Abstract
Immune checkpoint blockade therapy is perhaps the most important development in cancer treatment in recent memory. It is based on decades of investigation into the biology of immune cells and the role of the immune system in controlling cancer growth. While the molecular circuitry that governs the immune system in general—and antitumor immunity in particular—is intensely studied, far less attention has been paid to the role of cellular stress in this process. Proteostasis, intimately linked to cell stress responses, refers to the dynamic regulation of the cellular proteome and is maintained through a complex network of systems that govern the synthesis, folding, and degradation of proteins in the cell. Disruption of these systems can result in the loss of protein function, altered protein function, the formation of toxic aggregates, or pathologies associated with cell stress. However, the importance of proteostasis extends beyond its role in maintaining proper protein function; proteostasis governs how tolerant cells may be to mutations in protein-coding genes and the overall half-life of proteins. Such gene expression changes may be associated with human diseases including neurodegenerative diseases, metabolic disease, and cancer and manifest at the protein level against the backdrop of the proteostasis network in any given cellular environment. In this review, we focus on the role of proteostasis in regulating immune responses against cancer as well the role of proteostasis in determining immunogenicity of cancer cells.
Keywords: proteostasis, chaperones, cell stress, antigen processing, T cells, cancer, protein folding, protein degradation, major histocompatibility complex, proteasome
Abbreviations: APC, antigen-presenting cell; ATF6, activating transcription factor 6; CTL, cytotoxic T cell; DC, dendritic cell; ER, endoplasmic reticulum; HLA, human leukocyte antigens; Hsf1, heat shock factor 1; HSR, heat shock response; IRE1, inositol-requiring enzyme; MHCI, major histocompatibility complex type I; NK, natural killer; PD-1, programmed death 1; PD-L1, programmed death ligand 1; PERK, protein kinase R–like endoplasmic reticulum kinase; PLC, peptide-loading complex; TAP, transporter associated with antigen processing; UPR, unfolded protein response
Proteostasis
Cellular proteostasis is maintained through the integrated action of protein synthesis, folding, and degradation machinery (Fig. 1) (1, 2, 3). Ribosomes and molecular chaperones are directly responsible for the synthesis and folding of proteins (4, 5). Changes in transcription, metabolism, and the cellular environment provide the backdrop against which protein synthesis and stability must be achieved. When proteins have reached the end of their usefulness or have been damaged by environmental factors, they will be degraded by two main pathways: the ubiquitin-proteasome pathway or the autophagy lysosomal pathway (6, 7). Molecular chaperones play a key role in the determination of which proteins will be degraded as well as aiding the process of degradation itself (4, 8, 9).
Figure 1.
Overview of proteostasis pathways. 1. Proteins are synthesized on ribosomes, and molecular chaperones aid in synthesis and proper folding. 2. When proteins are misfolded and reach the end of their life or there is a disruption in proteostasis, two major pathways are responsible for protein degradation: A, the ubiquitin-proteasome pathway and B, the autophagy lysosomal pathway. A variety of stresses can promote the formation of misfolded proteins and toxic aggregates. The heat shock response (HSR) is regulated by the transcription factor, heat shock factor 1 (Hsf1). Hsf1 is normally held in an inactive, monomeric state by chaperones such as Hsp70 and Hsp90. When misfolded protein species become sufficiently abundant, Hsf1 can no longer efficiently compete for binding to chaperones and then undergoes trimerization and translocation to the nucleus. The unfolded protein response (UPR) is activated when misfolded proteins accumulate in the ER. The UPR sensors PERK, IRE1, and ATF6 are normally held in an inactive state by the ER chaperone, Grp78. Misfolded proteins titrate Grp78 away from these sensor proteins which are subsequently activated and trigger the UPR. ER, endoplasmic reticulum.
Proteostasis can be disrupted by a variety of extrinsic factors such as nutrient deprivation, thermal stress, hypoxia, oxidative stress, etc., as well as intrinsic factors such as mutations in protein-coding genes that can thermodynamically destabilize their protein products. Cancer cells, and the tumor microenvironments they define, are characterized by intrinsic and extrinsic challenges to proteostasis (10). Cancer cells are heavily dependent on molecular chaperone systems to maintain a functional proteome in the face of an ever-increasing burden of mutations in an environment that is often hypoxic, nutrient deprived, and flush with reactive oxygen species (11, 12, 13, 14, 15, 16). The tumor microenvironment also triggers unfolded protein response (UPR) activation in key immune cells that participate in antitumor immunity (17).
Cellular stress responses have evolved to transiently supply additional machinery to handle proteotoxic stress and avoid cell death. In eukaryotes, the heat shock response (HSR) and UPR are responsible for responding to proteotoxic stress and maintaining proteostasis. The HSR is triggered by cytoplasmic protein misfolding and is induced by the transcription factor, heat shock factor 1 (Hsf1) (18, 19, 20). Hsf1 is normally held in a monomeric state through interactions with chaperones such as Hsp70, TriC, and Hsp90 (21, 22, 23, 24). Elevated levels of misfolded proteins increase demand on chaperones, thereby titrating them away from Hsf1. This allows Hsf1 to undergo trimerization, activation, and translocation to the nucleus where it can act on target genes that aid in the response to protein misfolding (18, 19, 25, 26). The UPR can be induced by at least three different types of stress sensors in the endoplasmic reticulum (ER) (27, 28, 29). The inositol-requiring enzyme (IRE1), the activating transcription factor 6 (ATF6), and the protein kinase R–like endoplasmic reticulum kinase (PERK) all activate the UPR to allow cells a chance to adapt to challenges to proteostasis and modulate the activation of apoptosis (30, 31, 32). Each of these three sensors is activated in response to an elevated burden of misfolded proteins in the ER. IRE1, ATF6, and PERK are normally held in an inactive state by an abundant ER chaperone, Grp78 (also called BiP, Hspa5) (33, 34, 35). When the level of misfolded proteins increases in the ER, these misfolded proteins begin to compete with the stress sensors for binding to Grp78. Sufficient levels of misfolded proteins will eventually lead to the loss of Grp78 binding to PERK, IRE1, and ATF6, leading to their activation and the initiation of the UPR. Release of ATF6 from Grp78 allows for its traffic to the Golgi where it undergoes proteolysis to release a soluble and activate transcription factor domain (cATF6) that can then translocate to the nucleus (36, 37). In the case of IRE1, loss of Grp78 binding to its lumenal domain allows for dimerization, autophosphorylation, and activation of an RNase domain that then completes the splicing of the messenger RNA (mRNA) encoding the transcription factor XBP1 (38, 39). It has also been shown that direct binding of IRE1 with unfolded proteins is important for IRE1 oligomerization and activation (40, 41). Finally, in a manner very similar to IRE1, PERK activation results in autophosphorylation and subsequent phosphorylation of eIF2alpha (33, 42). Phosphorylation inactivates eIF2alpha and results in both a decrease in global translation as well as the translation of the ATF4 open reading frame (ORF) which is normally masked by translation of an overlapping ORF (43, 44).
Key players in antitumor immunity
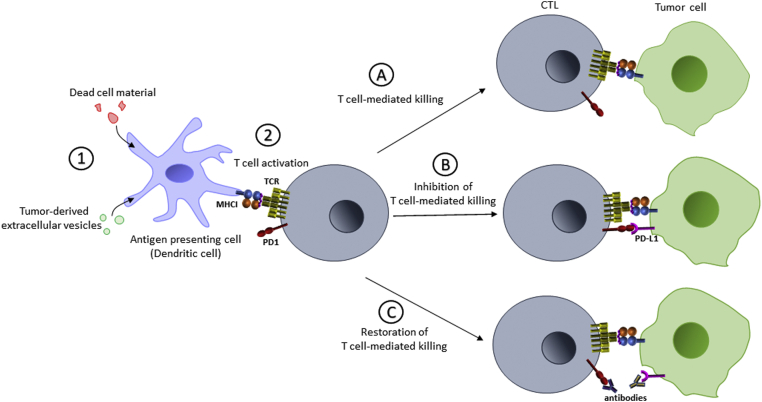
The immune system plays a critical role in detecting and eliminating cancer cells from the body (45, 46). However, it was not until the 1980s that the definitive link between the immune system and cancer was made despite the idea being proposed in the 19th century (47, 48, 49, 50, 51). This was in part owing to the difficulty associated with impairing specific immune cell lineages in animal models. Two of the most important immune cells for antitumor immunity are a type of antigen-presenting cell (APC) called dendritic cells (DCs) and cytotoxic T cells (CTLs) (Fig. 2A).
