Abstract
Conventional enrichment of microorganisms on branched nonylphenol (NP) as only carbon and energy source yielded mixed cultures able to grow on the organic compound. However, plating yielded no single colonies capable, alone or in combination with other isolates, of degrading the NP in liquid culture. Therefore, a special approach was used, referred to as “serial dilution-plate resuspension,” to reduce culture complexity. In this way, one isolate, TTNP3, tentatively identified as a Sphingomonas sp., was found to be able to grow on NP in liquid culture. Remarkably, this isolate was able to be filtered through a 0.45-μm-pore-diameter filter. Moreover, isolate TTNP3 did not form visible colonies on mineral medium with NP, and it formed visible colonies on R2A agar only after a prolonged incubation of 1 week. High-performance liquid chromatography and gas chromatography-mass spectroscopy analysis of the culture media indicated that the strain starts the degradation of NP with a fission of the phenol ring and preferably uses the para isomer of NP and not the ortho isomer. No distinct accumulation of an intermediary product could be observed.
The presence of nonylphenol (NP) in the aquatic environment is strongly related to the input of NP polyethoxylates (NPnEOs; with n designating the number of ethylene oxide units) through discharge of industrial effluents and sewage treatment plants. NPnEOs are an important group of nonionic surfactants that have been popular for their effectiveness, economy, and ease of handling and formulation for more than 40 years. They are used as detergents, emulsifiers, and wetting and dispersing agents and in the formulation of herbicides, spermicides, and cosmetics (9, 32, 35, 41). NPnEOs account for 80% of the total volume of alkylphenol polyethoxylates, with a worldwide production of about 600,000 metric tonnes a year (7, 23). During the last decade, NP has gained a lot of interest, since it has been designated as a member of the endocrine disrupters, more specifically, pseudoestrogens, which are suggested to be related to the observed decline of human and wildlife reproductive health (11, 14, 16, 24, 26, 29, 35, 39).
The biodegradability of these branched NPnEOs has been studied in activated sludge in laboratory-scale and full-scale situations. It has already been confirmed that primary degradation of NPnEOs proceeded easily and rapidly in laboratory-scale activated sludge units through shortening of the ethoxy chain, leaving NP, NP1EO, NP2EO, and their carboxylates as intermediates (19, 27). Surveys of full-scale biological wastewater treatment plants showed that NP occurs quite frequently as a stable intermediate in effluents and activated sludges in a concentration range of 2.2 to 330 μg of NP/liter and 1 to 7.2 g of NP/kg of dry matter, respectively (1, 4, 5, 13, 34). Analogous observations have been made for surface waters and their sediments (2, 10, 25).
The reports mentioned above demonstrate that primary degradation (i.e., shortening of the ethoxy chain) of NPnEOs is likely to occur in surface waters and activated sludge units. Further evidence was provided by the isolation of bacterial cultures able to grow solely on NPnEOs. Frassinetti et al. (12) isolated three different Gram-negative bacteria that can individually attack NPnEOs in axenic cultures effecting primary degradation. Pseudomonas sp. strains identified by Maki et al. (21) and John and White (17) were unable to mineralize NPnEO (average n = 9.5 ethoxy units) but were able to degrade its ethoxylated chain exclusively. The resulting dominant intermediate was an NP ethoxylate with 2 ethoxy units.
One step further is the establishment of a complete degradation of NPnEOs. It has been suggested by van Ginkel (38) that for a complete mineralization of compounds with surface active features (i.e., surfactants), mixed cultures of microorganisms are needed. The fact that in a surfactant molecule a hydrophilic moiety and hydrophobic moiety are joined can give rise to the fact that the microbial attack by a single strain often leads to the excretion of hydrophobic intermediate metabolites.
Relying on the reports mentioned above, the presence of a microbial consortium seems to be necessary to obtain a complete mineralization of surfactants. The latter is supported by Jiménez et al. (15), who reported the necessity of a four-member aerobic bacterial consortium to obtain significant mineralization of linear alkylbenzene sulfonates. The four components of the consortium had to be present together to result in a complete mineralization of both the alkyl chain and the benzene ring.
Taking into account the amphiphilic nature and the branched alkyl chain of NP as the metabolic intermediate of the NPnEOs, it can be assumed that a bacterial consortium is required to mineralize NP. A recent study showed that NP can be degraded in laboratory-scale activated sludge units, provided the operating temperature is high enough (i.e., above 15°C) (33). There is one report on the biodegradation of NP by a Candida maltosa isolate later designated as Candida aquaetextoris sp. nov. (6, 36), but the NP used as the sole energy and carbon source was synthesized with a linear alkyl chain. The latter is an important feature, because all commercial mixtures of NP ethoxylates contain isomers of NP having a branched nonyl chain. It is known that a branched alkyl chain does not facilitate microbial degradation (18, 20). A branched alkyl group, especially quaternary carbon (tert-butyl) structure at the end of the alkyl group, inhibits biological attack and biotransformation of the alkyl group (31). Transformation of compounds with quaternary carbons is said to be possible; however, only a few biotransformation pathways have been documented (3).
