Abstract
A patient in his late 40s presented after 1-year following below knee amputation and targeted muscle reinnervation (TMR) with new prosthesis intolerance and pinpoint pain, suspicious for neuroma. X-ray confirmed fibular heterotopic ossification (HO). Operative revision identified HO encompassing a TMR construct with a large neuroma requiring excision and neuroplasty revision. Now approximately 1-year post procedure, the patient remains active, pain-free and ambulating with a prosthetic. Amputated extremities can be at risk for development of HO. Although described in literature, the pathophysiology and timeline for HO development is not well understood. Preventative measures for HO have been described, yet results remain variable. The gold standard for existing HO remains to be operative excision. Due to the unpredictable nature and debilitating presentation, risk of HO should be incorporated into patient–physician discussions. Additionally, new prosthetic intolerance absent of prior trauma should raise suspicion for possible HO development.
Keywords: Plastic and reconstructive surgery, Orthopaedic and trauma surgery, Physiotherapy (rehabilitation)
Background
An amputated extremity can be at risk of developing chronic pain including phantom limb pain (PLP) and neuroma-induced pain with intolerance of prosthetic devices. Peripheral nerve interventions designed to decrease or eliminate the risk of these comorbidities and facilitate the use of bioamplified prosthetics include targeted muscle reinnervation (TMR) and regenerative peripheral nerve interface (RPNI). TMR is a technique designed to transfer proximal stumps of transected major peripheral nerves to redundant motor nerves within an amputated field.1–7 Its purpose is to provide a direct target for regenerating peripheral nerves, reducing the risk of PLP and neuroma development.1–7 Additionally, TMR enhances biological amplifiers for advanced myoelectric prosthetic use.1 7–9 The RPNI technique implants a transected peripheral nerve into a free muscle graft within the amputated field.10 11 The graft is re-vascularised and re-innervated by the peripheral nerve resulting in a nerve bioamplifier for advanced prosthetic control, also decreasing the risk of chronic pain, neuroma development and PLP.10
Heterotopic ossification (HO) is described as mature bone developing within soft tissue outside of the confines of normal periosteum.12–15 The development of HO is either acquired or genetic, with acquired being the most common.12–14 Acquired HO is often secondary to trauma, surgery, central nervous injury and burns.12 14 Although the exact pathogenesis of HO remains unknown,14 it is broadly secondary to an underlying inflammatory state.14 The risk of HO development following orthopaedic fracture is reported to be 20%–40%,13 16 and as high as 90% in hip-level fractures.16 The exact timeline for HO development following index injury is currently not well described. Herein, we present a unique case of TMR performed in the setting of lower extremity amputation, later compromised by HO and painful neuroma. To our knowledge, this is the first presented case of TMR failure with neuroma secondary to HO in the lower extremity, requiring reoperation. The purpose of this paper is to increase reconstructive surgeon awareness with this unique case presentation, report intraoperative findings and discuss recommendations for HO management and monitoring specifically in the TMR and/or RPNI population.
Case presentation
A Caucasian man presented in late 2019 following a motorcycle collision with left femoral shaft fracture and partial amputation of the left leg and foot. Following femur fracture fixation per orthopaedic colleagues, lower limb salvage was attempted with multistage washout and debridement, trial of antibiotic therapy and ongoing outpatient wound vacuum therapy. With minimal improvement, the patient agreed to left below knee amputation (BKA) with TMR in early 2020. A total of three nerve anastomoses were performed with interrupted 8–0 nylon; deep peroneal nerve to soleus muscle branch, tibial nerve to peroneal muscle motor branch and sural nerve to flexor digitorum longus motor branch. Coapted nerves were buried in local tissue, away from the distal end of the tibia and fibula. Available distal musculature was advanced over the distal tibia and secured to local fascia for purposes of soft tissue stump cushion prior to multilayered closure.
Five-months post-TMR, the patient presented with persistent localised distal tibia pain at the stump, directly overlying the point of contact of the prosthetic device. Limited soft tissue was noted over the area of the tibia, likely secondary to soft tissue atrophy and ongoing pressure of prosthetic use. A total of 75 cc of percutaneous fat grafting was injected to the distal stump for additional padding over the distal tibia in an effort to improve prosthetic tolerance. Following resolve of surgical pain, the patient did report improvement and resolve of prosthetic-related pain.
