeks showed a significant reduction in glycated hemoglobin, fasting blood glucose levels, and a reduction in the severity of diabetic sensorimotor polyneuropathy, which is a common complication of type 2 DM. Encapsulation of curcumin into the nanoformulation is reported to increase its bioavailability, resulting in increased antioxidant activity of curcumin and the occurrence of observed therapeutic effects. Furthermore, during the study period, the formulation was found to be safe and well tolerated.84
In another study, patients with type 2 DM received either curcumin (nano-micelle 80 mg/day) or placebo for 3 months as an add-on therapy to their usual medications in a double-blind randomized clinical trial. When the results of each patient before and after the treatment were compared (P < 0.05), a significant decrease in glycated hemoglobin, fasting blood glucose level, triglyceride level, and body mass index was found in the group that received nanocurcumin. The therapeutic effects observed in this study are suggested to be due to the increased bioavailability of curcumin when delivered as nano-micelles.85
Discussion
Many herbal preparations, such as plant extracts, partitions, fractions, and isolated compounds, have been formulated into nanoparticles and studied using in vitro and in vivo models of type 2 DM, and have demonstrated superior anti-DM effects when compared with the respective crude extracts or pure compounds.17,80 Only 2 nanoformulations on the other hand, have been explored in human beings.84 As a result, there is an urgent need to investigate anti-DM effects and toxicity levels of new nanoformulations to develop successful nanomedicines for the treatment and prevention of type 2 DM. According to the information in this review, medicinal plant extracts and isolated compounds (eg, scutellarin, quercetin, curcumin, resveratrol, polydatin, and wedelolactone) have been successfully used to synthesize nanoformulations. Anti-DM activity has primarily been investigated using in vitro and in vivo models. During the time period considered, only 2 clinical trials were discovered. Figure 8 depicts desirable anti-DM mechanisms investigated in nanoformulations. Nanoparticles have a larger surface area than microparticles, resulting in greater solubility, bioavailability, and improved controlled release, allowing for effective delivery of natural bioactive compounds at the site of action.86 Bioactive compounds can either be entrapped in the nanoparticle matrix or attached to nanoparticle surfaces.87 Many natural bioactive compounds are poorly soluble in aqueous media, resulting in poor absorption in the gastrointestinal tract. Natural bioactive compounds frequently have bitter or unpleasant tastes that reduce patient compliance, resulting in ineffectiveness. Unpleasant taste can be masked by entrapping in nanoparticles. The incorporation of bioactive compounds into solid lipid nanoparticles has demonstrated taste-masking properties.88 Nanosuspensions stabilized by surfactants are among the popular oral dosage forms, particularly for delivering drugs with poor solubility.87-89 Reduced absorption of natural bioactive compounds can occur as a result of the destruction by gastric acid and gastric enzymes, as well as mechanical obstruction imposed by the intestinal mucosa. Nanoparticles synthesized by encapsulating bioactive compounds in biodegradable polymers (eg, gelatin and PEG) offer a therapeutic option to overcome this reduced absorption, reduced bioavailability, and decreased therapeutic effectiveness. Furthermore, by targeting specific receptors in the intestinal epithelium, nanoparticles can be modified to increase intestinal absorption.
Figure 8.
Desirable properties and common therapeutic effects imparted by herbal nanoformulations targeting type 2 diabetes mellitus (type 2 DM).
The differences in morphological and morphometric properties of nanoformulations could significantly affect the characterization properties (eg, shape, size, zeta potential, and encapsulation efficiency) and anti-DM activity. The size and surface characteristics of the particles, for example, can influence their clearance by the lymphatic system. The optimum size for a nanoparticle is considered as approximately 100 nm to avoid immediate clearance by the lymphatic system. Hydrophilic nanoparticles would have a longer time in circulation than lipophilic nanoparticles. However, food-grade nanoparticles, as described in this review, typically exceed the size of 100 nm, and surface characteristics such as coatings, rather than particle size, influence their clearance. The increased half-life of such nanoformulations will require a reduced dosing frequency, thereby enhancing patient compliance. Coating nanoparticles with polymers or surfactants, or creating copolymers such as PEG, reduces opsonization and allows them to be in circulation for longer periods of time. The encapsulation of quercetin in a copolymer consisting of PEG demonstrated increased circulation time and sustained release of quercetin in vivo, resulting in improved anti-DM effects, including lower blood glucose levels and improved antioxidant activity when compared to free quercetin.21 Polymeric nanoparticle coatings also allow for the controlled release of therapeutic agents. In vitro, the starch coating of withanolides-encapsulated chitosan nanoparticles, demonstrated controlled release of withanolides.18 Polydatin incorporated in chitosan nanoparticles and myricitrin encapsulated in nanostructured lipid carriers also showed controlled release of the encapsulated compounds in vitro.19,80 The zeta potential can be altered to increase the physical stability of nanoparticles.90 However, the zeta potential should not be considered as the only parameter to determine stability because nanoparticles could also be stabilized by steric stabilization rather than electrostatic stabilization. As a result, zeta potential values plus stability testing would be useful for a better understanding of the stability. The stability of a nanoformulation is a critical factor for targeted delivery and to achieve the desired therapeutic effect/s. Targeted delivery is usually achieved by having polymers or ligands on nanoparticle surfaces, which are compatible and can bind to specific targets in the body, such as receptors on cell membranes, lipid components of the cell membrane, and antigens or proteins on the cell surface.75 Nanoformulations could also be modified to impart a combination of properties. For example, chitosan-deoxycholic acid-vitamin B–12 nanoparticles mentioned in this review showed increased water solubility as well as targeting epithelial cells of the small intestine to increase absorption from the intestinal epithelium. As a result, when scutellarin was formulated as nanoparticles, its bioavailability increased, as did its anti-DM effects.17 The effective delivery of encapsulated bioactive compounds by nanoformulations facilitates their anti-DM effects.