Figure 2.
Antitumor immunity and immune checkpoint blockade therapy. 1. Tumor-derived material is phagocytosed, processed, and presented by antigen-presenting cells (APCs) such as dendritic cells (DCs). 2. DCs presenting “nonself” peptides will activate cognate T cells. A, activated T cells will recognize MHCI complexes loaded with neoantigenic, nonself peptides on T cells, resulting in tumor cell killing. B, expression of PD-L1 on tumor cells will block T-cell action. C, neutralization of the PD-1–PD-L1 interaction with therapeutic antibodies will restore T cell–mediated killing. ER, endoplasmic reticulum; MHCI, major histocompatibility complex type I; PD-1, programmed death 1; PD-L1, programmed death ligand 1; PLC, peptide-loading complex; TAP, transporter associated with antigen processing.
DCs infiltrate the tumor in response to innate immune signaling such as inflammation (52, 53). These tumor-infiltrating DCs internalize material from dead cancer cells or tumor-derived extracellular vesicles, the protein components of which are then degraded into fragments for loading onto major histocompatibility complex type I (MHCI) and II (54, 55, 56). These complexes then translocate to the surface of the APC where they are “presented” to CTLs or helper T cells, respectively, in nearby lymph nodes (52). T cells that can recognize specific nonself antigens presented on the surface of these DCs will become activated and then launch an immune response against that particular epitope or antigen, in the tumor (57, 58). Normally nonself antigens are derived from pathogens, such as viral proteins, but in the context of cancer, peptides are derived from mutated gene products. Since all cancer cells possess mutations that encode potentially neoantigenic proteins, how can cancer cells escape the immune system at all?
Immune evasion via programmed death ligand 1
All nucleated vertebrate cells express MHCI on their surface that present peptide fragments derived from protein degradation for surveillance by the immune system. Unlike in APCs like DCs, which present antigens to activate immune cells, the presentation of neoantigens in MHCI by cancer cells renders them vulnerable to attack by CTLs in a manner that is often proportional to their mutagenic burden (59, 60). Complete loss of MHCI seems like an obvious mechanism for immune evasion, but this renders cancer cells vulnerable to killing by natural killer (NK) cells owing to the role that MHCI complexes play in inhibiting these cells (61, 62, 63, 64). However, the relentless evolution of new traits in tumors allows them to evade immune detection and alter immune cell function in the tumor microenvironment. The most important example of this is the transmembrane protein, programmed death ligand 1 (PD-L1) (65, 66). PD-L1 binds to another transmembrane protein called programmed death 1 (PD-1) that is expressed on CTLs (Fig. 2B) (67). PD-L1 is normally expressed by some macrophages, act9vated T and B cells, and DCs to maintain a balance between activation, tolerance, and immune-mediated tissue damage (68). Tumor cells express PD-L1 to protect them from CTL-mediated killing and are the focal point of immune checkpoint inhibition (ICI) (69, 70, 71, 72, 73). Antibodies directed against PD-1 (e.g., nivolumab, pembrolizumab) or PD-L1 (e.g., avelumab, atezolizumab) neutralize the PD-1–PD-L1 interaction, restore CTL-mediated killing, and lead to tumor regression in many settings (Fig. 2C) (74, 75, 76, 77, 78, 79).
The expression of immunomodulatory transmembrane proteins like PD-L1 can be influenced by different facets of the proteostasis network. Grp78 is an ER-resident member of the Hsp70 chaperone family that is important for the stability and trafficking of PD-L1 (80). Upregulation of Grp78 by the UPR is one mechanism through which tumors can promote the surface localization and accumulation of PD-L1 (81). Other chaperones have also been implicated in the regulation of PD-L1 stability and surface levels. Mammalian cells express four different Hsp90s (Hsp90ɑ and Hsp90β in the cytoplasm, Trap1 in the mitochondria, Grp94 in the ER) that can be inhibited by various highly specific pharmacological agents (82, 83). Inhibition of Hsp90 with ganetespib has been shown to decrease surface levels of PD-L1 in a mouse colon cancer cell line (84). However, another study examining the same cell line, albeit with a different Hsp90 inhibitor and at a lower concentration, did not show a change in PD-L1 levels but did result in an increase in MHCI complexes (85). These different studies may indicate that there may be different thresholds for changes in immunomodulatory systems with respect to proteostasis.
Immune evasion via changes in peptide generation
The proteasome is an important part of proteostasis, regulating the degradation of unneeded and damaged proteins. The 26S proteasome is responsible for the degradation of these polyubiquitinated proteins (86, 87). Polyubiquitinated proteins arise from the cascade of E1, E2, and E3 enzymes, which activate, conjugate, and transfer ubiquitin moieties to protein substrates (88, 89). The proteasome is a multisubunit complex that is composed of a 20S catalytic core and capped by two 19S regulator complexes on each end (90). The 20S core is composed of four stacked rings of seven subunits each, with seven α-subunits (α1–α7) forming the two outer rings and the two inner rings are composed of 7 different β-subunits (β1–β7) (91, 92). There are three specialized proteolytic subunits found in the heptameric β rings of the 20S core, β1, β2, and β5, which show different cleavage preferences. The proteasome produces peptides that are 8 to 10 residues in length, providing peptides that can be accommodated by the MHCI peptide-binding groove. Inhibition of the proteasome reduces peptide production and results in significantly reduced MHCI antigen presentation (93, 94). Interestingly, several of the components of the MHCI antigen presentation pathway are induced by the cytokine interferon-γ (IFNγ) (95, 96). All three of the β subunits with proteolytic activity have counterparts that are IFNγ inducible and define the so-called immunoproteasome. Specifically, immunoproteasomes contain the immunosubunits iβ1, iβ2, and iβ5 (also known as PSMB9/LMP2, PSMB10/MECL1, and PSMB8/LMP7, respectively) in place of the β1, β2, and β5 subunits contained in the standard proteasome. While both the standard proteasome and immunoproteasome are able to generate peptides that can be loaded onto MHCI complexes, immunoproteasomes have altered cleavage preferences that result in peptides with hydrophobic C termini and a different cleavage rate that support immune response (97, 98, 99, 100, 101). Peptides derived from immunoproteasomes are far more efficiently loaded into the MHCI peptide-binding groove. Mouce models show that deficiency in the immunoproteasome can reduce T-cell response and altered antigenic peptide presentation (102, 103, 104, 105). This difference in proteasome subtypes was again demonstrated in HeLa cells infected with hepatitis B virus, where only after stimulation with IFNγ did cells present hepatitis B virus epitopes (106, 107).
As the presentation of neoantigens by cancer cells is important for immune cell recognition, it makes sense that malignant cells suppress immunoproteasome function in order to avoid immune detection (108, 109). Low immunoproteasome expression in patients with non-small cell lung cancer is a prognostic marker for reduced survival and increased recurrence of metastases (110). Cancer cells need to limit immune recognition linked to peptide presentation while also relying heavily on the proteasome to maintain proteostasis due to protein imbalances caused by global expression changes (i.e., linked to aneuploidy, gene amplification, overexpression, mutation, and hyperactivation of oncogenes). Proteasome inhibitors, such as Velcade, have been in use for cancer treatments for almost 20 years as a way to induce apoptosis and cell death through a variety of different molecular effects (111, 112). While proteasome inhibition has been shown to alter peptide presentation, the two are not necessarily synonymous (93, 113, 114). Treatment of cells with the proteasome inhibitors has also been shown to increase the levels of intracellular peptides, presumably by shifting the task of degradation to different proteolytic systems (115, 116).
