Abstract
With emerging SARS-CoV-2 variants, vaccines approved so far are under scrutiny for long term effectiveness against the circulating strains. There is a prevalent obsession with humoral immunity as in vitro studies have indicated diminished effects of vaccine-induced neutralizing antibodies. However, this need not clinically translate to vaccine resistance as immune response against all forms of present vaccine preparations is T dependent unlike that against native viral particles which can induce T independent immune responses. Thus, we focused on this major correlate of protection against infections, T cell response. Using bioinformatics tools, we analyzed SARS-CoV-2 Spike protein T cell epitopes and their diversity across Delta plus/B.1.617.2.1, Gamma/P.1 (variant of concern), B.1.1.429, Zeta/P.2 and Mink cluster 5/B.1.1.298 variants as well as Omicron/B.1.1.529 (variant of concern). We also compared HLA restriction profiles of the mutant epitopes with that of the native epitopes (from Wuhan_hu_1 strain, used in vaccine formulations). Our observations show ~90% conservation of CD4+ and CD8+ epitopes across Delta plus/B.1.617.2.1, Gamma/P.1 (variant of concern), B.1.1.429, Zeta/P.2 and Mink cluster 5/B.1.1.298. For the Omicron/B.1.1.529 variant, ~75% of CD4+ and ~ 87% CD8+ epitopes were conserved. Majority of the mutated CD4+ and CD8+ epitopes of this variant were predicted to retain the HLA restriction pattern as their native epitopes. The results of our bioinformatics analysis suggest largely conserved T cell responses across the studied variants, ability of T cells to tackle new SARS-CoV-2 variants and aid in protection from COVID-19 post vaccination. In conclusion, the results suggest that current vaccines may not be rendered completely ineffective against new variants.
Abbreviations: CTLs, Cytotoxic T Lymphocytes; VoC, Variants of Concern; IEDB, Immune Epitope Database
Keywords: SARS-CoV-2, CD4+ T-cell epitopes, CD8+ T-cell epitopes, Spike protein, HLA-I, HLA-II
Graphical abstract
1. Introduction
Currently administered COVID-19 vaccines were originally designed to protect from the ancestral strain of SARS-CoV-2 (Wuhan_hu_1 strain – GenBank accession number MN908947) and except a few whole virus attenuated vaccines, majority are targeted to induce anti-spike protein antibody based immune response [1], [2]. Since the onset of COVID-19 pandemic, many new strains have evolved that harbor mutations in spike as well as other proteins of the virus [3]. Some of these new, circulating variants have higher transmissibility, pathogenicity with immune escape and have emerged as “variants of concerns” locally as well as globally [3]. According to the World Health Organization, variants of concern include Alpha/B.1.1.7 (former), Beta/B.1.351 (former), Gamma/P.1 (former), Delta/B.1.617.2 (current) and Omicron/B.1.1.529 (current) [4]. The delta plus variant was also dominant across the world with the status of “variant of concern” until recent times [4]. It was reported to have a distinct mutational profile compared to its parent (delta/B.1.617.2) variant, and the mutation K417N in its spike protein was thought to enable antibody evasion [5], [6]. Therefore, it is a matter of urgency to investigate the efficacy of existing vaccines against the new variants, in order to control and eventually eradicate the pandemic of COVID-19.
Recent studies have demonstrated that many of the new variants possess mutations that affect the binding and functioning of neutralizing antibodies against SARS-COV-2, hence facilitating its escape from infection/vaccination derived immunity [3], [7]. However, evading only humoral responses may not be sufficient to develop vaccine resistance and the same has been discussed recently by Cevik et al [8]. The authors point out that significant reduction in neutralizing activity of antibodies translates to only a modest decrease in vaccine efficacy (in clinical settings) against symptomatic and severe COVID-19. A multitude of factors other than neutralizing antibodies are involved in determining “vaccine efficacy” [9]. T cell response is one of them and yet to be delineated for its exact role in the phenomenon [9].
It is well established that T cells play a crucial role in eliciting a strong early immune response against viral pathogens and reducing disease severity. In the specific case of vaccines too, it is well established that T cells are crucial in assisting antibody production and generating long-term immune memory [9], [10], [11]. It is important to note that several studies have established, T cell independent antibody responses in vivo are elicited by native/live viral infection but not by immunization with viral proteins or virus-like particles [12], [13]. This emphasizes the importance of evaluating T cell responses in addition to neutralizing antibody responses in post-vaccination viral exposure/infection while assessing vaccine efficacy.
