Abstract
Background:
Mobility is important for independence in older age. While brain health correlates of objectively measured mobility-related features like gait and balance have been reported, we aimed to test neuroimaging and cognitive correlates of subjective measures of mobility-related confidence.
Methods:
We carried out a cross-sectional observational study comprised of N=29 cognitively unimpaired older adult participants, mean age 75.8±5.8, 52% female, 24% non-white. We measured cognition, hippocampal volume, white matter hyperintensities, cerebral amyloid-β (Aβ), and gait and balance confidence. We tested associations using unadjusted Spearman correlations and correlations partialling out covariates of interest one at a time.
Results:
Greater gait confidence was associated with better attention (unadjusted ρ=0.37, p=0.05; partially attenuated by adjustment for age, APOE4, anxiety, motivation, gait speed, and Aβ); executive performance (unadjusted ρ=0.35, p=0.06; partially attenuated by adjustment for age, APOE4, gait speed, or Aβ); and lower Aβ levels (unadjusted ρ= −0.40, p=0.04; partially attenuated by adjustment for age, depressive symptoms, motivation, or gait speed). Greater balance confidence was associated with better global cognition (unadjusted ρ=0.41, p=0.03; partially attenuated by adjustment for APOE4, gait speed, or Aβ); attention (unadjusted ρ=0.46, p=0.01; robust to adjustment); and lower Aβ levels (unadjusted ρ= −0.35, p=0.07; partially attenuated by adjustment for age, education, APOE4, depressive symptoms, anxiety, motivation, or gait speed).
Conclusions:
Self-reported mobility-related confidence is associated with neuroimaging and cognitive measures and would be easy for providers to use in clinical evaluations. These associations should be further evaluated in larger samples, and longitudinal studies can help determine temporality of declines.
Keywords: amyloid-β, cognition, hippocampal volume, white matter hyperintensities, mobility
1. INTRODUCTION
The ability to get where one would like to go—mobility—is a key feature of aging well and maintaining a good quality of life. Research into the neural and cognitive profiles associated with objectively measured mobility (e.g., gait speed and semi- and full-tandem stands) has suggested that mobility-related changes in aging may mark a subgroup of older adults at greater risk for cognitive impairment.1 Prior research has identified a number of neural correlates of poor mobility objectively measured in the lab, including greater white matter hyperintensity (WMH) burden, smaller hippocampal volume, and presence of amyloid-β (Aβ) in those who are cognitively unimpaired.2–4 In contrast, little is known overall about the neural and cognitive correlates of subjective mobility-related measures (gait and balance) which some have referred to in the mobility literature as “confidence” and some as “self-efficacy” based on Bandura’s Social Cognitive Theory5; we use the term “confidence”. For example, associations of self-reported balance confidence with whole brain gray and white matter volume have been reported in older women.6 Importantly, such subjective measures of mobility-related confidence are correlated with objective measures of mobility7,8 but also include psychological aspects such as insight and anxiety which may not be evident in objective measures. We include both gait and balance confidence because gait and balance represent complementary but distinct constructs necessary for mobility; balance, the ability to maintain upright posture, being necessary but not sufficient for gait, the pattern of walking.
While objective measures of performance have some advantages in comparison to subjective measures (e.g., not subject to recall bias due to imperfect memory or other biases inherent in self-report), subjective measures also bring many benefits to mobility assessment. First, they add important information beyond that provided by objective measures, integrating how well someone can perform with their perception of ability. Second, self-report measures capture perceived performance across a range of environmental conditions that are experienced regularly during daily life whereas objective measures are typically conducted without environmental context.9 Third, changes in subjective measures may in fact occur before objective impairments, particularly as people tend to over-perform under observation in clinical settings.10 Perceptions of poor balance or the presence of underlying neuropathology may lead to cautious gait patterns,11 which in itself can lead to falls.12 Similarly, subjective memory complaints predict conversion to mild cognitive impairment and dementia.13 Fourth, low mobility confidence may lead to reductions in activity, thus worsening mobility due to the bidirectional longitudinal links between physical function and physical activity.14 Finally, such self-reported measures are easy for clinicians to assess and for individuals to report on.
Given the clinical potential of mobility-related confidence measures, their associations with neuroimaging and cognitive variables merit further evaluation. Our aim was to explore the brain and cognitive correlates of mobility-related confidence measures including gait and balance confidence in a sample of older adults without cognitive impairment.
2. MATERIALS AND METHODS
2.1. Participants.
The Neural Mechanisms of Community Mobility (NMCM) Study was designed to evaluate brain health correlates of objective measures of mobility. NMCM participants were recruited from a longitudinal parent study of Aβ deposition in cognitively unimpaired older adults.15 The parent study participants are a convenience sample recruited through other studies of normal aging and newspaper advertisements; they were not recruited from a memory clinic. Exclusion criteria for the parent study included: mild cognitive impairment, dementia, and major psychiatric or neurological disorders. To be eligible for the NMCM Study, participants in the parent study had to 1) have neuropsychological testing completed preferably within four months of the NMCM visit; 2) have completed an MRI within 2 years of the NMCM visit; 3) be age 65 or older; and 4) be able to walk unassisted. Exclusion criteria for the NMCM Study included mild cognitive impairment or dementia diagnosis and history of stroke.
This study was reviewed and approved by the University of Pittsburgh Institutional Review Board, and informed consent was completed before any study procedures were initiated.