A majority of nonproteasomal peptidases are thought to play supportive roles to the proteasome, performing further trimming and degradation. While these pathways can ultimately lead to the destruction of peptides, there is evidence that both in conjunction with the proteasome and independently these peptidases play important roles in the processing of antigenic peptides and immune cell recognition (117, 118). Similar to the proteasome and other members of the proteostasis machinery, the expression of alternative peptidases has been shown to be induced in cancer and is often correlated with disease outcomes (119, 120, 121). The precise mechanisms through which these alternate proteolytic mechanisms become altered in cancer and how they can affect MHCI peptide trimming and loading are not known. However, one can speculate that shifts in the immunopeptidome, and resultant immunogenicity, could be linked to alternative proteolytic pathways.
One of the most fundamental players in regulating the targeting of proteins to degradation pathways are chaperones tasked with folding, and maintaining, proteins in the cells. As mentioned earlier, inhibition of Hsp90 has been shown to have varying effects on the stability and trafficking of PD-L1 in cancer cells (84, 85). In one of these studies, mild Hsp90 inhibition—that did not result in the induction of the HSR or UPR or changes in PD-L1 expression or localization—promoted the formation of MHCI complexes and their accumulation at the cell surface (85). This suggests that Hsp90 inhibition promotes the targeting of Hsp90 client proteins to the ubiquitin-proteasome pathway, has an effect on the MHCI assembly pathway, or both. Interestingly, this study showed in animal models that this mild Hsp90 inhibition resulted in an immune system–dependent reduction in tumor growth (85). This, together with their observation that mild Hsp90 inhibition resulted in changes in the immunopeptidome, suggests that chaperones like Hsp90 can have a profound effect on the immunopeptidome of cancer cells.
Immune evasion via changes in peptide transport, MHCI assembly, and peptide loading
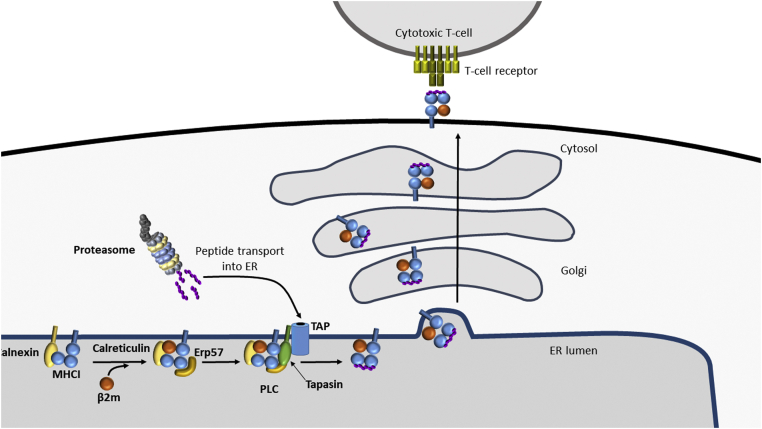
The assembly of MHCI molecules with peptide antigens involves the interaction and assembly of multiple components (Fig. 3). The sequential formation of the different interactions is difficult to dissect; however, several key interactors assist in peptide loading. Tapasin is an MHCI-specific chaperone that binds to peptide receptive MHCI heterodimers and to transporter associated with antigen processing (TAP), connecting MHCI to the source of peptides (122). ERp57 is a thiol oxidoreductase that functions together with the ER chaperones calnexin and calreticulin to stabilize MHCI and to stabilize the interaction of MHCI, calreticulin, tapasin, and TAP together as the peptide-loading complex (PLC) (123, 124). Tapasin plays a key role in bridging the components of the PLC together (125, 126).
Figure 3.
MHCI antigen presentation pathway. MHCI heavy chain is stabilized by the chaperone calnexin until it is replaced by its soluble counterpart, calreticulin, which helps to stabilize the binding of β2-microglobulin (β2m). This complex together with ERp57, Tapasin, and the TAP transporter form the peptide-loading complex (PLC). Peptides are transported through TAP and loaded onto MHCI. Kinetically stable MHCI–peptide complexes are released from the PLC and move through the secretory pathway to the cell surface for surveillance by cytotoxic T cells. ER, endoplasmic reticulum; MHCI, major histocompatibility complex type I; PD-1, programmed death 1; PD-L1, programmed death ligand 1; TAP, transporter associated with antigen processing.
Structural modulations and dysregulation of the components of the PLC can alter MHCI surface expression and change disease outcomes (127, 128, 129, 130). As an MHCI-specific chaperone, changes to tapasin expression can be directly linked to reduced MHCI antigen presentation. Loss of tapasin in cancer cells results in escape from recognition by CTLs and low levels of tapasin have been correlated with poor prognosis and patient survival time (131, 132, 133). In a similar way, the downregulation of TAP1 has been shown as a mechanism of tumor immune escape and implicated in tumorigenicity in several cancers (134, 135, 136, 137). Additional members of the PLC have more general roles in cellular function, and therefore, a direct line between MHCI antigen presentation and their regulation is more difficult to define. The ER chaperones calreticulin and calnexin are core components of the ER quality control pathway and are upregulated as part of the UPR (138). Induction of the UPR has been demonstrated to reduce expression of MHCI on the cell surface (139, 140). Reduction of MHCI levels due to the UPR has also been shown to promote NK-mediated killing in thyroid cells (141, 142, 143). In contrast, downregulation or mutation of calreticulin also decreases MHCI expression on the cell surface (144). In addition to a role in MHCI stability, calreticulin also functions in the folding and stabilization of tapasin and can bind to MHCI independent of the PLC (145, 146). In calreticulin-deficient cells, tapasin levels were reduced, indicating that calreticulin may influence MHCI complex formation both directly and indirectly (147). All of this suggests that the balance of chaperones in the ER is important to determine how efficiently MHCI complexes will form and how they will traffic to the cell surface. In addition to lower levels of MHCI complexes on the surface of cells when calreticulin activity is compromised, many of these complexes are suboptimally loaded with peptides. Interestingly, calreticulin plays an important role in the retrieval of such suboptimally loaded MHCI from post-ER compartments (148, 149). Loss or reduction of this retrieval function could explain the reduced MHCI antigen presentation observed in calreticulin-deficient cells.
Calreticulin itself can also be expressed on the cell surface as a marker for immunogenic cells (150). Levels of calnexin are known to alter antigen presentation and the cancer immune response (151, 152). Interestingly, the downregulation of calnexin was recently shown to be directly associated with lower expression of MHCI and enhanced cancer progression (153). Hsp90 has also been implicated in the loading of peptides into MHCI. Inhibition of Hsp90 has been shown to result in the appearance of unloaded MHCI on the surface of some cancer cells lines (154). To what degree alterations in proteostasis resulting in the appearance of unloaded MHC complexes at the cell surface of cancer cells influences recognition by the immune system remains to be examined.
Human leukocyte antigen expression and cancer
The type of MHCI complex on a cancer cell can also be as important for CTL-mediated killing as the peptide that is loaded within it. While the beta chain of a typical MHCI complex is always β2 microglobulin, there are several alpha chain genes, encoding different human leukocyte antigens (HLA), that can be expressed (155). In addition to the three classical alpha chains (HLA-A, HLA-B, and HLA-C in humans), there are three nonclassical HLA genes as well (HLA-E, HLA-F, and HLA-G). All of these HLA heavy chains differ in their peptide binding preferences and expression. Moreover, there are thousands of known alleles of HLA-A, HLA-B, and HLA-C and dozens of alleles of HLA-E, HLA-F, and HLA-G, giving each person a unique HLA profile and corresponding immunopeptidome in each cell type. HLA-A and HLA-B are the most well studied and have different peptide binding preferences (156). For example, HLA-A binds selectively to hydrophobic peptides, while HLA-B can bind to peptides with a wider range of properties. The nonclassical HLA-E binds to a limited number of peptides, usually derived from the signal sequences of other HLA molecules (A, B, C, and G). On the cell surface, these HLA-E MHCI complexes (loaded with MHCI signal peptides) can inhibit CTLs and NK cells (157, 158). Interestingly, the mitochondrial import sequence of Hsp60 can also be loaded onto HLA-E complexes and traffic to the cell surface (159). However, HLA-E complexes loaded with Hsp60-derived peptides do not inhibit NK cells and may provide a means of promoting immune clearance of stressed cells. It is intriguing to consider the possibility that the significance of HLA-E expression in disease severity can only be determined against the backdrop of the UPR status of the tumor. It may be that higher levels of stress, and concomitant expression of Hsp60, may cause a switch from an immune protective role to one that attracts NK cells. Another nonclassical MHCI, HLA-G, is often overexpressed in cancer and has been shown to facilitate immune evasion in melanoma (160). It has been proposed that HLA-G may constitute a novel immune checkpoint (161, 162). Similarly, high expression of the nonclassical MHCI, HLA-F, is associated with poor prognosis (163, 164).
