Abstract
Objectives
The renal activity index for lupus (RAIL) measures lupus nephritis (LN) activity considering urine levels of 6 biomarkers (neutrophil gelatinase-associated lipocalin, monocyte chemoattractant protein-1, kidney injury molecule-1, adiponectin, haemopexin, ceruloplasmin). We aimed to compare the accuracy of the RAIL and the renal domain-score of the SLE disease activity index (rSLEDAI) in detecting LN activity.
Methods
Random urine samples of patients with childhood-onset SLE with and without LN were assayed and scores of the RAIL, and RAIL standardised for urine creatinine (RAIL-Cr) were calculated. Clinical LN activity was measured by the rSLEDAI, and histological activity of LN was categorised as inactive/low-moderate/high for National Institute of Health-activity index scores of <2/2–10/>10, respectively.
Results
115 patients were included in the analysis (47 patients without and 68 with LN). RAIL, RAIL-Cr and rSLEDAI scores at the time (±3 months) of kidney biopsy were available for 32 patients. Median rSLEDAI, RAIL and RAIL-Cr values were 4, –0.04, 0.02 for inactive LN, 12, 0.7 and 0.9 for low-moderate LN activity and 12, 2 and 1.8 for high LN activity, respectively. The area under the receiver operating characteristic curve (AUC) to capture high LN activity was the lowest for the rSLEDAI (AUC=0.62), followed by the RAIL-Cr (AUC=0.73) and RAIL (AUC=0.79). Notably, when testing urine samples collected during routine clinic visits remote (>3 months) from a kidney biopsy, 50% patients with rSLEDAI scores of 0 had RAIL scores reflecting low-moderate LN activity.
Conclusion
Monitoring of renal inflammation in children and adolescents with SLE can be improved by the measurement of urine biomarkers. The RAIL may constitute important auxiliary tool for the surveillance of LN in a clinical setting and assist with the decision to obtain a kidney biopsy.
Keywords: lupus erythematosus, systemic; lupus nephritis; autoimmunity
WHAT IS ALREADY KNOWN ON THIS TOPIC
The renal activity index for lupus (RAIL) considers the concentration of six urinary biomarkers and has been developed to identify high lupus nephritis (LN) activity in an inception cohort.
WHAT THIS STUDY ADDS
The RAIL score can differentiate the degree of renal inflammation as graded by histological features.
We confirm that standardisation of the RAIL score for urine creatinine concentration is not needed to accurately capture LN activity in children.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
Irrespective of the renal domain score of the SLE disease activity index, elevated RAIL scores should raise the suspicion of LN activity.
Introduction
Renal involvement in childhood-onset SLE (cSLE), that is, lupus nephritis (LN), is associated with a significant burden of morbidity, with 10%–30% of patients with cSLE progressing to end-stage renal disease within 15 years of diagnosis.1 Indeed, renal damage is among the most important predictors of mortality in cSLE.2 3 Conventional tools, such as urine sediment and proteinuria are inferior to kidney biopsy to diagnose LN and determine the degree of renal inflammation, that is, LN activity.4 5 Although kidney biopsies are considered the gold standard to accurately capture the degree of activity inflammation with LN,6 7 the renal domain score of the SLE disease activity index (rSLEDAI) is among the most commonly used clinical measures of LN activity.5 8 This is of concern given that studies in adults with LN have shown that very low rSLEDAI scores can be present despite the persistence of renal inflammation as seen on kidney biopsy.9 10 Thus, additional tools or tests are needed to help clinicians monitor LN activity accurately in a clinical setting.
Urinary biomarkers have been in the spotlight as potential diagnostic and prognostic tools of LN activity.11 12 Most studies investigating urinary biomarkers in LN are focused on previously known protein targets. However, unbiased discovery proteomics has led to the identification of novel biomarkers that are potentially more clinically relevant. Based on the results of proteomics, the renal activity index in lupus (RAIL) was developed as a non-invasive tool to evaluate LN activity.13 The RAIL is a composite measure that is calculated from the natural log transformed concentrations of six urinary biomarkers, namely neutrophil gelatinase-associated lipocalin (NGAL), ceruloplasmin, monocyte chemoattractant protein-1 (MCP-1), adiponectin, haemopexin and kidney injury molecule-1 (KIM-1). Prior research showed that scores that are standardised for urine creatinine concentrations (RAIL-Cr) are 92% accurate in identifying high LN activity in a cohort of paediatric patients and young adult patients with LN.13 Furthermore, RAIL scores improve about 3 months prior to clinically observed response to LN therapy,14 hence can be used as early indicator of treatment response or LN flare. Validity of the RAIL biomarker to correctly capture LN throughout paediatric and adult age ranges has been confirmed,.14
The objectives of this study were to investigate in an independent cohort the potential of the RAIL biomarkers to estimate renal inflammation as seen on kidney biopsy; and to compare the performance RAIL and RAIL-Cr with that of the rSLEDAI in a paediatric clinical setting.
Methods
Patients and samples
Patients participating in this study constitute a convenience sample of patients with cSLE followed at Cincinnati Children’s Hospital or Texas Children’s Hospital (n=115). All patients met the American College of Rheumatology criteria for the classification of SLE prior to age 18 years.15 Patients were excluded if they had renal disease not attributed to SLE.16 The following information was extracted from their medical records at the time of urine sample collection: age, gender, systemic blood pressure (SBP), diastolic blood pressure (DBP), estimated glomerular filtration rate (eGFR),17 urine protein-to-creatinine ratio (UPCR), urine albumin-to-creatinine ratio (UACR) and disease activity as measured by the SLE disease activity index (SLEDAI; possible range 0–105). Renal domain score of the SLEDAI (rSLEDAI; possible range 0–16) was used calculated as a clinical measure of LN activity,5 and the sum of the remaining SLEDAI items was used to estimate extrarenal disease activity (extrarenal SLEDAI). Reports of kidney biopsies performed in the cSLE population were obtained and included the scores of the National Institute of Health-activity index (NIH-AI) and National Institute of Health-chronicity index (NIH-CI).18–20 Kidney biospies were performed in all patients with proteinuria of at least 500 mg/day not explained otherwise, and histological findings were interpreted by one of two experienced nephropathologists. As previously suggested, renal inflammation as seen on kidney biopsy (LN-activity status) was classified as inactive, low-moderate or high LN activity for NIH-AI scores of <2, 2–10 or >10, respectively.1 10 13 14 Patients in whom a kidney biopsy was never clinically indicated were considered as not having renal involvement with cSLE (no-LN). Patients contributed one or more urine samples to the study. Urine samples for biomarker measurement were collected mostly on the day of the rSLEDAI measurement (maximum: 4 weeks).
Biomarker measurement
The supernatant from spun spot-urine samples was stored at 4°C within 1 hour of collection, followed by long-term storage at −80°C within 24 hours, and batch testing of the RAIL biomarkers. NGAL, ceruloplasmin, MCP-1, adiponectin, haemopexin, KIM-1 and urine creatinine were all assayed as previously reported by our group.7 13 14 Laboratory technicians performing biomarker assays were blinded to clinical information.
Statistical analysis
Calculation of the RAIL score, or RAIL-Cr score, requires that the raw concentrations of urine creatinine (in mg/mL) or the urine biomarkers (in ng/mL for NGAL, ceruloplasmin, haemopexin, adiponectin; in pg/mL for KIM-1, MCP-1) to be natural log-transformed. The RAIL score is calculated as follows: −4.29–0.34×ln(NGAL)−0.06×ln(ceruloplasmin)+0.89×ln(MCP-1)+0.18×ln(adiponectin)−0.65×ln(haemopexin)+0.62×ln(KIM-1). The RAIL-Cr score necessitates each additional natural-log transformed biomarker levels to be divided by the natural-log transformed value of urine creatinine prior to applying the RAIL algorithm. RAIL and RAIL-Cr scores can assume negative and positive values, with higher, more positive scores indicating a higher degree renal inflammation.13
Descriptive analysis included medians and IQRs and frequencies for continuous and categorical variables, respectively. Only clinical data collected within 1 month and urine samples collected within 3 months of the kidney biopsy date were associated with histological activity as seen on kidney biopsy (LN-activity status). Clinical characteristics, RAIL scores, RAIL-Cr scores and select traditional measures of patients with cSLE with LN at the time of initial kidney biopsy and at the initial study visit of patients without LN (no-LN) were compared. We also compared traditional LN measures (SBP, DBP, eGFR, UPCR, UACR, NIH-AI, NIH-CI), biomarker concentrations, RAIL and RAIL-Cr scores by LN-activity status (inactive: NIH-AI ≤1, low-moderate: NIH-AI 2–10, high: NIH-AI >10) to the no-LN group.
Logistical regression and receiver operating characteristic curve (ROC) analyses for all patients with at least one biopsy was done to test the diagnostic accuracy assessments of the RAIL, RAIL-Cr and rSLEDAI to capture high LN activity status (NIH-AI >10) versus lower LN activity status (NIH-AI <10). Values of area under the ROC curve (AUC) can be interpreted as outstanding, excellent, good, fair or poor for values of 0.91–1.0, 0.81–0.90, 0.71–0.80, 0.61–0.70 and <0.6, respectively.21 The areas under the ROC curves between measures (RAIL, RAIL-Cr, rSLEDAI) were compared using the test by DeLong et al.22 Linear regression models were used to evaluate the ability for biomarker scores and/or rSLEDAI to predict NIH-AI and beta-coefficient for the standardised predictor and corresponding confidence were estimated and plotted; R-squares are reported. Lastly, only considering RAIL scores from urine samples collected >3 months of a kidney biopsy and follow-up urine samples of the no-LN group, we compared rSLEDAI scores to median RAIL scores measured at the time of kidney biopsy by LN-activity status. All analyses were conducted as two-sided tests with p<0.05 as statistically significant using SAS V.9.4 (SAS, Cary, North Carolina, USA).
Results
Demographics and clinical characteristics
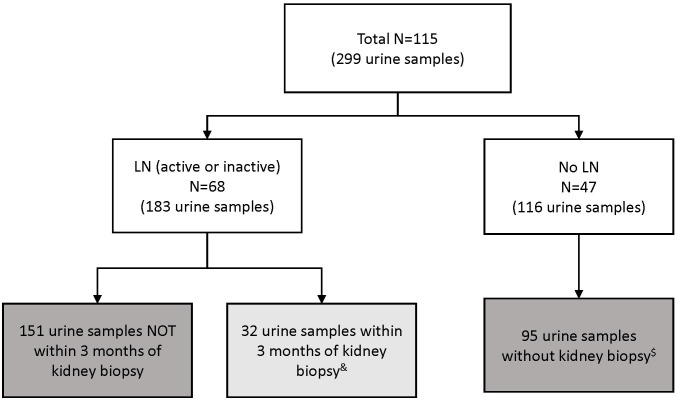
There was a total of 115 patients with cSLE and 299 samples available for this analysis (figure 1). The minimum age in the cohort was 7 years. Baseline characteristics of the study cohort are shown in table 1. There were 47 patients without renal involvement with cSLE (no-LN group) and 68 patients with LN, among them 32 with one or more visits at the time of a kidney biopsy. Indeed, 39 urine samples were collected at the time of kidney biopsies and NIH-AI scores ranged from 0 to 17. Median time since kidney biopsy was 2 days (IQR: 1–10.5). Besides having, as expected, significantly higher renal activity (rSLEDAI scores), patients with LN had markedly higher extrarenal disease activity. A minority of patients with LN were prescribed ACE inhibitor or an angiotensin receptor blocker as part of LN management.
Figure 1.
Flow chart of the urine samples used in the analyses. Light grey box indicates the 32 samples used in the initial analysis. Dark grey boxes indicate the 246 samples used in the last analysis. & From 32 patients with active lupus nephritis (LN). In a supplementary analysis, 39 samples proximal to kidney biopsy were analysed from the same 32 patients (some patients with >1 sample). $ Samples excluded (21 samples) due to missing renal domain-score of the SLE disease activity index.
Table 1.
Baseline clinical characteristics of study cohort by LN status*
| Variables | LN (n=68) | No-LN (n=47) | P value† |
| Clinical characteristics | |||
| Age, years | 17 (14.5, 20) | 18 (16, 20) | 0.3361 |
| Female | 55 (80.88%) | 41 (87.23%) | 0.3673 |
| Race | 0.2878 | ||
| White | 31 (46.97%) | 24 (52.17%) | . |
| Black | 32 (48.48%) | 17 (36.96%) | . |
| Other | 3 (4.55%) | 5 (10.87%) | . |
| Ethnicity (Hispanic) | 21 (30.88%) | 2 (4.26%) | 0.0004 |
| SBP (mm Hg) | 120 (110, 131.5) | 118 (108, 124) | 0.1102 |
| DBP (mm Hg) | 74 (63.5, 82) | 70 (61, 75) | 0.0571 |
| Use of ACEIs/ARBs | 14 (20.59%) | 0 (0%) | <0.0001 |
| Urine biomarker scores | |||
| Total RAIL score | 0.51 (−0.69, 1.6) | −0.21 (−1.69, 1.12) | 0.0277 |
| Total RAIL-Cr score | 0.68 (−0.68, 1.44) | −0.34 (−1.5, 0.96) | 0.0117 |
| Traditional cSLE measures | |||
| NIH-AI score | 4 (2, 12) | . | |
| NIH-CI score | 1 (0, 3) | . | |
| Total SLEDAI score | 13.5 (4, 22.5) | 2 (0, 4) | <0.0001 |
| Extrarenal SLEDAI score | 5.5 (2, 12) | 2 (0, 4) | <0.0001 |
| Renal SLEDAI score | 5 (0, 12) | 0 (0, 0) | <0.0001 |
*Continuous variables are demonstrated as median (25th, 75th percentile), categorical variables are demonstrated as frequency (percentage).
†P values are generated from Kruskal-Wallis test and χ2 test.
ACEI, ACE inhibitor; ARB, angiotensin receptor blocker; DBP, diastolic blood pressure; LN, lupus nephritis; NIH-AI, NIH-activity index; NIH-CI, NIH-chronicity index; RAIL, renal activity index for lupus; RAIL-Cr, RAIL standardised for urine creatinine; SBP, systolic blood pressure; SLEDAI, SLE disease activity index.
Urinary biomarker levels and traditional LN measures at the time of kidney biopsy
Among the traditional measures of LN considered in this study, only UPCR and UACR were significantly higher among patients with LN than without renal involvement (table 2). Notably, only the UACR but not the UPCR differed significantly with LN activity (as per kidney biopsy), and the UACR was more closely related to the NIH-AI score than the UPCR (Pearson’s correlation coefficient (r=0.47; p<0.0001 vs r=0.26; p=0.004)).
Table 2.
Traditional renal measures and biomarker concentrations at the time of kidney biopsy by LN activity category
| Variables* | (a) NIH-AI <2 (n=8) |
(b) NIH-AI 2–10 (n=12) | (c) NIH-AI >10 (n=12) | (d) No LN (n=47) |
(abcd) P value† |
(abc) P value |
(a) versus (d) P value |
| Urine biomarkers‡ and RAIL | |||||||
| NGAL (ng/mL) | 3.19 (2.66, 3.6) | 3.89 (3, 4.48) | 3.56 (3.16, 3.78) | 3.59 (3.15, 3.88) | 0.3130 | 0.2413 | 0.2518 |
| Ceruloplasmin (ng/mL) | 8.11 (6.66, 9.5) | 9 (8.11, 9.64) | 8.62 (7.63, 9.32) | 3.76 (2.99, 4.98) | <0.0001 | 0.6420 | <0.0001 |
| MCP-1 (pg/mL) | 6.34 (5.72, 7.04) | 7.4 (6.79, 7.87) | 8.33 (7.59, 8.84) | 5.9 (4.41, 6.92) | <0.0001 | 0.0103 | 0.3518 |
| Adiponectin (ng/mL) | 4.1 (3.79, 4.51) | 4.84 (4.19, 5.89) | 6.28 (4.73, 7.1) | 4.8 (3.6, 5.69) | 0.0096 | 0.0053 | 0.1974 |
| Haemopexin (ng/mL) | 7.21 (6.64, 8.13) | 8.38 (7.4, 8.73) | 7.92 (7.54, 8.15) | 6.49 (6.03, 6.89) | <0.0001 | 0.1930 | 0.0378 |
| KIM-1 (pg/mL) | 6.8 (5.94, 7.37) | 7.79 (6.71, 8.31) | 7.75 (6.9, 8.5) | 6.27 (4.93, 7.11) | 0.0007 | 0.1866 | 0.3771 |
| Urine creatinine (mg/mL) | 0.74 (0.62, 1.48) | 0.79 (0.56, 1.15) | 1.48 (0.74, 2.5) | 1.21 (0.5, 2.07) | 0.3344 | 0.2306 | 0.4034 |
| RAIL score | −0.04 (−1.13, 1) | 0.69 (0.09, 1.46) | 2.02 (1.03, 2.68) | −0.21 (−1.69, 1.12) | 0.0027 | 0.0190 | 0.8299 |
| RAIL-Cr score | 0.02 (−1.24, 1.01) | 0.9 (0.05, 1.45) | 1.83 (1.08, 2.43) | −0.34 (−1.5, 0.96) | 0.0003 | 0.0176 | 0.6849 |
| Traditional LN measures | |||||||
| SBP (mm Hg) | 122.5 (110, 127.5) | 125 (115, 136.5) | 122.5 (118.5, 133) | 118 (108, 124) | 0.2008 | 0.6556 | 0.5425 |
| DBP (mm Hg) | 63.5 (59.5, 77.5) | 77.5 (68, 89) | 76.5 (66.5, 87) | 70 (61, 75) | 0.1083 | 0.3301 | 0.6946 |
| eGFR | 105.5 (90, 146.5) | 97 (49, 128) | 82 (59, 133.5) | 109 (103, 130) | 0.1498 | 0.4488 | 0.5008 |
| UPCR | 1.39 (1.05, 3.79) | 4.49 (2.44, 9.21) | 2.17 (0.95, 3.83) | 0.14 (0.09, 0.23) | <0.0001 | 0.0898 | <0.0001 |
| UACR | 0.12 (0.07, 0.19) | 0.33 (0.21, 0.48) | 0.63 (0.14, 2.56) | 0.01 (0.01, 0.02) | <0.0001 | 0.0191 | <0.0001 |
| NIH-AI | 0.5 (0, 1) | 4.5 (4, 7.5) | 13.5 (12.5, 15) | – | <0.0001 | ||
| NIH-CI | 0 (0, 0) | 1.5 (0, 3) | 1 (1, 3) | – | 0.0119 | ||
| rSLEDAI score | 4 (2, 10) | 12 (12, 16) | 12 (8, 16) | 0 (0, 0) | <0.0001 | 0.0058 | <0.0001 |
*Values are median (25th, 75th percentile).
†P values are generated from Kruskal-Wallis test.
‡Concentration of urine proteins are natural log transformed.
§Standardisation by urine creatinine.
DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate as per modified Schwartz formula; KIM-1, kidney injury molecule 1; LN, lupus nephritis; MCP-1, monocyte chemotactic protein 1; NGAL, neutrophil gelatinase-associated lipocalin; NIH-AI, National Institute of Health activity index; NIH-CI, National Institute of Health chronicity index; SBP, systolic blood pressure; UACR, albumin-to-creatinine ratio; UPCR, urine protein-to-creatinine ratio.
Select individual urine biomarker levels (ceruloplasmin, haemopexin) included in the RAIL were significantly lower without LN than with LN, even if inactive (table 2). Both RAIL and RAIL-Cr scores significantly differed (p<0.019) among patients with different LN-activity status (inactive, low-moderate or high). As shown in table 2 and online supplemental figure S1, median (IQR) scores of the RAIL and RAIL-Cr increased with higher categories of LN activity. There was a trend towards lower RAIL and RAIL-Cr scores in patients without LN compared with those with inactive LN but differences did not reach statistical significance. Patients with inactive LN activity status had lower RAIL (median (IQR): −0.04 (-1.13, 1.00)) and RAIL-Cr scores (median (IQR): 0.02 (−1.24, 1.01)). Patients with high LN activity status had higher RAIL (median (IQR): 2.02 (1.03, 2.68)) and RAIL-Cr scores (median (IQR): 1.83 (1.08, 2.43)).
lupus-2021-000631supp001.pdf (89.7KB, pdf)
The rSLEDAI scores did not discriminate well patients with different categories of LN activity (NIH-AI score <2 vs 2–10 vs >10; p=0.76). However, median (IQR) scores of the rSLEDAI for patients with inactive LN (NIH-AI ≤1) were significantly lower than those with NIH-AI ≥2 (median (IQR): 4 (2, 10) vs 12 (10, 16); p=0.0014). Similar results for the rSLEDAI and RAIL (or RAIL-Cr) were observed when considering all 39 visits and a 3-month window around the time of available kidney biopsies (online supplemental figures S2 and S3).
lupus-2021-000631supp002.pdf (75.1KB, pdf)
lupus-2021-000631supp003.pdf (73.8KB, pdf)
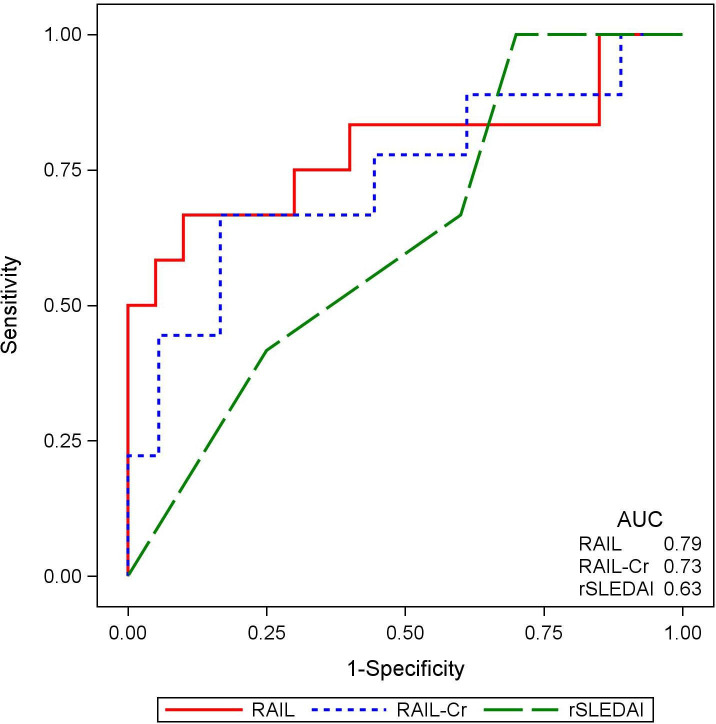
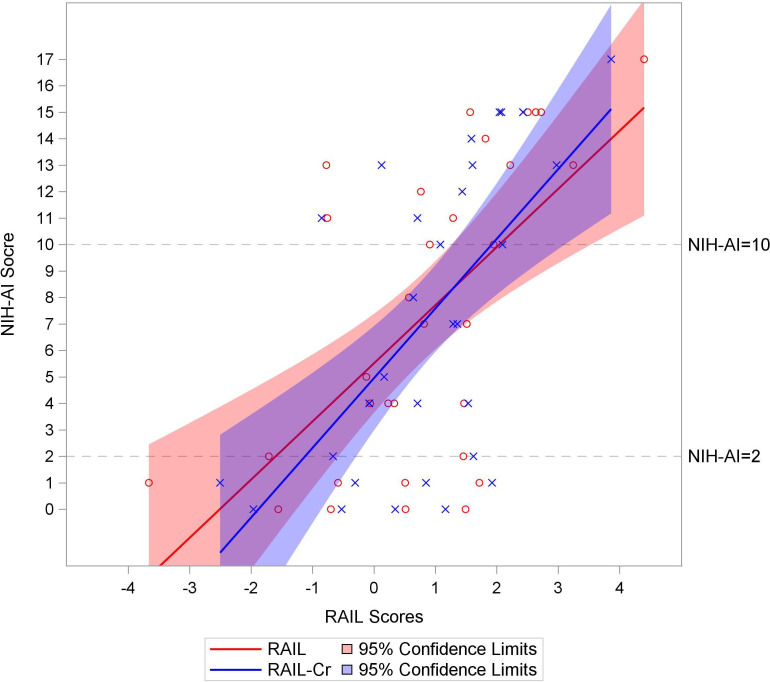
Figure 2 presents ROC curve for predicting high LN activity using RAIL, RAIL-Cr and rSLEDAI scores. The RAIL performed showed good accuracy in capturing high LN activity (NIH-AI >10) and performed best (AUC=0.79), followed by the RAIL-Cr (AUC=0.73) and the rSLEDAI (AUC=0.63), although differences in AUC values between measures did not reach statistical significance (AUC: RAIL vs rSLEDAI; p=0.26). Using all the kidney biopsy results (n=39) provided similar findings (see online supplemental figure S4). The distribution of RAIL and RAIL-Cr scores in relation to NIH-AI scores over the available range of 0–17 is shown in figure 3.
Figure 2.
The renal activity index for lupus (RAIL) and RAIL standardised for urine creatinine (RAIL-Cr) scores are superior in capturing high lupus nephritis (LN) activity. A receiver operating curve demonstrating the performance of the RAIL score (red solid line), RAIL-Cr (blue dotted line) and SLE disease activity index (rSLEDAI) score (green dashed line) in identifying patients with high activity LN (National Institute of Health-activity index >10) among 32 patients with active LN. The areas under the curves (AUC) are annotated for each score in the right lower corner.
Figure 3.
Renal activity index for lupus (RAIL) and RAIL standardised for urine creatinine (RAIL-Cr) scores above two correlate with high activity lupus nephritis (LN). Distribution of the RAIL and RAIL-Cr score (x-axis) represented by red and blue liner regression lines and 95% confidence limits, respectively, against the available range of National Institute of Health-activity index (NIH-AI) scores (y-axis) in 32 patients with active LN. Each NIH-AI score by RAIL (red circle) and RAIL-Cr scores (blue cross) is marked in the figure. The relationship between NIH-AI and two RAIL scores are presented in liner regression lines with 95% confidence limits (RAIL in red line and RAIL-Cr in blue line). Grey dash lines (NIH-AI=2 and 10) are reference cut points for inactive, low-moderate and high LN activity.
lupus-2021-000631supp004.pdf (90.8KB, pdf)
Clinical monitoring for active LN
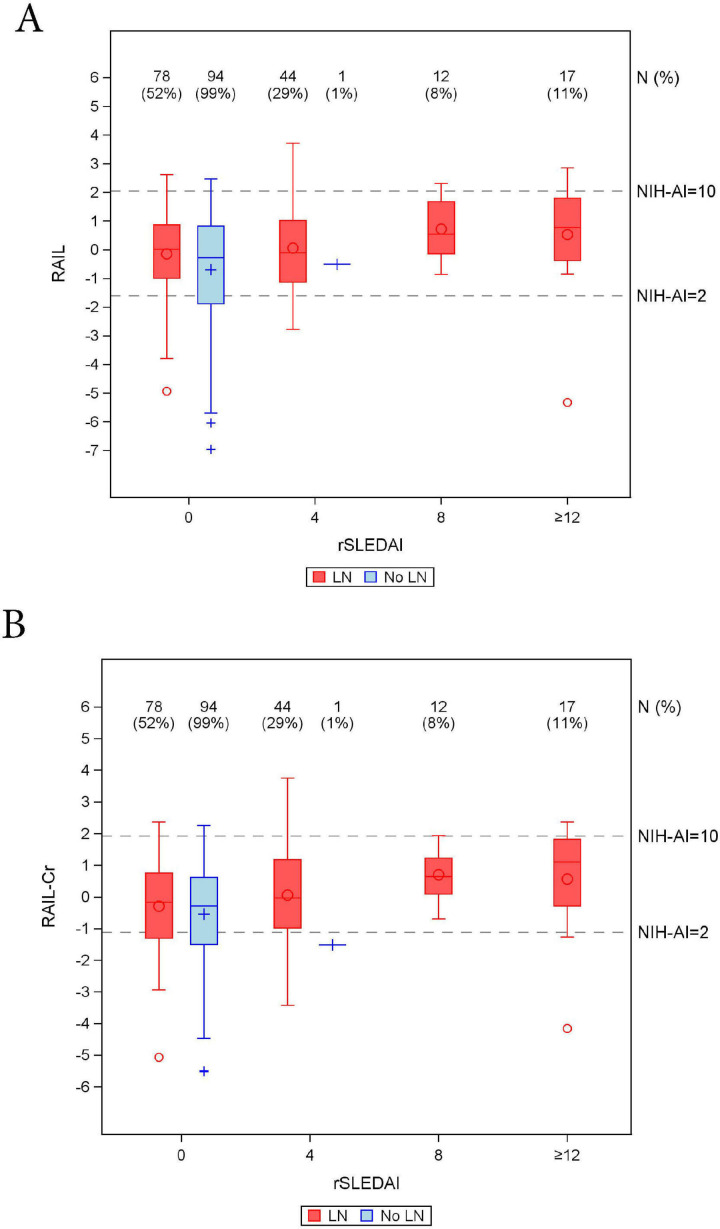
There were 246 urine samples from patients with or without LN that were collected >3 months apart from the date of a kidney biopsy. As shown in figure 4, in these urine samples not collected around the time of a kidney biopsy, the RAIL scores of patients with rSLEDAI scores of 0 or 4 supported the presence of at least low LN activity (NIH-AI >2) in 70% (n=171) of the samples. In this same category of patients with low rSLEDAI, 6% (n=16) of RAIL scores were suggestive of high activity LN (NIH-AI >10). Negative RAIL scores were present in 46% (n=114) of patients with rSLEDAI scores of 0 or 4.
Figure 4.
Distribution of renal activity index for lupus (RAIL) (A) and RAIL standardised for urine creatinine (RAIL-Cr) (B) scores in relation to SLE disease activity index (rSLEDAI) scores from 246 samples from patients collected >3 months before/after kidney biopsy. Box-Whisker plots of RAIL score (y-axis, A) or RAIL-Cr (y-axis, B) by rSLEDAI (x-axis) in patients with lupus nephritis (LN) (red colour) and no-LN (blue colour), where medians, IQR, maximum, minimum and outliers are shown. The number of urine samples (N%) for each box is outlined above the x-axis along with the percentage out of a total of 151 LN samples and 95 no-LN samples. Based on data in figure 3, the grey dash lines indicate the RAIL scores corresponding to NIH-AI of 2 and 10.
Discussion
We aimed to compare the accuracy of the RAIL and the renal domain-score of the rSLEDAI. While the RAIL provided a good estimate of the degree of inflammation with LN, rSLEDAI score proved to be a poor surrogate for biopsy-proven high LN activity. Furthermore, RAIL scores of patients with LN with rSLEDAI scores of 0 should raise the suspicion of the presence of at least minimal disease activity.
Like the amounts of urine albumin, concentrations of the RAIL biomarker could be influenced by the hydration status of the patient, although we have shown in the past that they are produced in the kidney rather than being filtered from the blood. In the current study, we confirm our prior research findings in that standardisation by urine creatinine does not improve the accuracy of the RAIL in capturing LN activity in paediatric cohorts.7 14 Notably, we have shown in the past that standardisation by urine creatinine but not urine albumin improves the accuracy with which the RAIL biomarkers reflect LN activity in adults with LN.14
Many urinary biomarkers have been proposed for monitoring LN and have been studied internationally.23–25 We have validated many of these biomarkers in the past, found them reflective of LN activity, and associated with LN histological findings. However, we were able to confirm that the six RAIL biomarkers were sufficient to capture LN activity accurately.13 26–28
Although we have firmly established NGAL as a biomarker of LN activity,14 26 using the current study design, NGAL levels did not add to the discrimination of the various categories of LN activity given that NGAL is a highly responsive biomarker and urine levels quickly decline with treatment. Conversely, especially levels of MCP-1 and adiponectin continued to differentiate between categories of LN activity, suggesting that these urine biomarkers normalise over a longer time frame.
Both patients with inactive LN and patients with cSLE without LN are expected to have rSLEDAI scores of 0. In this study, the level of ceruloplasmin significantly differed between patients with inactive LN and those without LN (no-LN), despite both having rSLEDAI-scores of 0. Ceruloplasmin is massively excreted in the urine with active LN,23 29 but in our prior research levels of urinary ceruloplasmin were not significantly different between the inactive LN group and the no-LN group.26 Additional studies will be needed to confirm this observation in an independent larger cohort.
In our cohort, we found higher extrarenal SLEDAI scores in patients with active LN compared with no-LN. We are limited by the small sample size of patients with no-LN with high extrarenal SLEDAI, but our previous work showed that the RAIL score is not influenced by extrarenal SLEDAI scores.14 30
Proteinuria and albuminuria are traditional measures of LN activity but may reflect renal damage in addition to renal inflammation.31 We have shown in the past that isolated traditional biomarkers of LN, including proteinuria cannot identify patients with high disease activity with high accuracy.13 The current study also expands on our prior observations5 and on those of other investigators10 32 that the rSLEDAI lacks the accuracy to monitor LN activity well. In this context, it seems important to point out that in cSLE, the renal domain score of The British Isles Lupus Assessment Group (BILAG) index does not seem to offer a better clinical alternative.5 Furthermore, although in this study the UACR seemed to perform somewhat better than the UPCR in capturing LN activity, differences were small and unlikely clinically important.31
Given the limitations of the traditional clinical measures of LN activity, that is, the rSLEDAI, the renal domain score of the BILAG, the UPCR and UACR, we propose to consider surveillance of LN to be informed by RAIL scores. Based on the findings of this study, a RAIL score equal to or under –1.6 provides strong support for the absence of LN activity, while scores >2 seem to indicate high LN activity. Scores between these values were common in our study and should raise the suspicion of ongoing mild-to-moderate LN activity. For RAIL scores within this range, active LN is likely, especially in conjunction with higher rSLEDAI scores. Owing to the predictive ability of the RAIL biomarkers to foresee LN flare, increasing RAIL score should prompt at least close monitoring of the patients.7 13 26 Despite numerically lower RAIL scores in the group of patients without known LN, we confirm our prior studies7 26 that the RAIL does not discriminate well patients without LN from those with inactive LN. Nonetheless, this study further demonstrates the potential of the RAIL score in supporting the surveillance of LN.
The performance of the RAIL and RAIL-Cr in capturing LN activity in this study was not as good as what was reported by us in our prior studies.7 14 The exact reasons contributing to this observation are unknown. In our prior research we restricted the time window to 4 weeks and this study we allowed for 3 months. Supported by our exploratory analyses (data not shown), we suspect that the more liberal definition of a urine sample that is collected at the time of biopsy contributed to the inferior performance of the RAIL in this validation study compared with our prior studies.13 14 Hence, we provide a conservative estimate of the benefits of measuring the RAIL biomarkers in clinical settings
A strength of our study is the prospective collection of clinical characteristics and standardised laboratory testing which adds to the quality of the results presented. Furthermore, many of the observations in this study are consistent with those of other investigators in larger LN cohorts.4 23 31
In conclusion, we confirm the validity of the RAIL as a biomarker-based non-invasive measure of LN activity in cSLE with LN. Our results support the benefits of routine measurement of the RAIL biomarkers to monitor LN.
Acknowledgments
We thank Rashmi D. Sahay and Chunyan Liu for participation in sample size estimation and an earlier analysis of the data. Qing Ma was essential for assaying the RAIL biomarker and Ms. Theresa Hennard, Catherin Robben and Megan Quinlan-Waters supported the assembly of the materials and data for this study. We are indebted to Ms. Lorie Lyrink for managing the biobanking of the urine samples used for this study. The project described was supported by the National Institutes of Arthritis and Musculoskeletal Skin Diseases under Award - Number P30AR076316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH
Footnotes
Twitter: @PedsLupusRenal
Contributors: NA, HB and PD conceptualised the study’s questions and design. NA, SEW, AMa, AMe and SJ planned data extraction and acquired the data. JR and PD carried out the urinary analyses and biomarker measurements. TQ and BH performed the statistical analyses and contributed to the interpretation of the results. HB supervised all stages of this study and is the guarantor of this project. All authors provided key contribution to the study and manuscript and approved the final draft of this manuscript.
Funding: This work has been supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under award numbers P30 AR076316 and P30 AR070549, a Pilot and Feasibility Project from the Pediatric Center of Excellence in Nephrology (NIH P50 DK096418; PD) and the CCTST at the University of Cincinnati is funded by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) programme, grant 2UL1TR001425-05A1.
Competing interests: SEW has a consultation agreement with Bristol-Myers Squibb in 2020 for an unrelated project on paediatric lupus nephritis.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data are available to be shared on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the CCHMC institutional review board (#2008-0635).
References
- 1.Costenbader KH, Desai A, Alarcón GS, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum 2011;63:1681–8. 10.1002/art.30293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danila MI, Pons-Estel GJ, Zhang J, et al. Renal damage is the most important predictor of mortality within the damage index: data from LUMINA LXIV, a multiethnic US cohort. Rheumatology 2009;48:542–5. 10.1093/rheumatology/kep012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerang K, Gilboe I-M, Steinar Thelle D, et al. Mortality and years of potential life loss in systemic lupus erythematosus: a population-based cohort study. Lupus 2014;23:1546–52. 10.1177/0961203314551083 [DOI] [PubMed] [Google Scholar]
- 4.Sule SD, Moodalbail DG, Burnham J, et al. Predictors of kidney disease in a cohort of pediatric patients with lupus. Lupus 2015;24:862–8. 10.1177/0961203315570162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mina R, Abulaban K, Klein-Gitelman MS, et al. Validation of the lupus nephritis clinical indices in childhood-onset systemic lupus erythematosus. Arthritis Care Res 2016;68:195–202. 10.1002/acr.22651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn BH, McMahon MA, Wilkinson A, et al. American College of rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res 2012;64:797–808. 10.1002/acr.21664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner HI, Bennett MR, Mina R, et al. Association of noninvasively measured renal protein biomarkers with histologic features of lupus nephritis. Arthritis Rheum 2012;64:2687–97. 10.1002/art.34426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunner HI, Feldman BM, Bombardier C, et al. Sensitivity of the systemic lupus erythematosus disease activity index, British Isles lupus assessment group index, and systemic lupus activity measure in the evaluation of clinical change in childhood-onset systemic lupus erythematosus. Arthritis Rheum 1999;42:1354–60. [DOI] [PubMed] [Google Scholar]
- 9.De Rosa M, Rocha AS, De Rosa G, et al. Low-Grade proteinuria does not exclude significant kidney injury in lupus nephritis. Kidney Int Rep 2020;5:1066–8. 10.1016/j.ekir.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarado AS, Malvar A, Lococo B, et al. The value of repeat kidney biopsy in quiescent Argentinian lupus nephritis patients. Lupus 2014;23:840–7. 10.1177/0961203313518625 [DOI] [PubMed] [Google Scholar]
- 11.Bennett M, Brunner HI. Biomarkers and updates on pediatrics lupus nephritis. Rheum Dis Clin North Am 2013;39:833–53. 10.1016/j.rdc.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyes-Thomas J, Blanco I, Putterman C. Urinary biomarkers in lupus nephritis. Clin Rev Allergy Immunol 2011;40:138–50. 10.1007/s12016-010-8197-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abulaban KM, Song H, Zhang X, et al. Predicting decline of kidney function in lupus nephritis using urine biomarkers. Lupus 2016;25:1012–8. 10.1177/0961203316631629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunner HI, Bennett MR, Gulati G, et al. Urine biomarkers to predict response to lupus nephritis therapy in children and young adults. J Rheumatol 2017;44:1239–48. 10.3899/jrheum.161128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 16.Anders H-J, Weening JJ. Kidney disease in lupus is not always 'lupus nephritis'. Arthritis Res Ther 2013;15:108. 10.1186/ar4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009;20:629–37. 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin HA, Boumpas DT, Vaughan EM, et al. Predicting renal outcomes in severe lupus nephritis: contributions of clinical and histologic data. Kidney Int 1994;45:544–50. 10.1038/ki.1994.70 [DOI] [PubMed] [Google Scholar]
- 19.Austin HA, Muenz LR, Joyce KM, et al. Prognostic factors in lupus nephritis. contribution of renal histologic data. Am J Med 1983;75:382–91. 10.1016/0002-9343(83)90338-8 [DOI] [PubMed] [Google Scholar]
- 20.Austin HA, Muenz LR, Joyce KM, et al. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int 1984;25:689–95. 10.1038/ki.1984.75 [DOI] [PubMed] [Google Scholar]
- 21.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36. 10.1148/radiology.143.1.7063747 [DOI] [PubMed] [Google Scholar]
- 22.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 23.Smith EMD, Jorgensen AL, Midgley A, et al. International validation of a urinary biomarker panel for identification of active lupus nephritis in children. Pediatr Nephrol 2017;32:283–95. 10.1007/s00467-016-3485-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moloi MW, Rusch JA, Omar F, et al. Urinary MCP-1 and TWEAK as non-invasive markers of disease activity and treatment response in patients with lupus nephritis in South Africa. Int Urol Nephrol 2021;53:1865–73. 10.1007/s11255-020-02780-9 [DOI] [PubMed] [Google Scholar]
- 25.Anania VG, Yu K, Pingitore F, et al. Discovery and qualification of candidate urinary biomarkers of disease activity in lupus nephritis. J Proteome Res 2019;18:1264–77. 10.1021/acs.jproteome.8b00874 [DOI] [PubMed] [Google Scholar]
- 26.Hinze CH, Suzuki M, Klein-Gitelman M, et al. Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum 2009;60:2772–81. 10.1002/art.24751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki M, Wiers KM, Klein-Gitelman MS, et al. Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatr Nephrol 2008;23:403–12. 10.1007/s00467-007-0685-x [DOI] [PubMed] [Google Scholar]
- 28.Suzuki M, Ross GF, Wiers K, et al. Identification of a urinary proteomic signature for lupus nephritis in children. Pediatr Nephrol 2007;22:2047–57. 10.1007/s00467-007-0608-x [DOI] [PubMed] [Google Scholar]
- 29.Davies JC, Carlsson E, Midgley A, et al. A panel of urinary proteins predicts active lupus nephritis and response to rituximab treatment. Rheumatology 2021;60:3747–59. 10.1093/rheumatology/keaa851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki M, Wiers K, Brooks EB, et al. Initial validation of a novel protein biomarker panel for active pediatric lupus nephritis. Pediatr Res 2009;65:530–6. 10.1203/PDR.0b013e31819e4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birmingham DJ, Rovin BH, Shidham G, et al. Relationship between albuminuria and total proteinuria in systemic lupus erythematosus nephritis: diagnostic and therapeutic implications. Clin J Am Soc Nephrol 2008;3:1028–33. 10.2215/CJN.04761107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christopher-Stine L, Siedner M, Lin J, et al. Renal biopsy in lupus patients with low levels of proteinuria. J Rheumatol 2007;34:332–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2021-000631supp001.pdf (89.7KB, pdf)
lupus-2021-000631supp002.pdf (75.1KB, pdf)
lupus-2021-000631supp003.pdf (73.8KB, pdf)
lupus-2021-000631supp004.pdf (90.8KB, pdf)
Data Availability Statement
All data are available to be shared on reasonable request.