Abstract
Previously we showed that only one phage-expressed protein (Orf1), a 425-bp region upstream of the orf1 gene (presumably encoding a promoter), and the attP region are necessary and also sufficient for integration of the bacteriophage TP901-1 genome into the chromosome of Lactococcus lactis subsp. cremoris (B. Christiansen, L. Brøndsted, F. K. Vogensen, and K. Hammer, J. Bacteriol. 178:5164–5173, 1996). In this work, a further analysis of the phage-encoded elements involved in integration was performed. Here we demonstrate that even when the orf1 gene is separated from the attP region, the Orf1 protein is able to promote site-specific integration of an attP-carrying plasmid into the attB site on the L. lactis subsp. cremoris chromosome. Furthermore, the first detailed deletion analysis of an attP region of a phage infecting lactic acid bacteria was carried out. We show that a fragment containing 56 bp of the attP region, including the core, is sufficient for the site-specific integration of a nonreplicating plasmid into the chromosome of L. lactis subsp. cremoris when the orf1 gene is donated in trans. The functional 56-bp attP region of TP901-1 is substantially smaller than minimal attP regions identified for other phages. Based on the deletion analysis, several repeats located within the attP region seem to be necessary for site-specific integration of the temperate bacteriophage TP901-1. By use of the integrative elements (attP and orf1) expressed by the temperate lactococcal bacteriophage TP901-1, a system for obtaining stable chromosomal single-copy transcriptional fusions in L. lactis was constructed. Two promoter-reporter integration vectors containing the reporter gene gusA or lacLM, encoding β-glucuronidase or β-galactosidase, respectively, were constructed. Immediately upstream of both genes are found translational stop codons in all three reading frames as well as multiple restriction enzyme sites suitable for cloning of the promoter of interest. By transformation of L. lactis subsp. cremoris MG1363 containing the integrase gene on a replicating plasmid, the promoter-reporter integration vectors integrated with a high frequency site specifically into the chromosomal attachment site attB used by bacteriophage TP901-1.
TP901-1 is a temperate phage for which Lactococcus lactis subsp. cremoris 3107 is the host. During infection, the phage genome can integrate site specifically into the bacterial chromosome by recombination between attachment sites attB and attP located on the bacterial and the phage genomes, respectively. This process leads to the formation of the hybrid attachment sites attL and attR at the junctions between the phage and the bacterial genomes. In all attachment sites (attB, attP, attR, and attL), the 5-bp core region in which recombination occurs is present. In addition, a 7-bp identical region is present in all four attachment sites, separated from the core region by a 1-bp mismatch (8).
Upstream of the attP region is located an open reading frame (orf1) which encodes a 485-amino-acid protein (Orf1). Orf1 is a member of a new family of site-specific recombinases which are more than twice the size of the resolvases but which show significant similarity to the resolvases in the N terminus of about 150 amino acids (9). The family of extended resolvases contains three more integrases encoded by bacteriophages: the Sre protein of phage R4 of Streptomyces parvulus, Orf613 of phage φC31 of Streptomyces fradia, and part of an open reading frame of Bacillus cereus bacteriophage TP21 (17, 21, 24). Also included are the site-specific recombinases from Bacillus subtilis and several Anabaena species involved in chromosomal inversion and deletion events occurring during differentiation into spores or heterocysts as well as the resolvase (TnpX) of the conjugative chloramphenicol resistance transposon Tn4451 from Clostridium perfringens (3, 7, 32). Identified integrases of other temperate lactococcal bacteriophages (Tuc2009, r1-t, φLC3, and BK5-T) are all of the Int type, showing homology to the integrase of Escherichia coli bacteriophage λ (5, 19, 34, 35). Orf1 is thus a unique type of integrase among temperate lactococcal bacteriophages.
The study of gene expression and gene regulation in lactic acid bacteria has been carried out mainly by use of transcriptional fusions located on replicating plasmids. In these studies, the variation in the copy number of the plasmids under different physiological conditions and in different mutants was not taken into account. By maintenance of the transcriptional fusions in single copies on the chromosome, the effects of plasmid copy number can be avoided. Several systems for the integration of genes into the chromosomes of lactic acid bacteria have been described, but none of these have been specifically designed for the study of gene expression and regulation (2, 4, 20). Only Sanders et al. (30) described a method for the construction of chromosomal lacZ transcriptional fusions by homologous recombination.
Previously we showed that only one phage-expressed protein (Orf1), a 425-bp region upstream of the orf1 gene (presumably encoding a promoter), and the attP region are necessary and also sufficient for integration of the phage TP901-1 genome into the chromosome of L. lactis subsp. cremoris (9). In this work, we performed a detailed deletion analysis of the attP region. Furthermore, we describe a method for stable site-specific integration of transcriptional fusions into the chromosome of L. lactis. The system is based on the phage-encoded elements (the attachment site attP and the integrase gene orf1) necessary for integration of the temperate lactococcal bacteriophage TP901-1 into the chromosome of L. lactis.
MATERIALS AND METHODS
Cell growth and enzyme assay.
Lactococcus strains were propagated at 30°C in M17 broth (Oxoid Limited, Basingstoke, Hampshire, United Kingdom) containing 0.5% (wt/vol) glucose without shaking (33). E. coli strains were grown with agitation at 37°C in Luria-Bertani broth (Difco Laboratories, Detroit, Mich.) (29). Bacto Agar (Difco) was used at 1.5% (wt/vol) in solid media. For determination of β-galactosidase activity, cells were permeabilized with sodium dodecyl sulfate (0.1%) and chloroform. Cell debris was removed by high-speed centrifugation. The assay was performed as described by Miller (25).
