Abstract
Rationale
Pulmonary arterial hypertension (PAH) is a rare disease characterised by limited survival despite remarkable improvements in therapy. The causes, clinical burden and outcomes of patients admitted to the intensive care unit (ICU) remain poorly characterised. The aim of this study was to describe patient characteristics, causes of ICU hospitalisation, and risk factors for ICU and 1-year mortality.
Methods
Data from patients enrolled in the Johns Hopkins Pulmonary Hypertension Registry were analysed for the period between January 2010 and December 2020. Clinical, functional, haemodynamic and laboratory data were collected.
Measurements and main results
102 adult patients with 155 consecutive ICU hospitalisations were included. The leading causes for admission were right heart failure (RHF, 53.3%), infection (17.4%) and arrhythmia (11.0%). ICU mortality was 27.1%. Mortality risk factors included Na <136 mEq·mL−1 (OR: 3.10, 95% CI: 1.41–6.82), elevated pro-B-type natriuretic peptide (proBNP) (OR: 1.75, 95% CI: 1.03–2.98), hyperbilirubinaemia (OR: 1.40, 95% CI: 1.09–1.80), hyperlactaemia (OR: 1.42, 95% CI: 1.05–1.93), and need for vasopressors/inotropes (OR: 5.29, 95% CI: 2.28–12.28), mechanical ventilation (OR: 3.76, 95% CI: 1.63–8.76) and renal replacement therapy (OR: 5.57, 95% CI: 1.25–24.76). Mortality rates at 3, 6 and 12 months were 17.5%, 27.6% and 39.0%, respectively. Connective tissue disease-associated PAH has lower 1-year survival compared to idiopathic PAH (51.4% versus 79.8%, log-rank test p=0.019).
Conclusions
RHF is the most common cause for ICU admission. In-hospital and 1-year mortality remain exceedingly high despite improved ICU care. Recognising specific risk factors on admission can help identifying patients at risk for poor outcomes.
Short abstract
PAH continues to have limited survival despite improvements in therapy. ICU and 1-year mortality risk factors include hyponatraemia, elevated proBNP, hyperbilirubinaemia, and need for vasopressors/inotropes and mechanical ventilation. https://bit.ly/3wCGGQg
Introduction
Pulmonary arterial hypertension (PAH) remains a disease with significant morbidity and mortality despite a plethora of modern medications which have improved general outcomes [1–3]. It is a complex disorder of the pulmonary vasculature leading to progressive adaptive and maladaptive morphological and functional changes of the right ventricle (RV), with RV failure being the single most important determinant of survival [4, 5].
The clinical course of PAH is characterised, in part, by a high risk of hospitalisation. Data from recent large multicentre trials and observational studies have identified right heart failure (RHF) as the primary cause of hospital admission and mortality in PAH patients [6–10]. However, patient characteristics and comorbidities, causes of admission and mortality risk factors of patients admitted to the intensive care unit (ICU) remain poorly defined, perhaps explaining the limited number of targeted clinical trials and therapeutic recommendations or guidelines from scientific societies for the management of critically ill PAH patients [11, 12].
The aims of this study were to analyse the causes, clinical characteristics, outcomes and mortality risk factors of PAH patients needing admission to the medical ICU, and to assess the 3-, 6- and 12-month mortality rates and associated risk factors upon hospital discharge.
Patients and methods
Patient population
From January 2010 to December 2020, data of adult PAH patients enrolled in the Johns Hopkins Pulmonary Hypertension Registry and hospitalised at the Johns Hopkins Hospital Medical ICU were collected. The diagnosis of PAH, group 1 pulmonary hypertension (PH), was confirmed by a mean pulmonary artery pressure (mPAP) >20 mmHg at rest, a pulmonary capillary wedge pressure (PCWP) ≤15 mmHg and a pulmonary vascular resistance (PVR) ≥3 Wood units [13]. Within group 1 PH, we limited our analysis to idiopathic PAH (IPAH), PAH due to connective tissue diseases (CTD-PAH), congenital heart diseases-PAH (CHD-PAH), portopulmonary hypertension-PAH (PoPH-PAH) and HIV-associated PAH (HIV-PAH). Other causes of group 1 PH were excluded due to a low number of subjects. This study was approved by the Johns Hopkins University Institutional Review Board.
Demographic data, including medical history, PAH characteristics at diagnosis and ICU admission and discharge information, were retrieved from electronic records. Charlson comorbidity index was calculated from the medical history [14]. Anaemia was defined as haemoglobin <13 g·dL−1 for males and <12 g·dL−1 for females [15]. Hyponatraemia was defined as a Na serum concentration ≤136 mEq·L−1 [6]. Estimated glomerular filtration rate (eGFR) was calculated using the Modified Diet in Renal Disease equation [16]. RHF was defined by symptoms and physical evidence of volume overload [17, 18].
Risk factors for in-hospital ICU mortality included demographics, underlying PAH diagnosis, World Health Organization functional class (WHO FC) at the latest outpatient assessment, physical signs and laboratory parameters on ICU admission, and ever need for vasopressors/inotropes, mechanical ventilation and renal replacement therapy during the ICU stay. These variables were selected based on prior studies that have addressed mortality related to hospital and ICU admissions in PAH patients [1, 6, 12, 19–21]. Among survivors, mortality at 3, 6 and 12 months was analysed from the index hospitalisation. Risk factors for mortality after discharge included similar variables. The latest clinic visit or contact with the patient was considered as censoring time for living patients. Death after discharge was determined from hospital records up to 31 December 2020.
Statistical methods
Group comparisons were made using the Chi-squared and Fisher exact tests as appropriate for categorical variables and ANOVA test for continuous variables. Patients were grouped as IPAH, CTD-PAH and other-PAH (including CHD-PAH, PoPH-PAH and HIV-PAH) for optimal statistical analysis and result presentation. Correlation analyses were performed to identify significant variables to be considered for regression analysis. The identification of prognostic factors for ICU mortality was performed using logistic regression with robust adjustment of the variance for repeated measurements to handle patients with multiple hospitalisations. Risk factors were adjusted for age and underlying PAH diagnosis in a multivariable model. Mortality after ICU discharge was assessed using the Kaplan–Meier method. Comparisons between groups were performed by log-rank test. Risk factors for mortality at 1 year were assessed via Cox proportional hazards models. Computations were completed using the Stata statistical software (version 16.1; Stata, College Station, TX, USA). A p-value <0.05 was considered statistically significant.