Figure 9 and Table 4 provide an overview of the findings from this review in terms of the utilization of various nanomaterials. Although a huge variety of nanomaterials are utilized in nanoformulations, only a small number of materials, have been certified for food and pharmaceutical applications known as generally recognized as safe. Polymers, such as carbohydrates, proteins, or lipids, are generally recognized as safe materials used for encapsulation. Inorganic materials (eg, tripolyphosphates) are also used as carriers or stabilizers.88
Figure 9.
Use of different nanomaterials in the preparation of herbal nanoformulations for type 2 diabetes mellitus.
Table 4.
Commonly used nanomaterials for nanoherbal formulations against type 2 diabetes mellitus.
| Type of nanoformulation | Nanomaterials used |
|---|---|
| Inorganic | Gold, silver, copper, zinc oxide, cerium oxide, selenium |
| Natural polymers | Chitosan, polygalacturonic acid |
| Synthetic polymers | Poly-ƹ-caprolactone PEG, polyvinyl alcohol, PLGA |
| Solid lipid nanoparticles | Compritol* 888 ATO, oleic acid, glyceryl monostearate |
| Nanoliposomes | soya phosphatidylcholine (eg, Lipoid† S45) |
ATO = xxxxx; PEG = polyethylene glycol; PLGA = poly (lactic-co-glycolic acid).
Compitrol * (888 ATO- Gattefosse, Saint-Priest, France).
Lipoid (Lipoid Kosmetik, Sennweidstrasse 44, Steinhausen 6312, Switzerland).
The abundance of inorganic nanoformulations could be attributed to their ease of preparation with a defined size when compared with other nanoformulations. Bioactive compounds are usually attached to the surface of the inorganic element via ionic or covalent bonds, and physical absorption occurs via the formation of a coating in the synthesis of inorganic nanoparticles. The secondary metabolites present in plant extracts, such as alkaloids, terpenoids, flavonoids, and other polyphenols, usually reduce metallic inorganic elements (eg, silver ion to Ag0), facilitating the formation of nanoparticles.91 It is also possible to modify the surfaces of these nanoparticles with multiple polymers and ligands to achieve targeted or controlled delivery of bioactive compounds. However, most inorganic elements have their own therapeutic effects that should be taken into account when determining doses. For example, zinc is essential for β-cell function, insulin action, glucose homeostasis, and the deficiency of zinc may be involved in the pathogenesis of diabetes and associated complications.92 Cerium oxide and sodium selenite nanoparticles have shown antioxidant potential in vivo, hence beneficial against diabetes-induced oxidative stress.93 Silver and gold nanoparticles have shown in vivo anti-DM effects in terms of reduction of blood glucose, oxidative stress, inflammation, and increase in insulin level.94 Silver nanoparticles have shown to exhibit inhibitory effects on the formation of advanced glycation end products.95 However, there are concerns of toxicity arising from inorganic elements that must be considered when synthesizing nanoformulations.96
When compared with the synthesis of inorganic nanoparticles, the formulation of polymeric and lipid-based nanoparticles is a more complex process. They could, however, be used for targeted and controlled delivery with or without surface modifications. In these formulations, bioactive compounds are usually encapsulated or entrapped within nanoparticles. Surface modifications such as PEGylation97 and coating with polymers such as starch18 impart important properties to nanoparticles, such as increased stability and resistance to destruction in gastric fluid. Biocompatible surfactants (eg, polysorbate 80) are also used frequently in these nanoformulations to increase stability. PEGylation is the term used for the process of covalent linking of PEG chains. PEGylation was used to increase the stability of nanoliposomes and to prolong their in vivo circulation time by inhibiting the adsorption of protein opsonins on liposome surfaces in circulation.97
Each of these drug delivery systems has its own unique chemical, physical, and morphological characteristics. Through different chemical interactions (eg, covalent bonds and hydrogen bonds) or physical interactions (eg, electrostatic and van der Waals interactions) the drug delivery system may have different affinities with the encapsulated/loaded drugs.13 The release of bioactive compounds depends on many factors, including pH, temperature, drug solubility, desorption of surface-bound or adsorbed drug, drug diffusion through the nanoparticle matrix, swelling of the nanoparticle matrix, erosion, and a combination of erosion and diffusion processes.90 All of these factors should be considered when developing a nanoformulation for a specific bioactive compound.