DNA technology.
Extraction of chromosomal DNA was performed as described for E. coli (29), with the modification that cells were treated with 20 μg of lysozyme per ml for 2 h before lysis. Recombinant plasmid DNA from E. coli was isolated by the alkaline lysis technique, and preparative portions were further purified on Qiagen (Hilden, Germany) columns as recommended by the supplier. Restriction endonuclease enzymes, DNA polymerase Klenow fragment, T4 DNA ligase, and buffer systems were supplied by Pharmacia Biotech. All enzymes were used as recommended by the supplier. The PCR was performed by use of a DNA thermal cycler (Perkin-Elmer Cetus) with Amplitaq polymerase and buffer supplied from Perkin-Elmer Cetus.
Plasmid DNA for sequencing was prepared from E. coli DH5α. The DNA sequences were determined by the method of Sanger and coworkers (31) with a Sequenase version 2.0 DNA sequencing kit (U.S. Biochemical Corp., Cleveland, Ohio).
Construction of plasmids.
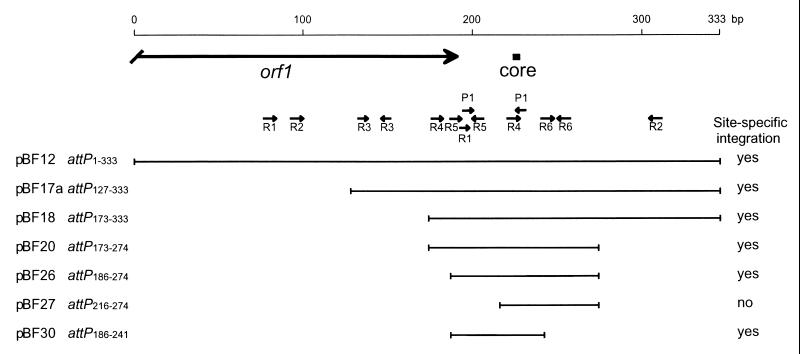
The plasmids used in this study are listed in Table 1. By use of TP901-1 DNA as a template and primers PB2 and PB3, a 333-bp attP PCR fragment was produced and cloned into the pMOSBlue vector (Amersham Life Science). Subsequently, the erm gene (1.1 kb) from pUC7,erm was cloned into the EcoRI site, resulting in plasmid pBF12 containing a 333-bp attP region (attP1–333) (Fig. 1). Plasmids pBF17a and pBF17b were constructed as follows. A purified 237-bp HincII fragment from pBF12 was cloned into a purified 3.9-kb pBF12 fragment digested with SmaI and XbaI, and Klenow polymerase was used for filling in. The orientation of the 207-bp attP region was the same in plasmid pBF17a (attP127–333) (Fig. 1) as in pBF12, whereas in pBF17b (attP127–333) (Fig. 1) the same attP region was cloned in the opposite orientation. Plasmid pBF18 (attP173–333) (Fig. 1) was constructed by religation of a 4.1-kb purified EcoRV- and SmaI-digested pBF12 fragment; pBF18 therefore contains 161 bp of the attP region. Plasmid pBF20 was constructed by cloning a 102-bp purified AseI- and KpnI-digested fragment from pBF12 into a digested (NdeI and KpnI) and purified 3.9-kb fragment from pBF12; plasmid pBF20 therefore contains 102 bp of the attP region (attP173–274) (Fig. 1). Plasmids pBF26, pBF27, and pBF30 contain 89 bp (attP186–274), 59 bp (attP216–274), and 56 bp (attP186–241) of the attP region, respectively (Fig. 1), and were constructed by cloning of PCR fragments produced with pBF12 as the template. For construction of plasmids pBF26 and pBF27, PCR fragments were produced with primers PB1 and PB4 or primers T7 and PB5, respectively. These PCR fragments were digested with AseI, purified, and cloned into a purified 3.9-kb SmaI- and NdeI-digested pBF12 fragment, resulting in plasmids pBF26 and pBF27. For construction of plasmid pBF30, a PCR fragment was produced with primers PB6 and PB4. This PCR fragment was digested with SphI and EcoRV, purified, and cloned into a purified 3.9-kb SphI- and SmaI-digested fragment from pBF12. All attP fragments, except those in pBF17b, were cloned in the same orientation within the vector, and the presence of the respective attP regions was confirmed by sequencing.
TABLE 1.