Results
Patient characteristics
From January 2010 to December 2020, 102 PAH patients were admitted to the Johns Hopkins Hospital Medical ICU. The general characteristics of the patient population are presented in table 1. 29 patients had IPAH, 57 patients CTD-PAH and 16 patients had other-PAH (CHD-PAH (3), PoPH-PAH (6) and HIV-PAH (7)). Compared to IPAH, the patient group with CTD-PAH were older (63.5±11.9 versus 53.7±13.5 years; p<0.001), had a lower proportion of females (89.5% versus 93.1%; p<0.001), included a higher proportion of White (63.1% versus 55.2%; p=0.05) and a lower proportion of African American individuals (35.1% versus 41.4%; p=0.05), presented with a higher Charlson comorbidity index (≥3) (77.2% versus 37.9%; p<0.001), and were more likely to have a history of cerebrovascular disease, peripheral vascular disease and depression.
TABLE 1.
Characteristics of PAH patients admitted to the ICU
| All | IPAH | CTD-PAH # | Other-PAH ¶ | p-value | |
| Subjects n | 102 | 29 | 57 | 16 | |
| Age upon first admission years | 58.7±13.6 | 53.7±13.5 | 63.5±11.9 | 50.6±12.8 | <0.001 |
| Female | 86 (84.3) | 27 (93.1) | 51 (89.5) | 8 (50.0) | <0.001 |
| Race n (White/AA/Hispanic/others) | 59/39/2/2 | 16/12/0/1 | 36/20/0/1 | 7/7/2/0 | 0.05 |
| WHO FC pre-admission n (II/III/IV) | 24/59/19 | 10/15/4 | 12/36/9 | 2/8/6 | 0.002 |
| Time since PAH diagnosis to first ICU admission months, median (IQR) | 38.9 (12.4–92.3) | 64.5 (25.9–147.3) | 39.5 (11.8–92.1) | 24.6 (9.7–33.9) | 0.002 |
| Haemodynamics at PAH diagnosis | |||||
| RAP mmHg | 11±6 | 12±6 | 9±6 | 12±7 | 0.14 |
| mPAP mmHg | 45±14 | 54±14 | 39±12 | 50±15 | <0.001 |
| PCWP mmHg | 10±4 | 10±3 | 11±4 | 11±4 | 0.97 |
| Cardiac output L·min−1 | 4.7±1.9 | 4.6±2.0 | 4.8±1.8 | 4.6±2.1 | 0.85 |
| Cardiac index L·min−1·m−2 | 2.19±0.64 | 2.11±0.53 | 2.23±0.69 | 2.12±0.58 | 0.61 |
| PVR Wood units, median (IQR) | 7.7 (4.8–11.8) | 10.0 (7.6–14.6) | 6.7 (3.0–9.6) | 9.4 (5.3−14.0) | 0.008 |
| Comorbidities | |||||
| Coronary artery disease | 10 (9.8) | 4 (13.8) | 4 (7.2) | 2 (13.0) | 0.56 |
| Systemic hypertension | 23 (22.6) | 8 (27.6) | 14 (24.6) | 1 (6.3) | 0.22 |
| Diabetes mellitus | 14 (13.7) | 9 (31.0) | 3 (5.3) | 2 (12.5) | 0.005 |
| Peripheral vascular disease | 3 (2.9) | 0 (0) | 2 (3.5) | 1 (6.3) | 0.45 |
| Cerebrovascular disease | 3 (2.9) | 0 (0) | 2 (3.5) | 1 (6.3) | 0.45 |
| History of depression | 9 (8.8) | 1 (3.5) | 5 (8.8) | 3 (18.8) | 0.68 |
| Charlson comorbidity index | |||||
| 0 | 9 (8.8) | 8 (27.6) | 0 (0) | 1 (6.3) | <0.001 |
| 1 | 10 (9.8) | 4 (13.8) | 6 (10.4) | 0 (0) | |
| 2 | 15 (14.7) | 6 (20.7) | 7 (12.3) | 2 (12.5) | |
| ≥3 | 68 (66.7) | 11 (37.9) | 44 (77.2) | 13 (81.2) | |
Data are expressed as mean±sd and n (%) unless otherwise indicated. PAH: pulmonary arterial hypertension; ICU: intensive care unit; IPAH: idiopathic PAH; CTD-PAH: connective tissue diseases PAH; AA: African American; WHO FC: World Health Organization functional class; IQR: interquartile range; RAP: right atrial pressure; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance. #: CTD-PAH includes: 46 scleroderma-associated PAH (SSc-PAH) patients and 11 patients with other CTD-associated PAH conditions; ¶: other-PAH includes: CHD-PAH (3), portopulmonary hypertension-PAH (6) and HIV-associated PAH (7) patients.
WHO FC, assessed during the last clinical encounter before admission, showed an overall high proportion of patients with functional limitation (WHO FC II: 23.5%, III: 57.9%, IV: 18.6%).
Haemodynamic data obtained at the time of PAH diagnosis indicated severe PAH across all groups, with a right atrial pressure (RAP) of 11±6 mmHg, mPAP of 45±14 mmHg, cardiac output (CO) of 4.7±1.9 L·min−1, cardiac index of 2.19±0.64 L·min−1·m−2 and PVR of 7.7 Wood units (IQR: 4.8–11.8). Patients with CTD-PAH had significantly lower mPAP compared with IPAH and other-PAH patients (39 versus 54 versus 50 mmHg; p<0.001), as well as a lower PVR (6.7 versus 10.0 versus 9.4 Wood units; p=0.008). Other haemodynamic parameters such as CO, cardiac index or RAP were similar between groups. The median time from PAH diagnosis to first ICU admission was 38.9 months (IQR: 12.4–92.3) and was significantly lower in CTD-PAH compared to IPAH patients (39.5 versus 64.5 months; p=0.002).