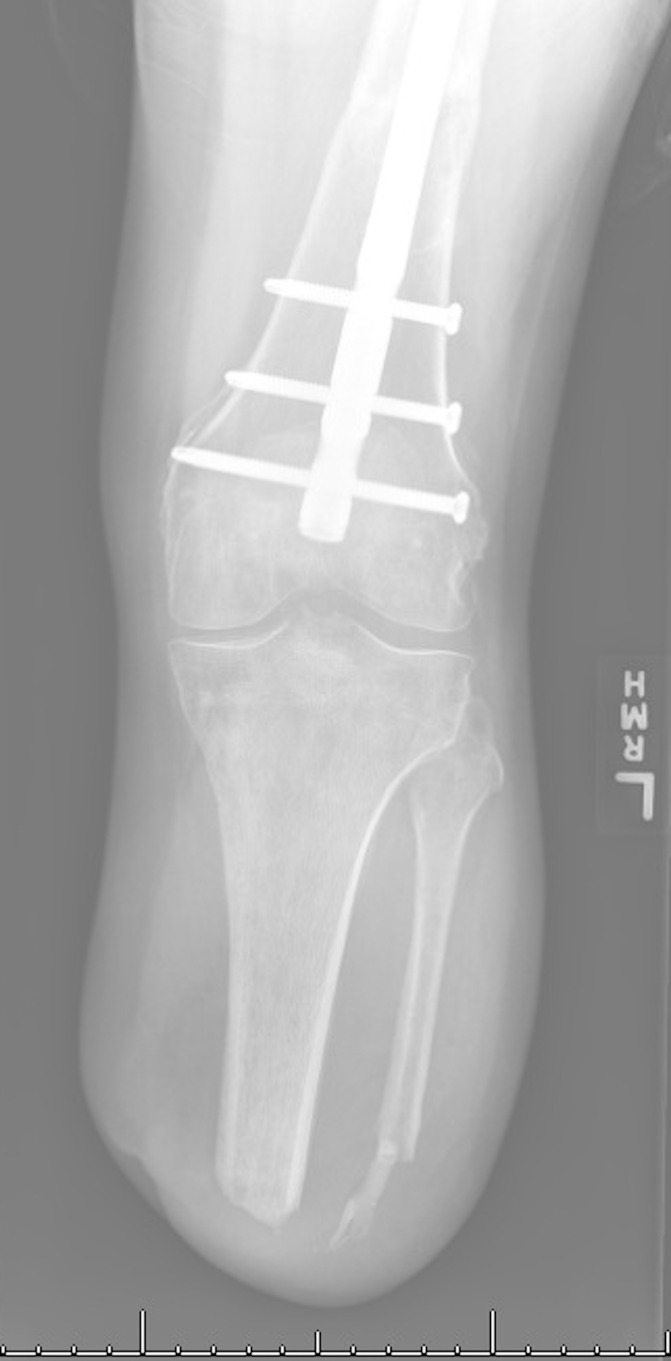
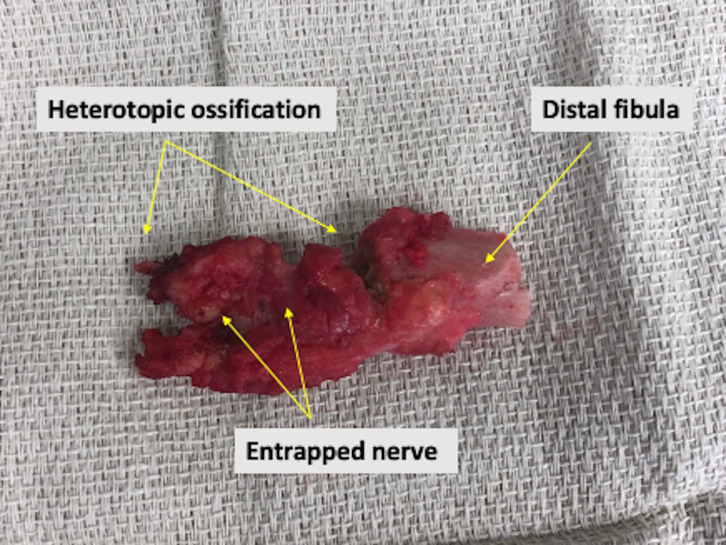
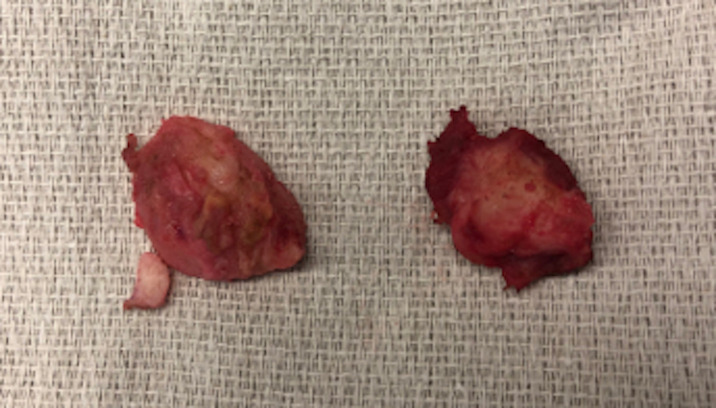
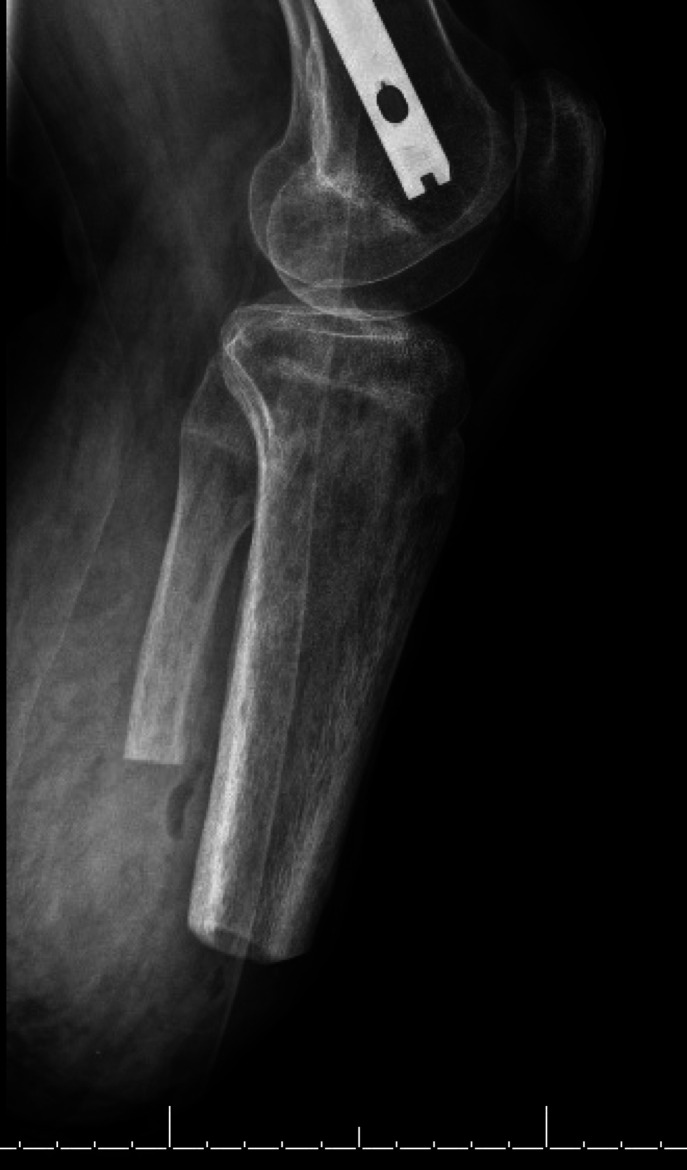
Six-months post fat grafting and nearly 1 year following initial TMR, the patient presented with new prosthetic intolerance, and severe pinpoint pain at the distal stump absent of any recent trauma. Clinical examination was suspicious for neuroma based laterally on the extremity. X-ray revealed approximately 3 cm of HO arising from the distal aspect of the fibula (figures 1 and 2). The patient underwent revision BKA, and the HO was noted to encompass a prior TMR anastomosis resulting in large neuroma development. The HO and associated entrapped TMR anastomosis with neuroma were excised. The entrapped TMR anastomosis was identified as the tibial nerve to peroneal muscle motor branch (figures 3 and 4). Due to the amount of nerve required for resection, suitable TMR targets could not be identified without undue tension, therefore, RPNI was performed to the remaining tibial nerve. The RPNI construct was secured in soft tissue, away from the remaining distal fibula. The stump exposure was closed in a multilayered fashion.