Plasmids used in this study
| Plasmid | Origina | Relevant genotypeb | Reference or source |
|---|---|---|---|
| pMOSBlue | lacZ bla | Amersham Life Science | |
| pUC7,erm | bla erm | Received from W. de Vos | |
| pCI372 | cat | 12 | |
| pGhost8 | tet orits | 22 | |
| pAK80 | lacLM erm | 15 | |
| pNZ273 | gusA cat | Received from W. de Vos | |
| pTRKH2 | erm | 27 | |
| pCP15 | CP15-lacLM | 16 | |
| pCP22 | CP22-lacLM | 16 | |
| pJM334 | Pupp-lacLM | 23 | |
| pAB201 | pGEM-3Zf(+)::1.4-kb PCR BamHI-SalI | orf1 bla | 6 |
| pLB45 | pGEM-7Zf(+)::2.2-kb EcoRI-XbaI TP901-1 1.1-kb erm | orf1 attP bla erm | 9 |
| pLB61 | pAB201 EcoRI-ClaI 3.4-kb::1.6-kb EcoRI-ClaI pLB45 | orf1 bla | This study |
| pLB65 | pCI372::1.9-kb EcoRI-SalI pLB61 | orf1 cat | This study |
| pLB85 | pBF17a::1.8-kb PstI-HindIII pNZ273 | attP127–333gusA bla erm | This study |
| pLB86 | pBF17b::4.0-kb HindIII-SalI pAK80 | attP127–333lacLM bla erm | This study |
| pLB87 | pBF17b::4.1-kb HindIII-SalI pCP22 | attP127–333 CP22-lacLM | This study |
| pLB88 | pBF17b::4.1-kb HindIII-SalI pCP15 | attP127–333 CP15-lacLM | This study |
| pLB89 | pBF17b::4.9-kb HindIII-SalI pJM334 | attP127–333 Pupp-lacLM | This study |
| pLB95 | pGhost8::1.9-kb EcoRI-SalI pLB61 | orf1 tet orits | This study |
| pBF12 | pMOSBlue::333-bp PCR attP 1.1-kb EcoRI erm | attP1–133bla erm | This study |
| pBF17a | pBF12 SmaI-XbaI Klenow 3.9-kb::237-bp HincII attP from pBF12 | attP127–333bla erm | This study |
| pBF17b | pBF12 SmaI-XbaI Klenow 3.9-kb::237-bp HincII attP from pBF12 | attP127–333bla erm | This study |
| pBF18 | pBF12 SmaI-EcoRV 4.1-kb | attP173–333bla erm | This study |
| pBF20 | pBF12 NdeI-KpnI 3.9-kb::107-bp AseI-KpnI attP from pBF18 | attP173–274bla erm | This study |
| pBF26 | pBF12 SmaI-NdeI 3.9-kb::97-bp AseI blunt PCR (PB1-PB4) | attP186–274bla erm | This study |
| pBF27 | pBF12 SmaI-NdeI 3.9-kb::67-bp AseI blunt PCR (T7-PB5) | attP216–274bla erm | This study |
| pBF30 | pBF12 SmaI-SphI 3.9-kb::56-bp SphI blunt PCR (PB6-PB4) | attP186–241bla erm | This study |
Kilobases and base pairs indicate sizes of fragments. The restriction endonuclease enzymes used for digestion are indicated. Ligation is shown by ::. For blunt PCR a polymerase that leaves blunt ends is used. Primers for PCR are indicated in parentheses.
The subscript numbers for the attP fragments refer to the base numbers in Fig. 1. orits, temperature-sensitive origin.
FIG. 1.
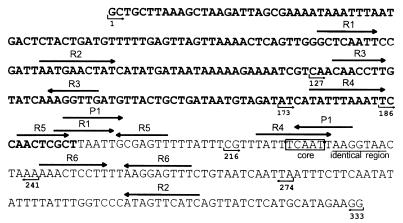
Sequence of the attP region of the temperate bacteriophage TP901-1. The bold sequence shows the coding region for the integrase of TP901-1 (Orf1). The core region is boxed, and the identical region is underlined. Direct and inverted repeats are indicated with black arrows above the sequence. Repeats identified by Christiansen et al. (8) are R1, R2, R3, R4, R5, and R6, whereas the recently identified inverted repeat is P1. The numbers and small arrows under the sequence indicate the borders of the different attP fragments. The largest attP fragment contains all bases shown, e.g., 1 to 333. Base 1 corresponds to base 2499 in the sequence deposited in GenBank.
Plasmids containing the orf1 gene without the attP region of TP901-1 were constructed as follows. Plasmid pAB201 contains the orf1 gene without the upstream sequence and the attP region. Plasmid pLB61 was constructed by ligation of a 3.4-kb EcoRI-ClaI fragment of pAB201 and a 1.6-kb EcoRI-ClaI fragment of pLB45. A 1.9-kb EcoRI-SalI fragment from pLB61 was cloned into shuttle vector (both E. coli and L. lactis) pCI372 (12), giving rise to plasmid pLB65, and into L. lactis vector pGhost8 (22), which carries a temperature-sensitive origin of replication, giving rise to plasmid pLB95. Plasmids pLB65 and pLB95 both contain the orf1 gene and a 425-bp region upstream of the orf1 gene but not the attP core region usually located downstream of the orf1 gene.
The promoter-reporter integration vector pLB85 was constructed as follows. A 1.8-kb purified PstI-HindIII fragment from pNZ273 carrying the gusA gene as well as stop codons in all three reading frames was cloned into PstI- and HindIII-digested pBF17a. The promoter-reporter integration vector pLB86 was constructed by cloning a purified 4.0-kb HindIII-SalI fragment from pAK80 containing the lacL and lacM genes (15) into plasmid pBF17b. Integration vectors carrying promoter regions were constructed as follows. From pCP15 and pCP22 (16), 4.1-kb HindIII-SalI fragments carrying the constitutive promoters and the reporter genes lacL and lacM were cloned into pBF17b, giving rise to pLB88 and pLB87, respectively. Plasmid pLB89 was constructed by cloning a 4.9-kb HindIII-SalI fragment from pJM334 (23) into pBF17b.