Characteristics of ICU hospitalisations
A total of 155 ICU hospitalisations in 102 patients were identified. The characteristics of the patients and hospitalisations, and differences between groups, are shown in table 2. The underlying diagnoses were IPAH (45 hospitalisations), CTD-PAH (85 hospitalisations) and other-PAH (25 hospitalisations, including CHD-PAH (3), PoPH-PAH (11) and HIV-PAH (11)). The most common causes for ICU admission were RHF (83; 53.5%), infection (27; 17.4%), arrhythmia (17; 11.0%), syncope (13; 8.4%) and bleeding disorders (8; 5.2%). Among infections, those related to pneumonia were the most frequent (15; 55.6%), followed by skin and soft tissue infections (6; 22.2%), urinary tract infections (4; 14.8%) and central line bloodstream infections (2; 7.4%).
TABLE 2.
Characteristics of ICU admissions
| All | IPAH | CTD-PAH | Other-PAH # | p-value | |
| Subjects n | 155 | 45 | 85 | 25 | |
| Causes of ICU admission | |||||
| Right heart failure | 83 (53.5) | 27 (60.0) | 45 (52.9) | 11 (44.0) | 0.27 |
| Infection | 27 (17.4) | 6 (13.3) | 16 (18.8) | 5 (20.0) | 0.54 |
| Arrhythmia | 17 (11.0) | 5 (11.1) | 11 (12.9) | 1 (4.0) | 0.61 |
| Syncope | 13 (8.4) | 1 (2.2) | 8 (9.4) | 4 (16.0) | 0.09 |
| Bleeding disorder | 8 (5.2) | 2 (4.4) | 3 (3.5) | 3 (12.0) | 0.19 |
| Others | 7 (4.5) | 4 (8.9) | 2 (2.4) | 1 (4.0) | 0.37 |
| Admission data | |||||
| Systolic BP mmHg | 105.9±22.1 | 105.3±15.5 | 106.8±24.3 | 103.6±26.6 | 0.85 |
| Diastolic BP mmHg | 62.4±12.9 | 63.2±9.0 | 62.9±15.0 | 58.3±9.9 | 0.37 |
| Heart rate beats per min | 93.7±17.7 | 94.9±15.1 | 93.7±18.7 | 91.1±19.8 | 0.75 |
| Creatinine mg·dL−1 | 1.8±1.4 | 1.8±1.3 | 1.5±1.2 | 2.6±2.0 | 0.006 |
| eGFR <60 mL·min−1 per 1.73 m2 | 97 (62.6) | 26 (57.8) | 52 (61.2) | 19 (76.0) | 0.009 |
| BUN mg·dL−1 | 34.5±22.3 | 33.0±21.3 | 36.2±23.1 | 31.7±22.0 | 0.57 |
| Na mEq·mL−1 | 135.7±6.1 | 135.2±6.8 | 136.5±5.9 | 134.1±4.7 | 0.16 |
| Na <136 mEq mL−1 | 74 (47.7) | 17 (37.8) | 37 (43.5) | 20 (80.0) | 0.002 |
| Bilirubin, total, mg·dL−1 | 1.5±1.6 | 1.7±2.0 | 1.0±0.9 | 2.5±2.0 | <0.001 |
| Lactate mmol·L−1 | 2.6±3.1 | 2.4±2.9 | 2.6±3.3 | 3.1±2.9 | 0.72 |
| Haemoglobin g·dL−1 | 11.0±2.4 | 11.6±2.9 | 10.9±2.1 | 10.6±2.5 | 0.15 |
| Anaemia | 115 (74.2) | 31 (68.9) | 63 (74.1) | 21 (84.0) | 0.38 |
| ProBNP pg·mL−1, median (IQR) | 3851 (2229–7000) | 3192 (1533–6874) | 4727 (2302–6945) | 4209 (921–8518) | 0.63 |
| ICU support | |||||
| Vasopressors/inotropes | 75 (48.4) | 19 (42.2) | 43 (50.6) | 13 (52.0) | 0.61 |
| Mechanical ventilation | 32 (20.1) | 9 (20.0) | 17 (20.0) | 6 (24.0) | 0.90 |
| Renal replacement therapy | 9 (5.8) | 2 (4.4) | 2 (2.4) | 5 (20.0) | 0.004 |
| Length of ICU stay days, median (IQR) | 5 (2–9) | 5 (2–8) | 5 (3–10) | 5 (3–8) | 0.60 |
| ICU mortality | 42 (27.1) | 10 (22.2) | 22 (25.9) | 10 (40.0) | 0.25 |
| Right heart failure, mortality | 26 (31.3) | 9 (90.0) | 12 (54.5) | 5 (50.0) | 0.31 |
| Infections, mortality | 6 (22.2) | 0 (0) | 5 (22.7) | 1 (10.0) | 0.76 |
| Vasopressors/inotropes, mortality | 32 (42.7) | 8 (80.0) | 15 (68.2) | 9 (90.0) | <0.001 |
| Mechanical ventilation, mortality | 16 (50.0) | 5 (50.0) | 7 (31.8) | 4 (40.0) | 0.001 |
Data are expressed as mean±sd and n (%) unless otherwise indicated. ICU: intensive care unit; PAH: pulmonary arterial hypertension; IPAH: idiopathic PAH; CTD-PAH: connective tissue diseases PAH; BP: blood pressure; eGFR: estimated glomerular filtration rate; BUN: blood urea nitrogen; Na: sodium; proBNP: pro-B-type natriuretic peptide; IQR: interquartile range. #: other-PAH includes: CHD-PAH (3), portopulmonary hypertension-PAH (11) and HIV-associated PAH (11) admissions.
Upon admission, systolic blood pressure (SBP), diastolic blood pressure, heart rate, blood urea nitrogen (BUN), lactate, haemoglobin and pro-B-type natriuretic peptide (proBNP) were not significantly different across groups. In contrast, creatinine was higher among other-PAH patients compared to IPAH and CTD-PAH (2.6 versus 1.8 versus 1.5 mg·dL−1; p=0.006). CTD-PAH patients had worse renal function overall based on eGFR <60 mL·min−1 per 1.73 m2 (p=0.009). IPAH presented the lowest proportion of patients with serum sodium concentrations <136 mEq·mL−1 (17; 37.8%). Patients with other-PAH had the highest mean levels of total bilirubin (2.5±2.0 mg·dL−1; p<0.001). During their ICU stay, 75 patients (48.4%) received vasopressors or inotropes, 32 (20.1%) received mechanical ventilation and 9 (5.8%) received renal replacement therapy. Among patients who received vasopressors or inotropes, norepinephrine was the most common vasopressor (55.8%), followed by dopamine (31.2%), with the latter including patients who only received low-dose dopamine to assist diuresis. Vasopressin was used in 21 patients (27.3%) and dobutamine in 12 patients (15.7%), usually as adjunct therapies to norepinephrine.