Initiation of T cell response is dependent on the recognition and Human Leukocyte Antigen (HLA) restriction of T cell epitopes sequences. Any amino acid mutations in the epitopes can lead to systemic immune effects of varying intensity. One of the potential consequences of mutations in T cell epitopes is immune evasion by one/more epitopes as demonstrated my multiple experimental studies so far [14]. Observed in both the arms of T cell immunity – CD4+ and CD8+, it is debatable whether such an escape can significantly affect the overall protection from COVID-19 (even in presence of retained humoral response) [15]. Thus, assessing the landscape of mutations in T cell epitopes is a primary step towards understanding the potential changes in T cell response and designing effective strategies for protection against COVID-19.
CD4+ T cells recognize Major Histocompatibility Complex (MHC) Class-II or Human Leukocyte Antigen (HLA) Class II bound viral peptides and function in activating the B cells as well as other immune cells and orchestrate further immune response to the recognized viral pathogen. Thus, CD4+ T cells help in establishing antigen specific immunity in both cellular and humoral arms [16]. In addition, cytotoxic T lymphocytes (CTLs) recognize viral epitopes presented by MHC-I molecules and aid in clearing virus infected cells playing a crucial role in viral load management. Along with neutralizing antibodies, SARS-CoV-2 specific CTL response has been recognized as a potential major correlate of protection from COVID-19 [17], [18].
Though humoral immunity is crucially involved in “prevention” of the disease, cellular immunity influences “severity, hospitalization and fatality” - important metrics of vaccine efficacy in addition to preventing infection [8]. Even vaccines that primarily rely on eliciting neutralizing antibodies for viral clearance need to elicit robust T cell responses that are necessary for achieving sufficient efficacy [19].
Moreover, the humoral immune response is more vulnerable to being bypassed by viral variants as neutralizing antibodies target a focused region of the spike protein. On the other hand, cellular immunity has a larger epitope recognition range/diversity. It has been reported that successful mounting of protective immune response requires recognition of multiple SARS-CoV-2 epitopes [20]. This may also mean that a few mutations may cause more disturbances/imbalances to humoral immunity than cellular immunity, suggesting that only diminished humoral response need not always mean immune escape.
Hence, investigating T cell epitopes across ancestral and variant strains is required to draw sound conclusions about efficacy of existing vaccines against new variants and to understand how we can improve the existing vaccine landscape.
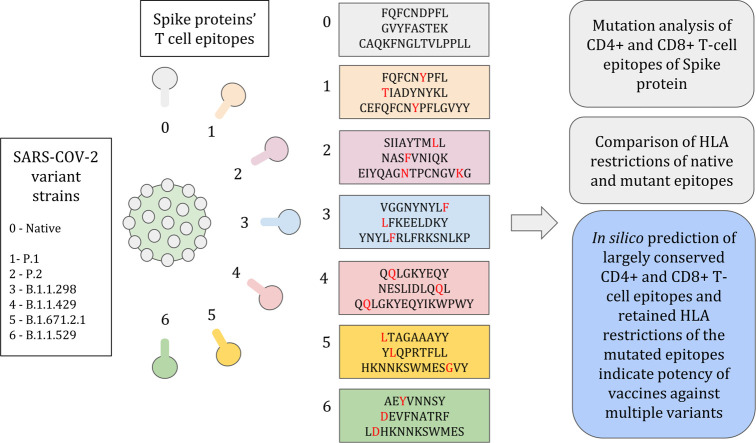
This study analyzes spike protein T cell epitope repertoire and their HLA restriction patterns in native and mutant strains of SARS-COV-2 (Delta plus/B.1.617.2.1, Gamma/P.1, Zeta/P.2, B.1.1.429, Mink Cluster 5/B.1.1.298 and Omicron/B.1.1.529) to gain insights into potential variation in T cell responses against new variants. Since majority vaccines are targeted to induce anti-spike protein antibody based immune response, we selectively focus only on spike protein T cell epitopes and the effects of mutations therein while computationally assessing the vaccine efficacy against new strains.
2. Methodology
2.1. Selection of representative genome sequences of SARS-CoV-2 variants and processing
SARS-COV-2 variant genome sequences were sourced from Global Initiative on Sharing All Influenza Data (GISAID [21]) (https://www.gisaid.org/) in July 2021. We selected a representative sequence for each variant. A quality control exercise (Supplementary File1, Supplementary Table 3) was performed to ensure fair selection and prove that such a selection will substantiate our purpose of analyzing T cell epitopes. Based on the results, it was inferred that the set of sequences for a particular variant deposited in GISAID does not have significant diversity in their T cell epitope pool assuring that our selection mode can faithfully represent the set of each variant. Hence, representative sequences for each variant were picked from the list of sequences obtained after applying the GISAID filters as mentioned in Supplementary File 1.
