Abstract
In this contribution, we provide a comprehensive overview of acyclic twisted amides, covering the literature since 1993 (the year of the first recognized report on acyclic twisted amides) through June 2020. The review focuses on classes of acyclic twisted amides and their key structural properties, such as amide bond twist and nitrogen pyramidalization, which are primarily responsible for disrupting nN to π*C=O conjugation. Through discussing acyclic twisted amides in comparison with the classic bridged lactams and conformationally-restricted cyclic fused amides, the Reader is provided with an overview of amidic distortion that results in novel conformational features of acyclic amides that can be exploited in various fields of chemistry ranging from organic synthesis and polymers to biochemistry and structural chemistry and the current position of acyclic twisted amides in modern chemistry.
Graphical Abstract
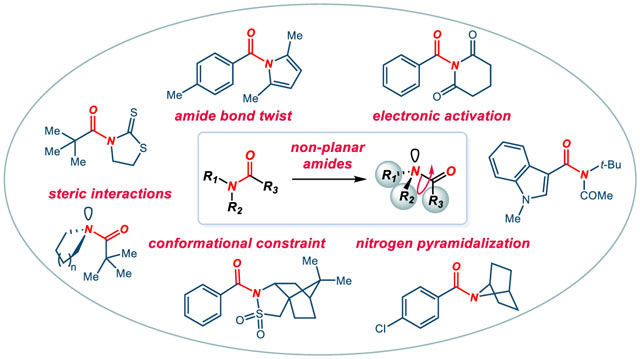
1. Introduction
The amide bond is a fundamental and arguably the most important functional group in chemistry and biology.1 It is well-accepted that the vast majority of amides are planar as a consequence of amidic resonance as vividly demonstrated by Pauling almost a century ago (nN to π*C=O conjugation; amidic resonance of 15–20 kcal/mol in planar amides) (Scheme 1).2–5 However, distortions of the amide bond from planarity6–24 have profound consequences on all major chemical properties of amides, which include (i) barrier to cis–trans rotation; (ii) planarity of the six atoms comprising the amide bond; (iii) geometric changes, such as shortening of the N–C(O) bond and elongation of the C=O bond; (iv) change of the thermodynamic protonation site from oxygen to nitrogen; (v) increased propensity to hydrolysis and nucleophilic acyl substitution; (vi) cleavage of σ N–C bonds; and more recently, (vii) oxidative addition of the N–C(O) bond to transition metals, among others.
Scheme 1.
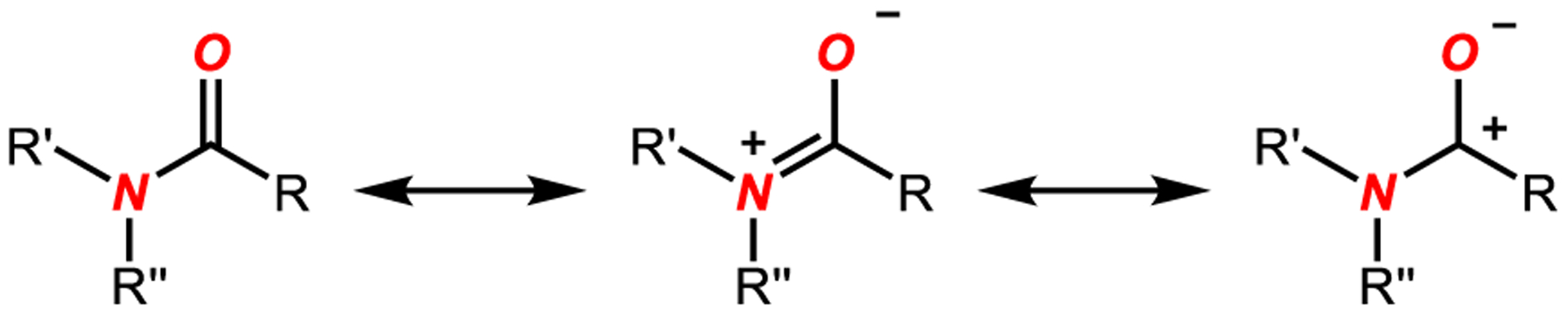
Amide Bond Resonance
The concept of amide bond distortion was first recognized in the 1930s.25 Following the studies by Pauling on amide bond planarity and the conclusion that typical amides are approximately 40% double bond in character,2–5 Lukeš proposed that restriction of the amide bond in a rigid bicyclic structure would have major implications on the properties of such twisted amides.26 The studies by Woodward and Robinson on the structure prediction of β-lactam antibiotics in the 1940s represented another early example that amide bond strain could produce the key driving force for the reactivity of amides.27 In the following years, many research groups reported significant studies on the structure and properties of non-planar amides enclosed in rigid bridged scaffolds.28–35 One of the most elegant of those is the now classic synthesis of a perfectly perpendicular 2-quinuclidonium tetrafluoroborate (2.32, Figure 4) accomplished by the Stoltz group in 2006,36–38 while the studies by Kirby39–43 and Greenberg44–48 on 1-aza-2-adamantanone (2.14, Figure 1) and 1-azabicyclo[3.3.1]nonan-2-one (such as 4.9, Figure 13), respectively, have enabled a greatly improved understanding of the properties of geometrically nonplanar amide bonds.
Figure 4.
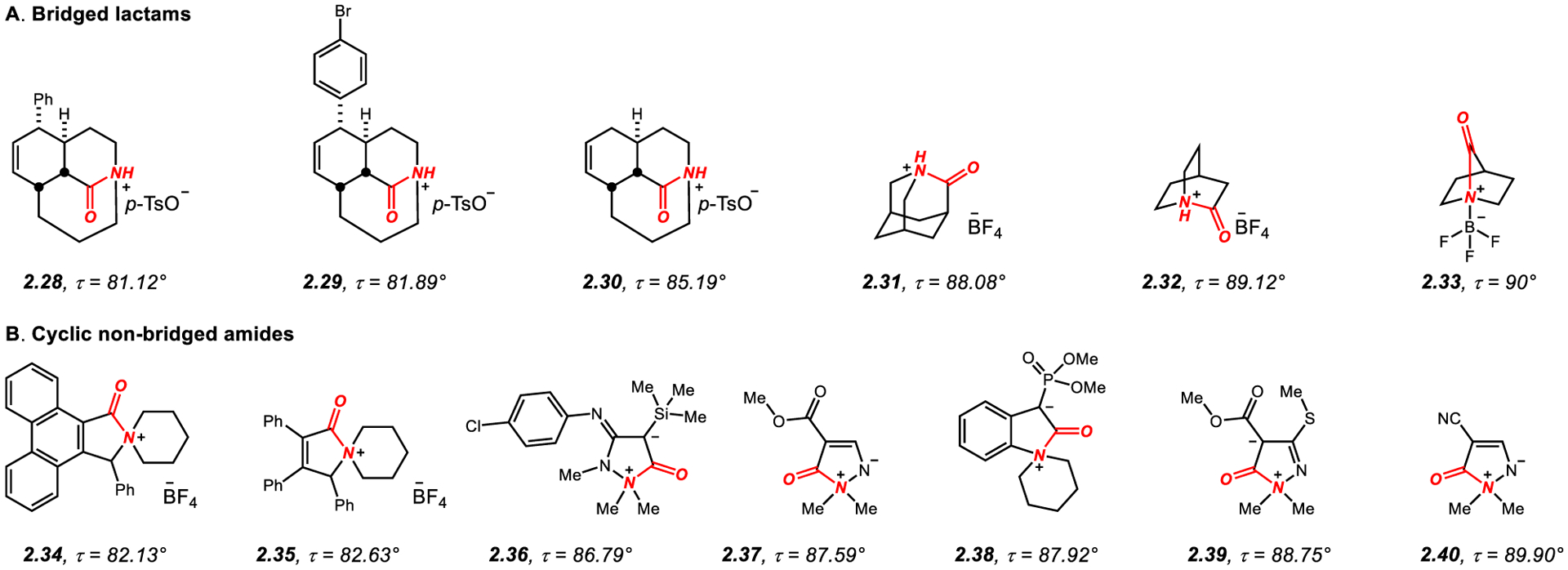
N-Quaternized Cyclic Amides with Twist Values of 40° to 90°.
Figure 1. Bicyclic Bridged Amides with Twist Values of 40° to 90°.

(See SI for details and expanded tables).
Figure 13.
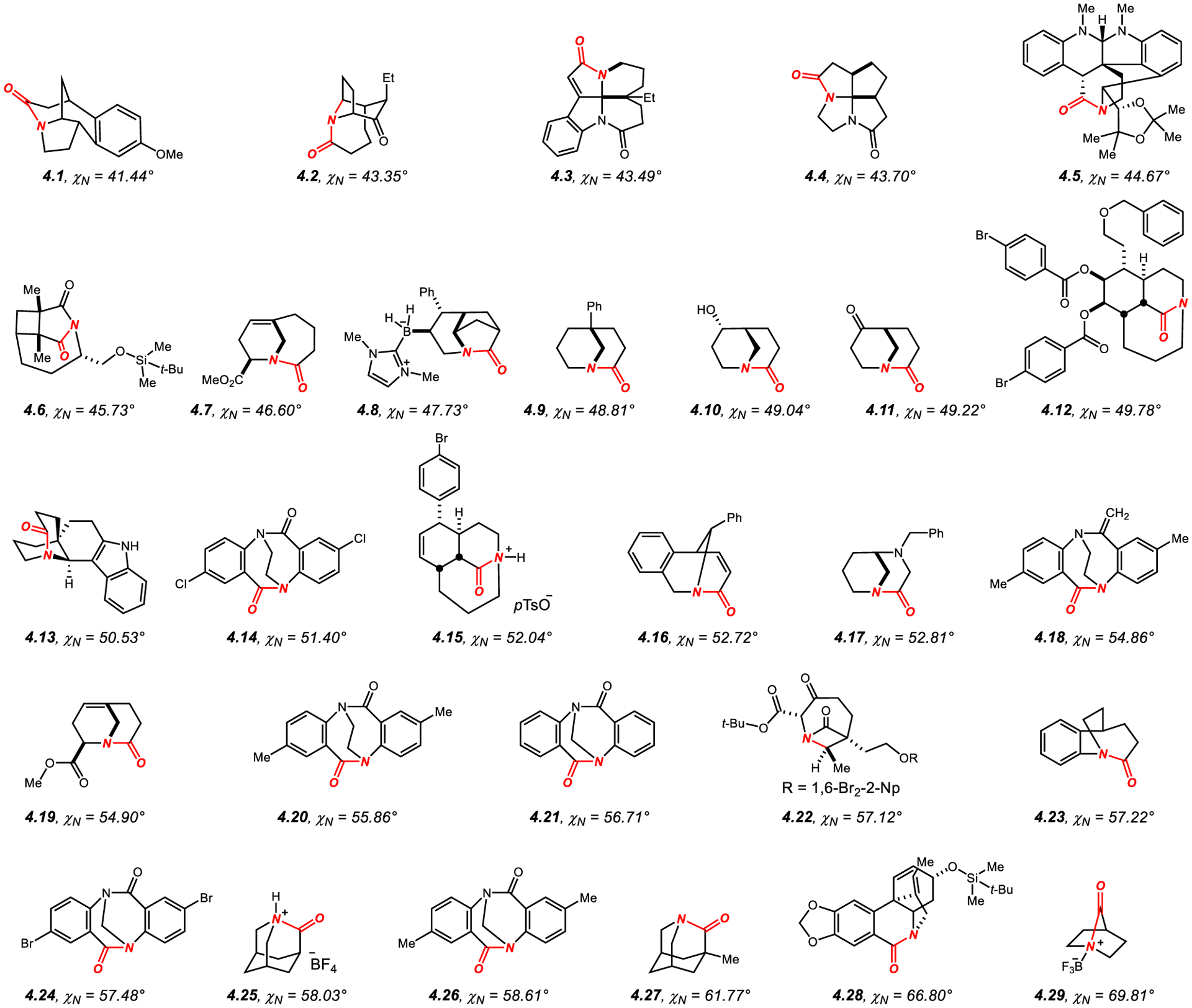
Bridged and Related Amides with Nitrogen Pyramidalization Values of 40° to 70°.
In contrast to the conformationally-restricted bridged lactams,28–48 recent years have witnessed an explosion of interest in acyclic twisted amides. Amide bond distortion in acyclic amides leads to conformational and electronic modifications of the properties of acyclic amides that are commonly encountered in organic chemistry.49–56 Recognized as early as in 1993 by Yamada,57–62 this ground-state-destabilization has recently resulted in the development of amide bond cross-coupling reactions, wherein the twisted amide N–C(O) bond undergoes oxidative addition to a low valent metal.63–78 Moreover, studies demonstrate that acyclic twisted amides can be effectively utilized in direct nucleophilic addition reactions, a class of processes that has a major impact on polymer modification, synthesis of pharmaceuticals, and peptide cleavage.79–82 Furthermore, acyclic amide bond twisting has been exploited in structural chemistry, showing that geometric changes around the amide bond could be applied to effectively control the conformation of molecules.83–90 Moreover, amide bond distortion of acyclic amides has been studied in the context of peptide cis-trans isomerization and peptide cleavage,91–102 wherein two mechanisms have been proposed: (i) hydrolysis via ketene intermediates, (ii) steric repulsion of N-substituents, both exploiting ground-state-destabilization and amide bond twist.103–105 Perhaps most importantly, numerous examples in synthetic chemistry demonstrate that acyclic twisted amides behave as carboxylic acid derivatives characterized by properties vastly different from classical amides.106–115 Thus, taken together with the fact that selective activation of planar amides to achieve distortion in acyclic amides is feasible,49,50 twisting of acyclic amide bonds results in a broadly applicable amide bond activation concept in small molecule synthesis.
Despite the fact that acyclic twisted amides represent a major class of amides in organic synthesis, structural chemistry and biochemistry and significant advances have been reported, a comprehensive review on acyclic twisted amides has not been published. In this manuscript, we provide a comprehensive overview of acyclic twisted amides, focusing on (i) classes of acyclic twisted amides, and (ii) their key structural properties, such as amide bond twist and nitrogen pyramidalization, which are primarily responsible for disrupting nN to π*C=O conjugation. By discussing acyclic twisted amides in comparison with the classic bridged lactams and conformationally-restricted cyclic fused amides, such as β-lactams, the reader will be provided with an overview of the area and the current position of acyclic twisted amides in modern chemistry. Twisted amides cover a broad range of amidic distortion that results in novel conformational features that can be exploited in various fields of chemistry ranging from organic synthesis and polymers to biochemistry and structural chemistry.
Amide bond distortion is typically defined by the Winkler-Dunitz distortion parameters (Scheme 2).116 Twist angle (τ) describes the magnitude of rotation around the N–C(O) bond, while pyramidalization parameters (χN) and (χC) describe pyramidalization at nitrogen and pyramidalization at carbon, respectively. Twist is 0° for planar amide bonds and 90° for fully orthogonal bonds, while χ parameters are 0° for planar bonds, and 60° for fully pyramidalized bonds. Since (χC) parameter is typically 0° or close to 0° irrespective of the geometry of the amide bond, twist angle (τ) and pyramidalization at nitrogen (χN) are used as the primary descriptors of non-planar amide bond geometry. In addition to Winkler-Dunitz distortion parameters, the additive distortion parameter (τ+χN) has been defined and it is particularly useful in comparing amide bond distortion within the same classes of non-planar amides.117,118 Furthermore, N–C(O) and C=O bond lengths, in particular, and to a lesser extent C–C(O) and C–NC(O) bond lengths typically give a very useful information about the structures and properties of acyclic non-planar amide bonds and should be considered when reporting new acyclic twisted amides and discussing their reactive properties.28–35 In terms of amidic resonance, resonance energies and barriers to rotation provide insight into the strength of the amide N–C(O) bond, and these values measured by spectroscopic or computational methods are available for numerous non-planar amides for comparison purposes.12,45,46,119–121
Scheme 2.

Winkler-Dunitz Distortion Parameters (τ, χN, χC) of Amide Bonds
Steric distortion by non-bonding interactions that is feasible in several classes of tertiary amides represents by far the most effective strategy for distortion of the amide bond planarity in acyclic amides (Scheme 3). While similar geometric alteration is not easily achievable in primary and secondary amides, from a synthetic standpoint, in many cases common primary and secondary amides can be readily and reversibly converted into sterically twisted tertiary amides,49,50 thus enabling the acyclic twisting concept to be applicable to all classes of amides.
Scheme 3.
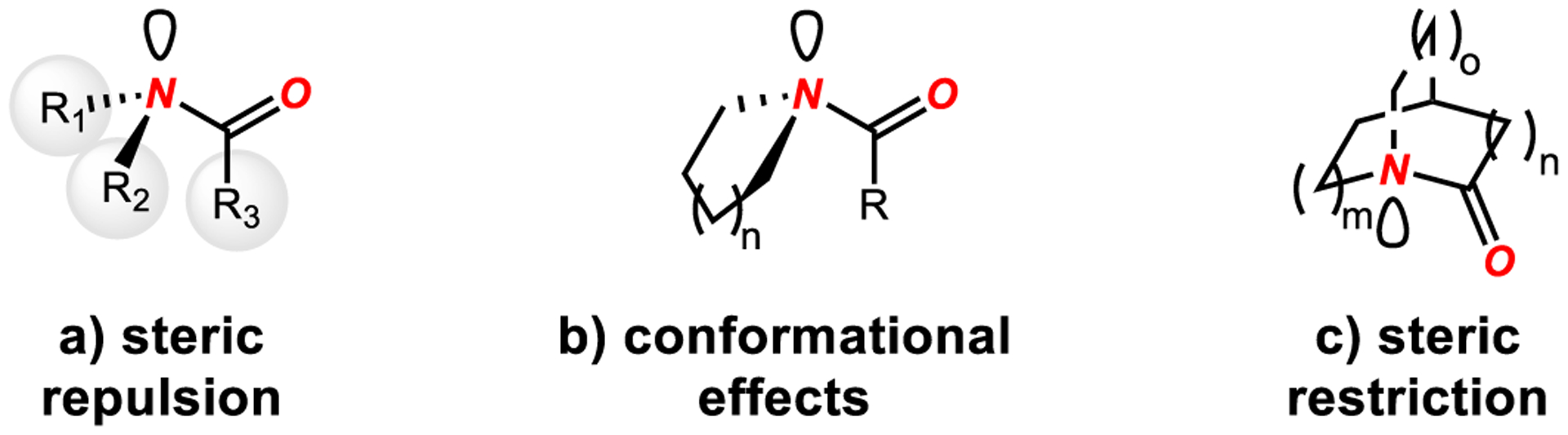
Types of Amide Bond Distortion. Note that Steric Restriction Applies to Both Bridged and Fused Ring Systems.
An additional point that should be discussed is the fact that in many cases steric distortion of the acyclic amide bond is associated with electronic activation through Nlp delocalization (lp = lone pair) on the substituents outside of the twisted N–C(O) bond. Depending on the class of amides, these effects may have a cooperative effect or be a consequence of one another. As such, in many instances acyclic twisted amides can also be considered as N-acyl, N-sulfonyl, N-carbamoyl or related derivatives. According to IUPAC (IUPAC = International Union of Pure and Applied Chemistry), amides are defined as carboxylic acid derivatives in which “acidic hydroxy group has been replaced by an amino or substituted amino group.” Thus, it is important to correctly assign the twisted N–C(O) amide bond when referring to non-planar amides and their derivatives. In this context, it is likely that more amides that have been synthesized over the years could be classified as twisted, but their twist remains unknown. In general, although DFT methods can be used to correctly predict amide bond distortion in bridged and related lactams,45,46,117,118 the accurate determination of the geometry of acyclic twisted amides is feasible only by x-ray crystallography, while DFT predictions in the absence of x-ray crystallographic analysis should be treated with caution.12,126,232 In general, DFT predictions of acyclic amides overestimate one or both distortion parameters (τ, χN) depending on the level used. In addition, it should be noted that DFT is unable to accurately predict the carbonyl bending angle of bridged lactams.38
With the aim of providing a comprehensive overview of acyclic twisted amides, we have conducted a comprehensive CCDC (Cambridge Structural Database) search of non-planar amides covering all years up to 2020. The analysis indicated >63,000 distinct tertiary amide and amide derivatives with reported structural parameters (63,071). For the purpose of the review, only amides without coordinated metal are included as it is well-established that metal-coordination to polar bonds changes their geometrical properties.122 These amides will be considered separately in the future studies. Similarly, polar derivatives of amides, such as ureas, carbamates and thiocarbamates as well as hydrazides and related compounds are not included.123–125 The polar derivatives will be the topic of our future studies. A summary of structurally-characterized amides as determined from the CCDC database is presented in Table 1.
Table 1.
Structurally Characterized Tertiary Amidesa
| entry | type | no. of amides and derivatives |
|---|---|---|
| 1 | All amides | 63,071 |
| 2 | Amides w/o metal | 48,024 |
| 3 | Ureas | 8,953 |
| 4 | Carbamates | 6,507 |
| 5 | Thiocarbamates | 266 |
| 6 | Anilides | 11,419 |
| 7 | Hydrazides | 3,988 |
| 8 | N-Acyl-hydroxylamines | 803 |
| 9 | N-Acyl-thiohydroxylamines | 1,298 |
| 10 | Acyclic amides | 16,505 |
| 11 | Acyclic anilides | 3,455 |
| 12 | Acyclic hydrazides | 1,749 |
| 13 | Acyclic N-acyl-hydroxylamines | 408 |
| 14 | Acyclic N-acyl-thiohydroxylamines | 585 |
| 15 | Acyclic N-acyl-azetidines | 108 |
| 16 | Acyclic N-acyl-aziridines | 53 |
CCDC ConQuest analysis, 05/05/2020. Note that for compounds in which two or more structures have been characterized in a single unit cell, the number of amides is one.
For comparison purposes, the total number of tertiary amides includes amides without coordinated metal (48,024) and amides with coordinated metal (15,047). Furthermore, the total number of structurally characterized tertiary amides includes ureas (8,953), carbamates (6,507) and thiocarbamates (266). It is further interesting to note that anilides (N–Ar) represent a major class of structurally-characterized tertiary amides to date (11,419). These amides are well-known to be electronically-activated due to nN → Ar conjugation with significantly reduced amidic resonance (RE, resonance energy, 13.5 kcal/mol of PhC(O)NPhMe, 1.1, Scheme 4).126 Furthermore, the total number includes hydrazides (3,988), N-acyl-hydroxylamines (803), and N-acyl-thiohydroxylamines (1,298). It should be noted that there is some overlap between the classes of amides in Table 1, entires 3–16.
Scheme 4.

Structures of Additional Amides Discussed in the Manuscript.
The total number of structurally-characterized tertiary amides (63,071) should be compared with the total number of structurally-characterized tertiary acyclic amides (16,505) with representative subclasses presented for comparison (anilides, 3,455; hydrazides, 1,749; N-acyl-hydroxylamines, 408; N-acyl-thiohydroxylamines, 585). It is worthwhile to note that there are only few structurally-characterized acyclic amides in which the nitrogen atom is contained in a small ring, such as N-azetidinyl amides (108), N-aziridinyl amides (53). These amides are well-established to contain pyramidalized nitrogen atom (e.g. χN = 32.5°, 4-Tol-C(O)-azetidine, 1.2, Scheme 4; χN = 54.9°, aziridinyl, 1,3-diadamantylaziridin-2-one, 1.3, Scheme 4). In this context, an important study on surveying crystallographically characterized amides by Chakrbarti and Dunitz should be noted.127 While the study by Dunitz focused on conformational preferences of planar amides with respect to bond lengths, C(O)–N–C–C(H) torsion and C(O)–N–C angles, the main conclusion of our study is that non-planarity of amide bond is commonly found in N-activated tertiary amides achieved by several methods of activation (Sections 2–5).
The evaluation of amide bond distortion distribution of structurally-characterized tertiary acyclic amides indicates a significant number of >200 amides with twist >40°, and >500 with pyramidalization >40° (Winkler-Dunitz distortion). Based on the experimental studies on twisted amides,28–35 the values of τ = 40° and χN = 40° are considered as threshold values that allow for unique reactivity of the amide bond that is distinct from typical planar amides. In many cases, these amides should be considered as “amino-ketones” or “activated amides” rather than classic amides, while the increasing continuum of changes in reactivity is enabled by steric and electronic activation.63–78,115 The Winkler-Dunitz parameters (τ, χN, χC) were calculated on the basis of the equation in Scheme 2.116 For classification purposes in the review, these values are given with the accuracy to two decimal places. In general, amide bond distortion parameters are given with the accuracy to three decimal places with respect to the bond lengths of the amide bond, while Winkler-Dunitz parameters are given with the accuracy to one or two decimal places. It should be noted that changes in the properties of the amide bond represent a continuum of change.
It is also worth noting that the additive Winkler-Dunitz parameter (τ+χN) represents a very accurate predictor of the twisted amide bond properties in conformationally-locked bridged lactams;117,118 however, in contrast to bridged systems, in which correlation between twist and pyramidalization is typically linear within the same scaffold, geometric distortions of acyclic amide bonds can be separately achieved by twist, pyramidalization and/or combination of twist and pyramidalization (i.e. twisted, pyramidalized and twisted pyramidalized amides by Yamada’s classification).30,57 As a result, twist angle (τ) and pyramidalization at nitrogen (χN) parameters are considered separately for acyclic geometrically distorted amide bonds, while the effects of the second parameter are discussed where relevant.
An additional parameter that should be discussed is the carbonyl bending angle (ξ).33,128 It has been noted by Bürgi and co-workers that strained lactones and lactams exhibit a compression of the amide NCO bond angle.128 Subsequently, Stoltz and co-workers made the same observation in their synthesis of 7-hypoquinuclidonium systems.38 The carbonyl bending angle has been mathematically defined as (ξ) = ((360° – CCN)/2 – OCN) (Scheme 5).38 This value has been proposed to correlate with the relative activation of amides as a trapped intermediate of the intramolecular elimination of the amine to form an acylium ion. For the most twisted bridged lactam, 7-hypoquinuclidone BF3 complex (2.33, Figure 4), ξ is 5.8°, which indicates early stage of acylium formation.38 For comparison, for the most twisted acyclic amides, such as N-benzoyl-glutarimide (3.100, Figure 9), ξ is 3.5°; for Yamada’s amide (3.56, Figure 8), ξ is 3.3°; for 4-Me2N-C6H4-C(O)N-Boc2 (3.88, Figure 9), ξ is 4.4°; for the fully twisted Ph-C(O)-N-Ts/Boc (3.139, Figure 11), ξ is 4.4°; and for benzoyl-2,5-dimethyl-pyrrole (3.107, Figure 10), ξ is 1.5°. Future studies on twisted amides should routinely report the carbonyl bending angle parameter (ξ).
Scheme 5.
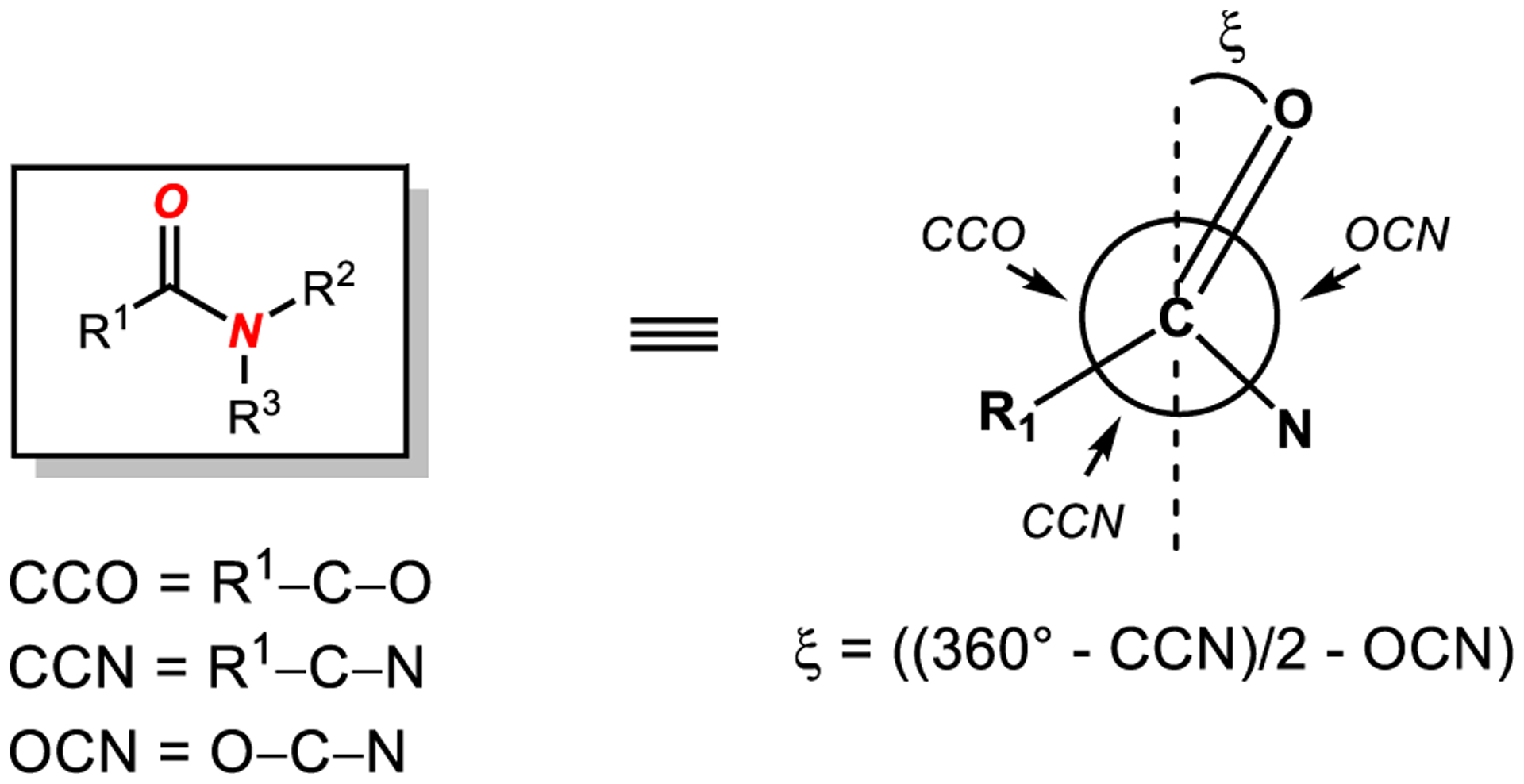
Carbonyl Bending Angle (ξ) of Amide Bonds
Figure 9.
N,N-Diacyl-Activated Acyclic Amides with Twist Values of 40° to 90°.
Figure 8.

N-Mono-Acyl-Activated Acyclic Amides with Twist Values of 65° to 90°.
Figure 11.
N-Sulfonyl-Activated Acyclic Amides with Twist Values of 40° to 90°.
Figure 10.
N-Heterocycle-Activated Acyclic Amides with Twist Values of 40° to 90°.
The reader should note that in order to allow for a broad overview and comparison of acyclic twisted amides with their more established bridged and cyclic counterparts, bridged lactams and cyclic amides are included in the review. As outlined in the section above, since it is well-established that twist and pyramidalization in acyclic twisted amides are typically independent of each other, these values are considered separately. As such, the review is arranged into the following sections: (i) cyclic amides with twist of 40° to 90°; (ii) acyclic amides with twist of 40° to 90°; (iii) cyclic amides with N-pyramidalization of 40° to 90°; (iv) acyclic amides with N-pyramidalization of 40° to 90°. Relevant examples of amide bond properties, computational characterization and amide bond reactivity are included along with the discussion of the structural properties of structurally-distorted amides. We hope that this review will stimulate the additional use of amide bond distortion by a range of interested chemists and lead to further progress in this highly important area of amide bond chemistry.
Note that detailed summary tables including Winkler-Dunitz distortion parameters are included in the Supporting Information (SI).
2. Cyclic Amides: Twist 40–90°
In this section, we present a comprehensive overview of structurally-characterized cyclic amides with twist values of 40° to 90°. In general, these amides can be divided into the following classes: (1) classic bridged lactams; (2) N-acyl-activated cyclic amides; (3) N-sulfonyl-activated cyclic amides; (4) N-quaternized cyclic amides; (5) N-aziridinyl cyclic amides; and (6) miscellaneous examples.
2.1. Bridged Cyclic Amides
Conformational-restriction of the amide bond geometry in a bicyclic ring with the nitrogen atom at the bridgehead position represents the most classic and historically relevant method for freezing out non-planar amide bond conformation (Figure 1). After the seminal proposal by Lukeš in 1938,26 many researchers became intrigued by the prospect of synthesizing these elusive amides, including very elegant studies by Yakhontov,129–132 Pracejus,133–135 Brown,136–138 and others,28–35 which after a clear misassignment by Yakhontov,129 culminated in the unambiguous synthesis of fully perpendicular 2-quinuclidonium tetrafluoroborate and 1-aza-2-adamantanone by Stoltz36–38 and Kirby,39–43 as well as the establishment of 1-azabicyclo[3.3.1]nonan-2-one as a model medium bridged twisted lactam characterized by the N-/O-protonation switch cross-over geometry by Greenberg.44–47
The most twisted of these bridged lactams show the reactive properties of “amino-ketones”, while additional unique reactivity can be achieved by differentiating distortion of planarity of the C–N–C–O bonds, such as σ N–C bond cleavage, which served as the basis the discovery of novel reactivity of acyclic twisted amides.49–78
In general, very few bridged lactams with twist values close to 90° have been reported. After early studies on increased rate of hydrolysis of bridged lactams by Pracejus and Brown,28–35 studies by Greenberg first quantified that the cross-over of the “amino-ketone” type reactivity can be expected with the τ values close to 40°.45,46 Studies by Aubé demonstrated the increased reactivity of the unactivated σ N–C bond to hydrogenolysis conditions, which represented one of the first examples of N–C bond scission of unactivated amide bonds.32,139 The amide bond geometry required for this type of reactions has been demonstrated to be close to 40°. These studies culminated in the demonstration of an instantaneous hydrolysis of N–C(O) bond in the perpendicular 2-quinuclidonium tetrafluoroborate (2.32, Figure 4) and 1-aza-2-adamantanone (2.14, Figure 1) systems by Stoltz36 and Kirby.39 Since several reviews on the properties of the bridged lactams have been published,28–35 this section briefly summarizes the geometry of bicyclic scaffolds.
Examination of amides in Figure 1140–152 reveals that highly rigid adamantanone (2.14–2.15),39,43 haemanthidine (2.10, 2.12)151 and tricyclic bridged stemona (2.7–2.9, 2.13)147,148 bicyclic frameworks are most effective for achieving high twist in bridged lactams. Note that the nomenclature that underlines the bridge with the C=O bond in bicyclic structures containing the lactam linkage is used. It is important to note that the position of the bridge determines the properties of amides in this class of lactams. It is interesting to note that related one-carbon bridged [6.3.1] (2.11)152 and [4.3.1] (2.3)142 systems result in a comparably high twist of the amide bond. Other ring systems that lead to τ > 40° include a [2.3.2] benzo-fused system (2.1),140 unique Tröger’s base bis-twisted amides (2.2, 2.5),144,145 a related [2.1.3] 1,5-diazabicyclo[3.2.1]octane system (2.4)143 and stemofoline alkaloid framework (2.6).146 Overall, it is rather surprising that more than 80 years after the original proposal by Lukeš only very few bridged lactams with appreciable twist have been structurally characterized.
2.2. N-Acyl-Activated Cyclic Twisted Amides
Activation of cyclic amides with N-acyl group represents another effective approach to achieve geometric distortion of the amide bond (Figure 2).153–162 Note that in contrast to acyclic amides and amide derivatives (Section 3), the twisted amide bond in examples in Figure 2 refers to the cyclic amide bond in lactams (cf. exo-cyclic amide bond). These examples also include related imidoyl-type activation as represented by 2.16.153 As shown in Figure 2, the activating group can be within the ring (endocyclic), such as amides 2.16,153 2.17,154 2.23,160 2.25162 or more commonly outside the lactam ring (exocyclic), such as 2.18,155 2.19,156 2.20,157 2.21,158 2.22.159 The twisted lactams feature 7-membered rings (2.16, 2.17, 2.19, 2.22, 2.23, 2.25), 8-membered rings (2.18, 2.20, 2.21) or macrocyclic rings (2.24).161 The latter compound is related to imide macrocycles (vide infra, Section 2.6.) The most recognized in this series is the eight-membered lactam 2.20, featuring a transoid amide bond, wherein the amide bond distortion arises from steric and electronic factors.157 The main distortion has been ascribed to the avoidance of allylic strain between the lactam N–C(O) bond and the N–acyl bond. Overall, N-acyl-activation appears as a highly effective way of distorting cyclic amide bonds, while the nN → π*C=O conjugation is accomplished through the presence of another carbonyl group (exo- or endocyclic).