Nanoformulations with a number of enhanced anti-DM effects were reported, compared with their free extracts or isolated compounds. The nanoformulations showed beneficial effects on the main therapeutic targets of type 2 DM using different in vivo and in vitro platforms. Inhibition of the formation of advanced glycation end products is another important mechanism of action of certain nanoformulations, which is important in preventing most diabetes-related complications. For example, zinc oxide nanoparticles synthesized using an aqueous extract of Morus indica L were beneficial in treating diabetic nephropathy in a preclinical study conducted using Wistar rats with type 2 DM.34 Gold nanoparticles coated with resveratrol showed positive effects in treating diabetic retinopathy in Wistar rats with type 2 DM,59 silver nanoparticles synthesized by a methanolic extract of seeds of Syzygium cumini showed therapeutic benefits in treating diabetic cardiomyopathy in in vitro cell studies.30 Almost all of the published studies on diabetes complications identified in this review involve inorganic/metallic nanoparticles. Metallic nanoparticles have antioxidant properties and have thus been reported to be useful in the formulation of nanomedicines against complications of diabetes, where massive generation of reactive oxygen species is observed.98 Materials constructed at the nanoscale level may exhibit properties not found at the macroscale level. Because nanoparticles enable targeted delivery of higher concentrations of compounds to tissues, unexpected effects or toxicity can occur even with generally recognized as safe materials. Although the concentrations of nanoparticles tested in vitro can be used to predict the optimum concentrations achievable in vivo, such conversions may not always produce expected therapeutic outcomes because pharmacokinetic processes affect the drug concentration achievable in vivo. Hence, further safety and efficacy tests are needed to determine optimal doses of nanoparticles.99 More focus on pharmacokinetic studies is needed for a better understanding of advantages of nanoformulations over the use of free extracts/isolated compounds and to investigate the mechanisms of action of these nanoformulations.
The literature search was limited to 3 online databases due to large amount of literature available for the topic of this review. Furthermore, the abundance of results received from Google Scholar and Science Direct databases led to the selection of only the first 100 articles from each of those databases. This is a major limitation of the present review. As a result, this review may not be based on all of the peer-reviewed literature on herbal nanoformulations targeting type 2 DM retrieved during the data collection period, which is a major limitation of this current review.
Challenges and future perspectives
The majority of synthesized nanoformulations have been evaluated in only preclinical studies,80 primarily using cell culture models28 and other in vitro models.31 In addition, almost all of the studies included in this review were of only acute or subacute duration and did not attempt to elucidate anti-DM mechanisms. As a result, future research should focus on detailed and long-term in vitro and in vivo investigations directed toward elucidation of anti-DM mechanisms, toxicity, pharmacokinetics, and stability of nanoformulations to ensure the safety and efficacy of the nanoformulations before advancing to clinical trials, and the path forward.
Conclusions
Medicinal plant extracts and isolated compounds have been nanoformulated using various methods. Inorganic compounds such as silver, gold, and zinc oxide are frequently used in the development of herbal nanoformulations for the management of type 2 DM. Natural polymeric nanomaterials such as chitosan, as well as synthetic polymeric nanomaterials, SLNs, and liposomes, are used as the matrix of the nanoformulations. Polymeric and lipid nanoformulations have been modified using various synthetic methods, with the goal of delivering natural bioactive compounds in a targeted and controlled manner. Furthermore, crude extracts/pure compounds achieve greater stability and higher resistance to destruction by gastric fluid through surface modifications. The majority of the bioactivity screening was performed in vitro using cellular and noncellular models and/or in vivo using animal models for acute or subacute durations. Important biological properties of nanoformulations that have been observed included improved glycemic control, improved insulin levels, inhibition of advanced glycation end products formation, and regeneration of pancreatic β cells. The effects of the nanoformulations observed were generally superior to those of the corresponding herbal extracts and isolated compounds. The literature reviewed suggests that a number of nanoformulations can improve many desirable biological properties of herbal products. Although the majority of the nanoformulations have been tested for cellular toxicity using cell cultures, detailed long-term toxicity studies are required to ensure the safe use of nanoformulations as therapeutic agents. At both the preclinical and clinical levels, there are a number of poorly explored and/or unexplored research areas in the development and bioactivity assessment of herbal nanoformulations.
CRediT author statement
Akurange Sujeevi Dammadinna Wickramasinghe: Methodology, Visualization, Writing - Original Draft, Data Curation, Investigation, Formal analysis. Pabasara Kalansuriya: Conceptualization, Methodology, Supervision, Visualization , Data curation, Writing - Review & Editing. Anoja Priyadarshani Attanayake: Conceptualization, Methodology, Supervision, Visualization, Writing - Review & Editing
Acknowledgments
Acknowledgments
This review was written in relation to a research project funded by World Bank under the Accelerating Higher Education Expansion and Development-AHEAD project (AHEAD/DOR STEM-15), Sri Lanka. The manuscript was checked for English grammar, punctuation, and for overall style and edited by Editrightly editorial service (https://www.editrightly.com/).
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2022.100672.
Appendix. Supplementary materials
Supplemental Figure. List of 64 publications used in the review.
References
- 1.International Diabetes Federation . ninth ed. 2019. IDF Diabetes Atlas. Brussels, Belgium. [Google Scholar]
- 2.World Health Organization, Noncommunicable Diseases Country Profiles 2018, https://apps.who.int/iris/handle/10665/274512.