2.2. Collating a library of native and mutant T cell epitopes
A master library of T cell epitopes of SARS-COV-2 native strain (Wuhan_hu_1 strain) was collated by sourcing the epitopes from literature [22], [23], [24]. Only the Spike protein native epitopes were used for further analysis in this study. The chosen sources report experimentally verified epitopes only. Collected epitopes were organized further into categories as CD4+ epitopes, CD8+ epitopes.
To locate and identify the T cell epitopes that are mutated in a given variant of SARS-COV-2, we compared the native T cell epitope sequences with the spike protein sequence of each variant. The spike protein sequence of each variant was obtained using Virus Pathogen Resource's VIGOR 4.0 genome annotator tool (https://www.viprbrc.org/) [25] that translated viral genome sequences into peptide sequences and resulting spike protein sequences were compared to native spike protein T cell epitope sequences to identify mutated epitopes using an in-house code (Supplementary File 2). The algorithm/code reads native T cell epitopes from a given excel sheet and spike protein sequence of a variant from a given fasta file. Then it compares the sequence strings of epitopes with the S protein sequence string, displaying only the epitope sequences that couldn't find a match in the S protein.
2.3. HLA-peptide binding analysis
As the T cell mediated response is always HLA restricted, we further analyzed and compared HLA binding profiles of the native and mutated epitopes. To assess the HLA allele binding and their affinities between native and mutated epitopes, CD4+ epitopes were analyzed using IEDB NetMHCIIPan 4.0 BA (http://tools.iedb.org/mhcii/) and CD8+ epitopes were analyzed using IEDB recommended 2020.09 NetMHCpan EL 4.1 (http://tools.iedb.org/mhci/) [26]. The reference sets of alleles used (recommended by IEDB) are provided in the Supplementary File 3. They provide population coverage of >97% for class I and >99% for class II and are representative of commonly shared binding specificities (Supplementary File 3). A cut off of 2 percentile was used to select MHC class-I alleles as binders (includes both strong and weak binders) [26]. For MHC class-II alleles, the binding affinity (BA) values were scored by the server in IC50 (nM) and in percentile rank. Binding affinity in IC-50 was used for predicting the binding of CD4+ epitopes with the alleles. For MHC-Class II, peptides binding to alleles with an IC50 below 1000 nM are reported to be binders and have immunogenicity [27] [28]. Therefore, a threshold of IC50 = 1000 nM was fixed. The alleles with binding affinity values less than 1000 nM were included and those above this threshold were eliminated.
3. Results
3.1. Minimal CD4+ and CD8+ T cell epitopes on the spike protein of SARS-CoV-2 are mutated in variants
Genome sequences were picked from GISAID for each variant (Table 1 ) as described in the methods section. A quality control exercise was performed to ensure that the sequence chosen is an appropriate representative to serve the purpose of this study (results in Supplementary File 1 and raw data in Supplementary File 5).