Figure 1.
Preoperative anterior X-ray showing the post-amputation stump with heterotopic ossification development extending from the fibula.
Figure 2.
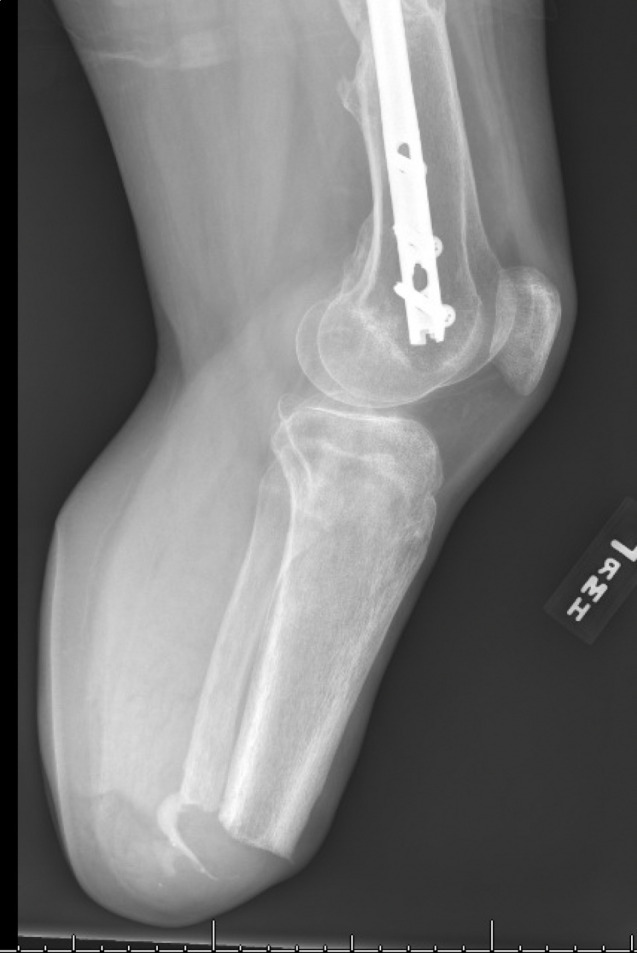
Preoperative lateral X-ray showing the post-amputation stump with heterotopic ossification development extending from the fibula.
Figure 3.
Intraoperative specimen post resection showing resected portion of distal fibula, heterotopic ossification and a segment of entrapped targeted muscle reinnervation anastomosis.
Figure 4.
Intraoperative divided neuroma specimen, post resection.
Outcome and follow-up
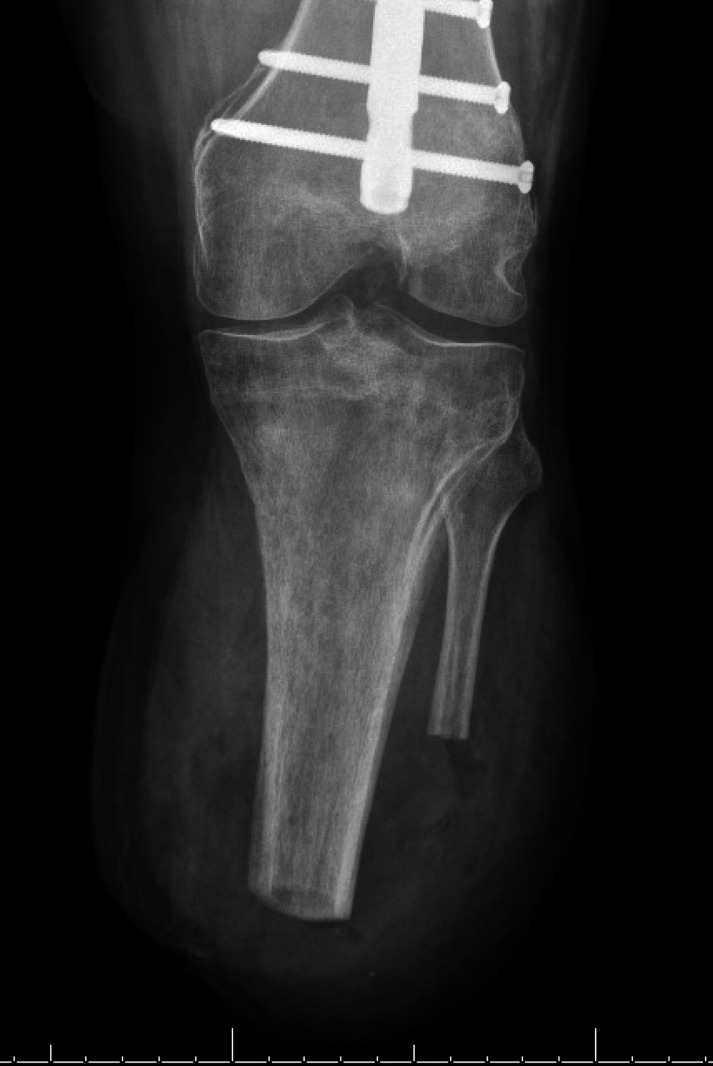
The patient was followed closely in the office, undergoing suture removal from the stump closure approximately 2 weeks post revision. Anterior and lateral post-revision X-ray is shown in figures 5 and 6. Stump shrinker was initiated approximately 4 weeks post procedure as well as consultation with the prosthetic team for re-fitting. At that time, the patient reported successful ambulation without pain or discomfort. The patient is now approximately 1 year post revision, and continues to actively ambulate on the prosthetic device without restriction or report of pain.
Figure 5.
Post-revision anterior X-ray.
Figure 6.
Post-revision lateral X-ray.
Discussion
The development of HO can be debilitating lending to pain and intolerance of prosthetics, impacting overall quality of life for the amputee, ultimately requiring definitive management. Described management of HO can be viewed broadly as two categories; prophylactic and therapeutic. Reported non-surgical prophylactic measures include the use of corticosteroids, non-steroidal anti-inflammatory drugs, radiation therapy and bisphosphonates.13 14 16 However, each prophylactic modality presents its own unique risk profile and should be evaluated as such. Despite reported benefits of prophylactic therapy, outcomes remain variable13 14 16 and are often not employed. Surgical prophylactic measures include softening rough or uneven bone edges with a rasp, and thorough washout of bone chips or dust particulate post amputation. In the setting of existing bony ectopy, surgical excision as performed in our described case currently remains the gold standard and sole definitive therapeutic treatment option.14