Figure 2.

N-Acyl-Activated Cyclic Amides with Twist Values of 40° to 90°.
2.3. N-Sulfonyl-Activated Cyclic Twisted Amides
N-sulfonyl activation represents a related method to N-acyl activation to twist cyclic amides bonds (Figure 3).163 The twist in the two lactams reported (2.26–2.27)164,165 results from a significant non-bonding interaction between the N-sulfonyl group and the adjacent C-substituents on both sides on the amide bond. It is interesting to note that both types of lactams are readily available by 1,7-enyne bicyclizations164 and enolate cyclizations.165
Figure 3.
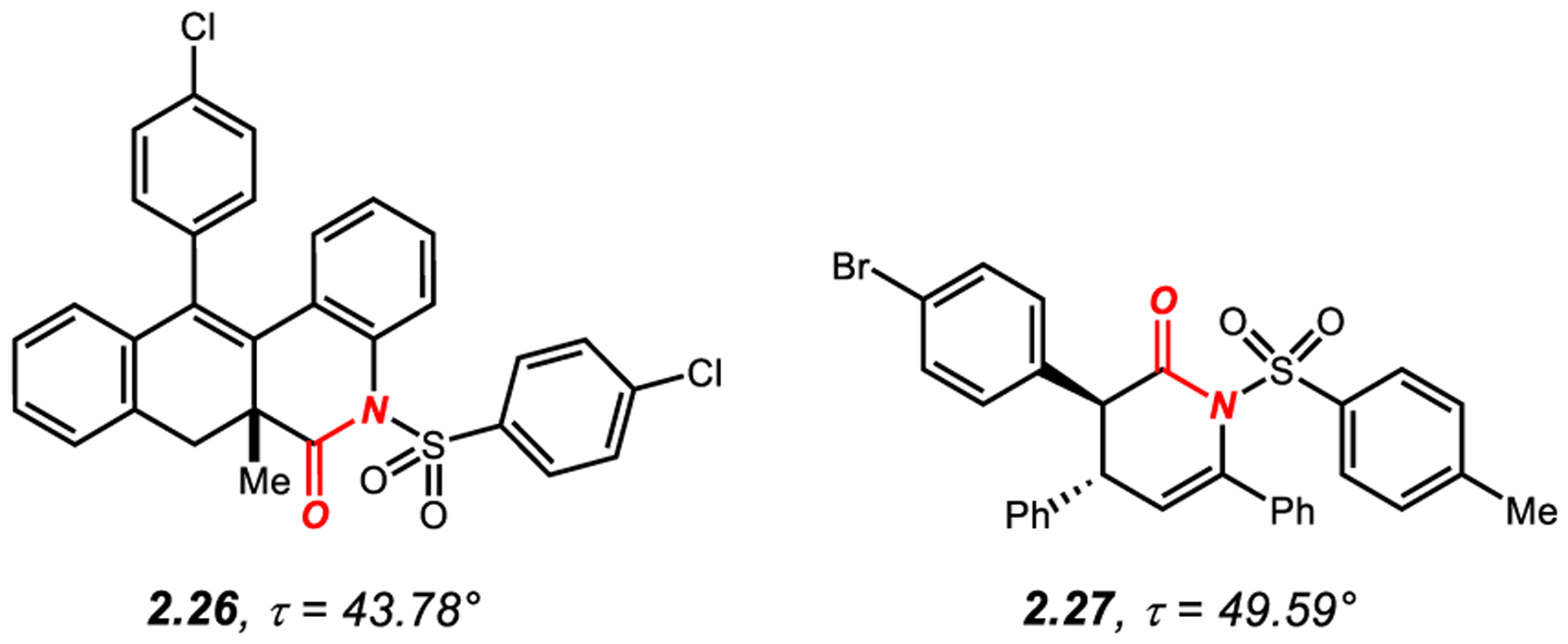
N-Sulfonyl-Activated Cyclic Amides with Twist Values of 40° to 90°.
2.4. N-Quaternized Cyclic Twisted Amides
Two classes of N-quaternized cyclic amides containing highly twisted amide bonds have been reported: (i) bridged lactams (Figure 4A); and (ii) cyclic non-bridged amides (Figure 4B).
It is particularly interesting from the standpoint of novel reactivity of N–C(O) bonds that amides 2.28–2.33 in Figure 4A have been prepared by the direct N-protonation of the corresponding bridged lactams.142,43,36,38 Note that this class also includes the incredibly strained bridged lactam 2.33 embedded in a one-carbon bridged [2.2.1] ring system with N-coordinated BF3 complex.38 The nitrogen atom in this particular lactam as well as in the archetypal 2-quinuclidonium tetrafluoroborate 2.32 featuring unsubstituted [2.2.2] system are protected in situ as quaternary salts after the ring forming intramolecular Schmidt reaction, which enables their facile isolation.36 In contrast, tricyclic lactam precursors to 2.28–2.30 are stable to the aqueous isolation conditions and undergo facile N-protonation by mild acids, such as p-TsOH.142 This class is also represented by the parent 1-aza-adamantanone 2.31 crystallized as HBF4 salt.43 In general, quaternization of the nitrogen atom in bridged lactams results in a significant increase of amide bond twist.166
N-Quaternized amides 2.34–2.35 feature close to perpendicular twist of the amide bond (Figure 4B).167–171 These amides have been prepared by the reaction of aminocarbene complexes of chromium with alkynes and demetallation. Of interest is the facile N–C(O) ring opening upon exposure to Et3N, consistent with the high reactivity of N-alkylated non-planar amides.167
Five-membered betaines, such as 2.36 has been isolated from the reaction of aryl isocyanate with an yne-hydrazines.168 Related compounds include pyrazolinium ylides 2.37 and 2.40 prepared from β-enaminoesters169 as well as 2-oxoindolinium enolate 2.38 from Wolff rearrangement/intramolecular nitrogen addition170 and pyrazolium betaines, such as 2.39 from the reaction of ketene ethylene acetals with N,N-dialkylhydrazines.171 Overall, these zwitterionic N-alkyl amides represent an attractive indirect way of accessing fully twisted (τ > 82°) cyclic amide bonds.
2.5. N-Aziridinyl-Fused Cyclic Twisted Amides
Amides 2.41–2.42 featuring fused [4.1.0] and [3.1.0] ring systems with the bridgehead nitrogen in a 3-membered ring contain significantly twisted amide bonds (τ > 50°) (Figure 5).172,173 It should be noted that in these examples, amide bond twist is accompanied by full pyramidalization of the nitrogen atom geometrically enforced by the 3-membered ring (χN = 68.1° and 61.9° for 2.40 and 2.41, respectively).17 Amide 2.40 undergoes facile aziridine ring opening with MeOH to give the seven-membered lactam; the reaction is likely initiated by N-protonation of the amide bond nitrogen.172
Figure 5.
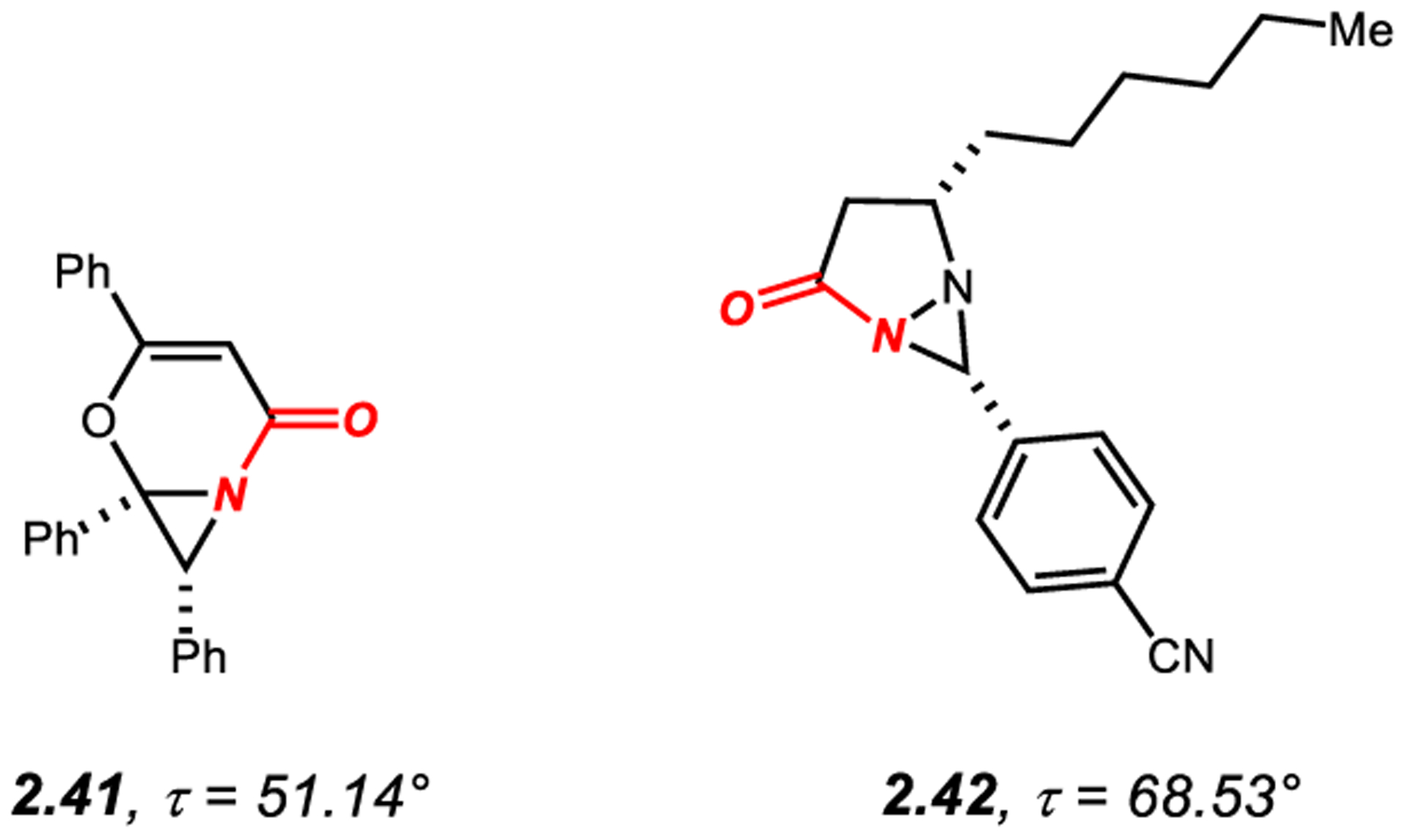
N-Aziridinyl Cyclic Amides with Twist Values of 40° to 90°.
2.6. Miscellaneous Cyclic Twisted Amides
Miscellaneous examples of cyclic amides with considerable twist of the amide bond include an intriguing BNC5 boracycle 2.43 reported by Martin (Figure 6A),174 imide macrocycles with 18-membered (2.44–2.50) and 24-membered (2.51–2.59) ring systems (Figure 6B–C)175–179 and azafulleroids, such as 2.60 (Figure 6D).180 In particular, the x-ray structure of boracycle 2.43 indicates Nlp delocalization into the boron atom (short B–N bond of 1.417 Å and short C=O bond of 1.209 Å).174 Resonance energies have not been reported. These intriguing compounds might find applications as boron Lewis acids in organic chemistry. Imide macrocycles 2.44–2.50 and 2.51–2.59 feature 3 and 4 sets of non-planar amide bonds, respectively, restricted by the imide-conformation.175–179 Azafulleroid 2.60 contains one-carbon bridged [4.3.1] ring system (vide supra, Section 2.1.) and readily reacts with basic alumina or BnNH2 to give the corresponding azafullerenes.180
Figure 6.

Miscellaneous Cyclic Amides with Twist Values of 40° to 90°.
3. Acyclic Amides: Twist 40–90°
Activation of acyclic tertiary amides by intramolecular steric repulsion between amide bond substituents results in disruption of amidic resonance, N–C(O) bond rotation and overall deformation of the amide bond geometry.163 The first to recognize that such geometric repulsion can be used to effectively twist acyclic amide bonds was Yamada in 1993,57–62 which resulted in an elegant investigation of 3-pivaloyl-1,3-thiazolidine-2-thiones (such as 3.56, Figure 8) benefiting from the large radius of the thiocarbonyl group in a compact 1,3-thiazolidine scaffold with a very significant τ of 74.3°.57 It was also noted that since in these acyclic systems, twist is generally disconnected from amide bond pyramidalization. As such, these amides depict the most accurate representation of twisted amides.
In general, acyclic twisted amides can be categorized into the following classes depending on the type of N-activating moiety: (1) N-mono-acyl-activated twisted amides; (2) N-di-acyl-activated twisted amides; (3) N-sulfonyl-activated twisted amides; (4) N-heterocycle-activated twisted amides; and (5) miscellaneous examples.
3.1. N-Acyl-Activated Acyclic Twisted Amides
At present, N-acyl-activation represents by far the most common method to achieve distortion of acyclic twisted amides with numerous examples of various amides, scaffolds and N-acyl activating groups approaching τ values of 80–90°. For clarity, N-acyl-activated amides have been divided into Nmono-acyl and N,N-di-acyl-activated twisted amides (sections 3.1.1. and 3.1.2.).
It should be noted that depending on the bond or substitution that are discussed, these amides can also be referred to as imides or derivatives. In these systems, steric distortion is closely related to the electronic activation of the amide bond owing to the presence of another carbonyl group that can participate in nN → π*C=O conjugation.49–78 In many cases, these amides represent extremely reactive twisted amides with “resonance-disconnected” N–C(O) bond conjugation. Importantly, these acyclic twisted are significantly more stable to storage and hydrolysis conditions than most of the highly twisted bridged lactams,28–35 which enables their application as acyl transfer reagents or, more recently, as resonance and geometry-tunable electrophilic cross-coupling reagents by N–C(O) oxidative addition to low valent metals.
3.1.1. N-Mono-Acyl-Activated Acyclic Twisted Amides
N-Mono-acyl-activated twisted amides with τ values of 40–90° are presented in Figures 7–8.57,60,181–230 Three points of amide bond geometry should be considered when discussing structures of acyclic twisted amides: (1) N-acyl-activating substituent; (2) the other N-substituent; (3) substitution at the α-carbon. It is important to note that when both of the N–C(O) groups are acyclic, geometric distortion of the more twisted bond represents a balance between the optimum geometry for the two acyl bonds, which often leads to the flattening of the other N-acyl bond.196
Figure 7.
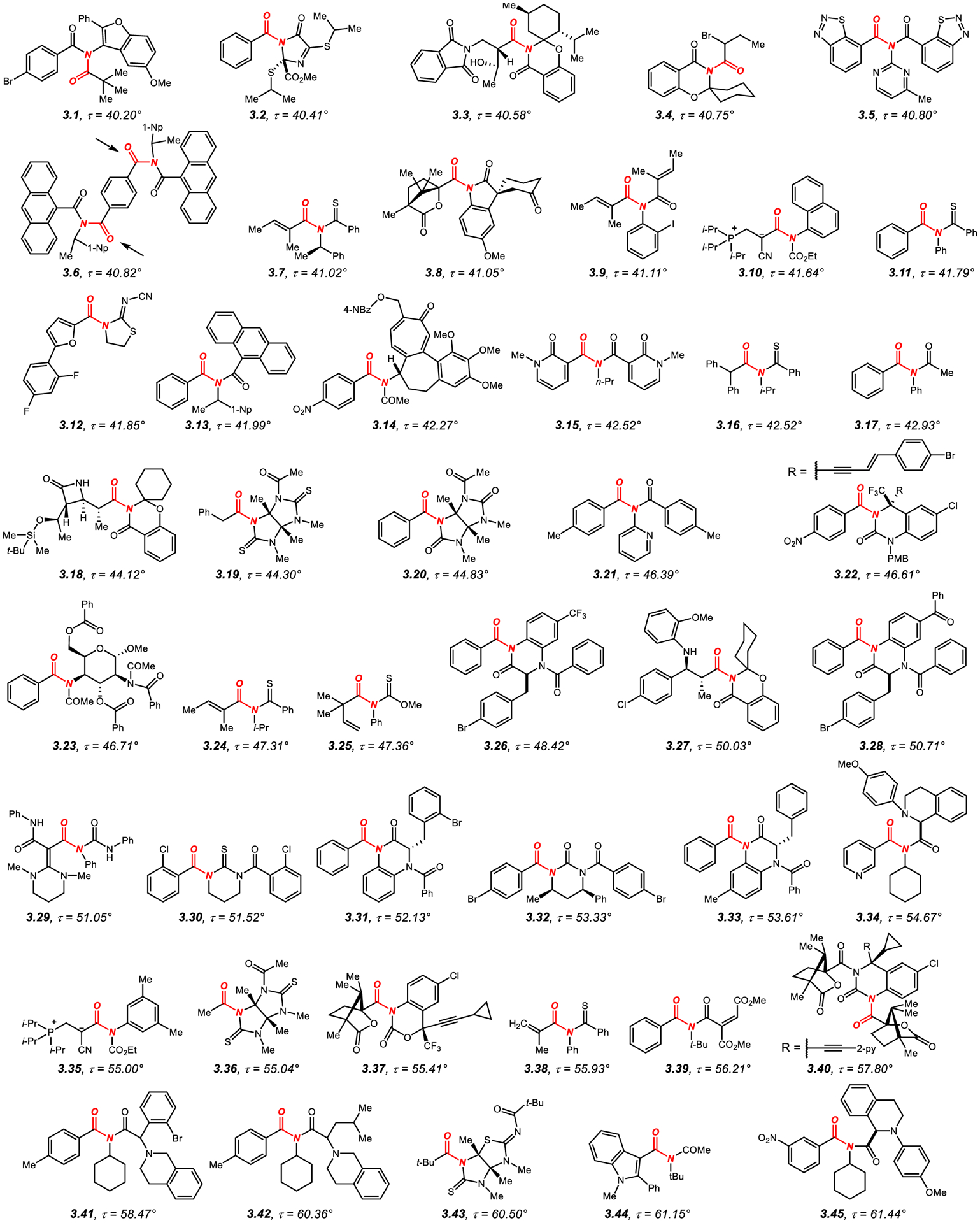
N-Mono-Acyl-Activated Acyclic Amides with Twist Values of 40° to 65°.
Examination of the examples in Figures 7–857,60,181–230 shows that N-acyl-substituents that result in a substantial twist of the amide bond include acyclic C(O)R, such as aromatic (aryl: 3.1, 3.21; anthracenyl: 3.6, 3.13), vinyl (3.9, 3.39, 3.59), heterocyclic (3.5, 3.15), 1° aliphatic (3.14, 3.17, 3.23, 3.44, 3.55, 3.60–3.61, 3.64), 2° aliphatic (3.34, 3.41–3.42, 3.45) and CF3 (3.51–3.52). Furthermore, the activating acyclic acyl group can be C(S)R (such as 3.7, 3.11, 3.16, 3.24, 3.38), CO2R (such as 3.10, 3.35, 3.48, 3.50, 3.54, 3.57–3.58, 3.62), C(S)OR (such as 3.25) or CONR2 (such as 3.29). Cyclic acyl groups include acyl heterocycles, such as imidazolidin-4-ones (3.2), 1,3-oxazinan-4-ones (3.3–3.4, 3.18, 3.27, 3.37), indolin-2-ones (3.8), thiazolidin-2-imines (3.12), imidazolidine-2-thiones (3.19–3.20, 3.36, 3.43, 3.47), 3,4-dihydroquinazolin-2(1H)-ones (3.22, 3.26, 3.28, 3.31, 3.33, 3.40), tetrahydropyrimidine-2(1H)-thiones (3.30, 3.53), tetrahydropyrimidin-2(1H)-ones (3.32), thiazolidine-2-thiones (3.46, 3.49, 3.56), 1,9-dihydro-6H-purin-6-ones (3.63), and 2H-benzo[b][1,4]oxazine-3(4H)-thiones (3.65).
There is also a significant variation in terms of the other N-substituent, which includes N-aryl (3.9–3.11, 3.17, 3.25, 3.29, 3.35, 3.38, 3.48, 3.54, 3.58, 3.60–3.62), N-heteroaryl (3.1, 3.5, 3.21), 1° alkyl (3.15, 3.50), 2° alkyl (3.6–3.7, 3.13, 3.14, 3.16, 3.23–3.24, 3.34, 3.41–3.42, 3.45, 3.55, 3.57) and 3° alkyl (3.39, 3.44, 3.51–3.52, 3.59, 3.64).
Similarly, the α-carbon substitution can be 1° alkyl (3.19, 3.36, 3.65), 2° alkyl (3.3–3.4, 3.10, 3.16, 3.18, 3.27, 3.35, 3.48–3.50, 3.54–3.55, 3.57–3.58, 3.60–3.62), aryl (3.2, 3.6, 3.11, 3.13–3.14, 3.17, 3.20–3.23, 3.26, 3.28, 3.30–3.33, 3.39, 3.41–3.42, 3.45, 3.51–3.53, 3.63), heteroaryl (3.5, 3.12, 3.15, 3.34, 3.44, 3.59, 3.64), vinyl (3.7, 3.24, 3.29, 3.38), and 3° alkyl (3.1, 3.8, 3.25, 3.37, 3.40, 3.43, 3.46–3.47, 3.56).
It has been recognized quite early on that increased steric substitution at the α-position leads to an increase in steric repulsion with the N-activating substituents, resulting in a general order of twist correlating with the increase of steric Charton and Taft parameters.57,60 In contrast, the substitution at the nitrogen atom typically represents a balance between the steric demand of the N-moieties, with the highest twist obtained with a large difference in steric hindrance between the substituents.
Several examples summarized in Figures 7–8 deserve additional discussion. Mono-twisted N-acetyl amides, such as 3.17, undergo selective N–C(O) scission of the more twisted ArC(O)–N amide bond (τ = 43.0° cf. N–Ac, τ = 5.1°) under Pd and Ni catalysis to give ketones and biaryls.196 These “mono-twisted” acyclic amides are readily synthesized from the corresponding 2° benzamides. Twisted amides embedded in 2,5-dithioglyucoluril scaffold, such as 3.19–3.20, 3.36 and 3.47 have been studied by Harrison and co-workers.198,199,213,219 These amides feature one of the exocyclic amide bonds significantly more twisted than the other exocyclic bond for small α-carbon substituents (e.g., R = Me, 3.36, τ = 55.0° vs. τ = 2.6°), and undergo further twisting with the increase of steric hindrance at the α-carbon (e.g., R = t-Bu, 3.47, τ = 66.4° vs. τ = 54.3°).213,219 N-Acyl-1,3-thiazolidine-2-thiones, such as 3.46, 3.49 and 3.56 have been pioneered by Yamada as the first models of the acyclic twisted amides.57,60 The most twisted in the series is N-pivaloyl derivative 3.56 (τ = 74.3°). These amides undergo selective hydrolysis with the rate correlated to the amide bond twist.59 Recent studies by Weng introduced N-trifluoroacetyl amides, such as 3.51–3.52.223 Facile synthesis from the corresponding nitrones and very high twist (τ = 72.9–73.6°) in the presence of electronically-activating trifluoroacetyl group are noteworthy. These amides are formally analogous to N-triflyl amides.231 Finally, amide 3.65 featuring a benzofused morpholine-3-thione system reported by Yamada represents one of the rare examples of exceptionally twisted amides (τ = 89.0°) with sterically unbiased 1° alkyl substituent at the α-carbon.230 The authors proposed that the steric interactions between the thiocarbonyl group and the alkyl substituent contribute to the high twist of the amide bond.
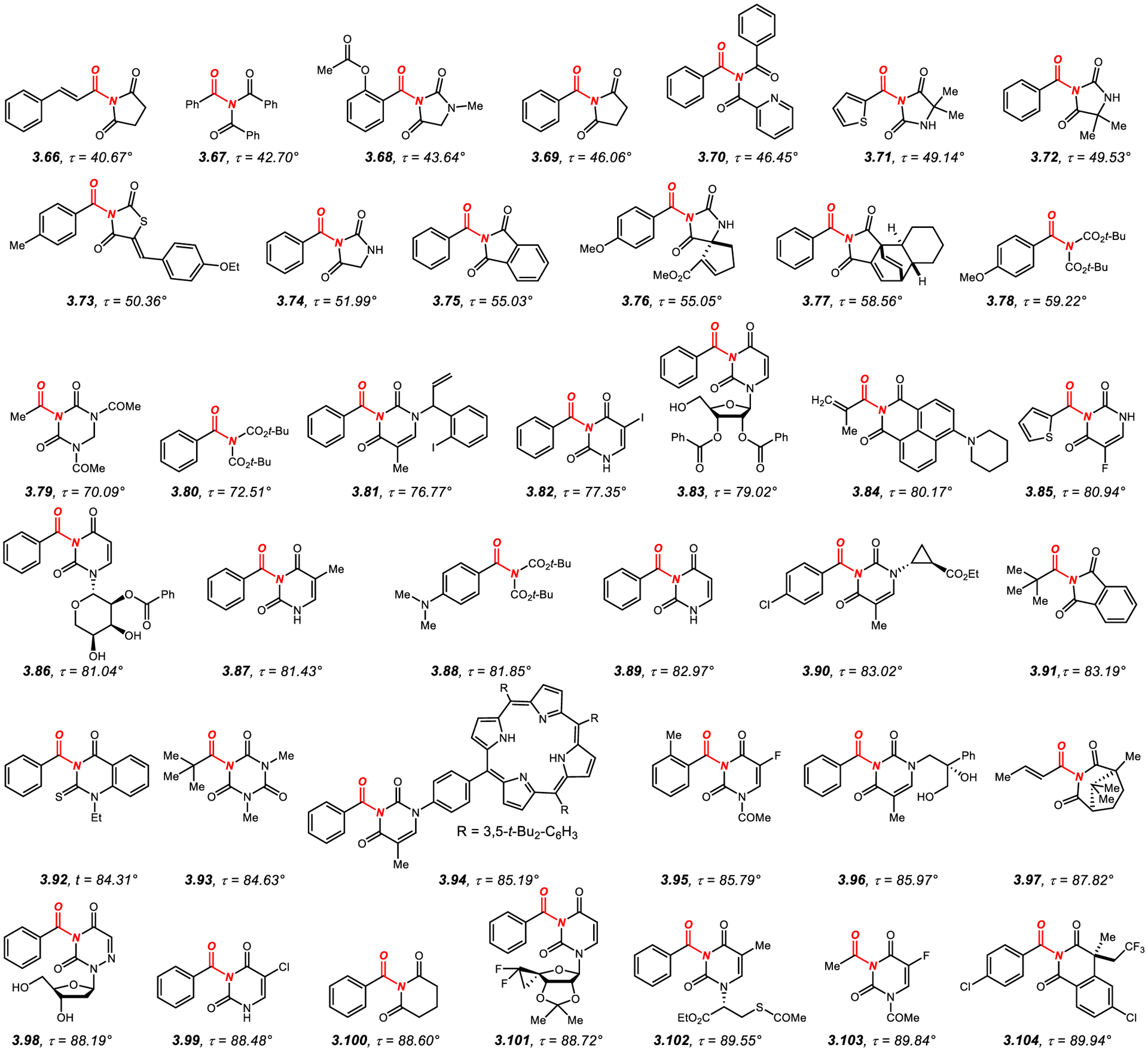
3.1.2. N,N-Di-Acyl-Activated Acyclic Twisted Amides
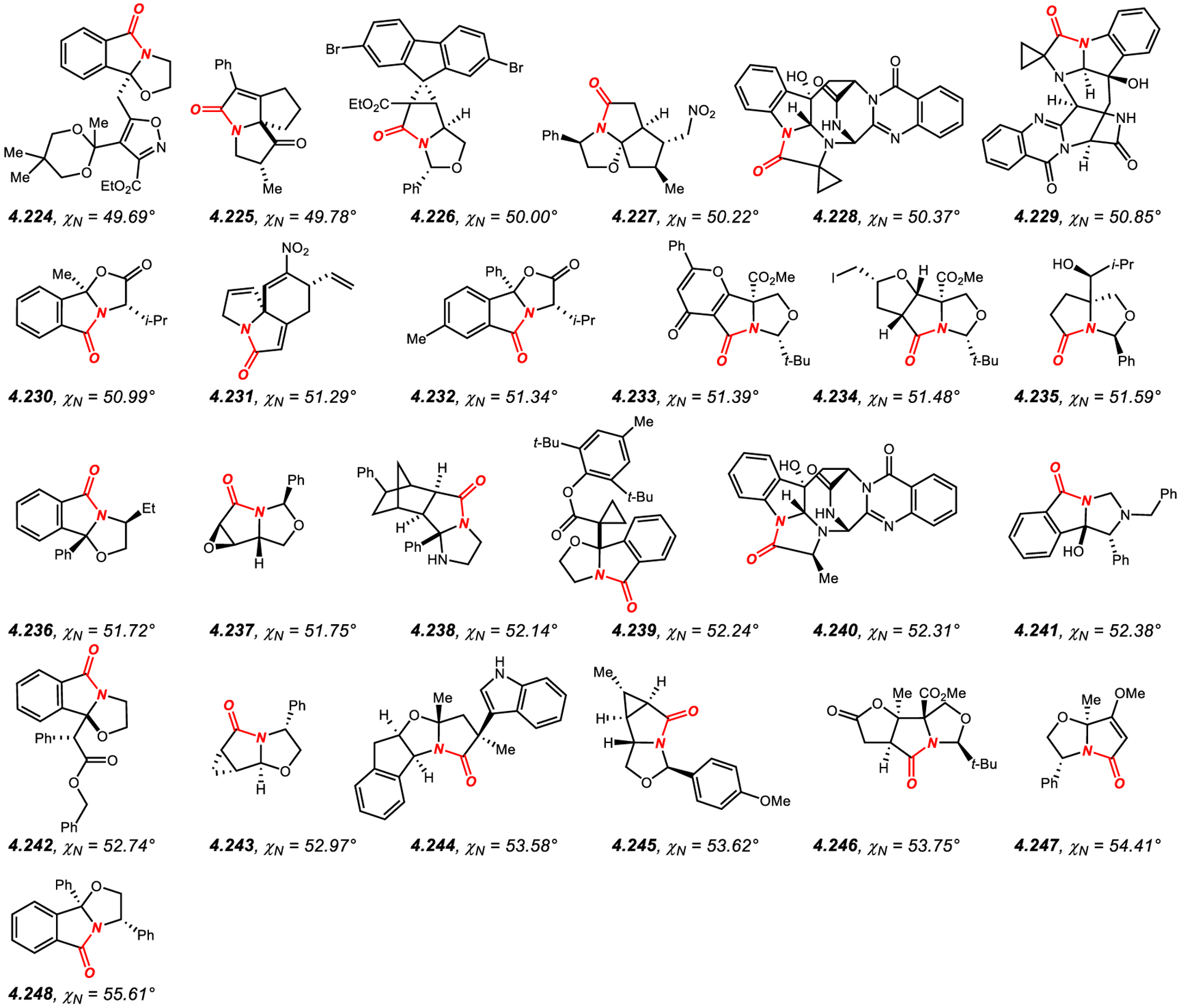
N,N-Di-acyl activation represents one of the most effective methods for twisting amide bonds (Figure 9).232–266 In this class of amides, nN to π*C=O conjugation is satisfied by delocalization onto two exo-cyclic carbonyl groups (cf. single C=O, section 3.1.1.), which leads to enhanced geometric distortion dependent primarily on steric and to a lesser extent on electronic properties of the activating group (cf. balanced effect of steric hindrance of all substituents comprising the amide bond, section 3.1.1.).
In particular, N,N-di-acyl-activation is notable for providing amide-based electrophilic reagents with reactivity exceeding acyl halides that have been exploited both in transition-metal-catalyzed cross-coupling chemistry and as acyl transfer reagents in transition-metal-free reactions.49–78 Computational studies on N,N-di-acyl-activated amides have been published, demonstrating that in many cases amidic resonance of the twisted amide bond is very low or virtually non-existent (e.g., N-acyl-glutarimides, RE < 2.4 kcal/mol depending on the R substituent at the α-position of the amide bond, 3.100, Figure 9).232,240,244 Furthermore, it is worth noting that in contrast to the typically less twisted N-mono-acylated amides which are generally synthesized from the corresponding acyl halides or other activated carboxylic acid derivatives, the direct N,N-di-acylation of fully planar 1° amide bonds is possible,49,50 which enables for twisting of otherwise planar bonds.
In general, N,N-di-acyl activation can be accomplished using N-acyclic activating groups,232–266 such as C(O)R where R is an aromatic or heteroaromatic ring (3.67, 3.70) or CO2R where R is t-Bu group (3.78, 3.80); however, more common is the use of cyclic N-activating groups, including heterocycles such as succinimide (3.66, 3.69, 3.77), hydantoin (3.68, 3.71–3.72, 3.74, 3.76), 2,4-thiazolidinedione (3.73), phthalimide (3.75, 3.91), 1,3,5-triazinane-2,4-dione (3.79), uracil (3.81–3.83, 3.85–3.87, 3.89–3.90, 3.94–3.96, 3.99, 3.101–3.103), 1,8-naphthalimide (3.84), thioquinazoline-2,4-dione (3.92), 1,3,5-triazinane-2,4,6-trione (3.93), 3-azabicyclo[3.2.1]octane-2,4-dione (3.97), 1,2,4-triazine-3,5-dione (3.98), glutarimide (3.100) and isoquinoline-1,3-dione (3.104).
