Introduction


The results of the phase 3 HIMALAYA trial were presented at ASCO-GI 2022 [1]. Durvalumab plus tremelimumab has achieved positive results in urothelial cancer and non-small cell cancer in phase 3 trials. A similar combination of the anti-programmed cell death-1 (PD-1) antibody nivolumab with the anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) antibody ipilimumab was approved for the treatment of melanoma, renal cell carcinoma, high microsatellite instability colorectal cancer, and non-small cell lung cancer. In the area of hepatocellular carcinoma (HCC), nivolumab plus ipilimumab was granted accelerated approval by the FDA as second-line therapy after sorafenib based on the results of a phase 1/2 trial [2], and a phase 3 trial is currently underway. The current durvalumab plus tremelimumab regimen is the first combination immunotherapy with anti-PD-L1 and anti-CTLA-4 antibodies that have been successful in the phase 3 setting. This editorial reviews the results of the phase 1/2 and phase 3 trials and discusses the role of durvalumab plus tremelimumab in the future treatment of advanced hepatocellular carcinoma.
Phase 1/2 Trial (Study 22) Results
In the phase 1/2 trial (Study 22), patients with HCC who had progressed on, were intolerant to, or declined sorafenib were randomly assigned to one of the following four groups: (1) T300 + D arm, tremelimumab 300 mg plus durvalumab 1,500 mg (one dose each during the first cycle) followed by durvalumab 1,500 mg once every 4 weeks; (2) D arm, durvalumab monotherapy (1,500 mg once every 4 weeks); (3) T arm, tremelimumab monotherapy (750 mg once every 4 weeks [seven doses] and then once every 12 weeks); and (4) T75 + D arm, tremelimumab 75 mg once every 4 weeks plus durvalumab 1,500 mg once every 4 weeks [four doses] followed by durvalumab 1,500 mg once every 4 weeks. Safety was the primary endpoint. Secondary endpoints included objective response rate (ORR) according to the Response Evaluation Criteria in Solid Tumors version 1.1 and overall survival (OS); exploratory endpoints included circulating lymphocyte profiles [3].
The ORR was highest at 24.0% in the T300 + D arm and was 10.6% in the D arm, 7.2% in the T arm, and 9.5% in the T75 + D arm. The median OS was 18.7 months in the T300 + D arm, 13.6 months in the D arm, 15.1 months in the T arm, and 11.3 months in the T75 + D arm (Table 1).
Table 1.
Efficacy outcomes in phase 1/2 randomized trial (study 22)
| Response | T300+D (n = 75) | Durvalumab (n = 104) | Tremelimumab (n = 69) | T75+D (n = 84) |
|---|---|---|---|---|
| Median OS, months (95% CI) | 18.7 (10.8–27.3) | 13.6 (8.7–17.6) | 15.1 (11.3–20.5) | 11.3 (8.4–15.0) |
| 18 months OS, % (95% CI) | 52.0 (38.9–63.6) | 35.3 (25.0–45.8) | 45.7 (32.8–57.7) | 34.7 (24.4–45.2) |
| ORR, % (95% CI) | 24.0 (14.9–35.3) | 10.6 (5.4–18.1) | 7.2 (2.4–16.1) | 9.5 (4.2–17.9) |
| CR, % | 1.3 | 0 | 0 | 2.4 |
| PR, % | 22.7 | 10.6 | 7.2 | 7.1 |
| SD, % | 21.3 | 26.9 | 42.0 | 27.4 |
| DCR, % | 45.3 | 37.5 | 49.3 | 36.9 |
| Median TTR, months | 1.86 | 3.65 | 1.81 | 2.86 |
| Median DOR, months | NR | 11.17 | 23.95 | 13.21 |
| Median PFS, months | 2.17 (1.91–5.42) | 2.07 (1.84–2.83) | 2.69 (1.87–5.29) | 1.87 (1.77–2.53) |
| Achieving SD >6 months, % | 8.0 | 3.8 | 14.5 | 4.8 |
Modified from ref. [3]. OS, overall survival; ORR; objective response rate; CR; complete response; PR, partial response; SD, stable disease; DCR, disease control rate; TTR, time to response; DOR, duration of response; PFS, progression-free survival; NR, not reached.