Cancer severity, and response to ICI, can be influenced in a variety of ways by HLA genetics and expression (165). Clear links between proteostasis and HLA expression profile have yet to be thoroughly investigated, let alone established, but there is good reason to pursue such lines of research. An excessive burden of MHC complex loading and folding can induce the UPR, and induction of the UPR can influence MHCI surface expression (141, 166). Taken together, it seems reasonable that proteostasis can play a role in determining which MHCI complexes reach the cell surface and what effect they have on tumor immunogenicity once they arrive.
Future prospects
The immune system may be our best ally in the fight against cancer. Some of the most dramatic treatment responses to immunotherapy have been seen in melanoma where the combination PD-L1 and CTLA-4 (another inhibitory transmembrane protein on T cells that normally prevents hyperproliferation and autoimmunity) blockade showed an overall median survival of longer than 60 months and a five-year overall survival of 60% (78, 167, 168). In non-small cell lung cancer, treatment with PD-1 blockade resulted in a significantly increased 4-year survival compared to chemotherapy (14% vs 5%) (169). Unfortunately, despite the tremendous advancements in immunotherapy, it is estimated that checkpoint inhibitors may only benefit approximately 13% of all patients with cancer (170). The reasons for why many tumors are “cold” to the immune system and thus do not respond to ICI is not well understood but is currently a major research focus (171, 172, 173). A cold tumor is often characterized by having little to no infiltration by immune cells or in which immune cells accumulate at the periphery of the tumor (174). “Hot” tumors are characterized by intense interferon gamma signaling, inflammation, and T-cell infiltration which in turn usually correlates with a positive response to ICI. While this is a general definition of cold and hot tumors, it is not absolute. A recent multiomic analysis of melanoma did not reveal a clear set of predictive elements for response to immunotherapy (175). Some tumors that appeared cold responded well to immunotherapy, and some tumors with all the hallmarks of a hot tumor did not. More focused analysis on cellular stress responses and proteostasis in the context of immunotherapy may lead to a more accurate definition for hot and cold tumor and lead to better strategies to treat patients.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Funding and additional information
The authors are supported by funding from the Alberta Cancer Foundation (27364), the Natural Sciences and Engineering Research Council (RGPIN-2019–04967), and the Canadian Institutes of Health Research (178282).
Author contributions
R. M. and P. L. writing-original draft; R. M. and P. L. writing-review and editing.
Edited by Peter Cresswell
References
- 1.Balchin D., Hayer-Hartl M., Hartl F.U. In vivo aspects of protein folding and quality control. Science. 2016;353:aac4354. doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- 2.Marinko J.T., Huang H., Penn W.D., Capra J.A., Schlebach J.P., Sanders C.R. Folding and misfolding of human membrane proteins in health and disease: From single molecules to cellular proteostasis. Chem. Rev. 2019;119:5537–5606. doi: 10.1021/acs.chemrev.8b00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers E.T., Gierasch L.M. The proteome folding problem and cellular proteostasis. J. Mol. Biol. 2021;433:167197. doi: 10.1016/j.jmb.2021.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartl F.U., Bracher A., Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 5.Javed A., Christodoulou J., Cabrita L.D., Orlova E.V. The ribosome and its role in protein folding: Looking through a magnifying glass. Acta Crystallogr. D Struct. Biol. 2017;73:509–521. doi: 10.1107/S2059798317007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa Marrero M., Barrio-Hernandez I. Toward understanding the biochemical determinants of protein degradation rates. ACS Omega. 2021;6:5091–5100. doi: 10.1021/acsomega.0c05318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg A.L. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 8.Kastle M., Grune T. Interactions of the proteasomal system with chaperones: Protein triage and protein quality control. Prog. Mol. Biol. Transl. Sci. 2012;109:113–160. doi: 10.1016/B978-0-12-397863-9.00004-3. [DOI] [PubMed] [Google Scholar]
- 9.Kettern N., Dreiseidler M., Tawo R., Hohfeld J. Chaperone-assisted degradation: Multiple paths to destruction. Biol. Chem. 2010;391:481–489. doi: 10.1515/BC.2010.058. [DOI] [PubMed] [Google Scholar]
- 10.Corazzari M., Gagliardi M., Fimia G.M., Piacentini M. Endoplasmic reticulum stress, unfolded protein response, and cancer cell fate. Front. Oncol. 2017;7:78. doi: 10.3389/fonc.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calderwood S.K., Gong J. Heat shock proteins promote cancer: It's a protection racket. Trends Biochem. Sci. 2016;41:311–323. doi: 10.1016/j.tibs.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciocca D.R., Arrigo A.P., Calderwood S.K. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch. Toxicol. 2013;87:19–48. doi: 10.1007/s00204-012-0918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trepel J., Mollapour M., Giaccone G., Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Workman P. Altered states: Selectively drugging the Hsp90 cancer chaperone. Trends Mol. Med. 2004;10:47–51. doi: 10.1016/j.molmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Lianos G.D., Alexiou G.A., Mangano A., Mangano A., Rausei S., Boni L., Dionigi G., Roukos D.H. The role of heat shock proteins in cancer. Cancer Lett. 2015;360:114–118. doi: 10.1016/j.canlet.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Murphy M.E. The HSP70 family and cancer. Carcinogenesis. 2013;34:1181–1188. doi: 10.1093/carcin/bgt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grootjans J., Kaser A., Kaufman R.J., Blumberg R.S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 2016;16:469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindquist S. The heat-shock response. Annu. Rev. Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 20.Pirkkala L., Nykanen P., Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- 21.Abravaya K., Myers M.P., Murphy S.P., Morimoto R.I. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 1992;6:1153–1164. doi: 10.1101/gad.6.7.1153. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y., Mosser D.D., Morimoto R.I. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou J., Guo Y., Guettouche T., Smith D.F., Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 24.Neef D.W., Jaeger A.M., Gomez-Pastor R., Willmund F., Frydman J., Thiele D.J. A direct regulatory interaction between chaperonin TRiC and stress-responsive transcription factor HSF1. Cell Rep. 2014;9:955–966. doi: 10.1016/j.celrep.2014.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabindran S.K., Haroun R.I., Clos J., Wisniewski J., Wu C. Regulation of heat shock factor trimer formation: Role of a conserved leucine zipper. Science. 1993;259:230–234. doi: 10.1126/science.8421783. [DOI] [PubMed] [Google Scholar]