Regarding therapy, 91 (89.2%) patients were already receiving PAH-specific therapy. The most commonly used medications included phosphodiesterase-5 inhibitors (PDE5-i) (78), followed by endothelin receptor antagonists (53) and prostanoids (35). Patients not receiving targeted therapy, included patients with a recent PAH diagnosis and who required immediate hospital admission.
The median length of ICU stay for all groups was 5 days (IQR: 2–9). Overall, ICU mortality was 27.1% (42 patients), and 42.7% and 50% among patients who required vasopressor/inotropes or mechanical ventilation, respectively. The in-hospital mortality was 31.3% for patients admitted for RHF, and 22.2% for those admitted due to infection.
Risk factors for in-hospital mortality during ICU hospitalisations
Significant risk factors for in-hospital mortality are shown in table 3. Several variables were shown by univariable logistic regression models to be potential risk factors. After adjusting for age and underlying PAH diagnosis, risk factors were Na <136 mEq·mL−1 (OR 3.10; p=0.005), elevated proBNP (OR 1.75; p=0.03), hyperbilirubinaemia (OR 1.40; p=0.009), hyperlactaemia (OR 1.42; p=0.023), use of vasopressors or inotropes (OR 5.29; p<0.001), mechanical ventilation (OR 3.76; p=0.002) and renal replacement therapy (OR 5.57; p=0.024). Although eGFR <60 mL·min−1 per 1.73 m2 was not statistically significant in the multivariable logistic regression model, its unadjusted significance is noteworthy (OR 5.27; p=0.04).
TABLE 3.
Risk factors for in-hospital mortality in PAH patients admitted to the ICU
|
Unadjusted
OR (95% CI) |
p-value |
Adjusted for PAH diagnosis and age
OR (95% CI) |
p-value | |
| Age per year | 1.01 (0.98–1.03) | 0.67 | NA | NA |
| Female versus male | 0.47 (0.20–1.10) | 0.08 | 0.57 (0.23–1.40) | 0.22 |
| CTD-PAH versus IPAH | 1.27 (0.52–2.90) | 0.64 | 1.11 (0.44–2.80)# | 0.83 |
| WHO FC pre-admission (III and IV) | 4.05 (0.56–29.27) | 0.16 | 3.46 (0.41–26.10) | 0.26 |
| Charlson index ≥2 | 1.94 (0.12–31.89) | 0.64 | 1.30 (0.22–7.80) | 0.29 |
| Data on admission | ||||
| Systolic BP per mmHg decrease | 0.99 (0.97–1.01) | 0.36 | 0.99 (0.97–1.01) | 0.28 |
| Systolic BP <100 mmHg | 1.05 (0.51–2.16) | 0.88 | 1.06 (0.51–2.20) | 0.87 |
| Anaemia | 0.70 (0.32–1.54) | 0.37 | 0.64 (0.29–1.43) | 0.28 |
| eGFR <60 mL·min−1 per 1.73 m2 | 5.27 (1.05–26.60) | 0.04 | 4.65 (0.86–25.10) | 0.07 |
| Na <136 mEq·mL−1 | 3.37 (1.58–7.19) | 0.002 | 3.10 (1.41–6.82) | 0.005 |
| ProBNP pg·mL−1 (log transformed) | 1.74 (1.04–2.93) | 0.036 | 1.75 (1.03–2.98) | 0.03 |
| Bilirubin, total, mg·dL−1 | 1.39 (1.08–1.78) | 0.01 | 1.40 (1.09–1.80) | 0.009 |
| Lactate mmol·L−1 | 1.40 (0.98–2.02) | 0.06 | 1.42 (1.05–1.93) | 0.023 |
| Previous hospitalisation | 1.10 (0.52–2.31) | 0.89 | 1.09 (0.52–2.31) | 0.81 |
| In-hospital support | ||||
| Need for vasopressors/inotropes | 5.21 (2.32–11.68) | <0.001 | 5.29 (2.28–12.28) | <0.001 |
| Need for mechanical ventilation | 3.73 (1.64–8.47) | 0.002 | 3.76 (1.63–8.67) | 0.002 |
| Need for renal replacement therapy | 6.11 (1.45–25.81) | 0.014 | 5.57 (1.25–24.76) | 0.024 |
PAH: pulmonary arterial hypertension; ICU: intensive care unit; CTD-PAH: connective tissue diseases PAH; IPAH: idiopathic PAH; WHO FC: World Health Organization functional class; BP: blood pressure; eGFR: estimated glomerular filtration rate; Na: sodium; proBNP: pro-B-type natriuretic peptide. #: adjusted only for age.
Outcomes after ICU discharge
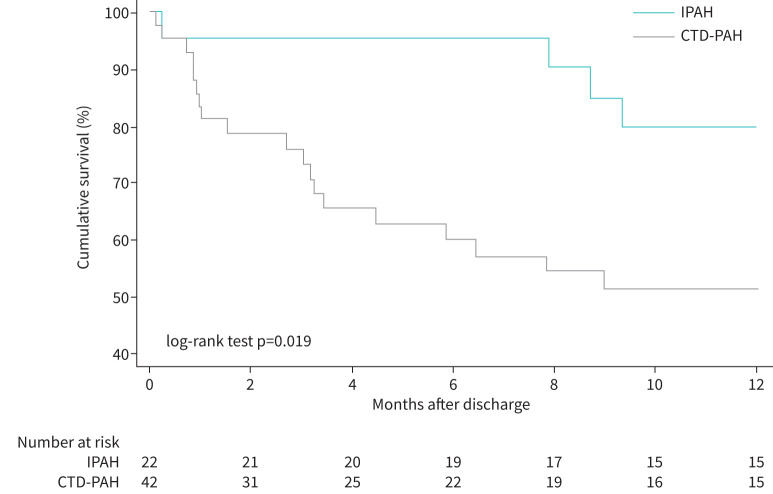
Among 77 patients who survived their first ICU admission, two patients underwent a lung transplant. Thus, outcomes were assessed in 75 patients. At the time of discharge from the ICU, 66 of these patients were on PAH-specific treatment, including 25 patients on monotherapy, 28 on dual therapy and 13 on triple therapy. Patients who were not started on targeted therapy, or therapy was not escalated, by the time they left the ICU were kept clinically and haemodynamically optimised before hospital discharge while awaiting initiation, or escalation, of PAH targeted therapy or pending insurance approval. Furthermore, the monotherapy cohort included patients with a long history of the disease who had remained stable on their respective regimen up until admission. The late mortality rates among hospital survivors at 3, 6 and 12 months were 17.5%, 27.6% and 39.0%, respectively (figure 1). When 1-year survival was compared across the three PAH groups, IPAH survival was 79.8% versus CTD-PAH 51.4% versus Other-PAH 59.7% (log-rank test p=0.06). Furthermore, the difference in survival was statistically significant when comparing the two largest groups, IPAH versus CTD-PAH (log-rank test p=0.019) (figure 2).