One possible reason for the poor ORR and OS in the T75 + D arm is that a single high priming dose of anti-CTLA-4 is sufficient, and repeated anti-CTLA-4 priming does not produce a significant priming effect. Another possible reason is that low-dose anti-CTLA-4 priming itself is not effective. In any case, even if low-dose anti-CTLA-4 priming is not effective, multiple high-dose anti-CTLA-4 priming is not a realistic option because it can increase toxicity. On the other hand, a single high priming dose of anti-CTLA-4 is sufficient and has fewer toxicities, supporting T300 + D as the ideal regimen [3].
In the T arm, OS was as long as 15.1 months despite the low ORR (7.2%). This discrepancy may be due to a higher rate of stable disease (42.0%) and a higher disease control rate (49.3%) in this group than in the other groups, and the ability of tremelimumab to promote a sustained, durable response by itself. The duration of response (DOR) in the T arm was 23.95 months, which is extremely long and may contribute to the prolonged OS [3].
Biomarker analysis in this phase 1/2 study showed that the T300 + D arm caused the highest induction of CD8+ T cells in peripheral blood and the second highest induction of CD4+ T cells after the T arm. The proliferative CD8+/Ki67+ T cell count (cells/mm3) was highest in the responder group (complete response [CR] or partial response) in all four treatment arms and was extremely high in the responder group of the T300 + D arm [3]. This increase in the number of proliferative CD8+/Ki67+ T cells in the peripheral blood may be due to the involvement of both anti-CTLA-4 antibody (tremelimumab) and anti-PD-L1 antibody (durvalumab) in the activation of naïve T cells during T cell priming (Fig. 1), which leads to an increase in activated cytotoxic T lymphocytes in blood. Moreover, tremelimumab improved the immunosuppressive tumor microenvironment by suppressing regulatory T cells (Treg), which enhanced the killing of cancer cells and resulted in the high ORR. The combination of anti-PD-L1 and anti-CTLA-4 antibodies not only activates and increases CD8+ T cells during the priming phase but also promotes CD8+ T cell invasion into the tumor and suppresses Tregs and myeloid-derived suppressor cells [4, 5, 6]. However, Fc-gamma receptor binding activity of the anti-CTLA-4 antibody is critical for the antitumor response and survival in humans [7]. Actually, inhibitory effect of Treg by anti-CTLA-4 antibody might be found only in 20–30% in human HCCs [7].
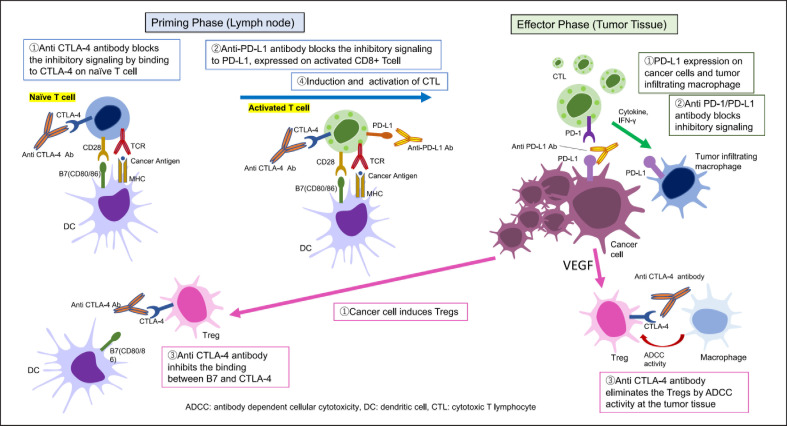
Fig. 1.
Restoring tumor immunity by blocking the CTLA-4 and PD-1/PD-L1 pathways. At the priming phase, Tregs inhibit the activation of naïve CD8+T cells by consuming interleukin-2, which is produced by CD4 + T cells. Effector Tregs suppress the maturation of antigen presenting cells through the CTLA-4/B7 pathway. In addition, naïve CD8+T cells are inactivated by expressing CTLA-4 on the surface, which binds to B7 on antigen presenting cells, resulting in the inhibition of CD8+ T cell activation. The anti-CTLA-4 antibody blocks inhibitory signaling by binding to CTLA-4 on naïve T cells (①). The anti-PD-L1 antibody blocks inhibitory signaling to PD-L1, expressed on activated CD8+ T cells (②). Anti-CTLA-4 inhibits the binding between B7 and CTLA-4 on the Treg (③). Finally, CD8+ T cells are induced and activated to become CTL (④). At the effector phase, PD-L1 is expressed on cancer cells and tumor infiltrating macrophages (①). The anti-PD-1/PD-L1 antibody blocks inhibitory signaling (②). The anti-CTLA-4 antibody eliminates Tregs through ADCC at the tumor tissue (③). These blockades of the CTLA-4 and PD-1/PD-L1 pathways restore the antitumor activity of CTL in the cancer immune microenvironment. ADCC, antibody-dependent cellular cytotoxicity; DC, dendritic cell; CTL, cytotoxic T cells; Treg, regulatory T cells.