As evidenced in our case, HO can disrupt TMR anastomoses lending to neuroma development and prosthetic intolerance, requiring operative revision. If local TMR anastomoses are compromised and require resection, revision neuroplasty is necessary. However, revision TMR may not be possible due to remaining nerve length post resection resulting in excessive tension prohibiting anastomosis and/or lack of sufficient motor nerve targets. Therefore, an alternative neuroplasty modality such as RPNI can be considered in this setting.
Despite reported risk factors including trauma and/or prior surgery,12 14 as well as surgical-based prophylactic measures, the appearance of acquired HO is essentially unpredictable. Therefore, patient history, physical and radiological examinations remain central for definitive post-amputation HO diagnosis. We recommend incorporating the risk of HO development in the preoperative and postoperative amputation patient–physician discussion. The patient must understand the importance of reporting localised pain or intolerance of a prosthesis to evaluate for the possibility of neuroma development or bony abnormality. The patient should understand this can arise at any time post procedure as presentation is often delayed. In addition, an X-ray of the stump should be performed if concern of a neuroma is noted on examination to investigate for suspicion of HO development. When present, operative bony excision is recommended with the possibility of revision TMR and/or RPNI. Whether TMR or RPNI is employed, it is paramount to ensure the neural construct is tension-free, securely positioned away from the ends of the bones and protected in surrounding soft tissue from compression or possible HO. Despite HO development with associated complication and comorbidity as shown in our case, successful operative revision can return an active lifestyle with prosthetic tolerance back to the amputee.
Learning points.
Heterotopic ossification (HO) is described as mature bone developing within soft tissue outside of the confines of normal periosteum and can be subcategorised as either acquired or genetic.