- 26.Sarge K.D., Murphy S.P., Morimoto R.I. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol. Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 28.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 29.Walter P., Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 30.Adams C.J., Kopp M.C., Larburu N., Nowak P.R., Ali M.M.U. Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Front. Mol. Biosci. 2019;6:11. doi: 10.3389/fmolb.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillary R.F., FitzGerald U. A lifetime of stress: ATF6 in development and homeostasis. J. Biomed. Sci. 2018;25:48. doi: 10.1186/s12929-018-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rozpedek W., Pytel D., Mucha B., Leszczynska H., Diehl J.A., Majsterek I. The role of the PERK/eIF2alpha/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 2016;16:533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 34.Hetz C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 35.Kopp M.C., Larburu N., Durairaj V., Adams C.J., Ali M.M.U. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat. Struct. Mol. Biol. 2019;26:1053–1062. doi: 10.1038/s41594-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida H., Okada T., Haze K., Yanagi H., Yura T., Negishi M., Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell Biol. 2000;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimata Y., Ishiwata-Kimata Y., Ito T., Hirata A., Suzuki T., Oikawa D., Takeuchi M., Kohno K. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J. Cell Biol. 2007;179:75–86. doi: 10.1083/jcb.200704166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pincus D., Chevalier M.W., Aragon T., van Anken E., Vidal S.E., El-Samad H., Walter P. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Credle J.J., Finer-Moore J.S., Papa F.R., Stroud R.M., Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18773–18784. doi: 10.1073/pnas.0509487102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gardner B.M., Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–1894. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harding H.P., Zhang Y., Zeng H., Novoa I., Lu P.D., Calfon M., Sadri N., Yun C., Popko B., Paules R., Stojdl D.F., Bell J.C., Hettmann T., Leiden J.M., Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 43.Fusakio M.E., Willy J.A., Wang Y., Mirek E.T., Al Baghdadi R.J., Adams C.M., Anthony T.G., Wek R.C. Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Mol. Biol. Cell. 2016;27:1536–1551. doi: 10.1091/mbc.E16-01-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pallmann N., Livgard M., Tesikova M., Zeynep Nenseth H., Akkus E., Sikkeland J., Jin Y., Koc D., Kuzu O.F., Pradhan M., Danielsen H.E., Kahraman N., Mokhlis H.M., Ozpolat B., Banerjee P.P., et al. Regulation of the unfolded protein response through ATF4 and FAM129A in prostate cancer. Oncogene. 2019;38:6301–6318. doi: 10.1038/s41388-019-0879-2. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan D.H., Shankaran V., Dighe A.S., Stockert E., Aguet M., Old L.J., Schreiber R.D. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shankaran V., Ikeda H., Bruce A.T., White J.M., Swanson P.E., Old L.J., Schreiber R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 47.Coley W.B. II. Contribution to the knowledge of sarcoma. Ann. Surg. 1891;14:199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorelik E. Concomitant tumor immunity and the resistance to a second tumor challenge. Adv. Cancer Res. 1983;39:71–120. doi: 10.1016/s0065-230x(08)61033-7. [DOI] [PubMed] [Google Scholar]
- 49.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 50.Ruggiero R.A., Bustuoadad O.D., Bonfil R.D., Sordelli D.O., Fontan P., Meiss R.P., Pasqualini C.D. [Antitumor concomitant immunity: A possible metastasis control mechanism] Medicina (B Aires) 1989;49:277–281. [PubMed] [Google Scholar]
- 51.Starnes C.O. Coley's toxins in perspective. Nature. 1992;357:11–12. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- 52.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 53.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 54.Heath W.R., Belz G.T., Behrens G.M., Smith C.M., Forehan S.P., Parish I.A., Davey G.M., Wilson N.S., Carbone F.R., Villadangos J.A. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 55.Mellman I., Steinman R.M. Dendritic cells: Specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 56.Savina A., Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 57.Gaudino S.J., Kumar P. Cross-Talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front. Immunol. 2019;10:360. doi: 10.3389/fimmu.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geijtenbeek T.B., van Vliet S.J., Engering A., t Hart B.A., van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu. Rev. Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 59.McGranahan N., Furness A.J., Rosenthal R., Ramskov S., Lyngaa R., Saini S.K., Jamal-Hanjani M., Wilson G.A., Birkbak N.J., Hiley C.T., Watkins T.B., Shafi S., Murugaesu N., Mitter R., Akarca A.U., et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S., Miller M.L., Rekhtman N., Moreira A.L., Ibrahim F., Bruggeman C., et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lanier L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez-Lostao L., Anel A., Pardo J. How do cytotoxic lymphocytes kill cancer cells? Clin. Cancer Res. 2015;21:5047–5056. doi: 10.1158/1078-0432.CCR-15-0685. [DOI] [PubMed] [Google Scholar]
- 63.Raulet D.H. Missing self recognition and self tolerance of natural killer (NK) cells. Semin. Immunol. 2006;18:145–150. doi: 10.1016/j.smim.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Raulet D.H., Vance R.E. Self-tolerance of natural killer cells. Nat. Rev. Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 65.Agata Y., Kawasaki A., Nishimura H., Ishida Y., Tsubata T., Yagita H., Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 66.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C., Horton H.F., Fouser L., Carter L., Ling V., Bowman M.R., et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharpe A.H., Wherry E.J., Ahmed R., Freeman G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 69.Curiel T.J., Wei S., Dong H., Alvarez X., Cheng P., Mottram P., Krzysiek R., Knutson K.L., Daniel B., Zimmermann M.C., David O., Burow M., Gordon A., Dhurandhar N., Myers L., et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 70.Hirano F., Kaneko K., Tamura H., Dong H., Wang S., Ichikawa M., Rietz C., Flies D.B., Lau J.S., Zhu G., Tamada K., Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 71.Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Latchman Y., Wood C.R., Chernova T., Chaudhary D., Borde M., Chernova I., Iwai Y., Long A.J., Brown J.A., Nunes R., Greenfield E.A., Bourque K., Boussiotis V.A., Carter L.L., Carreno B.M., et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 73.Strome S.E., Dong H., Tamura H., Voss S.G., Flies D.B., Tamada K., Salomao D., Cheville J., Hirano F., Lin W., Kasperbauer J.L., Ballman K.V., Chen L. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 74.Brahmer J.R., Drake C.G., Wollner I., Powderly J.D., Picus J., Sharfman W.H., Stankevich E., Pons A., Salay T.M., McMiller T.L., Gilson M.M., Wang C., Selby M., Taube J.M., Anders R., et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., Pitot H.C., Hamid O., Bhatia S., Martins R., Eaton K., et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garon E.B., Rizvi N.A., Hui R., Leighl N., Balmanoukian A.S., Eder J.P., Patnaik A., Aggarwal C., Gubens M., Horn L., Carcereny E., Ahn M.J., Felip E., Lee J.S., Hellmann M.D., et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 77.Powles T., Eder J.P., Fine G.D., Braiteh F.S., Loriot Y., Cruz C., Bellmunt J., Burris H.A., Petrylak D.P., Teng S.L., Shen X., Boyd Z., Hegde P.S., Chen D.S., Vogelzang N.J. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 78.Ribas A., Hamid O., Daud A., Hodi F.S., Wolchok J.D., Kefford R., Joshua A.M., Patnaik A., Hwu W.J., Weber J.S., Gangadhar T.C., Hersey P., Dronca R., Joseph R.W., Zarour H., et al. Association of Pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 79.Topalian S.L., Sznol M., McDermott D.F., Kluger H.M., Carvajal R.D., Sharfman W.H., Brahmer J.R., Lawrence D.P., Atkins M.B., Powderly J.D., Leming P.D., Lipson E.J., Puzanov I., Smith D.C., Taube J.M., et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chou C.W., Yang R.Y., Chan L.C., Li C.F., Sun L., Lee H.H., Lee P.C., Sher Y.P., Ying H., Hung M.C. The stabilization of PD-L1 by the endoplasmic reticulum stress protein GRP78 in triple-negative breast cancer. Am. J. Cancer Res. 2020;10:2621–2634. [PMC free article] [PubMed] [Google Scholar]
- 81.Chang L.C., Chen T.P., Kuo W.K., Hua C.C. The protein expression of PDL1 is highly correlated with those of eIF2alpha and ATF4 in lung cancer. Dis. Markers. 2018;2018:5068701. doi: 10.1155/2018/5068701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koren J., 3rd, Blagg B.S.J. The right tool for the job: An overview of Hsp90 inhibitors. Adv. Exp. Med. Biol. 2020;1243:135–146. doi: 10.1007/978-3-030-40204-4_9. [DOI] [PubMed] [Google Scholar]