The α-carbon substitution can be 1° alkyl (3.79, 3.103), 3° alkyl (3.91, 3.93), alkenyl (3.66, 3.84, 3.97) or most commonly aryl (3.67–3.70, 3.72–3.78, 3.80–3.83, 3.86–3.90, 3.92, 3.94–3.96, 3.98–3.102, 3.104) or heteroaryl (3.71, 3.85). In general, an increase of amide bond twist is observed with more sterically-demanding α-carbon substituents. Furthermore, six-membered N-acyl-activating groups result in a higher twist than their five-membered counterparts. A study of the series of glutarimides, succinimides and phthalimides demonstrated the following order of amide bond distortion (3° alkyl > aryl > 2° alkyl > 1° alkyl; glutarimide > succinimide > phthalimide).237 It is further interesting to note that heteroatom substitution of the activating ring has a noticeable but not a significant difference in amide bond twist (e.g., succinimides, 3.69 vs. hydantoins, 3.74).239,240
Several amides in this series deserve an additional comment. First, N-acyl-glutarimides and N-acyl-succinimides, such as 3.69 and 3.100, have emerged as highly reactive yet stable acyl- and aryl-electrophiles by metal-catalyzed N–C(O) bond oxidative addition.237 In many cases, the more twisted N-acyl-glutarimides are significantly more reactive than N-acyl-succinimides (3.100, τ = 88.6° vs. 3.69, τ = 46.1°); however, it should be noted that the use of both classes of these acyclic twisted amides is highly advantageous in metal-catalysis due to higher stability than that of the corresponding acyl halides and anhydrides.49–78 Second, twisted amides activated by exo-cyclic Boc groups, such as 3.88 (τ = 82.9°) permit for rapid synthesis from 1° benzamides.244 These amides have also been utilized in cross-coupling chemistry by acyl and decarbonylative mechanisms. Interestingly, while the amidic resonance is significantly reduced (RE = 6.3 kcal), steric distortion closely depends on the t-Bu groups.244 Third, many of the amides in this class based on the uracil and thymine frameworks have been synthesized with the goal of medicinal chemistry applications (e.g., 3.98, 3.101–3.103),261,263–265 and it is likely that amide bond twist plays a role in the biological activity of these compounds. Finally, the N-pivaloyl phthalimide derivative 3.91 synthesized by Yamada represents one of the classic examples of acyclic twisted amides, wherein the amide bond is almost fully perpendicular by the virtue of N-activating group and α-carbon substituent (τ = 83.2° vs. 3.75, τ = 55.0°).254
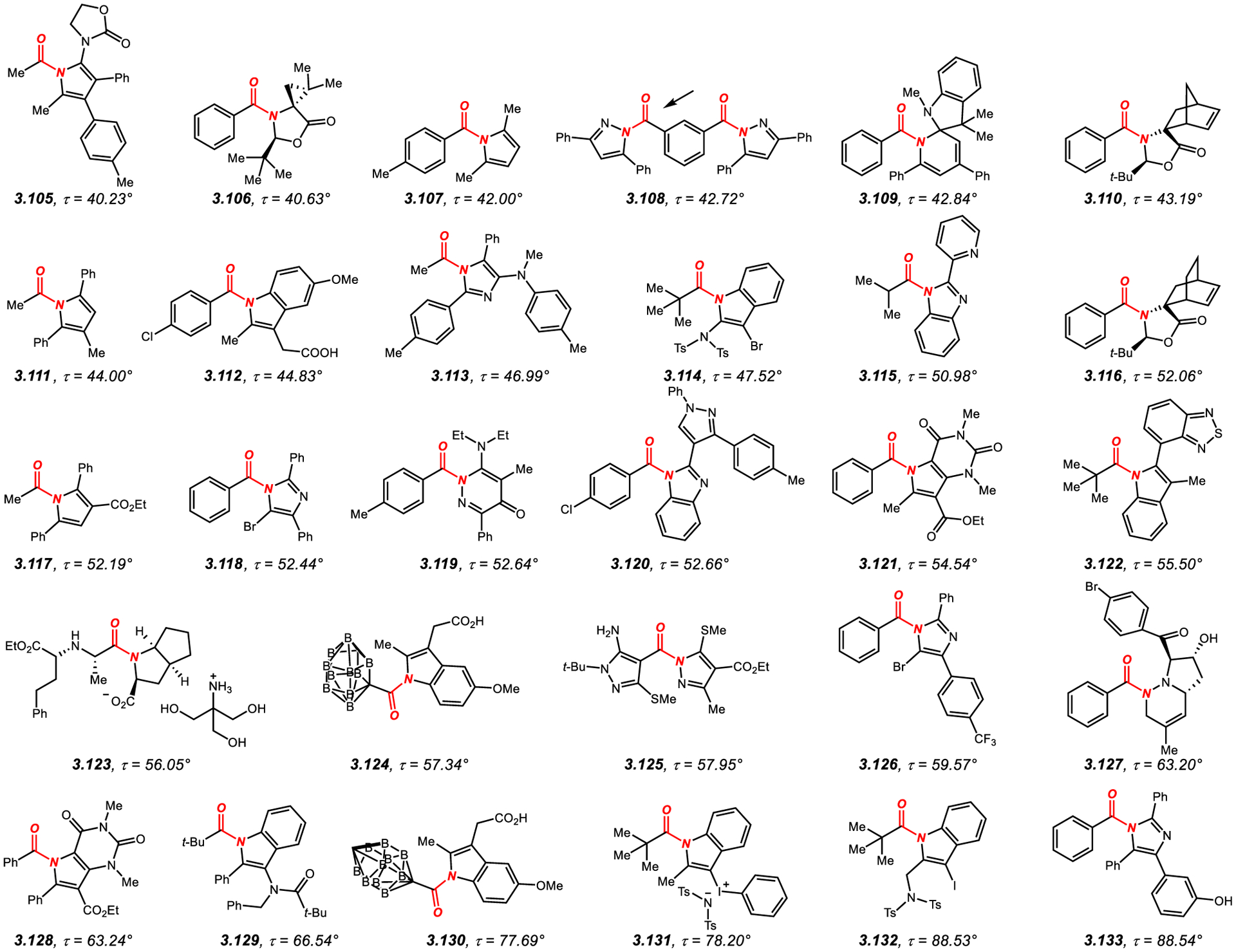
3.2. N-Heterocycle-Activated Acyclic Twisted Amides
N-Heterocyclic activation (i.e., activation by connecting the amide nitrogen atom to a heterocyclic system) represents another highly effective method of twisting amide bonds (Figure 10).267–288 In this method, heterocycles are either aromatic resulting in Nlp delocalization onto the aromatic ring system with a subsequent twisting of the amide bond, or non-aromatic, which leads to amide bond twisting due to steric repulsion in the absence of additional Nlp delocalization.
The most recognized amides in this class are N-benzoylpyrroles, such as 3.107, studied by Brown and co-workers.269 More recently, Miller and co-workers reported the synthesis and structural characterization of related imidazole analogues, such as 3.118, 3.126 and 3.133.278 In general, these N-acyl-azolides are well-established to undergo hydrolysis with the enhanced rate depending on the heterocycle and twist of the amide bond.289–292
The heterocyclic N-acyl twisted amides are of interest in medicinal chemistry as heterocyclic building blocks and target active compounds.293,294 Furthermore, cross-coupling of N-acyl-azolides by N–C(O) oxidative insertion has been reported.289,290 It should be noted that twisting in this class of amides is closely dependent on the steric impact of the heterocyclic ring system, which in all cases requires at least a single substitution at the adjacent C2-position to the amide nitrogen atom to achieve appreciable amide bond twist (Figure 10).267–288 As a consequence, the synthesis of these twisted amides is often is more challenging than N,N-di-acyl or N-mono-acyl-derivatives discussed in sections 3.1.1. and 3.1.2. Moreover, N-heterocyclic activated twisted amides are typically less hydrolytically stable than N-acyl or N,N-di-acyl counterparts since they cannot benefit from the nN to π*C=O delocalization on the adjacent carbonyl group.289–292
In general, twisting of the amide bond in this class of amides (Figure 10)267–288 can be achieved by using aromatic N-heterocycles, such as pyrroles (3.105, 3.107, 3.111, 3.117), pyrazoles (3.108, 3.125), indoles (3.112, 3.114, 3.122, 3.124, 3.129–3.132), imidazoles (3.113, 3.118, 3.126, 3.133), benzimidazoles (3.115, 3.120), pyridazin-4(1H)-ones (3.119) and pyrrolo[3,2-d]pyrimidines (3.121, 3.128) or saturated N-heterocycles, such as oxazolidin-5-ones (3.106, 3.110, 3.116), 1,2-dihydropyridines (3.109), octahydrocyclopenta[b]pyrroles (3.123) and 1,2,3,6-tetrahydropyridazines (3.127). An important difference is that in N-aromatic heterocycles the amide bond twisting has its origin in electronic delocalization of the lone pair at nitrogen on the aromatic ring269,289 in conjunction with steric hindrance at the ortho positions to the amide nitrogen. These N-acyl-azolides have been shown to have significantly reduced amidic resonance (e.g., N-benzoyl-pyrrole: RE = 9.3 kcal/mol, 1.4, Scheme 1; N-benzoyl-pyrazole: RE = 7.8 kcal/mol, 1.5, Scheme 1; N-benzoyl-imidazole: RE = 7.8 kcal/mol, 1.6, Scheme 1).289 As expected, the resonance is further decreased with steric substitution and the subsequent N–C(O) twisting (e.g., benzoyl-2,5-dimethyl-pyrrole, RE = 2.8 kcal/mol, such as 3.107, Figure 10).
In contrast, in non-aromatic N-heterocycles (Figure 10),267–288 the amide bond is twisted primarily due to steric repulsion with the adjacent substituents in the absence of additional Nlp delocalization. The α-carbon substitution can be 1° alkyl (3.105, 3.111, 3.113, 3.117), 2° alkyl (3.115, 3.123), 3° alkyl (3.114, 3.122, 3.129, 3.131–3.132), aryl (3.106–3.110, 3.112, 3.116, 3.118–3.121, 3.126–3.128, 3.133), heteroaryl (3.125) or carbaboranyl (3.124, 3.130). As expected, there is a good correlation between the amide bond twist and α-carbon substitution in the following order: 1° < 2° < aryl < 3°. Furthermore, there is the following order of N-heterocycles in amide bond twisting: pyrrole < pyrazole < indole < imidazole; however, specific ring substitution can often alter this trend.
There are several notable amides in this series that deserve additional discussion. Amide 3.107 was prepared by Brown and co-workers in a study of altered amidic resonance in acyclic and cyclic amides.269 The authors found that while the twist considerably increased in 3.107 in comparison with N-benzoyl-pyrrole (τ = 7.9° to 42.0°), the N–C(O) and C=O bond lengths have remained practically unchanged (1.409Å to 1.416 Å and 1.211 Å to 1.208 Å), indicative of significant Nlp to Ar delocalization. Twisted amides such as 3.111 and 3.117 are readily accessible by Pd(II)-catalyzed C–H annulation of enamides with alkynes, which in principle enables to activate otherwise unsubstituted 1° amides.273 Amide bonds in N-acyl-indoles undergo twisting due to steric repulsion with a C2-substituents (e.g., 3.112).274 In this respect, the direct oxidative C2-imidation of unsubstituted indoles such as 3.114 leads to moderate twist (τ = 47.5°),276 while the benzylic imidation, such as in 3.132, affords practically perpendicular amide bonds (τ = 88.5°).288 N-Acyl-imidazoles, such as 3.118 and 3.133 have been studied by Miller and co-workers.278 In this case, a significant increase of twist is observed by introducing 2,5-diphenyl substitution on the imidazole ring (τ = 52.4° to τ = 88.5°). Finally, N-acyl-imidazoles, such as 3.120 are potent bacterial FabH inhibitors,280 while 3.123 is a hydroxymethyl aminomethane salt of ramipril, an antihypertensive drug.283 Of medicinal interest are also twisted amide carbaboranes derivatives (3.124, 3.130) of indomethacin, a nonsteroidal antiinflammatory drug.284 The use of large carbaboranyl substituents instead of 4-chlorophenyl contributes to the high amide bond twist in these compounds. In this case, both the steric and the electronic effect of the carbaboranyl substituent should be considered; electronically, such an electropositive group on the amide bond would be expected to enhance amidic resonance.
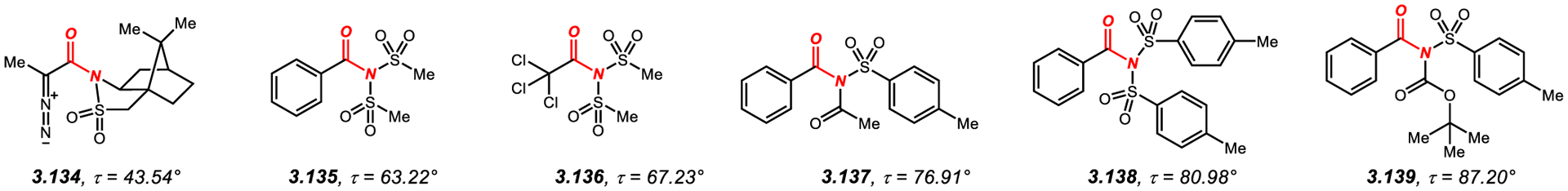
3.3. N-Sulfonyl-Activated and N,N-Di-Sulfonyl-Activated Twisted Amides
Several examples of N-sulfonyl-activated twisted amides have been reported (Figure 11).295–298 With the exception of the moderately twisted α-diazo-substituted amide 3.134 (τ = 43.5°),295 which is derived from Oppolzer’s sultam, amides in this class feature two activating substituents at the nitrogen atom. There are two types of activation: (1) bis-sulfonyl, such as in 3.135–3.136 and 3.138;296,297 and (2) combination of N-sulfonyl with N-acyl, such as in 3.137 and 3.139.298 The use of more sterically-hindered N-Ts substitution leads to a larger geometrical distortion than with N-Ms (Ms: 3.135, τ = 63.2°; Ts: 3.138, τ = 81.0°).296 Furthermore, it is noteworthy that N-Ts activation is more effective than the related N-Boc activation (Figure 9, 3.80, τ = 72.5°),244 which leads to practically perpendicular amide bonds (3.139, τ = 87.2°). These N-bis-sulfonyl-amides, such as N-Ms2 (3.135) and N-Ts2 (3.138) as well as N-Ts/Ac (3.137) and N-Ts/Boc amides (3.139) undergo Pd-catalyzed cross-coupling by oxidative addition of the N–C(O) bond.296,298
3.4. Miscellaneous Acyclic Twisted Amides
Amide 3.140 features N-Ph/N-1,3,5-triazin-2-yl substitution, which leads to moderate twist (τ = 44.4°) (Figure 12).299 It is interesting to note that this amide is significantly more twisted than the related N,N-diphenylbenzamide (PhCONPh2, τ = 11.2°). In contrast, amide 3.141 is a quaternary acyclic N-acyl ammonium salt (τ = 85.1°)300 that is related to the cyclic counterparts (section 2.4., 2.34);167 however, the lack of cyclic structure leads to low hydrolytic stability of this class of N-acyl quaternary ammonium salts.
Figure 12.

Miscellaneous Acyclic Amides with Twist Values of 40° to 90°.
4. Cyclic Amides: N-Pyramidalization 40–60°
In addition to twisting, amide bond geometric distortion can be achieved by pyramidalization of the nitrogen atom.28–35 In the extreme cases, these pyramidalized amides feature sp3 hybridization that is more characteristic to amines rather than amides.12,17 The most well-known examples of such pyramidalized amides include confining the amide bond nitrogen in a cyclic ring system, such as azetidine or aziridine, however in these moieties the inherent ring strain of the small-ring heterocycle contributes to the reactivity of these amides.301 Recent elegant studies by Ohwada and co-workers identified 7-azabicyclo[2.2.1]heptane amides (such as 5.7, Figure 21) as another class of fully pyramidalized amides.302–314
Figure 21.

N-Heterocycle-Activated Acyclic Twisted Amides with Nitrogen Pyramidalization Values of 40° to 65°.
It should be noted that with the exception of these inherently restricted ring systems,302–314 at present, it is not clear if N-pyramidalization alone is sufficient to engender new reactivity of amide bonds.28–35 In this respect, the case of bridged lactams is instructive; it has been shown in several studies that properties of twisted bridged lactams can be correlated with both twist and nitrogen pyramidalization when (1) comparing amide distortion within the same classes of N-alkyl non-planar bridged amides, and (2) the amide bond is sufficiently geometrically altered to promote N-amino-ketone type reactivity.45,46,117,118,315 By contrast, electronic activation by N-acyl or related substitution leads to redistribution of the nitrogen lone pair into the activating substituent,49–78 which in turn disconnects the amide bond conjugation within the N–C(O) moiety and results in Nlp being engaged in another nN to π*X=O delocalization.
Although thus far, with the exceptions noted above, clear correlations between N-pyramidalization and amide bond reactivity have not been found, these pyramidalized amides are fundamentally important as geometric probes for amide bond resonance,12 amide pyramidalization302 and cis/trans amide bond rotation.305 Applications of pyramidalized amides as peptidomimetics have been reported.308,310–314 Furthermore, N-pyramidalization is the key feature in the mechanism of action of β-lactam antibiotics.316–318
4.1. Bridged and Related Amides
Due to the geometric confinement of the amide bond in a rigid bicyclic ring structure, bridged amides are unique in the class of distorted amides in that typically twist and nitrogen pyramidalization are correlated with each other,45,46,117,118,315 while one effect follows the other depending on the ring size, type of the ring and peripheral substitution.28–35 This correlation is expressed by the additive distortion parameter (τ+χN) introduced recently using one-carbon bridged lactams,117,118 while earlier studies, in particular, by Greenberg and co-workers,45,46,48 demonstrated similar correlations in larger ring systems.
Since in this class of amides twist (τ) and nitrogen pyramidalization (χN) are connected to each other, the reader is encouraged to consider this section together with section 2.1.140–152, 36,38,43 Representative examples of bridged amides together with related amides featuring significant χN values of >40° are presented in Figure 13.319–336 Detailed summary of distortion parameters is presented in the Supporting Information. This section focuses on highlighting examples of bridged lactams that feature high χN in the absence of considerable twist, a property that is closely related to the specific ring system and can be potentially utilized to separate χN from twist in studying the properties of non-planar amide bonds.47,166
In this respect, amides 4.1 (χN = 41.4°, τ = 5.4°),319 4.2 (χN = 43.4°, τ = 1.2°),320 4.7 (χN = 46.6°, τ = 7.5°),325 4.8 (χN = 47.7°, τ = 16.7°),326 4.9 (χN = 48.8°, τ = 20.7°),327 4.10 (χN = 49.0°, τ = 21.9°),328 4.11 (χN = 49.2°, τ = 16.3°),329 4.13 (χN = 50.5°, τ = 23.5°),330 4.14 (χN = 51.4°, τ = 28.1°),331 4.16 (χN = 52.7°, τ = 30.8°),332 4.17 (χN = 52.8°, τ = 23.4°),328 4.18 (χN = 54.9°, τ = 30.2°),331 4.19 (χN = 54.9°, τ = 16.7°),333 4.20 (χN = 55.9°, τ = 29.8°),331 4.22 (χN = 57.1°, τ = 35.3°),334 4.23 (χN = 57.2°, τ = 35.6°),335 4.24 (χN = 57.5°, τ = 34.4°)336 and 4.26 (χN = 58.6°, τ = 39.1°)144,315 feature significantly larger χN values than τ and may be considered as bridged amide models for probing the effect of nitrogen pyramidalization on the properties of these amides under the proviso that in these systems both properties are still connected with each other.
In general, these amides include (1) constrained amides with additional bridging, such as 4.1, 4.2; (2) amides in [4.3.1] bridged systems, such as 4.7, and [3.3.1] bridged systems, such as 4.8–4.11, 4.13, 4.16–4.17, 4.19; (3) Tröger’s base twisted amides, such as 4.14, 4.18, 4.20, 4.24, 4.26; (4) azetidinyl bridged amide 4.22 in a [4.1.1] system; (5) amide 4.23 in a [2.2.3] ring system. In addition, amides 4.3 (χN = 43.5°, τ = 9.7°)321 and 4.4 (χN = 43.7°, τ = 10.2°)322 feature tetracyclic spirolactam scaffold that is structurally related to bridged lactams by an additional C–C bond connectivity.
In contrast, bridged lactams, such as tricyclic bridged 4.12 (χN = 49.8°, τ = 72.3°) and their N-protonated analogues, such as 4.15 (χN = 52.0°, τ = 81.9°),142,149 1-aza-2-adamantanone derivatives, such as 4.27 (χN = 61.7°, τ = 90.0°)39,43 and 2-quinuclidone derivatives, such as 4.29 (χN = 69.8°, τ = 90.0°)36,38 feature high pyramidalization and high twist. In particular, the reactivity of N-pyramidalized bridged amides in a [3.3.1] ring system has been studied, showing increased rates of hydrolysis,327,329 It is worth noting that the high rigidity of structures 4.25 and 4.29 means that little change in distortion is observed in going from the unprotonated lactam structure to the N-protonated salts, protonation at the nitrogen atom47 and σ N–C bond cleavage.166 While it may be assumed that nitrogen pyramidalization is the predominant amide bond distortion mechanism in these cases, further studies are needed to separate the effect of pyramidalization from twist in bridged bicyclic amides.
4.2. Fused Amides
In addition to bridged amides, significant nitrogen pyramidalization can also be achieved in fused ring systems. In general, these structurally-characterized amides can be categorized based on the ring system featuring the amide bond into the following classes: (1) four-membered ring twisted/pyramidalized amides; (2) five-membered ring twisted/pyramidalized amides; (3) six-membered ring twisted/pyramidalized amides; and (4) miscellaneous examples.
4.2.1. Four-Membered Ring N-Pyramidalized Amides
Constraining the amide bond in a β-lactam ring represents a classic example of enhancing the reactivity of the amide bond by ring strain.27 This increased amide bond distortion is critical for the mechanism of action of β-lactam antibiotics. Since comprehensive monographs on β-lactams337–339 and β-lactams316–318 antibiotics have been published, this section presents a summary of structurally-characterized pyramidalized amides embedded in a four-membered ring (Figures 14–15).
Figure 14.
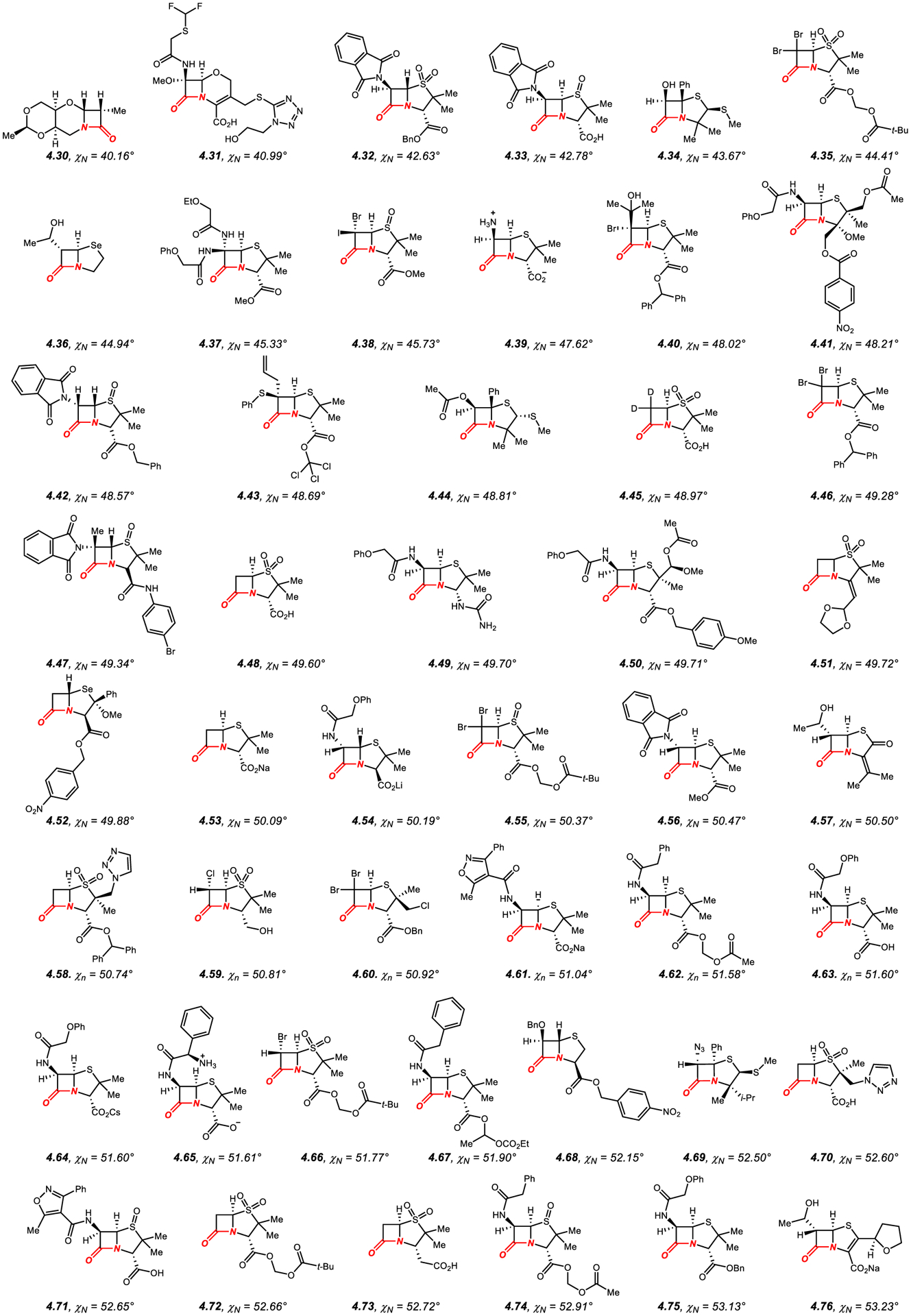
Amides in Four-Membered Rings with Nitrogen Pyramidalization Values of 40° to 53°.
Figure 15.
Amides in Four-Membered Rings with Nitrogen Pyramidalization Values of 53° to 69°.
In general, the amide bond geometry of structurally-characterized β-lactams presented in Figures 14–15340–416 can be characterized as N-pyramidalized (average χN of 54.4°), while twist is less significant (average τ of 19.2°), as expected from the geometry of the fused four-membered ring system. There is only a very scattered correlation between N-pyramidalization and twist of the amide bond, with the general trend of higher twist with increased nitrogen pyramidalization (R2 = 0.30).
The most common are [2.4.0] and [2.3.0] ring systems with the six-membered ring such as 1,3-oxazinane (e.g., 4.30),340 and more common five-membered ring, such as thiazolidine 1,1-dioxide (e.g., 4.32),342 thiazolidine 1-oxide (e.g., 4.33),343 thiazolidine (e.g., 4.34),344 1,3-selenazolidine (e.g., 4.36),346 pyrrolidine (e.g., 4.81),383 imidazolidine (e.g., 4.94),395 or oxazolidine (e.g., 4.101).401 In general, more dense substitution of the fused ring, in particular at the α-positions to the nitrogen atom and the carbonyl group and ring unsaturation result in higher N-pyramidalization.340–416 These N-pyramidalized amides are well known to be highly reactive as acylating reagents and are important pharmacophores in medicinal chemistry research.
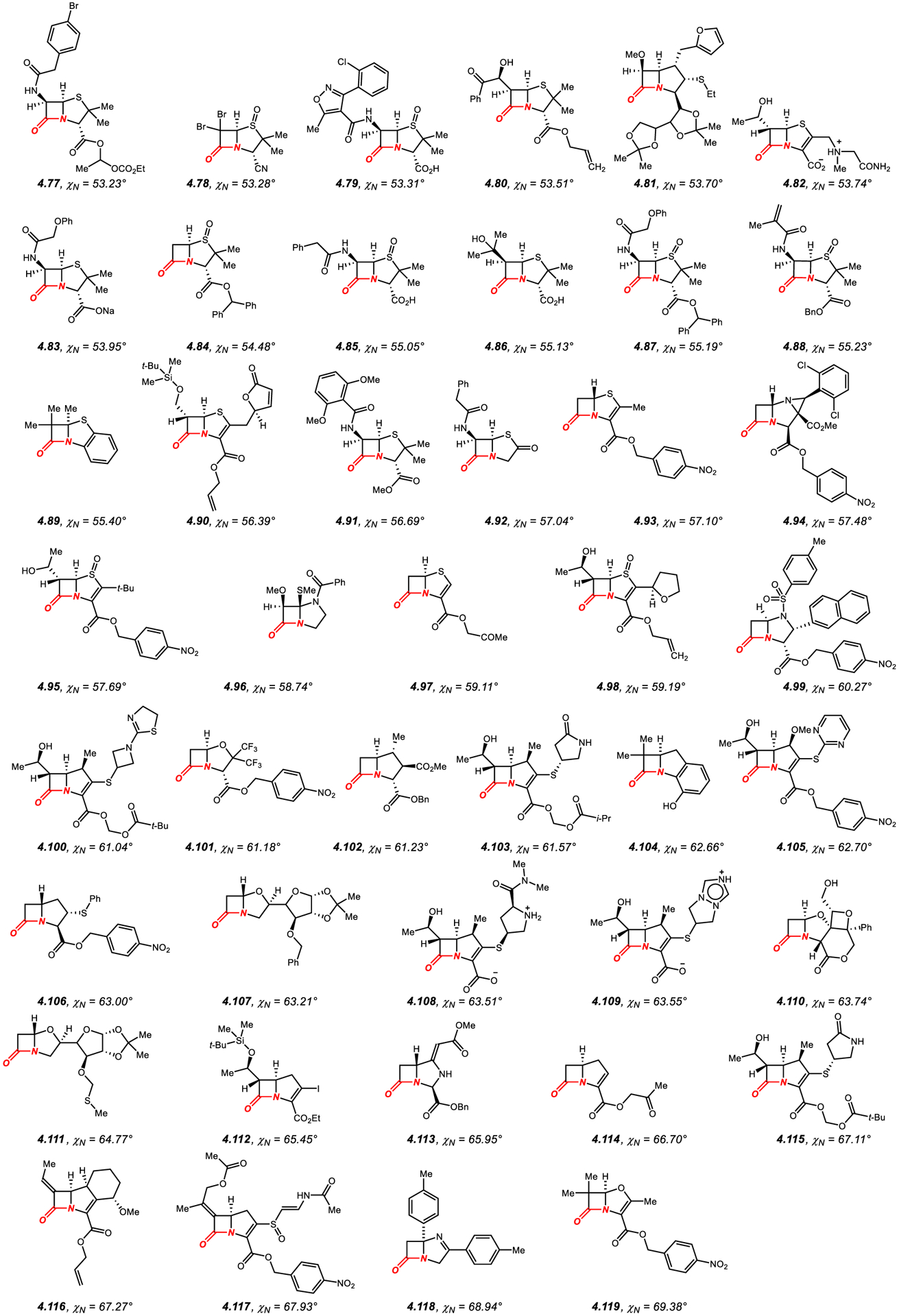
4.2.2. Five-Membered Ring N-Pyramidalized Amides
In contrast to the well-known β-lactams, it is much less recognized that constraining the amide bond in a five-membered fused ring system also leads to significant pyramidalization of the amide bond. This class of five-membered ring fused lactams plays a prominent role in heterocyclic chemistry293 and natural product synthesis417 en route to indolizidine, pyrrolizidine and related alkaloids.418–421 In these systems, it has been acknowledged that the reduction of lactam carbonyl groups often proceeds under mild reaction conditions, clearly a consequence of amide bond pyramidalization that weakens nN → π*C=O resonance.417–421
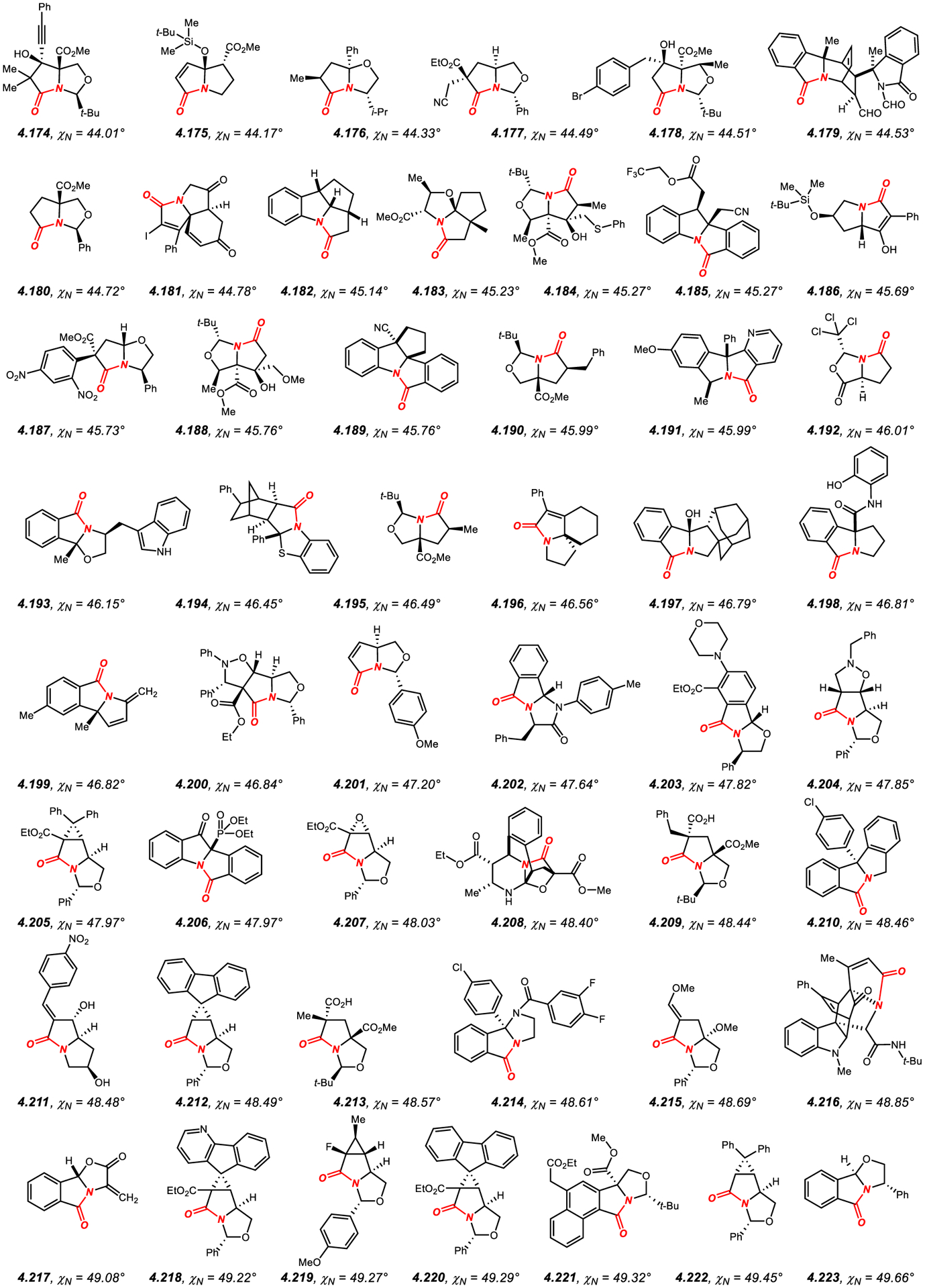
Similar to β-lactams, the amide bond geometry of structurally-characterized amides embedded in a fused five-membered ring system (Figures 16–18)422–522 can be characterized as N-pyramidalized (average χN of 45.9°) with minimal twist (average τ of 11.7°). As expected, the average values of N-pyramidalization and twist are slightly lower as compared to β-lactams by χN: 8.5° and τ: 7.5°, respectively,340–416 which is a consequence of less strained five-membered fused ring system. Similarly, the highest reported χN value for a five-membered fused lactam is lower than that of the most N-pyramidalized β-lactam (4.248: 55.6°;522 4.119: 69.4°,416 respectively); however, it clearly indicates a predominant sp3 character of the amide bond nitrogen atom in this ring system. Finally, there is no correlation between N-pyramidalization and amide bond twist in structurally-characterized amides constrained in fused five-membered ring systems.
Figure 16.
Amides in Five-Membered Rings with Nitrogen Pyramidalization Values of 40° to 44°.
Figure 18.
Amides in Five-Membered Rings with Nitrogen Pyramidalization Values of 50° to 60°.
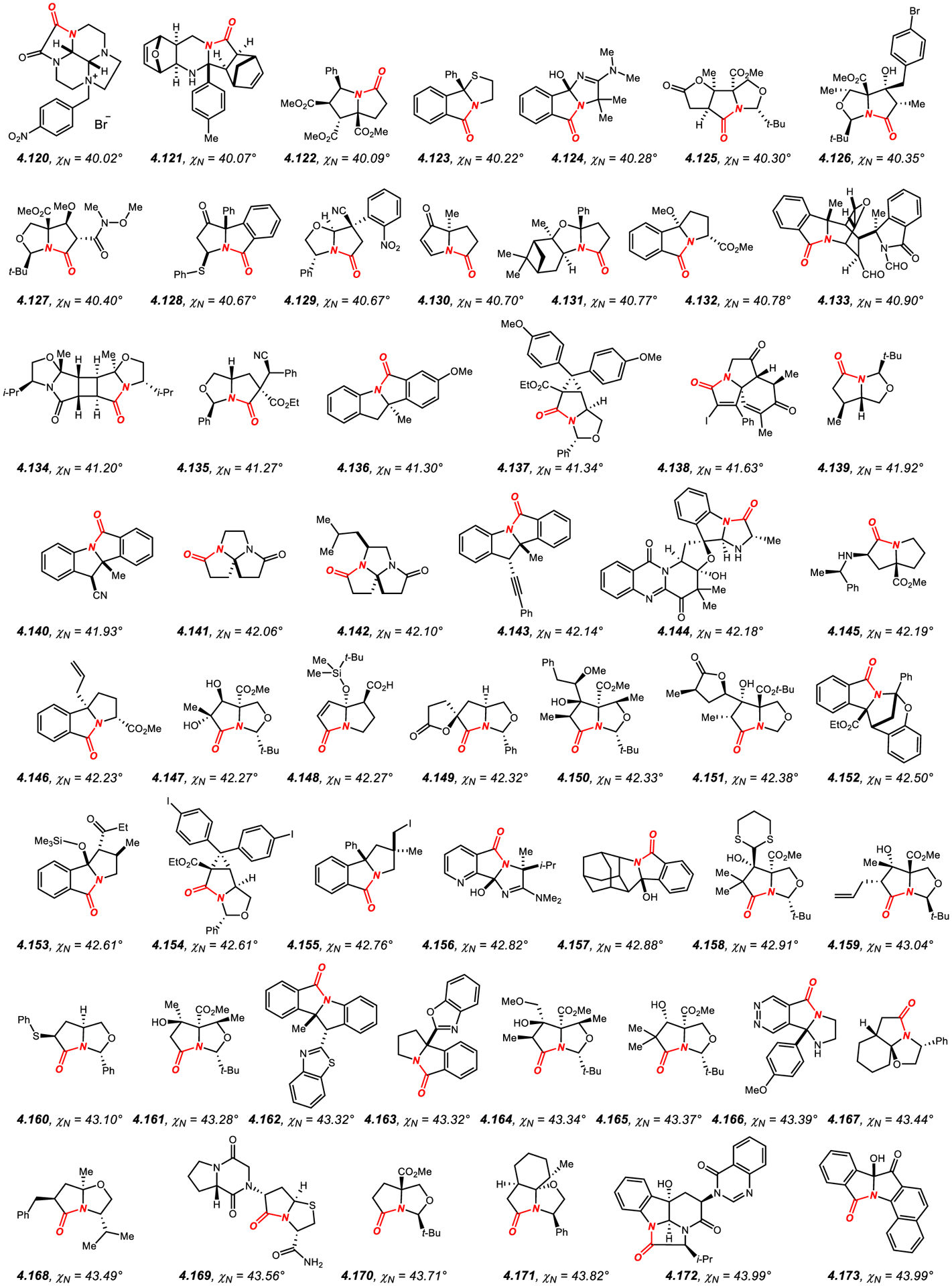
Most common in this class (Figures 16–18)422–522 is ring fusion to six-membered rings in [3.4.0] scaffold, including piperazine, such as 4.120,422 and hexahydropyrimidine, such as 4.121,423 and much more common [3.3.0] ring system with the ring fusion to five-membered rings, including pyrrolidine, such as 4.122,424 thiazolidine, such as 4.123,425 imidazolidine, such as 4.124,426 and oxazolidine, such as 4.125,427 as the most common ring scaffolds. This class also includes benzo-fused lactams, such as 4.140,442 4.146434 and 4.173,470 and tricyclic fused ring systems, such as 4.138,440 4.171,468 4.172469 and 4.181.474 In general, increased substitution at the α-position to the nitrogen atom and additional constraints of the five-membered ring, such as unsaturation, conformationally rigid ring systems and steric effects lead to higher N-pyramidalization.422–522 The high N-pyramidalization in five-membered fused lactams should be taken into account when studying the carbonyl addition reactions to amide bonds in this class of amides.