- 3.American Diabetes Association, 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes. Diabetes Care. 2020;43:S14–S31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 4.LeRoith D. Beta- cell dysfunction and insulin resistance in type 2 diabetes: role of metabolic and genetic abnormalities. Am J Med Sci. 2002;113:3–11. doi: 10.1016/s0002-9343(02)01276-7. [DOI] [PubMed] [Google Scholar]
- 5.Asmat U., Abad K., Ismail K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm J. 2016;24:547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association Standards of medical care in diabetes—abridged for primary care providers. Clinical Diabetes. 2020;38:10–13. doi: 10.2337/cd20-as01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar S., Mittal A., Babu D., Mittal A. Herbal medicines for diabetes management and its secondary complications. Curr Diabetes Rev. 2021;17(4):437–456. doi: 10.2174/1573399816666201103143225. [DOI] [PubMed] [Google Scholar]
- 8.Artasensi A., Pedretti A., Vistoli G., Fumagalli L. Type 2 diabetes mellitus: A review of multi-target drugs. Molecules. 2020;25(8):1987. doi: 10.3390/molecules25081987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen T. Type 2 diabetes mellitus-a multifactorial disease. Ann Univ Mariae Curie Sklodowska Med. 2002;57(1):544–549. [PubMed] [Google Scholar]
- 10.Thakur L., Ghodasra U., Patel N., Dabhi M. Novel approaches for stability improvement in natural medicines. Pharmacogn Rev. 2011;5(9):48–54. doi: 10.4103/0973-7847.79099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cano-Sarabia M., Maspoch D. In: Encyclopedia of Nanotechnology. Bhushan B, editor. Springer Netherlands; Dordrecht: 2014. Nanoencapsulation; pp. 1–16. [Google Scholar]
- 12.Jacob S., Nair A.B., Shah J. Emerging role of nanosuspensions in drug delivery systems. Biomater Res. 2020;24:3. doi: 10.1186/s40824-020-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.S. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonifácio B.V., Silva P.B., Ramos M.A.D.S., Negri K.M.S., Bauab T.M., Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomedicine. 2014;9:1–15. doi: 10.2147/IJN.S52634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammed M.A., Syeda J.T.M., Wasan K.M., Wasan E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017;9:53. doi: 10.3390/pharmaceutics9040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao C.F., Zhang X.R. Modulation of Diabetes mellitus-induced male rat reproductive dysfunction with micro-nanoencapsulated Echinacea purpurea ethanol extract. Biomed Res Int. 2018 doi: 10.1155/2018/4237354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Tan J., Luo J., Huang P., Zhou W., Chen L., Long L., Zhang L.M., Zhu B., Yang L., Deng D.Y. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J Nanobiotechnology. 2017;15:18. doi: 10.1186/s12951-017-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampathkumar K., Riyajan S., Tan C.K., Demokritou P., Chudapongse N., Loo S.C.J. Small-intestine-specific delivery of antidiabetic extracts from Withania coagulans using polysaccharide-based enteric-coated nanoparticles. ACS Omega. 2019;4:12049–12057. doi: 10.1021/acsomega.9b00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Moneim A., El-Shahawy A., Yousef A.I., Abd El-Twab S.M., Elden Z.E., Taha M. Novel polydatin-loaded chitosan nanoparticles for safe and efficient type 2 diabetes therapy: In silico, in vitro and in vivo approaches. Int J Biol Macromol. 2019;154:1496–1504. doi: 10.1016/j.ijbiomac.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Li J., Wang Z., Xu M.-Z., Zeng Z., Huang J.-P., Guan Y.-Q. Natural plant-derived polygalacturonic acid-oleanolic acid assemblies as oral-delivered nanomedicine for insulin resistance treatment. Chem Eng J. 2020;390 [Google Scholar]
- 21.Tong F., Liu S., Yan B., Li X., Ruan S., Yang S. Quercetin nanoparticle complex attenuated diabetic nephropathy via regulating the expression level of ICAM-1 on endothelium. Int J Nanomedicine. 2017;12:7799–7813. doi: 10.2147/IJN.S146978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S., Roy P., Pal R., Auddy R.G., Chakraborti A.S., Mukherjee A. Engineered silybin nanoparticles educe efficient control in experimental diabetes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101818. -e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul W., Sharma C.P. In: Biointegration of medical implant materials. second ed. Sharma C.P., editor. Woodhead Publishing; 2020. 13 - Inorganic nanoparticles for targeted drug delivery; pp. 333–373. [Google Scholar]
- 24.Vijayakumar S., Vinayagam R., Anand M.A.V., Venkatachalam K., Saravanakumar K., Wang M-H., Casimeer S., Gothandam K.M., David E. Green synthesis of gold nanoparticle using Eclipta alba and its antidiabetic activities through regulation of Bcl-2 expression in pancreatic cell line. J Drug Deliv Sci Tec. 2020;58 [Google Scholar]