In this paper, the isolation and characterization of a bacterial isolate capable of utilizing branched NP as the sole carbon and energy source are presented. To the best of our knowledge, this is the first report describing an axenic bacterial culture attacking branched NP.
MATERIALS AND METHODS
Sample collection.
Enrichment cultures were started with activated sludge samples obtained from laboratory-scale semicontinuous activated sludge units which had been fed with NP (∼0.5% based on chemical oxygen demand of influent) for a period of approximately 4 months (33).
Media.
Minimum mineral salts medium (MMO) was prepared in sterile MilliQ water (Millipore, Molsheim, France) according to the method of Stanier et al. (30). The MMO as such was used for the enrichment cultures after addition of NP (technical gradient; Aldrich Chemical Company, Inc., Deisenhofen, Germany) by using a sterile Pasteur pipette. For the preparation of plates with NP as the sole carbon source, the following procedure was used: MilliQ water and 20 g of Agar Noble (Difco Laboratories, Detroit, Mich.) per liter were autoclaved; after autoclavation, the mineral salts solutions were added through 0.22-μm-pore-diameter sterile filters (Millipore). Approximately 1.5 g of NP per liter was added with a sterile Pasteur pipette. Shaking of the mixture resulted in an emulsion of NP in the aqueous liquid agar (solubility of NP, ∼5 mg/liter). The emulsion was used to pour the plates. All chemicals used to prepare the MMO were of analytical grade and were purchased from Merck (Darmstadt, Germany) or Union Carbide (Vilvoorde, Belgium). R2A medium (Difco Laboratories, Detroit, Mich.) was used as a rich medium. All plates and liquid cultures were incubated at 28 ± 2°C. Liquid cultures were incubated on a shaker (40 to 50 rpm) in the dark. Luria-Bertani (LB) medium was composed of 10 g of tryptone, 5 g of yeast extract, and 10 g of sodium chloride per liter (Oxoid Ltd., Basingstoke, England; and VEL, Haasrode, Belgium) and used to culture the bacterial isolates, before they were stored at −70°C.
Enrichment.
To a sterile test tube, 5 ml of MMO, 100 μl of activated sludge (see above), and ∼15 mg of NP were added. From the moment that a dense culture was visible (optical density at 550 nm [OD550] of 1.0 to 1.4), 100 μl of the culture was transferred to newly prepared MMO-NP medium. Later, the enrichment cultures were scaled up to sterile Erlenmeyer flasks (250 ml) containing 100 ml of MMO and ∼300 mg of NP by transfer of 1 ml of inoculum. After eight transfers, the culture obtained was designated as enrichment culture I.
Serial dilutions of the enrichment culture.
A serial dilution technique according to the method of Maltseva and Oriel (22) was used to reduce the number of different phenotypes in the enrichment culture. For this, a 10-fold dilution series of enrichment culture I was made (sterile physiological solution; 0.85% NaCl in MilliQ water), and 100 μl of each dilution was surface plated on NP agar. Plates were incubated for 15 to 20 days, and then resuspended with 3 ml of sterile physiological solution, of which 1 ml was used to inoculate 100 ml of MMO containing NP. The liquid culture from the highest dilution giving growth was transferred and designated as enrichment culture II. This entire procedure, which is called “serial dilution-plate resuspension,” was sequentially repeated to further reduce culture complexity.
Identification of bacterial strains.
The isolated bacterial strains were tentatively identified by BCCM (Belgian Co-ordinated Collections of Micro-organisms, Ghent, Belgium) by fatty acid methyl ester analysis, the Biolog breathprint, and 16S ribosomal DNA (rDNA) sequence analysis.
Growth on NP-related compounds.
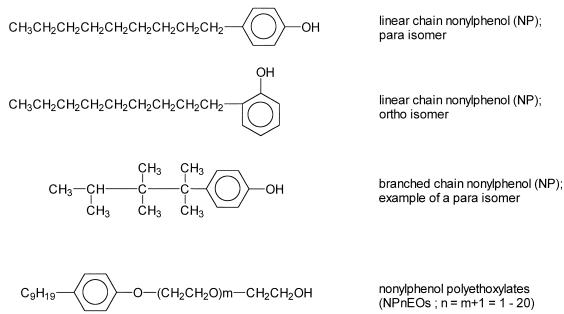
To evaluate their catabolic performance, the isolated strains were incubated in MMO administered with alkylphenolic compounds as the sole carbon source. The organic compounds were administered at concentrations of 100 to 200 mg/liter. NP2EO, NP12EO, octylphenol, octylphenol polyethoxylates (Triton X-100), and phenol were all of technical gradient quality and were purchased from Aldrich Chemical Company, Inc. The chemical structures of some alkylphenolic compounds are depicted in Fig. 1.