4.2.3. Six-Membered Ring N-Pyramidalized Amides
In addition to β-lactams and five-membered rings (sections 4.2.1. and 4.2.2.), amide bond pyramidalization can also be achieved in fused six-membered rings (Figure 19).422,523–531 As expected, comparatively fewer examples of structurally-characterized N-pyramidalized amides embedded in six-membered rings have been reported; however, these amides feature significant N-pyramidalization (average χN of 46.1°), while twist is much lower (average τ of 11.2°). These values compare well with the five-membered fused ring lactams (χN: 45.9° and τ: 11.7°),422–522 suggesting similar geometrical effects on the amide bond in these systems. Likewise, there is no correlation between N-pyramidalization and amide bond twist in six-membered fused amides.
Figure 19.
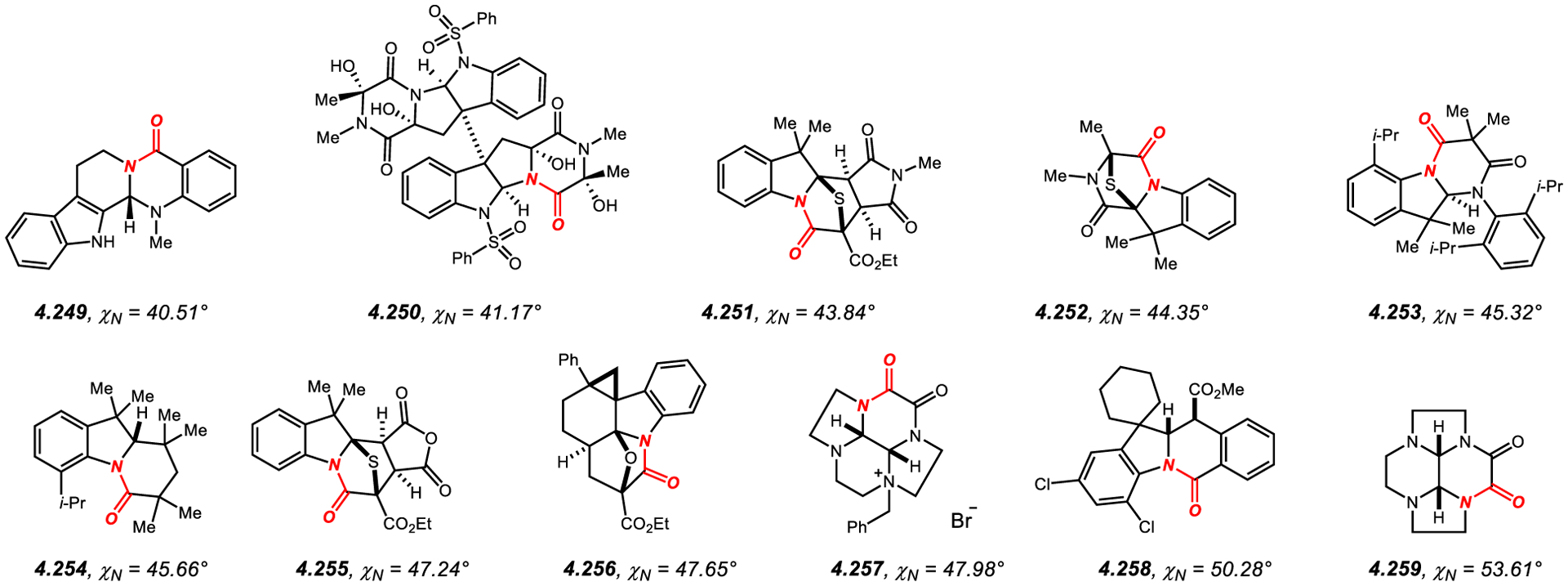
Amides in Six-Membered Rings with Nitrogen Pyramidalization Values of 40° to 60°.
In general, structurally-characterized six-membered fused amides that show significant pyramidalization of the amide bond422,523–531 feature [3.3.0] or [3.2.0] ring systems, wherein the six-membered ring is typically fused to piperidine, such as 4.249,523 pyrrolidine, such as 4.250,524 or imidazolidine, such as 4.257.422 Six-membered fused amides are important precursors in the syntheses of quinolizidine, indolizidine and 2,5-diketopiperazine alkaloids.532–534 Similar to the fused five-membered lactams, N-pyramidalization disrupts amidic resonance, which results in more facile electrophilic addition to the amide carbonyl group in these systems.418–421,532–534
4.3. Miscellaneous
Significant N-pyramidalization has been observed in saccharin-based imidoiodane 4.260 (χN = 40.1°) (Figure 20).535 This compound is synthesized from the direct reaction between saccharin and iodine acetate and serves as an aminating reagent using silyl enol ethers as nucleophiles.
Figure 20.

Miscellaneous Amides with Nitrogen Pyramidalization Values of 40° to 60°.
5. Acyclic Amides: N-Pyramidalization 40–60°
Nitrogen pyramidalization in acyclic amides leads to reduction of rotational barriers of the amide bond.12,28–35 The major methods to generate N-pyramidalization in acyclic amides are as follows: (1) N-heterocycle-activation; (2) N-sulfonyl-activation; (3) N-pyramidalization in aliphatic amides.
5.1. N-Heterocycle-Activated N-Pyramidalized Amides
Structurally-characterized N-pyramidalized N-heterocycle-activated amides with χN values >40° are summarized in Figure 21.268,302,305,306,536–547 In general, these amides can be divided into the following classes of amides: (1) conformationally-constricted N-acyl-7-azabicyclo[2.2.1]heptanes (5.4, 5.5, 5.7, 5.9–5.20)302,305,306 and related derivatives, such as N-acyl-8-azabicyclo[3.2.1]octanes (5.2)537 and N-acyl-2-azabicyclo[2.1.1]hexanes (5.8);540 (2) N-acyl-pyrrolidines (5.1, 5.3);536,538 and (3) N-acyl-oxazolidin-5-ones (5.6, 5.21).539,268 N-acyl-azetidines and N-acyl-azridines are not included since the ring strain of the small ring significantly contributes to the properties of these amides.17,301
It is interesting to note that N-acyl-7-azabicyclo[2.2.1]heptanes (Figure 21) can be classified as pyramidalized amides (average χN of 52.0°; average τ of 16.6°). The origin of nitrogen pyramidalization in N-acyl-7-azabicyclo[2.2.1]heptanes has been proposed to be due to small C–N–C angle and allylic strain between the amide substituents and the bridgehead hydrogen atoms.302–314 In agreement with this hypothesis, increased substitution of 7-azabicyclo[2.2.1]heptane results in an increase in nitrogen pyramidalization (e.g. 5.20, χN of 64.5°).547 Rotational barriers of 7-azabicyclo[2.2.1]heptane amides have been measured and are comparable to N-acyl-azetidines (5.12, 15.0 kcal/mol; N-4-toluoyl-azetidine, 15.7 kcal/mol).302 The intrinsic nitrogen pyramidalization in N-acyl 7-azabicyclo[2.2.1]heptanes provides an attractive scaffold for controlling cis/trans amide rotation.305,312
Similar to 7-azabicyclo[2.2.1]heptane amides, N-acyl-pyrrolidines 5.1 and 5.3 contain predominantly N-pyramidalized amide bonds cf. twist (5.1: χN = 40.2°, τ = 13.2°; 5.3: χN = 43.7°, τ = 13.2°),536,538 which originates from the steric interactions between amide bond substituents and pyrrolidine ring. The nitrogen pyramidalization leads to an increased electron density at the nitrogen and more electrophilic carbonyl groups in these amides.
In contrast, N-acyl-oxazolidin-5-ones 5.6 and 5.21 feature both N-pyramidalized and twisted amide bonds (5.6: χN = 44.4°, τ = 43.2°; 5.21: χN = 65.3°, τ = 40.6°).539,268 These amides represent very rare examples of twisted pyramidalized N-acyclic amides that do not require additional electronic activation to achieve high geometric distortion (cf. section 3.2.).
5.2. N-Sulfonyl-Activated N-Pyramidalized Amides
In addition to using heterocyclic ring systems (section 5.1.), N-pyramidalization of the amide bond can be achieved using N-sulfonyl activation (Figure 22).242,295,548–562 These N-acyl sulfonamides are derived from camphorsultam (Oppolzer’s sultam) and feature predominantly pyramidalized amide bonds cf. twist (average χN of 45.3°; average τ of 24.4°). Substitution at the α-carbon can be aliphatic (e.g., 5.23),549 alkenyl (e.g., 5.22),548 aromatic (e.g., 5.29)554 or heterocyclic (e.g., 5.24).550 In general, increased N-pyramidalization is observed with higher substitution at the α-carbon (e.g., 5.37: χN = 49.0°, τ = 22.4°),560 while the last three compounds in the series 5.38–5.40 feature both high pyramidalization and higher twist (5.38: χN = 49.3°, τ = 35.2°; 5.39: χN = 50.2°, τ = 43.5°; 5.40: χN = 51.6°, τ = 39.1°).561,295,562 These N-sulfonyl-activated N-pyramidalized amides are expected to undergo N–C(O) bond cleavage under mild conditions owing to the higher electron density at the nitrogen atom and nN to π*S=O conjugation with the sulfonyl group (cf. section 3.3.).
Figure 22.

N-Sulfonyl-Activated Acyclic Twisted Amides with Nitrogen Pyramidalization Values of 40° to 60°.
5.3. N-Aliphatic N-Pyramidalized Amides
Amide 5.41 features pyramidalized amide bond (χN = 46.7°, τ = 16.7°) (Figure 23).563 The pyramidalization originates from steric syn-pentane-type interactions between N-ethyl group and iso-butyl substituent at the α-carbon. Furthermore, acyclic quaternary N-acyl ammonium salts, such as 5.42, contain fully pyramidalized amide bonds (χN = 63.5°, τ = 85.1°) (cf. section 3.4.).300
Figure 23.
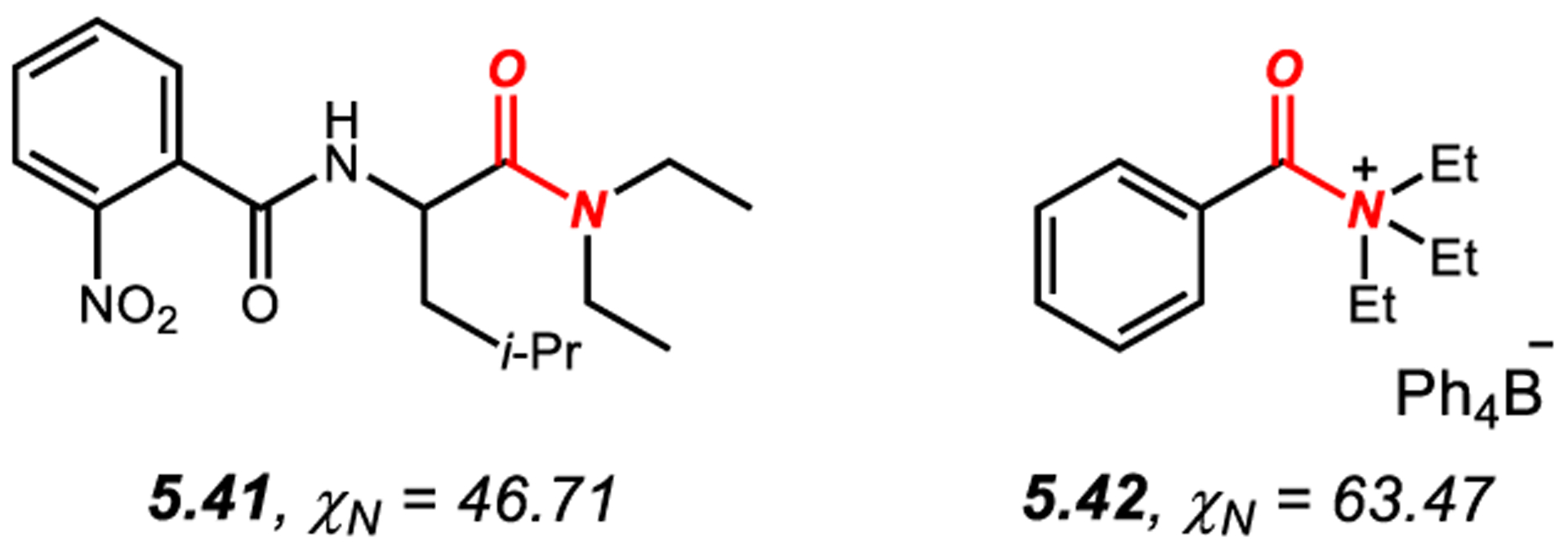
N-Aliphatic Acyclic Twisted Amides with Nitrogen Pyramidalization Values of 40° to 65°.
6. Application of Acyclic Twisted Amides in Bond Cleavage Reactions
An important point that should be addressed is synthetic application of acyclic twisted amides.63–82 In principle, N-activation of tertiary amides leads to geometric and electronic alteration of the amide bond, which disrupts amidic resonance through (1) twisting and N-pyramidalization; (2) channeling of the nN to π*C=O conjugation onto the external N-substituent of the amide bond. This permits for utilization of acyclic amides through selective bond cleavage processes that are beyond the scope of reactivity of classical amides. To date, the following classes of reactions of twisted amides have been developed: (1) N–C(O) acyl cleavage; (2) C–NCO decarbonylative cleavage; (3) NCO–C cleavage; (4) acyl nucleophilic addition; (5) generation of acyl radicals. These processes have been reviewed.63–82
An additional point that should be addressed in this context is the synthesis of acyclic twisted amides. In general, there are two main pathways for the synthesis of non-planar acyclic amides and derivatives, namely (1) amine acylation with carboxylic acids or derivatives; (2) N-acylation of 1° or 2° amides and related processes. For the major classes of acyclic twisted amides discussed, the synthetic pathways have now been well established, and these amides are readily available on preparative scale.63–82 From the standpoint of medicinal chemistry and late-stage derivatization, N-acylation of 1° or 2° amides has an advantage over amine acylation in that it permits to directly utilize planar amides as precursors to acyclic twisted amides. Since the synthesis of acyclic twisted amides directly affects their application, attention should be given to versatile, high yielding and practical methods of synthesis.
7. Conclusions and Outlook
In conclusion, amide bond planarity manifesting in the placement of all six atoms comprising the amide bond in a single plane is a fundamental and widely accepted property of amide bonds. Amide bond planarity has a major impact on application of amide bonds in chemical fields ranging from organic synthesis to polymers, medicinal chemistry, structural chemistry and biochemistry. Although classical studies on geometric constraint of amide bonds with the resulting decrease of amidic resonance and amino-ketone properties of amides have been focused on cyclic lactams, recent years have seen rapid developments of acyclic twisted amides.
In this review, we have presented a comprehensive overview of amide bond distortion in acyclic amides. Steric distortion in acyclic amides can be achieved by twist, nitrogen pyramidalization or a combination of both. Importantly, there are many different and complementary methods that result in high geometric distortion of acyclic amides. These methods include electronic activation, such as N-acylation, N-sulfonylation, or activation by aromatic N-heterocycles which leads to Nlp conjugation onto an exocyclic group as well as steric activation and N-pyramidalization in acyclic scaffolds. Remarkably, as demonstrated in this review, there are many examples of structurally-characterized acyclic amides that feature twist and N-pyramidalization values close to full twist (τ = 90°) and full pyramidalization (χN = 60°). Furthermore, comparison with the classic bridged lactams and conformationally-restricted cyclic fused lactams demonstrates many effective methods to achieve geometric alteration of the amide bond in acyclic amides.
Despite the undeniable progress in the last years, there are a number of challenges that need to be addressed, including: (1) development of rational models correlating amide bond distortion with the observed reactivity; (2) development of a better understanding of the properties of non-planar amide bonds, in particular, focused on the different impact of twist and pyramidalization; (3) development of considerably twisted acyclic amides that feature non-electronically-activated amide bonds; (4) development of new activation methods of acyclic amide bonds that cover broad scope and diverse structural variation of acyclic amides, including peripheral activation564 and mechanical twisting;565 and (5) expansion of the scope of activating groups used for twisting of acyclic amide bonds. Furthermore, there is clearly a need for studies merging the properties of classic cyclic twisted amides with their acyclic counterparts, including structure and reactivity.
We believe that the importance of amide bonds in various facets of chemistry and the inspiring journey of non-planar amide bonds since the seminal studies by Pauling will lead to the discovery of new and highly valuable twisted amides.
Supplementary Material
Figure 17.
Amides in Five-Membered Rings with Nitrogen Pyramidalization Values of 44° to 50°.
Acknowledgements
Rutgers University, the NSF (CAREER CHE-1650766), and the NIH (1R35GM133326) are gratefully acknowledged for support. J.Z. thanks the China Scholarship Council (201808610096). Additional support was provided by the Rutgers Graduate School in the form of Dean’s Dissertation Fellowship (G.M.).
Biographies
Guangrong Meng was born in Shandong Province, P.R. of China, and received his B.Sc. degree from Dalian Medical University in 2011. He received his M.Sc. from Fudan University in 2014. He completed his Ph.D. at Rutgers University in 2019 where he worked under the supervision of Professor Michal Szostak. Currently, he is a post-doctoral fellow in the group of Professor Jin-Quan Yu at The Scripps Research Institute. His research interests are focused on transition-metal-catalysis and C–H activation reactions.
Jin Zhang received his B.Sc. in Applied Chemistry in 2007 and Ph.D. in Organic Chemistry in 2012 both from Northwest University. He joined Shaanxi University of Science and Technology in 2012 and then worked as a postdoctoral fellow at SUST with Prof. Yangmin Ma from 2013 to 2016. He worked with Prof. Michal Szostak group at Rutgers as a visiting scholar in 2018–2019. His current research interests are focused on inert bond activation based on transition-metal-catalysis, carbonylation reactions and mechanochemistry.
Michal Szostak received his Ph.D. from the University of Kansas with Professor Jeffrey Aubé in 2009. After postdoctoral stints at Princeton University with Prof. David MacMillan and at the University of Manchester with Prof. David Procter, in 2014, he joined the faculty at Rutgers University. His research group is focused on the development of new synthetic methodology based on transition-metal-catalysis, amide bonds, C–H, C–O and C–N activation, decarbonylative coupling, and application to the synthesis of biologically active molecules.
Footnotes
The authors declare no competing financial interests.
Supporting Information
The Supporting Information is available free of charge via the Internet at http://pubs.acs.org. Detailed summary tables including Winkler-Dunitz distortion parameters for all amides discussed.
References
- 1.Greenberg A; Breneman CM; Liebman JF, Eds. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science; Wiley: New York, 2000. [Google Scholar]
- 2.Pauling L The Nature of the Chemical Bond; Cornell University Press, New York, 1940. [Google Scholar]
- 3.Pauling L; Corey RB; Branson HR The Structure of Proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain. Proc. Natl. Acad. Sci. USA 1951, 37, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edison AS Linus Pauling and the Planar Peptide Bond. Nat. Struct. Biol 2001, 8, 201–202. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg D The Discovery of the α-Helix and β-Sheet, the Principal Structural Features of Proteins. Proc. Natl. Acad. Sci. USA 2003, 100, 11207–11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemnitz CR; Loewen MJ “Amide Resonance” Correlates with a Breadth of C−N Rotation Barriers. J. Am. Chem. Soc 2007, 129, 2521–2528. [DOI] [PubMed] [Google Scholar]
- 7.Mujika JI; Matxain JM; Eriksson LA; Lopez X Resonance Structures of the Amide Bond: the Advantages of Planarity. Chem. Eur. J 2006, 12, 7215–7224. [DOI] [PubMed] [Google Scholar]
- 8.Jean Y; Demachy I; Lledos A; Maseras F Electronic against Steric Effects in Distorted Amides. J. Mol. Struc. (Theochem) 2003, 632, 131–144. [Google Scholar]
- 9.Mucsi Z; Tsai A; Szori M; Chass GA; Viskolcz B; Csizmadia IG A Quantitative Scale for the Extent of Conjugation of the Amide Bond. Amidity Percentage as a Chemical Driving Force. J. Phys. Chem. A 2007, 111, 13245–13254. [DOI] [PubMed] [Google Scholar]
- 10.Mucsi Z; Chass GA; Viskolcz B; Csizmadia IG Quantitative Scale for the Extent of Conjugation of Carbonyl Groups: “Carbonylicity” Percentage as a Chemical Driving Force. J. Phys. Chem. A 2008, 112, 9153–9165. [DOI] [PubMed] [Google Scholar]
- 11.Mucsi Z; Chass GA; Csizmadia IG Amidicity Change as a Significant Driving Force and Thermodynamic Selection Rule of Transamidation Reactions. A Synergy between Experiment and Theory. J. Phys. Chem. B 2008, 112, 7885–7893. [DOI] [PubMed] [Google Scholar]
- 12.Glover SA; Rosser AA Reliable Determination of Amidicity in Acyclic Amides and Lactams. J. Org. Chem 2012, 77, 5492–5502. [DOI] [PubMed] [Google Scholar]
- 13.Liebman JF; Greenberg A The Resonance Energy of Amides and Their Radical Cations. Struct. Chem 2019, 30, 1631–1634. [Google Scholar]
- 14.Mujika JI; Mercero JM; Lopez X Water-Promoted Hydrolysis of a Highly Twisted Amide: Rate Acceleration Caused by the Twist of the Amide Bond. J. Am. Chem. Soc 2005, 127, 44454453. [DOI] [PubMed] [Google Scholar]
- 15.Wang B; Cao Z Acid-Catalyzed Reactions of Twisted Amides in Water Solution: Competition between Hydration and Hydrolysis. Chem. Eur. J 2011, 17, 11919–11929. [DOI] [PubMed] [Google Scholar]
- 16.Matsubara T; Ueta C Computational Study of the Effects of Steric Hindrance on Amide Bond Cleavage. J. Phys. Chem. A 2014, 118, 8664–8675. [DOI] [PubMed] [Google Scholar]
- 17.Cho SJ; Cui C; Lee JY; Park JK; Suh SB; Park J; Kim BH; Kim KS N-Protonation vs O-Protonation in Strained Amides: Ab Initio Study. J. Org. Chem 1997, 62, 4068–4071. [Google Scholar]
- 18.Morgan J; Greenberg A; Liebman JF Paradigms and Paradoxes: O- and N-Protonated Amides, Stabilization Energy and Resonance Energy. Struct. Chem 2012, 23, 197–199. [Google Scholar]
- 19.Bednarova L; Malon P; Bour P Spectroscopic Properties of the Nonplanar Amide Group: a Computational Study. Chirality 2007, 19, 775–786. [DOI] [PubMed] [Google Scholar]
- 20.Wiberg KB; Laidig KE Barriers to Rotation Adjacent to Double Bonds. 3. The Carbon-Oxygen Barrier in Formic Acid, Methyl Formate, Acetic Acid, and Methyl Acetate. The Origin of Ester and Amide Resonance. J. Am. Chem. Soc 1987, 109, 5935–5943. [Google Scholar]
- 21.Wiberg KB; Breneman CM Resonance Interactions in Acyclic Systems. 3. Formamide Internal Rotation Revisited. Charge and Energy Redistribution along the C-N Bond Rotational Pathway. J. Am. Chem. Soc 1992, 114, 831–840. [Google Scholar]
- 22.Laidig KE; Cameron LM Barrier to Rotation in Thioformamide: Implications for Amide Resonance. J. Am. Chem. Soc 1996, 118, 1737–1742. [Google Scholar]
- 23.Wiberg KB The Interaction of Carbonyl Groups with Substituents. Acc. Chem. Res 1999, 32, 922–929. [Google Scholar]
- 24.Kovács E; Rózsa B; Csomos A; Csizmadia I; Mucsi Z Amide Activation in Ground and Excited States. Molecules 2018, 23, no. 2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasserman HH Chemistry: Synthesis with a Twist. Nature 2006, 441, 699–700. [DOI] [PubMed] [Google Scholar]
- 26.Lukeš R Collect. Sur une Nouvelle Application de la règle de Bredt. Czech., Chem. Commun 1938, 10, 148–152. [Google Scholar]
- 27.Clarke HT; Johnson JR; Robinson R, Eds. The Chemistry of Penicillin; Princeton University Press: Princeton, 1949. [Google Scholar]
- 28.Hall HK Jr.; El-Shekeil A Anti-Bredt Bridgehead Nitrogen Compounds in Ring-Opening Polymerization. Chem. Rev 1983, 83, 549–555. [Google Scholar]
- 29.Lease TG; Shea KJ A Compilation and Analysis of Structural Data of Distorted Bridgehead Olefins and Amides. In Advances in Theoretically Interesting Molecules; JAI Press: Greenwich, CT, 1992; Vol. 2. [Google Scholar]
- 30.Yamada S Chemistry of Highly Twisted Amides. Rev. Heteroat. Chem 1999, 19, 203–236. [Google Scholar]
- 31.Glover SA N-Acyloxy-N-alkoxyamides - Structure, Properties, Reactivity and Biological Activity. Adv. Phys. Org. Chem 2007, 42, 35–123. [Google Scholar]
- 32.Szostak M; Aubé, Medium-Bridged Lactams: a New Class of Non-Planar Amides. Org. Biomol. Chem 2011, 9, 27–35. [DOI] [PubMed] [Google Scholar]
- 33.Szostak M; Aubé J Chemistry of Bridged Lactams and Related Heterocycles. Chem. Rev 2013, 113, 5701–5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glover SA; Rosser AA Heteroatom Substitution at Amide Nitrogen-Resonance Reduction and HERON Reactions of Anomeric Amides. Molecules 2018, 23, no. 2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szostak R; Szostak M Chemistry of Bridged Lactams: Recent Developments. Molecules 2019, 24, no. 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tani K; Stoltz BM Synthesis and Structural Analysis of 2-Quinuclidonium Tetrafluoroborate. Nature 2006, 441, 731–734. [DOI] [PubMed] [Google Scholar]
- 37.Ly T; Krout M; Pham DK; Tani K; Stoltz BM; Julian RR Synthesis of 2-Quinuclidonium by Eliminating Water: Experimental Quantification of the High Basicity of Extremely Twisted Amides. J. Am. Chem. Soc 2007, 129, 1864–1865. [DOI] [PubMed] [Google Scholar]
- 38.Liniger M; VanderVelde DG; Takase MK; Shahgholi M; Stoltz BM Total Synthesis and Characterization of 7-Hypoquinuclidonium Tetrafluoroborate and 7-Hypoquinuclidone BF3 Complex. J. Am. Chem. Soc 2016, 138, 969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirby AJ; Komarov IV; Wothers PD; Feeder N The Most Twisted Amide: Structure and Reactions. Angew. Chem., Int. Ed 1998, 37, 785–786. [DOI] [PubMed] [Google Scholar]
- 40.Kirby AJ; Komarov IV; Feeder N Spontaneous, Millisecond Formation of a Twisted Amide from the Amino Acid, and the Crystal Structure of a Tetrahedral Intermediate. J. Am. Chem. Soc 1998, 120, 7101–7102. [Google Scholar]
- 41.Kirby AJ; Komarov IV; Feeder N Synthesis, Structure and Reactions of the Most Twisted Amide. J. Chem. Soc., Perkin Trans 2 2001, 522–529. [Google Scholar]
- 42.Morgan KM; Rawlins ML; Montgomery MN Influence of Methyl Substituents on the Stability of 1-Aza-2-Adamantanone, Kirby’s Most Twisted Amide. J. Phys. Org. Chem 2005, 18, 310–314. [Google Scholar]
- 43.Komarov IV; Yanik S; Ishchenko AY; Davies JE; Goodman JM; Kirby AJ The Most Reactive Amide as a Transition-State Mimic for Cis–Trans Interconversion. J. Am. Chem. Soc 2015, 137, 926–930. [DOI] [PubMed] [Google Scholar]
- 44.Greenberg A; Wu GL; Tsai JC; Chiu YY Improved Synthesis of 6,6,7,7-Tetramethyl-1-azabicyclop[2.2.2]octan-2-one and Its Stability Toward Base-Induced Methanolysis. Struct. Chem 1993, 4, 127–129. [Google Scholar]
- 45.Greenberg A; Venanzi CA Structures and Energetics of Two Bridgehead Lactams and Their N- and O-Protonated Forms: An Ab Initio Molecular Orbital Study. J. Am. Chem. Soc 1993, 115, 6951–6957. [Google Scholar]
- 46.Greenberg A; Moore DT; DuBois TD Small and Medium-Sized Bridgehead Bicyclic Lactams: a Systematic ab Initio Molecular Orbital Study. J. Am. Chem. Soc 1996, 118, 86588668. [Google Scholar]
- 47.Sliter B; Morgan J; Greenberg A 1-Azabicyclo[3.3.1]nonan-2-one: Nitrogen Versus Oxygen Protonation. J. Org. Chem 2011, 76, 2770–2781. [DOI] [PubMed] [Google Scholar]
- 48.Morgan J; Greenberg A Novel Bridgehead Bicyclic Lactams: Molecules Predicted to Have O-Protonated and N-Protonated Tautomers of Comparable Stability; Hyperstable Lactams and Their O-Protonated Tautomers. J. Chem. Thermodynamics 2014, 73, 206–212. [Google Scholar]
- 49.Zabicky J The Chemistry of Amides; Interscience: New York, 1970. [Google Scholar]
- 50.Larock RC Comprehensive Organic Transformations; Wiley: New York, 1999. [Google Scholar]
- 51.Pattabiraman VR; Bode JW Rethinking Amide Bond Synthesis. Nature 2011, 480, 471–479. [DOI] [PubMed] [Google Scholar]
- 52.Marchildon K Polyamides: Still Strong After Seventy Years. Macromol. React. Eng 2011, 5, 22–54. [Google Scholar]
- 53.Hughes AB Amino Acids, Peptides and Proteins in Organic Chemistry; Wiley: Weinheim, 2011. [Google Scholar]
- 54.Kaspar A, Reichert JM, Future M Directions for Peptide Therapeutics Development. Drug Discov. Today 2013, 18, 807–817. [DOI] [PubMed] [Google Scholar]
- 55.Roughley SD; Jordan AM The Medicinal Chemist’s Toolbox: an Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem 2011, 54, 3451–3479. [DOI] [PubMed] [Google Scholar]
- 56.Blakemore DC; Castro L; Churcher I; Rees DC; Thomas AW; Wilson DM; Wood A Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem 2018, 10, 383–394. [DOI] [PubMed] [Google Scholar]
- 57.Yamada S Structure and Reactivity of a Highly Twisted Amide. Angew. Chem., Int. Ed 1993, 32, 1083–1085. [Google Scholar]
- 58.Yamada S Relationship between C(O)–N Twist Angles and 17O NMR Chemical Shifts in a Series of Twisted Amides. Angew. Chem., Int. Ed 1995, 34, 1113–1115. [Google Scholar]
- 59.Yamada S; Sugaki T; Matsuzaki K Twisted Amides as Selective Acylating Agents for Hydroxyl Groups under Neutral Conditions: Models for Activated Peptides during Enzymatic Acyl Transfer Reaction. J. Org. Chem 1996, 61, 5932–5938. [Google Scholar]
- 60.Yamada S Effects of C(O)−N Bond Rotation on the 13C, 15N, and 17O NMR Chemical Shifts, and Infrared Carbonyl Absorption in a Series of Twisted Amides. J. Org. Chem 1996, 61, 941–946. [Google Scholar]
- 61.Yamada S; Nakamura M; Kawauchi I 13C–15N Coupling Constants in a Series of Twisted Amides: Relationships with C(O)–N Twist Angles. Chem. Commun 1997, 885–886. [Google Scholar]
- 62.Yamada S; Misono T; Iwai Y; Masumizu A; Akiyama Y New Class of Pyridine Catalyst Having a Conformation Switch System: Asymmetric Acylation of Various sec-Alcohols. J. Org. Chem 2006, 71, 6872–6880. [DOI] [PubMed] [Google Scholar]
- 63.Ruider S; Maulide N Strong Bonds Made Weak: Towards the General Utility of Amides as Synthetic Modules. Angew. Chem. Int. Ed 2015, 54, 13856–13858. [DOI] [PubMed] [Google Scholar]
- 64.Meng G; Shi S; Szostak M Cross-Coupling of Amides by N–C Bond Activation. Synlett 2016, 27, 2530–2540. [Google Scholar]
- 65.Liu C; Szostak M Twisted Amides: From Obscurity to Broadly Useful Transition-Metal Catalyzed Reactions by N–C Amide Bond Activation. Chem. Eur. J 2017, 23, 7157–7173. [DOI] [PubMed] [Google Scholar]
- 66.Dander JE; Garg NK Breaking Amides using Nickel Catalysis. ACS Catal. 2017, 7, 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takise R; Muto K; Yamaguchi J Cross-Coupling of Aromatic Esters and Amides. Chem. Soc. Rev 2017, 46, 5864–5888. [DOI] [PubMed] [Google Scholar]
- 68.Meng G; Szostak M N-Acyl-Glutarimides: Privileged Scaffolds in Amide N-C Bond Cross-Coupling. Eur. J. Org. Chem 2018, 20–21, 2352–2365. [Google Scholar]
- 69.Shi S; Nolan SP; Szostak M Well-Defined Palladium(II)-NHC (NHC = N-Heterocyclic Carbene) Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective Acyl CO–X (X = N, O) Cleavage. Acc. Chem. Res 2018, 51, 2589–2599. [DOI] [PubMed] [Google Scholar]
- 70.Kaiser D; Bauer A; Lemmerer M; Maulide N Amide Activation: an Emerging Tool for Chemoselective Synthesis. Chem. Soc. Rev 2018, 47, 7899–7925. [DOI] [PubMed] [Google Scholar]
- 71.Adachi S; Kumagai N; Shibasaki M Conquering Amide Planarity: Structural Distortion and its Hidden Reactivity. Tetrahedron Lett. 2018, 59, 1147–1158. [Google Scholar]
- 72.Li G; Ma S; Szostak M Amide Bond Activation: the Power of Resonance. Trends Chem. 2020, 2, 914–928. [Google Scholar]
- 73.Gooßen LJ; Rodriguez N; Gooßen K Carboxylic Acids as Substrates in Homogeneous Catalysis. Angew. Chem. Int. Ed 2008, 47, 3100–3120. [DOI] [PubMed] [Google Scholar]
- 74.Blangetti M; Rosso H; Prandi C; Deagostino A; Venturello P Suzuki-Miyaura Cross-Coupling in Acylation Reactions, Scope and Recent Developments. Molecules 2013, 18, 1188–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buchspies J; Szostak M Recent Advances in Acyl Suzuki Cross-Coupling. Catalysts 2019, 9, no. 53. [Google Scholar]
