Abstract
Hypoxic-ischemic encephalopathy is a common neonatal brain injury associated with significant morbidity and mortality despite the administration of therapeutic hypothermia (TH). Neonatal seizures and subsequent chronic epilepsy are frequent in this patient population and current treatments are partially effective. We used a neonatal murine hypoxia-ischemia (HI) model to test whether the severity of hippocampal and cortical injury predicts seizure susceptibility 8 days after HI and whether TH mitigates this susceptibility. HI at postnatal day 10 (P10) caused hippocampal injury not mitigated by TH in male or female pups. TH did not confer protection against flurothyl seizure susceptibility at P18 in this model. Hippocampal (R2 = 0.33, p = 0.001) and cortical (R2 = 0.33, p = 0.003) injury directly correlated with seizure susceptibility in male but not female pups. Thus, there are sex-specific consequences of neonatal HI on flurothyl seizure susceptibility in a murine neonatal HI model. Further studies are necessary to elucidate the underlying mechanisms of sex dimorphism in seizure susceptibility after neonatal HI.
Keywords: Hypoxic-ischemic encephalopathy, Neonatal seizures, Sex differences, Seizure susceptibility
Introduction
Hypoxic-ischemic encephalopathy (HIE) affects 2–6 per 1,000 live term births worldwide, and it is associated with significant mortality and poor neurologic outcomes including epilepsy [1]. Neonatal seizures occur in 33–75% of infants with HIE [2, 3] and in the majority of rodents after experimental neonatal hypoxia-ischemia (HI) [4]. Approximately 20% of infants with HIE-associated neonatal seizures and up to 50% of rodents exposed to neonatal HI develop epilepsy [5, 6]. Currently, first-line treatments control only about 50% of post-HI neonatal seizures [7]. In both animal models and humans, seizures exacerbate hypoxic-ischemic brain injury and are an independent predictor of worse neurodevelopmental outcomes in humans [8–10]. Better treatments are urgently needed.
Currently, therapeutic hypothermia (TH) is the standard of care for infants with HIE, decreasing the risk of death and moderate-to-severe disability by one-third [11, 12]. TH has also been shown to acutely decrease the electrographic seizure burden and the incidence of childhood epilepsy through unknown mechanisms [3, 13, 14]. While TH is an important therapy, it is only partially neuroprotective, with 45% of patients still suffering severe neurodevelopmental disability or death. Additionally, TH does not mitigate cognitive and memory deficits in grade school-aged HIE survivors or in rodent models [12, 15, 16]. The hippocampus is closely linked to both epileptogenesis after early life stressors [17, 18] and long-term cognitive and memory function. Thus, the development of preclinical models to understand the mechanisms of hippocampal injury and HI-associated seizures could lead to novel interventions for acute symptomatic seizures, epileptogenesis, and memory/learning deficits after neonatal HIE.
There is growing evidence that sex is an important biologic variable in HIE as well as in seizure occurrence and epileptogenesis after neonatal neurologic insults [19–25]. In humans, boys have a higher incidence and severity of perinatal neurologic insults and worse outcomes [19, 26]. These differences are paralleled in rodents after neonatal HI [15, 16]. In rodents, sexual dimorphism is documented in epilepsy after early life seizures and stress [21, 27], and in the therapeutic response after neonatal ischemic injury [25]. However, human and rodent studies have not examined differences according to sex in epileptogenesis or seizure susceptibility after neonatal HI.
In this study, we evaluated the effects of both TH and sex on seizure susceptibility to flurothyl after neonatal HI. Flurothyl is a commonly used convulsant to test neonatal seizure susceptibility [28]. Specifically, we examined an early age, postnatal day 18 (P18), when murine brain development resembles that in humans at 18–24 months of age [29]. We chose this age because post-HIE epileptogenesis typically occurs within the first 2 years of life [5]. We hypothesized that in rodents, neonatal HI would increase seizure susceptibility at P18 and that TH would mitigate this susceptibility. Regarding sex-stratification, we hypothesized that males would show higher seizure susceptibility than females after neonatal HI.
Methods
Animals
One hundred and four C57BL6 mouse pups (41 males and 36 females with seizure testing; 14 males and 13 females without seizure testing) were used in this study. The mortality rate for the model was 10% (3 males and 5 females). The mortality rate for the seizure testing was 9% (5 males and 1 female). This is similar to the rates in previously published studies [16, 30].
Hypoxic-Ischemic Injury and TH
Hypoxic-ischemic injury and TH were completed in C57BL6 mice (Charles River Laboratories, Wilmington, MA, USA) on P10 using the modified Vannucci model for mice as previously described [16]. Briefly, permanent unilateral right carotid artery ligation was performed while the pups were anesthetized with inhaled isoflurane (3% for induction and 1% for maintenance). The surgery took 5–8 min per pup. After a 1-h recovery period with the dam, the pups were exposed to 45 min of hypoxia (FiO2 0.08). Pups were then randomized to TH (31°C) or normothermia (NT; 36°C) for 4 h, and then returned to their dam. Control animals were littermates that underwent sham surgery with similar anesthesia exposure.
Flurothyl Seizure Susceptibility Testing
Flurothyl is a well-established inhaled convulsant that acts as a γ-aminobutyric acid (GABA)-type A receptor antagonist. It is not metabolized, produces a defined sequence of generalized seizures, and the resulting seizure thresholds are independent of body weight [30]. At P18, the pups were placed individually into a 30.5 × 30.5 × 24 cm Plexiglass chamber. Flurothyl ether (Synquest Laboratories, Lot 331700) was dripped from the top of the container at a rate of 40 μL/min onto filter paper at the base of the container (separated from the pup). All seizure tests were video-taped and the time to generalized seizures with a loss of posture (initial time point measured, approximates a stage 3 seizure [31]) and with tonic hindlimb extension (final time point measured, a stage 5 seizure [31]) were recorded. The time that elapsed from stimulation to seizure onset is called seizure latency. Of note, the characteristic clonic features of stage 3 seizures were often difficult to visualize. This phenomenon has been described previously when using flurothyl seizure testing in young rodent pups [30]. Some animals did not exhibit generalized stage 3 seizures, but all mice exhibited stage 5 seizures.