2.2. Neuroimaging.
MRI scanning was completed on a Siemens 3T TIM Trio scanner at the University of Pittsburgh Magnetic Resonance Research Center. Both hippocampal and intracranial volume (ICV) were measured via Magnetization Prepared – Rapid Gradient Echo MRI (MPRAGE; slice thickness=1.2mm, FOV=256mmx240mm, TR=2300ms, TI=900ms, TE=2.98ms, FA=9°). ICV was determined using an automatic mask applied to the MPRAGE image as described by Karim, et al.16 Whole hippocampal volume was processed using the Automated Labeling Pathway and normalized to ICV for analyses.17 WMH volume was extracted using an automated pipeline18 applied to the co-registered MPRAGE and T2-weighted FLAIR (slice thickness=3mm, FOV=256mmx212mm, TR=9160 ms, TI=2500ms, TE=90ms, FA=150°) and was normalized to whole brain volume. Aβ imaging with PET was performed using Pittsburgh Compound B (11C-PiB), which was synthesized as previously reported.19 The PET quantification approach has been fully reported elswewhere.20 Briefly, the PET image was co-registered to the individual MPRAGE MRI. FreeSurfer 5.3 was used for MRI region of interest (ROI) parcellations using a combination of the Imperial College London Clinical Imaging Centre (CIC) atlas21 (for striatum only) and the default Desikan-Killiany atlas.22,23 ROI sampling of the PET images was performed, where each ROI was the volume weighted average of the included FreeSurfer subregions. Standardized uptake value (SUV) measures were computed for each ROI based on a 50-70 minutes post-injection PET acquisition. SUV ratio (SUVR) was calculated by normalizing the SUV in each ROI to the SUV in cerebellar gray matter. To determine Aβ burden, we used a global ROI which is a volume-weighted average of anterior cingulate cortex, anterior ventral striatum, orbitofrontal cortex, superior frontal cortex, insula, lateral temporal cortex, parietal lobe, posterior cingulate cortex, and the precuneus.20 We treated global 11C-PiB SUVR as a continuous variable in these analyses, but also explored PiB positivity based on our global cutoff of 1.346, determined using our sparse k-means approach.24
2.3. Cognition.
The neuropsychological assessment battery used in the parent study has been previously described,15 and includes assessments of memory, visuospatial, attention, language, executive, and global domains which were administered by trained raters supervised by a neuropsychologist. Tests by domain are shown in Table 1. We z-scored the test scores based on means and standard deviations in the full parent study sample at study baseline when all parent study participants were cognitively unimpaired (N=128, age, mean (SD)= 73.1 (5.7), education, mean (SD)= 15.2 (2.5), female= 82 (64%)). If necessary, tests were reverse scored so the direction of all z-scores corresponds to higher representing better performance. Domain z-scores are the average z-score of the available tests in each domain, and the global z-score is the average of the available domain z-scores.
Table 1.
Neuropsychological tests comprising each cognitive domain.
| Domain | Tests |
|---|---|
| Memory | CERAD word list learning trials25 CERAD delayed recall25 Logical Memory—Delayed Recall26 Modified Rey Figure—Delayed Recall27 |
| Attention | Digit Span Forward Max Span26 Trails A Time28 |
| Visuospatial | Modified Rey Figure Copy27 Modified Block Design29 from the Wechsler Adult Intelligence Scale-Revised30 |
| Language | Boston Naming Test, spontaneously correct31 Fluency—Animals32 Fluency—Words starting with F, A, and S33 |
| Executive | Digit Span Backward Max Span26 Trails B Time28 Digit Symbol Coding Total Score30 Stroop Color Word Total Score34 |
2.4. Mobility-related confidence measures.
2.4.1. Gait confidence.
We used the modified Gait Efficacy Scale7 to assess participants’ confidence levels regarding walking under ten different circumstances with varying challenge levels such as walking on grass, over an obstacle, and up and down the stairs with and without a handrail. The score ranges from 10-100, with higher indicating greater confidence. The scale has demonstrated good test-retest reliability (intraclass correlation coefficient=0.93), internal consistency (Cronbach’s α=0.94), and validity for use in community-dwelling older adults.7 Specifically, the modified Gait Efficacy Scale is correlated with objective mobility-related measures such as gait speed (Spearman’s ρ=0.64) and 6-minute walk test (ρ=0.60) as well as other measures of confidence and fear such as the fear of falling subscale of the Survey of Activities and Fear of Falling in the Elderly (ρ= −0.71) and the Activities-specific Balance Confidence (ABC) Scale (ρ=0.88) 7
2.4.2. Balance confidence.
We used the ABC Scale8 to assess participants’ confidence that they could perform 16 different activities of varying difficulty levels without losing their balance (e.g., reaching at eye level, walking across a parking lot, sweeping, reaching on their tiptoes, reaching while standing on a chair, walking on an icy sidewalk, and the like). Confidence was rated from 0-100% for each item (greater percentages indicate greater confidence), and the overall score is the average. Evaluation of the ABC Scale’s psychometric properties has shown high test-retest reliability (r=0.92) and internal consistency (Cronbach’s α=0.96).8 It was able to more sensitively distinguish low-mobility from high-mobility older adults than the Falls Efficacy Scale, and it also has a wider range of variance than the Falls Efficacy Scale, making it more suitable to assess balance confidence in high-mobility older adults such as the NMCM study participants.8
2.5. Covariates and other variables.
Demographics (age, sex, race, and education) and hypertension diagnosis were self-reported. Body mass index (BMI) was calculated based on self-reported height and study-measured weight. Participants were tested for the APOE4 allele, which we treated as carrier (APOE4+)/non-carrier. We assessed depressive symptoms with the Geriatric Depression Scale (GDS).35 We characterized anxiety using a sum score of anxiety-related items from the GDS (items 6, 8, 11, 13, 26, 28, 29), which were reviewed with a geriatric psychiatrist. We also characterized motivation using a previously reported subscale of the GDS.36 Joint pain was assessed by asking participants if they had joint pain in the past lasting at least one month in their knees, hips, or ankles. Time to complete five chair stands and perform a full-tandem stand were evaluated based on the Short Physical Performance Battery.37 Standing balance assessment was ended at 30 seconds. Gait speed was calculated based on the average of four passes of a 15-meter walk.
2.6. Statistical Analysis.
Here, we report a cross-sectional exploratory analysis of N=29 community-dwelling older adults incorporating data collected both by the parent and NMCM studies. We calculated descriptive statistics including means and standard deviations and counts and percentages. Participant characteristics were compared using t-tests for normally distributed continuous variables, Wilcoxon-Mann-Whitney tests for continuous non-normally distributed variables, and Fisher’s exact tests for categorical variables. To provide information about the distributions of gait and balance confidence in this sample of healthy older adults, we also present ranges, medians, and first and third quartiles of these scores. Gait and balance confidence were treated as continuous in analyses. Because no clinical cut point has been established for gait confidence and our participants had higher scores than the previously suggested cut-points for balance confidence,38 for reporting purposes only in Table 2, we dichotomized based on median splits: <median=low and ≥median=high.