- 76.Liu C; Szostak M Decarbonylative Cross-Coupling of Amides. Org. Biomol. Chem 2018, 16, 7998–8010. [DOI] [PubMed] [Google Scholar]
- 77.Guo L; Rueping M Transition-Metal-Catalyzed Decarbonylative Coupling Reactions: Concepts, Classifications, and Applications. Chem. Eur. J 2018, 24, 7794–7809. [DOI] [PubMed] [Google Scholar]
- 78.Lu H; Yu TY; Xu PF; Wei H Selective Decarbonylation via Transition-Metal-Catalyzed Carbon–Carbon Bond Cleavage. Chem. Rev 2021, 121, 365–411. [DOI] [PubMed] [Google Scholar]
- 79.Marcia de Figueiredo R; Suppo JS; Campagne JM Nonclassical Routes for Amide Bond Formation. Chem. Rev 2016, 116, 12029–12122. [DOI] [PubMed] [Google Scholar]
- 80.Acosta-Guzmán P; Mateus-Gómez A; Gamba-Sánchez D Direct Transamidation Reactions: Mechanism and Recent Advances. Molecules 2018, 23, no. 2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li G; Szostak M Non-Classical Amide Bond Formation: Transamidation and Amidation of Activated Amides and Esters by Selective N–C/O–C Cleavage. Synthesis 2020, 52, 2579–2599. [Google Scholar]
- 82.Li G; Szostak M Transition-Metal-Free Activation of Amides by N–C Bond Cleavage. Chem. Rec 2020, 20, 649–659. [DOI] [PubMed] [Google Scholar]
- 83.Ramachandran GN Need for Nonplanar Peptide Units in Polypeptide Chains. Biopolymers 1968, 6, 1494–1496. [DOI] [PubMed] [Google Scholar]
- 84.MacArthur MW; Thornton JM Deviations from Planarity of the Peptide Bond in Peptides and Proteins. J. Mol. Biol 1996, 264, 1180–1195. [DOI] [PubMed] [Google Scholar]
- 85.Chalupsky J; Vondrasek J; Spirko V Quasiplanarity of the Peptide Bond. J. Phys. Chem. A 2008, 112, 693–699. [DOI] [PubMed] [Google Scholar]
- 86.Poteau R; Trinquier G All-Cis Cyclic Peptides J. Am. Chem. Soc 2005, 127, 13875–13889. [DOI] [PubMed] [Google Scholar]
- 87.Shin SBY; Yoo B; Todaro LJ; Kirshenbaum K Cyclic Peptoids. J. Am. Chem. Soc 2007, 129, 3218–3225. [DOI] [PubMed] [Google Scholar]
- 88.Roy O; Caumes C; Esvan Y; Didierjean C; Faure S; Taillefumier C The tert-Butyl Side Chain: A Powerful Means to Lock Peptoid Amide Bonds in the Cis Conformation. Org. Lett 2013, 15, 2246–2249. [DOI] [PubMed] [Google Scholar]
- 89.Metrano AJ; Abascal NC; Mercado BQ; Paulson EK; Hurtley AE; Miller SJ Diversity of Secondary Structure in Catalytic Peptides with β-Turn-Biased Sequences. J. Am. Chem. Soc 2017, 139, 492–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roy O; Dumonteil G; Faure S; Jouffret L; Kriznik A; Taillefumier C Homogeneous and Robust Polyproline Type I Helices from Peptoids with Nonaromatic α-Chiral Side Chains. J. Am. Chem. Soc 2017, 139, 13533–13540. [DOI] [PubMed] [Google Scholar]
- 91.Somayaji V; Brown RS Distorted Amides as Models for Activated Peptide N-C(O) Units Produced During Enzyme-Catalyzed Acyl Transfer Reactions. 1. The Mechanism of Hydrolysis of 3,4-Dihydro-2-oxo-1,4-ethanoquinoline and 2,3,4,5-Retrahydro-2-oxo-1,5-ethanobenzazepine. J. Org. Chem 1986, 51, 2676–2686. [Google Scholar]
- 92.Perrin CL Proton Exchange in Amides: Surprises from Simple Systems. Acc. Chem. Res 1989, 22, 268–275. [Google Scholar]
- 93.Mihaylov TT; Parac-Vogt TN; Pierloot K A Mechanistic Study of the Spontaneous Hydrolysis of Glycylserine as the Simplest Model for Protein Self-Cleavage. Chem. Eur. J 2014, 20, 456–466. [DOI] [PubMed] [Google Scholar]
- 94.Scalvini L; Ghidini A; Lodola A; Callegari D; Rivara S; Piomelli D; Mor M N-Acylethanolamine Acid Amidase (NAAA): Mechanism of Palmitoylethanolamide Hydrolysis Revealed by Mechanistic Simulations. ACS Catal. 2020, 10, 11797–11813. [Google Scholar]
- 95.Poland BW; Xu MQ; Quiocho FA Structural Insights into the Protein Splicing Mechanism of PI-SceI. J. Biol. Chem 2000, 275, 16408–16413. [DOI] [PubMed] [Google Scholar]
- 96.Romanelli A; Shekhtman A; Cowburn D; Muir TW Semisynthesis of a Segmental Isotopically Labeled Protein Splicing Precursor: NMR Evidence for an Unusual Peptide Bond at the N-Extein–Intein Junction. Proc. Natl. Acad. Sci. U. S. A 2004, 101, 6397–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shemella P; Pereira B; Zhang YM; Van Roey P; Belfort G; Garde S; Nayak SK Mechanism for Intein C-Terminal Cleavage: A Proposal from Quantum Mechanical Calculations. Biophys. J 2007, 92, 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lizak C; Gerber S; Numao S; Aebi M; Locher KP X-ray Structure of a Bacterial Oligosaccharyltransferase. Nature 2011, 474, 350–355. [DOI] [PubMed] [Google Scholar]
- 99.Lizak C; Gerber S; Michaud G; Schubert M; Fan YY; Bucher M; Darbare T; Aebi M; Reymond JL; Locher KP Unexpected Reactivity and Mechanism of Carboxamide Activation in Bacterial N-Linked Protein Glycosylation. Nat. Commun 2013, 4, no. 2627. [DOI] [PubMed] [Google Scholar]
- 100.Elashai HE; Raj M Site-Selective Chemical Cleavage of Peptide Bonds. Chem. Commun 2016, 52, 6304–6307. [DOI] [PubMed] [Google Scholar]
- 101.Elashal HE; Cohen RD; Elashal HE; Raj M Oxazolidinone-Mediated Sequence Determination of One-Bead One-Compound Cyclic Peptide Libraries. Org. Lett 2018, 20, 2374–2377. [DOI] [PubMed] [Google Scholar]
- 102.Mahesh S; Tang KC; Raj M Amide Bond Activation of Biological Molecules. Molecules 2018, 23, no. 2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hutchby M; Houlden CE; Haddow MF; Tyler SN; Lloyd-Jones GC; Booker-Milburn KI Switching Pathways: Room-Temperature Neutral Solvolysis and Substitution of Amides. Angew. Chem., Int. Ed 2012, 51, 548–551. [DOI] [PubMed] [Google Scholar]
- 104.Aubé J A New Twist on Amide Solvolysis. Angew. Chem., Int. Ed 2012, 51, 3063–3065. [DOI] [PubMed] [Google Scholar]
- 105.Bollu A; Sharma NK Cleavable Amide Bond: Mechanistic Insight into Cleavable 4-Aminopyrazolyloxy Acetamide at Low pH. J. Org. Chem 2019, 84, 5596–5602. [DOI] [PubMed] [Google Scholar]
- 106.Hie L; Nathel NFF; Shah TK; Baker EL; Hong X; Yang YF; Liu P; Houk KN; Garg NK Conversion of Amides to Esters by the Nickel-Catalysed Activation of Amide C–N Bonds. Nature 2015, 524, 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ji C-L; Hong X Factors Controlling the Reactivity and Chemoselectivity of Resonance Destabilized Amides in Ni-Catalyzed Decarbonylative and Nondecarbonylative Suzuki-Miyaura Coupling. J. Am. Chem. Soc 2017, 139, 15522–15529. [DOI] [PubMed] [Google Scholar]
- 108.Wang H; Zhang S-Q; Hong X Computational Studies on Ni-Catalyzed Amide C–N Bond Activation. Chem. Commun 2019, 55, 11330–11341. [DOI] [PubMed] [Google Scholar]
- 109.Ni S; Zhang W; Mei H; Han J; Pan Y Ni-Catalyzed Reductive Cross-Coupling of Amides with Aryl Iodide Electrophiles via C–N Bond Activation. Org. Lett 2017, 19, 2536–2539. [DOI] [PubMed] [Google Scholar]
- 110.Wybon CCD; Mensch C; Hollanders K; Gadals C; Herrebout WA; Ballet S; Maes BUW Zn-Catalyzed tert-Butyl Nicotinate-Directed Amide Cleavage as a Biomimic of Metallo-Exopeptidase Activity. ACS Catal. 2018, 8, 203–218. [Google Scholar]
- 111.Zhuo J; Li Z; Li C Nickel-Catalyzed Direct Acylation of Aryl and Alkyl Bromides with Acylimidazoles. ACS Catal. 2020, 10, 3895–3903. [Google Scholar]
- 112.Kerackian T; Reina A; Bouyssi D; Monteiro N; Amgoune A Silyl Radical Mediated Cross-Electrophile Coupling of N-Acyl-imides with Alkyl Bromides under Photoredox/Nickel Dual Catalysis. Org. Lett 2020, 22, 2240–2245. [DOI] [PubMed] [Google Scholar]
- 113.Zhang ZB; Yang Y; Yu ZX; Xia JB Lewis Base-Catalyzed Amino-Acylation of Arylallenes via C–N Bond Cleavage: Reaction Development and Mechanistic Studies. ACS Catal. 2020, 10, 5419–5429. [Google Scholar]
- 114.Long Y; Su Z; Zheng Y; He S; Zhong J; Xiang H; Zhou X Rhodium-Catalyzed Transarylation of Benzamides: C–C Bond vs C–N Bond Activation. ACS Catal. 2020, 10, 3398–3403. [Google Scholar]
- 115.Ielo L; Pace V; Holzer W; Rahman M; Meng G; Szostak R; Szostak M Electrophilicity Scale of Activated Amides: 17O NMR and 15N NMR Chemical Shifts of Acyclic Twisted Amides in N−C(O) Cross-Coupling. Chem. Eur. J 2020, 26, 16246–16250. [DOI] [PubMed] [Google Scholar]
- 116.Winkler FK; Dunitz JD The Non-Planar Amide Group. J. Mol. Biol 1971, 59, 169–182. [DOI] [PubMed] [Google Scholar]
- 117.Szostak R; Aubé J; Szostak M An Efficient Computational Model to Predict Protonation at the Amide Nitrogen and Reactivity along the C–N Rotational Pathway. Chem. Commun 2015, 51, 6395–6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Szostak R; Aubé J; Szostak M Determination of Structures and Energetics of Small- and Medium-Sized One-Carbon Bridged Twisted Amides using ab Initio Molecular Orbital Methods. Implications for Amidic Resonance along the C–N Rotational Pathway. J. Org. Chem 2015, 80, 7905–7927. [DOI] [PubMed] [Google Scholar]
- 119.Kessler H Detection of Hindered Rotation and Inversion by NMR Spectroscopy. Angew. Chem. Int. Ed 1970, 9, 219–235. [Google Scholar]
- 120.Drakenberg T; Dahlqvist KI; Forsen S Barrier to Internal Rotation in Amides. IV. N,N-Dimethylamides. Substituent and Solvent Effects. J. Phys. Chem 1972, 76, 2178–2183. [Google Scholar]
- 121.Kleinpeter E Effect of the Variation of the Ring Size of Cyclic NR2 Substituents on the Barrier to Rotation in Amides, Thioamides and Related Compounds. J. Mol. Struct 1996, 380, 139–156. [Google Scholar]
- 122.Sigel H; Martin RB Coordinating Properties of the Amide Bond. Stability and Structure of Metal Ion Complexes of Peptides and Related Ligands. Chem. Rev 1982, 82, 385–426. [Google Scholar]
- 123.Ghosh AK Brindisi M Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem 2015, 58, 2895–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Volz N; Clayden J The Urea Renaissance. Angew. Chem. Int. Ed 2011, 50, 12148–12155. [DOI] [PubMed] [Google Scholar]
- 125.Majumdar P; Pati A; Patra M; Behera RK; Behera AK Acid Hydrazides, Potent Reagents for Synthesis of Oxygen-, Nitrogen-, and/or Sulfur-Containing Heterocyclic Rings. Chem. Rev 2014, 114, 2942–2977. [DOI] [PubMed] [Google Scholar]
- 126.Szostak R; Meng G; Szostak M Resonance Destabilization in N-Acylanilines (Anilides): Electronically-Activated Planar Amides of Relevance in N–C(O) Cross-Coupling. J. Org. Chem 2017, 82, 6373–6378. [DOI] [PubMed] [Google Scholar]
- 127.Chakrabarti P; Dunitz JD Structural Characteristics of the Carboxylic Amide Group. Helv. Chim. Acta 1982, 65, 1555–1562. [Google Scholar]
- 128.Nørskov-Lauritsen L; Bürgi HB; Hofmann P; Schmidt HR Bond Angles in Lactones and Lactams Helv. Chim. Acta 1985, 68, 76–82. [Google Scholar]
- 129.Yakhontov LN; Rubtsov MV The Synthesis of Quinuclidone-2. J. Gen. Chem. USSR 1957, 27, 83–87. [Google Scholar]
- 130.Levkoeva EI; Nikitskaya ES; Yakhontov LN Reactions of 2-Quinuclidones with and without Scission of C–N Bonds. Dokl. Akad. Nauk 1970, 192, 342–345 [Google Scholar]
- 131.Kostyanovsky RG; Mikhlina EE; Levkoeva EI; Yakhontov LN Mass Spectra of the Substituted Quinuclidines. Org. Mass Spectrom 1970, 3, 1023–1029. [Google Scholar]
- 132.Levkoeva EI; Nikitskaya ES; Yakhontov LN Synthesis and Transformations of 6,6,7,7-Tetramethyl-2-quinuclidone. Chem. Heterocycl. Compd 1971, 7, 349–354. [Google Scholar]
- 133.Pracejus H 2.2-Dimethyl-chinuclidon-(6), ein Mesomeriefreies Säureamid. Chem. Ber 1959, 92, 988–998. [Google Scholar]
- 134.Pracejus H; Kehlen M; Kehlen H; Matschiner H Neues zur Sterischen Mesomeriehinderung bei Lactamen vom Typ des α-Chinuclidons. Tetrahedron 1965, 21, 2257–2270. [Google Scholar]
- 135.Pracejus H Bicyclische Basen mit einem Asymmetrischen N-Atom, IV: Die Stereoisomeren 2.2.6-Trimethyl-chinuclidine und -chinuclidone-(7). Chem. Ber 1965, 98, 2897–2905 [Google Scholar]
- 136.Somayaji V; Brown RS Hydrolysis of a Distorted Amide Facilitated by Diacids: a Phenomenological Model for the Aspartate Proteinases. J. Am. Chem. Soc 1987, 109, 4738–4739. [Google Scholar]
- 137.Bennet AJ; Wang QP; Slebocka-Tilk H; Somayaji RS; Brown RS; Santarsiero BD Relationship between Amidic Distortion and Ease of Hydrolysis in Base. If Amidic Resonance does not Exist, Then What Accounts for the Accelerated Hydrolysis of Distorted Amides? J. Am. Chem. Soc 1990, 112, 6383–6385. [Google Scholar]
- 138.Wang QP; Bennet AJ; Brown RS; Santarsiero BD Distorted Amides as Models for Activated Peptide N-C(O) Units. 3. Synthesis, Hydrolytic Profile, and Molecular Structure of 2,3,4,5-Tetrahydro-2-oxo-1,5-propanobenzazepine. J. Am. Chem. Soc 1991, 113, 5757–5765. [Google Scholar]
- 139.Golden J; Aubé JA Combined Intramolecular Diels–Alder/Intramolecular Schmidt Reaction: Formal Synthesis of (±)-Stenine. Angew. Chem. Int. Ed 2002, 41, 4316–4318. [DOI] [PubMed] [Google Scholar]
- 140.Wang Q; Bennet AJ; Brown RS; Santarsiero BD Distorted Amides as Models for Activated Peptide N-C=O Units. 2. The Synthesis, Hydrolytic Profile, and Molecular Structure of 3,4-Dihydro-2-oxo-1,4-propanoquinoline. Can. J. Chem 1990, 68, 1732–1739. [Google Scholar]
- 141.Gardarsson H; Schweizer B; Diederich F 5,11-Methanodibenzo[b,f][1,5]diazocine-6,12-dione. Experimental Crystal Structure Determination 2014, DOI: 10.5517/cc13kmx6. [DOI] [Google Scholar]
- 142.Szostak M; Yao L; Day VW; Powell DR; Aubé J Structural Characterization of N-Protonated Amides: Regioselective N-Activation of Medium-Bridged Twisted Lactams. J. Am. Chem. Soc 2010, 132, 8836–8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alcaide B; Casarrubios L; Dominguez G; Sierra MA; Monge A Chromium(0) Carbene Complexes Bearing Imino Tethers: Synthesis and Photochemical Reactivity. J. Am. Chem. Soc 1995, 117, 5604–5605. [Google Scholar]
- 144.Artacho J; Ascic E; Rantanen T; Karlsson J; Wallentin CJ; Wang R; Wendt OF; Harmata M; Snieckus V; Wärnmark K Twisted Amide Analogues of Tröger’s Base. Chem. Eur. J 2012, 18, 1038–1042. [DOI] [PubMed] [Google Scholar]
- 145.Rúnarsson ÖV; Benkhäuser C; Christensen NJ; Ruiz JA; Ascic E; Harmata M; Snieckus V; Rissanen K; Fristrup P; Lützen A; Wärnmark K Resolution and Determination of the Absolute Configuration of a Twisted Bis-Lactam Analogue of Tröger’s Base: a Comparative Spectroscopic and Computational Study. J. Org. Chem 2015, 80, 8142–8149. [DOI] [PubMed] [Google Scholar]
- 146.Baylis AM; Davies MPH; Thomas EJ Synthetic Approaches to the Polycyclic Alkaloid Stemofoline. Org. Biomol. Chem 2007, 5, 3139–3155. [DOI] [PubMed] [Google Scholar]
- 147.Jiang R; Hon P; But PP; Chung H; Lin G; Ye W; Mark TCW Isolation and Stereochemistry of Two New Alkaloids from Stemona Tuberose. Tetrahedron 2002, 58, 6705–6712. [Google Scholar]
- 148.Dong JW; Ding T; Zhang SY; Chen ZM; Tu YQ A Facile Approach to Oximes and Ethers by a Tandem NO+-Initiated Semipinacol Rearrangement and H-Elimination. Angew. Chem. Int. Ed 2018, 57, 13192–13196. [DOI] [PubMed] [Google Scholar]
- 149.Lei Y; Wrobleski AD; Golden JE; Powell DR; Aubé J Facile C–N Cleavage in a Series of Bridged Lactams. J. Am. Chem. Soc 2005, 127, 4552–4553. [DOI] [PubMed] [Google Scholar]
- 150.Szostak M; Yao L; Aube J Stability of Medium-Bridged Twisted Amides in Aqueous Solutions. J. Org. Chem 2009, 74, 1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ma X; Gao N; Banwell MG; Carr PD; Willis AC A Total Synthesis of (±)-3-O-Demethylmacronine through Rearrangement of a Precursor Embodying the Haemanthidine Alkaloid Framework. J. Org. Chem 2017, 82, 4336–4341. [DOI] [PubMed] [Google Scholar]
- 152.Bremner JB; Jaturonrusmee W; Engelhardt LM; White AH Photolysis of Chloroacetamides as a Route to New 2,8-Bridged Isoquinoline Derivatives. X-ray Crystal Structure of 8,13-Dihydro-2-methoxy-4,6-ethanodibenz[c,ƒ]azonine-5,7-dione. Tetrahedron Lett. 1989, 30, 3213–3216. [Google Scholar]
- 153.Biegger P; Schaffroth M; Brödner K; Tverskoy O; Rominger F; Bunz UHF Bisalkynylated 3,6-diiminocyclohexa-1,4-diene-1,4-diamine. Chem. Commun 2015, 51, 14844–14847. [DOI] [PubMed] [Google Scholar]
- 154.Kwit M; Rychlewska U; Gawroński J Induced Homohelicity of Diphenimide BisPropellers. New J. Chem 2002, 26, 1714–1717. [Google Scholar]
- 155.Jones DS; Karle IL The Crystal and Molecular Structures of Two Photodimers from N-Chloroacetyltyramine. Acta Cryst. 1974, B30, 617–623. [Google Scholar]
- 156.Thiering S; Thiem J; Kopf J Reactions of Glycosan-Annelated Oxolactams. Heterocycles 2007, 74, 533–543. [Google Scholar]
- 157.Evans PA; Holmes AB; Collins I; Raithby PR; Russell K The First Example of a Transoid Amide (Imide) in an Eight-Membered Lactam. Chem. Commun 1995, 22, 2325–2326. [Google Scholar]
- 158.Kuti M; Rábai J; Kapovits I; Jalsovszky I; Argay G; Kálmán A; Párkányi L Transannular Sulfur-Nitrogen Interaction in 1,5-Thiazocine Derivatives: An X-ray Study. J. Mol. Struct 1996, 382, 1–11. [Google Scholar]
- 159.Benzeid H; Saffon N; Garrigues B; Essassi EM Ng, S. W. 1-Acetyl-4-phenyl-5a,6,7,8,9,9a-hexa-hydro-5H-1,5-benzodiazepin-2(1H)-one. Acta Cryst. 2009, E65, o2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zheng C; Chen J; Fan R Dearomatization Strategy and Palladium-Catalyzed Domino Reaction: Construction of Azepino[5,4,3-cd]indoles from 2-Alkynylanilines. Org. Lett 2014, 16, 816–819. [DOI] [PubMed] [Google Scholar]
- 161.Bishara A; Rudi A; Goldberg I; Aknin M; Neumann D; Ben-Califa N; Kashman Y Tausalarin C: A New Bioactive Marine Sponge-Derived Nitrogenous Bismacrolide. Org. Lett 2009, 11, 3538–3541. [DOI] [PubMed] [Google Scholar]
- 162.Peng H; Xie W; Otterness DM; Cogswell JP; McConnell RT; Carter HL; Powis G; Abraham RT; Zalkow LH Syntheses and Biological Activities of a Novel Group of Steroidal Derived Inhibitors for Human CDC25A Protein Phosphatase. J. Med. Chem 2001, 44, 834–848. [DOI] [PubMed] [Google Scholar]
- 163.Szostak R; Shi S; Meng G; Lalancette R; Szostak M Ground-State Distortion in NAcyl-tert-butyl-carbamates (Boc) and N-Acyl-tosylamides (Ts): Twisted Amides of Relevance to Amide N−C Cross-Coupling. J. Org. Chem 2016, 81, 8091–8094. [DOI] [PubMed] [Google Scholar]
- 164.Zhu Y; Jiang B; Hao J; Qiu J; Sun J; Wang D; Wei P; Wang A; Li G; Tu S Catalytic Arylsulfonyl Radical Triggered 1,7-Enyne Bicyclizations. Org. Lett 2015, 17, 60786081. [DOI] [PubMed] [Google Scholar]
- 165.Hao L; Lv H; Chen X; Jiang H; Shao Y; Chi YR Enantioselective Activation of Stable Carboxylate Esters as Enolate Equivalents via N-Heterocyclic Carbene Catalysts. Org. Lett 2012, 14, 2154–2157. [DOI] [PubMed] [Google Scholar]
- 166.Hu F; Lalancette R; Szostak M Structural Characterization of N-Alkylated Twisted Amides: Consequences for Amide Bond Resonance and N–C Cleavage. Angew. Chem. Int. Ed 2016, 55, 5062–5066. [DOI] [PubMed] [Google Scholar]
- 167.Rosoff M; Rudler M; Vaissermann J Aminocarbene Complexes of Chromium. VII. Modification of the Reactivity of Nitrogen-Ylide Complexes Derived Therefrom upon Removal of the Metal. J. Organomet. Chem 1997, 541, 77–87. [Google Scholar]
- 168.Gerulat O; Himbert G; Bergsträβer U Five-Membered Betaines from Reaction of N,N’,N’-Trimethyl-N-(trimethylsilyl-ethynyl)hydrazine with Aryl Isocyanates. Synlett 1995, 8, 835–836. [Google Scholar]
- 169.Coqueret X; Bourelle-Wargnier F; Chuche J; Toupet L Synthesis of Prazolinones from β-N,N-Dimethylhydrazinopropenoates: an Example of a Thermally Induced [1,4]-Alkyl Shift. J. Chem. Soc., Chem. Commun 1983, 20, 1144–1145. [Google Scholar]
- 170.Chantegrel B; Deshayes C; Faure R Tandem Wolff Rearrangement-“tert-Amino Effect” Sequence: Synthesis of 2-Oxoindolinium Enolate and 1H-2-Benzopyrane Derivatives. Tetrahedron Lett. 1995, 36, 7859–7862. [Google Scholar]
- 171.Neidlein R; Schröder G; Krieger C; Kikelj D Heterocycles Starting from Bis(alkoxycarbonyl)ketene Ethylene Acetals (= Dialkyl 2-(1,3-dioxolan-2-ylidene)propane-1,3-dioate). Synthesis and Properties of a New Class of Pyrazolium Betaines. Helv. Chim. Acta 1992, 75, 1039–1051. [Google Scholar]
- 172.Khlebnikov AF; Novikov MS; Pakalnis VV; Lakovenko RO; Yufit DS Domino Reactions of 2H-Azirines with Acylketenes from Furan-2,3-diones: Competition between the Formation of ortho-Fused and Bridged Heterocyclic Systems. Beilstein J. Org. Chem 2014, 10, 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kulpe S; Seidel I; Menz I; Geissler G; Tomaschewski G The Structure of 4-N-Hexyle-6-(4-cyanophenyl)-1,5-diazobicyclo[3.1.0]-hexanone-(2), C17H21N3O. Cryst. Res. Technol 1986, 21, 635–640. [Google Scholar]
- 174.Bluer KR; Laperriere LE; Pujol A; Yruegas S; Adiraju VA; Martin CD Coordination and Ring Expansion of 1,2-Dipolar Molecules with 9-Phenyl-9-Borafluorene. Organometallics 2018, 37, 2917–2927. [Google Scholar]
- 175.Mocilac P; Gallagher JF Halogenated Tennimides and Trezimides: Impact of Halogen Bonding and Solvent Role on Porous Network Formation and Inclusion. CrystEngComm 2016, 18, 2375–2384. [Google Scholar]
- 176.Mocilac P; Gallagher JF Entry Point into New Trimeric and Tetrameric Imide-Based Macrocyclic Esters Derived from Isophthaloyl Dichloride and Methyl 6-Aminonicotinate. Acta Cryst. 2013, B69, 62–69. [DOI] [PubMed] [Google Scholar]
- 177.Mocilac P; Gallagher JF Trezimides and Tennimides: New Imide-Based Macrocycles. J. Org. Chem 2013, 78, 2355–2361. [DOI] [PubMed] [Google Scholar]
- 178.Mocilac P; Gallagher JF Halogen Bonding Directed Supramolecular Assembly in Bromo-Substituted Trezimides and Tennimides. CrystEngComm 2014, 16, 1893–1903. [Google Scholar]
- 179.Evans LS; Gale PA Imide Linked ‘4 + 4’ Macrocycles Formed by Condensation of Isophthaloyl Dichloride and Tetra- or Penta-Fluoroaniline. Chem. Commun 2004, 11, 1286–1287. [DOI] [PubMed] [Google Scholar]
- 180.Zhang G; Huang S; Xiao Z; Chen Q; Gan L; Wang Z Preparation of Azafullerene Derivatives from Fullerene-Mixed Peroxides and Single Crystal X-Ray Structures of Azafulleroid and Azafullerene. J. Am. Chem. Soc 2008, 130, 12614–12615. [DOI] [PubMed] [Google Scholar]
- 181.Fan Y; Das U; Hsiao M; Liu M; Lin W Chemoselective Intramolecular Wittig Reactions for the Synthesis of Oxazoles and Benzofurans. J. Org. Chem 2014, 79, 11567–11582. [DOI] [PubMed] [Google Scholar]
- 182.Schwarz T; Steglich W; Polborn K Methyl 1-Benzoyl-2,4-bis(isopropylsulfanyl)-5-oxo-2,5-dihydro-1H-imidazole-2-carboxylate. Experimental Crystal Structure Determination 2005, DOI: 10.5517/cc8c13r. [DOI] [Google Scholar]
- 183.Seki M; Miyake T; Yamanaka T; Ohmizu H Practical Synthesis of Penems and Carbapenems Key Intermediate. Synlett 1996, 5, 455–456. [Google Scholar]
- 184.Jian S; Lei M 3-(2-Bromo-butano-yl)spiro-[2H-1,3-benzoxazine-2,1’-cyclo-hexan]-4(3H)-one. Acta Cryst. 2005, E61, o3196–o3197. [Google Scholar]
- 185.Ai Y; Zhang Y; Liu F; Song H; Fan Z N-(4-Methyl-pyrimidin-2-yl)bis(1,2,3-benzothia-diazole-7-carbonyl)amine. Acta Cryst. 2006, E62, o101–o103. [Google Scholar]
- 186.Kohmoto S; Takeichi H; Kishikawa K; Masu H; Azumaya I Conformation of S-Shaped Aromatic Imide Foldamers and Their Induced Circular Dichroism. Tetrahedron Lett. 2008, 49, 1223–1227. [Google Scholar]
- 187.Sakamoto M; Takahashi M; Fujita T; Watanabe S; Iida I; Nishio T; Aoyama H Solid-State Photochemistry: Absolute Asymmetric Oxetane Synthesis from an Achiral Acyclic Imide Using the Chiral Crystal Environment. J. Org. Chem 1993, 58, 3476–3477. [Google Scholar]
- 188.Feldman KS; Karatjas AG Extending Pummerer Reaction Chemistry. Asymmetric Synthesis of Spirocyclic Oxindoles via Chiral Indole-2-Sulfoxides. Org. Lett 2006, 8, 4137–4140. [DOI] [PubMed] [Google Scholar]
- 189.McDermott MC; Stephenson GR; Hughes DL; Walkington AJ Intramolecular Asymmetric Heck Reactions: Evidence for Dynamic Kinetic Resolution Effects. Org. Lett 2006, 8, 2917–2920. [DOI] [PubMed] [Google Scholar]
- 190.Gololobov YG; Galkin VI; Petrovskii PV; Linchenko OA; Zueva EM; Mubarakova LG; Cherkasov RA; Schutzler R; Ernst L; Jones PG; Freytag M Atropisomerism of Phosphorus-Containing N-Aryl Carbamates. Experimental and Computational Data. Russ. Chem. Bull 2003, 52, 1920–1927. [Google Scholar]
- 191.Fu TY; Scheffer JR; Trotter J N-Phenyl-N-(phenylthioxomethyl)benzamide. Acta Cryst. 1998, C54, 101–102. [Google Scholar]
- 192.Xiang X; Tao H; Jiang S; Zhang L; Cui Z Synthesis and Bioactivity of Thiazolidin-2-Cyanamide Derivatives Against Type III Secretion System of Xanthomonas Oryzae on Rice. Pestic. Biochem. Phys 2018, 149, 89–97. [DOI] [PubMed] [Google Scholar]
- 193.Chen B; Hu Y; Zhang D; Deng L; Lu J; Min D; Ye W; Li C Enantioselective Total Synthesis of (−)-Colchicine, (+)-Demecolcinone and Metacolchicine: Determination of the Absolute Configurations of the Latter Two Alkaloids. Chem. Sci 2017, 8, 4961–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hyuma M; Ken O; Keiki K; Makoto Y; Kentaro Y; Shigeo K Creation of Concave-Shaped Conformation in Crystal Structures Using an Iminodicarbonyl Linker. An Application to Solid-State Intramolecular [4 + 4] Photocycloaddition Reactions of 2-Pyridone Derivatives. Bull. Chem. Soc. Jpn 2005, 78, 1127–1131. [Google Scholar]
- 195.Sakamoto M; Takahashi M; Shimizu M; Fujita T; Nishio T; Iida I; Yamaguchi K; Watanabe S “Absolute” Asymmetric Synthesis Using the Chiral Crystal Environment: Photochemical Hydrogen Abstraction from Achiral Acyclic Monothioimides in the Solid State. J. Org. Chem 1995, 60, 7088–7089. [Google Scholar]
- 196.Liu C; Li G; Shi S; Meng G; Lalancette R; Szostak R; Szostak M Acyl and Decarbonylative Suzuki Coupling of N-Acetyl Amides: Electronic Tuning of Twisted, Acyclic Amides in Catalytic Carbon-Nitrogen Bond Cleavage. ACS Catal 2018, 8, 9131–9139. [Google Scholar]
- 197.Kondo K; Seki M; Kuroda T; Yamanaka T; Iwasaki T 2-Substituted 2,3-Dihydro-4H-1,3-Benzoxazin-4-Ones: a Novel Auxiliary for Stereoselective Synthesis of 1-Beta-Methylcarbapenems. J. Org. Chem 1995, 60, 1096–1097. [DOI] [PubMed] [Google Scholar]
- 198.Chen M; Harrison HM; Gu LQ; Yu HH 1-Acetyl-3,4-dimethyl-6-phenyl-acetyl-3a,4,6,6a-tetra-hydro-imidazo[4,5-d]imidazole-2,5(1H,3H)-dithione. Acta Cryst. 2007, E63, o613–o614. [Google Scholar]
- 199.Matta CF; Cow CN; Harrison PHM Twisted Amides: X-Ray Crystallographic and Theoretical Study of Two Acylated Glycolurils with Aromatic Substituents. J. Mol. Struct 2003, 660, 81–97. [Google Scholar]
- 200.Mocilac P; Farrell M; Lough AJ; Jelsch C; Gallagher JF Aggregation in Isomeric Imides: Analysis of the Weak Interactions in Six N-(Benzoyl)-N-(2-Pyridyl)Benzamides. Struct. Chem. 2018, 29, 1153–1164. [Google Scholar]
- 201.Zhang Y; Nie J; Zhang F; Ma J Zinc-Mediated Enantioselective Addition of Terminal 3-En-1-Ynes to Cyclic Trifluoromethyl Ketimines. J. Fluor. Chem 2018, 208, 1–9. [Google Scholar]
- 202.Luger P; Paulsen H Die 1C4-Konformation beim Methyl-2,4-Bis(N-Acetyl-N-Benzoylamino)-3,6-Di-O-Benzoyl-2,4-Didesoxy-α-D-Idopyranosid zur Vermeidung 1,3Diaxialer Wechselwirkungen. Acta Cryst. 1978, B34, 1254–1259. [Google Scholar]
- 203.Sakamoto M; Takahashi M; Mino T; Fujita T Absolute Asymmetric β-Lactam Synthesis via the Solid-State Photoreaction of Acyclic Monothioimides and the Reaction Trajectory in The Chiral Crystalline Environment. Tetrahedron 2001, 57, 6713–6719. [Google Scholar]
