Abstract
During malolactic fermentation in wine by Oenococcus oeni, the degradation of citric acid was delayed compared to the degradation of malic acid. The maximum concentration of diacetyl, an intermediary compound in the citric acid metabolism with a buttery or nutty flavor, coincided with the exhaustion of malic acid in the wine. The maximum concentration of diacetyl obtained during malolactic fermentation was strongly dependent on the oxygen concentration and the redox potential of the wine and, to a lesser extent, on the initial citric acid concentration. The final diacetyl concentration in the wine was also dependent on the concentration of SO2. Diacetyl combines rather strongly with SO2 (Kf = 7.2 × 103 M−1 in 0.1 M malate buffer [pH 3.5] at 30°C). The reaction is exothermic and reversible. If the concentration of SO2 decreases during storage of the wine, the diacetyl concentration increases again.
One of the most difficult steps to control in winemaking is the so-called malolactic fermentation (MLF), which normally occurs after completion of the alcoholic fermentation. It is conducted by lactic acid bacteria (LAB), preferably Oenococcus oeni (formerly known as Leuconostoc oenos [4]), which deacidify the wine by converting malic acid, a dicarboxylic acid, to lactic acid, a monocarboxylic acid, resulting in a wine with a softer mouth feel. In addition, the MLF also affects the final aroma and taste balance by modifying fruit-derived aromas and producing aroma-active compounds (8).
In recent years, the introduction of commercial freeze-dried bacterial cultures of O. oeni for direct inoculation into wine has improved the control of MLF (17). Commercial cultures ensure better control of the time of onset and the rate of MLF, reduce the potential for spoilage by other bacteria, and reduce potential interference by bacteriophages (6). The winemaker can now pay more attention to the control of the flavor modifications induced by O. oeni.
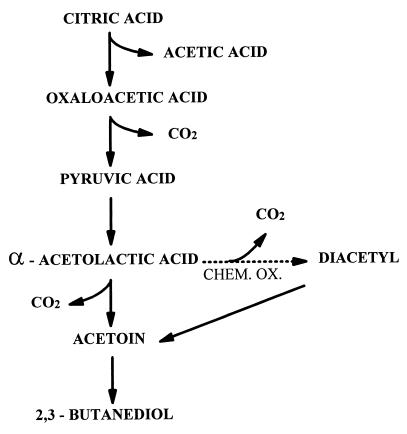
Not only malic acid but also citric acid in the wine is metabolized by O. oeni (Fig. 1). One of the intermediary compounds in the metabolism of citric acid is diacetyl, which is considered one of the most important flavors produced during MLF (8, 21). When present at a concentration above the sensory threshold, diacetyl gives the wine an aroma which can be characterized as buttery or nutty. It has been demonstrated that threshold values in different wines vary from 0.2 mg/liter in Chardonnay wine to 0.9 mg/liter in Pinot Noir and 2.8 mg/liter in Cabernet Sauvignon wine (11). The source of diacetyl is α-acetolactic acid (ALA), an unstable compound that besides the enzymatic decarboxylation by the bacteria also may decarboxylate spontaneously to acetoin and, in oxidizing conditions, also to diacetyl. In LAB-fermented dairy products, the latter reaction is now generally believed to be the only source of diacetyl (7). Diacetyl is reduced further by O. oeni to acetoin and 2,3-butanediol, which in normal concentrations has no influence on the wine aroma (18).
FIG. 1.
Main pathways for citric acid metabolism by O. oeni. CHEM. OX., chemical oxidation.
Understanding the factors influencing the diacetyl concentration is critical for the control of the final flavor in wine. The objective of this paper is to describe the dynamics of the citrate metabolism during MLF by O. oeni and how the concentration of the intermediary compound, diacetyl, is influenced by different physicochemical factors.
MATERIALS AND METHODS
Wine.