Table 2.
Participant characteristics by gait and balance confidence.
| Characteristic | Overall | Gait confidence | Balance confidence | ||||
|---|---|---|---|---|---|---|---|
| Low, n=13 | High, n=16 | p | Low, n=14 | High, n=15 | p | ||
| Mini-Mental State Examination | 28.6 (1.5) | 28.8 (1.5) | 28.5 (1.6) | 0.68 | 28.7 (1.5) | 28.5 (1.6) | 0.78 |
| Age | 75.8 (5.8) | 77.6 (5.9) | 74.3 (5.4) | 0.12 | 76.7 (5.7) | 74.9 (5.9) | 0.40 |
| Female sex | 15 (51.7) | 7 (53.9) | 8 (50.0) | >0.99 | 7 (50.0) | 8 (53.3) | >0.99 |
| Non-white race | 7 (24.1) | 3 (23.1) | 4 (25.0) | >0.99 | 3 (21.4) | 4 (26.7) | >0.99 |
| Education, years | 15.6 (2.3) | 15.5 (2.6) | 15.6 (2.0) | 0.98 | 15.1 (2.6) | 15.9 (1.9) | 0.35 |
| APOE4+ | 12 (48.0) | 6 (54.6) | 6 (42.9) | 0.70 | 6 (54.6) | 6 (42.9) | 0.70 |
| BMI | 26.6 (4.5) | 26.4, (4.0) | 26.7, (5.1) | 0.86 | 25.7, (4.4) | 27.4, (4.6) | 0.32 |
| Hypertension | 18 (62.1) | 9 (69.2) | 9 (56.3) | 0.70 | 9 (64.3) | 9 (60.0) | >0.99 |
| Depressive symptoms | 2.5 (2.5) | 3.4, (2.7) | 1.7, (2.2) | 0.10 | 3.4 (2.7) | 1.6 (2.2) | 0.07 |
| Anxiety | 0.8 (1.2) | 1.0 (1.4) | 0.6 (1.0) | 0.51 | 1.1 (1.3) | 0.5 (0.9) | 0.10 |
| Motivation | 1.3 (1.7) | 1.9 (1.8) | 0.8 (1.6) | 0.06 | 1.9 (1.9) | 0.7 (1.4) | 0.03 |
| Joint pain | 8 (27.59) | 4, (30.77) | 4, (25.00) | >0.99 | 3, (21.43) | 5, (33.33) | 0.68 |
| Chair stand time (s) | 12.3 (2.8) | 13.6 (2.9) | 11.0 (2.2) | 0.02 | 13.0 (2.1) | 11.6 (3.2) | 0.22 |
| Full tandem time (s) | 28.7 (4.3) | 28.3 (4.8) | 29.0 (3.8) | 0.43 | 28.4 (4.6) | 29.0 (4.0) | 0.51 |
| Gait speed (m/s) | 1.0 (0.1) | 0.9 (0.1) | 1.0 (0.2) | 0.20 | 0.9 (0.1) | 1.0 (0.2) | 0.13 |
| Balance confidence | 93.5 (5.4) | 90.4, (5.6) | 96.1, (3.7) | 0.01 | -- | -- | -- |
| Gait confidence | 93.2 (6.8) | -- | -- | -- | 90.3 (6.1) | 95.9 (6.6) | 0.01 |
Note: Numbers are N (%) or mean (SD). Low (<median) vs high (≥ median) gait confidence based on median split at 94.0. Low (<median) vs high (≥ median) balance confidence based on median split at 94.3. P-values are from Fisher’s exact tests for categorical variables or t-tests (for normally distributed) or Wilcoxon-Mann-Whitney (for non-normally distributed) for continuous variables. m=meters, s=seconds.
Due to both non-normal distributions of gait and balance confidence, assessed via visual inspection of the distributions and Shapiro-Wilk tests of normality, and the small sample size, we tested associations of gait and balance confidence with cognition and brain pathology using Spearman correlations. We present results of unadjusted correlations followed by correlations partialling out one covariate at a time, due to small sample size, as follows: age, sex, race, education, APOE4, depressive symptoms, anxiety, motivation, gait speed, Aβ, and the time between assessments of mobility and brain pathology / cognition. In sensitivity analyses, we repeated the analysis for any correlations that, upon visual inspection of scatterplots, appeared potentially driven by outliers.
We set alpha to 0.10, and we did not correct for multiple comparisons. We took this approach in these exploratory analyses because we wished to keep any promising associations for future confirmatory hypothesis driven testing; therefore, we wished to minimize false negative results.39 Statistical analyses were carried out in SAS version 9.440 and R version 4.1.0.41
3. RESULTS
Participant characteristics overall and by gait and balance confidence are presented in Table 2. On average, participants were 76 years of age with nearly a college education. They were likelier to be white (76%) than non-white, in a proportion which was similar to that for the local population overall (82%42), and they were nearly evenly split by sex (local population age 65+ = 58% female42). The sample was enriched for APOE4 beyond typical US population levels (48% sample vs. typically 7-33% US43,44).
Both gait and balance confidence were left skewed. Scores on gait confidence ranged from 78.0-100.0, with first and third quartiles of 90.0 and 100.0 and mean and median of 93.2 and 94.0. Balance confidence scores ranged from 81.9-100.0, with first and third quartiles of 90.6 and 98.1 and mean and median of 93.5 and 94.4.
Participants with high gait confidence (≥ the median) had faster chair stand time (p=0.02), better motivation score (p=0.06), and higher balance confidence (p=0.01) than those with low gait confidence (Table 2). While differences were not significant, those with high gait confidence were younger (p=0.12) and had fewer depressive symptoms (p=0.10) than those with low gait confidence (Table 2). Compared with those with low balance confidence, participants with high balance confidence (≥ the median) had fewer depressive symptoms (p=0.07), better motivation score (p=0.03), and higher gait confidence (p=0.01; Table 2). While not significantly different, those with high balance confidence had faster gait speed than those with low balance confidence (p=0.13; Table 2).
In Table 3, we show descriptive statistics of neuroimaging and cognitive variables in the sample overall. Overall, mean Aβ levels fell below the PiB positive threshold and cognitive performance was within 1 SD of the mean.