Histopathologic Analysis of Injury
After seizure testing at P18, animals were euthanized with an overdose of isoflurane and exsanguinated with 0.1 M cold PBS (pH 7.4) via intracardiac perfusion. Brains were perfusion-fixed with 4% paraformaldehyde/0.1 m PBS for 30 min at 4 mL/min. Tissues were cryoprotected with graded immersion in 15 and 30% sucrose in PBS until they sank, and then frozen and stored at −8°C until cut at 50 μm on a freezing microtome [16]. Glial fibrillary acid protein (GFAP) immunohistochemistry (IHC) was used to grade astrogliosis as previously described with whole rabbit antisera anti-GFAP antibody (DAKO North America, Carpinteria, CA, cat. No. Z0334 at 1:1,000) [16, 32]. Goat anti-rabbit antibody (1:200) was used as the secondary antibody and DAB as the chromagen. Brain slices were stained with cresyl violet and Perl’s iron stain to evaluate the cell loss and iron deposition per standard protocol.
Two injury scores were quantitated. The first was based on a previously published grading system [33] in which visual analysis is performed on sequential coronal brain slices of 3 regions to determine hippocampal and cortical injury scores (ranging from 0 to 9). The 3 hippocampal subfields scored included CA1, CA3, and the dentate gyrus (DG), and the 3 cortical sections scored included the anterior, middle, and posterior cortical slices. Representative cresyl violet-stained hippocampal and cortical samples are shown in Figure 1B, 4C. Two blinded assessors (M.A.M. and F.J.N.) independently scored each sample. Any discrepancy was rectified by discussion and re-review by the 2 assessors. A second injury analysis was completed using hippocampal GFAP IHC grading as previously published [32]. Briefly, the amount of GFAP staining, the presence of glial scarring, and astrocyte morphology were graded in the anterior, middle, and posterior sections of hippocampal CA1, CA3, and DG subfields. The cumulative score for these different indices ranged from 3 to 21.
Fig. 1.
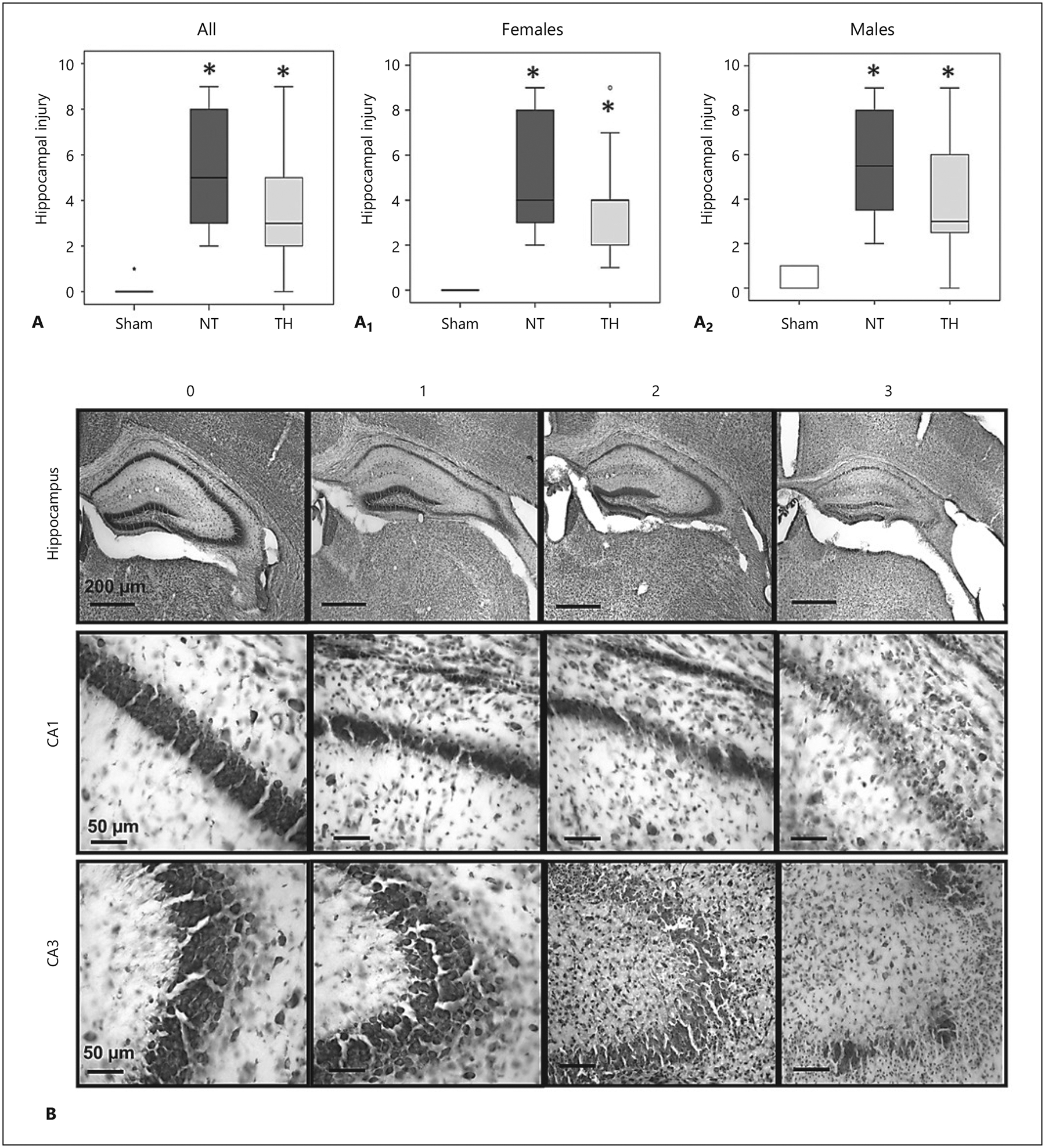
Neonatal hypoxia-ischemia (HI) causes hippocampal injury that is not mitigated by therapeutic hypothermia (TH). Hippocampal injury is greater in HI-injured animals independent of sex or TH. Injury scores of the sham, normothermia (NT), and TH groups for all animals (A), females (A1), and males (A2). Results are presented as box and whisker plots, where the box is limited by the 25th and 75th percentiles and the solid line represents the median. Kruskal-Wallis ANOVA with Dunn-Bonferroni post hoc analysis, * p < 0.05 (sham 7 females and 6 males; NT 9 females and 12 males; TH 9 females and 12 males). B Representative cresyl violet-stained hippocampal sections for injury scores of 0–3.