- 25.Badeggi U.M., Ismail E., Adeloye A.O., Botha S., Badmus J.A., Marnewick J.L., Cupido C.N., Hussein A.A. Green synthesis of gold nanoparticles capped with procyanidins from Leucosidea sericea as potential antidiabetic and antioxidant agents. Biomolecules. 2020;10:452. doi: 10.3390/biom10030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govindaraju K., Suganyaab K.S.U. In vitro anti-diabetic assessment of guavanoic acid functionalized gold nanoparticles in regulating glucose transport using L6 rat skeletal muscle cells. RSC Med. Chem. 2020;11:814–822. doi: 10.1039/d0md00125b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smina C.S., Lalitha P., Nagabhushana H., Sharma S.C. Terminalia bellirica dried fruit and seed extract offers alpha-amylase inhibitory potential in tackling diabetes. Appl Nanosci. 2020;10:4325–4339. [Google Scholar]
- 28.Ramachandran V., Arokia V.A.M. Antidiabetic activity of gold nanoparticles synthesized using wedelolactone in RIN-5F cell line. Antioxidants. 2019;9(1):8. doi: 10.3390/antiox9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia C.A.H., Perez G.R.M., Manriquez-Alvirde G., Muñiz R.A. Protection of silver nanoparticles using Eysenhardtia polystachya in peroxide-induced pancreatic β-Cell damage and their antidiabetic properties in zebrafish. Int J Nanomedicine. 2018;13:2601–2612. doi: 10.2147/IJN.S163714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atale N., Saxena S., Nirmala J.G., Narendhirakannan R.T., Mohanty S., Rani V. Synthesis and characterization of Sygyzium cumini nanoparticles for its protective potential in high glucose-induced cardiac stress: a green approach. Appl Biochem Biotechnol. 2017;181:1140–1154. doi: 10.1007/s12010-016-2274-6. [DOI] [PubMed] [Google Scholar]
- 31.Jini D., Sharmila S. Green synthesis of silver nanoparticles from Allium cepa and its in vitro antidiabetic activity. Mater Today Proc. 2020;22:432–438. [Google Scholar]
- 32.Prabhu S., Vinodhini S., Elanchezhiyan C., Rajeswari D. Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles using Pouteria sapota in streptozotocin-induced diabetic rats. J Diabetes. 2018;10:28–42. doi: 10.1111/1753-0407.12554. [DOI] [PubMed] [Google Scholar]
- 33.Shwetha U.R., Latha M.S., Kumar C.R.R., Kiran M.S., Betageriet V.S. Facile synthesis of zinc oxide nanoparticles using novel Areca catechu leaves extract and their in vitro antidiabetic and anticancer studies. J Inorg Organomet Polym. 2020;30:4876–4883. [Google Scholar]
- 34.Anandan S., Mahadevamurthy M., Ansari M.A., Alzohairy M.A., Alomary M.N. Biosynthesized ZnO-NPs from Morus indica attenuates methylglyoxal-induced protein glycation and RBC damage: in-vitro, in-vivo and molecular docking study. Biomolecules. 2019;9(12):882. doi: 10.3390/biom9120882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L., Li C., Huang Q., Fu X. Biofunctionalization of selenium nanoparticles with a polysaccharide from Rosa roxburghii fruit and their protective effect against H2O2-induced apoptosis in INS-1 cells. Food Funct. 2019;10:539–553. doi: 10.1039/c8fo01958d. [DOI] [PubMed] [Google Scholar]
- 36.Daisy P., Saipriya K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int J Nanomedicine. 2012;7:1189–1202. doi: 10.2147/IJN.S26650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ponnanikajamideen M., Rajeshkumar S., Vanaja M., Annadurai G. In vivo type 2 diabetes and wound-healing effects of antioxidant gold nanoparticles synthesized using the insulin plant Chamaecostus cuspidatus in albino rats. Can J Diabetes. 2019;43:82–89. doi: 10.1016/j.jcjd.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Kouame K., Peter A.I., Akang E.N., Moodley R., Naidu E.C., Azu O.O. Histological and biochemical effects of Cinnamomum cassia nanoparticles in kidneys of diabetic Sprague-Dawley rats. Bosn J Basic Med Sci. 2019;19:138–145. doi: 10.17305/bjbms.2019.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rathee S., Kamboj A. Optimization and development of antidiabetic phytosomes by the Box-Behnken design. J Liposome Res. 2018;28:161–172. doi: 10.1080/08982104.2017.1311913. [DOI] [PubMed] [Google Scholar]
- 40.Sengani M., Devi R.V. Identification of potential antioxidant indices by biogenic gold nanoparticles in hyperglycemic Wistar rats. Environ Toxicol Pharmacol. 2017;50:11–19. doi: 10.1016/j.etap.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Paul K., Bhattacharjee P., Chatterjee N., Pal T.K. Nanoliposomes of supercritical carbon dioxide extract of small cardamom seeds redresses type 2 diabetes and hypercholesterolemia. Recent Pat Biotechnol. 2019;13:284–303. doi: 10.2174/1872208313666190404101336. [DOI] [PubMed] [Google Scholar]
- 42.Shanker K., Krishna M.G., Mayasa V., Pravallika L. Antihyperglycemic and anti-hyperlipidemic effect of biologically synthesized silver nanoparticles and G. sylvestre extract on streptozotocin induced diabetic rats-an in vivo approach. Mater Lett. 2017;195:240–244. [Google Scholar]
- 43.Bala N., Saha S., Chakraborty M., Maiti M., Das S., Basu R., Nandy P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Advances. 2015;5:4993–5003. [Google Scholar]