Table 3.
Neuroimaging and cognitive characteristics in the full study sample (N=29).
| Neuroimaging Measures | Mean | SD |
|---|---|---|
| Hippocampal volume* | 0.007 | 0.001 |
| White matter hyperintensity volume** | 0.006 | 0.009 |
| Amyloid SUVR*** | 1.30 | 0.35 |
| Cognitive Measures | Mean | SD |
| Global cognition z-score | 0.20 | 0.52 |
| Memory z-score | 0.37 | 0.72 |
| Attention z-score | 0.15 | 0.82 |
| Visuospatial z-score | 0.22 | 0.69 |
| Language z-score | 0.22 | 0.60 |
| Executive z-score | 0.03 | 0.83 |
In N=23.
In N=26.
In N=28.
SUVR=standardized uptake value ratio.
Note: Hippocampal volume was normalized to intracranial volume and white matter hyperintensity volume was normalized to whole brain volume. These measures are therefore unitless and represent the proportion of the head or brain which are hippocampus or white matter hyperintensities, respectively.
In Table 4, we show results of correlation analyses of neuroimaging and cognitive measures and subjective mobility confidence. Greater gait confidence was associated with better attention and executive performance (Table 4 and Figure 1), as well as with lower Aβ levels (Table 4 and Figure 2). Although the association was not significant, greater gait confidence was associated with better global cognition (Table 4). One at a time adjustment for covariates weakened associations of gait confidence with neuroimaging and cognitive variables as follows: attention adjusted for age, APOE4, anxiety, motivation, gait speed, or Aβ; executive function adjusted for age, APOE4, gait speed, or Aβ; and Aβ adjusted for age, depressive symptoms, motivation, or gait speed (Table 4). A similar pattern with covariate adjustment was seen with global cognition (Table 4). No other associations were significant.
Table 4.
Unadjusted and partial Spearman correlations and p-values of gait and balance confidence with neuroimaging and cognition (N=29).
| Unadjusted | Age | Sex | Race | Education | APOE4+ | Depressive Symptoms | Anxiety | Motivation | Gait Speed | Amyloid SUVR | Time Between Function & MRI, PET, or NP | Time Between PET & MRI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values are presented as Spearman’s ρ, p-value. | |||||||||||||
| Hippocampal volume * | |||||||||||||
| Gait confidence | 0.12, 0.59 | 0.02, 0.92 | 0.17, 0.44 | 0.12, 0.60 | 0.12, 0.60 | 0.14, 0.58 | 0.02, 0.94 | 0.05, 0.82 | −0.02, 0.92 | 0.08, 0.72 | 0.08, 0.72 | 0.14%, 0.54 | 0.15, 0.52 |
| Balance confidence | −0.09, 0.69 | −0.14, 0.52 | −0.002, 0.99 | −0.09, 0.70 | −0.07, 0.76 | 0.0001, >0.99 | −0.19, 0.40 | −0.20, 0.37 | −0.27, 0.22 | −0.12, 0.61 | −0.15, 0.50 | −0.10%, 0.65 | −0.13, 0.59 |
| WMH ** | |||||||||||||
| Gait confidence | 0.06, 0.76 | 0.18, 0.39 | 0.07, 0.73 | 0.08, 0.71 | 0.09, 0.65 | −0.03, 0.90 | 0.05, 0.80 | 0.05, 0.80 | 0.14, 0.50 | 0.07, 0.74 | 0.15, 0.47 | −0.05%, 0.80 | 0.06, 0.78 |
| Balance confidence | −0.12, 0.56 | −0.05, 0.80 | −0.11, 0.59 | −0.10, 0.64 | −0.08, 0.72 | −0.14, 0.54 | −0.15, 0.48 | −0.15, 0.48 | −0.06, 0.79 | −0.12, 0.54 | −0.05, 0.80 | −0.07%, 0.74 | −0.13, 0.53 |
| Amyloid *** | |||||||||||||
| Gait confidence | −0.40, 0.04 | −0.26, 0.20 | −0.40, 0.04 | −0.39, 0.04 | −0.40, 0.04 | −0.46, 0.02 | −0.32, 0.10 | −0.34, 0.08 | −0.31, 0.12 | −0.22, 0.27 | −0.41^, 0.03 | −0.44, 0.02 | |