Fig. 4.
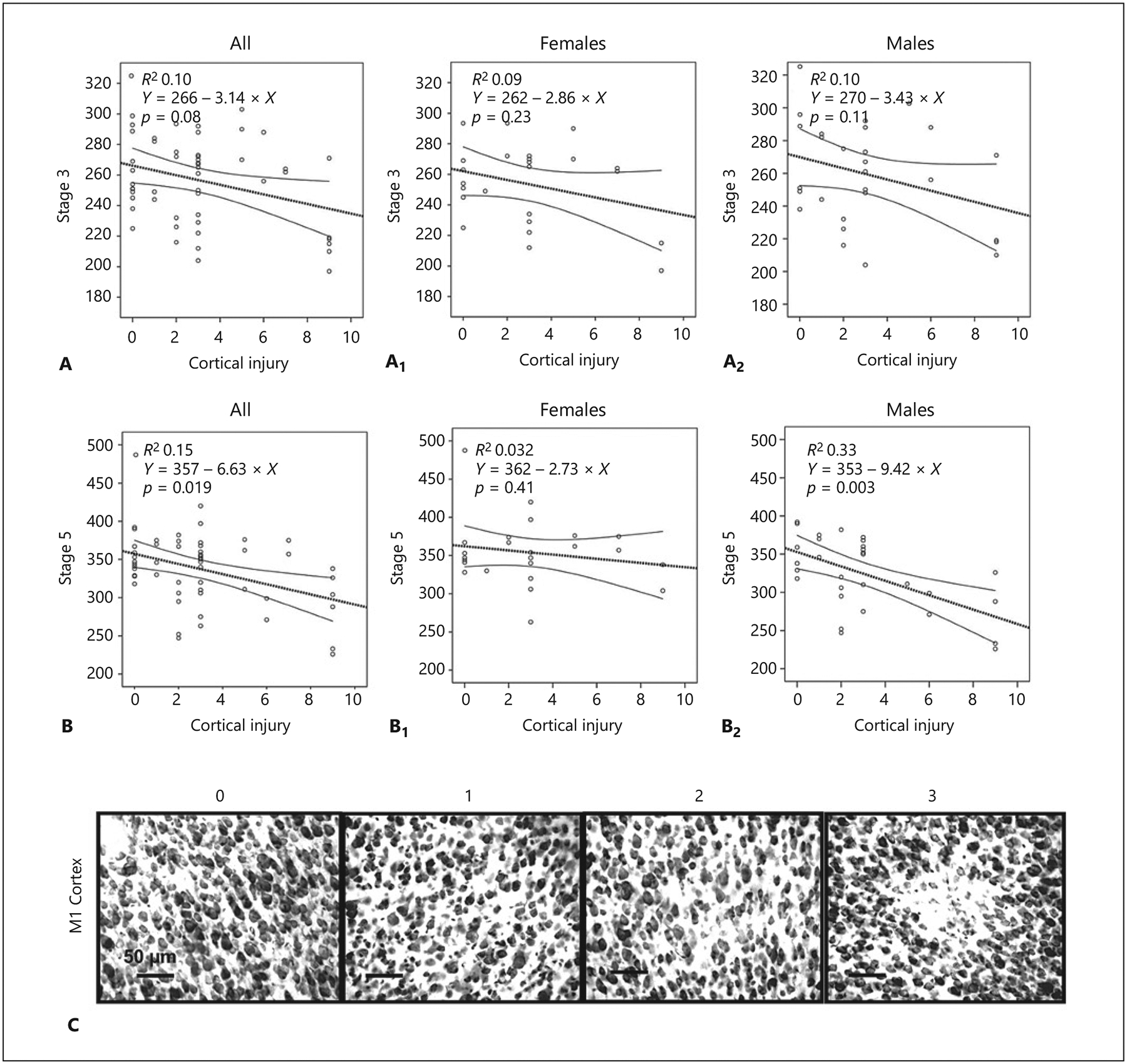
Cortical injury inversely correlates with flurothyl seizure susceptibility after neonatal hypoxia-ischemia in a sex-specific manner. Correlation of cortical injury with latency (in seconds) to stage 3 seizure (A) and stage 5 seizure (B) is shown for the whole group, and in females (A1, B1), and males (A2, B2). When stratified by sex, cortical injury correlates with latency to stage 5 seizure in male pups only (B1 vs. B2). Nonparametric Spearman Rho correlation statistics were used for data analysis. C Representative cresyl violet-stained cortical samples for injury scores of 0–3.