Table 1.
SARS-COV-2 variants genome sequences selected for analysis.
| Variant | Sequence ID |
|---|---|
| B.1.1.429 | hCoV-19/Latvia/1122/2020 |
| B.1.1.298 | hCoV-19/Denmark/DCGC-53391/2020 |
| B.1.617.2.1 | hCoV-19/England/ALDP-153B839/2021 |
| P.1 | hCoV-19/Japan/PG-58436/2021 |
| P.2 | hCoV-19/Brazil/MG-LBI185/2021 |
| B.1.1.529 | hCoV-19/South Africa/LAZ-AMC-211204828-DS/2021 |
To identify the mutated CD4+ and CD8+ epitopes of the spike protein in the variants, the CD4+ or CD8+ epitopes of the native spike protein mapped through multiple experimental studies (Supplementary Table 1, Supplementary Table 2) were searched for in the variants' spike protein sequences. This exercise was carried out using a locally developed script (Supplementary File 2) that returns the epitopes that are not found due to character mismatches, thus indicating mutation (Table 2, mutated residues highlighted in red). Among 97 CD4+ epitopes of the native spike protein (Wuhan_Hu_1 strain), only three epitopes of the variant B.1.1.298, one epitope of B.1.1.429, eight epitopes of delta plus/B.1.617.2.1, nine epitopes of gamma/P.1, two epitopes of zeta/P.2 and 24 epitopes of omicron/B.1.1.529 were found to be mutated. The mutated epitopes are listed in Table 2. Likewise, among 187 native CD8+ epitopes, 17 epitopes were mutated in delta plus/B.1.617.2.1, 10 were mutated in gamma/P.1, 10 in B.1.1.298, 5 in zeta/P.2, 4 in B.1.1.429 and 24 in omicron/B.1.1.529 respectively. Further, out of the 97 native CD4+ T cell epitopes, 35 distinct epitopes were found to be mutated across the studied variants. And in case of CD8+ T cell epitopes too, 40 distinct epitopes out of 187 native epitopes were found to be mutated across the studied variants. It was interesting to note that some epitopes were consistently mutated in multiple variants, while some were specific to each variant. As can be seen from Table 2, the native CD8+ T cell epitope YQDVNCTEV mutates to YQGVNCTEV and the epitope YFQPRTFLL mutates to YLQPRTFLL in all the studied variants. Furthermore, the native CD4+ T cell epitope EIYQAGSTPCNGVEG mutates to EIYQAGSTPCNGVKG, EIYQAGNTPCNGVKG, EIYQAGSKPCNGVEG and EIYQAGNKPCNGVAG in P.1, P.2, B.1.617.2.1 and B.1.1.529 respectively. Also, the native CD4+ T cell epitope CEFQFCNDPFLGVYY mutates to CEFQFCNYPFLGVYY, CEFQFCNDPFLDVYY and CEFQFCNDPFLD in P.1, B.1.617.2.1 and B.1.1.529 respectively. Other than these, we observed a few CD4 and CD8 native epitopes to undergo same or different mutations among utmost two different variants (Table 2). Also, it is important to note that at least 90% of CD4+ epitopes (~90% in P.1, ~97% in P.2, ~91% in B.1.617.2.1, ~98% in B.1.1.429, ~96% in B.1.1.298) as well as at least 90% of CD8+ epitopes (~90% in B.1.617.2.1, ~94% in P.1, ~97% in P.2 and B.1.1.429, ~94% in B.1.1.298) were conserved across all the selected variants except omicron/B.1.1.529. Omicron/B.1.1.29 displayed comparatively lower conservation (~75% in CD4+ epitopes and ~87% in CD8 epitopes) due to the remarkably higher number of mutations in its spike protein.