FIGURE 1.
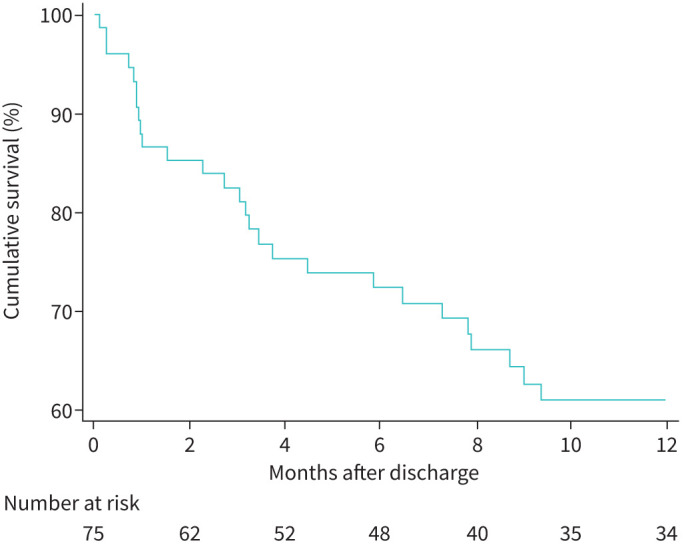
Cumulative survival after discharge at 3, 6 and 12 months of pulmonary arterial hypertension (PAH) patients admitted to the intensive care unit.
FIGURE 2.
Kaplan–Meier curve of 1-year survival after discharge of pulmonary arterial hypertension (PAH) patients admitted to the intensive care unit by underlying diagnosis (idiopathic PAH (IPAH) versus PAH due to connective tissue diseases (CTD-PAH)).
Risk factors for 1-year mortality
Univariable and multivariable Cox proportional hazards models for 1-year mortality are presented in table 4. Several variables were identified by univariable Cox models to be potential risk factors. After adjusting for age and underlying PAH diagnosis, risk factors were Na <136 mEq·mL−1 (HR 1.71; p=0.021), elevated proBNP (HR 1.35; p=0.04), hyperbilirubinaemia (HR 1.19; p=0.006) and use of vasopressors or inotropes (HR 2.57; p<0.001) and mechanical ventilation (HR 2.06; p=0.005). After adjusting for age, CTD-PAH patients had a higher risk of death compared to IPAH patients (HR 1.89; p=0.05).
TABLE 4.
Risk factors for mortality within the first year after discharge in PAH patients admitted to the ICU
|
Unadjusted
HR (95% CI) |
p-value |
Adjusted for PAH diagnosis and age
HR (95% CI) |
p-value | |
| Age per year | 1.02 (1.01–1.04) | 0.01 | NA | NA |
| Female versus male | 0.89 (0.49–1.62) | 0.70 | 0.98 (0.53–1.81) | 0.93 |
| CTD-PAH versus IPAH | 2.31 (1.28–4.16) | 0.005 | 1.89 (1.01–3.59)# | 0.05 |
| WHO FC pre-admission (III and IV) | 3.24 (0.95–11.02) | 0.06 | 2.99 (0.84–10.63) | 0.09 |
| Charlson index ≥2 | 4.41 (0.53–36.67) | 0.17 | 1.25 (0.35–4.52) | 0.72 |
| Data on admission | ||||
| Systolic BP <100 mmHg | 1.08 (0.70–1.68) | 0.72 | 1.06 (0.69–1.64) | 0.78 |
| Anaemia | 0.91 (0.55–1.49) | 0.70 | 0.86 (0.53–1.40) | 0.55 |
| eGFR <60 mL·min−1 per 1.73 m2 | 1.91 (0.76–4.78) | 0.16 | 1.52 (0.59–3.92) | 0.38 |
| Na <136 mEq·mL−1 | 1.87 (1.21–2.89) | 0.005 | 1.71 (1.08–2.69) | 0.021 |
| ProBNP pg·mL−1 (log transformed) | 1.48 (1.10–1.98) | 0.009 | 1.35 (1.01–1.83) | 0.04 |
| Bilirubin, total, mg·dL−1 | 1.12 (1.00–1.26) | 0.06 | 1.19 (1.05–1.34) | 0.006 |
| In-hospital support | ||||
| Need for vasopressors/inotropes | 2.73 (1.73–4.30) | <0.001 | 2.57 (1.62–4.08) | <0.001 |
| Need for mechanical ventilation | 2.05 (1.24–3.38) | 0.005 | 2.06 (1.25–3.40) | 0.005 |
| Need for renal replacement therapy | 1.80 (0.68–4.76) | 0.23 | 1.91 (0.68–5.38) | 0.21 |
PAH: pulmonary arterial hypertension; ICU: intensive care unit; CTD-PAH: connective tissue diseases PAH; IPAH: idiopathic PAH; WHO FC: World Health Organization functional class; BP: blood pressure; eGFR: estimated glomerular filtration rate; Na: sodium; proBNP: pro-B-type natriuretic peptide. #: adjusted only for age.
Discussion
This study identified specific features related to PAH patients requiring ICU admission. First, RHF continues to be the single most common reason for hospitalisation to an ICU setting. Second, common risk factors for ICU and 1-year mortality after discharge include hyponatraemia, elevated proBNP, hyperbilirubinaemia and need for vasopressors or inotropes and mechanical ventilation. Third, among all PAH subgroups, CTD-PAH is associated with worse outcomes and survival. Finally, in-hospital and 1-year mortality remain exceedingly high despite a general improvement in clinical management and targeted therapeutics for PAH.