- 44.Kalakotla S., Jayarambabu N., Mohan G.K., Mydin R.B.S.M.N., Gupta V.R. A novel pharmacological approach of herbal mediated cerium oxide and silver nanoparticles with improved biomedical activity in comparison with Lawsonia inermis. Colloids Surf B Biointerfaces. 2019;174:199–206. doi: 10.1016/j.colsurfb.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Shanker K., Naradala J., Mohan G.K., Kumar G.S., Pravallika P.L. A sub-acute oral toxicity analysis and comparative in vivo anti-diabetic activity of zinc oxide, cerium oxide, silver nanoparticles, and Momordica charantia in streptozotocin-induced diabetic Wistar rats. RSC Advances. 2017;7:37158–37167. [Google Scholar]
- 46.Nouri Z., Hajialyani M., Izadi Z., Bahramsoltani R., Farzaei M.H., Abdollahi M. Nanophytomedicines for the prevention of metabolic syndrome: a pharmacological and biopharmaceutical review. Front. Bioeng. Biotechnol. 2020;8:425. doi: 10.3389/fbioe.2020.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu L., Li W., Shi Q., Li H., Yang Z., Liao D., Li L., Yang X., Zhang J. Synthesis of mulberry leaf extract mediated gold nanoparticles and their ameliorative effect on Aluminium intoxicated and diabetic retinopathy in rats during perinatal life. J Photochem Photobiol B. 2019;196 doi: 10.1016/j.jphotobiol.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Anbazhagan P., Murugan K., Jaganathan A., Sujitha V., Samidoss C.M., Jayashanthani S., Amuthavalli P., Higuchi A., Kumar S., Wei H., Nicoletti M., Canale A., Benelli G. Mosquitocidal, antimalarial and antidiabetic potential of Musa paradisiaca-synthesized silver nanoparticles: In vivo and in vitro approaches. J Clust Sci. 2017;28:91–107. [Google Scholar]
- 49.Aldawsari H.M., Hanafy A., Labib G.S., Badr J.M. Antihyperglycemic activities of extracts of the mistletoes Plicosepalus acaciae and P. curviflorus in comparison to their solid lipid nanoparticle suspension formulations. Z Naturforsch C J Biosci. 2014;69:391–398. doi: 10.5560/znc.2014-0047. [DOI] [PubMed] [Google Scholar]
- 50.Shanker K., Mohan G.K., Hussain M.A., Jayarambabu N., Pravallika P.L. Green biosynthesis, characterization, in vitro antidiabetic activity, and investigational acute toxicity studies of some herbal-mediated silver nanoparticles on animal models. Pharmacogn Mag. 2017;13:188–192. doi: 10.4103/0973-1296.197642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manna K., Mishra S., Saha M., Mahapatra S., Saha C., Yenge G., Gaikwad N., Pal R., Oulkar D., Banerjee K., Das-Saha K. Amelioration of diabetic nephropathy using pomegranate peel extract-stabilized gold nanoparticles: assessment of NF-κB and Nrf2 signaling system. Int J Nanomedicine. 2019;14:1753–1777. doi: 10.2147/IJN.S176013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Opris R., Tatomir C., Olteanu D., Moldovan R., Moldovan B., David L., Nagy A., Decea N., Kiss M.L., Filip G.A. The effect of Sambucus nigra L. extract and phytosinthesized gold nanoparticles on diabetic rats. Colloids Surf B Biointerfaces. 2017;150:192–200. doi: 10.1016/j.colsurfb.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 53.Ansari S.A., Bari A., Ullah R., Mathanmohun M., Veeraraghavan V.P., Sun Z. Gold nanoparticles synthesized with Smilax glabra rhizome modulates the anti-obesity parameters in high-fat diet and streptozotocin induced obese diabetes rat model. J Photochem Photobiol B. 2019;201 doi: 10.1016/j.jphotobiol.2019.111643. [DOI] [PubMed] [Google Scholar]
- 54.Perumal V., Manickam T., Bang K.S., Velmurugan P., Oh B.T. Antidiabetic potential of bioactive molecules coated chitosan nanoparticles in experimental rats. Int J Biol Macromol. 2016;92:63–69. doi: 10.1016/j.ijbiomac.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Bitencourt P.E., Cargnelutti L.O., Stein C.S., Lautenchleger R., Ferreira L.M., Sangoi M., Denardi L., Borges R.M., Boligon A., Moresco R.N., Cruz L., Zanette R.A., Alves S.H., Moretto M.B. Nanoparticle formulation increases Syzygium cumini antioxidant activity in Candida albicans-infected diabetic rats. Pharm Biol. 2017;55:1082–1088. doi: 10.1080/13880209.2017.1283338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garg A., Pandey P., Sharma P., Shukla A. Synthesis and characterization of silver nanoparticle of ginger rhizome (Zingiber officinale) extract: Synthesis, characterization and anti diabetic activity in streptozotocin induced diabetic rats. European J Biomed Pharm Sci. 2016;3:605–611. [Google Scholar]
- 57.Abdel-Mageid A.D., Abou-Salem M.E.S., Salaam N., El-Garhy S. The potential effect of garlic extract and curcumin nanoparticles against complication accompanied with experimentally induced diabetes in rats. Phytomedicine. 2018;43:126–134. doi: 10.1016/j.phymed.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 58.Panwar R., Raghuwanshi N., Srivastava A.K., Sharma A.K., Pruthi V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater Sci Eng C. 2018;92:381–392. doi: 10.1016/j.msec.2018.06.055. [DOI] [PubMed] [Google Scholar]