Table 2.
Mutated T cell epitopes in spike protein from variants of SARS-COV-2.
| CD8 native epitope | CD8 Mutant epitope | Variants |
|---|---|---|
| AEHVNNSY | AEYVNNSY | P.1, B.1.1.529 |
| DGVYFASTEK | DGVYFASIEK | B.1.617.2.1, B.1.1.529 |
| DSKVGGNYNY | DSKVSGNYNY | B.1.1.529 |
| GEVFNATRF | DEVFNATRF | B.1.1.529 |
| FQFCNDPFL | FQFCNYPFL | P.1 |
| GVYFASTEK | GVYFASIEK | B.1.617.2.1, B.1.1.529 |
| GVYYHKNNK | DVYYHKNNK | B.1.617.2.1 |
| DHKNNK | B.1.1.529 | |
| HVSGTNGTK | SGTNGTK | B.1.1.298 |
| HVISGTNGTK | B.1.1.529 | |
| IYKTPPIKDF | IYKTPPIKYF | B.1.1.529 |
| KIADYNYKL | NIADYNYKL | B.1.617.2.1 |
| TIADYNYKL | P.1 | |
| KVGGNYNYLY | KVGGNYNYRY | B.1.617.2.1 |
| KVGGNYNYLF | B.1.1.298 | |
| KVSGNYNYLY | B.1.1.529 | |
| LGAENSVAY | LGVENSVAY | B.1.1.529 |
| LPPAYTNSF | LPSAYTNSF | P.1 |
| NASVVNIQK | NASFVNIQK | P.1, P.2 |
| NESLIDLQEL | NESLIDLQQL | B.1.1.429 |
| NSASFSTFK | NLAPFFTFK | B.1.1.529 |
| NYNYLYRLF | NYNYLFRLF | B.1.1.298 |
| NYNYRYRLF | B.1.617.2.1 | |
| QELGKYEQY | QQLGKYEQY | B.1.1.429 |
| QIAPGQTGK | QIAPGQTGN | B.1.617.2.1 |
| QIAPGQTGT | P.1 | |
| QTNSPRRAR | QTNSRRRAR | B.1.617.2.1 |
| QTKSHRRAR | B.1.1.529 | |
| RASANLAATK | RASANLAAIK | P.1 |
| SFKEELDKY | LFKEELDKY | B.1.1.298 |
| SIIAYTMSL | SIIAYTMLL | P.2 |
| SPRRARSV | SRRRARSV | B.1.617.2.1 |
| SHRRARSV | B.1.1.529 | |
| SPRRARSVA | SRRRARSVA | B.1.617.2.1 |
| SHRRARSVA | B.1.1.529 | |
| SVLNDILSR | SVLNDIFSR | B.1.1.529 |
| SWMESEFRVY | SWMESGVY | B.1.617.2.1 |
| TEKSNIIRGW | IEKSNIIRGW | B.1.617.2.1, B.1.1.529 |
| TPINLVRDL | TPIIVREPEDL | B.1.1.529 |
| TVYDPLQPELDSFK | TVYDPLQPELDLFK | B.1.1.298 |
| VGGNYNYLY | VGGNYNYLF | B.1.1.298 |
| VGGNYNYRY | B.1.617.2.1 | |
| VSGNYNYLY | B.1.1.529 | |
| VLNDILSRL | VLNDIFSRL | B.1.1.529 |
| VYDPLQPELDSF | VYDPLQPELDLF | B.1.1.298 |
| WTAGAAAYY | LTAGAAAYY | B.1.617.2.1 |
| YFQPRTFLL | YLQPRTFLL | B.1.617.2.1, B.1.1.298, P.1, B.1.1.429, P.2, B.1.1.529 |
| YGFQPTNGV | YGFQPTYGV | P.1 |
| YSFRPTYGV | B.1.1.529 | |
| YNYLYRLFR | YNYLFRLFR | B.1.1.298 |
| YNYRYRLFR | B.1.617.2.1 | |
| YQDVNCTEV | YQGVNCTEV | B.1.617.2.1, B.1.1.298, P.1, B.1.1.429, P.2, B.1.1.529 |
| YTMSLGAENSVAY | YTMLLGAENSVAY | P.2 |
| YTMSLGVENSVAY | B.1.1.529 | |
| YYHKNNKSW | DHKNNKSW | B.1.1.529 |
| AENSVAYSNNSIAIP | VENSVAYSNNSIAIP | B.1.1.529 |
| ARDLICAQKFNGLTV | ARDLICAQKFKGLTV | B.1.1.529 |
| CAQKFNGLTVLPPLL | CAQKFKGLTVLPPLL | B.1.1.529 |
| CEFQFCNDPFLGVYY | CEFQFCNYPFLGVYY | P.1 |
| CEFQFCNDPFLDVYY | B.1.617.2.1 | |
| CEFQFCNDPFLD | B.1.1.529 | |
| CPFGEVFNATRFASV | CPFDEVFNATRFASV | B.1.1.529 |
| CVADYSVLYNSASFS | CVADYSVLYNLAPFF | B.1.1.529 |
| EIYQAGSTPCNGVEG | EIYQAGSTPCNGVKG | P.1 |
| EIYQAGNTPCNGVKG | P.2 | |
| EIYQAGSKPCNGVEG | B.1.617.2.1 | |
| EIYQAGNKPCNGVAG | B.1.1.529 | |
| FKIYSKHTPINLVRD | FKIYSKHTPIIVREPED | B.1.1.529 |
| FNCYFPLQSYGFQPT | FNCYFPLRSYSFRPT | B.1.1.529 |
| FNGLTVLPPLLTDEM | FKGLTVLPPLLTDEM | B.1.1.529 |