The Johns Hopkins Hospital Medical ICU is a closed unit with standardised protocols designed to provide optimal evidence-based patient care, particularly related to the use of intravenous diuretics, vasopressors or inotropes, mechanical ventilation and renal replacement therapy as clinically indicated [11, 22, 23]. The Johns Hopkins PH specialists are directly involved in the care of PAH patients admitted to the ICU to provide multidisciplinary management along with the ICU providers. Therefore, the goal of this study was to explore the clinical characteristics, causes of ICU admission, general practices and determinants of survival, and to assess improvement in care and survival, if any, compared to our experience from a decade ago [6] and other recent studies.
In 2010, Sztrymf et al. [21] reported findings in a PAH population of 46 patients admitted to an ICU setting. Mortality was 41%; however, no significant differences were found between survivors and non-survivors across demographics, clinical features and haemodynamic data collected on admission. SBP, proBNP, C-reactive protein, Na and creatinine correlated significantly with survival [21]. In contrast, Campo et al. [6] and Haddad et al. [12] described statistically significant differences across PAH groups regarding demographics, outcomes and survival of patients admitted to the hospital due to RHF; however, only 25% and 17% of these admissions required ICU care, for each study respectively. More recently, Ambroz et al. [24] reported predictors of survival in 70 PH patients (59 PAH and 11 chronic thromboembolic pulmonary hypertension) admitted with RHF. Their cohort included 96 admissions to cardiology wards and 21 to the ICU, with overall hospital and ICU mortality rates of 12.8% and 52.4%, respectively. Hyponatraemia, younger age, CTD-PAH and need for PAH combination therapy were described as in-hospital mortality risk factors [24]. Moreover, Bauchmuller and colleagues recently published a retrospective observational study based on the ASPIRE registry. They included 242 critical care PH admissions involving 206 patients (162 PAH admissions, including 96 IPAH and 40 CTD-PAH). Hospital survival was 59.3%, 94% and 92% for patients admitted for medical, surgical or obstetric reasons, respectively. Higher APACHE II (Acute Physiology and Chronic Health Evaluation) score, age and lactate levels, and lower oxygen saturation, platelet count and sodium levels were identified as independent predictors of hospital mortality [25], similar to risk factors described in our study.
Observational studies and registry data have demonstrated RHF to be the leading cause of hospitalisation in PAH patients, which is associated with a shorter time to clinical worsening after discharge, including PAH-related readmissions, deterioration in FC or decrease in 6-min walk test distance by 20% from baseline, need for interventional procedures (i.e., lung transplantation, atrial septostomy) and increased mortality rates [6, 19, 21, 24, 26]. Respiratory failure frequently complicates the hospitalisation course with hypoxia, acidosis and hypercapnia contributing to worsening pulmonary vasoconstriction and increased RV afterload [27]. The latter is also increased by the effect of positive pressure from mechanical ventilation [28]. In this study, significant predictors of poor outcomes included the use of vasopressors or inotropes and mechanical ventilation. In particular, we observed that 42.7% of patients treated with either vasopressors or inotropes and 50% of patients who needed mechanical ventilation died during their hospitalisation, compared to 45.7% and 100% as we previously reported, respectively [6]. This improvement of outcomes in PAH patients receiving mechanical ventilation may derive from recent advances in evidence-based practices focused on addressing pathophysiological pathways with emphasis on low tidal volume ventilation [29], appropriate sedation, early mobilisation [30] and the implementation of ICU care bundles [31]. Furthermore, a multidisciplinary team-based approach between PAH providers and cardiac anaesthesia at our institution [32] may have contributed to the education and awareness among ICU and anaesthesia providers regarding the specific challenges surrounding induction and intubation, and other invasive procedures in the ICU.
Several metabolic and electrolyte derangements were identified as ICU mortality risk factors. Aside from hyponatraemia, elevated proBNP, hyperbilirubinaemia and hyperlactaemia, the need for renal replacement therapy was a strong risk factor for ICU adverse outcomes. Renal dysfunction has been associated with abnormal cardiopulmonary haemodynamics, increased in-hospital mortality and poor survival in PAH patients enrolled in the Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL). Participants with a ≥10% decline in eGFR from baseline over ≥1 year had a significantly increased risk of death (HR 1.66; p<0.0001) and the composite of all-cause hospitalisation and death (HR 1.33; p=0.002) [33]. In the present study, renal dysfunction, defined as eGFR <60 mL·min−1 per 1.73 m2 at the time of ICU admission, was a significant risk factor for ICU mortality (OR 5.3; p=0.04) and highlights the significance of the cardiorenal axis. The primary mechanism through which PH leads to renal dysfunction is due to increased central venous pressure and consequent renovascular congestion, mediated by RV dysfunction [34]. This results in decreased effective renal perfusion due to reduced trans-glomerular pressure gradients. Furthermore, a cascade of myogenic and neural reflexes, baroreceptor stimulation, activation of the renin-angiotensin-aldosterone system (RAAS), and pro-inflammatory pathways lead to a rapid decline in the glomerular fraction rate. The activation of the RAAS and production of angiotensin-II has been shown to activate NADPH oxidase, leading presumably to progressive oxidative kidney injury [35]. In this context, as renal function declines and diuretics become ineffective, renal replacement therapy is the only available option for fluid removal. Hence, we found that patients requiring renal replacement therapy had a poor prognosis and increased mortality (OR 5.6; p=0.024).
Overall in-hospital mortality in PAH patients who required ICU care was lower in this study compared with previous reports (27.1% versus 38% [12] versus 41% [21]). It is noteworthy that admission for RHF portends a worse prognosis (31.3% present study versus 48% [6] versus 52.4% [24] ICU mortality) relative to left heart failure (LHF) (4%–17%) [20, 36, 37]. Compared with patients with LHF, patients with PAH are younger, are more often women and have a higher prevalence of connective tissue disease. Comorbid conditions, such as systemic hypertension, diabetes mellitus or peripheral vascular disease, are also less common. Despite high in-hospital mortality rates, we noted a remarkable improvement compared to our previous experience between 2000 and 2009. The cohort then included 61 patients with 115 hospitalisations, including 25.2% ICU admissions [6]. Of the latter group, 48.3% of patients admitted with RHF died compared to 31.3% patients in the present study. Within the largest subgroups of this population, IPAH (45 hospitalisations) and CTD-PAH (85 hospitalisations), in-hospital mortality was 22.2% and 25.9%, respectively, which is considerably lower compared to rates from the prior epoch (30% and 57.9%, respectively) [6]. This important trend in outcomes could be the reflection of a better understanding of the disease and improved overall care.