The incidence of serious treatment-related adverse events (TRAEs) was highest in the T arm (24.6%) and was 17.6%, 10.9%, and 14.6% in the T300 + D, D, and T75 + D arms, respectively. TRAEs requiring systemic steroids were more frequent in the tremelimumab-containing arms (T300 + D: 24.3%; tremelimumab: 26.1%; T75 + D: 24.4%) than in the D arm (9.9%). Immune-mediated AEs also occurred at a higher frequency in the tremelimumab-containing arms (T300 + D: 31.1%; T: 24.6%; T75 + D: 26.8%) than in the D arm (15.8%). TRAEs requiring discontinuation occurred in 6–13% of patients across arms and were highest in the T arm. The incidence of hepatitis or hepatic failure as immune-mediated AE was low in all arms (≤2 patients per arm).
In the T300 + D arm, the incidence of TRAEs requiring systemic steroids was 24.3%, and the safety profile appeared favorable: only 10.8% of patients in this arm discontinued treatment because of TRAEs [3]. These results demonstrate an acceptable tolerability profile of the T300 + D regimen.
Phase 3 HIMALAYA Trial Results
The HIMALAYA trial started with four arms [1]: arm 1, tremelimumab (300 mg, one dose) plus durvalumab (1,500 mg every 4 weeks, STRIDE regimen = T300 + D arm in the phase 1/2 study); arm 2, durvalumab (1,500 mg every 4 weeks); arm 3, tremelimumab (75 mg every 4 weeks, 4 doses) plus durvalumab (1,500 mg every 4 weeks; T75 + D); and arm 4, sorafenib (400 mg twice daily). However, enrollment into the T75 + D arm (arm 3) was discontinued midway through the study because of the poor results of the phase 1/2 trial (Study 22) [3]. Consequently, 1,171 patients were randomized to the STRIDE arm (n = 393), durvalumab arm (n = 389), or sorafenib arm (n = 389).
The median OS (95% CI) in the STRIDE arm was 16.4 (95% CI 14.2–19.6) months, and that in the sorafenib arm was 13.8 (95% CI, 12.3–16.1) months, demonstrating the statistically significant superiority of the STRIDE regimen over sorafenib in terms of OS (HR = 0.78 [96.02% CI, 0.65–0.92]; p = 0.0035). The noninferiority of durvalumab to sorafenib was also demonstrated (HR = 0.86 [95.67% CI, 0.73–1.03], prespecified noninferiority margin, 1.08) (Table 2).
Table 2.
Results of phase 3 HIMALAYA trial: efficacy outcome
| HIMALAYA trial | |||
|---|---|---|---|
| STRIDE (D+T300) (n = 393) | durvalumab (n = 389) | sorafenib (n = 389) | |
| Median follow-up, months | 33.2 | 32.6 | 32.2 |
| Median OS, months (95% CI) | 16.4 (14.2–19.6) | 16.6 (14.1–19.1) | 13.8 (12.3–16.1) |
| OS HR | 0.78 (96.02% CI, 0.65–0.92) | 0.86 (95.67% CI, 0.73–1.03) | |
| p value | 0.0035 | ||
| Median PFS, months (95% CI) | 3.8 (3.7–5.3) | 3.7 (3.2–3.8) | 4.1 (3.8–5.5) |
| PFS HR (95% CI) | 0.90 (0.77–1.05) | 1.02 (0.88–1.19) | |
| ORR, % | 20.1 | 17.0 | 5.1 |
| CR, n (%) | 12 (3.1) | 6 (1.5) | 0 |
| PR, n (%) | 67 (17.0) | 60 (15.4) | 20 (5.1) |
| SD, n (%) | 157 (39.9) | 147 (37.8) | 216 (55.5) |
| PD, n (%) | 157 (39.9) | 176 (45.2) | 153 (39.3) |
| DCR, n (%) | 236 (60.1) | 213 (54.8) | 23.6 (60.7) |
| Median DOR, months (IQR) | 22.34 (8.54–NR) | 16.82 (7.43–NR) | 18.43 (6.51–25.99) |
| Median TTR, months (95% CI) | 2.17 (1.84–3.98) | 2.09 (1.87–3.98) | 3.78 (1.89–8.44) |
Modified from ref. [1]. STRIDE, Single Tremelimumab Regular Interval Durvalumab; D+T300, durvalumab plus high dose tremelimumab; OS, overall survival; HR, hazard ratio; PFS, progression-free survival; ORR, objective response rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate; DOR, duration of response; TTR, time to response; N/R, not reached.