| GINASVVNIQKEIDR | GINASFVNIQKEIDR | P.1, P.2 |
| GKQGNFKNLREFVFK | GKQGNFKNLSEFVFK | P.1 |
| HKNNKSWMESEFRVY | HKNNKSWMESGVY | B.1.617.2.1 |
| IVRFPNITNLCPFGE | IVRFPNITNLCPFDE | B.1.1.529 |
| KHTPINLVRDLPQGF | KHTPIIVREPEDLPQGF | B.1.1.529 |
| LGVYYHKNNKSWMES | LDVYYHKNNKSWMES | B.1.617.2.1 |
| LDHKNNKSWMES | B.1.1.529 | |
| LMDLEGKQGNFKNLR | LMDLEGKQGNFKNLS | P.1 |
| LPFFSNVTWFHAIHV | LPFFSNVTWFHAI | B.1.1.298 |
| LPFFSNVTWFHVI | B.1.1.529 | |
| LQPELDSFKEELDKY | LQPELDLFKEELDKY | B.1.1.298 |
| LTGTGVLTESNKKFL | LKGTGVLTESNKKFL | B.1.1.529 |
| NLLLQYGSFCTQLNR | NLLLQYGSFCTQLKR | B.1.1.529 |
| NLVRDLPQGFSALEP | IVREPEDLPQGFSALEP | B.1.1.529 |
| NPVLPFNDGVYFAST | NPVLPFNDGVYFASI | B.1.617.2.1, B.1.1.529 |
| PINLVRDLPQGFSAL | PIIVREPEDLPQGFSAL | B.1.1.529 |
| QELGKYEQYIKWPWY | QQLGKYEQYIKWPWY | B.1.1.429 |
| QLIRAAEIRASANLAATK | QLIRAAEIRASANLAAIK | P.1 |
| SASFSTFKCYGVSPT | LAPFFTFKCYGVSPT | B.1.1.529 |
| SVLYNSASFSTFKCY | SVLYNLAPFFTFKCY | B.1.1.529 |
| TPPIKDFGGFNFSQI | TPPIKYFGGFNFSQI | B.1.1.529 |
| TQLNRALTGIAVEQD | TQLKRALTGIAVEQD | B.1.1.529 |
| WNSNNLDSKVGGNYN | WNSNKLDSKVSGNYN | B.1.1.529 |
| VNLTTRTQLPPAYTN | VNFTNRTQLPSAYTN | P.1 |
| VNLRTRTQLPPAYTN | B.1.617.2.1 | |
| VSSQCVNLTTRTQLP | VSSQCVNFTNRTQLP | P.1 |
| VSSQCVNLRTRTQLP | B.1.617.2.1 | |
| VVIKVCEFQFCNDPF | VVIKVCEFQFCNYPF | P.1 |
| YNYLYRLFRKSNLKP | YNYRYRLFRKSNLKP | B.1.617.2.1 |
| YNYLFRLFRKSNLKP | B.1.1.298 |
Footnote: Positions with mutations are highlighted in red. Red highlighted residues in native epitopes indicate the deletions. Yellow highlighted mutated epitopes were not used for further HLA analysis due to their length (less than 8 or more than 15 residues).
3.2. Most mutated and native CD4+ T-cell epitopes of spike protein show similar HLA-II restrictions
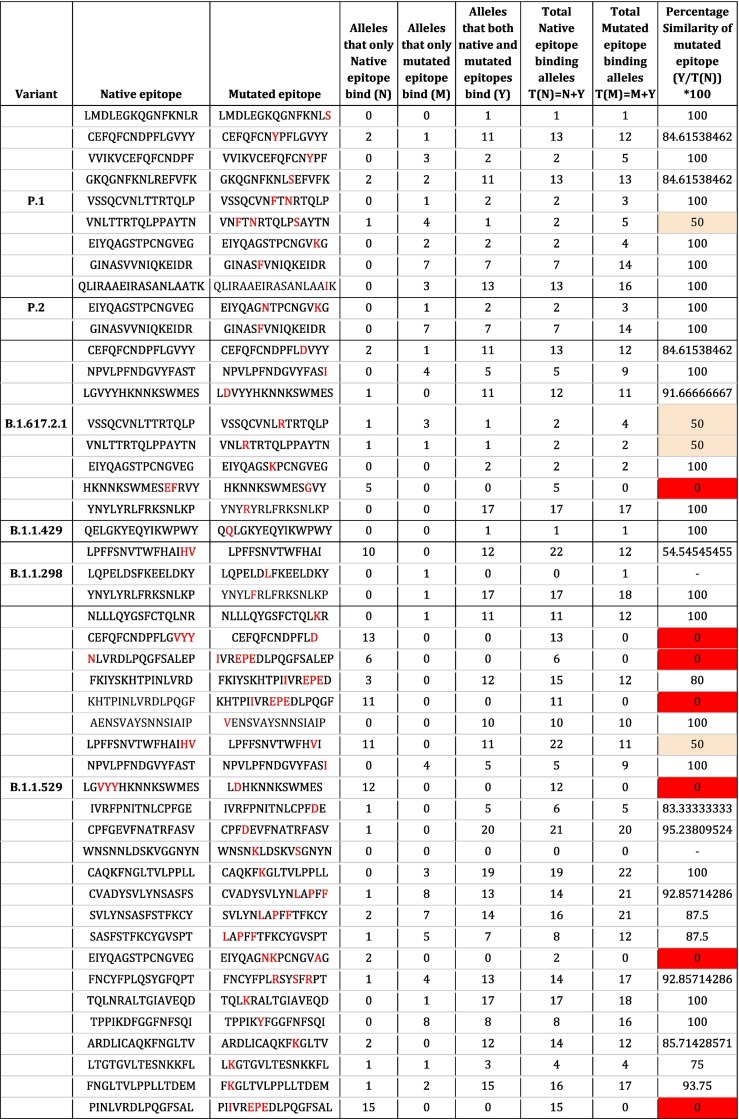
Although most of the CD4+ T-cell epitopes of the spike protein are conserved, it is necessary to check whether the mutated epitopes escape CD4+ T-cell recognition by not binding to HLA-II of antigen presenting cells, which is a crucial step in the initiation of T-cell response and also necessary for T -dependent B-cell activation. To investigate this, we subjected the mutated epitopes of the variants as well as their native counterparts to HLA-II binding prediction to a set of 27 alleles (reference set) using NetMHCIIPan 4.0 _ BA. Since an IC50 value <1000 nM indicates binding of the epitopes to the alleles, a threshold of IC50 = 1000 nM was fixed. Binding of the mutated epitopes to alleles similar to their native counterparts (i.e. similar HLA restriction) would indicate that these epitopes can be recognized by the CD4+ T-cells despite their mutations resulting in activation. The HLA-II allele restriction patterns of native and mutated CD4+ epitopes are shown in Fig. 1 . The number of HLA-II alleles that native and mutated epitopes bind is given in Table 4. The raw data for binding affinity scores for native and mutated epitopes of the variant are given in Supplementary File 4.
Fig. 1.