- 83.Neckers L., Workman P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clin. Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zavareh R.B., Spangenberg S.H., Woods A., Martinez-Pena F., Lairson L.L. HSP90 inhibition enhances cancer immunotherapy by modulating the surface expression of multiple immune checkpoint proteins. Cell Chem. Biol. 2021;28:158–168.e155. doi: 10.1016/j.chembiol.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 85.Jaeger A.M., Stopfer L., Lee S., Gaglia G., Sandel D., Santagata S., Lin N.U., Trepel J.B., White F., Jacks T., Lindquist S., Whitesell L. Rebalancing protein homeostasis enhances tumor antigen presentation. Clin. Cancer Res. 2019;25:6392–6405. doi: 10.1158/1078-0432.CCR-19-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bard J.A.M., Goodall E.A., Greene E.R., Jonsson E., Dong K.C., Martin A. Structure and function of the 26S proteasome. Annu. Rev. Biochem. 2018;87:697–724. doi: 10.1146/annurev-biochem-062917-011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naujokat C., Hoffmann S. Role and function of the 26S proteasome in proliferation and apoptosis. Lab. Invest. 2002;82:965–980. doi: 10.1097/01.lab.0000022226.23741.37. [DOI] [PubMed] [Google Scholar]
- 88.Hershko A., Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 89.Swatek K.N., Komander D. Ubiquitin modifications. Cell Res. 2016;26:399–422. doi: 10.1038/cr.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Voges D., Zwickl P., Baumeister W. The 26S proteasome: A molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 91.Groll M., Ditzel L., Lowe J., Stock D., Bochtler M., Bartunik H.D., Huber R. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 92.Harshbarger W., Miller C., Diedrich C., Sacchettini J. Crystal structure of the human 20S proteasome in complex with carfilzomib. Structure. 2015;23:418–424. doi: 10.1016/j.str.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 93.Rock K.L., Gramm C., Rothstein L., Clark K., Stein R., Dick L., Hwang D., Goldberg A.L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 94.Schwartz A.L., Ciechanover A. The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annu. Rev. Med. 1999;50:57–74. doi: 10.1146/annurev.med.50.1.57. [DOI] [PubMed] [Google Scholar]
- 95.Aki M., Shimbara N., Takashina M., Akiyama K., Kagawa S., Tamura T., Tanahashi N., Yoshimura T., Tanaka K., Ichihara A. Interferon-gamma induces different subunit organizations and functional diversity of proteasomes. J. Biochem. 1994;115:257–269. doi: 10.1093/oxfordjournals.jbchem.a124327. [DOI] [PubMed] [Google Scholar]
- 96.Gaczynska M., Rock K.L., Goldberg A.L. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993;365:264–267. doi: 10.1038/365264a0. [DOI] [PubMed] [Google Scholar]
- 97.Boes B., Hengel H., Ruppert T., Multhaup G., Koszinowski U.H., Kloetzel P.M. Interferon gamma stimulation modulates the proteolytic activity and cleavage site preference of 20S mouse proteasomes. J. Exp. Med. 1994;179:901–909. doi: 10.1084/jem.179.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heink S., Ludwig D., Kloetzel P.M., Kruger E. IFN-gamma-induced immune adaptation of the proteasome system is an accelerated and transient response. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9241–9246. doi: 10.1073/pnas.0501711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marchingo J.M., Cantrell D.A. Protein synthesis, degradation, and energy metabolism in T cell immunity. Cell Mol. Immunol. 2022;19:303–315. doi: 10.1038/s41423-021-00792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Princiotta M.F., Finzi D., Qian S.B., Gibbs J., Schuchmann S., Buttgereit F., Bennink J.R., Yewdell J.W. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–354. doi: 10.1016/s1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 101.Toes R.E., Nussbaum A.K., Degermann S., Schirle M., Emmerich N.P., Kraft M., Laplace C., Zwinderman A., Dick T.P., Muller J., Schonfisch B., Schmid C., Fehling H.J., Stevanovic S., Rammensee H.G., et al. Discrete cleavage motifs of constitutive and immunoproteasomes revealed by quantitative analysis of cleavage products. J. Exp. Med. 2001;194:1–12. doi: 10.1084/jem.194.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Basler M., Groettrup M. Recent insights how combined inhibition of immuno/proteasome subunits enables therapeutic efficacy. Genes Immun. 2020;21:273–287. doi: 10.1038/s41435-020-00109-1. [DOI] [PubMed] [Google Scholar]
- 103.Fehling H.J., Swat W., Laplace C., Kuhn R., Rajewsky K., Muller U., von Boehmer H. MHC class I expression in mice lacking the proteasome subunit LMP-7. Science. 1994;265:1234–1237. doi: 10.1126/science.8066463. [DOI] [PubMed] [Google Scholar]
- 104.Pang K.C., Sanders M.T., Monaco J.J., Doherty P.C., Turner S.J., Chen W. Immunoproteasome subunit deficiencies impact differentially on two immunodominant influenza virus-specific CD8+ T cell responses. J. Immunol. 2006;177:7680–7688. doi: 10.4049/jimmunol.177.11.7680. [DOI] [PubMed] [Google Scholar]
- 105.Van Kaer L., Ashton-Rickardt P.G., Eichelberger M., Gaczynska M., Nagashima K., Rock K.L., Goldberg A.L., Doherty P.C., Tonegawa S. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity. 1994;1:533–541. doi: 10.1016/1074-7613(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 106.Sijts A.J., Ruppert T., Rehermann B., Schmidt M., Koszinowski U., Kloetzel P.M. Efficient generation of a hepatitis B virus cytotoxic T lymphocyte epitope requires the structural features of immunoproteasomes. J. Exp. Med. 2000;191:503–514. doi: 10.1084/jem.191.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sijts A.J., Standera S., Toes R.E., Ruppert T., Beekman N.J., van Veelen P.A., Ossendorp F.A., Melief C.J., Kloetzel P.M. MHC class I antigen processing of an adenovirus CTL epitope is linked to the levels of immunoproteasomes in infected cells. J. Immunol. 2000;164:4500–4506. doi: 10.4049/jimmunol.164.9.4500. [DOI] [PubMed] [Google Scholar]
- 108.Heink S., Fricke B., Ludwig D., Kloetzel P.M., Kruger E. Tumor cell lines expressing the proteasome subunit isoform LMP7E1 exhibit immunoproteasome deficiency. Cancer Res. 2006;66:649–652. doi: 10.1158/0008-5472.CAN-05-2872. [DOI] [PubMed] [Google Scholar]
- 109.McCarthy M.K., Weinberg J.B. The immunoproteasome and viral infection: A complex regulator of inflammation. Front. Microbiol. 2015;6:21. doi: 10.3389/fmicb.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tripathi S.C., Peters H.L., Taguchi A., Katayama H., Wang H., Momin A., Jolly M.K., Celiktas M., Rodriguez-Canales J., Liu H., Behrens C., Wistuba, Ben-Jacob E., Levine H., Molldrem J.J., et al. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E1555–E1564. doi: 10.1073/pnas.1521812113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chauhan D., Catley L., Li G., Podar K., Hideshima T., Velankar M., Mitsiades C., Mitsiades N., Yasui H., Letai A., Ovaa H., Berkers C., Nicholson B., Chao T.H., Neuteboom S.T., et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 112.Ito S. Proteasome inhibitors for the treatment of multiple myeloma. Cancers (Basel) 2020;12:265. doi: 10.3390/cancers12020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Basler M., Lauer C., Beck U., Groettrup M. The proteasome inhibitor bortezomib enhances the susceptibility to viral infection. J. Immunol. 2009;183:6145–6150. doi: 10.4049/jimmunol.0901596. [DOI] [PubMed] [Google Scholar]