- 59.Dong Y., Wan G., Yan P., Qian C., Li F., Peng G. Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J Photochem Photobiol B. 2019;195:51–57. doi: 10.1016/j.jphotobiol.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Singh A.K., Pandey H., Ramteke P.W., Mishra S.B. Nano-suspension of ursolic acid for improving oral bioavailability and attenuation of type II diabetes: A histopathological investigation. Biocatal Agric Biotechnol. 2019;22 [Google Scholar]
- 61.Das G., Patra J.K., Debnath T., Ansari A., Shin H.S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.) PLoS One. 2019;14 doi: 10.1371/journal.pone.0220950. -e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajakumar G., Thiruvengadam M., Mydhili G., Gomathi T., Chung I.M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst Eng. 2018;41:21–30. doi: 10.1007/s00449-017-1840-9. [DOI] [PubMed] [Google Scholar]
- 63.Saratale G.D., Saratale R.G., Benelli G., Kumar G., Pugazhendhi A., Kim D.S., Shin H.S. Anti-diabetic potential of silver nanoparticles synthesized with Argyreia nervosa leaf extract high synergistic antibacterial activity with standard antibiotics against foodborne bacteria. J Clust Sci. 2017;28:1709–1727. [Google Scholar]
- 64.Rehana D., Mahendiran D., Kumar R.S., Rahiman A.K. In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts. Bioprocess Biosyst Eng. 2017;40:943–957. doi: 10.1007/s00449-017-1758-2. [DOI] [PubMed] [Google Scholar]
- 65.Johnson P., Krishnan V., Loganathan C., Govindhan K., Raji V., Sakayanathan P., Vijayan S., Sathishkumar P., Palvannan T. Rapid biosynthesis of Bauhinia variegata flower extract-mediated silver nanoparticles: an effective antioxidant scavenger and α-amylase inhibitor. Artif Cells Nanomed Biotechnol. 2018;46:1488–1494. doi: 10.1080/21691401.2017.1374283. [DOI] [PubMed] [Google Scholar]
- 66.Govindappa M., Hemashekhar B., Arthikala M.K., Ravishankar R.V., Ramachandra Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018;9:400–408. [Google Scholar]
- 67.Manam V.K., Murugesan S. Biological Synthesis of silver nanoparticles from marine alga Colpomenia sinuosa and its in vitro anti-diabetic activity. AJBBL. 2014;3:1–7. [Google Scholar]
- 68.Vinotha V., Iswarya A., Thaya R., Govindarajan M., Alharbi N.S., Kadaikunnan S., Khaled J.M., Al-Anbr M.N., Vaseeharan B. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm and anti-oxidant properties. J Photochem Photobiol B. 2019;197 doi: 10.1016/j.jphotobiol.2019.111541. [DOI] [PubMed] [Google Scholar]
- 69.Ajithadas A., Nandhini S.R., Karthikeyan V., Bose P. Comparative in-vitro antioxidant screening of methanolic extract of Costus pictus and its silver nanoparticles. IJPSDR. 2014;6(4):334–340. [Google Scholar]
- 70.Ghosh S., More P., Nitnavare R., Jagtap S., Chippalkatti R., Derle A., Kitture R., Asok A., Kale S., Singh S., Shaikh M.L., Ramanamurthy B., Bellare J., Chopade B.A. Antidiabetic and antioxidant properties of copper nanoparticles synthesized by medicinal plant Dioscorea bulbifera. J Nanomed Nanotechnol. 2015;S6:007. [Google Scholar]
- 71.Jamdade D.A., Rajpali D., Joshi K.A., Kitture R., Kulkarni A.S., Shinde V.S., Bellare J., Babiya K.R., Ghosh S. Gnidia glauca- and Plumbago zeylanica-mediated synthesis of novel copper nanoparticles as promising antidiabetic agents. Adv Pharmacol Sci. 2019:1–11. doi: 10.1155/2019/9080279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balan K., Qing W., Wang Y., Liu X., Palvannan T., Wang Y., Ma F., Zhang Y. Antidiabetic activity of silver nanoparticles from green synthesis using Lonicera japonica leaf extract. RSC Advances. 2016;6:40162–40168. [Google Scholar]
- 73.Thiruvengadam M., Chung I.M., Gomathi T., Ansari M.A., Gopiesh-Khanna V., Babu V., Rajakumar G. Synthesis, characterization and pharmacological potential of green synthesized copper nanoparticles. Bioprocess Biosyst Eng. 2019;42:1769–1777. doi: 10.1007/s00449-019-02173-y. [DOI] [PubMed] [Google Scholar]
- 74.Malapermal V., Botha I., Krishna S.B.N., Mbatha J.N. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J Biol Sci. 2017;24:1294–1305. doi: 10.1016/j.sjbs.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patra J.K., Das G., Shin H.S. Facile green biosynthesis of silver nanoparticles using Pisum sativum L. outer peel aqueous extract and its antidiabetic, cytotoxicity, antioxidant, and antibacterial activity. Int J Nanomedicine. 2019;14:6679–6690. doi: 10.2147/IJN.S212614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kitture R., Chordiya K., Gaware S., Ghosh S., More P.A., Kulkarni P., Chopade B.A., Kale S.N. ZnO Nanoparticles-red sandalwood conjugate: A promising anti-diabetic agent. J Nanosci Nanotechnol. 2015;15:4046–4051. doi: 10.1166/jnn.2015.10323. [DOI] [PubMed] [Google Scholar]
- 77.Rajaram K., Aiswarya D.C., Sureshkumar P. Green synthesis of silver nanoparticle using Tephrosia tinctoria and its antidiabetic activity. Mater Lett. 2015;138:251–254. [Google Scholar]