Statistical Analysis
Nonparametric statistical methods were applied in all cases. For the analysis of 2 groups, the Mann-Whitney U test was applied with stratification by sex. For multiple groups, Kruskal-Wallis (KW) H one-way ANOVA stratified by sex was applied with post hoc pair analysis using the Dunn-Bonferroni test. All results were presented as box and whisker plots, where the box is limited by the 25th and 75th percentiles and the solid line represents the median. Significance was represented by p ≤ 0.05 in all cases, with adjustment for multiple comparisons as needed. Nonparametric Spearman Rho correlations were applied between: (i) GFAP scoring and the hippocampal injury score, to assess coherence between both assessments, and (ii) the severity of hippocampal or cortical injury and latency to stage 3 or stage 5 seizure. Analysis was performed using IBM SPSS Statistics v24 (IBM Corp., Armonk, NY, USA).
Results
Hippocampal Injury after Neonatal HI
Significant histological hippocampal injury was documented 8 days after HI injury at P10 (KW p < 0.001, H 21.2, df 2; Dunn-Bonferroni p < 0.001 vs. sham), and TH did not mitigate the injury (p < 0.001, vs. sham, Fig. 1A). A similar degree of hippocampal injury was documented at P18 in both female (KW p = 0.001, H 13.8, df 2; Fig. 1A1) and male (KW p < 0.001, H 14.3, df 2; Fig. 1A2) mice subjected to NT (p = 0.001 [females, n = 9] and p < 0.001 [males, n = 12] vs. sham). Again, hippocampal injury was not mitigated by TH in either female (n = 9, p = 0.008 vs. sham) or male (n = 12, p = 0.019 vs. sham) mice (Fig. 1). Representative photomicrographs exemplifying the grading of hippocampal injury are shown in Figure 1B.
Since seizures are known to increase hippocampal inflammation [34], flurothyl exposure at P18 may have independently worsened the inflammation produced by HI and thus confounded the effects of neonatal HI and TH on this process. So, in P18 HI-injured mice, we compared the degree of hippocampal astrogliosis produced by HI alone vs. that produced with flurothyl exposure and HI (Fig. 2). The hippocampus of HI-injured pups subjected to NT and not exposed to flurothyl had greater evidence of astrogliosis by GFAP scoring in the CA1 (KW p = 0.001, H 14.6, df 2; Fig. 2A), CA3 (KW p < 0.001, H 15.7, df 2; Fig. 2B), and DG (KW p = 0.004, H 11.2, df 2; Fig. 2C) subfields, which compounded into a greater hippocampal GFAP score in the whole hippocampus (KW p < 0.001, H 15.3, df 2; Fig. 2D). Astrogliosis was not attenuated by TH. For the most part, these effects were similar in female (Fig. 2A1–D1) and male (Fig. 2A2–D2) pups. Additional exposure to flurothyl did not significantly change hippocampal GFAP scoring in either female or male pups.
Fig. 2.
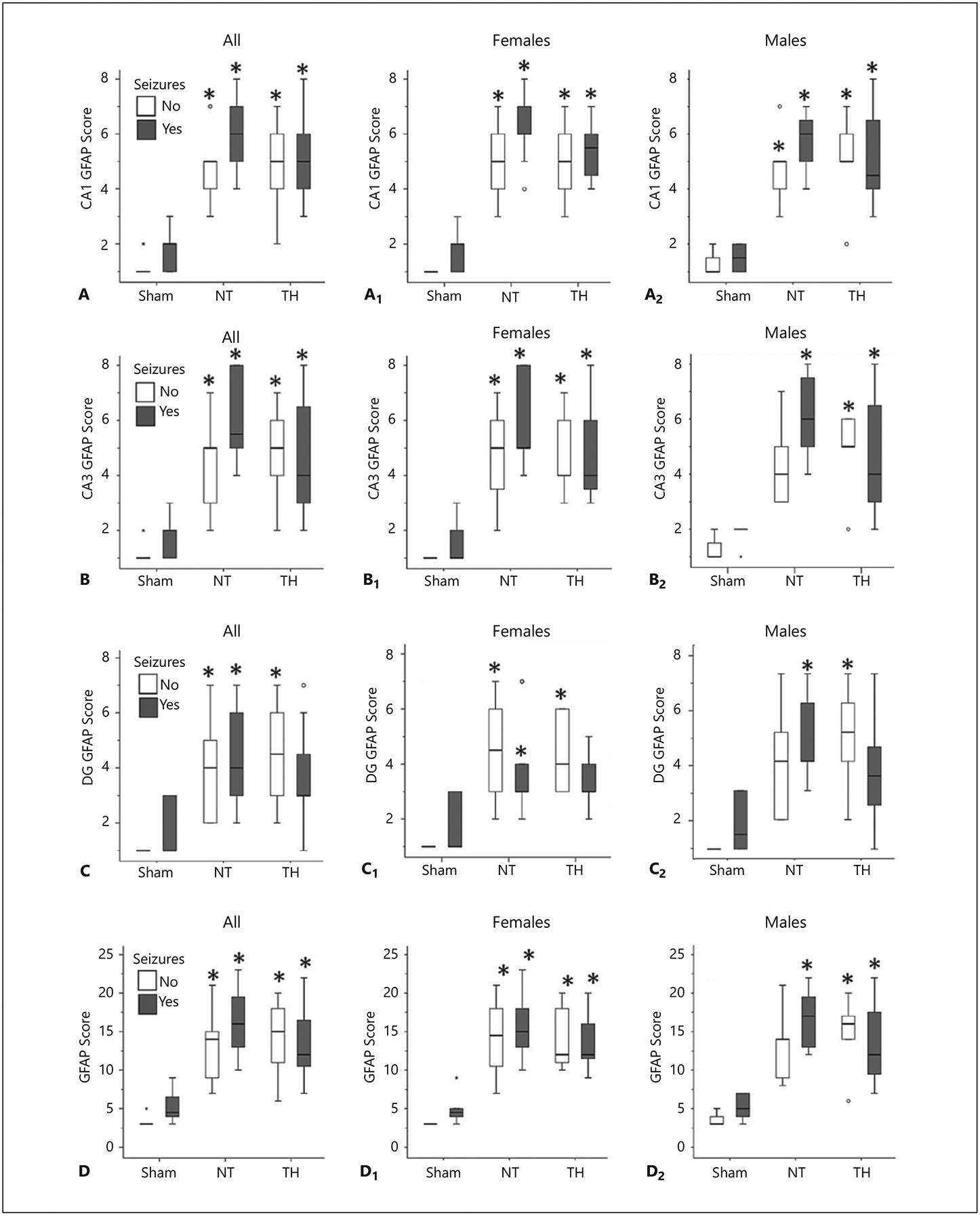
Hippocampal astrogliosis after hypoxia-ischemia is not significantly changed by flurothyl-induced seizures. Comparison of astrogliosis, as determined by GFAP score, in CA1 (A), CA3 (B), the dentate gyrus (DG, C), and total hippocampus (D) in the sham, normothermia (NT), and therapeutic hypothermia (TH) groups. Results are presented as box and whisker plots, where the box is limited by the 25th and 75th percentiles and the solid line represents the median. Kruskal-Wallis ANOVA with Dunn Bonferroni post hoc pairwise analysis. * p < 0.05 vs. sham for females (A1–D1) and males (A2–D2). n = 6–14 pups in injured groups and 4–6 pups in sham groups.