- 114.Finn J.D., Hui D., Downey H.D., Dunn D., Pien G.C., Mingozzi F., Zhou S., High K.A. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol. Ther. 2010;18:135–142. doi: 10.1038/mt.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dasgupta S., Castro L.M., Dulman R., Yang C., Schmidt M., Ferro E.S., Fricker L.D. Proteasome inhibitors alter levels of intracellular peptides in HEK293T and SH-SY5Y cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liang Y.H., Chen K.H., Tsai J.H., Cheng Y.M., Lee C.C., Kao C.H., Chan K.Y., Chen Y.T., Hsu W.L., Yeh K.H. Proteasome inhibitors restore the STAT1 pathway and enhance the expression of MHC class I on human colon cancer cells. J. Biomed. Sci. 2021;28:75. doi: 10.1186/s12929-021-00769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lazaro S., Gamarra D., Del Val M. Proteolytic enzymes involved in MHC class I antigen processing: A guerrilla army that partners with the proteasome. Mol. Immunol. 2015;68:72–76. doi: 10.1016/j.molimm.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 118.Oliveira C.C., van Hall T. Alternative antigen processing for MHC class I: Multiple roads lead to rome. Front. Immunol. 2015;6:298. doi: 10.3389/fimmu.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Firat E., Tsurumi C., Gaedicke S., Huai J., Niedermann G. Tripeptidyl peptidase II plays a role in the radiation response of selected primary cell types but not based on nuclear translocation and p53 stabilization. Cancer Res. 2009;69:3325–3331. doi: 10.1158/0008-5472.CAN-08-3269. [DOI] [PubMed] [Google Scholar]
- 120.He S., Xu M., Xiong Z., Hu Y., Huo Q., Lu J., Lin Y., Yang L. Predictive value of protease-activated receptor-2 (PAR2 ) in cervical cancer metastasis. J. Cell Mol. Med. 2021;25:1415–1424. doi: 10.1111/jcmm.16227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hsu F.F., Chou Y.T., Chiang M.T., Li F.A., Yeh C.T., Lee W.H., Chau L.Y. Signal peptide peptidase promotes tumor progression via facilitating FKBP8 degradation. Oncogene. 2019;38:1688–1701. doi: 10.1038/s41388-018-0539-y. [DOI] [PubMed] [Google Scholar]
- 122.Bangia N., Lehner P.J., Hughes E.A., Surman M., Cresswell P. The N-terminal region of tapasin is required to stabilize the MHC class I loading complex. Eur. J. Immunol. 1999;29:1858–1870. doi: 10.1002/(SICI)1521-4141(199906)29:06<1858::AID-IMMU1858>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 123.Garbi N., Hammerling G., Tanaka S. Interaction of ERp57 and tapasin in the generation of MHC class I-peptide complexes. Curr. Opin. Immunol. 2007;19:99–105. doi: 10.1016/j.coi.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 124.Lindquist J.A., Jensen O.N., Mann M., Hammerling G.J. ER-60, a chaperone with thiol-dependent reductase activity involved in MHC class I assembly. EMBO J. 1998;17:2186–2195. doi: 10.1093/emboj/17.8.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chambers J.E., Jessop C.E., Bulleid N.J. Formation of a major histocompatibility complex class I tapasin disulfide indicates a change in spatial organization of the peptide-loading complex during assembly. J. Biol. Chem. 2008;283:1862–1869. doi: 10.1074/jbc.M708196200. [DOI] [PubMed] [Google Scholar]
- 126.Fisette O., Schroder G.F., Schafer L.V. Atomistic structure and dynamics of the human MHC-I peptide-loading complex. Proc. Natl. Acad. Sci. U. S. A. 2020;117:20597–20606. doi: 10.1073/pnas.2004445117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dierssen J.W., de Miranda N.F., Ferrone S., van Puijenbroek M., Cornelisse C.J., Fleuren G.J., van Wezel T., Morreau H. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer. 2007;7:33. doi: 10.1186/1471-2407-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noblejas-Lopez M.D.M., Nieto-Jimenez C., Morcillo Garcia S., Perez-Pena J., Nuncia-Cantarero M., Andres-Pretel F., Galan-Moya E.M., Amir E., Pandiella A., Gyorffy B., Ocana A. Expression of MHC class I, HLA-A and HLA-B identifies immune-activated breast tumors with favorable outcome. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2019.1629780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reeves E., James E. Antigen processing and immune regulation in the response to tumours. Immunology. 2017;150:16–24. doi: 10.1111/imm.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dhatchinamoorthy K., Colbert J.D., Rock K.L. Cancer immune evasion through loss of MHC class I antigen presentation. Front. Immunol. 2021;12:636568. doi: 10.3389/fimmu.2021.636568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shionoya Y., Kanaseki T., Miyamoto S., Tokita S., Hongo A., Kikuchi Y., Kochin V., Watanabe K., Horibe R., Saijo H., Tsukahara T., Hirohashi Y., Takahashi H., Sato N., Torigoe T. Loss of tapasin in human lung and colon cancer cells and escape from tumor-associated antigen-specific CTL recognition. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2016.1274476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sokol L., Koelzer V.H., Rau T.T., Karamitopoulou E., Zlobec I., Lugli A. Loss of tapasin correlates with diminished CD8(+) T-cell immunity and prognosis in colorectal cancer. J. Transl. Med. 2015;13:279. doi: 10.1186/s12967-015-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thuring C., Follin E., Geironson L., Freyhult E., Junghans V., Harndahl M., Buus S., Paulsson K.M. HLA class I is most tightly linked to levels of tapasin compared with other antigen-processing proteins in glioblastoma. Br. J. Cancer. 2015;113:952–962. doi: 10.1038/bjc.2015.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ling A., Lofgren-Burstrom A., Larsson P., Li X., Wikberg M.L., Oberg A., Stenling R., Edin S., Palmqvist R. TAP1 down-regulation elicits immune escape and poor prognosis in colorectal cancer. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1356143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maeurer M.J., Gollin S.M., Martin D., Swaney W., Bryant J., Castelli C., Robbins P., Parmiani G., Storkus W.J., Lotze M.T. Tumor escape from immune recognition: Lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/melan-A antigen. J. Clin. Invest. 1996;98:1633–1641. doi: 10.1172/JCI118958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seliger B., Dunn T., Schwenzer A., Casper J., Huber C., Schmoll H.J. Analysis of the MHC class I antigen presentation machinery in human embryonal carcinomas: Evidence for deficiencies in TAP, LMP and MHC class I expression and their upregulation by IFN-gamma. Scand. J. Immunol. 1997;46:625–632. doi: 10.1046/j.1365-3083.1997.d01-176.x. [DOI] [PubMed] [Google Scholar]
- 137.Seliger B., Maeurer M.J., Ferrone S. TAP off--tumors on. Immunol. Today. 1997;18:292–299. doi: 10.1016/s0167-5699(97)01052-9. [DOI] [PubMed] [Google Scholar]
- 138.Hebert D.N., Molinari M. In and out of the ER: Protein folding, quality control, degradation, and related human diseases. Physiol. Rev. 2007;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- 139.Granados D.P., Tanguay P.L., Hardy M.P., Caron E., de Verteuil D., Meloche S., Perreault C. ER stress affects processing of MHC class I-associated peptides. BMC Immunol. 2009;10:10. doi: 10.1186/1471-2172-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shaikh S.R., Mitchell D., Carroll E., Li M., Schneck J., Edidin M. Differential effects of a saturated and a monounsaturated fatty acid on MHC class I antigen presentation. Scand. J. Immunol. 2008;68:30–42. doi: 10.1111/j.1365-3083.2008.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Almeida S.F., Fleming J.V., Azevedo J.E., Carmo-Fonseca M., de Sousa M. Stimulation of an unfolded protein response impairs MHC class I expression. J. Immunol. 2007;178:3612–3619. doi: 10.4049/jimmunol.178.6.3612. [DOI] [PubMed] [Google Scholar]
- 142.Oberg L., Johansson S., Michaelsson J., Tomasello E., Vivier E., Karre K., Hoglund P. Loss or mismatch of MHC class I is sufficient to trigger NK cell-mediated rejection of resting lymphocytes in vivo - role of KARAP/DAP12-dependent and -independent pathways. Eur. J. Immunol. 2004;34:1646–1653. doi: 10.1002/eji.200424913. [DOI] [PubMed] [Google Scholar]