| Balance confidence | −0.35, 0.07 | −0.26, 0.18 | −0.35, 0.08 | −0.35, 0.08 | −0.32, 0.10 | −0.31, 0.14 | −0.26, 0.18 | −0.22. 0.26 | −0.23, 0.25 | −0.16, 0.43 | −0.34^, 0.08 | −0.38, 0.06 | |
| Global cognition z-score | |||||||||||||
| Gait confidence | 0.31, 0.11 | 0.14, 0.49 | 0.33, 0.09 | 0.34, 0.07 | 0.32, 0.10 | 0.23, 0.28 | 0.31, 0.11 | 0.29, 0.13 | 0.24, 0.22 | 0.11, 0.58 | 0.19, 0.35 | 0.32#, 0.10 | |
| Balance confidence | 0.41, 0.03 | 0.32, 0.09 | 0.41, 0.03 | 0.42, 0.02 | 0.33, 0.09 | 0.32, 0.13 | 0.42, 0.03 | 0.41, 0.03 | 0.36, 0.06 | 0.26, 0.19 | 0.33, 0.10 | 0.41#, 0.03 | |
| Memory z-score | |||||||||||||
| Gait confidence | 0.01, 0.98 | −0.14, 0.49 | −0.005, 0.98 | 0.01, 0.95 | −0.01, 0.94 | −0.13, 0.56 | −0.01, 0.95 | 0.0005, >0.99 | 0.003, 0.99 | −0.16, 0.42 | −0.01, 0.96 | 0.01#, 0.97 | |
| Balance confidence | 0.19, 0.32 | 0.12, 0.53 | 0.19, 0.33 | 0.19, 0.32 | 0.13, 0.51 | 0.19, 0.36 | 0.20, 0.32 | 0.20, 0.30 | 0.23, 0.25 | 0.07, 0.71 | 0.20, 0.31 | 0.19#, 0.33 | |
| Visuospatial z-score | |||||||||||||
| Gait confidence | −0.03, 0.88 | −0.10, 0.65 | −0.03, 0.89 | −0.01, 0.94 | −0.09, 0.65 | −0.05, 0.81 | 0.01, 0.96 | 0.009, 0.96 | −0.07, 0.71 | −0.21, 0.29 | −0.01, 0.96 | −0.02#, 0.90 | |
| Balance confidence | 0.12, 0.52 | 0.01, 0.97 | 0.12, 0.53 | 0.13, 0.52 | −0.04, 0.85 | 0.10, 0.64 | 0.18, 0.36 | 0.20, 0.30 | 0.10, 0.60 | −0.02, 0.94 | 0.16, 0.42 | 0.12#, 0.54 | |
| Attention z-score | |||||||||||||
| Gait confidence | 0.37, 0.05 | 0.22, 0.25 | 0.40, 0.04 | 0.38, 0.05 | 0.37, 0.06 | 0.33, 0.12 | 0.32, 0.09 | 0.31, 0.11 | 0.24, 0.21 | 0.28, 0.16 | 0.26, 0.19 | 0.38#, 0.04 | |
| Balance confidence | 0.46, 0.01 | 0.38, 0.04 | 0.46, 0.01 | 0.46, 0.01 | 0.42, 0.03 | 0.35, 0.09 | 0.42, 0.03 | 0.37, 0.06 | 0.33, 0.08 | 0.37, 0.06 | 0.38, 0.05 | 0.46#, 0.01 | |
| Language z-score | |||||||||||||
| Gait confidence | 0.08, 0.67 | 0.13, 0.51 | 0.17, 0.38 | 0.14, 0.49 | 0.07, 0.73 | 0.006, 0.98 | 0.18, 0.37 | 0.13, 0.51 | 0.13, 0.50 | 0.02, 0.92 | 0.03, 0.88 | 0.09#, 0.65 | |
| Balance confidence | 0.14, 0.47 | 0.17, 0.39 | 0.17, 0.38 | 0.16, 0.42 | 0.09, 0.65 | 0.06, 0.76 | 0.24, 0.22 | 0.23, 0.25 | 0.21, 0.28 | 0.11, 0.59 | 0.11, 0.57 | 0.14#, 0.49 | |
| Executive z-score | |||||||||||||
| Gait confidence | 0.35, 0.06 | 0.14, 0.48 | 0.33, 0.08 | 0.37, 0.05 | 0.35, 0.07 | 0.28, 0.18 | 0.37, 0.05 | 0.34, 0.08 | 0.37, 0.06 | 0.25, 0.20 | 0.29, 0.15 | 0.35#, 0.07 | |
| Balance confidence | 0.26, 0.17 | 0.13, 0.50 | 0.26, 0.18 | 0.27, 0.17 | 0.19, 0.34 | 0.27, 0.20 | 0.27, 0.16 | 0.25, 0.20 | 0.28, 0.15 | 0.17, 0.41 | 0.21, 0.30 | 0.27#, 0.17 | |
In N=23.
In N=26.
In N=28.
Adjusted for time between assessment of function and MRI.
Adjusted for time between assessment of function and PET.
Adjusted for time between assessment of function and neuropsychological exam (NP).
SUVR=standardized uptake value ratio.
Figure 1. Scatterplots of mobility-related confidence measures by cognition for significant correlations.
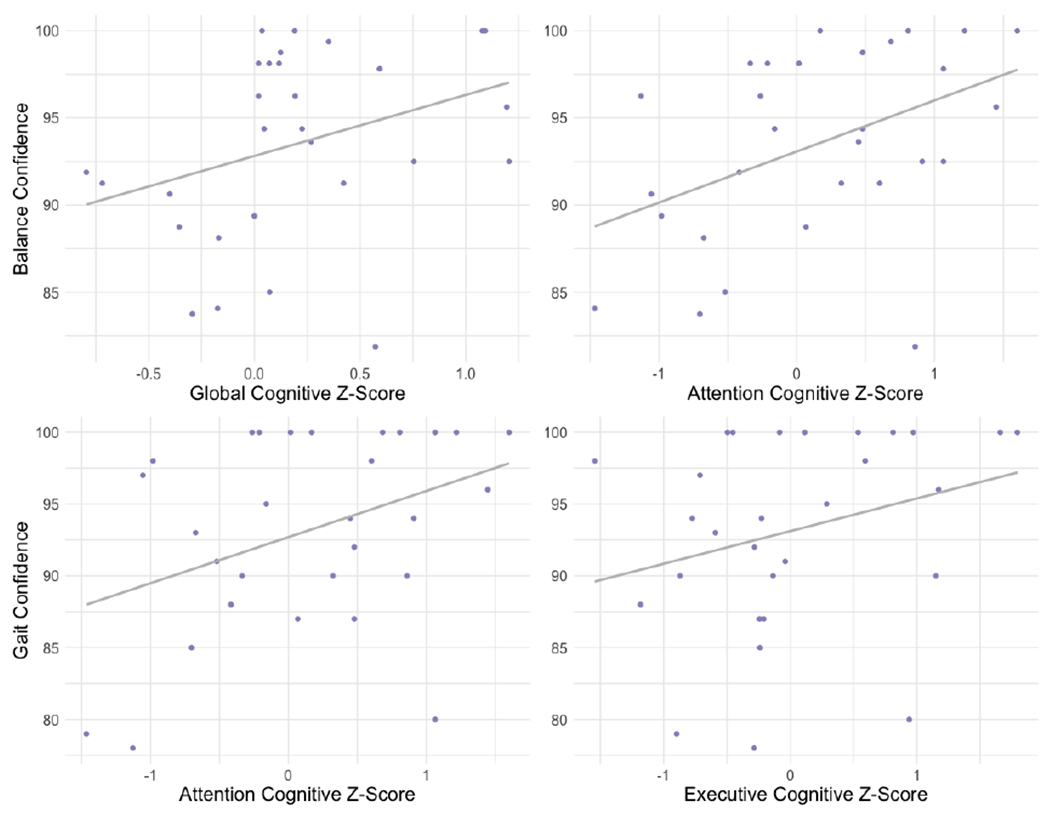
Correlation of balance confidence with global cognition (top left) and attention (top right) and of gait confidence with attention (bottom left) and executive function (bottom right). Note: Regression lines are to aid visualization only. For Spearman correlation coefficients, refer to text and Table 4.