The wine used in the experiments was prepared from Chardonnay grape juice which was ultrahigh temperature treated (120°C for 10 s) just after the pressing. The juice was inoculated with Saccharomyces cerevisiae (Saint-George S101, Fould-Springer, France), and the alcoholic fermentation was conducted at 20°C in 5-liter glass containers fitted with fermentation locks. After the alcoholic fermentation, the yeast was removed from the wine by being filtered through a 0.45-μm-pore-size filter (Sartobran capsule from Sartorius). After filtration, the wine data were as follows: 12.0% (vol/vol) ethanol, 2.4 g of l-malic acid per liter, 0.35 g of citric acid per liter, and pH 3.4. The MLF in the wine was initiated by direct inoculation with freeze-dried O. oeni (Viniflora oenos; Chr. Hansen) to a concentration of 5 × 106 CFU/ml. Unless stated otherwise, all handling of the wine was conducted without access by atmospheric air by flushing bottles, filters, tubes, etc., with N2.
Experimental conditions.
The experiments with MLF under anaerobic and semiaerobic conditions were conducted in 2-liter wide-mouth bottles (Schott Glaswerke, Mainz, Germany). The semiaerobic conditions were obtained by placing a loose plastic lid on the bottles, leaving access by air to the wine while the anaerobic conditions were maintained by use of rubber stoppers on the bottles. Redox potential and O2 concentration were measured by continuously pumping wine from the bottles into a small container equipped with a redox and O2 electrode until the readings from the electrodes were constant. The wine removed from the anaerobic bottles during the measurements was replaced by N2.
The wine with completed MLF used in the physicochemical experiments was filtered through a 0.45-μm-pore-size filter (Sartobran capsule from Sartorius) before use in order to remove the bacteria. Wine storage experiments were conducted in 2-liter wide-mouth bottles fitted with rubber stoppers and fermentation locks.
The SO2 added to the wine was from a 10,000-mg/liter stock solution prepared from K2S2O5, and the diacetyl added was from a 5,000-mg/liter stock solution. All experiments were conducted at 20°C.
Bacterial enumeration.
The enumeration of viable bacteria was performed after appropriate dilution in water containing 0.1% peptone and 0.9% NaCl, followed by pour plate seeding in MRS agar (Oxoid Ltd.) with pH 5.0. Viable counts were obtained as the number of CFU after incubation at 30°C for 7 days.
Physicochemical analyses.
The redox potential and the O2 concentration were measured respectively with a combination platinum electrode (Pt 4865-50-SC-S7) with the Xerolyt Ag:AgCl reference system and with an O2 electrode (inst. type: MO 128) calibrated in wine at 0% O2 with N2, and then at 21% O2 with air (both electrodes from Mettler Toledo, Urdorf, Switzerland). Wine samples for analyses were stored at −20°C until analyzed. l-Malic acid, citric acid, and acetic acid were determined by the enzymatic test kits of Boehringer Mannheim. Ethanol and total SO2 were determined according to the method of Schmitt (23).
Diacetyl, ALA, and acetoin were determined by headspace gas chromatography as described by Richelieu et al. (22), except for the fact that the oven temperature was held at 60°C for 2 min, the total cycle time was 20 min, and the sample temperature for diacetyl was 30°C.
The two isomer forms of acetoin and three isomer forms of 2,3-butanediol were determined by gas chromatography. The gas chromatograph (HP 5890, Hewlett-Packard, Palo Alto, Calif.) was equipped with a mass selective detector (HP 5972; Hewlett-Packard) with a 25-m fused silica capillary column (0.25-mm inside diameter, CP Chirasil-Dex coating; part no 7502; Chrompack International BV, 4330 EA Middelburg, The Netherlands). The gas chromatograph was connected to a Hewlett-Packard autosampler (model HP 7673A). The operating parameters of the gas chromatograph were as follows: 1.0-μl samples injected in a split ratio of 1:30, 25 KPa of head pressure, 21-ml/min helium as carrier gas, and 250°C injector and detector temperatures. The oven temperature was held at 60°C for 3 min; the temperature was then increased in increments of 20°C/min up to 220°C, which was held for 5 min. To avoid any possible systematic effects on the final results, all samples for gas chromatography were randomized prior to analysis. The total 2,3-butanediol concentration was calculated from the sum of the three isomer forms.