- 143.Ulianich L., Terrazzano G., Annunziatella M., Ruggiero G., Beguinot F., Di Jeso B. ER stress impairs MHC Class I surface expression and increases susceptibility of thyroid cells to NK-mediated cytotoxicity. Biochim. Biophys. Acta. 2011;1812:431–438. doi: 10.1016/j.bbadis.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 144.Arshad N., Cresswell P. Tumor-associated calreticulin variants functionally compromise the peptide loading complex and impair its recruitment of MHC-I. J. Biol. Chem. 2018;293:9555–9569. doi: 10.1074/jbc.RA118.002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Del Cid N., Jeffery E., Rizvi S.M., Stamper E., Peters L.R., Brown W.C., Provoda C., Raghavan M. Modes of calreticulin recruitment to the major histocompatibility complex class I assembly pathway. J. Biol. Chem. 2010;285:4520–4535. doi: 10.1074/jbc.M109.085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wearsch P.A., Peaper D.R., Cresswell P. Essential glycan-dependent interactions optimize MHC class I peptide loading. Proc. Natl. Acad. Sci. U. S. A. 2011;108:4950–4955. doi: 10.1073/pnas.1102524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jeffery E., Peters L.R., Raghavan M. The polypeptide binding conformation of calreticulin facilitates its cell-surface expression under conditions of endoplasmic reticulum stress. J. Biol. Chem. 2011;286:2402–2415. doi: 10.1074/jbc.M110.180877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gao B., Adhikari R., Howarth M., Nakamura K., Gold M.C., Hill A.B., Knee R., Michalak M., Elliott T. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity. 2002;16:99–109. doi: 10.1016/s1074-7613(01)00260-6. [DOI] [PubMed] [Google Scholar]
- 149.Howe C., Garstka M., Al-Balushi M., Ghanem E., Antoniou A.N., Fritzsche S., Jankevicius G., Kontouli N., Schneeweiss C., Williams A., Elliott T., Springer S. Calreticulin-dependent recycling in the early secretory pathway mediates optimal peptide loading of MHC class I molecules. EMBO J. 2009;28:3730–3744. doi: 10.1038/emboj.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gardai S.J., McPhillips K.A., Frasch S.C., Janssen W.J., Starefeldt A., Murphy-Ullrich J.E., Bratton D.L., Oldenborg P.A., Michalak M., Henson P.M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 151.Chen Y., Ma D., Wang X., Fang J., Liu X., Song J., Li X., Ren X., Li Q., Li Q., Wen S., Luo L., Xia J., Cui J., Zeng G., et al. Calnexin impairs the antitumor immunity of CD4(+) and CD8(+) T cells. Cancer Immunol. Res. 2019;7:123–135. doi: 10.1158/2326-6066.CIR-18-0124. [DOI] [PubMed] [Google Scholar]
- 152.Kobayashi M., Nagashio R., Jiang S.X., Saito K., Tsuchiya B., Ryuge S., Katono K., Nakashima H., Fukuda E., Goshima N., Satoh Y., Masuda N., Saegusa M., Sato Y. Calnexin is a novel sero-diagnostic marker for lung cancer. Lung Cancer. 2015;90:342–345. doi: 10.1016/j.lungcan.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 153.Alam A., Taye N., Patel S., Thube M., Mullick J., Shah V.K., Pant R., Roychowdhury T., Banerjee N., Chatterjee S., Bhattacharya R., Roy R., Mukhopadhyay A., Mogare D., Chattopadhyay S. SMAR1 favors immunosurveillance of cancer cells by modulating calnexin and MHC I expression. Neoplasia. 2019;21:945–962. doi: 10.1016/j.neo.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Callahan M.K., Garg M., Srivastava P.K. Heat-shock protein 90 associates with N-terminal extended peptides and is required for direct and indirect antigen presentation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1662–1667. doi: 10.1073/pnas.0711365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wieczorek M., Abualrous E.T., Sticht J., Alvaro-Benito M., Stolzenberg S., Noe F., Freund C. Major histocompatibility complex (MHC) class I and MHC class II proteins: Conformational plasticity in antigen presentation. Front. Immunol. 2017;8:292. doi: 10.3389/fimmu.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.van Deutekom H.W., Kesmir C. Zooming into the binding groove of HLA molecules: Which positions and which substitutions change peptide binding most? Immunogenetics. 2015;67:425–436. doi: 10.1007/s00251-015-0849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Borst L., van der Burg S.H., van Hall T. The NKG2A-HLA-E Axis as a novel checkpoint in the tumor microenvironment. Clin. Cancer Res. 2020;26:5549–5556. doi: 10.1158/1078-0432.CCR-19-2095. [DOI] [PubMed] [Google Scholar]
- 158.Gooden M.J., van Hall T. Infiltrating CTLs are bothered by HLA-E on tumors. Oncoimmunology. 2012;1:92–93. doi: 10.4161/onci.1.1.17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Michaelsson J., Teixeira de Matos C., Achour A., Lanier L.L., Karre K., Soderstrom K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J. Exp. Med. 2002;196:1403–1414. doi: 10.1084/jem.20020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Paul P., Rouas-Freiss N., Khalil-Daher I., Moreau P., Riteau B., Le Gal F.A., Avril M.F., Dausset J., Guillet J.G., Carosella E.D. HLA-G expression in melanoma: A way for tumor cells to escape from immunosurveillance. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4510–4515. doi: 10.1073/pnas.95.8.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kochan G., Escors D., Breckpot K., Guerrero-Setas D. Role of non-classical MHC class I molecules in cancer immunosuppression. Oncoimmunology. 2013;2 doi: 10.4161/onci.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krijgsman D., Roelands J., Hendrickx W., Bedognetti D., Kuppen P.J.K. HLA-G: A new immune checkpoint in cancer? Int. J. Mol. Sci. 2020;21:4528. doi: 10.3390/ijms21124528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Harada A., Ishigami S., Kijima Y., Nakajo A., Arigami T., Kurahara H., Kita Y., Yoshinaka H., Natsugoe S. Clinical implication of human leukocyte antigen (HLA)-F expression in breast cancer. Pathol. Int. 2015;65:569–574. doi: 10.1111/pin.12343. [DOI] [PubMed] [Google Scholar]
- 164.Ishigami S., Arigami T., Okumura H., Uchikado Y., Kita Y., Kurahara H., Maemura K., Kijima Y., Ishihara Y., Sasaki K., Uenosono Y., Natsugoe S. Human leukocyte antigen (HLA)-E and HLA-F expression in gastric cancer. Anticancer Res. 2015;35:2279–2285. [PubMed] [Google Scholar]
- 165.Chowell D., Morris L.G.T., Grigg C.M., Weber J.K., Samstein R.M., Makarov V., Kuo F., Kendall S.M., Requena D., Riaz N., Greenbaum B., Carroll J., Garon E., Hyman D.M., Zehir A., et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359:582–587. doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lemin A.J., Saleki K., van Lith M., Benham A.M. Activation of the unfolded protein response and alternative splicing of ATF6alpha in HLA-B27 positive lymphocytes. FEBS Lett. 2007;581:1819–1824. doi: 10.1016/j.febslet.2007.03.069. [DOI] [PubMed] [Google Scholar]
- 167.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., Ferrucci P.F., Smylie M., Hogg D., Hill A., Marquez-Rodas I., et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 168.Maio M., Grob J.J., Aamdal S., Bondarenko I., Robert C., Thomas L., Garbe C., Chiarion-Sileni V., Testori A., Chen T.T., Tschaika M., Wolchok J.D. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J. Clin. Oncol. 2015;33:1191–1196. doi: 10.1200/JCO.2014.56.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gettinger S.N., Wurtz A., Goldberg S.B., Rimm D., Schalper K., Kaech S., Kavathas P., Chiang A., Lilenbaum R., Zelterman D., Politi K., Herbst R.S. Clinical features and management of acquired resistance to PD-1 Axis inhibitors in 26 patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 2018;13:831–839. doi: 10.1016/j.jtho.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Haslam A., Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Borresen-Dale A.L., Boyault S., Burkhardt B., Butler A.P., Caldas C., Davies H.R., et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bonaventura P., Shekarian T., Alcazer V., Valladeau-Guilemond J., Valsesia-Wittmann S., Amigorena S., Caux C., Depil S. Cold tumors: A therapeutic challenge for immunotherapy. Front. Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 174.Liu Y.T., Sun Z.J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11:5365–5386. doi: 10.7150/thno.58390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Newell F., Pires da Silva I., Johansson P.A., Menzies A.M., Wilmott J.S., Addala V., Carlino M.S., Rizos H., Nones K., Edwards J.J., Lakis V., Kazakoff S.H., Mukhopadhyay P., Ferguson P.M., Leonard C., et al. Multiomic profiling of checkpoint inhibitor-treated melanoma: Identifying predictors of response and resistance, and markers of biological discordance. Cancer Cell. 2022;40:88–102.e107. doi: 10.1016/j.ccell.2021.11.012. [DOI] [PubMed] [Google Scholar]