Figure 2. Scatterplots of mobility-related confidence measures by PiB SUVR.
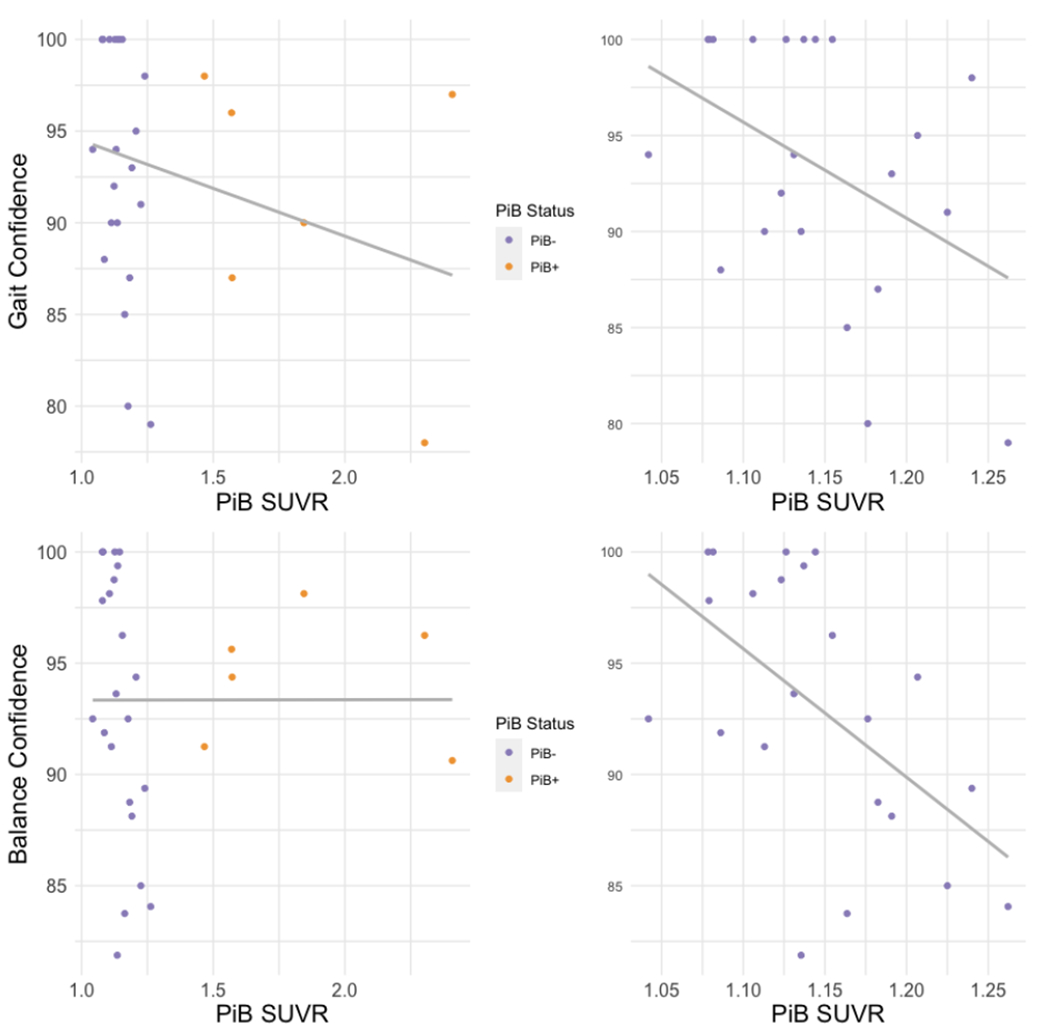
Correlation of gait confidence with amyloid-β burden in all participants (top left) and those who are PiB negative (top right) and of balance confidence with amyloid-β burden in all participants (bottom left) and those who are PiB negative (bottom right). Note: Regression lines are to aid visualization only. For Spearman correlation coefficients, refer to text and Table 4. SUVR=standardized uptake value ratio.
Greater balance confidence was associated with better global cognition and attention, as well as lower Aβ levels (Table 4, Figure 1, and Figure 2). One at a time adjustment for covariates weakened these associations of balance confidence with neuroimaging and cognitive variables as follows: global cognition adjusted for APOE4, gait speed, or Aβ; and Aβ adjusted for age, education, APOE4, depressive symptoms, anxiety, motivation, or gait speed (Table 4). Associations of balance confidence with attention were robust to all adjustments (Table 4). No other associations were significant (Table 4). Partialling out time between assessments did not alter significant associations for either measure of mobility-related confidence (Table 4).
Because relatively few participants (N=6) were PiB positive by our cut-off, and associations of gait and balance confidence with Aβ appeared affected by those with high Aβ burden, we conducted sensitivity analyses withholding those who were PiB positive (Figure 2). The inverse association of gait confidence with Aβ remained similar (N=22, unadjusted ρ= −0.41, p=0.06), and the negative correlation of balance confidence with Aβ was strengthened (N=22, unadjusted ρ= −0.54, p=0.01).
4. DISCUSSION
We found that better subjective ratings of mobility-related confidence were associated with better cognition, specifically in attention and executive domains, and with lower Aβ levels. With correlation absolute values ranging from 0.35 to 0.46, these represent moderate to large effect sizes.45 Given the associations of subjective and objective measures of mobility, objective mobility marking a group of older adults at risk for cognitive impairment, and the possibility of subjective mobility to decline before objective mobility, this raises the intriguing possibility that lower mobility-related confidence may similarly mark a sub-group of older adults at greater risk for cognitive impairment. This is further supported by our findings here. Most of these associations were partially explained by age, APOE4, gait speed, or Aβ levels. Mood or motivation also partially attenuated many of these relationships, suggesting that these relationships are related both to subjective physical performance and psychological factors.