FIG. 1.
Chemical structures in relation to NP.
Determination of degradation kinetics.
The determination of the oxygen consumption of incubated cultures with NP as the sole carbon source was performed by using the Sapromat respirometer (Voith, Heidenheim, Germany). In this device, cultivation occurs in vessels stirred and kept at the required temperature and fitted with a carbon dioxide absorber (soda lime pellets; Merck 6839.1000) and a gas-proof connection with a pressure switch and an electrochemical oxygen supply. The carbon dioxide formed is absorbed, and the resulting underpressure is compensated for by electrochemical oxygen production of the same volume. The cumulative oxygen consumption and production are recorded as oxygen consumption curves by a computer. Measurements of NP concentrations at the beginning and the end of the incubation of 250-ml (MMO-NP) cultures of the different bacterial isolates were performed.
To monitor NP disappearance in and growth of the NP-degrading culture, the following experiment was designed. Twenty-four Erlenmeyer flasks (250 ml) containing 100 ml of MMO and ∼67 mg of NP were simultaneously inoculated with 1 ml of a dense culture of TTNP3 (12 days of incubation in NP medium at 28°C; OD550 of 0.56). Every 2 days, three flasks were sacrificed. Two of them were sampled (2 ml) for growth determination, and the remaining content of the flask was used (including the plastic tips used to take the 2-ml sample) for extraction and NP analysis. The content of the third flask was filter sterilized (0.22-μm pore diameter; Millipore), and the filtrate was used for further analysis of intermediate products.
Growth determination.
Determination of the biomass of bacterial cultures was impaired by various factors. Application of a protein assay (Bio-Rad Detergent Compatible Protein Assay; Bio-Rad Laboratories, Eke, Belgium) was not feasible, because the bacterial cells could not be lysed by bead beating lysis with glass beads 0.10 to 0.11 mm in diameter (B. Braun Biotech International, Meisungen, Germany) and addition of sodium dodecyl sulfate (SDS) (BDH Laboratory Supplies, Poole, England) (37). Determination of biomass content by means of dry weight was not reliable, because NP was also partly retained on the filters and thus influenced the dry weight measurements. Assessment of biomass growth by plate counts (CFU per milliliter) was impaired by floc formation in the bacterial cultures. The best available method to monitor the bacterial growth was the measurement of the OD550.
NP analysis.
NP was extracted from samples by an exhaustive steam distillation-extraction technique with n-hexane as the extraction solvent. The steam distillation method had a method detection limit of ∼1 μg of NP per liter. The relative standard deviation on duplicate analysis was smaller than 20%, and the percent recovery on laboratory spike samples was always >80%. The amount of NP present in the collected extracts was determined by high-performance liquid chromatography (HPLC) using an Adsorbosphere XL Silica 90A 5U column (250 by 4.6 mm) from Alltech (Laarne, Belgium). Isocratic elution was performed with a 98/2 n-hexane–ethanol mixture as the mobile phase (liquid chromatography, Union Carbide Belgium; absolute grade, Merck). Calibration of the HPLC was performed with primary standards in the range of 1 to 1,000 μg of NP per ml in hexane. UV detection was performed at 230 and 277 nm; for the fluorescence detector, the excitation and emission wavelengths were set at 230 and 295 nm, respectively. An extensive description of the methodology used for NP analysis is given by Tanghe et al. (33). The extracts were also analyzed with gas chromatography-mass spectroscopy (GC-MS) technology to distinguish between the different NP isomers (different branched nonyl chains) present. A Varian STAR 3400 capillary gas chromatograph (Varian Associates, Walnut Creek, Calif.) equipped with a J & W Scientific (Folsom, Calif.) (30 m by 0.25 mm) fused silica capillary column (DB-5MS) was directly coupled to an ion-trap detector. Helium N60 was used as a carrier gas with a linear flow velocity of 30 cm/s. Injections were performed with a Varian split-splitless capillary injector equipped with a straight tubular glass insert. A volume of 1 μl was injected in the injector in the split-splitless mode. The splitter was opened 30 s after injections in a split ratio of 40:1. A good separation was obtained with the following GC program. The initial temperature of 100°C was maintained for 4 min. The temperature was increased with a gradient of 8°C/min up to 300°C. This maximal temperature was maintained for 1 min. A Varian Saturn type II mass spectrometer was used with a manifold temperature kept at 220°C. The filament current was 40 μA. The temperatures of the injector and transfer line were kept at 270 and 285°C, respectively. This GC-MS method was only used for quantitative purposes.