Flurothyl Seizure Susceptibility after Neonatal HI
Eight days after neonatal HI, TH did not attenuate flurothyl seizure susceptibility in either male or female pups (KW p > 0.05 for all comparisons, n = 9–14 pups per group). The median latency to stage 3 and 5 seizures in the NT group was 262 and 357 s in females, respectively, and 250 and 336 s in males, respectively. With TH, seizure latencies were not significantly different in either group (265 and 347 s in females and 256 and 311 s in males, for stage 3 and 5, respectively). Given the known variability in injury severity in our HI model [15], we evaluated the relationship between injury severity and seizure susceptibility.
Hippocampal injury and GFAP scores were directly correlated in male and female mice (R2 0.86, p < 0.001 for both), demonstrating coherence between the 2 injury assessments (Fig. 3A, A1, A2). Scores were thus combined to create a composite hippocampal injury score for a more refined assessment of injury in that region. This composite hippocampal injury score did not correlate with latency to stage 3 seizure in male or female pups (Fig. 3B, B1, B2), although there was a trend for an inverse correlation between them when pooling all pups together (Spearman Rho p = 0.06; Fig. 3B). However, composite hippocampal injury inversely correlated with latency to stage 5 seizure (p = 0.01, Fig. 3C). When stratified by sex, hippocampal injury inversely correlated with stage 5 latency only in male pups (R2 0.33, p = 0.001; Fig. 3C1 vs. C2).
Fig. 3.
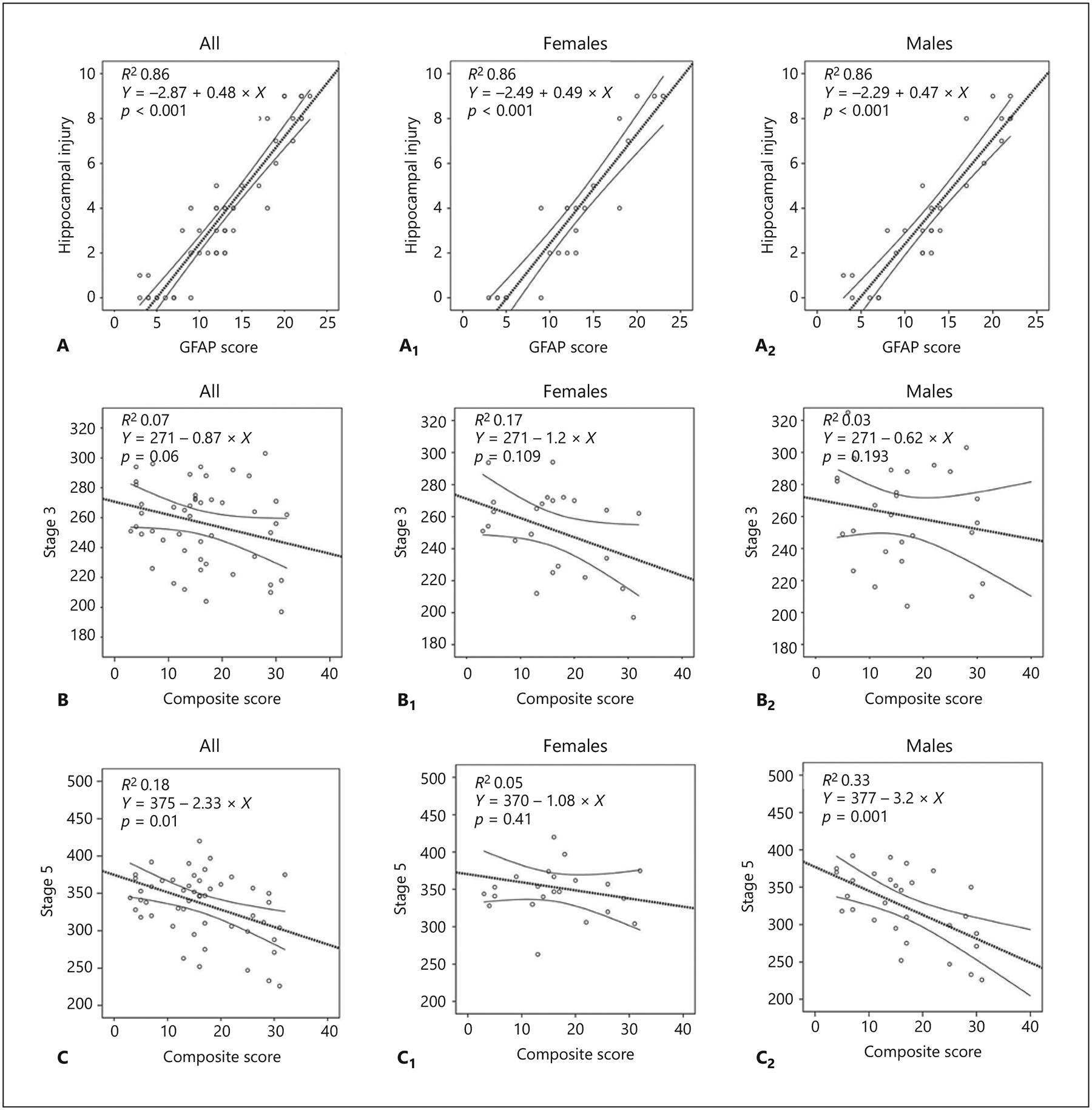
Hippocampal injury inversely correlates with flurothyl seizure susceptibility after neonatal hypoxia-ischemia in a sex-specific manner. A Hippocampal injury and GFAP scores are directly correlated in the whole group of pups, and stratified by sex (A1, A2). B, B1, B2 Composite hippocampal injury does not correlate with latency (in seconds) to stage 3 seizure. C Composite hippocampal injury correlates with latency (in seconds) to stage 5 seizure. When stratified by sex, this correlation is seen in male pups only (C1 vs. C2). Nonparametric Spearman Rho correlation statistics were used.
We also investigated the correlation between cortical injury and seizure susceptibility (Fig. 4). We observed a similar inverse correlation between stage 5 seizure latency and cortical injury in male mice (R2 0.33, p = 0.003; Fig. 4B2). Representative photomicrographs exemplifying grading of cortical injury are shown in Figure 4C.
Discussion
In our murine model, there were sex-specific responses to neonatal HI on flurothyl seizure susceptibility. Despite the consistent hippocampal astrogliosis and hippocampal and cortical histopathologic injury that we found after neonatal HI in both male and female pups, hippocampal and cortical injury severity correlated with increased seizure susceptibility in male mice only. In contrast, seizure susceptibility in females was not related to the severity of injury in the cortex and hippocampus. Finally, we found that TH did not modify flurothyl seizure susceptibility in our model.