The mean and median scores for both gait and balance confidence were high in this sample. No clinical cut point has been established for gait confidence, but the balance confidence median of 94.0 is within the highest functioning range and is representative of older adults who are generally healthy and typically physically active.38 By design, the NMCM study participants were free from mild cognitive impairment, dementia, and stroke and were able to walk without assistance. They are community-dwelling older adults who are performing well rather than exhibiting clinical impairment in gait and balance. Furthermore, the strengths of the correlations of gait and balance confidence with Aβ levels remained similar or stronger when the PiB positive participants were withheld indicating they were driven by the PiB negative participants. If mobility-related confidence is correlated with Aβ levels in those below the Aβ positivity threshold who are free from clinical cognitive and mobility impairment, this adds support to the possibility of early detection of a group at risk of cognitive impairment with these measures.
We extend prior work in both objectively and subjectively measured mobility. Our results are in line with evidence demonstrating a longitudinal association of better objectively measured gait speed with lower Aβ levels.4 An association of Aβ with anxiety has been reported, which may in part explain our findings.46 Prior work has found associations of balance confidence with whole brain gray and white matter volume in older women.6 Our study extended evaluation of neuroimaging and cognitive correlates of subjective mobility and balance to an older sample (mean age 76 (our sample) vs 69) including both men and women, and we evaluated more specific markers of brain pathology (hippocampal volume, WMH, and Aβ). While we failed to find associations of mobility-related confidence with hippocampal volume or WMH as has been reported with objective markers of mobility,2–4 this could be a true result or may be due to low power due to small sample size. Hippocampal volume and WMH remain plausible correlates of subjective gait and balance and should be evaluated in larger samples.
We found that higher gait confidence was significantly associated with better attention and executive z-scores and nearly so with global cognition and that greater balance confidence was associated with better attention and global z-scores. This is consistent with results testing associations of cognition with objectively assessed mobility measures. A prior meta-analysis of objective measures of gait, lower extremity function, and balance in relation to cognitive function found that executive function was most strongly related to mobility, though consistent associations with attention, memory, and global cognitive function were also observed.47 These associations are thought to arise from degradation of neural networks important for both cognitive function and mobility.1
Physical activity and fitness, loss of muscle mass and strength, and common comorbidities of aging may confound the relationships of mobility-related markers and neuroimaging / cognitive measures.48 We did not have measures of physical activity and fitness in this study, and given the bidirectional relationship of physical activity and physical function, which we have previously shown,14 these factors should be included in future studies. While we did assess BMI and did not find an association with gait and balance confidence, we did not have measures of central adiposity such as waist circumference or waist to hip ratio, nor did we have other measures of body composition. We did have measures of BMI, hypertension, depressive symptoms, anxiety, and joint pain, but our small sample size prevented us from controlling for multiple relevant confounders at once. Being able to control for multiple common comorbidities of aging at once would strengthen future larger studies in this area.
Our analyses were cross-sectional, and as such we cannot clarify the temporality of changes in subjective mobility, neuroimaging, and cognition; our analyses were also exploratory by design. Several relationship structures between mobility-related confidence and neuroimaging / cognitive features could exist: a non-causal relationship whereby mobility-related confidence could simply be a marker of brain and cognitive integrity, a causal relationship in which mobility-related confidence is a cause of neuroimaging / cognitive outcomes, or a causal relationship in which neuroimaging / cognitive factors are causes of mobility-related confidence. Therefore, our results should be tested in larger studies and whether declines in gait and balance confidence may precede or occur early in the course of neuroimaging and cognitive declines should be evaluated in longitudinal studies. If temporality of events is confirmed in future studies, with changes in confidence potentially preceding changes in objective performance, this suggests that, if the relationship is causal, building mobility-confidence may be a good intervention strategy to improve objectively measured mobility. Indeed, Bandura conceived of self-efficacy as a determinant of motivation and behavioral action.5
Our approach in this early and exploratory analysis of associations between self-reported mobility-related confidence and neuroimaging / cognitive markers was to minimize false negative results and preserve candidate associations to test more definitively in future, well-powered studies.39 We anticipate that the descriptive statistics and effect sizes we have reported here will lay the groundwork for investigators planning future studies to conduct power analyses for sample size planning and estimation of minimally detectable effects.
4.1. Conclusions
Overall, our results are consistent with those from the literature on changes in objective measures of mobility and neuroimaging / cognitive measures, which suggest changes in mobility can be early markers of brain and cognitive declines.1 Further, if lower mobility-related confidence is a marker of amyloid deposition, this could have beneficial consequences because assessments of subjective mobility are simpler to conduct than objective measures and could be easily implemented and quickly performed during clinical evaluations to identify at-risk individuals.
Highlights.
Subjective measures of mobility and neuroimaging/cognition assessed in older adults
Greater gait confidence associated with better attention and executive function
Greater balance confidence associated with better global cognition and attention
Greater gait and balance confidence associated with lower brain amyloid levels
ACKNOWLEDGMENTS
The authors would like to thank Carmen Andreescu, MD, for review of the GDS anxiety-related items.
FUNDING
This work was supported by the National Institute on Aging at the National Institutes of Health (grant numbers K01AG053431 to ALR, RF1AG025516 originally to HJA and WEK and presently to HJA and VLV, and CES was supported by T32AG055381).