Extraction and determination of degradation products.
Cultures of TTNP3 were filtered over 0.22-μm-pore-size filters (Millipore). To 6 ml of filtrate, 1 ml of 1/1 sulfuric acid-water (95 to 96%; VEL), ∼0.8 g of NaCl (VEL), and 2 ml of diethyl ether (VEL) were added. This mixture was vortexed for 15 min and centrifuged for 5 min at 3,000 × g. About 1.5 ml of the diethyl ether layer was transferred to a test tube and dried with ∼0.5 g of anhydrous sodium sulfate (VEL). This diethyl ether extract was used for injection in the GC-MS. The following GC program was used. The initial temperature of 50°C was increased with a gradient of 6°C/min up to 240°C. The other GC-MS conditions were as described above.
RESULTS
Enrichment of NP-degrading culture—conventional approach.
An enrichment in MMO-NP medium starting from activated sludge from laboratory-scale reactors was carried out. Control tubes containing only MMO medium and NP did not show any bacterial growth, excluding the presence of NP-degrading bacteria in the nonsterile NP used. There were five transfers to liquid medium (100 μl to 5 ml of MMO-NP) with a time interval of 8 to 12 days. Dense cultures developed after 6 to 7 days (OD550 of 1.0 to 1.4). Plate counts on R2A medium performed after the third and fifth transfers showed cell densities of about 108 CFU/ml. The culture was scaled up to Erlenmeyer flasks (250 ml) containing 100 ml of liquid NP medium. The culture was transferred three times with a time interval of 15 days. Cell densities of about 109 CFU/ml were measured (plate counts on R2A medium, 6 days of incubation). On the latter plates, four colony types with different morphologies could be distinguished. The subculturing did not alter the diversity of the culture. It was designated as enrichment culture I and was used for further purification and isolation of NP-degrading bacterial strains.
The four different colonies were picked up and transferred to test tubes with NP medium in all possible combinations of one, two, three, and four colonies (i.e., 15 tubes in total). Even after an extra transfer and incubation of 15 days, no significant growth occurred in any of the tubes (<103 CFU/ml; R2A medium). These results suggested the presence of nonplatable components in a bacterial consortium necessary to degrade NP. The number of bacterial components was thus expected to be larger than four.
Purification of NP-degrading enrichment cultures.
To reveal unknown culture members, the serial dilution-plating resuspension approach was used. This sequential procedure was applied to enrichment culture I, yielding enrichment culture II and resulting in enrichment culture III. Enrichment culture III was serially diluted and plated on R2A, MMO-NP, and MMO, respectively. The latter was done to verify whether the bacterial colonies growing on the plates were using NP as carbon source and not some impurities present in the Agar Noble. No colonies appeared on the MMO plates, while on both the R2A and MMO-NP plates, two colonies with different morphologies were visible (17 days of incubation). According to these platings, a decrease in the bacterial morphotypes from four down to two was observed.
Repeated incubation of the two easily distinguishable colony types (picked from the MMO-NP plates) in liquid MMO-NP medium, individually and combined, sometimes resulted in growth, while the plate resuspension technique with the same plates always rendered growth. This suggested the presence on the plates of very tiny bacterial colonies, invisible to the eye, which were apparently necessary to induce growth in medium with NP as the sole carbon source. Prolonged incubation of the R2A plates (>20 days) revealed the presence of a third strain with a different morphotype. Apparently the latter is a slow-growing bacterium resulting in very small colonies on rich medium. No such colonies were observed on the MMO-NP plates, on which only two kinds of colonies were visible. (Resuspension of the plates in question resulted in growth in MMO-NP medium.)
Identification and degradative capacities of the isolated bacteria.
After purification on R2A plates of the three different strains isolated, they were cultured in LB medium and stored in glycerol (∼22%) at −70°C. The three strains were designated TTNP1, TTNP2, and TTNP3. The three isolates were identified by fatty acid analysis and Biolog breathprints. TTNP1 and TTNP2 were determined to be a Pseudomonas putida strain and an Alcaligenes sp. (probably A. piechaudii) strain, respectively. The results of the 16S rDNA sequencing indicate that TTNP3 represents a new, not previously described genomic species of the genus Sphingomonas. The morphological and biochemical characteristics of the three strains are shown in Table 1. A remarkable feature of isolate TTNP3 was that it was able to pass through a 0.45-μm-pore-size filter. Also, when a mixed culture was filtered, TTNP1 and TTNP2 were retained on the filter, while TTNP3 could pass through and be cultured again in liquid MMO-NP medium. All three strains were incubated in liquid MMO-NP medium (100 ml; ∼3 g of NP/liter) in various combinations to check for growth on NP (three subsequent transfers to fresh MMO-NP medium). Growth occurred in the mixed cultures in which TTNP3 was present, as well as in the axenic culture with TTNP3 alone. After 5 to 7 days of incubation, all cultures turned from white into a pink color.