Scleroderma-associated PAH (SSc-PAH) was the most common cause of PAH in our cohort due to referral bias, and the second most commonly identified PAH aetiology in most PH registries. It is the leading cause of death in SSc [5, 38, 39]. Dual upfront combination therapy has certainly been a significant therapeutic advance in this disease and offers new hopes for this population [7, 40]. The current CTD-PAH cohort included 46 (81%) SSc-PAH patients, who were older and had a higher prevalence of comorbidities compared to other groups, which is similar to prior observations [41]. Most patients had a Charlson comorbidity index ≥2 (89.5%), suggesting that the presence of numerous comorbidities carries a higher in-hospital mortality risk, although this association was not statistically significant. After discharge, CTD-PAH patients had the worst 1-year survival (48.6% mortality), followed by other-PAH (40.3%) and IPAH (20.2%). Similar risk factors predicted 1-year mortality, including hyponatraemia, elevated proBNP and hyperbilirubinaemia, as well as the need for vasopressors or inotropes and mechanical ventilation during the initial ICU admission. Carrying a diagnosis of CTD-PAH compared to IPAH is by itself a strong mortality risk factor. This difference in outcomes in CTD-PAH patients can be attributed to a complex interaction between older age, higher prevalence of comorbidities and more refractory PAH [41–43]. In addition, we have previously identified profound sarcomere dysfunction (i.e., depressed RV contractility and contractile reserve) in SSc-PAH compared to IPAH [44], which could potentially be a major contributory factor to RHF, and hence worse clinical outcomes.
Some limitations of this study must be noted. First, we analysed the predictive value of clinical and laboratory parameters on admission, but not the effect of their changes during the ICU stay. Second, given that we only included patients cared for at a single centre, and considering the high proportion of patients with CTD-PAH, our findings cannot be generalised to other centres where PAH subgroups may differ.
Conclusion
In summary, in-hospital mortality for PAH patients remains unacceptably high, and when patients need advanced care in the ICU, outcomes can be devastating. This trend is carried over after discharge, and despite a plethora of new therapeutics, short-term survival remains dismal. These findings are especially concerning for CTD-PAH patients, suggesting that the need for ICU hospitalisation itself portends a poor prognosis. Common risk factors for ICU and 1-year mortality include hyponatraemia, elevated proBNP and total bilirubin, and the need for vasopressors/inotropes and mechanical ventilation. Abnormal renal function or need for renal replacement also convey a poor prognosis. Improvements in long-term outcomes of stable PAH patients are now reflected in a considerable, but still far from acceptable, decrease of in-hospital and post-discharge mortality rates. Strategies aimed at identifying patients at higher risk early in the hospitalisation course should help guide management and optimise treatment.
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: M. Naranjo, V. Mercurio, H. Hassan, S.K. Sahetya, M. Mukherjee, S. Hsu, A. Balasubramanian, C.E. Simpson, R.L. Damico, T.M. Kolb, S.C. Mathai and P.M. Hassoun were involved in conceptual design, data acquisition, analysis, interpretation and manuscript preparation. N. Alturaif, A. Cuomo, U. Attanasio and N. Diab were involved in data acquisition, analysis and interpretation. All authors listed have been involved in revising the manuscript for important intellectual content, approved the final version submitted for publication, and are in agreement with the accuracy and integrity of the submitted work.
Conflict of interest: M. Naranjo has nothing to disclose. V. Mercurio has nothing to disclose. H. Hassan has nothing to disclose. N. Alturaif has nothing to disclose. A. Cuomo has nothing to disclose. U. Attanasio has nothing to disclose. N. Diab has nothing to disclose. S.K. Sahetya has nothing to disclose. M. Mukherjee has nothing to disclose. S. Hsu has nothing to disclose. A. Balasubramanian has nothing to disclose. C.E. Simpson has nothing to disclose. R.L. Damico has nothing to disclose. T.M. Kolb has nothing to disclose. S.C. Mathai has received consulting fees from Actelion, Bayer, Acceleron and United Therapeutics. P.M. Hassoun served on a scientific advisory board for Merck & Co.