Progression-free survival (PFS) (95% CI) was not prolonged in the STRIDE (3.8 months [3.7–5.3]) or durvalumab (3.7 months [3.2–3.8]) arm compared with the sorafenib arm (4.1 months [3.8–5.5]). ORR and CR rates (Response Evaluation Criteria in Solid Tumors ver. 1.1) were 20.1% and 3.1% with the STRIDE regimen, 17.0% and 1.5% with durvalumab, and 5.1% and 0% with sorafenib, respectively. The disease control rate was 60.1% with STRIDE, 54.8% with durvalumab, and 60.7% with sorafenib. The DOR was 22.34 months with STRIDE, 16.82 months with durvalumab, and 18.43 months with sorafenib.
The incidence rates of any grade TRAEs in the STRIDE, durvalumab, and sorafenib arms were 75.8%, 52.1%, and 84.8%, respectively. There was no significant difference in the incidence of grade 3/4 TRAEs between the three arms (25.8% in STRIDE, 12.9% in durvalumab, and 36.9% in sorafenib). Serious TRAEs and TRAEs leading to death were slightly more common in the STRIDE arm (Table 3). There was no significant difference in the incidence of TRAEs leading to discontinuation, grade 3/4 hepatic TRAEs, or grade 3/4 hemorrhage TRAEs between the three arms (Table 3). Grade 3/4 immune-mediated TRAEs, immune-mediated AEs requiring treatment with high-dose steroids, and immune-mediated AEs leading to discontinuation of treatment were only slightly more common in the STRIDE arm, which did not raise any concerns about tolerability (Table 3). The incidence of these grade 3/4 TRAEs in the STRIDE arm (25.8%) was somewhat lower than that previously reported (>50%) in arm A of the CheckMate 040 study (nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks [four doses] followed by nivolumab 240 mg flat dose every 2 weeks) [2]. This difference may be due to the different regimens, as only a single initial priming dose of anti-CTLA-4 antibody was given in the HIMALAYA trial. Furthermore, there were few incidences of events that commonly occur in combination immunotherapy with anti-VEGF/TKI and anti-PD-1/PD-L1 antibodies, such as hypertension, proteinuria, hand-foot skin reactions, and bleeding events [8, 9].
Table 3.
Results of phase 3 HIMALAYA trial: safety outcome
| Event | STRIDE (n = 388) | Durvalumab (n = 388) | Sorafenib (n = 374) |
|---|---|---|---|
| TRAE, n (%) | 294 (75.8) | 202 (52.1) | 317 (84.8) |
| Grade 3/4 TRAE, n (%) | 100 (25.8) | 50 (12.9) | 138 (36.9) |
| Serious TRAE, n (%) | 68 (17.5) | 32 (8.2) | 35 (9.4) |
| TRAE leading to death, n (%) | 9 (2.3)a | 0 | 3 (0.8)b |
| TRAE leading to discontinuation, n (%) | 32 (8.2) | 16 (4.1) | 41 (11.0) |
| Grade 3/4 hepatic SMQ TRAE, n (%) | 23 (5.9) | 20 (5.2) | 17 (4.5) |
| Grade 3/4 hemorrhage SMQ TRAE, n (%) | 2 (0.5) | 0 | 4 (1.1) |
| Grade 3/4 immune-mediated TRAE, n (%) | 49 (12.6) | 24 (6.2) | 9 (2.4) |
| Immune-mediate AE requiring treatment with high-dose steroids, n (%) | 78 (20.1) | 37 (9.5) | 7 (1.9) |
| Immune-mediated AE leading to discontinuation of study treatment, n (%) | 22 (5.7) | 10 (2.6) | 6 (1.6) |
Modified from ref. [1]. SMQ, Standardized MedDRA Query; STRIDE, Single Tremelimumab Regular Interval Durvalumab; TRAE, treatment-related adverse event. a Nervous system disorder (n = 1), acute respiratory distress syndrome (n = 1), hepatitis (n = 1), myocarditis (n = 1), immune-mediated hepatitis (n = 2), pneumonitis (n = 1), hepatic failure (n = 1), myasthenia gravis (n = 1). b Hematuria (n = 1), cerebral hematoma (n = 1), hepatic failure (n = 1).