- 204.Sakamoto M; Takahashi M; Arai T; Shimizu M; Mino T; Watanabe S; Fujita T; Yamaguchi K Solid State Photochemical Reaction of Achiral N-(β,γ-Unsaturated Carbonyl)Thiocarbamate to Optically Active Thiolactone in the Chiral Crystalline Environment. Chem. Commun 1998, 21, 2315–2316. [Google Scholar]
- 205.Abraham CJ; Paull DH; Scerba MT; Grebinski JW; Lectka T Catalytic, Enantioselective Bifunctional Inverse Electron Demand Hetero-Diels-Alder Reactions of Ketene Enolates and O-Benzoquinone Diimides. J. Am. Chem. Soc 2006, 128, 13370–13371. [DOI] [PubMed] [Google Scholar]
- 206.Jian S; Gu J Wang Y 3-[(2R*,3S*)-3-(4-Chloro-phenyl)-3-(2-methoxy-anilino)-2-methyl-propionyl]-spiro-[2H-1,3-benzoxazine-2,1’-cyclo-hexan]-4(3H)-one. Acta Cryst. 2005, E61, o814–o815. [Google Scholar]
- 207.Ye G; Chatterjee S; Li M; Zhou A; Song Y; Barker BL; Chen C; Beard DJ; Henry WP; Pittman CU Push-Pull Alkenes From Cyclic Ketene-N,N’-Acetals: a Wide Span of Double Bond Lengths and Twist Angles. Tetrahedron 2010, 66, 2919–2927. [Google Scholar]
- 208.Yildirim SÖ; Akkurt M; Genc M; Sekerci M; Fun HK 1,3-Bis(2-chloro-benzo-yl)-3,4,5,6-tetra-hydro-pyrimidine-2(1H)-thione. Acta Cryst. 2006, E62, o3697–o3698. [Google Scholar]
- 209.Huang R; Chen X; Mou C; Luo G; Li Y; Li X; Chi YR Carbene-Catalyzed α-Carbon Amination of Chloroaldehydes for Enantioselective Access to Dihydroquinoxaline Derivatives. Org. Lett 2019, 21, 4340–4344. [DOI] [PubMed] [Google Scholar]
- 210.Feng GS; Chen MW; Shi L; Zhou YG Facile Synthesis of Chiral Cyclic Ureas Through Hydrogenation of 2-Hydroxypyrimidine/Pyrimidin-2-(1H)-One Tautomers. Angew. Chem. Int. Ed 2018, 57, 5853–5857. [DOI] [PubMed] [Google Scholar]
- 211.Chen Y; Feng G Visible Light Mediated Sp3 C–H Bond Functionalization of N-Aryl-1,2,3,4-Tetrahydroisoquinolines via Ugi-Type Three-Component Reaction. Org. Biomol. Chem 2015, 13, 4260–4265. [DOI] [PubMed] [Google Scholar]
- 212.Gololobov YG; Petrovskii PV; Ivanova EM; Linchenko OA; Schutzler R; Ernst L; Jones PG; Karacar A; Freytag M Okucu, S. C-N Migrations of the Ethoxycarbonyl Group in Reactions of Ortho-Substituted Aryl Isocyanates with the 1,3-Zwitterion Derived from Triisopropylphosphine and Ethyl 2-Cyanoacrylate. Russ. Chem. Bull 2003, 52, 427–436. [Google Scholar]
- 213.Cow CN; Britten JF; Harrison PHM X-Ray Crystal Structure of 1,6-Diacetyl-3,4,7,8-Tetramethyl-2,5-Dithioglycoluril, A Highly Twisted Acetamide. Chem. Commun 1998, 10, 1147–1148. [Google Scholar]
- 214.Young SB; Britcher SF; Tran LO; Payne LS; Lumma WC; Lyle TA; Huff JR; Anderson PS; Olsen DB; Carroll SS; Pettibone DJ; O’Brien JA; Ball RG; Balani SK; Lin JH; Chen W; Schleif WA; Sardana VV; Long WJ; Byrnes VW; Emini EA L-743, 726 (DMP-266): a Novel, Highly Potent Nonnucleoside Inhibitor of the Human Immunodeficiency Virus Type 1 Reverse Transcriptase. Antimicrob. Agents Chemother 1995, 39, 2602–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sakamoto M; Hokari N; Takahashi M; Fujita T; Watanabe S; Iida I; Nishio T Chiral Thietane-Fused Beta-Lactam from an Achiral Monothioimide Using the Chiral Crystal Environment. J. Am. Chem. Soc 1993, 115, 818–818. [Google Scholar]
- 216.Alizadeh A; Rostamnia S; Zhu L Reaction between tert-Butyl Isocyanide, Dialkyl Acetylenedicarboxylates, and Aromatic Carboxylic Acids: an Efficient Method for the Synthesis of Dialkyl (E)-2-{[Benzoyl(Tert-Butyl)Amino]Carbonyl}−2-Butenedioate Derivatives. Tetrahedron 2006, 62, 5641–5644. [Google Scholar]
- 217.Tucker TJ; Lyle TA; Wiscount CM; Britcher SF; Young SD; Sanders WM; Lumma WC; Goldman ME; O’Brien JA; Ball RG; Homnick CF; Schleif WA; Emini EA; Huff JR; Anderson PS Synthesis of a Series of 4-(Arylethynyl)-6-Chloro-4-Cyclopropyl-3,4-Dihydroquinazolin-2(1H)-Ones as Novel Non-Nucleoside Hiv-1 Reverse Transcriptase Inhibitors. J. Med. Chem 1994, 37, 2437–2444. [DOI] [PubMed] [Google Scholar]
- 218.Yan J; Bai Q; Xu C; Feng G Orthogonal Sp3 C1-H and N-H Bond Functionalization of 1,2,3,4-Tetrahydroisoquinolines via the Ugi Four-Component Reaction. Synthesis 2006, 48, 3730–3742. [Google Scholar]
- 219.Duspara PA; Matta CF; Jenkins SI; Harrison PHM Twisted Amides: Synthesis and Structure of 1,6-Dipivaloyl-3,4,7,8- Tetramethyl-2,5-Dithioglycoluril. Org. Lett 2001, 3, 495–498. [DOI] [PubMed] [Google Scholar]
- 220.Qiu G; Chen C; Yao L; Wu J An Efficient Route to 3-Amidylindoles via a Palladium-Catalyzed Tandem Reaction of 2-Alkynylanilines with Isocyanides. Adv. Synth. Catal 2013, 355, 1579–1584. [Google Scholar]
- 221.Yamada S; Morita C Regio- and Stereoselective Addition of Ketene Silyl Acetals to Quinolinium Salts by Way of an Intramolecular C=O⋯Qu+ Or C=S⋯Qu+ Interaction. Chem. Lett 2001, 30, 1034–1035. [Google Scholar]
- 222.Gololobov YG; Pinchuk VA; Thonnessen H; Jones PG; Schmutzler R Zwitterionic Species from Triisopropylphosphine and 2-Cyanoacrylates: Synthesis, Structure and Properties. Phosphorus Sulfur Silicon Relat. Elem 1996, 115, 19–37. [Google Scholar]
- 223.Wang J; Weng Z Borrowing and Returning Oxygen Atom in Trifluoroacetic Anhydride Transfer to Nitrones: a Versatile Route for the Synthesis of N-Trifluoroacetyl Amides. Eur. J. Org. Chem 2019, 2019, 1330–1334. [Google Scholar]
- 224.Kazak C; Yilmaz VT; Servi S; Koca M; Heinemann FW 1,3-Dibenzoylimidazolidine-2-thione and 1,3-Dibenzo-yl-3,4,5,6-tetra-hydro-pyrimidine-2(1H)-thione. Acta Cryst. 2005, C61, o348–o350. [DOI] [PubMed] [Google Scholar]
- 225.Krylova TO; Shishkin OV; Strechkov YT; Kolomnikova GD; Gololobov YG; New Reaction of Phenylisocyanate Intercalation. 1. Structure and Spectral Properties of Phenylisocyanate and Betaines Adducts Prepared on the Basis of 2-Cyanoacrylates and Tertiary Phosphines. Russ. J. Org. Chem 1995, 65, 1393–1397. [Google Scholar]
- 226.Gololobov YG; Galkina MA; Dovgan OV; Krasnova IY; Petrovskii PV; Schmutzler R; Kapacar A; Freytag M; Jones PG Intramolecular Electrophilic Rearrangements in Saturated Acyclic Systems. C→N Migrations of Acetyl Group. Russ. J. Org. Chem 2001, 37, 1061–1067. [Google Scholar]
- 227.Chen S; Wei W; Wang J; Xia Y; Shen Y; Wu X; Jing H; Liang Y Palladium-Catalyzed Isocyanide Insertion With Allylic Esters: Synthesis of N-(But-2-enoyl)-N-(tert-Butyl)Benzamide Derivatives Via Intramolecular Acyl Transfer Termination. Adv. Synth. Catal 2017, 359, 3538–3544. [Google Scholar]
- 228.Madre M; Ikaunieks M; Belyakov S A Convenient Method for the Modification of 8-Bromoguanine via Its N9-Tetrahydrofuranyl Derivative. Synthesis 2007, 9, 1325–1332. [Google Scholar]
- 229.Peng J; Liu L; Hu Z; Huang J; Zhu Q Direct Carboxamidation of Indoles by Palladium-Catalyzed C–H Activation and Isocyanide Insertion. Chem. Commun 2012, 48, 3772–3774. [DOI] [PubMed] [Google Scholar]
- 230.Yamada S; Matsuda K Remarkable Thiocarbonyl and Ring-Size Effects on the Amide Bond Twisting. Chem. Lett 2001, 30, 750–751. [Google Scholar]
- 231.Shi S; Lalancette R; Szostak R; Szostak M Triflamides: Highly Reactive, Electronically Activated N–Sulfonyl Amides in Catalytic N–C(O) Amide Cross-Coupling. Org. Lett 2019, 21, 1253–1257. [DOI] [PubMed] [Google Scholar]
- 232.Szostak R; Szostak M N-Acyl-Glutarimides: Resonance and Proton Affinities of Rotationally-Inverted Twisted Amides Relevant to N−C(O) Cross-Coupling. Org. Lett 2018, 20,1342–1345. [DOI] [PubMed] [Google Scholar]
- 233.Rahman M; Liu C; Bisz E; Dziuk B; Lalancette R; Wang Q; Chen H; Szostak R; Szostak M N-Acyl-glutarimides: Effect of Glutarimide Ring on the Structures of Fully Perpendicular Twisted Amides and N–C Bond Cross-Coupling. J. Org. Chem 2020, 85, 5475–5485. [DOI] [PubMed] [Google Scholar]
- 234.Soloshonok VA; Cai C; Hruby VJ; Meervelt LV; Yamazaki T Rational Design of Highly Diastereoselective, Organic Base-Catalyzed, Room-Temperature Michael Addition Reactions. J. Org. Chem 2000, 65, 6688–6696. [DOI] [PubMed] [Google Scholar]
- 235.Caron A; Riche C; Pascard-Billy C; Gramain JC La Structure Cristalline et Moléculaire du Tribenzamide, N(COC6H5)3. Acta Cryst. 1977, B33, 3786–3792. [Google Scholar]
- 236.Xu Y; Wang F; Guo H; Wang S; Ni S; Zhou Y; Wang Y Antitussive and Anti-Inflammatory Dual-Active Agents Developed from Natural Product Lead Compound 1-Methylhydantoin. Molecules 2019, 24, 2355–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pace V; Holzer W; Meng G; Shi S; Lalancette R; Szostak R; Szostak M Structures of Highly Twisted Amides Relevant to Amide N−C Cross-Coupling: Evidence for Ground-State Amide Destabilization. Chem. Eur. J 2016, 22, 14494–14498. [DOI] [PubMed] [Google Scholar]
- 238.Gasser G; Stoeckli-Evans H N-Benzoyl-N-(2-pyridylcarbon-yl)benzamide. Acta Cryst. 2007, E63, o1518–o1520. [Google Scholar]
- 239.Luo Z; Liu T; Guo W; Wang Z; Huang J; Zhu Y; Zeng Z N-Acyl-5,5-Dimethylhydantoin, A New Mild Acyl-Transfer Reagent in Pd Catalysis: Highly Efficient Synthesis of Functionalized Ketones. Org. Process Res. Dev 2018, 22, 1188–1199. [Google Scholar]
- 240.Szostak R; Liu C; Lalancette R; Szostak M Twisted N-Acyl-Hydantoins: Rotationally Inverted Urea-Imides of Relevance in N–C(O) Cross-Coupling. J. Org. Chem 2018, 83, 14676–14682. [DOI] [PubMed] [Google Scholar]
- 241.Popov-Pergal KM; Poleti D; Rančić MP; Meden A; Pergal MV Synthesis And Structure of New 5-(Arylidene)-3-(4-Methylbenzoyl)Thiazolidine-2,4-Diones. J. Heterocycl. Chem 2010, 47, 224–228. [Google Scholar]
- 242.Pham TQ; Pyne SG; Skelton BW; White AH Synthesis of Carbocyclic Hydantocidins via Regioselective and Diastereoselective Phosphine-Catalyzed [3 + 2]-Cycloadditions to 5-Methylenehydantoins. J. Org. Chem 2005, 70, 6369–6377. [DOI] [PubMed] [Google Scholar]
- 243.McSweeney N; Pratt AC; Long C; Howie RA (1RS,2SR,7RS,8RS)-N-Benzoyl-tri-cyclo-[6.2.2.02,7]-dodeca-9,11-diene-1,10-dicarbox-imide. Acta Cryst. 2005, E61, o547–o549. [Google Scholar]
- 244.Meng G; Shi S; Lalancette R; Szostak R; Szostak M Reversible Twisting of Primary Amides via Ground State N–C(O) Destabilization: Highly Twisted Rotationally Inverted Acyclic Amides. J. Am. Chem. Soc 2018, 140, 727–734. [DOI] [PubMed] [Google Scholar]
- 245.Klepp KO; Stähr M; Schmidt H Crystal Structure of 1,3,5-Triacetyl-2,4-Dioxohexahydro-1,3,5-Triazine, (СН3СO)3С3H2N3O2. Z. Kristallogr. New Cryst. Struct 2000, 215, 151–152. [Google Scholar]
- 246.Liang L; Xie M; Qin T; Zhu M; Qu G; Guo H Regio- and Enantioselective Synthesis of Chiral Pyrimidine Acyclic Nucleosides via Rhodium-Catalyzed Asymmetric Allylation of Pyrimidines. Org. Lett 2017, 19, 5212–5215. [DOI] [PubMed] [Google Scholar]
- 247.Meščić A; Harej A; Klobučar M; Glavač D; Cetina M; Pavelić SK; Raić-Malić S Discovery of New Acid Ceramidase-Targeted Acyclic 5-Alkynyl and 5-Heteroaryl Uracil Nucleosides. ACS Med. Chem. Lett 2015, 11, 1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kolappan S; Seshadri TP N3-Benzoyl-2’,3’-di-O-benzoyluridine. Acta Cryst. 1999, C55, 604–606. [Google Scholar]
- 249.Hanton LR; Moratti SC; Shi Z; Simpson J N-Methacryloyl-4-(piperidin-1-yl)-1,8-naphthalimide. Acta Cryst. 2010, E66, o1476–o1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhu Y; Li M; Cai X; Hu M Crystal Structure of 5-Fluoro-3-(Thiophene-2-Carbonyl)Pyrimidine-2,4(1H,3H)-Dione, C9H5FN2O3S. Z. Kristallogr. New Cryst. Struct 2011, 226, 107–108. [Google Scholar]
- 251.Bats JW; Quinkert G 2-(3-Benzoyl-5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4,5-dihydroxytetrahydro-2H-pyran-3-yl benzoate. Experimental Crystal Structure Determination 2016, DOI: 10.5517/ccdc.csd.cczk16r. [DOI] [Google Scholar]
- 252.Parvez M; Phillips SE; Sutherland TC 3-Benzoylthymine. Acta Cryst. 2007, E63, o733–o734. [Google Scholar]
- 253.Xie M; Zhou P; Niu H; Qu G; Guo H Enantioselective Intermolecular Cyclopropanations for the Synthesis of Chiral Pyrimidine Carbocyclic Nucleosides. Org. Lett 2016, 18, 4344–4347. [DOI] [PubMed] [Google Scholar]
- 254.Yamada S; Noriko N; Kayako H A Nonresonated Orthogonally Twisted Amide. Chem. Lett 1998, 27, 451–452. [Google Scholar]
- 255.Pink M; Sieler J; Gutschow M Crystal Structure of 3-Benzoyl-1-Ethyl-2,3-Dihydro-2-Thioxoquinazolin-4(1H)-One, C17H14N2O2S. Z. Kristallogr. Cryst. Mater 1993, 207, 316–318. [Google Scholar]
- 256.Kaminski ZJ; Glowka ML; Olczak A; Martynowski D Thermal Isomerization of 2-Acyloxy-4,6-Dimethoxy-1,3,5-Triazines to 1-Acyl-3,5-Dimethyl-1,3,5-Triazin-2,4,6(1H,3H,5H)-Triones. Crystal Structure of 1-(2,2-Dimethylpropanoyloxy)-3,5-Dimethyl-1,3,5-Triazin-2,4,6(1H,3H,5H)-Trione. Pol. J. Chem 1996, 70, 1316–1323. [Google Scholar]
- 257.Slater AG; Hu Y; Yang L; Argent SP; Lewis W; Blunt MO; Champness NR Thymine Functionalized Porphyrins, Synthesis and Heteromolecular Surface-Based Self-Assembly. Chem. Sci 2015, 6, 1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jiang A; Hu S; Wang Y; Chen Q Crystal and Molecular Structure of N1-Acetyl-N3-O-Toluyl-5-Fluorouracil. Chem. J. Chinese U 1988, 9, 307–309. [Google Scholar]
- 259.Qin T; Li JP; Xie MS; Qu GR; Guo HM Synthesis of Chiral Acyclic Nucleosides by Sharpless Asymmetric Dihydroxylation: Access to Cidofovir and Buciclovir. J. Org. Chem 2018, 83, 15512–15523. [DOI] [PubMed] [Google Scholar]
- 260.Matraszek J; Mieczkowski J; Cyranski MK Synthesis of Crotonoyl, Cynamoyl and p-Methoxycynamoyl Derivatives of Camphoric Imide. Crystal and Molecular Structure of (1R,5S)-3-[(E)-2’-Butenoyl]-1,8,8-Trimethyl-3-Azabicyclo[3.2.1]Octane-2,4-Dione. Pol. J. Chem 2000, 74, 477–482. [Google Scholar]
- 261.Seela F; Chittepu P Oligonucleotides Containing 6-Aza-2’-Deoxyuridine: Synthesis, Nucleobase Protection, pH-Dependent Duplex Stability, and Metal-DNA Formation. J. Org. Chem 2007, 72, 4258–4366. [DOI] [PubMed] [Google Scholar]
- 262.Gainsford GJ; Clinch K 3-Benzoyl-5-chloro-uracil. Acta Cryst. 2009, E65, o342–o342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Nowak I; Robins MJ Addition of Difluorocarbene to 4’,5’-Unsaturated Nucleosides: Synthesis and Deoxygenation Reactions of Difluorospirocyclopropane Nucleosides. J. Org. Chem 2006, 71, 8876–8883. [DOI] [PubMed] [Google Scholar]
- 264.Li J; Tuo H; Xie M; Kang B; Qu G; Guo H Synthesis of Chiral Acyclic Pyrimidine Nucleosides with a Sulfur-Containing Side Chain via Enantioselective Tandem Conjugate Addition/Protonation. Asian J. Org. Chem 2018, 7, 128–132. [Google Scholar]
- 265.Beall HD; Prankerd RJ; Todaro LJ; Sloan KB Structure of 3-Acetyl-5-Fluorouracil (5-FU): Implication for Its Rearrangements During Hydrolysis and Upon Heating. Pharm. Res 1993, 10, 905–912. [DOI] [PubMed] [Google Scholar]
- 266.Kong W; Casimiro M; Fuentes N; Merino E; Nevado C Metal-Free Aryltrifluoromethylation of Activated Alkenes. Angew. Chem. Int. Ed 2013, 52, 13086–13090. [DOI] [PubMed] [Google Scholar]
- 267.Xiao X; Zhou A; Shu C; Pan F; Li T; Ye L Atom-Economic Synthesis of Fully Substituted 2-Aminopyrroles via Gold-Catalyzed Formal [3+2] Cycloaddition Between Ynamides and Isoxazoles. Chem. Asian J 2015, 10, 1854–1858. [DOI] [PubMed] [Google Scholar]
- 268.Chinchilla R; Nájera C; García-Granda S; Menéndez-Velázquez A Synthesis of (R)- and (S)-2,3-Methanovaline from (2S)-N-Benzoyl-2-Tert-Butyl-4-Methylene-1,3-Oxazolidin-5-One. Tetrahedron Lett. 1993, 34, 5799–5802. [Google Scholar]
- 269.Bennet AJ; Somayaji V; Brown RS; Santarsiero BD The Influence of Altered Amidic Resonance on the Infrared and Carbon-13 and Nitrogen-15 NMR Spectroscopic Characteristics and Barriers to Rotation about the N-C(O) Bond in Some Anilides and Toluamides. J. Am. Chem. Soc 1991, 113, 7563–7571. [Google Scholar]
- 270.Li K; Mohlala MS; Segapelo TV; Shumbula PM; Guzei IA; Darkwa J Bis(Pyrazole)- and Bis(Pyrazolyl)-Palladium Complexes as Phenylacetylene Polymerization Catalysts. Polyhedron 2008, 27, 1017–1023. [Google Scholar]
- 271.Zimmermann T; Abram U Ring Transformations of Heterocyclic Compounds. XIX. Spiro[Dihydropyridine-Indolines] Novel Heterocycles with Two Spiro-Condensed N-Containing Subunits Easy Accessible by 1,3-Oxazinium Ring Transformation. J. Heterocycl. Chem 2000, 37, 1241–1245. [Google Scholar]
- 272.Pyne SG; Dikic B; Gordon PA; Skelton BW; White AH Asymmetric Synthesis of Chiral Cyclic Amino Acids by Diels-Alder Reactions of (2S)- and (2R)-4-Methyleneoxazolidin-5-Ones. Aust. J. Chem 1993, 46, 73–93. [Google Scholar]
- 273.Zhao M; Ren Z; Wang Y; Guan Z Pd-Catalyzed Oxidative Coupling of Enamides and Alkynes for Synthesis of Substituted Pyrroles. Org. Lett 2014, 16, 608–611. [DOI] [PubMed] [Google Scholar]
- 274.Majumder M; Buckton G; Rawlinson-Malone R; Williams AC; Spillman MJ; Pidcock E; Shankland K Application of Hydrogen-Bond Propensity Calculations to an Indomethacin–Nicotinamide (1:1) Co-Crystal. CrystEngComm 2013, 15, 4041–4044. [Google Scholar]
- 275.Zeng Z; Jin H; Xie J; Tian B; Rudolph M; Rominger F; Hashmi ASK α-Imino Gold Carbenes from 1,2,4-Oxadiazoles: Atom-Economical Access to Fully Substituted 4-Aminoimidazoles. Org. Lett 2017, 19, 1020–1023. [DOI] [PubMed] [Google Scholar]
- 276.Moriyama K; Ishida K; Togo H Regioselective Csp2–H Dual Functionalization of Indoles Using Hypervalent Iodine(III): Bromo-Amination via 1,3-Migration of Imides on Indolyl(Phenyl)Iodonium Imides. Chem. Commun 2015, 51, 2273–2276. [DOI] [PubMed] [Google Scholar]
- 277.White JM; Skene CE 2-Methyl-1-(2-(pyridin-2-yl)-1H-benzimidazol-1-yl)propan-1-one. Experimental Crystal Structure Determination 2015, DOI: 10.5517/cc1js2n1. [DOI] [Google Scholar]
- 278.Stone EA; Mercado BQ; Miller SJ Structure and Reactivity of Highly Twisted N-Acylimidazoles. Org. Lett 2019, 21, 2346–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Peters K; Peters EM; Feineis E; Christl M Crystal Structure of 6-Diethylamino-1,4-Dihydro-5-Methyl-4-Oxo-3-Phenyl-1-(4-Toluoyl)Pyridazine, C4N2O(CH3)(COC6H4CH3)(N(C2H5)2)(C6H5). Z. Kristallogr. New Cryst. Struct 1998, 213, 769–770. [Google Scholar]
- 280.Wang YT; Shi TQ; Fu J; Zhu HL Discovery of Novel Bacterial Fabh Inhibitors (Pyrazol-Benzimidazole Amide Derivatives): Design, Synthesis, Bioassay, Molecular Docking and Crystal Structure Determination. Eur. J. Med. Chem 2019, 171, 209–220. [DOI] [PubMed] [Google Scholar]
- 281.Kanazawa H; Ichiba M; Shimizu N; Tamura Z; Senga K Further Studies on the Ring Transformation of Pyrimido[5,4-e]-Triazine 4-Oxides to Pyrrolo[3,2-d]Pyrimidines Involving 1,3-Dipolar Cycloaddition Reaction. J. Org. Chem 1985, 50, 2413–2416. [Google Scholar]
- 282.Ito S; Taguchi T; Yamada T; Ubukata T; Yamaguchi Y; Asami M Indolylbenzothiadiazoles with Varying Substituents on the Indole Ring: a Systematic Study on the Self-Recovering Mechanochromic Luminescence. RSC Adv. 2017, 7, 16953–16962. [Google Scholar]
- 283.Bhattacharya A; Chattopadhyay B; Chakraborty S; Roy BN; Singh GP; Godbole HM; Rananaware UB; Mukherjee AK Tris(Hydroxymethyl) Aminomethane Salt of Ramipril: Synthesis, Structural Characterization from X-Ray Powder Diffraction and Stability Studies. J. Pharmaceut. Biomed 2012, 70, 280–287. [DOI] [PubMed] [Google Scholar]
- 284.Scholz M; Blobaum AL; Marnett LJ; Hey-Hawkins E ortho-Carbaborane Derivatives of Indomethacin as Cyclooxygenase (COX)-2 Selective Inhibitors. Bioorg. Med. Chem 2012, 20, 4830–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Li J; Song H; Zhu Y; Yang H Ethyl 1-[5-Amino-1-tert-butyl-3-(methyl-sulfan-yl)-1H-pyrazole-4-carbon-yl]-5-methyl-3-(methyl-sulfan-yl)-1H-pyrazole-4-carboxylate. Acta Cryst. 2006, E62, o1679–o1681. [Google Scholar]
- 286.Li Y; Barløse C; Jørgensen J; Carlsen BD; Jørgensen KA Asymmetric Catalytic Aza-Diels-Alder/Ring-Closing Cascade Reaction Forming Bicyclic Azaheterocycles by Trienamine Catalysis. Chem. Eur. J 2017, 23, 38–41. [DOI] [PubMed] [Google Scholar]
- 287.Neue B; Reiermann R; Gerdes K; Frohlich R; Wibbeling B; Wurthwein E Ring Closure Reactions of 2,6-Diazaheptatrienyl Metal Compounds: Synthesis of 3-Aminoindole Derivatives and 14-Membered Macrocyclic Dimers. J. Org. Chem 2011, 76, 8794–8806. [DOI] [PubMed] [Google Scholar]
- 288.Moriyama K; Hamada T; Ishida K; Togo H 1,3-Iodo-Amination of 2-Methyl Indoles via Csp2–Csp3 Dual Functionalization with Iodine Reagent. Chem. Commun 2018, 54, 4258–4261. [DOI] [PubMed] [Google Scholar]
- 289.Meng G; Szostak R; Szostak M Suzuki–Miyaura Cross-Coupling of N-Acylpyrroles and Pyrazoles: Planar, Electronically Activated Amides in Catalytic N–C Cleavage. Org. Lett 2017, 19, 3596–3599. [DOI] [PubMed] [Google Scholar]
- 290.Buchspies J; Rahman M; Szostak R; Szostak M N-Acylcarbazoles and N-Acylindoles: Electronically Activated Amides for N–C(O) Cross-Coupling by Nlp to Ar Conjugation Switch. Org. Lett 2020, 22, 4703–4709. [DOI] [PubMed] [Google Scholar]
- 291.Cipciani A; Linda P; Savelli G; Bunton CA Acid-Catalyzed Hydrolyses of Acylpyrroles and Acylindoles. Noninvolvement of Protonated Substrates. J. Am. Chem. Soc 1981, 103, 4874–4879. [Google Scholar]
- 292.Linda P; Stener A; Cipiciani A; Savelli G Hydrolysis of Amides. Kinetics and Mechanism of the Basic Hydrolysis of N-Acylpyrroles, N-Acylindoles and N-Acylcarbazoles. J. Heterocycl. Chem 1983, 20, 247–248. [Google Scholar]
- 293.Joule JA; Mills K Heterocyclic Chemistry; Wiley: Chichester, 2010. [Google Scholar]
- 294.Li JJ Heterocyclic Chemistry in Drug Discovery; Wiley: New York, 2013. [Google Scholar]
- 295.Hashimoto T; Miyamoto H; Naganawa Y; Maruoka K Stereoselective Synthesis of α-Alkyl-β-Keto Imides via Asymmetric Redox C-C Bond Formation Between α-Alkyl- α-Diazocarbonyl Compounds and Aldehydes. J. Am. Chem. Soc 2009, 131, 11280–11281. [DOI] [PubMed] [Google Scholar]
- 296.Lim M; Kim H; Ban J; Son J; Lee JK; Min S; Lee SU; Rhee H Palladium-Catalyzed Carbonylative Coupling Reactions of N,N-Bis(Methanesulfonyl)Amides through C-N Bond Cleavage. Eur. J. Org. Chem 2018, 41, 5717–5724. [Google Scholar]
- 297.Blaschette A; Dalluhn J; Fischer A; Jones PG Polysulfonylamines. XLVIII. Structure of N-Trichloroacetyldimesylamine. Z. Kristallogr 1994, 209, 445–447. [Google Scholar]
- 298.Liu C; Shi S; Liu Y; Liu R; Lalancette R; Szostak R; Szostak M The Most Twisted Acyclic Amides: Structures and Reactivity. Org. Lett 2018, 20, 7771–7774. [DOI] [PubMed] [Google Scholar]
- 299.Kunishima M; Kato D; Kimura N; Kitamura M; Yamada K; Hioki K Potent Triazine-Based Dehydrocondensing Reagents Substituted by an Amido Group. Beilstein J. Org. Chem 2016, 12, 1897–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.King JA Jr; Bryant GL Jr Structures of Two N-Acyl Triethyl Ammonium Salts and One Simple Triethyl Ammonium Salt. Acta Cryst. 1991, C47, 2249–2252. [Google Scholar]
- 301.Dudev T; Lim C Ring Strain Energies from ab Initio Calculations. J. Am. Chem. Soc 1998, 120, 4450–4458. [Google Scholar]
- 302.Otani Y; Nagae O; Naruse Y; Inagaki S; Ohno M; Yamaguchi K; Yamamoto G; Uchiyama M; Ohwada T An Evaluation of Amide Group Planarity in 7-Azabicyclo[2.2.1]Heptane Amides. Low Amide Bond Rotation Barrier in Solution. J. Am. Chem. Soc 2003, 125, 15191–15199. [DOI] [PubMed] [Google Scholar]
- 303.Ohwada T; Kojima D; Kiwada T; Futaki S; Sugiura Y; Yamaguchi K; Nishi Y; Kobayashi Y α,α-Disubstituted Amino Acids Bearing a Large Hydrocarbon Ring. Peptide Self-Assembly through Novel Hydrophobic Recognition. Chem. Eur. J 2004, 10, 617–626. [DOI] [PubMed] [Google Scholar]
- 304.Otani Y; Futaki S; Kiwada T; Sugiura Y; Muranaka A; Kobayashi N; Uchiyama M; Yamaguchi K; Ohwada T Oligomers of beta-Amino Acid Bearing Nonplanar Amides form Ordered Structures. Tetrahedron 2006, 62, 11635–11644. [Google Scholar]
- 305.Hosoya M; Otani Y; Kawahata M; Yamaguchi K; Ohwada T Water-Stable Helical Structure of Tertiary Amides of Bicyclic β-Amino Acid Bearing 7-Azabicyclo[2.2.1]Heptane. Full Control of Amide Cis-Trans Equilibrium By Bridgehead Substitution. J. Am. Chem. Soc 2010, 132, 14780–14789. [DOI] [PubMed] [Google Scholar]
- 306.Wang S; Otani Y; Liu X; Kawahata M; Yamaguchi K; Ohwada T Robust trans-Amide Helical Structure of Oligomers of Bicyclic Mimics of β-Proline: Impact of Positional Switching of Bridgehead Substituent on Amide cis-trans Equilibrium. J. Org. Chem 2014, 79, 5287–5300. [DOI] [PubMed] [Google Scholar]
- 307.Wang S; Taniguchi T; Monde K; Kawahata M; Yamaguchi K; Otani Y; Ohwada T Hydrogen Bonding to Carbonyl Oxygen of Nitrogen-Pyramidalized Amide-Detection of Pyramidalization Direction Preference by Vibrational Circular Dichroism Spectroscopy. Chem. Commun 2016, 52, 4018–4021. [DOI] [PubMed] [Google Scholar]
- 308.Otani Y; Watanabe S; Ohwada T; Kitao A Molecular Dynamics Study of Nitrogen-Pyramidalized Bicyclic β-Proline Oligomers: Length-Dependent Convergence to Organized Structure. J. Phys. Chem. B 2017, 121, 100–109. [DOI] [PubMed] [Google Scholar]
- 309.Gutierrez de Velasco DAO; Su A; Zhai L; Kinoshita S; Otani Y; Ohwada T Unexpected Resistance to Base-catalyzed Hydrolysis of Nitrogen Pyramidal Amides Based on the 7-Azabicyclic[2.2.1]heptane Scaffold. Molecules 2018, 23, no. 2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Zhai L; Wang S; Nara M; Takeuchi K; Shimada I; Otani Y; Ohwada T Application of C-Terminal 7-Azabicyclo[2.2.1]Heptane to Stabilize β-Strand-Like Extended Conformation of a Neighboring α-Amino Acid. J. Org. Chem 2018, 83, 13063–13079. [DOI] [PubMed] [Google Scholar]
- 311.Wang S; Otani Y; Zhai L; Su A; Nara M; Kawahata M; Yamaguchi K; Sada A; Ohki R; Ohwada T Overall Shape Constraint of Alternating α/β-Hybrid Peptides Containing Bicyclic β-Proline. Org. Lett 2019, 21, 7813–7817. [DOI] [PubMed] [Google Scholar]
- 312.Otani Y; Liu X; Ohno H; Wang S; Zhai L; Su A; Kawahata M; Yamaguchi K; Ohwada T Amide Nitrogen Pyramidalization Changes Lactam Amide Spinning. Nat. Commun 2019, 10, no. 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Otani Y; Park S; Ohwada T Conformational Preference of Bicyclic β-Amino Acid Dipeptides. Chirality 2020, 32, 790–807. [DOI] [PubMed] [Google Scholar]
- 314.Zhai L; Otani Y; Hori Y; Tomita T; Ohwada T Peptide-based Short Single β-Strand Mimics without Hydrogen Bonding or Aggregation. Chem. Commun 2020, 56, 1573–1576. [DOI] [PubMed] [Google Scholar]
- 315.Szostak R; Szostak M Tröger’s Base Twisted Amides: High Amide Bond Twist and N/-O-Protonation Aptitude. J. Org. Chem 2019, 84, 1510–1516. [DOI] [PubMed] [Google Scholar]
- 316.Georg GI The Organic Chemistry of Beta-Lactams; Wiley-VCH: New York, 1992. [Google Scholar]
- 317.Alcaide B; Almendros P Beta-Lactams as Versatile Synthetic Intermediates for the Preparation of Heterocycles of Biological Interest. Curr. Med. Chem 2004, 11, 1921–1949. [DOI] [PubMed] [Google Scholar]
- 318.Fernandes R; Amador P; Prudêncio C β-Lactams Chemical Structure, Mode of Action and Mechanisms of Resistance. Rev. Med. Microbiol 2013, 24, 7–17. [Google Scholar]
- 319.Fang B; Zheng H; Zhao C; Jing P; Li H; Xie X; She X Synthesis of the Tetracyclic Core (ABCE Rings) of Daphenylline. J. Org. Chem 2012, 77, 8367–8373. [DOI] [PubMed] [Google Scholar]
- 320.Chen Z; Tian J; Chen Z; Tu Y Total Synthesis of (±)-Parvineostemonine. Chem. Asian J 2012, 7, 2199–2202. [DOI] [PubMed] [Google Scholar]
- 321.Low Y; Hong F; Lim K; Thomas NF; Kam T-S. Transformations of the 2,7-Seco Aspidosperma Alkaloid Leuconolam, Structure Revision of Epi-Leuconolam, and Partial Syntheses of Leuconoxine and Leuconodines A and F. J. Nat. Prod 2014, 77, 327–338. [DOI] [PubMed] [Google Scholar]
- 322.Ealick SE; Washecheck DM; Helm DV The Crystal Structures of Two Tetracyclic Spirodilactams Containing Non-Planar Amide Bonds. Acta Cryst. 1976, B32, 895–900. [Google Scholar]
- 323.Zuo Z; Ma D Enantioselective Total Syntheses of Communesins A and B. Angew. Chem. Int. Ed 2011, 50, 12008–12011. [DOI] [PubMed] [Google Scholar]
- 324.Roscini C; Cubbage KL; Berry M; Orr-Ewing AJ; Booker-Milburn KI Reaction Control in Synthetic Organic Photochemistry: Switching Between [5+2] and [2+2] Modes of Cycloaddition. Angew. Chem. Int. Ed 2009, 48, 8716–8720. [DOI] [PubMed] [Google Scholar]
- 325.Lease TG; Shea KJ The Type 2 Intramolecular Imino Diels-Alder Reaction. Synthesis and Structural Characterization of Bicyclo[n.3.1] Bridgehead Olefin/Bridgehead Lactams. J. Am. Chem. Soc 1993, 115, 2248–2260. [Google Scholar]
- 326.Qi J; Zhang FL; Huang YS; Xu AQ; Ren SC; Yi ZY; Wang YF Radical Borylative Cyclization of 1,6-Dienes: Synthesis of Boron-Substituted Six-Membered Heterocycles and Carbocycles. Org. Lett 2018, 20, 2360–2364. [DOI] [PubMed] [Google Scholar]
- 327.Buchanan GL; Kitson DH; Mallinson PR; Sim GA; White DNJ; Cox PJ Conformational Study of the 1-Azabicyclo[3.3.1]Nonan-2-One System. Molecular-Mechanics Calculations and X-Ray Structure of 5-Phenyl-1-Azabicyclo[3.3.1]Nonan-2-One. J. Chem. Soc., Perkin Trans. 2 1983, 9, 1709–1712. [Google Scholar]
- 328.Hassan H; Marsden SP; Nelson A Design and Synthesis of a Fragment Set Based on Twisted Bicyclic Lactams. Bioorg. Med. Chem 2018, 26, 3030–3033. [DOI] [PubMed] [Google Scholar]
- 329.McCabe PH; Milne NJ; Sim GA Conformational Study of Bridgehead Lactams. Preparation and X-Ray Structural Analysis of 1-Azabicyclo[3.3.1]Nonane-2,6-Dione. J. Chem. Soc., Perkin Trans. 2 1989, 10, 1459–1462. [Google Scholar]
- 330.Pritchett BP; Donckele EJ; Stoltz BM Enantioselective Catalysis Coupled with Stereodivergent Cyclization Strategies Enables Rapid Syntheses of (+)-Limaspermidine and (+)-Kopsihainanine A. Angew. Chem. Int. Ed 2017, 56, 12624–12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Pereira R; Pfeifer L; Gouverneur V; Cvengroš J Twisting the Ethano-Tröger’s Base: the Bisamide. Org. Biomol. Chem 2017, 15, 628–633. [DOI] [PubMed] [Google Scholar]
- 332.Satyanarayana G; Helmchen G Enantioselective Syntheses of Bicyclic Lactams Based on Iridium-Catalyzed Asymmetric Allylic Substitution and Heck Cyclization. Eur. J. Org. Chem 2014, 11, 2242–2252. [Google Scholar]
- 333.Shea KJ; Lease TG; Ziller JW Synthesis and X-Ray Crystal Structure of a Highly Strained Anti-Bredt Olefin/Anti-Bredt Lactam. Exo-2-Carbomethoxy-1-Aza-8-Oxobicyclo[3.3.1]Non-4-Ene. J. Am. Chem. Soc 1990, 112, 8627–8629. [Google Scholar]
- 334.Williams RM; Lee BH; Miller MM; Anderson OP Synthesis and X-Ray Crystal Structure Determination of 1,3-Bridged Beta-Lactams: Novel, Anti-Bredt Beta.-Lactams. J. Am. Chem. Soc 1989, 111, 1073–1081. [Google Scholar]
- 335.Somayaji V; Skorey KI; Brown RS; Ball RG Molecular Structure of 3,4,5-Trihydro-2-Oxo-1,5-Ethanobenzazepine and Its Reaction with β-Amino Alcohols as a Model for the Acylation Step of the Serine Proteases. J. Org. Chem 1986, 51, 4866–4872. [Google Scholar]
- 336.Gardarsson H; Schweizer B; Diederich F 2,8-Dibromo-5,11-methanodibenzo[b,f][1,5]diazocine-6,12-dione. Experimental Crystal Structure Determination 2014, DOI: 10.5517/cc13kmy7. [DOI] [Google Scholar]
- 337.Alcaide B; Almendros P; Aragoncillo C β-Lactams: Versatile Building Blocks for the Stereoselective Synthesis of Non-β-Lactam Products. Chem. Rev 2007, 107, 4437–4492. [DOI] [PubMed] [Google Scholar]
- 338.Brandi A; Cicchi S; Cordero FM Novel Syntheses of Azetidines and Azetidinones. Chem. Rev 2008, 108, 3988–4035. [DOI] [PubMed] [Google Scholar]
- 339.Pitts CR; Lectka T Chemical Synthesis of β-Lactams: Asymmetric Catalysis and Other Recent Advances. Chem. Rev 2014, 114, 7930–7953. [DOI] [PubMed] [Google Scholar]
- 340.Urbanczyk-Lipkowska Z (3R,4R,6R,7S)-5-Dethia-4,7-dimethyl-3,4’-(S)-methylmethylenedioxy-5-oxacepham. Experimental Crystal Structure Determination 2008, DOI: 10.5517/cc7jt1l. [DOI] [Google Scholar]
- 341.Nakai H; Takasuka M; Ide Y; Hamada Y; Shiro M Structure of a 7-α-Methoxy-1-oxacephem: Dichloromethane Solvated (−)-(6R,7R)-7-{2-[(Difluoromethyl)thio]acetamido}−3-({[1-(2-hydroxyethyl)-1H-tetrazol-5-yl]thio}methyl)-7-methoxy-8-oxo-5-oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid. Acta Cryst 1987, C43, 2153–2155. [Google Scholar]
- 342.Prout K; Baldwin JE; Adlington RM; Fekner T; Jones TW; Schofield CJ Benzyl (2S,5S,6R)-6-Phthalimidopenicillinate-4,4-dioxide. Experimental Crystal Structure Determination 2000, DOI: 10.5517/cc4ywjg. [DOI] [Google Scholar]
- 343.Urbanczyk-Lipkowska Z; Eda P Structures of Three Derivatives of 6-Phthalimidopenicillanic Acid. Acta Cryst 1992, C48, 2167–2172. [Google Scholar]
- 344.Shi J; Linden A; Heimgartner H Reactions of Acid Chlorides/Ketenes with 2-Substituted 4,5-Dihydro-4,4-Dimethyl-1,3-Thiazoles: Formation of Penam Derivatives. Helv. Chim. Acta 2013, 96, 1462–1481. [Google Scholar]
- 345.Alzari PM; Rivero BE; Punte G; Ronco AE Structure of Pivaloyloxymethyl (3S,5R)-6,6-Dibromopenicillanate 1,1-Dioxide. Acta Cryst. 1986, C42, 1029–1032. [Google Scholar]
- 346.Garud DR; Ando H; Kawai Y; Ishihara H; Koketsu M Synthesis of Novel Selenapenams, Selenacephems, and Selenazepines Using a 2-(Trimethylsilyl) Ethyl Protection Approach. Org. Lett 2007, 9, 4455–4458. [DOI] [PubMed] [Google Scholar]
- 347.Cameron AF; McElhatton J; Campbell MM; Johnson G Methyl 6α-Ethoxyformamido-6β-phenoxyacetamidopenicillanate. Acta Cryst. 1979, B35, 1263–1266. [Google Scholar]
- 348.Simon K; Jászberényi JC; Párkányi L Crystal and Molecular Structure of Methyl 6-Bromo-6-Iodopenicillanate-1-Oxide. J. Mol. Struct 1985, 127, 369–374. [Google Scholar]
- 349.Saouane S; Buth G; Fabbiani FP Crystal Structure and Packing Energy Calculations Of (+)-6-Aminopenicillanic Acid. Acta Cryst. 2013, C69, 1238–1242. [DOI] [PubMed] [Google Scholar]
- 350.Testero SA; O’Daniel PI; Shi Q; Lee M; Hesek D; Ishiwata A; Mobashery S Regiospecific Syntheses of 6α-(1 R-Hydroxyoctyl) Penicillanic Acid and 6β-(1 R-Hydroxyoctyl) Penicillanic Acid as Mechanistic Probes of Class D β-Lactamases. Org. Lett 2009, 11, 2515–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Domiano P; Balsamo A; Giorgi I; Macchia B; Macchia F; Rossello A Crystal and Molecular Structure of 2-β-Acetoxymethyl-3-α-Methoxy-2- α-Methyl-3- β-(p-Nitrobenzoyloxymethyl)-6-β-Phenoxyacetamidopenam. Gazz. Chim. Ital 1987, 117, 155–159. [Google Scholar]
- 352.Fekner T; Baldwin JE; Adlington RM; Jones TW; Prout CK; Schofield CJ Syntheses of (6S)-Cephalosporins from 6-Aminopenicillanic Acid. Tetrahedron 2000, 56, 6053–6074. [Google Scholar]
- 353.Giddings PJ; John DI; Thomas EJ; Williams DJ Preparation of 6α-Monosubstituted and 6,6-Disubstituted Penicillanates from 6-Diazopenicillanates: Reactions of 6-Diazopenicillanates with Alcohols, Thiols, Phenylseleninyl Compounds, and Allylic Sulphides, and Their Analogues. J. Chem. Soc., Perkin Trans. 1 1982, 0, 2757–2766. [Google Scholar]
- 354.Brenner DG; Knowles JR; Rihs G Penicillanic Acid Sulfone: an Unexpected Isotope Effect in the Interaction of 6-α and 6-β-Monodeuterio and of 6,6-Dideuterio Derivatives with RTEM. β-Lactamase from Escherichia Coli. Crystal Structure of Penicillanic Acid Sulfone. Biochemistry 1981, 20, 3680–3687. [DOI] [PubMed] [Google Scholar]
- 355.Baldwin JE; Herchen SR; Clardy JC; Hirotsu K; Chou TS Preparation of 6-β-Imidopenicillinate-1-(S)-oxides. J. Org. Chem 1978, 43, 1342–1346. [Google Scholar]
- 356.Dauter Z; Bogucka-Ledóchowska M; Borowski E; Dreissig W; Barnickel G; Bradaczek H The Structure Of 2,2-Dimethyl-3-Ureido-6-Phenoxyacetamidopenam. Acta Cryst. 1981, B37, 2179–2183. [Google Scholar]
- 357.Domiano P; Nardelli M; Balsamo A; Macchia B; Macchia F Crystal and Molecular Structure of 4-Methoxybenzyl-2α-methyl-2β-[(R)-acetoxy(methoxy)methyl]-6β-phenoxyacetamidopenam-3α-carboxylate. Acta Cryst. 1979, B35, 1363–1372. [Google Scholar]
- 358.Buynak JD; Ghadachanda VR; Vogeti L; Zhang H; Chen H Synthesis and Evaluation of 3-(Carboxymethylidene) and 3-(Carboxymethyl) Penicillinates as Inhibitors of β-Lactamase. J. Org. Chem 2005, 70, 4510–4513. [DOI] [PubMed] [Google Scholar]
- 359.Brown GA; Anderson KM; Murray M; Gallagher T; Hales NJ The Azomethine Ylid Strategy in β-Lactam Synthesis. Application to Selenapenams. Tetrahedron 2000, 56, 5579–5586. [Google Scholar]
- 360.Gibon V; Norberg B; Evrard G; Durant F Structure of the Sodium Salt of Penicillanic Acid. Acta Cryst. 1988, C44, 652–654. [Google Scholar]
- 361.Wendeler M; Fattah J; Twyman JM; Edwards AJ; Dobson CM; Heyes SJ; Prout K Combination of Cp/Mas Nmr Spectroscopy and X-Ray Crystallography: Structure and Dynamics in Molecular Crystals of Hydrogen, Lithium, Sodium, Rubidium, and Cesium Penicillin V. J. Am. Chem. Soc 1997, 119, 9793–9803. [Google Scholar]
- 362.Alzari PM; Punte G; Ronco AE; Rivero BE Structure of Pivaloyloxymethyl (1S,3S,5R)-6,6-Dibromopenicillanate 1-oxide. Acta Cryst. 1986, C42, 1034–1036. [Google Scholar]
- 363.Hou D; Mas JL; Chan TM; Wong YS; Steinman M; McPhail AT Novel, Stereoselective Syntheses of Penem Antibiotics: Efficient, Formal Syntheses of SCH 34343. Bioorg. Med. Chem. Lett 1993, 3, 2171–2176. [Google Scholar]
- 364.Bai GY; Peng HW; Qin XY; Zhang YC; Zeng T (2S,3S,5R)-Diphenylmethyl 4,4-Dioxo-3-(1, 2, 3-triazol-1-ylmethyl)-2-penicillanate. Acta Cryst. 2006, E62, o4997–o4998. [Google Scholar]
- 365.Goeta AE; Boschetti CE; Mascaretti O; Punte G 6α-Chloro-3α-hydroxymethyl-2, 2-dimethylpenam 1,1-dioxide. Acta Cryst. 1998, C54, 242–244. [Google Scholar]
- 366.Wang XZ; Pang LN; Liu JZ; Sun FG; Wang JW Benzyl 6,6-Dibromo-2β-chloromethyl-2α-methylpenicillanate. Acta Cryst. 2007, E63, o1184–o1185. [Google Scholar]
- 367.Blanpain P; Laurent G; Durant F Étude de la Structure Moléculaire de L’oxacilline et de l‚ Acide Penicilloique Correspondant. Bull. Soc. Chim. Belg 1977, 86, 767–775. [Google Scholar]
- 368.Labischinski H; Naumann D; Barnickel G; Dreißig W; Gruszecki W; Hofer A; Bradaczek H Comparison Between the Molecular and Crystal Structures of a Benzylpenicillin Ester and Its Corresponding Sulfoxide with Drastically Reduced Biological Activity. Z. Naturforsch. B Chem. Sci 1987, 42, 367–375. [Google Scholar]
- 369.Luger P; Dittrich B; Koritsanszky T; Paulmann C; Scheins S; Wagner A 3,3-Dimethyl-7-oxo-6-((phenoxyacetyl)amino)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid. Experimental Crystal Structure Determination 2009, DOI: 10.5517/cc7y37h. [DOI] [Google Scholar]
- 370.Burley JC; Streek J; Stephens PW Ampicillin Trihydrate from Synchrotron Powder Diffraction Data. Acta Cryst. 2006, E62, o797–o799. [Google Scholar]
- 371.Alzari PM; Ronco AE; Rivero BE; Punte G Structure of Pivaloyloxymethyl (3S,5R,6S)-6-bromopenicillanate. Acta Cryst. 1986, C42, 1037–1038. [Google Scholar]
- 372.Csöregh I; Palm TB The Crystal and Molecular Structure of Benzylpenicillin 1’-Diethyl Carbonate Ester. Acta Cryst. 1977, B33, 2169–2175. [Google Scholar]
- 373.Rheingold AL (4-Nitrophenyl)methyl-6-(benzyloxy)-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate. Experimental Crystal Structure Determination 2019, DOI: 10.5517/ccdc.csd.cc221rtq. [DOI] [Google Scholar]
- 374.Jenny C; Prewo R; Bieri JH; Heimgartner H Synthese von (Methylthio) Penam-Derivaten durch Keten-Addition an 4,5-Dihydro-5-(Methylthio)-1,3-Thiazole. Helv. Chim. Acta 1986, 69, 1424–1434. [Google Scholar]
- 375.Toomer CA; Schwalbe CH; Ringan NS; Lambert PA; Lowe PR; Lee VJ Structural Studies on Tazobactam. J. Med. Chem 1991, 34, 1944–1947. [DOI] [PubMed] [Google Scholar]
- 376.Gibon V; Szafraniak K; Evrard G; Durant F Molecular Structure of 3-Phenyl-5-Methyl-4-Isoxazolyl-Penicillin Sulfone (Oxacillin Sulfone): C19H19N3O8S. J. Chem. Crystallogr 1987, 17, 751–760. [Google Scholar]
- 377.Punte G; Rivero BE; Alzari PM Structure of Pivaloyloxymethyl (3S,5R)-Penicillanate 1,1-Dioxide. Acta Cryst. 1988, C44, 1327–1328. [Google Scholar]
- 378.Shin W; Cho SW Structure of Penicillin V Benzyl Ester. Acta Cryst. 1992, C48, 1447–1449. [Google Scholar]
- 379.Shi D; Hou C Bis(μ−6-(1-Hydroxyethyl)-7-(oxo)-3-(tetrahydrofuran-2-yl)-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylato)-tetra-aqua-di-sodium Monohydrate. Experimental Crystal Structure Determination 2017, DOI: 10.5517/ccdc.csd.cc131z26. [DOI] [Google Scholar]
- 380.Salomon CJ; Mascaretti OA; Strouse CE; Punte G Stereospecific Synthesis,1H and 13C NMR Spectroscopy, and X-Ray Crystallographic Studies of 6,6-Dibromo-3α-Cyano-2, 2-Dimethylpenam-(1 R)-S-Oxide. Can. J. Chem 1991, 69, 578–583. [Google Scholar]
- 381.Blanpain P; Durant F 3-(2-Chlorophenyl)-5-Methyl-4-Isoxazolyl-Penicillin Sulfoxide (Cloxacillin Sulfoxide) Dioxane Monohydrate, C19H18ClO6N3S. C4H8O2. H2O. Cryst. Struct. Commun 1976, 5, 89–94. [Google Scholar]
- 382.Reed LA III; Charleson DA; Volkmann RA Addition of Penicillin Grignards to Glyoxals: Synthesis of Novel Penam Ketoalcohols. Tetrahedron Lett. 1987, 28, 3431–3434. [Google Scholar]
- 383.Chiaroni A; Riche C; Adonias M; Anaya J; Géro SD; Tachdjian C Two Methyl-Substituted Carbapenem Antibiotic Precursors. Acta Cryst. 1995, C51, 1306–1310. [Google Scholar]
- 384.Dapporto P; Paoli P; Rossi P; Altamura M; Perrotta E X-Ray Structures of Three Penem Antibiotics: Molecular Mechanical and Dynamic Aspects. Struct. Chem 1999, 10, 311–319. [Google Scholar]
- 385.Bai GY; Peng HW; Qin XY; Zhang YC; Zeng T Diphenylmethyl 1-Oxo-1-penicillanate. Acta Cryst. 2006, E62, o4391–o4392. [Google Scholar]
- 386.Duan EH; Zhao DS; Wang J; Li ML 1-Oxo-6-(2-phenylacetylamino)-1-penicillanic Acid. Acta Cryst. 2006, E62, o3249–o3250. [Google Scholar]
- 387.Yoon TS; Shin W Penicillin V Benzhydryl Ester Sulfoxide Monohydrate. Acta Cryst. 1996, C52, 3142–3144. [Google Scholar]
- 388.Tashiro M; Saotome Y Structure of Benzyl 6-Methacryloylaminopenicillanate 1-Oxide. Acta Cryst. 1991, C47, 1338–1340. [Google Scholar]
- 389.Santer G; Ongania KH; Hofer K; Gieren A Tricyclic β-Lactames, VI. Synthesis and Structure of 5,6,6-Trimethyl-2,3-Benzo-4-Thia-1-Aza-Bicyclo [3.2.0] Heptane-7-One, a New Basic Skeleton of β-Lactams. Z. Naturforsch. B Chem. Sci 1988, 43, 758–762. [Google Scholar]
- 390.Santer G; Ongania KH Synthesis of Benzanellated Carbacephames. Monatsh. Chem 1994, 125, 71–78. [Google Scholar]
- 391.Caparo HG; Francotte E; Kohler B; Rihs G; Schneider P; Scartazzinni R; Tosch W Synthesis and Biological Activity of 2-Lactonyl Penems. J. Antibiot 1988, 41, 759–770. [DOI] [PubMed] [Google Scholar]
- 392.Blanpain P; Melebeck M; Durant F (2,6-Dimethoxyphenyl) Penicillin Methyl Ester (Methicillin Methyl Ester). Acta Cryst. 1977, B33, 580–582. [Google Scholar]
- 393.Declercq JP; Piccinnileopardi C; Marchand-Brynaert J X-Ray-Diffraction Analysis of 2-Oxo-Penam Derivatives, Precursors of Penems-6-Beta-Phenylacetamido-2-Oxo-Penam (7) and 2-Oxo-Bisnorpenicillin G-3-(Allyl) Carboxylate (8). New J. Chem 1987, 11, 499–502. [Google Scholar]
- 394.Beels CM; Abu-Rabie MS; Murray-Rust P; Murray-Rust J Chiral Conversion of 6-Aminopenicillanic Acid into an Antibacterial Pen-2-Em-3-Carboxylic Acid Derivative: Absolute Structure from X-Ray Analysis. J. Chem. Soc., Chem. Commun 1979, 15, 665–666. [Google Scholar]
- 395.Brown D; Brown GA; Andrews M; Large JM; Urban D; Butts CP; Gallagher T The Azomethine Ylide Strategy for β-Lactam Synthesis. Azapenams and 1-Azacephams. J. Chem. Soc., Perkin Trans. 1 2002, 17, 2014–2021. [Google Scholar]
- 396.Weishaupt R; Pfaendler HR; Polborn K 4-Nitrobenzyl 3-t-butyl-6-(1-hydroxyethyl)-4,7-dioxo-1-aza-5-thiabicyclo[3.2.0]hept-2-ene-2-carboxylate. Experimental Crystal Structure Determination 2005, DOI: 10.5517/cc8q223. [DOI] [Google Scholar]
- 397.Krajewski JW; Gluziński P; Grochowski E; Pupek K; Mishnyov A; Kemme A Synthesis and X-Ray Structural Investigation Of (5R*,6S*)-1-Benzoyl-5-Methylthio-6-Methoxy-1-Azapenam. J. Mol. Struct 1992, 271, 191–196. [Google Scholar]
- 398.Pfaendler HR; Gosteli J; Woodward RB; Rihs G Structure, Reactivity, and Biological Activity of Strained Bicyclic β-Lactams. J. Am. Chem. Soc 1981, 103, 4526–4531. [Google Scholar]
- 399.Tanaka R; Oyama Y; Ishiguro M Structure of Penem Sulphoxide. J. Chem. Soc., Chem Commun 1990, 12, 853–854. [Google Scholar]
- 400.Tang C; Cai L; Liu S; Zheng Z; Li G; Chen J; Sui Q Crystal Structure of Tebipenem Pivoxil. Acta Cryst. 2018, E74, 1215–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Andrews MD; Brown GA; Charmant JP; Peakman TM; Rebello A; Walsh KE; Ales NJ Aldehydes and Ketones as Dipolarophiles: Application to the Synthesis of Oxapenams. Chem. Commun 1999, 3, 249–250. [Google Scholar]
- 402.Martel SR; Wisedale R; Gallagher T; Hall LD; Mahon MF; Bradbury RH; Hales NJ β-Lactam-Based Azomethine Ylide Reactivity. Expedient Synthesis of Carbapenams and Carbapenems. J. Am. Chem. Soc 1997, 119, 2309–2310. [Google Scholar]
- 403.Seki M; Yamanaka T; Kondo K Practical Synthesis of (R)-4-Mercaptopyrrolidine-2-Thione from L-Aspartic Acid. Preparation of a Novel Orally Active 1-β-Methylcarbapenem, TA-949. J. Org. Chem 2000, 65, 517–522. [DOI] [PubMed] [Google Scholar]
- 404.Coulton S; Gilchrist TL; Graham K Benzocarbapenems from Indoles 1. J. Chem. Soc., Perkin Trans.1 1998, 7, 1193–1202. [Google Scholar]
- 405.Nagao Y; Abe T; Shimizu H; Kumagai T; Inoue Y Asymmetric Total Synthesis of a New Non-Natural 1β-Methoxycarbapenem. J. Chem. Soc., Chem Commun 1989, 13, 821–823. [Google Scholar]
- 406.Furman B; Krajewski P; Urbańczyk-Lipkowska Z; Frelek J; Kałuża Z; Kozerski L; Chmielewski M A Simple Method for the Assignment of the Relative Stereochemistry of 2-Substituted Clavams. J. Chem. Soc., Perkin Trans. 2 1998, 8, 1737–1742. [Google Scholar]
- 407.Yanagi K; Takeuchi Y; Sunagawa M Structure of a Novel Carbapenem Antibiotic, Meropenem. Acta Cryst. 1992, C48, 1737–1739. [Google Scholar]
- 408.Kumagai T; Tamai S; Abe T; Matsunaga H; Hayashi K; Kishi I; Nagao Y New Straightforward Synthesis and Characterization of a Unique 1β-Methylcarbapenem Antibiotic Biapenem Bearing a σ-Symmetric Bicyclotriazoliumthio Group as the Pendant Moiety. J. Org. Chem 1998, 63, 8145–8149. [Google Scholar]
- 409.Brown AG; Corbett DF; Goodacre J; Harbridge JB; Howarth TT; Ponsford RJ; King TJ Clavulanic Acid and Its Derivatives. Structure Elucidation of Clavulanic Acid and the Preparation of Dihydroclavulanic Acid, Isoclavulanic Acid, Esters and Related Oxidation Products. J. Chem. Soc., Perkin Trans. 1 1984, 635–650. [Google Scholar]
- 410.Jiang B; Tian H; Huang ZG; Xu M Successive Copper (I)-Catalyzed Cross-Couplings in One Pot: a Novel and Efficient Starting Point for Synthesis of Carbapenems. Org. Lett 2008, 10, 2737–2740. [DOI] [PubMed] [Google Scholar]
- 411.Branch CL; Pearson MJ Synthesis of Novel Fused β-Lactams by Intramolecular 1, 3-Dipolar Cycloadditions. Part 9. Preparation of the 7-Oxo-1,3-Diazabicyclo [3.2.0]-Heptane-2-Carboxylate and 8-Oxo-1,3-Diazabicyclo [4.2.0] Octane-2-Carboxylate Ring Systems. J. Chem. Soc., Perkin Trans. 1 1986, 1077–1095. [Google Scholar]
- 412.Kobayashi K; Fukuhara H; Kawamoto I; Ito S; Hata T Crystal Structure of a 1 β-Methylcarbapenem Antibiotic, Pivaloyloxymethyl (1R,5S,6S)-6-[(R)-1-Hydroxyethyl]-1-Methyl-2-[[(R)-5-Oxopyrrolidin-3-Yl] Thio]-1-Carbapen-2-Em-3-Carboxylate, CS-834 Dihydrate. Anal. Sci 2001, 17, 357–358. [DOI] [PubMed] [Google Scholar]
- 413.Leban I; Selič L; Mesar T; Čopar A; Šolmajer T Precursor of a β-Lactamase Inhibitor: Allyl (4S,8S,9R)-10-[(E)-Ethylidene]-4-Methoxy-11-Oxo-1-Azatricyclo [7.2.0.0.3.8] Undec-2-Ene-2-Carboxylate. Acta Cryst. 2002, C58, o367–o369. [DOI] [PubMed] [Google Scholar]
- 414.Tsuji N; Nagashima K; Kobayashi M; Shoji J; Kato T; Terui Y; Shiro M Asparenomycins A, B and C, New Carbapenem Antibiotics. J. Antibiot 1982, 35, 24–31. [DOI] [PubMed] [Google Scholar]
- 415.Ahmed FR Structure of a β-Lactam, 2,6a-Di-P-Tolyl-6,6a-Dihydro-3H,5H-Azeto [2, 1-b] Imidazol-5-One, C19H18N2O. Acta Cryst. 1983, C39, 735–737. [Google Scholar]
- 416.Pfaendler HR; Hendel W; Nagel U Stable Oxapenem-3-Carboxylic Acids: a New Class of β-Lactam Antibiotics. Influence of 2- and 6-Alkyl Substituents. Z. Naturforsch. B Chem. Sci 1992, 47, 1037–1050. [Google Scholar]
- 417.Nicolaou KC; Sorensen EJ Classics in Total Synthesis; Wiley-VCH: Weinheim, 1996. [Google Scholar]
- 418.Daly JW; Spande TF; Garraffo HM Alkaloids from Amphibian Skin: a Tabulation of over Eight-Hundred Compounds. J. Nat. Prod 2005, 68, 1556–1575. [DOI] [PubMed] [Google Scholar]
- 419.Michael JP Indolizidine and Quinolizidine Alkaloids. Nat. Prod. Rep 2008, 25, 139–165. [DOI] [PubMed] [Google Scholar]
- 420.Robertson J; Stevens K Pyrrolizidine Alkaloids: Occurrence, Biology, and Chemical Synthesis. Nat. Prod. Rep 2017, 34, 62–89 [DOI] [PubMed] [Google Scholar]
- 421.Ratmanova NK; Andreev IA; Leontiev AV; Momotova D; Novoselov AM; Ivanova OA; Trushkov IV Strategic Approaches to The Synthesis of Pyrrolizidine and Indolizidine Alkaloids. Tetrahedron 2020, 76, no. 131031. [Google Scholar]
- 422.Delépine AS; Tripier R; Bernard H; Le Bris N; Handel H Selective Mono-N-Alkylation of Triethylenetetraamine. A New Versatile Route to Polylinear Aza-Ligands. Tetrahedron Lett. 2009, 50, 2521–2524. [Google Scholar]
- 423.Kanizsai I; Miklós F; Sohár P; Csámpai A; Sillanpää R; Stájer G Preparation and Structure of Pyrrolo [2,1-b] and Isoindolo [1, 2-b][3,1] Epoxyquinazolines. J. Mol. Struct 2007, 831, 37–45. [Google Scholar]
- 424.Kudryavtsev KV; Nukolova NV; Kokoreva OV; Smolin ES Stereoselective Synthesis of Functional Derivatives of 2-(2-Carboxyethyl) Pyrrolidine-2-Carboxylic Acid. Russ. J. Org. Chem 2006, 42, 412–422. [Google Scholar]
- 425.Schaefer W; Friebe WG; Leinert H; Mertens A; Poll T; Von der Saal W; Ziegler ML Non-Nucleoside Inhibitors of HIV-1 Reverse Transcriptase: Molecular Modeling and X-Ray Structure Investigations. J. Med. Chem 1993, 36, 726–732. [DOI] [PubMed] [Google Scholar]
- 426.Schlapferdahler M; Prewo R; Bieri JH; Germain G Heimgartner H Synthesis of an Azacyclol by Trans-Annular Ring Contraction. Chimia 1988, 42, 25–26. [Google Scholar]
- 427.Rheingold AL Methyl 6-t-Butyl-8b-methyl-2,4-dioxotetrahydro-4H,6H-furo[2’,3’:3,4]pyrrolo[1,2-c][1,3]oxazole-8a(8H)-carboxylate. Experimental Crystal Structure Determination 2019, DOI: 10.5517/ccdc.csd.cc21zrwp. [DOI] [Google Scholar]
- 428.Heaviside EA; Moloney MG; Thompson AL Diastereoselective Intramolecular Aldol Ring Closures of Threonine Derivatives Leading to Densely Functionalised Pyroglutamates Related to Oxazolomycin. RSC Adv. 2014, 4, 16233–16249. [Google Scholar]
- 429.Josa-Culleré L; Christensen KE; Moloney MG Diastereoselective Reduction of the Tricarbonyl Moiety in Bicyclic Tetramates Giving Pyroglutamates. Org. Biomol. Chem 2018, 16, 2705–2710. [DOI] [PubMed] [Google Scholar]
- 430.Parsons S; Shannon D; McNab H; Wood PA 9b-Phenyl-3-phenylsulfanyl-2,3-dihydro-9bH-pyrrolo(2,1-a)isoindole-1,5-dione. Experimental Crystal Structure Determination 2004, DOI: 10.5517/cc8766w. [DOI] [Google Scholar]
- 431.Le Goff R; Martel A; Sanselme M; Lawson AM; Daich A; Comesse S Simple Access to Highly Functional Bicyclic Γ- and Δ-Lactams: Origins of Chirality Transfer to Contiguous Tertiary/Quaternary Stereocenters Assessed by DFT. Chem. Eur. J 2015, 21, 2966–2979. [DOI] [PubMed] [Google Scholar]
- 432.Parsons S; Shannon D; McNab H; Wood PA 7a-Methyl-7,7a-dihydro-6H-pyrrolizine-1,5-dione. Experimental Crystal Structure Determination 2004, DOI: 10.5517/cc8769z. [DOI] [Google Scholar]
- 433.Roth GP; Leonard SF; Tong L Complementary Selectivity in the Alkylation of Chiral Bicyclic Lactam Enolates. J. Org. Chem 1996, 61, 5710–5711. [Google Scholar]
- 434.Griesbeck AG; Nerowski F; Lex J Decarboxylative Photocyclization: Synthesis of Benzopyrrolizidines and Macrocyclic Lactones. J. Org. Chem 1999, 64, 5213–5217. [DOI] [PubMed] [Google Scholar]
- 435.Paquette LA; Dura RD; Modolo I Contrasting Responses of Pyrido [2,1-a] Isoindol-6-Ones and Their Sultam Counterparts to Photochemical Activation. J. Org. Chem 2009, 74, 1982–1987. [DOI] [PubMed] [Google Scholar]
- 436.Barra L; Dickschat JS Sceptrin-Enantioselective Synthesis of a Tetrasubstituted All-Trans Cyclobutane Key Intermediate. Eur. J. Org. Chem 2017, 31, 4566–4571. [Google Scholar]
- 437.Cowley AR; Hill TJ; Kocis P; Moloney MG; Stevenson RD; Thompson AL Spirocyclic Systems Derived from Pyroglutamic Acid. Org. Biomol. Chem 2011, 9, 7042–7056. [DOI] [PubMed] [Google Scholar]
- 438.Qin X; Lee MWY; Zhou JS Nickel-Catalyzed Asymmetric Reductive Heck Cyclization of Aryl Halides to Afford Indolines. Angew. Chem. Int. Ed 2017, 56, 12723–12726. [DOI] [PubMed] [Google Scholar]
- 439.Harris L; Gilpin M; Thompson AL; Cowley AR; Moloney MG A Novel Class of Azatricyclononanes: Pentasubstituted Cyclopropanes from an Uncatalysed Reaction. Tetrahedron Asymmetry 2009, 20, 726–729. [Google Scholar]
- 440.Jia MQ; Liu C; You SL Diastereoselective and Enantioselective Desymmetrization of α-Substituted Cyclohexadienones via Intramolecular Stetter Reaction. J. Org. Chem 2012, 77, 10996–11001. [DOI] [PubMed] [Google Scholar]
- 441.Wright SW; Choi C; Chung S; Boscoe BP; Drozda SE; Mousseau JJ; Trzupek JD Reversal of Diastereoselection in the Conjugate Addition of Cuprates to Unsaturated Lactams. Org. Lett 2015, 17, 5204–5207. [DOI] [PubMed] [Google Scholar]
- 442.Petrone DA; Yen A; Zeidan N; Lautens M Dearomative Indole Bisfunctionalization via a Diastereoselective Palladium-Catalyzed Arylcyanation. Org. Lett 2015, 17, 4838–4841. [DOI] [PubMed] [Google Scholar]
- 443.Ealick ST; Van der Helm D The Crystal and Molecular Structure of 5,8-Diaza-4,9-Dioxotricyclo[6.3.0.0.1.5]Undecane, a Non-Planar Tertiary Amide. Acta Cryst. 1975, B31, 2676–2680. [Google Scholar]
- 444.Bláha K; Buděšínský M; Koblicová Z; Maloň P; Tichý M; Baker JR; Van der Helm D Optically Active Tricyclic Dilactams with Non-Planar Cis-Amide Groups: Synthesis, X-Ray, NMR and CD Studies. Collect. Czech. Chem. C 1982, 47, 1000–1019. [Google Scholar]
- 445.Liu RR; Xu TF; Wang YG; Xiang B; Gao JR; Jia YX Palladium-Catalyzed Dearomative Arylalkynylation of Indoles. Chem. Commun 2016, 52, 13664–13667. [DOI] [PubMed] [Google Scholar]
- 446.Huang LH; Xu MY; Li HJ; Li JQ; Chen YX; Ma WZ; Lan WJ Amino Acid-Directed StrategyfFor Inducing the Marine-Derived Fungus Scedosporium Apiospermum F41–1 to Maximize Alkaloid Diversity. Org. Lett 2017, 19, 4888–4891. [DOI] [PubMed] [Google Scholar]
- 447.Cordero FM; Pisaneschi F; Meschini Batista K; Valenza S; Machetti F; Brandi A A New Bicyclic Dipeptide Isostere with Pyrrolizidinone Skeleton. J. Org. Chem 2005, 70, 856–867. [DOI] [PubMed] [Google Scholar]
- 448.Andrews M; Brewster A; Crapnell K; Ibbett A; Moloney M Regioselective Dieckmann Cyclisations Leading to Enantiopure Highly Functionalised Tetramic Acid Derivatives. J. Chem. Soc., Perkin Trans. 1 1998, 2, 223–236. [Google Scholar]
- 449.de Figueiredo RM; Fröhlich R; Christmann M Efficient Synthesis and Resolution of Pyrrolizidines. Angew. Chem. Int. Ed 2007, 46, 2883–2886. [DOI] [PubMed] [Google Scholar]
- 450.Makino K; Shintani K; Yamatake T; Hara O; Hatano K; Hamada Y Stereoselective Synthesis of (S)-(+)-Lycoperdic Acid through an Endo Selective Hydroxylation of the Chiral Bicyclic Lactam Enolate with MoOPH. Tetrahedron 2002, 58, 9737–9740. [Google Scholar]
- 451.Kim JH; Kim I; Song Y; Kim MJ; Kim S Asymmetric Total Synthesis of (+)-Neooxazolomycin Using a Chirality-Transfer Strategy. Angew. Chem. Int. Ed 2019, 131, 11134–11138. [DOI] [PubMed] [Google Scholar]
- 452.Jin S; Guo J; Fang D; Huang Y; Wang Q; Bu Z A Brønsted Acid-Catalyzed Michael Addition/Cyclization Sequence for the Diastereoselective Assembly of Chroman-Bridged Polycyclic Isoindolinones. Adv. Synth. Catal 2019, 361, 456–461. [Google Scholar]
- 453.Yoon UC; Kim DU; Lee CW; Choi YS; Lee YJ; Ammon HL; Mariano PS Novel and Efficient Azomethine Ylide Forming Photoreactions of N-(Silylmethyl) Phthalimides and Related Acid and Alcohol Derivatives. J. Am. Chem. Soc 1995, 117, 2698–2710. [Google Scholar]
- 454.Newman SG; Howell JK; Nicolaus N; Lautens M Palladium-Catalyzed Carbohalogenation: Bromide to Iodide Exchange and Domino Processes. J. Am. Chem. Soc 2011, 133, 14916–14919. [DOI] [PubMed] [Google Scholar]
- 455.Obrech JP; Schönholzer P; Jenny CJ; Prewo R; Heimgartner H The Reaction of 3-(Dimethylamino)-2H-Azirines with 2, 3-Pyridinedicarboximide. Helv. Chim. Acta 1988, 71, 1319–1327. [Google Scholar]
- 456.Basarić N; Horvat M; Franković O; Mlinarić-Majerski K; Neudörfl J; Griesbeck AG Photoinduced Hydrogen Atom Abstraction in N-(Adamantyl) Phthalimides: Structure–Reactivity Study in the Solid State. Tetrahedron, 2009, 65, 1438–1443. [Google Scholar]
- 457.Moloney MG; Yaqoob M Equilibration in Bicyclic Pyroglutamates by Ring Opening-Reclosure. Tetrahedron Lett 2008, 49, 6202–6204. [Google Scholar]
- 458.Ling T; Macherla VR; Manam RR; McArthur KA; Potts BC Enantioselective Total Synthesis of (−)-Salinosporamide A (NPI-0052). Org. Lett 2007, 9, 2289–2292. [DOI] [PubMed] [Google Scholar]
- 459.Gainsford GJ; Luxenburger A; Woolhouse AD (3R,6S,7aS)-3-Phenyl-6-(Phenylsulfanyl) Perhydropyrrolo [1,2-c] Oxazol-5-One. Acta Cryst. 2009, E65, o943–o943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Anwar M; Cowley AR; Moloney MG Novel Chiral Pyrrolidinone Scaffolds Derived from Threonine with Antibacterial Activity. Tetrahedron Asymmetry 2010, 21, 1758–1770. [Google Scholar]
- 461.Zeidan N; Beisel T; Ross R; Lautens M Palladium-Catalyzed Arylation/Heteroarylation of Indoles: Access to 2,3-Functionalized Indolines. Org. Lett 2018, 20, 7332–7335. [DOI] [PubMed] [Google Scholar]
- 462.Salcedo A; Neuville L; Zhu J Palladium-Catalyzed Intramolecular C-Arylation of Benzylic Carbon: Synthesis of 3-Benzoxazolylisoindolinones by a Sequence of Ugi-4CR/Postfunctionalization. J. Org. Chem 2008, 73, 3600–3603. [DOI] [PubMed] [Google Scholar]
- 463.Barth B; Dierich M; Heinisch G; Matuszczak B; Mereiter K; Soder J; Stoiber H Novel Oxazolo [3’,2’:1,2] Pyrrolo [3,4-d] Pyridazines And Imidazolo [1’,2’:1,2] Pyrrolo [3,4-d] Pyridazines: Synthesis and Biological Evaluation. Arch. Pharm 1996, 329, 403–407. [DOI] [PubMed] [Google Scholar]
- 464.Viveros-Ceballos JL; Martínez-Toto EI; Eustaquio-Armenta C; Cativiela C; Ordóñez M First and Highly Stereoselective Synthesis of Both Enantiomers of Octahydroindole-2-Phosphonic Acid (OicP). Eur. J. Org. Chem 2017, 45, 6781–6787. [Google Scholar]
- 465.Meyers AI; Wanner KT Chiral Quaternary Carbon Compounds. II. An Asymmetric Synthesis of (R) or (S)-4, 4-Dialkyl-2-Cyclopentenones. Tetrahedron Lett. 1985, 26, 2047–2050. [Google Scholar]
- 466.Baures PW; Ojala WH; Costain WJ; Ott MC; Pradhan A; Gleason WB; Johnson RL Design, Synthesis, and Dopamine Receptor Modulating Activity of Diketopiperazine Peptidomimetics of L-Prolyl-L-Leucylglycinamide. J. Med. Chem 1997, 40, 3594–3600. [DOI] [PubMed] [Google Scholar]
- 467.Zhang J; Flippen-Anderson JL; Kozikowski AP A Tandem Michael Addition Ring-Closure Route to the Metabotropic Receptor Ligand α-(Hydroxymethyl) Glutamic Acid and Its γ-Alkylated Derivatives. J. Org. Chem 2001, 66, 7555–7559. [DOI] [PubMed] [Google Scholar]
- 468.Ghirardi E; Griera R; Piccichè M; Molins E; Fernández I; Bosch J; Amat M Stereocontrolled Access to Enantiopure 7-Substituted Cis and Trans-Octahydroindoles. Org. Lett 2016, 18, 5836–5839. [DOI] [PubMed] [Google Scholar]
- 469.Mao ZY; Geng H; Zhang TT; Ruan YP; Ye JL; Huang PQ Stereodivergent and Enantioselective Total Syntheses of Isochaetominines A-C and Four Pairs of Isochaetominine C Enantiomers: a Six-Step Approach. Org. Chem. Front 2016, 3, 24–37. [Google Scholar]
- 470.Hashemian S; Notash B 10-Hydroxy-2-azapentacyclo [10.8. 0.02, 10.04, 9.015, 20] icosa-1 (12), 4 (9), 5, 7, 13, 15 (20), 16, 18-octaene-3, 11-dione. Acta Cryst. 2011, E67, o680–o680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Angelov P; Hosamani KM; Jeong YC; Moloney MG; Thompson AL; Yaqoob M Synthesis and Antibacterial Activity of Bicyclic Lactam-Lactones. Synlett 2011, 15, 2181–2184. [Google Scholar]
- 472.Meyers AI; Harre M; Garland R Asymmetric Synthesis of Quaternary Carbon Centers. J. Am. Chem. Soc 1984, 106, 1146–1148. [Google Scholar]
- 473.Hameed A; Blake AJ; Hayes CJ An Enantioselective Formal Synthesis of (+)-Lactacystin from Hydroxymethyl Glutamic Acid (Hmg). Synlett 2010, 04, 535–538. [Google Scholar]
- 474.Jia MQ; You SL Desymmetrization of Cyclohexadienones via D-Camphor-Derived Triazolium Salt Catalyzed Intramolecular Stetter Reaction. Chem. Commun 2012, 48, 6363–6365. [DOI] [PubMed] [Google Scholar]
- 475.Capretz Agy A; Rodrigues MT Jr; Zeoly LA; Simoni DA; Coelho F Palladium-Mediated Oxidative Annulation of δ-Indolyl-α,β-Unsaturated Compounds Toward the Synthesis of Cyclopenta[b]Indoles and Heterogeneous Hydrogenation to Access Fused Indolines. J. Org. Chem 2019, 84, 5564–5581. [DOI] [PubMed] [Google Scholar]
- 476.Jida M; Deprez-Poulain R; Malaquin S; Roussel P; Agbossou-Niedercorn F; Deprez B; Laconde G Solvent-Free Microwave-Assisted Meyers’ Lactamization. Green Chem 2010, 12, 961–964. [Google Scholar]
- 477.Douki K; Ono H; Taniguchi T; Shimokawa J; Kitamura M; Fukuyama T Enantioselective Total Synthesis of (+)-Hinckdentine A via a Catalytic Dearomatization Approach. J. Am. Chem. Soc 2016, 138, 14578–14581. [DOI] [PubMed] [Google Scholar]
- 478.Wagner T; Schönleber A A Non-Mathematical Introduction to the Superspace Description of Modulated Structures. Acta Cryst. 2009, B65, 249–268. [DOI] [PubMed] [Google Scholar]
- 479.Sen S; Potti VR; Surakanti R; Murthy YLN; Pallepogu R Enantioselective Synthesis of Spirooxoindoles via Chiral Auxiliary (Bicyclic Lactam) Controlled SNAr Reactions. Org. Biomol. Chem 2011, 9, 358–360. [DOI] [PubMed] [Google Scholar]
- 480.Bahajaj AA; Vernon JM; Wilson GD Stereoselectivity of Cyclisations via N-Acyliminium Ions to Form Pyrido [2’,3’:3,4] Pyrrolo [2,1-a] Isoindole,-Isoquinoline and- Benz[c]Azepine Ring Systems. J. Chem. Soc., Perkin Trans. 1 2001, 12, 1446–1451. [Google Scholar]
- 481.Köhn U; Schramm A; Kloß F; Görls H; Arnold E; Anders E Synthesis and Characterization of Chiral 1,2-Diamines From 5-Oxo-Pyrrolidine-(S)-2-Carboxylic Acid. Tetrahedron Asymmetry 2007, 18, 1735–1741. [Google Scholar]
- 482.Pereira NA; Monteiro Â; Machado M; Gut J; Molins E; Perry MJ; Santos MM Enantiopure Indolizinoindolones with in Vitro Activity Against Blood- and Liver-Stage Malaria Parasites. ChemMedChem 2015, 10, 2080–2089. [DOI] [PubMed] [Google Scholar]
- 483.Pihlaja K; Sillanpaeae R; Stájer G; Frimpong-Manso S X-Ray Study of a Pentacyclic Partially Saturated Benzothiazolo [2,3-a] Isoindolone. Acta Chem. Scand 1992, 46, 1021–1021. [Google Scholar]
- 484.Chelain E; Parlier A; Audouin M; Rudler H; Daran JC; Vaissermann J Reaction of Aminocarbene Complexes of Chromium with Alkynes. 2. Intramolecular Insertions Leading to Polycyclic Lactams. J. Am. Chem. Soc 1993, 115, 10568–10580. [Google Scholar]
- 485.Horvat M; Görner H; Warzecha KD; Neudörfl J; Griesbeck AG; Mlinaric-Majerski K; Basaric N Photoinitiated Domino Reactions: N-(Adamantyl) Phthalimides and N-(Adamantylalkyl) Phthalimides. J. Org. Chem 2009, 74, 8219–8231. [DOI] [PubMed] [Google Scholar]
- 486.Yang P; You SL Palladium-Catalyzed Asymmetric Intramolecular Dearomative Heck Reaction of Pyrrole Derivatives. Org. Lett 2018, 20, 7684–7688. [DOI] [PubMed] [Google Scholar]
- 487.Bailey JH; Cherry DT; Crapnell KM; Moloney MG; Shim SB; Bamford MJ; Lamont RB Functionalised Pyrrolidinones Derived from (S)-Pyroglutamic Acid by Cycloaddition Reactions. Tetrahedron 1997, 53, 11731–11744. [Google Scholar]
- 488.Katritzky AR; Xu YJ; He HY; Steel PJ Stereoselective Syntheses of 1H-Imidazo [2,1-a] Isoindole-2,5(3H,9bH)-Diones. J. Chem. Soc., Perkin Trans. 1 2001, 15, 1767–1770. [Google Scholar]
- 489.Medimagh R; Marque S; Prim D; Marrot J; Chatti S Concise Synthesis of Tricyclic Isoindolinones via One-Pot Cascade Multicomponent Sequences. Org. Lett 2009, 11, 1817–1820. [DOI] [PubMed] [Google Scholar]
- 490.Chiaroni A; Deyine A; Griffard-Brunet D; Langlois N; Riche C (3aS,3bR,6R,7aR)-2-Benzyl-1,2,3a,3b,4,7a-Hexahydro-6-phenyl-3,5-dioxa-2,6a-Diazacyclopenta[a]Pentalen-7-one. Acta Cryst. 1995, C51, 91–93. [Google Scholar]
- 491.Duncanson P; Cheong YK; Motevalli M; Griffiths DV A Novel Approach to Isoindolo [2,1-a] Indol-6-Ones. Org. Biomol. Chem 2012, 10, 4266–4279. [DOI] [PubMed] [Google Scholar]
- 492.Cottrell IF; Davis PJ; Moloney MG Stereoselective Oxygenation of Bicyclic Lactams. Tetrahedron Asymmetry 2004, 15, 1239–1242. [Google Scholar]
- 493.Jauk B; Belaj F; Kappe CO Synthesis and Reactions of Biginelli-Compounds. Part 14. A Rhodium-Induced Cyclization–Cycloaddition Sequence for the Construction of Conformationally Rigid Calcium Channel Modulators of the Dihydropyrimidine Type. J. Chem. Soc., Perkin Trans. 1 1998, 3, 307–314. [Google Scholar]
- 494.Nishimura T; Noishiki A; Ebe Y; Hayashi T Hydroxorhodium/Chiral Diene Complexes as Effective Catalysts for the Asymmetric Arylation of 3-Aryl-3-Hydroxyisoindolin-1-Ones. Angew. Chem. Int. Ed 2013, 52, 1777–1780. [DOI] [PubMed] [Google Scholar]
- 495.Oliveira FL; Freire KRL; Aparicio R; Coelho F (1S,2E,6R,7aR)-1,6-Dihydroxy-2-(4-Nitrobenzylidene)-2,3,5,6,7,7a-Hexahydro-1H-Pyrrolizin-3-one. Acta Cryst. 2012, E68, o1570–o1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Gentry PR; Kokubo M; Bridges TM; Kett NR; Harp JM; Cho HP; Daniels JS Discovery of the First M5-Selective and CNS Penetrant Negative Allosteric Modulator (NAM) of a Muscarinic Acetylcholine Receptor: (S)-9b-(4-Chlorophenyl)-1-(3,4-Difluorobenzoyl)-2,3-Dihydro-1H-Imidazo [2,1-a] Isoindol-5(9bH)-One (ML375). J. Med. Chem 2013, 56, 9351–9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Chiaroni A (2R,5R)-5-Methoxy-7-(methoxymethylene)-8-oxo-2-phenyl-1-aza-3-oxabicyclo(3.3.0)octane. Experimental Crystal Structure Determination 2004, DOI: 10.5517/cc7zdvf. [DOI] [Google Scholar]
- 498.He Y; Liu Z; Wu D; Li Z; Robeyns K; Van Meervelt L; Van der Eycken EV Modular Access to Diverse Bridged Indole Alkaloid Mimics via a Gold-Triggered Cascade Dearomative Spirocarbocyclization/[4+ 2] Cycloaddition Sequence. Org. Lett 2019, 21, 4469–4474. [DOI] [PubMed] [Google Scholar]
- 499.Melo TMP; Santos CI; Gonsalves AMAR; Paixão JA; Beja AM; Silva MR Synthesis of Novel Tricyclic Isoindole Derivatives. Tetrahedron Lett. 2003, 44, 8285–8287. [Google Scholar]
- 500.Lee KL; Ambler CM; Anderson DR; Boscoe BP; Bree AG; Brodfuehrer JI; Chang JS; Choi C; Chung S; Curran KJ et al. Discovery Of Clinical Candidate 1-{[(2S,3S,4S)-3-Ethyl-4-Fluoro-5-Oxopyrrolidin-2-yl]Methoxy}−7-Methoxyisoquinoline-6-Carboxamide (PF-06650833), a Potent, Selective Inhibitor of Interleukin-1 Receptor Associated Kinase 4 (IRAK4), by Fragment-Based Drug Design. J. Med. Chem 2017, 60, 5521–5542. [DOI] [PubMed] [Google Scholar]
- 501.Andrews MD; Brewster AG; Chuhan J; Ibbett AJ; Moloney MG; Prout K; Watkin D A Short Synthesis of an Enantiopure Benzo[e]Isoindolinone. Synthesis 1997, 3, 305–308. [Google Scholar]
- 502.Allin SM; Northfield CJ; Page MI; Slawin AM A Highly Diastereoselective Synthesis of Tricyclic Lactams and Their Application as Novel N-Acyl Iminium Ion Precursors in the Synthesis of Isoindolinone Derivatives. Tetrahedron Lett. 1997, 38, 3627–3630. [Google Scholar]
- 503.Nelson J; Twamley B; Natale NR Ethyl 5-(5-oxo-2,3-dihydro-5H-oxazolo [2,3-a] isoindol-9b-yl-methyl)-4-(2,5,5-trimethyl-1,3-dioxan-2-yl) Isoxazole-3-Carboxylate: the Product of a Novel Synthetic Method. Acta Cryst. 2004, E60, o2255–o2257. [Google Scholar]
- 504.Deprez-Poulain R; Willand N; Boutillon C; Nowogrocki G; Azaroual N; Deprez B A Simple Reaction to Produce Small Structurally Complex and Diverse Molecules. Tetrahedron Lett. 2004, 45, 5287–5290. [Google Scholar]
- 505.Cheng Z; Lou L; Liu D; Li X; Proksch P; Yin S; Lin W Versiquinazolines A–K, Fumiquinazoline-Type Alkaloids from the Gorgonian-Derived Fungus Aspergillus Versicolor LZD-14–1. J. Nat. Prod 2016, 79, 2941–2952. [DOI] [PubMed] [Google Scholar]
- 506.Zhuang Y; Teng X; Wang Y; Liu P; Li G; Zhu W New Quinazolinone Alkaloids Within Rare Amino Acid Residue from Coral-Associated Fungus, Aspergillus Versicolor LCJ-5–4. Org. Lett 2011, 13, 1130–1133. [DOI] [PubMed] [Google Scholar]
- 507.Mao MK; Webber RK Reaction of Amino Acids with O-Acetylbenzoic Acid. J. Chem. Soc., Chem. Commun 1990, 9, 679–680. [Google Scholar]
- 508.Lam JK; Schmidt Y; Vanderwal CD Complex Polycyclic Scaffolds by Metathesis Rearrangement of Himbert Arene/Allene Cycloadducts. Org. Lett 2012, 14, 5566–5569. [DOI] [PubMed] [Google Scholar]
- 509.Jing K; Wang XN; Wang GW Diastereoselective Synthesis of Oxazoloisoindolinones via Cascade Pd-Catalyzed ortho-Acylation of N-Benzoyl α-Amino Acid Derivatives and Subsequent Double Intramolecular Cyclizations. J. Org. Chem 2018, 84, 161–172. [DOI] [PubMed] [Google Scholar]
- 510.Josa-Culleré L; Towers C; Thompson AL; Moloney MG Chemoselective Formation and Reaction of Densely Functionalised Bicyclic Tetramic Acids and Their Biological Activity. Eur. J. Org. Chem 2017, 47, 7055–7059. [Google Scholar]
- 511.Matthews CJ; Moloney MG; Thompson AL; Winiarska H; Winney HT Access to the Bicyclic Core of Isatisine, and an Investigation of Its Antibacterial Activity. Synlett 2011, 3, 378–382. [Google Scholar]
- 512.Nair DS; Pauranik V; Shah AC Synthesis of Phenyl and Substituted Phenyl 3-Ethyl-2,3,5,9b-Tetrahydro[1,3]Oxazolo [2,3-a]Isoindol-5-ones. J. Chem. Res 2003, 12, 772–774. [Google Scholar]
- 513.Herdeis C; Hubmann HP; Lotter H Chiral Pool Synthesis of Trans-(2S,3S)-3-Hydroxyproline and Castanodiol from S-Pyroglutamic Acid. Tetrahedron Asymmetry 1994, 5, 119–128. [Google Scholar]
- 514.Stájer G; Sillanpaa R; Pihlaja K X-Ray Structure Determination of a Saturated Methylene-Bridged Diphenylimidazo 2,1-Aisoindolone. Acta Chem. Scand 1994, 48, 603–605. [Google Scholar]
- 515.Petter RC; Banerjee S; Englard S Inhibition of Gamma-Butyrobetaine Hydroxylase by Cyclopropyl-Substituted Gamma-Butyrobetaines. J. Org. Chem 1990, 55, 3088–3097. [Google Scholar]
- 516.Coyle JD; Smart LE; Challiner JF; Haws EJ Photocyclization of N-(Dialkylaminoalkyl) Aromatic 1,2-Dicarboximides. X-Ray Molecular Structure of a Stereoisomer of 4-Benzyl-2-Hydroxy-3-Phenyl-4,6-Diazatricyclo [6.4.0.0] Dodeca-1 (12), 8, 10-Trien-7-One. J. Chem. Soc., Perkin Trans. 1 1985, 121–129. [Google Scholar]
- 517.Calmes M; Juan E; Rolland M; Martinez J Influence of the Base Stoichiometry on Cyclocondensation of N-(2-Bromoethyl) Phthalimide with Lithium Ester Enolates. J. Heterocycl. Chem 2002, 39, 849–852. [Google Scholar]
- 518.Vargas A; Orea ML; Gnecco D; Aparicio DM; Juárez JR; Terán JL Diastereospecific Intramolecular Cyclopropanation of Enantiopure 8-Bromo-3-Phenylhexahydrooxazolo[3,2-a]pyridine-5-ones. Heterocycles 2018, 96, 152–157. [Google Scholar]
- 519.Łyżwa D; Dudzinski K; Kwiatkowski P High-Pressure Accelerated Asymmetric Organocatalytic Friedel-Crafts Alkylation of Indoles With Enones: Application to Quaternary Stereogenic Centers Construction. Org. Lett 2012, 14, 1540–1543. [DOI] [PubMed] [Google Scholar]
- 520.Ling T; Potts BC; Macherla VR Concise Formal Synthesis of (−)-Salinosporamide A (Marizomib) Using a Regio- and Stereoselective Epoxidation and Reductive Oxirane Ring-Opening Strategy. J. Org. Chem 2010, 75, 3882–3885. [DOI] [PubMed] [Google Scholar]
- 521.Jiang LJ; Lan HQ; Zheng JF; Ye JL; Huang PQ A Flexible Approach to Methyl (5S)-5-Alkyltetramate Derivatives. Synlett 2009, 2, 297–301. [Google Scholar]
- 522.Allin SM; Northfield CJ; Page MI; Slawin AM Approaches to the Synthesis of Non-Racemic 3-Substituted Isoindolinone Derivatives. J Chem. Soc., Perkin Trans. 1 2000, 11, 1715–1721. [Google Scholar]
- 523.Hirayama N; Fujii I; Kobayashi Y Molecular Structures of Two Indole Alkaloids, Evodiamine and Rutecarpine, from Evodia Fruit. Z. Kristallogr. Cryst. Mater 2000, 215, 762–765. [Google Scholar]
- 524.Kim J; Ashenhurst JA; Movassaghi M Total Synthesis of (+)-11,11’-Dideoxyverticillin A. Science 2009, 324, 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Moody CJ; Slawin AM; Willows D Dirhodium(II) Tetraacetate Catalysed Reactions of Diazo Thioamides: Isolation and Cycloaddition of Anhydro-4-Hydroxy-1,3-Thiazolium Hydroxides (Thioisomünchnones), an Approach to Analogues of Dehydrogliotoxin. Org. Biomol. Chem 2003, 1, 2716–2722. [DOI] [PubMed] [Google Scholar]
- 526.Noordik JH; Beurskens PT; Ottenheijm HCJ; Herscheid JDM; Tijhuis MW 9,9a-Dihydro-1,2,9,9-Tetramethyl-2,9a-Epitho-3,10-Diketopiperazino[1,2-a] Indole, C15H16N2O2S, Absolute Configuration. Cryst. Struct. Comm 1978, 7, 669–670. [Google Scholar]
- 527.Hudnall TW; Bielawski CW An N, N’-Diamidocarbene: Studies in C-H Insertion, Reversible Carbonylation, and Transition-Metal Coordination Chemistry. J. Am. Chem. Soc 2009, 131, 16039–16041. [DOI] [PubMed] [Google Scholar]
- 528.McCarty ZR; Lastovickova DN; Bielawski CW A Cyclic (Alkyl)(Amido) Carbene: Synthesis, Study and Utility as a Desulfurization Reagent. Chem. Commun 2016, 52, 5447–5450. [DOI] [PubMed] [Google Scholar]
- 529.Bonderoff SA; Padwa A Rh(II)-Catalyzed Reactions of Differentially Substituted Bis (Diazo) Functionalities. Org. Lett 2013, 15, 4114–4117. [DOI] [PubMed] [Google Scholar]
- 530.Bakulina O; Ivanov A; Suslonov V; Dar’in D; Krasavin M A Speedy Route to Sterically Encumbered, Benzene-Fused Derivatives of Privileged, Naturally Occurring Hexahydropyrrolo[1,2-b]Isoquinoline. Beilstein J. Org. Chem 2017, 13, 1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Hervé G; Bernard H; Toupet L; Handel H Condensation of Glyoxal with Triethylenetetraamine; Isomerization and Cyclization. Eur. J. Org. Chem 2000, 1, 33–35. [Google Scholar]
- 532.Bunsupa S; Yamazaki M; Saito K Quinolizidine Alkaloid Biosynthesis: Recent Advances and Future Prospects. Front. Plant. Sci 2012, 3, no. 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Michael JP Simple Indolizidine and Quinolizidine Alkaloids. Alkaloids Chem. Biol 2016, 75, 1–498. [DOI] [PubMed] [Google Scholar]
- 534.Borthwick AD 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev 2012, 112, 3641–3716. [DOI] [PubMed] [Google Scholar]
- 535.Yoshimura A; Koski SR; Fuchs JM; Saito A; Nemykin VN; Zhdankin VV Saccharin-Based μ-Oxo Imidoiodane: a Readily Available and Highly Reactive Reagent for Electrophilic Amination. Chem. Eur. J 2015, 21, 5328–5331. [DOI] [PubMed] [Google Scholar]
- 536.Journot G; Neier R; Stoeckli-Evans H 4-Methoxybenzoyl-meso-octamethylcalix[2]pyrrolidino[2]pyrrole: an Acyl Chloride Derivative of a Partially Reduced Calix[4]pyrrole. Acta Cryst. 2012, E68, o929–o930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Casabona D; Jiménez AI; Cativiela C A New Constrained Proline Analogue with an 8-Azabicyclo[3.2.1]Octane Skeleton. Tetrahedron 2007, 63, 5056–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Raikar SN; Malinakova HC Divergent Reaction Pathways of Homologous and Isosteric Propargyl Amides in Sequential Ru/Pd-Catalyzed Annulations for the Synthesis of Heterocycles. J. Org. Chem 2013, 78, 3832–3846. [DOI] [PubMed] [Google Scholar]
- 539.Pyne SG; Dikic B; Gordon PA; Skelton BW; White AH Highly Exo-Diastereoselective Diels–Alder Reactions of (2S)-N-Benzoyl-2-tert-Butyl-4-Methylene-1,3-Oxazolidin-5-One. J. Chem. Soc., Chem. Commun 1991, 21, 1505–1506. [Google Scholar]
- 540.Mykhailiuk PK; Kubyshkin V; Bach T; Budisa N Peptidyl-Prolyl Model Study: How Does the Electronic Effect Influence the Amide Bond Conformation? J. Org. Chem 2017, 82, 8831–8841. [DOI] [PubMed] [Google Scholar]
- 541.Avenoza A; Busto JH; Peregrina JM; Rodríguez F Incorporation of Ahc into Model Dipeptides as an Inducer of a β-Turn with a Distorted Amide Bond. Conformational Analysis. J. Org. Chem 2002, 67, 4241–4249. [DOI] [PubMed] [Google Scholar]
- 542.Gil AM; Buñuel E; Cativiela C A New Approach to Enantiopure β-Endo-Substituted Azabicyclic Proline Analogues by Base Induced Epimerization of a Formyl Derivative. Arkivoc 2007, 4, 157–169. [Google Scholar]
- 543.Öztürk S; Akkurt M; Tepe E; Heinemann FW; Altundas A; Kara Y 1-(12-Benzoyl-10-oxa-12-aza-tetra-cyclo-[6.3.1.02,7.09,11]-dodeca-2,4,6-trien-1-yl)-ethan-1-one. Acta Cryst. 2003, E59, o635–o637. [Google Scholar]
- 544.Gil AM; Buñuel E; Jiménez AI; Cativiela C Stabilisation of the Type I β-Turn Conformation by a Bicyclic Analogue of Proline. Tetrahedron Lett. 2003, 44, 5999–6002. [Google Scholar]
- 545.Gil AM; Buñuel E; Díaz-de-Villegas MD; Cativiela C Olefination of Methyl (1S,2R,4R)-N-Benzoyl-2-Formyl-7-Azabicyclo[2.2.1]Heptane-1-Carboxylate, a Synthetic Approach to New Conformationally Constrained Prolines. Tetrahedron Asymmetry 2003, 14, 1479–1488. [Google Scholar]
- 546.Avenoza A; Busto JH; Cativiela C; Peregrine JM Synthesis of 7-Azabicyclo[2.2.1]Heptane Derivatives via Bridgehead Radicals. Tetrahedron 2002, 58, 1193–1197. [Google Scholar]
- 547.Gil AM; Orús E; López-Carrillo V; Buñuel E; Cativiela C New Enantiopure 7-Azanorbornane β-Substituted Prolines by SN2 Displacements at the Cγ of the Side Chain. Tetrahedron Asymmetry 2005, 16, 3115–3123. [Google Scholar]
- 548.Oppolzer W; Poli G; Starkemann C; Bernardinelli G Stable and Reactive Conformations of N-Enoyl-Bornane-10,2-Sultams in the Absence of Lewis Acids: Asymmetric 1,4-Hydride Additions. Tetrahedron Lett. 1988, 29, 3559–3562. [Google Scholar]
- 549.Lumbierres M; Marchi C; Moreno-Manas M; Sebastian RM; Vallribera A; Lago E; Molins E The Contribution Made by Triphenylphosphane in the Putative Catalysis by Ruthenium Species in Conjugate Additions of β-Dicarbonyl Compounds. Eur. J. Org. Chem 2001, 2001, 2321–2328. [Google Scholar]
- 550.Hashimoto T; Nakatsu H; Watanabe S; Maruoka K Stereoselective Synthesis of Trisubstituted Aziridines with N-α-Diazoacyl Camphorsultam. Org. Lett 2010, 12, 1668–1671. [DOI] [PubMed] [Google Scholar]
- 551.Toyota S; Akinaga T; Kojima H; Aki M; Oki M Absolute Conformation and Substituent Effects on Chiroptical Properties of 9-(2-Halo-1,1-dimethylethyl)-11,12-bis(methoxycarbonyl)-9,10-dihydro-9,10-ethenoanthracenes. J. Am. Chem. Soc 1996, 118, 11460–11466. [Google Scholar]
- 552.Josien H; Chassaing G Asymmetric Synthesis of the Diastereoisomers of L-1-Indanylglycine and L-1-Benz[f]Indanylglycine, χ1,χ2-Constrained Side-Chain Derivatives of L-Phenylalanine and L-2-Naphthylalanine. Tetrahedron Asymmetry 1992, 3, 1351–1354. [Google Scholar]
- 553.Shinji T; Tsutomu M; Yasutaka M; Tamami M; Michinori O Absolute Conformation and Chiroptical Properties. III. Optically Active Methyl sc-3-Methyl-3-(Substituted 9-Triptycyl)Butanoate Rotamers. Bull. Chem. Soc. Jpn 1994, 67, 1680–1693. [Google Scholar]
- 554.Koszewska K; Piątek A; Chapuis C; Jurczak J X-Ray Structure Analyses of Syn/Anti-Conformers of N-Furfuroyl-, N-Benzoyl-, and N-Picolinoyl-Substituted (2R)-Bornane-10,2-Sultam Derivatives. Helv. Chim. Acta 2008, 91, 1409–1418. [Google Scholar]
- 555.Nobuyuki K; Tetsutaro H; Ayanobu T; Yoshikazu O; Sotaro M Ester-Mediated Nucleophilic Aromatic Substitution of 2,3-Alkylidenedioxybenzoic Esters by Aryl Lithium Reagents. Chem. Lett 1997, 26, 641–642. [Google Scholar]
- 556.Harada N; Soutome T; Nehira T; Uda H; Oi S; Okamura A; Miyano S Revision of the Absolute Configurations of [8]-Paracyclophane-10-Carboxylic and 15-Methyl[10]Paracyclophane-12-Carboxylic Acids. J. Am. Chem. Soc 1993, 115, 7547–7548. [Google Scholar]
- 557.Ma M; Peng L; Li C; Zhang X; Wang J Highly Stereoselective [2,3]-Sigmatropic Rearrangement of Sulfur Ylide Generated Through Cu(I) Carbene And Sulfides. J. Am. Chem. Soc 2005, 127, 15016–15017. [DOI] [PubMed] [Google Scholar]
- 558.Piatek AM; Sadowska A; Chapuis C; Jurczak J Diastereoselective Alkyl Grignard 1,4-Additions To para-Substituted (2R)-N-Cinnamoylbornane-10,2-Sultam Derivatives: Influence of N-Atom Pyramidalization. Helv. Chim. Acta 2011, 94, 2141–2167. [Google Scholar]
- 559.Yamaguchi M; Okubo H; Hirama M Synthesis of Optically Active Macrocycles Consisting of Helical Chiral Unit 1,12-Dimethylbenzo[c]Phenanthrene-5,8-Dicarboxylate as a Novel Chiral Building Block. Chem. Commun 1996, 15, 1771–1772. [Google Scholar]
- 560.Hiroki S; Daisuke S; Harunori O; Kazuhiro T; Yusuke T; Ryo A; Masahiko Y Synthesis and Structure of Optically Active 1,12-Diethyl- and 1,12-Diisopropylbenzo[c]Phenanthrenes: an Isopropyl Group Can Be Smaller than a Methyl Group. Chem. Lett 2007, 36, 72–73. [Google Scholar]
- 561.Amat M; Coll MD; lior N; Escolano C; Molins E; Miravitlles C; Bosch J Asymmetric Synthesis of Tetracyclic Substructures of Strychnos Indole Alkaloids. Tetrahedron Asymmetry 2003, 14, 1691–1699. [Google Scholar]
- 562.Aller E; Brown DS; Cox GG; Miller DJ; Moody CJ Diastereoselectivity in the O-H Insertion Reactions of Rhodium Carbenoids Derived from Phenyldiazoacetates of Chiral Alcohols. Preparation of Alpha-Hydroxy and Alpha-Alkoxy Esters. J. Org. Chem 1995, 60, 4449–4460. [Google Scholar]
- 563.Snyder SE; Huang BS; Chu YW; Lin HS; Carey JR The Effects of Substituents on the Geometry of π–π Interactions. Chem. Eur. J 2012, 18, 12663–12671. [DOI] [PubMed] [Google Scholar]
- 564.Adachi S; Kumagai N; Shibasaki M Pyramidalization/Twisting of the Amide Functional Group via Remote Steric Congestion Triggered by Metal Coordination. Chem. Sci 2017, 8, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Takezawa H; Shitozawa K; Fujita M Enhanced Reactivity of Twisted Amides Inside a Molecular Cage. Nat. Chem 2020, 12, 574–578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