CONFLICTS OF INTEREST
GE Healthcare holds a license agreement with the University of Pittsburgh based on the PiB-PET technology described in this manuscript. Dr. Klunk is a co-inventor of PiB and, as such, has a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this manuscript. All other authors have no conflicts of interest with PiB-PET and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All other authors have no conflicts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(11):1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosso AL, Studenski SA, Longstreth WT Jr., Brach JS, Boudreau RM, Rosano C. Contributors to Poor Mobility in Older Adults: Integrating White Matter Hyperintensities and Conditions Affecting Other Systems. The journals of gerontology Series A, Biological sciences and medical sciences. 2017;72(9):1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosso AL, Verghese J, Metti AL, et al. Slowing gait and risk for cognitive impairment: The hippocampus as a shared neural substrate. Neurology. 2017;89(4):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan KJ, Ranadive R, Su D, et al. Imaging-based indices of Neuropathology and gait speed decline in older adults: the atherosclerosis risk in communities study. Brain imaging and behavior. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandura A Human agency in social cognitive theory. Am Psychol. 1989;44(9):1175–1184. [DOI] [PubMed] [Google Scholar]
- 6.Nagamatsu LS, Hsu CL, Davis JC, Best JR, Liu-Ambrose T. White Matter Volume Mediates the Relationship Between Self-Efficacy and Mobility in Older Women. Experimental aging research. 2016;42(5):460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newell AM, VanSwearingen JM, Hile E, Brach JS. The modified Gait Efficacy Scale: establishing the psychometric properties in older adults. Physical therapy. 2012;92(2):318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. The journals of gerontology Series A, Biological sciences and medical sciences. 1995;50a(1):M28–34. [DOI] [PubMed] [Google Scholar]
- 9.Patla AE, Shumway-Cook A. Dimensions of mobility: defining the complexity and difficulty associated with community mobility. Journal of Aging and Physical Activity. 1999;7(1):7–19. [Google Scholar]
- 10.Hillel I, Gazit E, Nieuwboer A, et al. Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. Eur Rev Aging Phys Act. 2019;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman T, Giladi N, Gurevich T, Hausdorff JM. Gait instability and fractal dynamics of older adults with a “cautious” gait: why do certain older adults walk fearfully? Gait & posture. 2005;21(2):178–185. [DOI] [PubMed] [Google Scholar]
- 12.van Schooten KS, Pijnappels M, Rispens SM, et al. Daily-Life Gait Quality as Predictor of Falls in Older People: A 1-Year Prospective Cohort Study. PloS one. 2016;11(7):e0158623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta psychiatrica Scandinavica. 2014;130(6):439–451. [DOI] [PubMed] [Google Scholar]
- 14.Metti AL, Best JR, Shaaban CE, Ganguli M, Rosano C. Longitudinal changes in physical function and physical activity in older adults. Age and ageing. 2018;47(4):558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of neurology. 2008;65(11):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karim HT, Wang M, Andreescu C, et al. Acute trajectories of neural activation predict remission to pharmacotherapy in late-life depression. NeuroImage Clinical. 2018;19:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu M, Carmichael O, Lopez-Garcia P, Carter CS, Aizenstein HJ. Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Human brain mapping. 2006;27(9):747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry research. 2006;148(2-3):133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25(11):1528–1547. [DOI] [PubMed] [Google Scholar]
- 20.Snitz BE, Tudorascu DL, Yu Z, et al. Associations between NIH Toolbox Cognition Battery and in vivo brain amyloid and tau pathology in non-demented older adults. Alzheimer’s & dementia (Amsterdam, Netherlands). 2020;12(1):e12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tziortzi AC, Searle GE, Tzimopoulou S, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. NeuroImage. 2011;54(1):264–277. [DOI] [PubMed] [Google Scholar]
- 22.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 23.Tudorascu DL, Minhas DS, Lao PJ, et al. The use of Centiloids for applying [(11)C]PiB classification cutoffs across region-of-interest delineation methods. Alzheimer’s & dementia (Amsterdam, Netherlands). 2018;10:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen AD, Mowrey W, Weissfeld LA, et al. Classification of amyloid-positivity in controls: comparison of visual read and quantitative approaches. NeuroImage. 2013;71:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D WMS-R: Wechsler memory scale-revised. Psychological Corporation; 1987. [Google Scholar]
- 27.Becker JT, Boller F, Saxton J, McGonigle-Gibson KL. Normal rates of forgetting of verbal and non-verbal material in Alzheimer’s disease. Cortex; a journal devoted to the study of the nervous system and behavior. 1987;23(1):59–72. [DOI] [PubMed] [Google Scholar]
- 28.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and motor skills. 1958;8(3):271–276. [Google Scholar]
- 29.Lopez OL, Becker JT, Jagust WJ, et al. Neuropsychological characteristics of mild cognitive impairment subgroups. Journal of neurology, neurosurgery, and psychiatry. 2006;77(2):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler D Wechsler adult intelligence scale-revised (WAIS-R). Psychological Corporation; 1981. [Google Scholar]
- 31.Saxton J, Ratcliff G, Newman A, et al. Cognitive test performance and presence of subclinical cardiovascular disease in the cardiovascular health study. Neuroepidemiology. 2000;19(6):312–319. [DOI] [PubMed] [Google Scholar]
- 32.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5(2):135–140. [Google Scholar]
- 33.Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6(1):53–60. [Google Scholar]
- 34.Golden CJ. A manual for the clinical and experimental use of the Stroop color and word test. 1978. [Google Scholar]
- 35.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research. 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 36.Sheikh JI, Yesavage JA, Brooks JO, 3rd, et al. Proposed factor structure of the Geriatric Depression Scale. International psychogeriatrics / IPA. 1991;3(1):23–28. [DOI] [PubMed] [Google Scholar]
- 37.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 38.Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. The journals of gerontology Series A, Biological sciences and medical sciences. 1998;53(4):M287–294. [DOI] [PubMed] [Google Scholar]
- 39.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology (Cambridge, Mass). 1990;1(1):43–46. [PubMed] [Google Scholar]
- 40.The SAS system for Windows [computer program]. Cary, NC: SAS Institute; 2013. [Google Scholar]
- 41.R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 42.American Community Survey, 2019. American Community Survey 5-Year Estimates, Allegheny County, Pennsylvania. Table DP05. https://data.census.gov/. Accessed May, 14, 2021.
- 43.Rajan KB, Barnes LL, Wilson RS, et al. Racial Differences in the Association Between Apolipoprotein E Risk Alleles and Overall and Total Cardiovascular Mortality Over 18 Years. Journal of the American Geriatrics Society. 2017;65(11):2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh PP, Singh M, Mastana SS. APOE distribution in world populations with new data from India and the UK. Ann Hum Biol. 2006;33(3):279–308. [DOI] [PubMed] [Google Scholar]
- 45.Cohen J Statistical power analysis for the behavioral sciences. 2nd ed: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 46.Ng KP, Chiew H, Rosa-Neto P, Kandiah N, Ismail Z, Gauthier S. Associations of AT(N) biomarkers with neuropsychiatric symptoms in preclinical Alzheimer’s disease and cognitively unimpaired individuals. Transl Neurodegener. 2021;10(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demnitz N, Esser P, Dawes H, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait & posture. 2016;50:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salive ME. Multimorbidity in older adults. Epidemiologic reviews. 2013;35:75–83. [DOI] [PubMed] [Google Scholar]