The sex-specific differences in the correlation of cortical and hippocampal injury to flurothyl seizure susceptibility that we observed may reflect baseline differences in cerebral inhibitory systems and how they respond to neonatal HI. Neonatal HI disrupts the development of cerebral inhibition in both humans and rodents, giving rise to an inhibitory dysmaturation phenotype [32, 35]. In both humans and animal models, inhibitory dysmaturation has been shown to increase the susceptibility to seizures in later life [36–38]. However, the influence of sex on this process is unclear.
The in vitro and in vivo evidence to date suggests that female rodent pups show an earlier maturation of inhibitory GABAergic systems than males [27, 32, 39]. In patch-clamp studies of acute rat hippocampal slices, GABAAergic postsynaptic currents are depolarizing in males until about P14, while in females they are often hyperpolarizing as early as P4 [40]. Sex differences are also seen in the depolarizing properties of the GABAA receptor as well as in resting intracellular calcium levels in cultured rat hippocampal neurons [41]. The relevance of these sex-specific processes is highlighted by evidence that, after neonatal seizures on P4–P6, male rat pups showed an earlier GABAAR shift to hyperpolarization, whereas females showed a transient shift to depolarizing GABAAR responses [40]. Recent work from our laboratory has also identified sex differences in the maturation of hippocampal parvalbumin-positive interneurons, with more advanced expression of parvalbumin and GAD65/67 in female than male mice as well as differences in the molecular response to HI at P10 [32]. Thus, the baseline hippocampal inhibitory tone appears distinct between sexes in neonatal rodents, and the maturational pattern and molecular response to injury varies.
In our model, TH did not attenuate hippocampal injury or seizure susceptibility, even though recent clinical data suggest that TH decreases the incidence of long-term epilepsy in patients after HIE [3, 14]. Both preclinical and clinical data suggest that TH may only be partially protective in the hippocampus [15, 22, 42, 43]. Also, previous work has shown that epileptogenesis after experimental neonatal HI in rats correlates with the severity of brain injury [6]. Given the significant variability of injury produced by our model, therefore, significant findings may have been diluted and we did not have the statistical power to compare different injury subgroups. Additionally, we utilized only one method of seizure induction (flurothyl exposure) and it is possible that different results would be observed with other methods of seizure induction (e.g., maximal electroshock or pentylenetetrazol exposure). Finally, it is plausible that the timing of seizure susceptibility testing in our study was not optimal for capturing the effects of TH, and an investigation of later time points could yield different results.