Post-treatment immunotherapies may have contributed to the tail plateau in the sorafenib arm, as found in the CheckMate 459 trial [10].
Positioning of Durvalumab Plus Tremelimumab for Unresectable HCC in Real-World Clinical Practice
The STRIDE regimen showed clear superiority over sorafenib, with a favorable 3-year survival rate of 30.7% in the Kaplan-Meier curve. The regimen was also superior to durvalumab monotherapy in terms of ORR, OS, and CR rate, clearly demonstrating that a single high priming dose of anti-CTLA-4 antibody has an add-on effect when combined with anti-PD-L1 antibody and functions as theory suggests in patients with HCC. This regimen is the first combination of anti-PD-L1 and anti-CTLA4 antibodies for the treatment of HCC and has an acceptable and manageable toxicity profile. Because this regimen has a different mode of action from the currently used anti-PD-L1 plus anti-VEGF regimen, sequential therapy with both regimens is worthy of consideration. Moreover, the regimen is associated with a low incidence of AEs and no bleeding risk despite the use of an anti-CTLA-4 antibody. Thus, it may eliminate the need for endoscopy immediately before treatment.
Another major advantage of this regimen is that it does not show the AEs associated with ICI + anti-VEGF/TKI combination therapy in advanced HCC related to the anti-VEGF effect of molecular targeted agents, such as proteinuria, hypertension, ascites, or encephalopathy. However, the HIMALAYA trial excluded HCC patients with Vp4 (tumor thrombus involving the main trunk and/or contralateral branch of the portal vein), and the lack of data on these patients is one of the limitations of this regimen. In addition, although combination immunotherapy with anti-PD-1/PD-L1 antibody and anti-VEGF antibody or a TKI shows a certain level of efficacy against WNT/β-catenin mutation [11] and NASH-related HCC [12], whether the combination of anti-PD-L1 and anti-CTLA-4 antibodies is effective against these diseases remains unclear. This combination may not be effective for the treatment of these diseases, which may explain the high rate of PD (39.9%) with the STRIDE regimen. The lack of an anti-VEGF effect may also decrease the efficacy of the regimen for improving the immunosuppressive microenvironment compared with the anti-PD-L1 plus anti-VEGF regimen [13].
Atezolizumab plus bevacizumab, which is currently the first-line therapy of choice, is effective against Vp4. In a comparison of the atezolizumab plus bevacizumab combination regimen with the durvalumab plus tremelimumab regimen, the hazard ratio for OS of the IMbrave150 (updated) regimen versus sorafenib was 0.66, which is better than that of the STRIDE regimen versus sorafenib (0.78). The PD rate was 19% with atezolizumab plus bevacizumab versus 40% with the STRIDE regimen, indicating that twice as many patients treated with the STRIDE regimen did not respond to treatment at all. A similar trend was observed for PFS; the hazard ratio for PFS was 0.65 for atezolizumab plus bevacizumab, and 0.90 for the STRIDE regimen versus sorafenib. Atezolizumab plus bevacizumab was also superior to STRIDE in terms of ORR (30% vs. 20%) and CR rate (8% vs. 3%). However, the DOR was slightly longer, with the STRIDE regimen at 22.34 months versus 18.1 months with atezolizumab plus bevacizumab.
One interpretation of the results of the phase 3 IMbrave 150 and HIMALAYA studies suggests that the first choice of first-line therapy should generally be atezolizumab plus bevacizumab. However, patients who had PD on atezolizumab plus bevacizumab could be treated with durvalumab plus tremelimumab, a combination therapy with a different mode of action, as second-line therapy. Because the atezolizumab plus bevacizumab regimen does not deteriorate liver functional reserve (Kudo M., ILCA2021), most of the patients who received atezolizumab plus bevacizumab can move on to durvalumab plus tremelimumab (A+B→ D+T sequence).
Another option is to start with durvalumab plus tremelimumab, as anti-CTLA-4 antibody priming is only needed once, and then sequentially switch to atezolizumab plus bevacizumab as soon as PD is confirmed. This enables substantial triple regimen with anti-CTLA4, anti-PD-L1, and anti-VEGF antibodies. This triple therapy sequence may be another potentially effective treatment strategy (D+T → A+B sequence).