Binding of Native and Mutated CD4 epitopes from spike protein of SARS-CoV-2 to HLA class -II alleles.
The native CD4 epitopes of the Wuhan strain and their mutated counterparts for each variant were subjected to NetMHCIIPan 4.0 BA to predict their binding to 27 HLA Class-II alleles (reference set) with a threshold of IC50 < 1000 nM. The epitopes in the left are native epitopes while the peptides in the right are Mutated epitopes. The Mutated amino acids in the mutated epitopes are colored red. The alleles to which only the native epitopes bind are marked as’N′ (light yellow), while the alleles to which only the mutated epitopes bind are marked as ‘M’ (light magenta). The alleles to which both the native and their mutated counterparts bind are marked as ‘Y’ (light green). The native epitopes that are mutated among the variants are highlighted in different colors for each epitope.
Table 4.
Comparison of HLA allele restrictions of native and mutant CD4+ T-cell epitopes from spike protein of SARS CoV-2.
Footnote: Positions with mutations are highlighted in red. Red highlighted residues in native epitopes indicate the deletions.
It can be seen from Table 4 that more than half of the mutated epitopes of all the variants show ≥80% similarity in HLA restriction to their corresponding native epitopes except B.1.1.298. However, the mutated epitopes VNFTNRTQLPSAYTN of gamma/P.1 variant, VSSQCVNLRTRTQLP and VNLRTRTQLPPAYTN of delta plus/B.1.617.2.1 variant showed a varied HLA-II binding pattern. These three epitopes bind to alleles that their native counterparts do not bind (Fig. 1). Notably, some mutated epitopes, including VSSQCVNLRTRTQLP of delta plus/ B.1.617.2.1 variant and VNFTNRTQLPSAYTN of gamma/P.1 variant are predicted to be binding to additional alleles than their corresponding native epitopes (Table 4). It is striking to observe that the mutated epitope HKNNKSWMESGVY, from the delta plus variant, did not bind to any of the HLA-II alleles under study.
Mutation analysis of the B.1.1.529 (Omicron) variant's CD4+ T-cell epitopes reveals the presence of 24 mutated epitopes among the 97 native epitopes analyzed, which is higher than the other variants studied. The HLA-II binding prediction shows that 15 out of the 24 mutated epitopes of this variant have ≥80% similar HLA-II binding pattern as their native counterparts (Table 4). Notably, like HKNNKSWMESGVY of the delta plus variant (B.1.617.2), the mutated epitopes CEFQFCNDPFLD, IVREPEDLPQGFSALEP, KHTPIIVREPEDLPQGF, LDHKNNKSWMES, EIYQAGNKPCNGVAG and PIIVREPEDLPQGFSAL of the omicron variant do not bind to any of the native epitope binding alleles. (Figure-1). Also, the mutated epitope LPFFSNVTWFHVI shows a 50% reduction in HLA-II binding. Overall, since we observe majority of the mutated epitopes from studied SARS-CoV-2 variants including the omicron show similar HLA-II restriction (≥80%) to their corresponding native epitopes in our HLA-II binding prediction, we indicate that these variants may not escape the CD4+ T-cell response elicited by the current mRNA spike protein vaccines.