Primers used in this study.
The primers used for PCR amplification of fragments for cloning were as follows: PB1 (5′-CCTTCTATGCATGAGATAAC-3′), PB2 (5′-GCTGCTTAAAGCTAAGATT-3′), PB3 (5′-GCAAATTTCACAGATCGATA-3’), PB4 (5′-GGGGGCTCGAGTCCAACTCGCTTAATTGC-3′), PB5 (5′-GGGGGCTCGAGCGTTTATTTCAATTAAGGTAAC-3′), PB6 (5′-GGGGGGCATGCTTTAGTTACCTTAATTGAAAT-3′), and T7 (5′-TATACGACTCACTATAGGG-3′).
The primers used for PCR amplification of attB were as follows: pattBL (5′-CTACTGCTGCTTCACCAG-3′) and BI-POB1inv (5′-GTATGCAGCGATGTCGTTACCC-3′). The primers used for PCR amplification of attL and attR of integrated pLB85 and derivatives thereof were as follows: pattBL and gusArev (5′-GTCGAGTTTTTTGATTTCACGGG-3′) (for attL) and BI-POB1inv and 1211 (5′-GTAAAACGACGGCCATG-3′) (for attR). The primers used for PCR amplification of attL and attR of integrated pLB86 and derivatives thereof were as follows: pattBL and 1211 (for attL) and BI-POB1inv and PB5 (5′-CGTTTATTTCAATTAAGGTAAC-3′) (for attR).
Transformation and selection in E. coli and L. lactis subsp. cremoris.
E. coli DH5α [φ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1] (laboratory strain) was made competent with CaCl2 and transformed as described by Sambrook et al. (29). When the erm cassette was introduced, selection was performed with 100 μg of ampicillin per ml and 150 μg of erythromycin per ml.
L. lactis subsp. cremoris MG1363 (10) was transformed by electroporation by the method of Holo and Nes (13) with 0.03 to 0.5 μg of DNA per electroporation. Transformants were selected on plates containing 2 μg of erythromycin per ml. Integration was analyzed as the presence of attL and attR and the absence of attB in chromosomal DNA from representative transformants by PCR as previously described (8). The DNA concentration of the plasmid preparations was determined by comparing the digested plasmid DNA with known amounts of λ DNA digested with HindIII (Boehringer Mannheim GmbH).
Nucleotide sequence accession number.
The attP sequence reported in this study has been deposited in GenBank under accession no. X85213.
RESULTS
Deletion analysis of the attP region of TP901-1.
In E. coli bacteriophage λ, several proteins involved in the recombination process bind to repeats present within the attP region. One of these proteins is the phage-expressed integrase responsible for the actual crossing over of the DNA strands, whereas other proteins present in the host are involved in changing the DNA conformation: IHF (integration host factor), FIS (factor for inversion stimulation), and XIS (excise) (for a review, see reference 18). The attP region of the temperate bacteriophage TP901-1 contains, in addition to the 5-bp core region and a 7-bp region also present in the attachment sites attB, attL, and attR, several direct and inverted repeats surrounding and overlapping the core region (Fig. 1). These repeats could be binding sites for proteins involved in recombination between the attP and attB sites during integration of the TP901-1 phage genome into the bacterial chromosome. By deletion analysis, the importance of the repeats located in the attP region for the integration process was investigated. Several of the repeats are located within orf1 (Fig. 1), encoding the integrase of TP901-1, which mediates recombination between the attP and attB sites. In order to make deletions in the attP region without interfering with orf1, the attP region was separated from the orf1 gene.
All plasmids which carry different parts of the attP region are derivatives of E. coli vectors containing a selectable marker (erm) functional in Lactococcus but no origin of replication functional in gram-positive bacteria. The integrase is donated in trans by plasmid pLB65, which carries both the expressed orf1 gene without the attP region and the cat gene and is able to replicate in L. lactis subsp. cremoris. The ability of all attP plasmids to integrate into the chromosomal attB site was investigated by transformation of L. lactis subsp. cremoris MG1363 containing pLB65 with the respective attP plasmids and selection for erythromycin resistance. As a control, L. lactis subsp. cremoris MG1363 containing the vector (pCI372) used for the construction of pLB65 was transformed. Therefore, the frequency of transformation of L. lactis subsp. cremoris MG1363 in the presence of either pLB65 (orf1) or pCI372 (vector) could be calculated for all attP-carrying plasmids (Table 2). In the presence of pLB65 (orf1), the frequency of transformation varied from 5 × 106 to 2 × 107 CFU/μg of DNA for all attP plasmids, except pBF27, which resulted in a frequency of transformation of 104 CFU/μg of DNA. In the presence of pCI372 (vector), the frequency of transformation varied from 2 × 102 to 2 × 104 CFU/μg of DNA for all attP plasmids (Table 2).
TABLE 2.