Amputated extremities can be at risk for development of HO, which may lead to disruption of targeted muscle reinnervation anastomoses via painful neuroma formation.
Despite known risk factors, the appearance of acquired HO is essentially unpredictable, therefore, we recommended incorporation of the risk of HO development in a preoperative and postoperative patient discussion.
Regardless of the neuroplasty technique employed, it is paramount to ensure the neural construct is securely positioned away from the distal ends of the bones and protected in surrounding soft tissue to reduce the risk of compression or possible compromise by HO.
Footnotes
Contributors: SRA: Operative procedure, organisation, chart review, literature review, rough draft, revision, final draft and submission. NG: Chart review, literature review, rough draft and final draft. EAJ: Literature review and rough draft. RMJ: Preoperative assessment, operative procedure, postoperative care and final draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Cheesborough JE, Smith LH, Kuiken TA, et al. Targeted muscle reinnervation and advanced prosthetic arms. Semin Plast Surg 2015;29:062–72. 10.1055/s-0035-1544166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheesborough JE, Souza JM, Dumanian GA, et al. Targeted muscle reinnervation in the initial management of traumatic upper extremity amputation injury. Hand 2014;9:253–7. 10.1007/s11552-014-9602-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wimalawansa S, Lygrisse D, Anderson SR. Targeted muscle reinnervation in partial hand amputation: an anatomical description of technique and clinical case correlates. J Hand Surg 2021;9:e3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valerio I, Dumanian G, Jordan S. Preemptive Treatmetn of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J Am Coll Surg 2019;22:217–26. [DOI] [PubMed] [Google Scholar]
- 5.Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg 2019;270:238–46. 10.1097/SLA.0000000000003088 [DOI] [PubMed] [Google Scholar]
- 6.Alexander JH, Jordan SW, West JM, et al. Targeted muscle reinnervation in oncologic amputees: early experience of a novel institutional protocol. J Surg Oncol 2019;120:348–58. 10.1002/jso.25586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson SR, Wimalawansa SM, Roubaud MS, et al. Targeted muscle reinnervation following external hemipelvectomy or hip disarticulation: an anatomic description of technique and clinical case correlates. J Surg Oncol 2020;122:1693–710. 10.1002/jso.26189 [DOI] [PubMed] [Google Scholar]
- 8.Kuiken TA, Li G, Lock BA, et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA 2009;301:619–28. 10.1001/jama.2009.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuiken TA, Barlow AK, Hargrove L, et al. Targeted muscle reinnervation for the upper and lower extremity. Tech Orthop 2017;32:109–16. 10.1097/BTO.0000000000000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vu PP, Vaskov AK, Irwin ZT, et al. A regenerative peripheral nerve interface allows real-time control of an artificial hand in upper limb amputees. Sci Transl Med 2020;12. 10.1126/scitranslmed.aay2857. [Epub ahead of print: 04 03 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo SL, Kung TA, Brown DL, et al. Regenerative peripheral nerve interfaces for the treatment of Postamputation neuroma pain: a pilot study. Plast Reconstr Surg Glob Open 2016;4:e1038. 10.1097/GOX.0000000000001038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson GJ, Lee MY, Mehta MK. Heterotopic ossification in the residual lower limb in an adult nontraumatic amputee patient. Am J Phys Med Rehabil 2010;89:245–8. 10.1097/PHM.0b013e3181c5657c [DOI] [PubMed] [Google Scholar]
- 13.Dey D, Wheatley BM, Cholok D, et al. The traumatic bone: trauma-induced heterotopic ossification. Transl Res 2017;186:95–111. 10.1016/j.trsl.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mujtaba B, Taher A, Fiala MJ, et al. Heterotopic ossification: radiological and pathological review. Radiol Oncol 2019;53:275–84. 10.2478/raon-2019-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minarelli J, Davis EL, Dickerson A, et al. Characterization of neuromas in peripheral nerves and their effects on heterotopic bone formation. Mol Pain 2019;15:174480691983819. 10.1177/1744806919838191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranganathan K, Loder S, Agarwal S, et al. Heterotopic ossification: Basic-Science principles and clinical correlates. J Bone Joint Surg Am 2015;97:1101–11. 10.2106/JBJS.N.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]