- 78.Musthaba S.M., Baboota S., Ahmed S., Ahuja A., Ali J. Status of novel drug delivery technology for phytotherapeutics. Expert Opin Drug Deliv. 2009;6:625–637. doi: 10.1517/17425240902980154. [DOI] [PubMed] [Google Scholar]
- 79.Paliwal R.., Paliwal S.R., Kenwat R., Kurmi B.D., Sahu M.K. Solid lipid nanoparticles: a review on recent perspectives and patents. Expert Opin Ther Pat. 2020;30:179–194. doi: 10.1080/13543776.2020.1720649. [DOI] [PubMed] [Google Scholar]
- 80.Ahangarpour A., Oroojan A.A. Solid lipid nanoparticles of myricitrin have antioxidant and antidiabetic effects on streptozotocin-nicotinamide-induced diabetic model and myotube cell of male mouse. Oxid Med Cell Longev. 2018 doi: 10.1155/2018/7496936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su L., Zhou F., Yu M., Ge R., He J., Zhang B., Zhang Y., Fan J. Solid lipid nanoparticles enhance the resistance of oat-derived peptides that inhibit dipeptidyl peptidase IV in simulated gastrointestinal fluids. J Funct Foods. 2020;65 [Google Scholar]
- 82.Li J., Wang X., Zhang T., Wang C., Huang Z., Luo X., Deng Y. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 2015;10:81–98. [Google Scholar]
- 83.Mozafari M.R. In: Liposomes: Methods and protocols, Volume 1: Pharmaceutical Nanocarriers. Weissig V, editor. Humana Press; Totowa, NJ: 2010. Nanoliposomes: Preparation and Analysis; pp. 29–50. [Google Scholar]
- 84.Asadi S., Gholami M.S., Siassi F., Qorbani M., Khamoshian K., Sotoudeh G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo- controlled clinical trial. Complement Ther Med. 2019;43:253–260. doi: 10.1016/j.ctim.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 85.Rahimi H.R., Mohammadpour A.H., Dastani M., Jaafari M.R., Abnous K., Ghayour M.M., Kazemi O.R. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed. 2016;6:567–577. [PMC free article] [PubMed] [Google Scholar]
- 86.Fang Z., Bhandari B. Encapsulation of polyphenols – a review. Trends Food Sci Technol. 2010;21:510–523. [Google Scholar]
- 87.Ravichandran R. Nanoparticles in drug delivery: Potential green nanobiomedicine applications. Int J Green Nanotechnol Biomed. 2009;1:B108–BB30. [Google Scholar]
- 88.Zheng X., Wu F., Hong Y., Shen L., Lin X., Feng Y. Developments in taste-masking techniques for traditional chinese medicines. Pharmaceutics. 2018;10 doi: 10.3390/pharmaceutics10030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wandrey C., Bartkowiak A., Harding S.E. Materials for Encapsulation. Encapsulation Technologies for Active Food Ingredients and Food Processing. Zuidam, N. J., Nedovic,V, Springer. 2010:31–100. [Google Scholar]
- 90.Rizvi S.A.A., Saleh A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J. 2018;26:64–70. doi: 10.1016/j.jsps.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mittal A.K., Chisti Y., Banerjee U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31:346–356. doi: 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 92.Ranasinghe P., Pigera S., Galappatthy P., Katulanda P., Constantine G.R. Zinc and diabetes mellitus: understanding molecular mechanisms and clinical implications. Daru. 2015;23:44. doi: 10.1186/s40199-015-0127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pourkhalili N., Hosseini A., Nili-Ahmadabadi A., Hassani S., Pakzad M., Baeeri M., Mohammadirad A., Abdollahi M. Biochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic rats. World J Diabetes. 2011;2:204–210. doi: 10.4239/wjd.v2.i11.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shaheen T.I., El-Naggar M.E., Hussein J.S., El-Bana M., Emara E., El-Khayat Z., Fouda M.M.G., Ebaid H., Hebeish A. Antidiabetic assessment; in vivo study of gold and core-shell silver-gold nanoparticles on streptozotocin-induced diabetic rats. Biomed Pharmacother. 2016;83:865–875. doi: 10.1016/j.biopha.2016.07.052. [DOI] [PubMed] [Google Scholar]
- 95.Ashraf J.M., Ansari M.A., Khan H.M., Alzohairy M.A., Choi I. Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci Rep. 2016;6:20414. doi: 10.1038/srep20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murakami T., Tsuchida K. Recent advances in inorganic nanoparticle-based drug delivery systems. Mini Rev Med Chem. 2008;8:175–183. doi: 10.2174/138955708783498078. [DOI] [PubMed] [Google Scholar]
- 97.Mohamed M., Abu-Lila A.S., Shimizu T., Alaaeldin E., Hussein A., Sarhan H.A., Szebeni J., Ishida T. PEGylated liposomes: immunological responses. Sci Technol Adv Mater. 2019;20:710–724. doi: 10.1080/14686996.2019.1627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lushchak O., Zayachkivska A., Vaiserman A. Metallic nanoantioxidants as potential therapeutics for type 2 diabetes: A hypothetical background and translational perspectives. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/3407375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Silva S., Veiga M., Costa E.M., Oliveira A.L.S., Madureira A.R., Pintado M. Nanoencapsulation of polyphenols towards dairy beverage incorporation. Beverages. 2018;4:61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. List of 64 publications used in the review.