All experiments were conducted in duplicate. Unless stated otherwise, the chemicals used were of analytical grade.
RESULTS
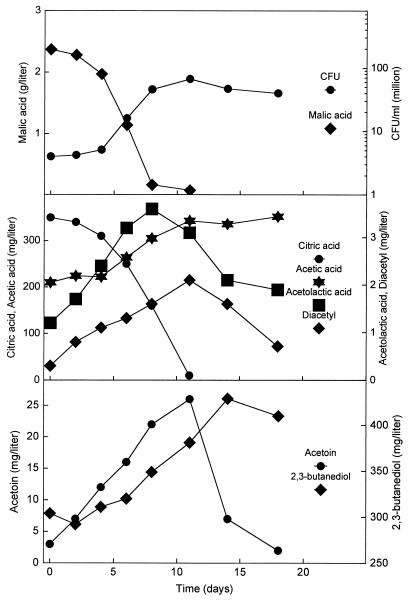
Figure 2 shows the MLF and the dynamics of the citrate fermentation induced in wine by direct inoculation with freeze-dried O. oeni. There was no loss of viability after inoculation, and the bacteria started to grow after 4 days and completed the degradation of malic acid in 8 days. The bacteria also degraded the citric acid. However, this degradation was delayed for several days compared to the degradation of the malic acid. The concentration of ALA increased during the catabolism of citric acid, and maximum was obtained just before the exhaustion of the citric acid. The concentration of diacetyl followed the same pattern, resulting in a maximum concentration just after completion of the MLF. ALA and diacetyl were further degraded by the bacteria to acetoin (Fig. 2). Both R and S isomers of acetoin were measured, although it was not possible to identify which was the R and which was the S form. One of the isomers dominated in the first part of the citric acid metabolism, and the other dominated toward the end (results not shown). This fluctuation probably reflects different formation pathways which may be by diacetyl reductase and ALA decarboxylase of the bacteria and by chemical decarboxylation of ALA. Acetoin was further reduced to 2,3-butanediol by the bacteria, about 80% of it as the mesoisomer.
FIG. 2.
The dynamics of the citrate fermentation during MLF in Chardonnay wine. The MLF was initiated by direct inoculation of freeze-dried O. oeni and conducted under anaerobic conditions.
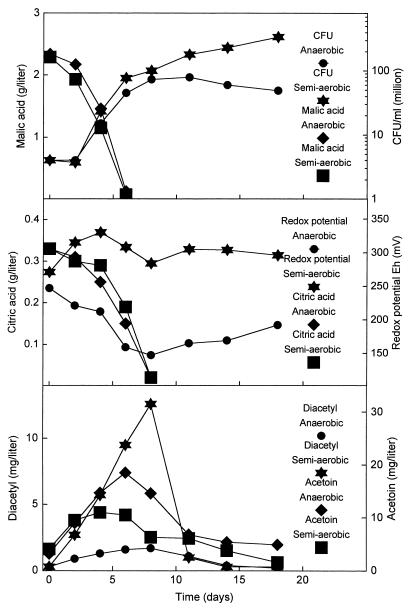
In practice, the physicochemical parameters for the MLF, including the redox potential and oxygen concentration, vary according to the local and individual vinification techniques. To examine in more detail the influence of the oxygen concentration and redox potential, MLF was conducted under anaerobic and semiaerobic conditions (Fig. 3). Under the anaerobic conditions, the redox potential decreased below 150 mV when the bacteria started to grow, and the oxygen concentration was less than 0.2 mg/liter throughout the fermentation. In the semiaerobic fermentation, the redox potential stabilized above 300 mV and the oxygen concentration stabilized between 2 and 4 mg/liter. The results in Fig. 3 show that the two different fermentation conditions had no influence on the degradation of malic and citric acid by the bacteria and that the growth of the bacteria during the degradation was almost the same in both fermentation conditions. However, large differences were observed for the diacetyl and acetoin concentrations. Under semiaerobic conditions, the diacetyl concentration reached 13 mg/liter, which was considerably higher than the 2 mg/liter obtained under anaerobic conditions. The acetoin concentrations showed the opposite behavior. A maximum of 20 mg/liter was obtained under anaerobic conditions, and 12 mg/liter was obtained under semiaerobic conditions. After exhaustion of malic and citric acid from the wine, the bacteria in the semiaerobic wine continued to grow, while the anaerobic wine did not support any further growth.
FIG. 3.
The effect of anaerobic and semiaerobic conditions on the citrate fermentation during MLF in Chardonnay wine. The MLF was initiated by direct inoculation of freeze-dried O. oeni.
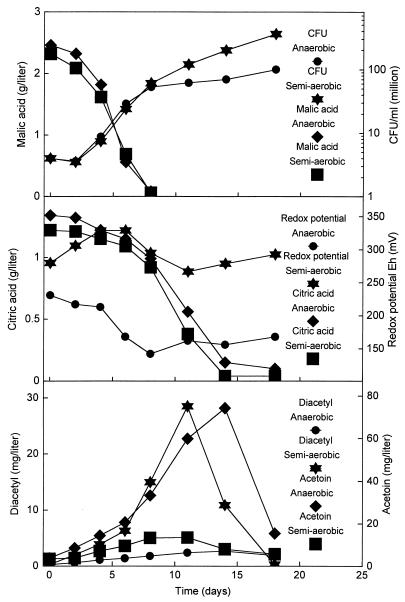
The same fermentations were conducted with addition of 1 g of citric acid per liter to the wine (Fig. 4). The redox potentials and oxygen concentrations showed the same pattern as in the fermentations without citric acid addition. Likewise, the bacterial population and degradation of malic and citric acid were again very similar under both the anaerobic and the semiaerobic conditions. The results clearly confirm that the degradation of citric acid is delayed compared to the degradation of malic acid. The extra citric acid had a pronounced effect on the diacetyl and acetoin concentrations. Under semiaerobic conditions, the diacetyl concentration reached a maximum of 29 mg/liter, more than twice the concentration obtained without addition of citric acid (Fig. 3). Under anaerobic conditions, the extra citric acid did not result in an increased diacetyl production. The maximum concentration of 2 mg/liter was similar to that for the wine without addition (Fig. 3 and 4). The maximum acetoin concentration obtained under semiaerobic conditions was not affected by citric acid addition (Fig. 3 and 4). However, under anaerobic conditions, the citric acid addition resulted in an increase in the maximum concentration of acetoin from 20 mg/liter (Fig. 3) to 75 mg/liter (Fig. 4). Figures 2, 3, and 4 show that O. oeni very effectively reduced both the diacetyl and the acetoin concentrations in the wine once the citric acid had been exhausted.
FIG. 4.
The effect of anaerobic and semiaerobic conditions on the citrate fermentation during MLF in Chardonnay wine supplemented with 1 g of citric acid per liter. The MLF was initiated by direct inoculation of freeze-dried O. oeni.
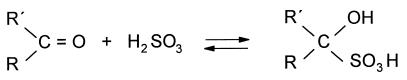
Besides the microbial activity, the final diacetyl concentration in wine is also affected by the concentration of SO2. The main sources of SO2 in wine are from the addition to the grape juice before the alcoholic fermentation, from SO2 produced by the yeast during the alcoholic fermentation, and from the addition after completion of the MLF. The last-mentioned addition, which normally stops all further microbiological activity, is the largest. SO2, which exists predominantly as the bisulfite ion at the pH observed in wine, has the ability to react with many different compounds in the wine, including carbonyl compounds like diacetyl (2). Generally, the reaction of SO2 with carbonyl compounds can be written as shown in Fig. 5.