Our results also show that there was not a significant change in hippocampal astrogliosis after flurothyl seizures. It is known that hippocampal inflammation after seizures can occur for several days [44], so it is possible that the flurothyl-induced seizures affected inflammation and injury in a manner that was not reflected by astrogliosis at such a short survival time. Despite these limitations, the documented sex-specific differences may represent important pathophysiologic differences in how male and female pups respond to neonatal HI. Given the differences in the incidence of neonatal brain injury and outcomes that can be observed clinically in the sexes, this notion warrants further study as it might have therapeutic implications.
In summary, we found sex-specific responses to neonatal HI on flurothyl seizure susceptibility in a murine neonatal HI model. Future work should delineate any baseline differences in cerebral inhibitory tone in male and female pups and also how this changes after neonatal HI. Additionally, long-term EEG monitoring for the development of spontaneous seizures will provide a better understanding of the timing and incidence of epilepsy in male versus female animals. Previous work in a rat neonatal HI model found that epileptogenesis was dependent on the severity of cerebral injury [6]; the study was not, however, powered to assess sex differences. It is indeed possible that both sexes develop epilepsy, but at different rates and via different pathophysiologic mechanisms. Identifying these differences and defining the pathways involved will be important for developing individualized adjuvant therapies and improving long-term outcomes after neonatal HIE.
Funding Sources
Experiments and investigators were funded by NIH grants (KO8NS096115 to R.C.-V.; RO1HD070996 and RO1HD086058 to F.J.N.; KO8NS097704 to R.J.F.; R25NS065729 to M.A.M.), the Johns Hopkins University-SOM Clinician Scientist Award (to R.C.-V.), the Sutland-Pakula Endowment for Neonatal Research (to R.C.-V.), the Mathias Koch Memorial Fund of the Community Foundation of Southern Wisconsin (to C.E.S.) and the Sandra and Malcom Berman Foundation (to C.E.S.).
Footnotes
Statement of Ethics
The authors have no ethical conflicts to disclose. Animal experiments conformed to internationally accepted standards, were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University School of Medicine and carried out with standards of care and housing in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services 85-23, 2011.
Disclosure Statement
The authors have no conflicts of interests to declare.
References
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010. Jun;86(6):329–38. [DOI] [PubMed] [Google Scholar]
- 2.Massaro AN, Murthy K, Zaniletti I, Cook N, DiGeronimo R, Dizon M, et al. Short-term outcomes after perinatal hypoxic ischemic encephalopathy: a report from the Children’s Hospitals Neonatal Consortium HIE focus group. J Perinatol. 2015. Apr;35(4):290–6. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Jary S, Cowan F, Thoresen M. Reduced infancy and childhood epilepsy following hypothermia-treated neonatal encephalopathy. Epilepsia. 2017. Nov;58(11):1902–11. [DOI] [PubMed] [Google Scholar]
- 4.Cuaycong M, Engel M, Weinstein SL, Salmon E, Perlman JM, Sunderam S, et al. A novel approach to the study of hypoxia-ischemia-induced clinical and subclinical seizures in the neonatal rat. Dev Neurosci. 2011;33(3–4): 241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisani F, Facini C, Pavlidis E, Spagnoli C, Boylan G. Epilepsy after neonatal seizures: literature review. Eur J Paediatr Neurol. 2015. Jan;19(1):6–14. [DOI] [PubMed] [Google Scholar]
- 6.Kadam SD, White AM, Staley KJ, Dudek FE. Continuous electroencephalographic monitoring with radio-telemetry in a rat model of perinatal hypoxia-ischemia reveals progressive post-stroke epilepsy. J Neurosci. 2010. Jan;30(1):404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rooij LG, van den Broek MP, Rademaker CM, de Vries LS. Clinical management of seizures in newborns: diagnosis and treatment. Paediatr Drugs. 2013. Feb;15(1):9–18. [DOI] [PubMed] [Google Scholar]
- 8.Wirrell EC, Armstrong EA, Osman LD, Yager JY. Prolonged seizures exacerbate perinatal hypoxic-ischemic brain damage. Pediatr Res. 2001. Oct;50(4):445–54. [DOI] [PubMed] [Google Scholar]
- 9.Miller SP, Weiss J, Barnwell A, Ferriero DM, Latal-Hajnal B, Ferrer-Rogers A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002. Feb;58(4):542–8. [DOI] [PubMed] [Google Scholar]
- 10.Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr. 2009. Sep;155(3): 318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfister RH, Soll RF. Hypothermia for the treatment of infants with hypoxic-ischemic encephalopathy. J Perinatol. 2010. Oct;30(S1 Suppl):S82–7. [DOI] [PubMed] [Google Scholar]
- 12.Azzopardi D, Strohm B, Marlow N, Brockle-hurst P, Deierl A, Eddama O, et al. ; TOBY Study Group. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014. Jul;371(2):140–9. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr. 2013. Aug;163(2):465–70. [DOI] [PubMed] [Google Scholar]
- 14.Lugli L, Balestri E, Berardi A, Guidotti I, Cavalleri F, Todeschini A, et al. Brain cooling reduces the risk of post-neonatal epilepsy in newborns affected by moderate to severe hypoxic-ischemic encephalopathy. Minerva Pediatr. 2018. DOI: 10.23736/S0026-4946.18.05224-6. [DOI] [PubMed] [Google Scholar]
- 15.Burnsed JC, Chavez-Valdez R, Hossain MS, Kesavan K, Martin LJ, Zhang J, et al. Hypoxia-ischemia and therapeutic hypothermia in the neonatal mouse brain – a longitudinal study. PLoS One. 2015. Mar;10(3):e0118889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz J, Abiola S, Kim N, Avaritt O, Flock D, Yu J, et al. Therapeutic hypothermia provides variable protection against behavioral deficits after neonatal hypoxia-ischemia: a potential role for brain-derived neurotrophic factor. Dev Neurosci. 2017;39(1–4):257–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang LT. Early-life stress impacts the developing hippocampus and primes seizure occurrence: cellular, molecular, and epigenetic mechanisms. Front Mol Neurosci. 2014. Feb; 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ, Brunson KL. Stressed-out, or in (utero)? Trends Neurosci. 2002. Oct;25(10): 518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007. Jan;49(1):74–8. [DOI] [PubMed] [Google Scholar]
- 20.Galanopoulou AS. Sex and epileptogenesis, introduction to the special issue. Neurobiol Dis. 2014. Dec;72 Pt B:123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desgent S, Duss S, Sanon NT, Lema P, Lévesque M, Hébert D, et al. Early-life stress is associated with gender-based vulnerability to epileptogenesis in rat pups. PLoS One. 2012;7(8):e42622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavez-Valdez R, O’Connor M, Perin J, Reyes M, Armstrong J, Parkinson C, et al. Sex-specific associations between cerebrovascular blood pressure autoregulation and cardiopulmonary injury in neonatal encephalopathy and therapeutic hypothermia. Pediatr Res. 2017. May;81(5):759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chavez-Valdez R, Martin LJ, Razdan S, Gauda EB, Northington FJ. Sexual dimorphism in BDNF signaling after neonatal hypoxia-ischemia and treatment with necrostatin-1. Neuroscience. 2014. Feb;260:106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chavez-Valdez R, Martin LJ, Northington FJ. Programmed necrosis: a prominent mechanism of cell death following neonatal brain injury. Neurol Res Int. 2012;2012:257563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter BM, Sullivan BJ, Landers JR, Kadam SD. Dose-dependent reversal of KCC2 hypo-function and phenobarbital-resistant neonatal seizures by ANA12. Sci Rep. 2018. Aug; 8(1):11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis S, Glinianaia SV, Arnaud C, Fauconnier J, Johnson A, McManus V, et al. ; SCPE collaboration of European Cerebral Palsy Registers. Case gender and severity in cerebral palsy varies with intrauterine growth. Arch Dis Child. 2005. May;90(5):474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akman O, Moshé SL, Galanopoulou AS. Sex-specific consequences of early life seizures. Neurobiol Dis. 2014. Dec;72 Pt B:153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid R, Tandon P, Stafstrom CE, Holmes GL. Effects of neonatal seizures on subsequent seizure-induced brain injury. Neurology. 1999. Nov;53(8):1754–61. [DOI] [PubMed] [Google Scholar]
- 29.Yager JY, Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 2009. Mar;40(3):156–67. [DOI] [PubMed] [Google Scholar]
- 30.Prichard JW, Gallagher BB, Glaser GH. Experimental seizure-threshold testing with fluorthyl. J Pharmacol Exp Ther. 1969. Mar; 166(1):170–8. [PubMed] [Google Scholar]
- 31.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972. Mar; 32(3):281–94. [DOI] [PubMed] [Google Scholar]
- 32.Chavez-Valdez R, Emerson P, Goffigan-Holmes J, Kirkwood A, Martin LJ, Northington FJ. Delayed injury of hippocampal interneurons after neonatal hypoxia-ischemia and therapeutic hypothermia in a murine model. Hippocampus. 2018. Aug;28(8):617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheldon RA, Jiang X, Francisco C, Christen S, Vexler ZS, Täuber MG, et al. Manipulation of antioxidant pathways in neonatal murine brain. Pediatr Res. 2004. Oct;56(4):656–62. [DOI] [PubMed] [Google Scholar]
- 34.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011. Jan;7(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson S, Li Q, Dechant A, Cohen ML. Neonatal loss of gamma-aminobutyric acid pathway expression after human perinatal brain injury. J Neurosurg. 2006. Jun;104(6 Suppl):396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013. Feb;77(3):388–405. [DOI] [PubMed] [Google Scholar]
- 37.Failor S, Nguyen V, Darcy DP, Cang J, Wend-land MF, Stryker MP, et al. Neonatal cerebral hypoxia-ischemia impairs plasticity in rat visual cortex. J Neurosci. 2010. Jan;30(1):81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwaller B, Tetko IV, Tandon P, Silveira DC, Vreugdenhil M, Henzi T, et al. Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol Cell Neurosci. 2004. Apr;25(4): 650–63. [DOI] [PubMed] [Google Scholar]
- 39.Giorgi FS, Galanopoulou AS, Moshé SL. Sex dimorphism in seizure-controlling networks. Neurobiol Dis. 2014. Dec;72 Pt B:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA(A) receptors. J Neurosci. 2008. Feb; 28(7):1557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nuñez JL, McCarthy MM. Resting intracellular calcium concentration, depolarizing Gamma-Aminobutyric Acid and possible role of local estradiol synthesis in the developing male and female hippocampus. Neuroscience. 2009. Jan;158(2):623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allard J, Paci P, Vander Elst L, Ris L. Regional and time-dependent neuroprotective effect of hypothermia following oxygen-glucose deprivation. Hippocampus. 2015. Feb;25(2): 197–207. [DOI] [PubMed] [Google Scholar]
- 43.Kasdorf E, Engel M, Heier L, Perlman JM. Therapeutic hypothermia in neonates and selective hippocampal injury on diffusion-weighted magnetic resonance imaging. Pediatr Neurol. 2014. Jul;51(1):104–8. [DOI] [PubMed] [Google Scholar]
- 44.Guo D, Zou J, Wong M. Rapamycin attenuates acute seizure-induced astrocyte injury in mice in vivo. Sci Rep. 2017. Jun;7(1):2867. [DOI] [PMC free article] [PubMed] [Google Scholar]