A third option would be to start with atezolizumab plus bevacizumab, followed by a scheduled, short-term treatment with durvalumab plus tremelimumab, and then return to atezolizumab plus bevacizumab (A+B → D+T → A+B sequence). In fact, advanced melanoma, CheckMate 064 study clearly showed PD-1 antibody(nivolumab) followed by anti-CTLA-4(ipilimumab) sequence showed better efficacy than the first ipilimumab followed by nivolumab [14]. Similarly, anti-CTLA-4 could be more effective after anti-PD-1/PD-L1 plus anti-VEGF combination therapy in HCC as well.
Both the immuno-oncology (IO)-IO combination and sequential therapy with IO and anti-VEGF antibody are viable treatment strategies, regardless of which regimen is used first. In the future, it will be common practice to move on to targeted therapy only after PD occurs with both regimens.
In the HIMALAYA trial, durvalumab showed noninferiority to sorafenib. If durvalumab monotherapy is also approved, it would be the first single-agent PD-L1 antibody to be tested in the phase 3 setting, excluding nivolumab and pembrolizumab, which were both approved in an accelerated manner by the FDA.
The indication for single-agent PD-L1 antibody treatment in clinical practice is a matter of debate. As anti-PD-L1 monotherapy, single-agent durvalumab could be used in patients who are intolerant to atezolizumab plus bevacizumab because of AEs such as proteinuria, bleeding risk, and hypertension; elderly patients who may be susceptible to toxicities associated with anti-CTLA-4 antibody; patients with poor PS; patients with severe complications; or patients with Child-Pugh B liver function. However, it should be kept in mind there is a trade-off between toxicity and efficacy, and treatments associated with fewer AEs tend to be less effective [15]. Most valuable aspect in IO-IO combination therapy and IO monotherapy in clinical practice is that they do not cause proteinuria, the biggest adverse event to prevent successful sequential therapy using IO plus anti-VEGF and effective molecular targeted agents, all of which do have adverse event of proteinuria owing to anti-VEGF activity.
Statement of Ethics
Statement of Ethics is not applicable.
Conflict of Interest Statement
Lecture: Eisai, Bayer, MSD, BMS, EA Pharma, Eli Lilly, Chugai; Grants: Eisai, Takeda, Otsuka, Taiho, EA Pharma, Gilead Sciences, Abbvie, Sumitomo Dainippon Pharma, Chugai, Ono Pharma; Advisory Consulting: Eisai, Ono, MSD, BMS, Roche. Masatoshi Kudo is Editor-in-Chief of Liver Cancer.
Funding Sources
No funding was received for this study.
Author Contributions
M. Kudo conceived, wrote, and approved the final manuscript.
Data Availability Statement
Data availability is not applicable.
References
- 1.Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab and durvalumab as first-line therapy in patients with unresectable hepatocellular carcinoma: HIMALAYA. J Clin Oncol. 2022;40((4)):379. [Google Scholar]
- 2.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol. 2020 Oct 1;6((11)):e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a Phase I/II Study. J Clin Oncol. 2021 Sep 20;39((27)):2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010 Mar 2;107((9)):4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015 Feb 1;194((3)):950–9. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha D, Tanaka A, Kibayashi T, Tanemura A, Sugiyama D, Wing JB, et al. Differential control of human Treg and effector T cells in tumor immunity by Fc-engineered anti-CTLA-4 antibody. Proc Natl Acad Sci U S A. 2019 Jan 8;116((2)):609–18. doi: 10.1073/pnas.1812186116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E, et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell. 2018 Apr 9;33((4)):649–63. doi: 10.1016/j.ccell.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib Study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020 Sep 10;38((26)):2960–70. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020 May 14;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 10.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022 Jan;23((1)):77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 11.Kudo M. Gd-EOB-DTPA-MRI could predict WNT/β-catenin mutation and resistance to immune checkpoint inhibitor therapy in hepatocellular carcinoma. Liver Cancer. 2020;9((5)):479–90. doi: 10.1159/000509554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo M. Impaired response to immunotherapy in non-alcoholic steatohepatitis-related hepatocellular carcinoma? Liver Cancer. 2021 Jul;10((4)):289–95. doi: 10.1159/000517841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudo M. A new era in systemic therapy for hepatocellular carcinoma: atezolizumab plus bevacizumab combination therapy. Liver Cancer. 2020 Apr;9((2)):119–37. doi: 10.1159/000505189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL, Jr, Lawrence DP, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. 2016 Jul;17((7)):943–55. doi: 10.1016/S1470-2045(16)30126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudo M. Limited impact of anti-PD-1/PD-L1 monotherapy for hepatocellular carcinoma. Liver Cancer. 2020 Dec;9((6)):629–39. doi: 10.1159/000512170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable.