Abstract
Objective
Among the most significant challenges in SLE are the excessive diagnosis delay and the lack of coordinated care. The aim of the study was to investigate patient pathways in SLE in order to improve clinical and organisational challenges in the management of those with suspected and confirmed SLE.
Methods
We conducted a cross-sectional study of patients with SLE, healthcare providers and other representative stakeholders. Focus groups were conducted, and based on the collected data the most impactful disruption points in SLE patient pathways were identified. A novel framework to improve individual patient pathways in SLE was developed, discussed and validated during a consensus meeting with representative stakeholders.
Results
Six thematic clusters regarding disruption in optimal patient pathways in SLE were identified: appropriate and timely referral strategy for SLE diagnosis; the need for a dedicated consultation during which the diagnosis of SLE would be announced, and following which clarifications and psychological support offered; individualised patient pathways with coordinated care based on organ involvement, disease severity and patient preference; improved therapeutic patient education; prevention of complications such as infections, osteoporosis and cancer; and additional patient support. During the consensus meeting, the broader panel of stakeholders achieved consensus on these attributes and a framework for optimising SLE patient pathways was developed.
Conclusions
We have identified significant disruption points and developed a novel conceptual framework to improve individual patient pathways in SLE. These data may be of valuable interest to patients with SLE, their physicians, health organisations as well as policy makers.
Keywords: systemic lupus erythematosus, autoimmune diseases, patient care team, qualitative research, psychology
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Excessive diagnosis delay and lack of coordinated care remain major challenges in SLE.
WHAT THIS STUDY ADDS
We have identified significant disruption points in SLE patient pathways, including additional needs in the field of referral strategy, dedicated consultation for diagnosis announcement, coordinated care, therapeutic patient education, prevention of complications and psychosocial patient support.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
Based on these data, we have developed a novel conceptual framework to improve individual patient pathways in SLE.
Introduction
SLE is a chronic systemic autoimmune disease with multiple and heterogeneous clinical phenotypes and variations in disease severity and damage accrual across the whole spectrum of patients with SLE.1 Earlier diagnosis and treatment advances have resulted in improved outcomes over the past decades.2 However, increased morbidity and mortality persists in SLE, indicating that several unmet needs impact the optimal management of the disease.3 4 Among the most significant are the excessive diagnosis delay and the common lack of coordinated care for SLE.5 A patient pathway (PP) is the patient experience from the first symptom through the initial referral for diagnosis, treatment and follow-up, and includes diverse aspects of disease management, such as holistic support and prevention of complications. According to the European Pathway Association, a care pathway is a complex intervention for mutual decision making and organisation of care processes for a well-defined group of patients during a well-defined period. The general concept behind that of PP is that the healthcare systems should ensure faster diagnosis and, for patients diagnosed with SLE, result in a rapid initiation of treatment through streamlined, standardised strategies. Clinical studies have long proven that patient-centred PPs reduce variability in clinical practice, improve outcomes and reduce the overall cost of disease management.6–8 In the context of SLE, early diagnosis is both a priority and a challenge, as prolonged diagnostic delay may lead to worse outcomes, including damage accrual9 and socioprofessional disinsertion. The paradigm of primary healthcare, under which general practitioners (GPs) are involved in the initial diagnosis and therapeutic management of symptoms, is largely challenged by the rare and highly polymorphic nature of the disease. Also, virtually all patients with SLE have significant levels of ANA, but this essential laboratory test is not specific to the diagnosis, yielding a high number of inappropriate referral to secondary-care or tertiary-care specialists.3 Several attempts to optimise the care of patients with SLE have resulted in the implementation of several national and international guidelines,1 but individual trajectories of autoimmune patients within healthcare systems remain largely suboptimal. Both diagnosed and undiagnosed patients with SLE typically present complex problems, often requiring multiple interventions provided concurrently from several partners in their care.10 This emphasises the need for an integrated and holistic approach to SLE diagnosis and treatment.
The aim of the present study was to analyse PPs in SLE in order to overcome organisational challenges in the management of suspected and confirmed SLE cases.
Methods
Study design and area
We conducted a cross-sectional qualitative study using a content analytic approach to identify both disruption points and strategies to improve PPs in SLE in the French region of Alsace, an 8280 km² territory in northeastern France. This area has previously been used for epidemiological studies of autoimmune or inflammatory diseases by our group.11 12 According to the national census, the population of Alsace was 1 898 533 inhabitants in 2020, with an age distribution close to the global metropolitan French population. Based on a nationwide population-based study of the prevalence of SLE in France using national administrative databases,13 the number of prevalent SLE cases in Alsace was 776 (yielding a crude prevalence rate of 46.3 per 100 000) and the number of incident cases was 66 (crude incidence rate of 3.92 per 100 000) for the year 2010. Using focus groups involving patients with SLE and healthcare professionals separately, followed by consensus meetings involving a broader panel of key stakeholders, we came to an agreement regarding the most disruptive and optimisable aspects of PP in SLE.
Participant sample
Healthcare professionals and patients with SLE were invited to participate in face-to-face physical or virtual (online) focus group meetings during the year 2020. These focus groups involved six healthcare professionals representative of medical specialties most commonly involved in SLE care (primary care, rheumatology, internal medicine and clinical immunology, dermatology, nephrology) from different types of practice (academic and non-academic centres, as well as private practice), academic occupational physicians, six patients with SLE from different backgrounds, a representative from the French SLE patient association (Association Française du Lupus et des autres maladies auto-immunes, aFL+), a methodology consultant specialised in the analysis of PP and a fellow in rheumatology. All participants gave their consent to the study.
Focus groups
Healthcare professionals and patients with SLE met separately as small groups of two to six people in a physical room or within virtual rooms using the Zoom software (during national lockdowns due to COVID-19). They were presented with an introductory lecture on general aspects of SLE, including its epidemiology, main clinical manifestations, diagnosis and treatment strategies, as well as the general methodology of the study. Using a standardised template of open-ended questions, participants were asked to describe the current PP for SLE and to point out any significant disruption point they could think of. What is the typical pathway of a patient with lupus? Are there any disruption points in current lupus pathways? What could be an optimal PP for SLE? What are the most challenging or controversial aspects for optimisation of PP for SLE? Using a semistructured approach, one facilitator (IP-R) asked each participant in turn to share their perspectives with the group.
Analysis
The characteristics of the focus group participants were summarised using descriptive statistics. Notes from the focus groups were analysed and elements which captured key thoughts or underlying concepts in the most optimal manner were extracted. These concepts were organised into relevant themes, which described the most significant disruptive points and potential optimisation strategies for PP in SLE.
Consensus meeting
During a final meeting held both physically and online in March 2021, the aggregate results of the focus groups were presented to a larger and representative panel of stakeholders (n=22), including lupus patient associations, healthcare professionals (from academic centres, non-academic centres and private practice), representatives of the regional professional unions of pharmacists, biologists and GPs, and institutional representatives of healthcare system payers (representative of the board of hospital directors and of the regional agencies for healthcare organisation). Following a general presentation on SLE, the stakeholders were presented with the key disruption points identified during the focus group as well as the thematic clusters. The stakeholders were offered the possibility to provide detailed feedback and discuss these thematic clusters. The inclusive process ensured that all participants had an opportunity to contribute, avoiding the potential effect of strong personalities which may be encountered during consensus groups.
Results
Various disruptive points and challenges regarding PP in SLE were reported during the focus groups with patients with SLE and healthcare professionals (table 1).
Table 1.
Disruptive points and challenges reported by healthcare professionals and patients with SLE
| Potential disruption points to an optimal patient pathway in SLE | Physicians | Patients |
| Undertraining of physicians (including primary care physicians) about SLE. | ✓ | ✓ |
| Excessive diagnosis delay for SLE (in some cases). | ✓ | ✓ |
| Indications for ANA testing. | ✓ | ✗ |
| Lack of adequate management and/or referral strategy for patients with ANA positivity. | ✓ | ✓ |
| Lack of dedicated consultation for announcing the diagnosis. | ✗ | ✓ |
| Lack of proper support following diagnosis announcement. | ✗ | ✓ |
| Need for more coordinated healthcare. | ✓ | ✓ |
| Need for interoperable data management systems between healthcare professionals. | ✓ | ✗ |
| Use of innovating tools and technologies to ensure privacy and high quality of care. | ✓ | ✗ |
| Need for clearly defined and individualised SLE patient pathways. | ✓ | ✓ |
| Lack of detailed information about how to prepare the consultation with the specialist. | ✗ | ✓ |
| Need to clarify the role of secondary healthcare professionals. | ✓ | ✓ |
| Management and referral strategy for SLE flares. | ✓ | ✓ |
| Lack of access to an SLE care coordinator (such as a specialised nurse). | ✓ | ✓ |
| Written personalised therapeutic management plan for patients. | ✗ | ✓ |
| Regular SLE cases review by a multidisciplinary expert panel. | ✓ | ✗ |
| Individualised treatment strategies based on patient characteristics. | ✓ | ✗ |
| Lack of detailed feedback on laboratory tests results. | ✓ | ✓ |
| Therapeutic education about SLE. | ✓ | ✓ |
| Prevention of work disability, including the use of appropriate social support. | ✗ | ✓ |
| Increased interprofessional interactions with coordinated care. | ✓ | ✓ |
| Access to psychologists and dietitians (outside inpatient care settings). | ✗ | ✓ |
| Specific management of paediatric and adolescent SLE, including transition towards adult care. | ✓ | ✓ |
| Improved pain management. | ✗ | ✓ |
| The need for more coordinated care between in-hospital and outpatient pharmacies. | ✗ | ✓ |
| Lack of detailed assessment of SLE impact over personal life. | ✗ | ✓ |
Disruptive points and challenges marked with a tick (✓) were spontaneously reported by the stakeholders during the focus groups while those marked with a cross (✗) were not.
As reported by healthcare professionals
Healthcare professionals underlined the following aspects of SLE diagnosis and management: the general undertraining of physicians (including of primary care physicians) about autoimmune diseases such as SLE, uncertainties about how to manage or to whom shall be referred patients with ANA positivity, and the lack of clearly defined and individualised SLE-PP (taking into account organ manifestations and disease severity), the need for increased access to appropriate and timely diagnosis for all potential patients with SLE, independent of their socioeconomic background or language barrier, if any, was also highlighted. They have also underlined the importance of individualised treatment based on the characteristics of patients with SLE, organ involvement, severity and predictors of response, including therapeutic adherence, with the necessity to incorporate innovating tools and technologies to ensure privacy and high quality of care, while favouring interprofessional interactions for coordinated care and shared decision making. Finally, the promotion of therapeutic education and the need for additional support throughout the patient journey, including the prevention of work disability with the help of occupational physicians and the use of appropriate social support, were strengthened.
As reported by patients with SLE and patient associations
Patients with SLE and patient associations underlined several key disruptive elements in line with those reported by healthcare professionals (table 1). While patients with SLE did not specifically discuss the issue of ANA testing or the need for more interoperable and secured data management systems between healthcare professionals, they were specifically concerned about the lack of specific consultation for announcing the diagnosis of SLE and of adequate support following this announcement. They were also concerned about the need for more detailed information on how to prepare the consultation with the specialist, as well as the need for a written and personalised therapeutic management plan. Finally, they underlined the lack of coordinated care.
Thematic clusters
Based on the disruptive points and challenges identified during the focus groups, six thematic clusters emerged regarding facets underlying the optimisation of PP in SLE:
The need for an appropriate and timely referral strategy for SLE diagnosis, clarifying the indications of ANA testing and management of ANA positivity, as well as the subsequent referral strategy to specialists for proper SLE diagnosis.
The need for a dedicated consultation, during which the diagnosis of SLE would be announced and psychological support offered (and reiterated, if needed).
The need for individualised pathways with coordinated care based on organ involvement, disease activity (flares), severity and patient preference. Access to an SLE care coordinator (such as a specialised nurse) and implementation of interoperable data management systems between healthcare professionals should improve interprofessional management. Specific populations such as paediatric patients with SLE or pregnant women with SLE should benefit from dedicated pathways.
The need for improved patient education, with specific emphasis over the natural course of the disease and general therapeutic strategy, should be emphasised.
The need for preventing complications such as infections, osteoporosis, cancer and cardiovascular diseases.
The need for additional patient support, especially psychological, social and occupational support.
Conceptual framework for an optimised SLE pathway
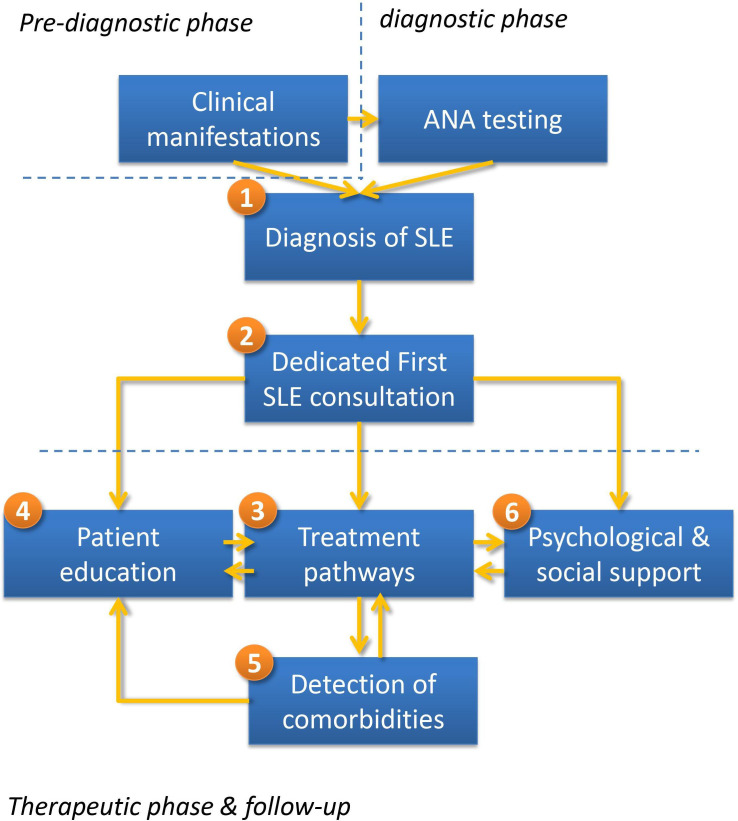
During consensus meeting, stakeholders discussed these six thematic clusters and came to a consensus on the key elements of an optimised SLE pathway. Based on focus group discussions and the expert panel consensus, a unifying framework for an optimised SLE-PP is presented in figure 1. This framework is modulated by individual patient characteristics, such as the socioeconomic background and employment status, as well as by urgency and severity of organ involvement and the need for a referral to an expert centre.
Figure 1.
Conceptual framework for an optimised SLE pathway: (1) appropriate and timely referral strategy for SLE diagnosis; (2) dedicated consultation, during which the diagnosis of SLE is announced; (3) individualised pathways with coordinated care based on organ involvement, disease severity and patient preference; (4) improved patient education; (5) prevention and detection of complications such as infections, osteoporosis and cancer; (6) additional patient support.
Discussion
We have developed a framework for optimising PPs in SLE which integrates the following elements: appropriate and timely referral strategy for SLE diagnosis; a dedicated lupus consultation, during which the diagnosis is formally announced, and following which psychological support is offered; individualised pathways with coordinated care based on organ involvement, disease severity and patient preference; improved patient education; prevention of complications such as infections, osteoporosis and cancer; as well as additional patient support, in particular from the social and employment perspectives. It is interesting to point out that five disruptive points and challenges which were reported by the stakeholders were not spontaneously reported by patients with SLE and that nine of these points were reported by patients but not stakeholders. Reconciling the different points of view of healthcare professionals and patients is obviously a major challenge when optimising PP.
Appropriate and timely diagnosis
A recent study has shown that the median diagnosis delay for SLE in Europe is ≈2 years and ≈1 year in France.5 This is largely due to the rarity of the disease, its highly polymorphic nature and the lack of a highly specific biomarker. Importantly, diagnosis delay may vary according to ethnic background and/or socioeconomic background. It is common feeling that the early diagnosis of SLE can be beneficial by allowing early intervention and potentially improving short-term and long-term outcomes. However, there is still limited evidence supporting this assumption, which mainly derives from administrative database analysis showing that patients with early diagnosis (<6 months between probable SLE onset and diagnosis) had lower rates of flares and hospitalisations compared with patients with late diagnosis (≥6 months).14 During focus groups, the GPs (primary care physicians) have underlined the lack of general training in the field of autoimmune diseases as well as the complexity of ANA interpretation. Hence, the indications for ANA testing and the adequate referral procedure following ANA positivity should be optimised within the SLE-PP. In the USA, it has been shown that test ordering is significantly influenced by hidden cognitive processes related to the physician’s calculation of patient resources and a health insurance system that requires certain types of evidence in order to permit further tests or particular interventions.15 In France, as well as in most European countries, the healthcare system is publicly funded and access to ANA testing is not a significant limitation. However, in most European countries, there are insufficient physicians in primary care for timely and adequate patient detection and monitoring,16 as well as significant imbalance between resource and demand for specialised care, which may result in additional delay in referral from primary to secondary or tertiary care.
Dedicated first SLE confirmation consultation
Due to the chronic nature of the disease and its potential complications, including the impact on pregnancy, patients have underlined the need for a dedicated first SLE consultation, during which the diagnosis would be announced formally and the natural course of the disease, general therapeutic strategy as well as the potential impact on various life aspects, including occupational issues, would be explained. This concept is very much related to patient education (see patient education), but the initial SLE consultation should be considered a separate intervention.
Personalised therapeutic strategy with integrated care ensuring multiprofessional collaboration
Finding the right treatment for the right patient remains one of the most important challenges in SLE. The markers that have been known for decades, such as anti-double-stranded (ds)DNA IgG antibodies, complement factor consumption or leucopenia, are now insufficient to progress in the management of the disease.3 The era of multiomics (genomics, transcriptomics, proteomics or metabolomics), by means of high-throughput tools, opens the door for integrated and individualised approach, but these tools have not entered daily medical care.3
Also, patients with SLE have underlined the lack of coordinated care from various healthcare providers. The consequences may include duplicate diagnosis testing, contradicting information about SLE diagnosis or treatment, and time wasted on avoidable consultations, with the corresponding work absence, loss of productivity and healthcare expenditures. The most adequate therapeutic strategy should be documented by healthcare professionals who are competent and qualified to make a diagnosis of SLE and/or recommend treatment for the patient’s problems. A key element is the need to communicate with all healthcare professionals involved in SLE patient care.10 The need for patients with SLE to be referred directly to SLE specialists for confirmation of diagnosis and assessment for disease-modifying therapies has been underlined. Also, the importance of having access to specialised SLE teams and clinics with different Healthcare Professionals (HCPs) working together was highlighted.17 Finally, the strategy for access to secondary and tertiary care should be better defined. The stakeholders suggested that disease flare and symptom management should be triaged and managed by a dedicated SLE nurse. Also, pregnancy is challenging for patients with SLE and their treating physicians. In general, a multidisciplinary team, consisting of a rheumatologist or an internist and an obstetrician with significant experience in high-risk pregnancies, manages the care of pregnant patients with SLE. Patients should be informed that pregnancies in SLE should be carefully anticipated and that prepregnancy multidisciplinary counselling is important to determine the risk of both maternal and fetal complications.
Detection and prevention of comorbidities
Due to its chronic nature, patients with SLE may develop cardiovascular complications, infections and osteoporosis, which may in part be preventable by modifying or treating the associated risk factors.18 19 Rigorous assessment and modification of traditional and disease-related Cardiovascular disease (CVD risk factors in patients with SLE are warranted, especially in high-risk populations such as those with baseline disease severity, renal involvement, high cumulative corticosteroid dose and positive antiphospholipid antibodies.20 21 Good control of disease activity,22 minimisation of corticosteroid exposure and lifestyle optimisation are of high importance. The role of hydroxychloroquine in the reduction of CVD risk has also been demonstrated based on its pleiotropic mode of action.23 24 In addition, implementation strategies for risk factor prevention are also needed. Serious infections, defined as those requiring hospitalisation or resulting in death, constitute one of the leading causes of morbidity and mortality in SLE, along with CVD.25 In a study of all-cause SLE readmissions using hospital discharge databases (2008–2009) from five geographically dispersed US states, one in six patients with SLE were readmitted within 30 days, with significant hospital-level and state-level variations in readmission rates.6 Interestingly, lower risk-adjusted readmission rates were observed in a state with high concentration of dedicated SLE centres. A recent study using national population-based data on the outcomes of adults with SLE admitted with sepsis (2002–2011) showed a wide variation in mortality rates between hospitals, with lower rates in hospitals treating more patients with SLE.7 Together, these data reinforce the need for expert centres dedicated to the care of autoimmune diseases, including SLE.
Patient education
Recent guidelines recognise the importance of patient involvement in the management of their own healthcare needs.26 The routine time available for medical appointment is clearly insufficient for indepth patient education16 27 and empowerment for self-management, such as by providing advice and education on lifestyle and risk factor management, and ultimately for shared decision making.28 Dedicated patient education programmes should be developed, taking into account individualised patient profiles, education level and disease severity. Digital health offers the perspective of online patient education for remote patients with SLE.
Psychological, social and occupational support
An important challenge in SLE is to favour holistic medicine, which is the use of therapeutic strategies to treat the patient as a whole person. In the last decades, with improvement in the life expectancy of patients with SLE, the relationship between social support and health in this population has received a considerable amount of attention in behavioural medicine and health psychology.29 A number of different potential psychological issues can affect those with SLE; difficulties may arise from the disease itself, which may affect the central nervous system (neuropsychiatric lupus), from the general effects of having a chronic condition with a variable course or from adverse events related to medications. Severe fatigue, depression and generalised anxiety can occur as a reaction to these symptoms.30 31 Access to psychological support should be encouraged and streamlined. Also, approximately half of respondents in the recent Lupus Europe survey felt that SLE had impacted their studies or their employment status.5 Further, previous studies have shown that presenteeism, absenteeism and work disability are high in SLE.32 However, adaptations of workstations are often possible if necessary, by means of occupational physicians and his pluriprofessional team, which may be contacted early in order to find the most accurate way to retain the individual in employment. Work disability is associated with a complex array of health factors, including comorbidity, physical and mental health limitations, and clinical features of lupus, which warrant increased attention in future research33 and a dedicated consultation which should be incorporated in the PP.
The strength of this study is the use of both qualitative and consensus methods involving SLE experts as well as representative stakeholders from patients, physicians and payers. This study design facilitated constructive debate while reducing the potential bias of influential opinions. The presentation of the results for review and comment to patients and healthcare professionals and then back to the whole group of stakeholders further supports the validity of our findings. It is however important to underline that wider involvement of different stakeholders from different countries may further contribute to patients’ care pathways. The involvement of different healthcare systems can ensure the inclusion of different barriers and challenges. The ERN ReCONNET (European Reference Network on Rare and Complex Connective Tissue Diseases) is a framework under which several initiatives are ongoing on patients’ care pathways (including SLE), and specific methodologies have been designed and adopted (RarERN Path methodology) to enable the design of patients’ care pathways based on a deep sharing of expertise on high-quality care and characterised by a strong patient-centred approach.34
In summary, we have identified points of disruptions in SLE-PP and developed a unifying conceptual framework for optimising these pathways. The results may prove useful at several levels, including that of patients, physicians and healthcare organisations. Among the challenges are the practical implementation of this optimal PP at the regional level and its subsequent evaluation and generalisation at the national level, incorporating cost-effectiveness and the timely initiation of diagnostic strategy and treatments, tailored at the individual patient level.
Acknowledgments
The authors wish to acknowledge the crucial role of Ms Marianne Rivière from the patient association aFL+ (Association Française du Lupus et des autres maladies auto-immunes) in the study, as well as the patients who participated in this study. The authors also wish to thank Ms Baumgaertner for the invaluable assistance in the preparation of the manuscript.
Footnotes
Contributors: LA, TM and IP-R designed the study. All authors contributed to data collection. LA performed the data analysis. LA drafted the manuscript. All authors reviewed the manuscript for intellectual content and approved the final version. LA is the guarantor of the study.
Funding: This work is funded by GSK.
Competing interests: LA has acted as a consultant for Alexion, Amgen, AstraZeneca, Biogen, BMS, Boehringer Ingelheim, GSK, Grifols, Janssen-Cilag, LFB, Lilly, Menarini France, Medac, Novartis, Pfizer, Roche-Chugaï and UCB. Médiation Conseil Santé has been hired as a consulting agency for the organisation of the patient pathway study.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Ethics Committee of Strasbourg Faculty of Medicine, Strasbourg, France.
References
- 1.Tamirou F, Arnaud L, Talarico R, et al. Systemic lupus erythematosus: state of the art on clinical practice guidelines. RMD Open 2018;4:e000793. 10.1136/rmdopen-2018-000793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scherlinger M, Mertz P, Sagez F, et al. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun Rev 2020;19:102531. 10.1016/j.autrev.2020.102531 [DOI] [PubMed] [Google Scholar]
- 3.Felten R, Sagez F, Gavand P-E, et al. 10 most important contemporary challenges in the management of SLE. Lupus Sci Med 2019;6:e000303. 10.1136/lupus-2018-000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piga M, Arnaud L. The main challenges in systemic lupus erythematosus: where do we stand? J Clin Med 2021;10:243. 10.3390/jcm10020243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornet A, Andersen J, Myllys K, et al. Living with systemic lupus erythematosus in 2020: a European patient survey. Lupus Sci Med 2021;8:e000469. 10.1136/lupus-2020-000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yazdany J, Marafino BJ, Dean ML, et al. Thirty-day Hospital readmissions in systemic lupus erythematosus: predictors and hospital- and state-level variation. Arthritis Rheumatol 2014;66:2828–36. 10.1002/art.38768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tektonidou MG, Dasgupta A, Ward MM. Interhospital variation in mortality among patients with systemic lupus erythematosus and sepsis in the USA. Rheumatology 2019;58:1794–801. 10.1093/rheumatology/kez103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maritaz C, Gault N, Roy C, et al. [Impact of a coordinated regional organization to secure the management of patients on oral anticancer drugs: CHIMORAL, a comparative trial]. Bull Cancer 2019;106:734–46. 10.1016/j.bulcan.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 9.Chasset F, Richez C, Martin T, et al. Rare diseases that mimic systemic lupus erythematosus (lupus mimickers). Joint Bone Spine 2019;86:165–71. 10.1016/j.jbspin.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 10.Walsh DA, Kelly C, Bosworth A, et al. Provisional guidelines for applying the Department of health (England) 18-week-patient pathway to specialist rheumatology care. Rheumatology 2007;46:1200–6. 10.1093/rheumatology/kem125 [DOI] [PubMed] [Google Scholar]
- 11.Spielmann L, Arnaud L, Severac F, et al. Population-Based prevalence of eosinophilic fasciitis (Shulman syndrome): a capture-recapture study. Br J Dermatol 2018;179:516–7. 10.1111/bjd.16535 [DOI] [PubMed] [Google Scholar]
- 12.Giorgiutti S, Dieudonne Y, Hinschberger O, et al. Prevalence of antineutrophil cytoplasmic antibody-associated vasculitis and spatial association with Quarries in a region of northeastern France: a capture-recapture and Geospatial analysis. Arthritis Rheumatol 2021;73:2078–85. 10.1002/art.41767 [DOI] [PubMed] [Google Scholar]
- 13.Arnaud L, Fagot J-P, Mathian A, et al. Prevalence and incidence of systemic lupus erythematosus in France: a 2010 nation-wide population-based study. Autoimmun Rev 2014;13:1082–9. 10.1016/j.autrev.2014.08.034 [DOI] [PubMed] [Google Scholar]
- 14.Oglesby A, Korves C, Laliberté F, et al. Impact of early versus late systemic lupus erythematosus diagnosis on clinical and economic outcomes. Appl Health Econ Health Policy 2014;12:179–90. 10.1007/s40258-014-0085-x [DOI] [PubMed] [Google Scholar]
- 15.Tritter JQ, Lutfey K, McKinlay J. What are tests for? the implications of stuttering steps along the US patient pathway. Soc Sci Med 2014;107:37–43. 10.1016/j.socscimed.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 16.Alves da Costa F, Rydant S, Antoniou S. The patient pathway in cardiovascular care: a position paper from the International pharmacists for anticoagulation care Taskforce (iPACT). J Eval Clin Pract 2020;26:670–81. 10.1111/jep.13316 [DOI] [PubMed] [Google Scholar]
- 17.Talarico R, Aguilera S, Alexander T, et al. The impact of COVID-19 on rare and complex connective tissue diseases: the experience of ERN ReCONNET. Nat Rev Rheumatol 2021;17:177–84. 10.1038/s41584-020-00565-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chasset F, Francès C, Barete S, et al. Influence of smoking on the efficacy of antimalarials in cutaneous lupus: a meta-analysis of the literature. J Am Acad Dermatol 2015;72:634–9. 10.1016/j.jaad.2014.12.025 [DOI] [PubMed] [Google Scholar]
- 19.Parisis D, Bernier C, Chasset F, et al. Impact of tobacco smoking upon disease risk, activity and therapeutic response in systemic lupus erythematosus: a systematic review and meta-analysis. Autoimmun Rev 2019;18:102393. 10.1016/j.autrev.2019.102393 [DOI] [PubMed] [Google Scholar]
- 20.Arnaud L, Mathian A, Devilliers H, et al. Patient-Level analysis of five international cohorts further confirms the efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies. Autoimmun Rev 2015;14:192–200. 10.1016/j.autrev.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 21.Moulis G, Audemard-Verger A, Arnaud L, et al. Risk of thrombosis in patients with primary immune thrombocytopenia and antiphospholipid antibodies: a systematic review and meta-analysis. Autoimmun Rev 2016;15:203–9. 10.1016/j.autrev.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 22.Chessa E, Piga M, Floris A, et al. Use of physician global assessment in systemic lupus erythematosus: a systematic review of its psychometric properties. Rheumatology 2020;59:3622–32. 10.1093/rheumatology/keaa383 [DOI] [PubMed] [Google Scholar]
- 23.Dima A, Jurcut C, Arnaud L. Hydroxychloroquine in systemic and autoimmune diseases: where are we now? Joint Bone Spine 2021;88:105143. 10.1016/j.jbspin.2021.105143 [DOI] [PubMed] [Google Scholar]
- 24.Petitdemange A, Felten R, Sibilia J, et al. Prescription strategy of antimalarials in cutaneous and systemic lupus erythematosus: an international survey. Ther Adv Musculoskelet Dis 2021;13:1759720X211002595. 10.1177/1759720X211002595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moghaddam B, Marozoff S, Li L, et al. All-Cause and cause-specific mortality in systemic lupus erythematosus: a population-based study. Rheumatology 2021;61:367–76. 10.1093/rheumatology/keab362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergier H, Duron L, Sordet C, et al. Digital health, big data and smart technologies for the care of patients with systemic autoimmune diseases: where do we stand? Autoimmun Rev 2021;20:102864. 10.1016/j.autrev.2021.102864 [DOI] [PubMed] [Google Scholar]
- 27.Irving G, Neves AL, Dambha-Miller H, et al. International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open 2017;7:e017902. 10.1136/bmjopen-2017-017902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toupin-April K, Décary S, de Wit M, et al. Endorsement of the OMERACT core domain set for shared decision making interventions in rheumatology trials: results from a multi-stepped consensus-building approach. Semin Arthritis Rheum 2021;51:593–600. 10.1016/j.semarthrit.2021.03.017 [DOI] [PubMed] [Google Scholar]
- 29.Mazzoni D, Cicognani E. Social support and health in patients with systemic lupus erythematosus: a literature review. Lupus 2011;20:1117–25. 10.1177/0961203311412994 [DOI] [PubMed] [Google Scholar]
- 30.Arnaud L, Mertz P, Amoura Z, et al. Patterns of fatigue and association with disease activity and clinical manifestations in systemic lupus erythematosus. Rheumatology 2021;60:2672–7. 10.1093/rheumatology/keaa671 [DOI] [PubMed] [Google Scholar]
- 31.Arnaud L, Gavand PE, Voll R, et al. Predictors of fatigue and severe fatigue in a large international cohort of patients with systemic lupus erythematosus and a systematic review of the literature. Rheumatology 2019;58:987–96. 10.1093/rheumatology/key398 [DOI] [PubMed] [Google Scholar]
- 32.Utset TO, Baskaran A, Segal BM, et al. Work disability, lost productivity and associated risk factors in patients diagnosed with systemic lupus erythematosus. Lupus Sci Med 2015;2:e000058. 10.1136/lupus-2014-000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al Dhanhani AM, Gignac MAM, Su J, et al. Work disability in systemic lupus erythematosus. Arthritis Rheum 2009;61:378–85. 10.1002/art.24347 [DOI] [PubMed] [Google Scholar]
- 34.Talarico R, Cannizzo S, Lorenzoni V, et al. RarERN path: a methodology towards the optimisation of patients' care pathways in rare and complex diseases developed within the European reference networks. Orphanet J Rare Dis 2020;15:347. 10.1186/s13023-020-01631-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.