Frequency of transformation of L. lactis subsp. cremoris MG1363
| attP plasmid | Frequency of transformation a
|
% of site-specific recombinationb | |
|---|---|---|---|
| pLB65 (orf1 plasmid) | pCI372 (vector) | ||
| pBF12 | 1 × 107 | 5 × 103 | 99.9 |
| pBF17 | 2 × 107 | 2 × 104 | 99.9 |
| pBF18 | 9 × 106 | 7 × 103 | 99.9 |
| pBF20 | 1 × 107 | 4 × 103 | 99.9 |
| pBF26 | 1 × 107 | 1 × 104 | 99.9 |
| pBF27 | 1 × 104 | 1 × 104 | 0.0 |
| pBF30 | 5 × 106 | 5 × 103 | 99.9 |
| pTRKH2c | 1 × 107 | 1 × 107 | 0.0 |
| None | 0 | 0 | ND |
Number of erythromycin-resistant transformants of L. lactis subsp. cremoris MG1363 containing pLB65 or pCI372 per microgram of attP plasmid DNA.
Percentage of insertions of attP plasmids due to site-specific recombination, calculated as (1 − B/A × 100, where A is the number of transformants containing pLB65 and B is the number of transformants containing pCI372. ND, not done.
Plasmid pTRKH2 is able to replicate in L. lactis subsp. cremoris MG1363 and was used to compare the competence of cells containing pLB65 and pCI372.
The percentage of insertions due to site-specific integration was calculated for all attP plasmids. This value was found to be 99.9% for all attP plasmids, except pBF27, for which this value was 0%. This result suggests that in the case of pBF27, all transformants obtained in the presence of Orf1 were caused by the presence of the vector DNA, indicating that pBF27 was not able to integrate site specifically. It is possible that these transformants arose from homologous recombination between almost identical regions located in both the vector (pCI372) and the vector part of pBF27. The same could also be true for transformants that arose from the transformation of L. lactis subsp. cremoris containing pCI372 (vector) with any of the attP plasmids. By PCR performed on chromosomal DNA extracted from chloramphenicol- and erythromycin-resistant transformants carrying either pLB65 (orf1) or pCI372 (vector), site-specific integration of the attP plasmids was verified by the presence of attL and attR as well as the absence of attB (data not shown). When L. lactis subsp. cremoris MG1363 carried only the vector (pCI372), none of the attP plasmids was able to integrate site specifically, whereas in the presence of pLB65 (orf1), site-specific integration could be verified for all attP plasmids, except pBF27.
The results concerning the site-specific integration of the attP plasmids are summarized in Fig. 2, which combines the structure of the plasmids containing various deletions of the attP region and their ability to integrate site specifically into the chromosome of L. lactis subsp. cremoris MG1363. Plasmid pBF30 harbors the smallest functional attP fragment (attP186–241) (Fig. 1), containing the inverted R5 repeats and the overlapping R1 and P1 repeats, as well as the R4 and P1 repeats overlapping the core region; in contrast, 14 repeats are found in the largest plasmid (pBF12). When pBF26 (attP186–274) (Fig. 1) and pBF27 (attP216–274) (Fig. 1) were compared, the former was able to integrate, while the latter was not. This result strongly indicates that the R5 repeats and/or the overlapping R1 and P1 repeats are necessary for site-specific integration.
FIG. 2.
Deletion analysis of the attP region of the temperate bacteriophage TP901-1. For each plasmid, the nucleotides of the cloned attP fragment are indicated by numbers, which correspond to the numbers in Fig. 1. The cloned attP fragment is indicated by a black line, and the ability of each fragment to promote site-specific integration of the plasmid is indicated (yes or no). The large black arrow indicates the 3′ end of the orf1 gene, the black box indicates the core region, and small arrows indicate direct and inverted repeats.
Promoter-reporter vectors for site-specific integration.
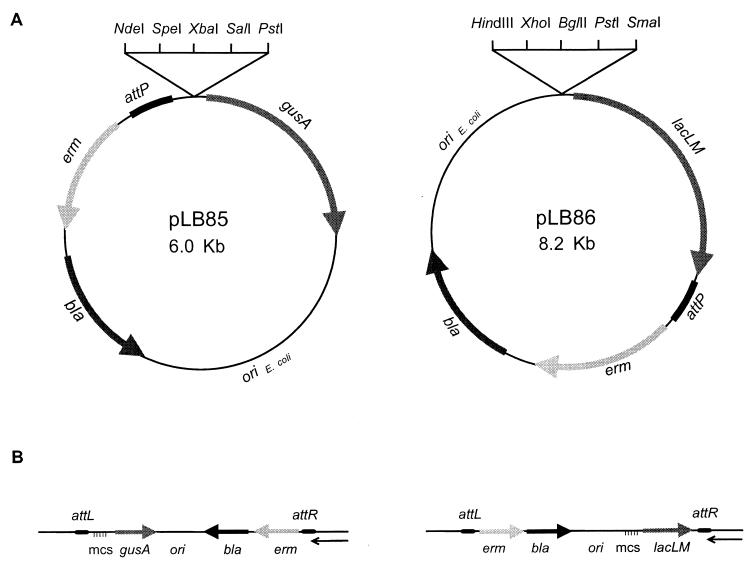
Two promoter-reporter integration vectors containing the reporter gene gusA or lacLM, encoding β-glucuronidase or β-galactosidase, respectively, were constructed. Immediately upstream of both genes, translational stop codons in all three reading frames are found. These genes have been used for the study of gene expression and regulation on multicopy plasmids in L. lactis (15, 28). The integration vectors contain an E. coli origin of replication, a selectable marker (the erm gene) for Lactococcus, the bla gene, and a 207-bp fragment carrying the attP region (attP127–333) (Fig. 1) of the temperate bacteriophage TP901-1. In addition, multiple cloning sites, which are suitable for cloning of the promoter of interest, are located immediately upstream of the reporter genes (Fig. 3A).