Support statement: This study was supported by funding from the National Institutes of Health National Heart, Lung and Blood Institute grants HL114910 (P.M. Hassoun) and T32HL007534 (M. Naranjo). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Galie N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. doi: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 3.Mercurio V, Bianco A, Campi G, et al. New drugs, therapeutic strategies, and future direction for the treatment of pulmonary arterial hypertension. Curr Med Chem 2019; 26: 2844–2864. doi: 10.2174/0929867325666180201095743 [DOI] [PubMed] [Google Scholar]
- 4.Hassoun PM. The right ventricle in scleroderma (2013 Grover Conference Series). Pulm Circ 2015; 5: 3–14. doi: 10.1086/679607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naranjo M, Hassoun PM. Systemic sclerosis-associated pulmonary hypertension: spectrum and impact. Diagnostics (Basel) 2021; 11: 911. doi: 10.3390/diagnostics11050911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campo A, Mathai SC, Le Pavec J, et al. Outcomes of hospitalisation for right heart failure in pulmonary arterial hypertension. Eur Respir J 2011; 38: 359–367. doi: 10.1183/09031936.00148310 [DOI] [PubMed] [Google Scholar]
- 7.Galie N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. doi: 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 8.Jansa P, Pulido T. Macitentan in pulmonary arterial hypertension: a focus on combination therapy in the SERAPHIN trial. Am J Cardiovasc Drugs 2018; 18: 1–11. doi: 10.1007/s40256-017-0260-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. doi: 10.1056/NEJMoa1503184 [DOI] [PubMed] [Google Scholar]
- 10.Vachiery JL, Galie N, Barbera JA, et al. Initial combination therapy with ambrisentan+tadalafil on pulmonary arterial hypertension related hospitalization in the AMBITION trial. J Heart Lung Transplant 2019; 38: 194–202. doi: 10.1016/j.healun.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 11.Konstam MA, Kiernan MS, Bernstein D, et al. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation 2018; 137: e578–e622. doi: 10.1161/CIR.0000000000000560 [DOI] [PubMed] [Google Scholar]
- 12.Haddad F, Peterson T, Fuh E, et al. Characteristics and outcome after hospitalization for acute right heart failure in patients with pulmonary arterial hypertension. Circ Heart Fail 2011; 4: 692–699. doi: 10.1161/CIRCHEARTFAILURE.110.949933 [DOI] [PubMed] [Google Scholar]
- 13.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 15.Le CH. The prevalence of anemia and moderate-severe anemia in the US population (NHANES 2003–2012). PLoS One 2016; 11: e0166635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich JD, Rich S. Clinical diagnosis of pulmonary hypertension. Circulation 2014; 130: 1820–1830. doi: 10.1161/CIRCULATIONAHA.114.006971 [DOI] [PubMed] [Google Scholar]
- 18.Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet 2003; 361: 1533–1544. doi: 10.1016/S0140-6736(03)13167-4 [DOI] [PubMed] [Google Scholar]
- 19.Burger CD, Long PK, Shah MR, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest 2014; 146: 1263–1273. doi: 10.1378/chest.14-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chioncel O, Ambrosy AP, Filipescu D, et al. Patterns of intensive care unit admissions in patients hospitalized for heart failure: insights from the RO-AHFS registry. J Cardiovasc Med (Hagerstown) 2015; 16: 331–340. doi: 10.2459/JCM.0000000000000030 [DOI] [PubMed] [Google Scholar]
- 21.Sztrymf B, Souza R, Bertoletti L, et al. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J 2010; 35: 1286–1293. doi: 10.1183/09031936.00070209 [DOI] [PubMed] [Google Scholar]
- 22.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 23.Johns Hopkins University School of Medicine. Hopkins Policy and Document Library 2021. www.hpo.johnshopkins.edu/hopkins/?event=public.view
- 24.Ambroz D, Jansa P, Kuchar J, et al. Predictors of survival in patients with pulmonary hypertension and acute right heart failure. Bratisl Lek Listy 2020; 121: 230–235. [DOI] [PubMed] [Google Scholar]
- 25.Bauchmuller K, Condliffe R, Southern J, et al. Critical care outcomes in patients with pre-existing pulmonary hypertension: insights from the ASPIRE registry. ERJ Open Res 2021; 7: 00046-2021. doi: 10.1183/23120541.00046-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preston IR, Suissa S, Humbert M. New perspectives in long-term outcomes in clinical trials of pulmonary arterial hypertension. Eur Respir Rev 2013; 22: 495–502. doi: 10.1183/09059180.00006413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price LC, Wort SJ, Finney SJ, et al. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care 2010; 14: R169. doi: 10.1186/cc9264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowroozpoor A, Malekmohammad M, Seyyedi SR, et al. Pulmonary hypertension in intensive care units: an updated review. Tanaffos 2019; 18: 180–207. [PMC free article] [PubMed] [Google Scholar]
- 29.Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–1308. doi: 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 30.Hashem MD, Nelliot A, Needham DM. Early mobilization and rehabilitation in the ICU: moving back to the future. Respir Care 2016; 61: 971–979. doi: 10.4187/respcare.04741 [DOI] [PubMed] [Google Scholar]
- 31.Aryal S, King CS. Critical care of patients with pulmonary arterial hypertension. Curr Opin Pulm Med 2020; 26: 414–421. doi: 10.1097/MCP.0000000000000713 [DOI] [PubMed] [Google Scholar]
- 32.Steppan J, Diaz-Rodriguez N, Barodka VM, et al. Focused review of perioperative care of patients with pulmonary hypertension and proposal of a perioperative pathway. Cureus 2018; 10: e2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakinala MM, Coyne DW, Benza RL, et al. Impact of declining renal function on outcomes in pulmonary arterial hypertension: a REVEAL registry analysis. J Heart Lung Transplant 2018; 37: 696–705. doi: 10.1016/j.healun.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 34.Naranjo M, Lo KB, Mezue K, et al. Effects of pulmonary hypertension and right ventricular function in short and long-term kidney function. Curr Cardiol Rev 2019; 15: 3–11. doi: 10.2174/1573403X14666181008154215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2019; 139: e840–e878. doi: 10.1161/CIR.0000000000000664 [DOI] [PubMed] [Google Scholar]
- 36.Fonarow GC, Adams KF Jr, Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005; 293: 572–580. doi: 10.1001/jama.293.5.572 [DOI] [PubMed] [Google Scholar]
- 37.Padkins M, Breen T, Anavekar N, et al. Age and shock severity predict mortality in cardiac intensive care unit patients with and without heart failure. ESC Heart Fail 2020; 7: 3971–3982. doi: 10.1002/ehf2.12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avouac J, Airo P, Meune C, et al. Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies. J Rheumatol 2010; 37: 2290–2298. doi: 10.3899/jrheum.100245 [DOI] [PubMed] [Google Scholar]
- 39.Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005; 52: 3792–3800. doi: 10.1002/art.21433 [DOI] [PubMed] [Google Scholar]
- 40.Hassoun PM, Zamanian RT, Damico R, et al. Ambrisentan and tadalafil up-front combination therapy in scleroderma-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192: 1102–1110. doi: 10.1164/rccm.201507-1398OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher MR, Mathai SC, Champion HC, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum 2006; 54: 3043–3050. doi: 10.1002/art.22069 [DOI] [PubMed] [Google Scholar]
- 42.Hassoun PM. Pulmonary arterial hypertension complicating connective tissue diseases. Semin Respir Crit Care Med 2009; 30: 429–439. doi: 10.1055/s-0029-1233312 [DOI] [PubMed] [Google Scholar]
- 43.Kawut SM, Taichman DB, Archer-Chicko CL, et al. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest 2003; 123: 344–350. doi: 10.1378/chest.123.2.344 [DOI] [PubMed] [Google Scholar]
- 44.Hsu S, Kokkonen-Simon KM, Kirk JA, et al. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension. Circulation 2018; 137: 2360–2370. doi: 10.1161/CIRCULATIONAHA.117.033147 [DOI] [PMC free article] [PubMed] [Google Scholar]