FIG. 5.
Reaction of SO2 with a carbonyl compound.
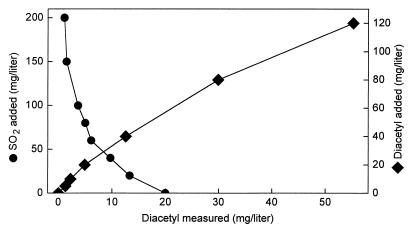
The reaction, which is reversible, is very important for the diacetyl concentration in wine. The formation equilibrium constant, Kf, of the reaction at different temperatures was calculated from headspace gas chromatography determinations of the diacetyl concentration in 0.1 M malate buffer (pH 3.5) to which were added variable concentrations of diacetyl and SO2. At 30, 45, and 70°C, the Kfs were calculated to be 7.2 × 103, 2.2 × 103, and 0.6 × 103 M−1, respectively, indicating that the reaction is exothermic, i.e., the equilibrium moves to the left with increasing temperatures. For practical illustration, Fig. 6 shows the diacetyl concentrations measured in wine with completed MLF and different amounts of SO2 and diacetyl added. The measured diacetyl concentrations are somewhat higher than those expected from the Kf determined in the malate buffer. Most probably this is because SO2 also reacts with compounds other than diacetyl in the wine. Nonetheless, Fig. 6 illustrates how SO2 rather effectively reduces the diacetyl concentration in a wine with completed MLF. For instance, addition of 80 mg of SO2 per liter, which is within the range used in the wine industry, reduced the diacetyl concentration from initially 20 to 5 mg/liter, i.e., by 75%.
FIG. 6.
The diacetyl concentration measured in Chardonnay wine with completed MLF and addition initially of the following: 20 mg of diacetyl per liter and different amounts of SO2 (•) or 80 mg of SO2 per liter and different amounts of diacetyl (♦).
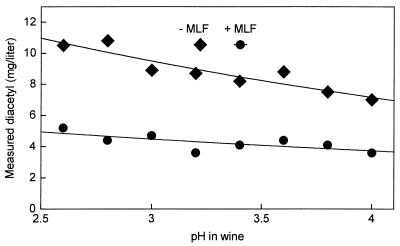
Figure 7 shows the influence of pH on the diacetyl concentration in wine to which were added initially 20 mg of diacetyl/liter and 80 mg of SO2/liter. The influence was determined both before and after MLF in the wine. The results show that the pH has only a weak influence on the reaction between diacetyl and SO2 in the pH range of 4.0 to 2.6, which covers most wines. What is more important for the diacetyl concentration is whether the wine has been taken through the MLF. In the wine without MLF, the diacetyl concentrations were about twice the concentrations measured in the wine with MLF.
FIG. 7.
The effect of pH in Chardonnay wine on the diacetyl concentration measured after addition of 20 mg of diacetyl per liter and 80 mg of SO2 per liter. The effect was examined in the wine before MLF (−MLF) and after MLF (+MLF).
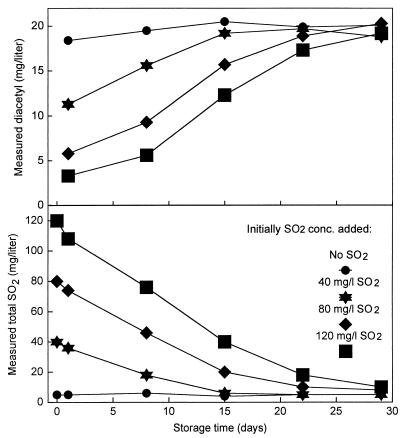
After sulfitation, most wines are stored in tanks, barrels, or bottles in a span ranging from a few months to several years. During this storage, some of the SO2 will evaporate and some will react, reversibly or irreversibly, with different compounds in the wine, including oxygen diffusing into the wine from the surroundings. This affects the diacetyl concentration in the wine because of the reversible nature of the reaction between diacetyl and SO2. Figure 8 shows results of an accelerated storage experiment where wine with diacetyl and different concentrations of SO2 added was stored in 2-liter glass bottles fitted with rubber stoppers and fermentation locks. The results show that immediately after the addition some of the SO2 combined with part of the diacetyl. However, during the 4 weeks of the experiment, the total SO2 concentration, i.e., the sum of uncombined and reversibly combined SO2, was reduced in all bottles and at the same time a concomitant increase in the diacetyl concentration in the wines was observed. It was beyond the scope of our work to identify the fate of the missing SO2 during the experiment.
FIG. 8.
The effect of storage time on the diacetyl and total SO2 concentrations in Chardonnay wine with completed MLF and initial addition of 20 mg of diacetyl per liter and different amounts of SO2. conc., concentration.
DISCUSSION
The catabolism of malic and citric acid in the wine by O. oeni was not concomitant but sequential. The observations seem to be very consistent because similar results have been obtained with different strains of O. oeni in wines from different parts of the world (16, 18). It is likely that the presence of malic acid inhibits the activity of one or more of the enzymes in the citric acid metabolism of the bacteria. Martineau and Henick-Kling (12) found that the presence of malic acid in the culture medium inhibited citric acid transport, and Lonvaud-Funel et al. (9) found that the activity of citrate lyase was maximal after the malic acid had disappeared. At many wineries, the exhaustion of malic acid in the wine is used as the criterion for completion of the MLF and thereby for the time of sulfitation, which stops all further microbiological activity. Our results show that this criterion may result in an incomplete citric acid degradation in the wine.
The concentration of ALA measured in wine was very low compared to the concentration normally observed in dairy products fermented with citrate-fermenting LAB, and only a new sensitive detection method (22) made the determination in wine possible. There may be several reasons for the low concentration. Ramos et al. (20) found that the ALA decarboxylase level in O. oeni is 10 times higher than the ALA synthetase level. Besides, ALA is an unstable compound that may decarboxylate spontaneously to either diacetyl or acetoin, especially at a low pH such as the pH in wine (14, 22).
Fluctuations in the diacetyl concentration caused by O. oeni have been reported by several persons (3, 19). In accordance with our previous observations (16), the maximum diacetyl concentration was obtained around the time when malic acid was exhausted from the wine followed by degradation again by the bacteria. For control of the final diacetyl concentration in the wine, it is important to be aware of this coincidence of maximum diacetyl concentration and exhaustion of malic acid. If the wine is sulfited at this point, which is common at many wineries, all further microbiological activity stops. And so does the irreversible reduction of the diacetyl, because this can be accomplished only by living bacteria and yeast (16). If the buttery note from diacetyl is too overwhelming after exhaustion of the malic acid, it is advisable to hesitate with sulfitation until the diacetyl concentration has been reduced by the bacteria and yeast.
The total production of diacetyl and acetoin during the MLF by O. oeni was stimulated by increased citric acid concentrations in the wine. However, the production of the two compounds was not equally but strongly dependent on the redox potential and O2 concentration of the wine. Similar results have been reported for skim milk fermented with the ALA-accumulating strain of Lactococcus lactis subsp. lactis biovar diacetylactis (15). An examination of synthetic ALA shows that the spontaneous decarboxylation of ALA to diacetyl is strongly favored by high redox potential and O2 concentration and low pH, such as the pH in wine (14, 22). From this and the fact that diacetyl synthetase has never been isolated from LAB, it seems most likely that the diacetyl in wine is the result of a pure chemical reaction between ALA and O2. At low redox potentials and O2 concentrations, the ALA is converted almost exclusively, either chemically or by the bacterial ALA decarboxylase, to acetoin.
After the malic and citric acids were degraded, the bacteria in the semiaerobic wine continued to grow in contrast to the bacteria in the anaerobic wine. It is possible that certain compounds in the wine can function as a substrate for O. oeni only when O2 is available. Certain substrates, such as polyols, have been reported to be fermented by some LAB only when O2 is available (1, 5).
Sulfite added to wine reacts fast and rather strongly with diacetyl and thereby reduces the buttery flavor. However, in contrast to the microbiological reduction by bacteria and yeast, this reaction is reversible. If some of the SO2 evaporates or combines with other compounds in the wine, the concentration of the flavor may later increase in the wine again. This should be kept in mind when the time of sulfitation after the MLF is decided and when the wine is stored in tanks or barrels and later bottled.
The concentration of free diacetyl in wine containing SO2 may also depend on the concentration of other SO2-binding compounds, such as acetaldehyde, α-ketoglutaric acid, and pyruvic acid. Mayer et al. (13) found that these compounds are substantially reduced during MLF. Accordingly, we measured a decrease in the concentration of acetaldehyde, which combines very strongly with SO2 (Kf = 7 × 105 M−1 [2]), from initially 17 mg/liter to 1.5 mg/liter at the end of the MLF. The proportion of SO2 available for reaction with diacetyl is therefore higher in wine after the MLF than before, and this probably explains the observed lower proportion of free diacetyl in wine after the MLF than before (Fig. 7).
Several publications have described the influence of the diacetyl concentration on the wine flavor (11, 21). The present results show that if the wine contains SO2, it is important to be aware of the reversible and exothermic reaction of SO2 with diacetyl when the actual diacetyl concentration in the wine is determined and correlated with an organoleptic evaluation. If, e.g., headspace gas chromatography is used for the determination, as in this publication, the sample equilibration temperature should not be higher than 30°C in order not to overestimate the actual diacetyl concentration. The exothermic nature of the reaction also indicates that if one wishes to accentuate the buttery-nutty aroma of diacetyl in, e.g., a bottle of Chardonnay, it should be consumed at 20 rather than at 10°C.
REFERENCES
- 1.Brown J P, VanDemark P J. Respiration of Lactobacillus casei. Can J Microbiol. 1968;14:829–835. doi: 10.1139/m68-141. [DOI] [PubMed] [Google Scholar]
- 2.Burroughs L F, Sparks A H. Sulphite-binding power of wines and ciders. II. Theoretical consideration and calculation of sulphite-binding equilibria. J Sci Food Agric. 1973;24:199–206. doi: 10.1002/jsfa.2740240212. [DOI] [PubMed] [Google Scholar]
- 3.de Revel G, Bertrand A, Lonvaud-Funel A. Synthèse des substances acétoïniques par Leuconostoc oenos. Reduction du diacétyle. Connaiss Vigne Vin. 1989;1:39–45. [Google Scholar]
- 4.Dicks L M T, Dellaglio F, Collins M D. Proposal to reclassify Leuconostoc oenos as Oenococcus oeni [corrig.] gen. nov., comb. nov. Int J Syst Bacteriol. 1995;45:395–397. doi: 10.1099/00207713-45-2-395. [DOI] [PubMed] [Google Scholar]
- 5.Dobrogosz W J, Stone R W. Oxidative metabolism in Pediococcus pentosaceus. I. Role of oxygen and catalase. J Bacteriol. 1962;84:716–723. doi: 10.1128/jb.84.4.716-723.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henick-Kling T. Malolactic fermentation. In: Fleet G H, editor. Wine microbiology and Biotechnology. Chur, Switzerland: Harwood Academic Publishers; 1993. pp. 289–326. [Google Scholar]
- 7.Hugenholtz J. Citrate metabolism in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:165–178. [Google Scholar]
- 8.Laurent M H, Henick-Kling T, Acree T E. Changes in the aroma and odor of Chardonnay due to malolactic fermentation. Wein-Wiss. 1994;49:3–10. [Google Scholar]
- 9.Lonvaud-Funel A, Zmirou C, Larue F. Le métabolisme de l’acide citrique par les bactéries lactiques de la fermentation malolactique des vins. Sci Aliments 4 hors série. 1984;3:81–85. [Google Scholar]
- 10.Martineau B, Henick-Kling T. Performance and diacetyl production of commercial strains of malolactic bacteria in wine. J Appl Bacteriol. 1995;78:526–536. [Google Scholar]
- 11.Martineau B, Acree T E, Henick-Kling T. Effect of wine type on threshold for diacetyl. Food Res In. 1995;28:139–143. [Google Scholar]
- 12.Martineau B, Henick-Kling T. Effect of malic acid on citric acid metabolism in Leuconostoc oenos. Am J Enol Vitic. 1996;47:229. . (Abstract.) [Google Scholar]
- 13.Mayer K, Pause G, Vetsch U. Gehalte an SO2-bindenden Stoffen in Wein: Einfluss von Garüng und biologischem Säureabbau. Schweiz Z Obst Weinbau. 1976;112:309–313. [Google Scholar]
- 14.Monnet C, Schmitt P, Divies C. Method for assaying volatile compounds by headspace gas chromatography and application to growing starter cultures. J Dairy Sci. 1994;77:1809–1815. [Google Scholar]
- 15.Monnet C, Schmitt P, Divies C. Diacetyl production in milk by an α-acetolactic acid accumulating strain of Lactococcus lactis ssp. lactis biovar. diacetylactis. J Dairy Sci. 1994;77:2916–2924. [Google Scholar]
- 16.Nielsen J C, Prahl C. Metabolism of citric acid by Leuconostoc oenos in direct inoculation. Effect on wine flavour. In: Lonvaud-Funel A, editor. Oenologie 95, 5e Symposium international d’oenologie. Paris, France: Tec & Doc; 1995. pp. 317–320. [Google Scholar]
- 17.Nielsen J C, Prahl C, Lonvaud-Funel A. Malolactic fermentation in wine by direct inoculation with freeze-dried Leuconostoc oenos cultures. Am J Enol Vitic. 1996;47:42–48. [Google Scholar]
- 18.Nielsen J C, Prahl C. The dynamics of citrate metabolism during malolactic fermentation by Leuconostoc oenos. In: Henick-Kling T, Wolf T E, Harkness E M, editors. Proceedings of the Fourth International Symposium on Cool Climate Viticulture & Enology, VI. New York, N.Y: American Society for Enology and Viticulture; 1996. pp. 72–73. [Google Scholar]
- 19.Postel W, Meier B. Verhalten von 2-Acetolactat, 2-Acetohydroxybutyrat, Diacetyl, 2,3-Pentandion und Acetoin während des bakteriellen Apfelsäureabbaus in Wein. Z Lebensm Unters Forsch. 1983;176:356–359. [Google Scholar]
- 20.Ramos A, Lolkema J S, Konings W N, Santos H. Enzyme basis for pH regulation of citrate and pyruvate metabolism by Leuconostoc oenos. Appl Environ Microbiol. 1995;61:1303–1310. doi: 10.1128/aem.61.4.1303-1310.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rankine B C, Fornachon J C M, Bridson D A. Diacetyl in Australian dry red wines and its significance in wine quality. Vitis. 1969;8:129–134. [Google Scholar]
- 22.Richelieu M, Houlberg U, Nielsen J C. Determination of α-acetolactic acid and volatile compounds by headspace gas chromatography. J Dairy Sci. 1997;80:1918–1925. [Google Scholar]
- 23.Schmitt A. Aktuelle Weinanalytik. 2nd ed. Schwäbisch Hall, Germany: Verlag Heller Chemie- und Verwaltungsgesellschaft mbH; 1983. [Google Scholar]