FIG. 3.
(A) Structures of the promoter-reporter integration vectors pLB85 and pLB86. The direction of transcription is indicated by arrows. The black box indicates the 207-bp attP region of TP901-1. (B) Results of site-specific integration of the promoter-reporter vectors in the chromosome of L. lactis subsp. cremoris. The multiple cloning sites (mcs) are indicated by horizontal lines, and the direction of transcription is indicated by arrows. The black box indicates the attP part of attL and attR of TP901-1, and the thin black arrow indicates the orientation of transcription of chromosomal genes located within the attB region (8).
The promoter-reporter integration vectors could integrate into the attB site of L. lactis subsp. cremoris MG1363 when the integrase of TP901-1 was present. L. lactis subsp. cremoris MG1363 containing the integrase gene on a replicating plasmid (pLB65) was transformed with the promoter-reporter integration vectors (pLB85 and pLB86). In addition, L. lactis subsp. cremoris MG1363 containing the integrase gene on a plasmid carrying a temperature-sensitive origin of replication (pLB95) was transformed with the promoter-reporter integration vectors (pLB85 and pLB86). By selection for erythromycin-resistant transformants, approximately 5 × 106 CFU/μg of DNA was obtained in the presence of the integrase gene in pLB65, whereas in the presence of pLB95 (temperature-sensitive origin), approximately 5 × 104 CFU/μg of DNA was found. A strain carrying pLB95 can be cured of this plasmid by growth without selection at 37°C. Subsequently, the loss of pLB95 can be confirmed, since the strain becomes sensitive to tetracycline.
In the presence of the integrase of TP901-1, the promoter-reporter integration vectors were integrated site specifically into the chromosomal attachment site attB used by bacteriophage TP901-1. This finding was verified by the presence of attL and attR as well as the absence of attB in a PCR analysis performed on chromosomal DNA extracted from eight independent erythromycin-resistant transformants (data not shown). Furthermore, no PCR product was found with primers annealing at each side of the attP site, showing that only one copy of the vector was integrated site specifically into the chromosome (data not shown). When the promoter-reporter vectors were integrated into the attB site of the chromosome, transcription of the reporter genes was divergent relative to the transcription of the adjacent chromosomal gene (Fig. 3B) (8).
To test the system, different promoter regions were cloned into the promoter-reporter vector pLB86 and integrated site specifically into the attB site on the chromosome of L. lactis subsp. cremoris MG1363. The promoter activity of the integrated fusions was measured as β-galactosidase activity in overnight cultures. The activity of the chromosomal single-copy promoter-reporter fusions was decreased six- to ninefold compared with that of the plasmid-borne promoter-reporter fusions (Table 3).
TABLE 3.
Specific activity of β-galactosidase of integrated and plasmid-borne promoter fusions
| Promoter | Sp act of β-galactosidase (U/ml × OD600b)
|
Fold activitya | |
|---|---|---|---|
| Plasmid borne | Integrated | ||
| None | 0.24 | 0.04 | 6.2 |
| CP22 | 92.7 | 10.2 | 9.1 |
| CP15 | 98.9 | 15.0 | 6.6 |
| upp | 194 | 24.9 | 7.8 |
Specific activity of β-galactosidase of plasmid-borne fusions/specific activity of β-galactosidase of integrated fusions.
OD600, optical density at 600 nm.
To test the stability of the integrated fusions, L. lactis subsp. cremoris MG1363 containing plasmid pLB89 (the upp promoter cloned in front of the lacLM gene) integrated into the attB site on the chromosome was grown without selection. To be able to screen for the loss of the integrated promoter-lacLM transcriptional fusion, cultures were plated on plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) at selected times. After growth for more than 100 generations, no white colonies were found among the approximately 10,000 colonies screened, showing that the integrated fusion was stably maintained within the attB site of L. lactis subsp. cremoris MG1363.
DISCUSSION
In this study, we performed a detailed deletion analysis of the attP region of the temperate lactococcal bacteriophage TP901-1. This deletion analysis is the first reported for phages infecting lactic acid bacteria. In our analysis, the smallest functional attP region found was a 56-bp fragment carrying the core region and several repeats.
The 56-bp attP region contains the R5 inverted repeats, one repeat each of R4 and R1, and the P1 inverted repeats. Deletion of the R5 inverted repeats and the overlapping P1 and R1 repeats leads to a nonfunctional attP region, suggesting that one or more of these repeats are necessary for site-specific integration of the temperate bacteriophage TP901-1. Furthermore, a second P1 repeat located within the core region is expected to bind the integrase of TP901-1 during recombination. Thus, the P1 repeats could be binding sites for the integrase of TP901-1. Sequences showing homology to the P1 repeats were also found close to and within the attB core region on the L. lactis subsp. cremoris chromosome (6).
The 56-bp attP region of TP901-1 is substantially smaller than identified minimal attP regions reported for other phages, such as bacteriophage λ (235 bp), bacteriophage P2 (220 bp), and bacteriophage HP1 (418 bp) (11, 14, 26, 36). These three minimal functional attP regions all contain several sites for the binding of different proteins involved in integration. These proteins are, in addition to the integrase which catalyzes the crossing over of the DNA strands, DNA binding proteins which introduce bends in the DNA. In bacteriophage λ, the DNA-bending proteins, the integrase, and the attP region form a complex, called the intasome, which makes recombination between the attP and attB regions possible (for a review, see reference 18). The small size of a functional attP region of TP901-1 suggests that a limited number of proteins are involved in the integration process. Furthermore, the integrase of TP901-1 does not show homology to the integrase of λ but belongs to a new family of recombinases (the extended resolvases) which contains a region showing homology to the catalytic site of resolvases and invertases but which contains an extended C terminus (9). These details suggest that recombination between the attP and attB sites catalyzed by the TP901-1 integrase, representing the new family of recombinases, requires the binding of less auxiliary proteins than the λ integrase. However, as has been found for the λ integrase, site-specific integration mediated by the TP901-1 integrase can be performed when the integrase is donated in trans.
Based on the phage-encoded elements (the attachment site attP and the integrase gene orf1) necessary for integration of the temperate bacteriophage TP901-1 into the chromosome of L. lactis, we have developed a method for the site-specific integration of transcriptional fusions into the chromosome of L. lactis. A similar system based on the integrative elements for bacteriophage λ for use in E. coli has been developed by Atlung et al. (1). Two promoter-reporter integration vectors containing the reporter gene gusA or lacLM, encoding β-glucuronidase or β-galactosidase, respectively, were constructed. The two vectors integrated site specifically into the chromosomal attB site used by TP901-1 in the presence of the TP901-1 integrase. The system is suitable for the study of gene expression and regulation in L. lactis, since the transcriptional fusion is stably maintained in a single copy within the chromosome, thereby eliminating the effects of variation of plasmid copy number. Furthermore, by integration of promoter-reporter transcriptional fusions in the TP901-1 phage attachment site on the chromosome, the promoter fragments are not located in the usual context. With this system, it is therefore possible to study the effect of deletions on promoter activity and regulation; such study is not straightforward when the construction of chromosomal transcriptional fusions is based on homologous recombination (30).
ACKNOWLEDGMENTS
We are grateful to Willem M. de Vos for providing plasmid pNZ273 prior to publication. We thank Anne Breüner for providing plasmid pAB201 and for stimulating discussions. Bjarne Faurholm is acknowledged for construction of the pBF plasmids, and we sincerely appreciate the expert technical assistance of Lotte Bredahl.
This work was supported by grants from the EC BIOTECH-G program (BIO2-CT94-3055) and the Carlsberg Foundation.
REFERENCES
- 1.Atlung T, Nielsen A, Rasmussen L J, Nellemann L J, Holm F. A versatile method for integration of genes and gene fusions into the λ attachment site of Escherichia coli. Gene. 1991;107:11–17. doi: 10.1016/0378-1119(91)90291-i. [DOI] [PubMed] [Google Scholar]
- 2.Auvray F, Coddeville M, Ritzenthaler P, Dupont L. Plasmid integration in a wide range of bacteria mediated by the integrase of Lactobacillus delbrueckii bacteriophage mv4. J Bacteriol. 1997;179:1837–1845. doi: 10.1128/jb.179.6.1837-1845.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannam T L, Crellin P K, Rood J I. Molecular genetics of the chloramphenicol-resistance transposon Tn4451 from Clostridium perfringens: the TnpX site-specific recombinase excises a circular transposon molecule. Mol Microbiol. 1995;16:535–551. doi: 10.1111/j.1365-2958.1995.tb02417.x. [DOI] [PubMed] [Google Scholar]
- 4.Biswas I, Gruss A, Ehrlich S D, Maguin E. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J Bacteriol. 1993;175:3628–3635. doi: 10.1128/jb.175.11.3628-3635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce J D, Davidson B E, Hillier A J. Spontaneous deletion mutants of the Lactococcus lactis temperate bacteriophage BK5-T and localization of the BK5-T attP site. Appl Environ Microbiol. 1995;61:4105–4109. doi: 10.1128/aem.61.11.4105-4109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breüner A. Factors involved in site-specific recombination in the temperate lactococcal bacteriophage TP901-1. Ph.D. thesis. Lyngby, Denmark: Technical University of Denmark; 1998. [Google Scholar]
- 7.Carrasco C D, Ramaswamy K S, Ramasubramanian T S, Golden J W. Anabaena xisF gene encodes a developmentally regulated site-specific recombinase. Genes Dev. 1994;8:74–83. doi: 10.1101/gad.8.1.74. [DOI] [PubMed] [Google Scholar]