TABLE 1.
Morphological and biochemical characteristics of the bacterial isolates TTNP1, TTNP2, and TTNP3a
| Parameter | Characteristic of
|
||
|---|---|---|---|
| TTNP1 | TTNP2 | TTNP3 | |
| Colony morphology | Smooth | Smooth | Smooth |
| Colony color | Cream | Cream | Yellowish |
| Gram reaction | Negative | Negative | Negative |
| Morphology | Rod (1 by 2–4 μm), single or in pairs, motile | Rod (0.8 by 2–4 μm); single or in pairs, motile | Rod (0.8 by 2–3 μm); single or in pairs, motile |
| Growth on NP-related compound | |||
| NP | − | − | + |
| NP2EO | + | − | + |
| NP12EO | − | − | − |
| OPb | +/− | + | + |
| OPnEOsc (Triton X-100) | − | − | − |
| Phenol | + | + | + |
The substrates were used at a concentration of 100 to 200 mg/liter.
OP, octylphenol.
OPnEOs, octylphenol polyethoxylates.
Degradation kinetics and intermediate metabolites.
To evaluate the degradation kinetics of the different cultures, they were incubated in the Sapromat system to record the oxygen consumption curve at 28°C. Axenic culture TTNP3 took off somewhat faster than the mixed cultures. This difference is also expressed by the rate constants of the fitted curves’ exponential rise to maximum, which was largest for the TTNP3 culture. All cultures reached about the same level of cumulative oxygen consumption at the end of incubation. The stoichiometry of NP conversion for all cultures was in the range of 6.2 to 7.4 mol of O2/mol of NP. The amounts of NP added and remaining after incubation, as well as the oxygen consumption registered by the Sapromat system, for each culture are listed in Table 2.
TABLE 2.
NP concentration and kinetics of oxygen demand before and after 13 days of incubation in the Sapromat system
| Culture | NP concn (mg/liter) at:
|
% NP removed | Cumulative oxygen consumption curve-fitting valuesa
|
||
|---|---|---|---|---|---|
| 0 h | 312 h | A (mg of O2/liter) | B (1/t) | ||
| TTNP3 | 388.4 | 6.3 | 98 | 347 | 0.017 |
| TTNP1 + TTNP3 | 349.6 | 6.7 | 98 | 366 | 0.007 |
| TTNP2 + TTNP3 | 352.8 | 4.8 | 99 | 393 | 0.011 |
| TTNP1 + TTNP2 + TTNP3 | 368.0 | 8.8 | 98 | 348 | 0.009 |
Values represent curve fitting (exponential rise to maximum) of the oxygen consumption curves of the Sapromat system as determined by the equation OC = A (1 − e−Bt), where OC is cumulative oxygen consumption and t is time in hours at the rate constant B. For all curves r2 > 0.99.
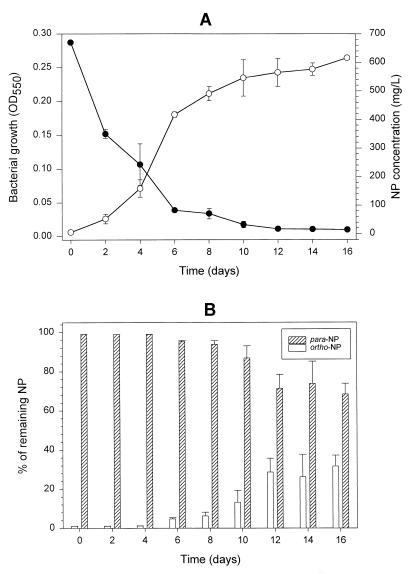
The growth curve measured by turbidity (OD550) and NP disappearance for the TTNP3 culture (separate experiment) are shown in Fig. 2. The disappearance coincided with growth. The ratio of para-NP over ortho-NP of the remaining NP decreased from about 99/1 to 68/32 during the incubation period of 16 days (Fig. 2).
FIG. 2.
Utilization of a commercial mixture of NP as a growth substrate for TTNP3 (Sphingomonas sp. strain). (A) Growth was assessed by OD550. NP concentrations were determined by HPLC analysis of steam distillation extracts of the entire culture medium. (B) Change in the percentage of ortho-NP and para-NP of the remaining NP in the culture media during the incubation of TTNP3. Values represent the mean ± standard deviation (n = 2); in some cases, the standard deviations were too small to illustrate.
Analysis of the diethyl ether extracts of the TTNP3 culture media by GC-MS showed the presence of a variety of branched-chain alcohols, ethers, and esters (C5 to C10). Full-scan GC-MS and computer-aided single-ion analysis were performed to check the presence of alkyl-substituted phenolic compounds. No alkylated aromatic rings (m/z, 107) could be detected, except for the peaks of NP. There was no distinct evolution in the amount of these compounds (surface area of peaks) over the course of incubation of the TTNP3 culture. Apparently, no accumulation of distinct degradation products occurred.
The extraction procedure was evaluated by extraction of water samples spiked with free fatty acids (acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, caproic acid, and isocaproic acid [VEL]) and alcohols (n-butanol, n-pentanol, and n-octanol; Sigma-Aldrich S.A., Bornem, Belgium). The levels of recovery for both types of compounds were satisfactory, from which we can conclude that, if present in the culture medium, similar compounds can be extracted by this method.
DISCUSSION
By using the conventional transfer technique, an enrichment culture of bacteria able to grow on NP as the sole carbon source was quite easily obtained. Reduction of the culture complexity by the serial dilution-plate resuspension technique (22) and further purification resulted in the isolation of three bacterial strains. Characterization of the three strains showed that TTNP3 was the only strain which could initiate growth on NP. These results suggest that, when the enrichment culture was grown on NP plates, TTNP3 happened to be nearby or underneath the main colonies of TTNP1 or TTNP2. The latter might consume some metabolites excreted by TTNP3, which induces primary degradation of NP. In liquid NP medium, an analogous situation occurred. It was assumed that the other two strains could bring about a more complete degradation of NP, since the omission of TTNP3 from the consortium resulted in a culture unable to grow on NP as the only carbon source. However, the oxygen consumption curves of the different cultures support the concept that the two strains TTNP1 and TTNP2 only grow on intermediate metabolites excreted by strain TTNP3, which initiates degradation of NP, but cannot accomplish a more complete degradation compared with that of strain TTNP3.
The fact that all strains, especially TTNP3, can grow on phenol as the sole carbon source may suggest that the degradation of NP starts at the phenolic moiety of the molecule. Because nonylbenzene, a structurally related compound, is attacked via ω- or β-oxidation of the side chain (28), it has been suggested by van Ginkel and Kroon (38) that the degradation of NP probably proceeds through the breakdown of the alkyl chain. However, ω- or β-oxidation of the alkyl chain only occurs when it is not highly branched (25) or when dealing with a linear alkyl chain (6). Because commercially available NPnEOs have highly branched nonyl chains which prevent the β-oxidation, the primary degradation of these surfactants is assumed to start at the ethoxy chain. Nevertheless, characterization of intermediates from biotransformation of branched NPnEOs has shown that compounds having both side chains (alkyl and ethoxy chains) oxidized can occur, but were presumably generated from less extensively branched isomers (8).
According to the GC-MS and HPLC analyses, the cultures did not have any preference for any of the nonyl chain isomers of NP present in the commercial mixture. On the other hand, the data suggest that the ortho isomers of NP, of which about 1 to 2% was present in the technical mixture of NP used, were less degraded by the enriched culture. Of the ortho isomers, only 30 to 60% was removed (depending on strains and experiment used), while over 98% of the para isomer disappeared. Steric hindrance of the enzymatic system could be at the basis of the recalcitrance of the ortho isomer. Moreover, next to NP, there were no compounds with an aromatic moiety present in the extracts. This indicates that the degradation of NP by bacterial strain TTNP3 starts with a fission of the phenol ring leaving intermediates of branched alkyl chains with different lengths.
The search for and identification of metabolites are impaired by the presence of a mixture of NP isomers (different branched alkyl chains) at the onset. Each isomer can give rise to a series of metabolites. Further fractionation of the extracts and the application of nuclear magnetic resonance and GC-MS techniques are needed to identify the chemical structures of all metabolites and to further elucidate the degradation.
ACKNOWLEDGMENTS
This research was funded by a doctoral fellowship of the Flemish Institute for the Promotion of Scientific-Technological Research in the Industry (IWT) and a doctoral fellowship of the Special Research Fund (BOF) of the University of Ghent, Ghent, Belgium.
We are indebted to Olga Maltseva for sharing her experience with the isolation techniques used.
REFERENCES
- 1.Ahel M, Giger W, Koch M. Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment. I. Occurrence and transformation in sewage treatment. Water Res. 1994;28:1131–1142. [Google Scholar]
- 2.Ahel M, Giger W, Schaffner C. Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment. II. Occurrence and transformation in rivers. Water Res. 1994;28:1143–1152. [Google Scholar]
- 3.Ball H A, Reinhard M, McCarty P L. Biotransformation of halogenated and nonhalogenated octylphenol polyethoxylate residues under aerobic and anaerobic conditions. Environ Sci Technol. 1989;23:951–961. [Google Scholar]
- 4.Blackburn M A, Waldock M J. Concentrations of alkylphenols in rivers and estuaries in England and Wales. Water Res. 1995;29:1623–1629. [Google Scholar]
- 5.Brunner P H, Capri S, Marcomini A, Giger W. Occurrence and behaviour of linear alkylbenzenesulphonates, nonylphenol, nonylphenol mono- and nonylphenol diethoxylates in sewage and sewage treatment. Water Res. 1988;22:1465–1472. [Google Scholar]
- 6.Corti A, Frassinetti S, Vallini G, D’Antone S, Fichi C, Solaro R. Biodegradation of nonionic surfactants. I. Biotransformation of 4-(1-nonyl)phenol by a Candida maltosa isolate. Environ Pollut. 1995;90:83–87. doi: 10.1016/0269-7491(94)00080-w. [DOI] [PubMed] [Google Scholar]
- 7.Cox C. Masculinity at risk, pesticides and male fertility. J Pestic Reform. 1996;16:2–7. [Google Scholar]
- 8.Di Corcia A, Costantino A, Marinoni E, Sampero R. Characterization of recalcitrant intermediates from biotransformation of the branched alkyl side chain of nonylphenol ethoxylate surfactants. Environ Sci Technol. 1998;32:2401–2409. [Google Scholar]
- 9.Dunmire E N, Katz D F. Alteration of human sperm kinematics in cervical mucus due to nonoxynol-9. Contraception. 1997;55:209–217. doi: 10.1016/s0010-7824(97)00009-7. [DOI] [PubMed] [Google Scholar]
- 10.Espadaler I, Caixach J, Om J, Ventura F, Cortina M, Pauné F, Rivera J. Identification of organic pollutants in Ter river and its system of reservoirs supplying water to Barcelona (Catalonia, Spain): a study by GC/MS and FAB/MS. Water Res. 1997;31:1996–2004. [Google Scholar]
- 11.Facemire C F, Gross T S, Guillette L J., Jr Reproductive impairment in the Florida panther: nature or nurture? Environ Health Perspect. 1995;103:79–86. doi: 10.1289/ehp.103-1519283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frassinetti S, Isoppo A, Corti A, Vallini G. Bacterial attack of non-ionic aromatic surfactants: comparison of degradative capabilities of new isolates from nonylphenol polyethoxylate polluted wastewaters. Environ Technol. 1996;17:199–205. [Google Scholar]
- 13.Giger W, Brunner P H, Schaffner C. 4-Nonylphenol in sewage sludge: accumulation of toxic metabolites from nonionic surfactants. Science. 1984;225:623–625. doi: 10.1126/science.6740328. [DOI] [PubMed] [Google Scholar]
- 14.Gray M A, Metcalfe C D. Induction of testis-ova in Japanese medaka (Oryzias latipes) exposed to p-nonylphenol. Environ Toxicol Chem. 1997;16:1082–1086. [Google Scholar]
- 15.Jiménez L, Breen A, Thomas N, Federle T W, Sayler G S. Mineralization of linear alkylbenzene sulfonate by a four-member aerobic bacterial consortium. Appl Environ Microbiol. 1991;57:1566–1569. doi: 10.1128/aem.57.5.1566-1569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jobling S, Sumpter J P. Detergent components in sewage effluents are weakly oestrogenic to fish: an in vitro study using rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat Toxicol. 1993;27:361–372. [Google Scholar]
- 17.John D M, White G F. Mechanism for biotransformation of nonylphenol polyethoxylates to xenoestrogens in Pseudomonas putida. J Bacteriol. 1998;180:4332–4338. doi: 10.1128/jb.180.17.4332-4338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolbener P, Baumann U, Leisinger T, Cook A M. Linear alkylbenzenesulfonate (LAS) surfactants in a simple test to detect refractory organic carbon (ROC): attribution of recalcitrants to impurities in LAS. Environ Toxicol Chem. 1995;14:571–577. [Google Scholar]
- 19.Kravetz L, Chung H, Guin K F, Shebs W T, Smith L S. Ultimate biodegradation of an alcohol ethoxylate and a nonylphenol ethoxylate. Household Personal Prod Ind. 1982;March:48–72. , April:62–70. [Google Scholar]
- 20.Madsen T, Petersen G, Seiero C, Torslov J. Biodegradability and aquatic toxicity of glycoside surfactants and a nonionic alcohol ethoxylate. J Am Oil Chem Soc. 1996;73:929–933. [Google Scholar]
- 21.Maki H, Masuda N, Fujiwara Y, Ike M, Fujita M. Degradation of alkylphenol ethoxylates by Pseudomonas sp. strain TR01. Appl Environ Microbiol. 1994;60:2265–2271. doi: 10.1128/aem.60.7.2265-2271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maltseva O, Oriel P. Monitoring of an alkaline 2,4,6-trichlorophenol-degrading enrichment culture by DNA fingerprinting methods and isolation of the responsible organism, haloalkaliphilic Nocardioides sp. strain M6. Appl Environ Microbiol. 1997;63:4145–4149. doi: 10.1128/aem.63.11.4145-4149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naylor C G. Environmental fate and safety of nonylphenol ethoxylates. Text Chem Color. 1995;27:29–33. [Google Scholar]
- 24.Nimrod A C, Benson W H. Environmental estrogenic effects of alkylphenol ethoxylates. Crit Rev Toxicol. 1996;26:335–364. doi: 10.3109/10408449609012527. [DOI] [PubMed] [Google Scholar]
- 25.Osburn Q W, Benedict J H. Polyethoxylated alkyl phenols: relationship of structure to biodegradation mechanism. J Am Oil Chem Soc. 1966;43:141–146. [Google Scholar]
- 26.Routledge E J, Sumpter J P. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ Toxicol Chem. 1996;15:241–248. [Google Scholar]
- 27.Rudling L, Solyom P. The investigation of biodegradability of branched nonyl phenol ethoxylates. Water Res. 1974;8:115–119. [Google Scholar]
- 28.Sariaslani F S, Harper D B, Higgins I J. Microbial degradation of hydrocarbons. Catabolism of 1-phenylalkanes by Nocardia salmonicolor. Biochem J. 1974;140:31–45. doi: 10.1042/bj1400031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharpe R M, Skakkebaek N E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- 30.Stanier R Y, Palleroni J J, Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 31.Swisher R D. Surfactant biodegradation. New York, N.Y: Marcel Dekker, Inc.; 1987. [Google Scholar]
- 32.Talmage S S. Environmental and human safety of major surfactants. 2. Nonionic surfactants, alcohol ethoxylates and alkylphenol ethoxylates, a report to The Soap and Detergent Association, New York. Boca Raton, Fla: Lewis Publishers; 1994. [Google Scholar]
- 33.Tanghe T, Devriese G, Verstraete W. Nonylphenol degradation in lab scale activated sludge units is temperature dependent. Water Res. 1998;32:2889–2896. [Google Scholar]
- 34.TemaNord. TemaNord 1996:580. Copenhagen, Denmark: Nordic Council of Ministers; 1996. Chemicals with estrogen-like effects; p. 278. [Google Scholar]
- 35.Toppari J, Larsen J C, Christiansen P, Giwercman A, Grandjean P, Guillette L J, Jégou B, Jensen T K, Jouannet P, Keiding N, Leffers H, McLachlan J A, Meyer O, Müller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek N E. Miljøprojekt nr. 290. Male reproductive health and environmental chemicals with estrogenic effects. Copenhagen, Denmark: Ministry of Environment and Energy, Denmark, Danish Environmental Protection Agency; 1995. p. 166. [Google Scholar]
- 36.Vallini G, Frassinetti S, Scorzetti G. Candida aquaetextoris sp. nov., a new species of yeast occurring in sludge from a textile industry wastewater treatment plant in Tuscany, Italy. Int J Syst Bacteriol. 1997;47:336–340. doi: 10.1099/00207713-47-2-336. [DOI] [PubMed] [Google Scholar]
- 37.Van Elsas J D, Smalla A K. Extraction of microbial community DNA from soils. In: Akkermans A D L, Van Elsas J D, De Bruin F J, editors. Molecular microbial ecology manual. London, United Kingdom: Kluwer Academic Publishers; 1995. pp. MMEM-1.3.3:1–11. [Google Scholar]
- 38.Van Ginkel C G, Kroon A G M. Metabolic pathway for the biodegradation of octadecylbis(2-hydroxyethyl)amine. Biodegradation. 1993;3:435–443. [Google Scholar]
- 39.Van Waeleghem K, De Clercq N, Vermeulen L, Schoonjans F, Comhaire F. Deterioration of sperm quality in young healthy Belgian men. Hum Reprod. 1996;11:325–329. doi: 10.1093/humrep/11.2.325. [DOI] [PubMed] [Google Scholar]
- 40.Verstraete W, Voets J P, Vanloocke R. Three-step measurement by the Sapromat to evaluate BOD5, the mineral imbalance and the toxicity of water samples. Water Res. 1974;8:1077–1081. [Google Scholar]
- 41.Vethaak D, Opperhuizen A. Xeno-oestrogene stoffen in het aquatische milieu in Nederland: een verkennende studie. 1996. p. 73. . Directoraat-Generaal Rijkswaterstaat, Rapport RIKZ 96.015. The Hague, The Netherlands. [Google Scholar]