- 8.Christiansen B, Johnsen M G, Stenby E, Vogensen F K, Hammer K. Characterization of the lactococcal temperate phage TP901-1 and its site-specific integration. J Bacteriol. 1994;176:1069–1076. doi: 10.1128/jb.176.4.1069-1076.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiansen B, Brøndsted L, Vogensen F K, Hammer K. A resolvase-like protein is required for the site-specific integration of the temperate lactococcal bacteriophage TP901-1. J Bacteriol. 1996;178:5164–5173. doi: 10.1128/jb.178.17.5164-5173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauser M A, Scocca J J. Site-specific integration of the Haemophilus influenzae bacteriophage HP1: location of the boundaries of the phage attachment site. J Bacteriol. 1992;174:6674–6677. doi: 10.1128/jb.174.20.6674-6677.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes F, Daly C, Fitzgerald G. Identification of the minimal replicon of Lactococcus lactis subsp. lactis UC317 plasmid pCI305. Appl Environ Microbiol. 1990;56:202–209. doi: 10.1128/aem.56.1.202-209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holo H, Nes I F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu P L, Ross W, Landy A. The lambda phage att site: functional limits and interaction with Int protein. Nature. 1980;285:85–91. doi: 10.1038/285085a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Israelsen H, Madsen S M, Vrang A, Hansen E B, Johansen E. Cloning and partial characterization of regulated promoters from Lactococcus lactis Tn917-lacZ integrants with the new promoter probe vector pAK80. Appl Environ Microbiol. 1995;61:2540–2547. doi: 10.1128/aem.61.7.2540-2547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen P R, Hammer K. The sequence of spacers between the consensus sequences modulates the strength of procaryotic promoters. Appl Environ Microbiol. 1998;64:82–87. doi: 10.1128/aem.64.1.82-87.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhstoss S, Rao R N. Analysis of the integration function of the streptomycete bacteriophage ΦC31. J Mol Biol. 1991;222:897–908. doi: 10.1016/0022-2836(91)90584-s. [DOI] [PubMed] [Google Scholar]
- 18.Landy A. Dynamic, structural, and regulatory aspects of λ site-specific recombination. Annu Rev Biochem. 1989;58:913–949. doi: 10.1146/annurev.bi.58.070189.004405. [DOI] [PubMed] [Google Scholar]
- 19.Lillehaug D, Birkeland N-K. Characterization of genetic elements required for site-specific integration of the temperate lactococcal bacteriophage φLC3 and construction of integration-negative φLC3 mutants. J Bacteriol. 1993;175:1745–1755. doi: 10.1128/jb.175.6.1745-1755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lillehaug D, Nes I F, Birkeland N-K. A highly efficient and stable system for site-specific integration of genes and plasmids into the phage φLC3 attachment site (attB) of the Lactococcus lactis chromosome. Gene. 1997;188:129–136. doi: 10.1016/s0378-1119(96)00798-6. [DOI] [PubMed] [Google Scholar]
- 21.Loessner M J, Maier S K, Daubek-Puza H, Wendlinger G, Scherer S. Three Bacillus cereus bacteriophage endolysins are unrelated but reveal high homology to cell wall hydrolases from different bacilli. J Bacteriol. 1997;179:2845–2851. doi: 10.1128/jb.179.9.2845-2851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maguin E, Prévost H, Ehrlich S D, Gruss A. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J Bacteriol. 1996;178:931–935. doi: 10.1128/jb.178.3.931-935.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinussen J, Hammer K. Cloning and characterization of upp, a gene encoding uracil phosphoribosyltransferase from Lactococcus lactis. J Bacteriol. 1994;176:6457–6463. doi: 10.1128/jb.176.21.6457-6463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuura M, Noguchi T, Yamaguchi D, Aida T, Asayama M, Takahashi H, Shirai M. The sre gene (ORF469) encodes a site-specific recombinase responsible for integration of the R4 phage genome. J Bacteriol. 1996;178:3374–3376. doi: 10.1128/jb.178.11.3374-3376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 26.Mizuuchi M, Mizuuchi K. Integrative recombination of bacteriophage lambda: extent of the DNA sequence involved in attachment site function. Proc Natl Acad Sci USA. 1980;77:3220–3224. doi: 10.1073/pnas.77.6.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Sullivan D J, Klaenhammer T R. High- and low-copy-number Lactococcus shuttle cloning vectors with features for clone screening. Gene. 1993;137:227–231. doi: 10.1016/0378-1119(93)90011-q. [DOI] [PubMed] [Google Scholar]
- 28.Platteeuw C, Simons G, de Vos W M. Use of the Escherichia coli β-glucuronidase (gusA) gene as a reporter gene for analyzing promoters in lactic acid bacteria. Appl Environ Microbiol. 1994;60:587–593. doi: 10.1128/aem.60.2.587-593.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Sanders J W, Venema G, Kok J, Leenhouts K. Identification of a sodium chloride-regulated promoter in Lactococcus lactis by single-copy chromosomal fusion with a reporter gene. Mol Gen Genet. 1998;257:681–685. doi: 10.1007/s004380050697. [DOI] [PubMed] [Google Scholar]
- 31.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato T, Samori Y, Kobayashi Y. The cisA cistron of Bacillus subtilis sporulation gene spoIVC encodes a protein homologous to a site-specific recombinase. J Bacteriol. 1990;172:1092–1098. doi: 10.1128/jb.172.2.1092-1098.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Guchte M, Daly C, Fitzgerald G F, Arendt E. Identification of int and attP on the genome of lactococcal bacteriophage Tuc2009 and their use for site-specific plasmid integration in the chromosome of Tuc2009-resistant Lactococcus lactis MG1363. Appl Environ Microbiol. 1994;60:2324–2329. doi: 10.1128/aem.60.7.2324-2329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Sinderen D, Karsens H, Kok J, Terpstra P, Ruiters M H J, Venema G, Nauta A. Sequence analysis and molecular characterization of the temperate lactococcal bacteriophage r1-t. Mol Microbiol. 1996;19:1343–1355. doi: 10.1111/j.1365-2958.1996.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 36.Yu A, Haggård-Ljungquist E. Characterization of the binding sites of two proteins involved in the bacteriophage P2 site-specific recombination system. J Bacteriol. 1993;175:1239–1249. doi: 10.1128/jb.175.5.1239-1249.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]