3.3. Similar HLA restrictions of native and mutant CD8+ T cell epitopes of spike protein
As CD8+ T cell responses are driven by HLA-I restriction, mutant (and their corresponding native) CD8+ T cell epitopes on variants' spike proteins were analyzed for their differences in HLA allele binding, using IEDB recommended 2020.09 NetMHCpan EL 4.1. A threshold of 2% is known to include both strong and weak binders [26]. Hence, a cut-off of 2 percentile (or % rank) was used to differentiate ‘binders’ from ‘non-binders’. The resultant HLA allele sets exhibiting binding for each native and mutant epitope were compared and the results are presented in the Table 3 . The data in a variant-specific manner has been presented in Table 5. It can be seen that for the gamma/P.1 variant, ~80% (7 out of 8) mutated spike protein CD8+ T cell epitopes were observed to have ≥60% similarity in the set of binding HLA alleles. Similarly, all (100%) epitopes from zeta/P.2 and B.1.1.429, ~70% (12 out of 17) from delta plus/B.1.617.2.1, ~77% (7 out of 9) from B.1.1.298 and ~ 86% (20 out of 23) from omicron/B.1.1.529 displayed ≥60% similar HLA allele restriction.
Table 3.
HLA Class I restriction as predicted by NetMHCpan 4.1 EL.
| Native epitope | HLA restriction* | Mutated epitope | HLA restriction* |
|---|---|---|---|
| AEHVNNSY | HLA-B*44:03 HLA-B*44:02 HLA-B*15:01 HLA-B*40:01 HLA-A*01:01 |
AEYVNNSY | HLA-B*44:03 HLA-B*44:02 HLA-B*15:01 HLA-B*40:01 HLA-A*01:01 |
| DGVYFASTEK | HLA-A*68:01 HLA-A*03:01 HLA-A*11:01 |
DGVYFASIEK | HLA-A*68:01 HLA-A*03:01 HLA-A*11:01 |
| DSKVGGNYNY | HLA-A*26:01 HLA-A*01:01 HLA-A*30:02 HLA-B*35:01 |
DSKVSGNYNY | HLA-A*01:01 HLA-A*30:02 HLA-B*35:01 |
| GEVFNATRF | HLA-B*44:03 HLA-B*44:02 HLA-B*40:01 HLA-B*15:01 |
DEVFNATRF | HLA-B*44:03 HLA-B*44:02 HLA-B*40:01 |
| FQFCNDPFL | HLA-A*02:06 HLA-A*02:01 HLA-A*02:03 HLA-B*40:01 |
FQFCNYPFL | HLA-A*02:06 HLA-A*02:01 HLA-A*02:03 |
| GVYFASTEK | HLA-A*11:01 HLA-A*03:01 HLA-A*68:01 HLA-A*30:01 |
GVYFASIEK | HLA-A*11:01 HLA-A*03:01 HLA-A*68:01 HLA-A*30:01 |
| GVYYHKNNK | HLA-A*03:01 HLA-A*11:01 HLA-A*30:01 HLA-A*68:01 HLA-A*31:01 |
DVYYHKNNK | HLA-A*68:01 HLA-A*33:01 HLA-A*11:01 HLA-A*03:01 HLA-A*26:01 |
| HVSGTNGTK | HLA-A*68:01 HLA-A*03:01 HLA-A*11:01 HLA-A*30:01 |
HVISGTNGTK | HLA-A*68:01 HLA-A*11:01 HLA-A*03:01 HLA-A*30:01 |
| IYKTPPIKDF | HLA-A*24:02 HLA-A*23:01 HLA-A*30:02 |
IYKTPPIKYF | HLA-A*24:02 HLA-A*23:01 HLA-A*30:02 HLA-B*57:01 |
| KIADYNYKL | HLA-A*02:06 HLA-A*02:01 HLA-A*32:01 HLA-A*02:03 HLA-A*68:02 HLA-A*30:02 HLA-B*58:01 HLA-A*24:02 HLA-A*23:01 |
NIADYNYKL | HLA-A*68:02 HLA-A*02:06 HLA-A*02:01 HLA-A*02:03 HLA-A*26:01 HLA-B*08:01 HLA-A*32:01 |
| TIADYNYKL | HLA-A*02:06 HLA-A*68:02 HLA-A*02:01 HLA-A*02:03 HLA-A*26:01 HLA-A*32:01 |
||
| KVGGNYNYLY | HLA-A*30:02 HLA-B*57:01 HLA-A*01:01 HLA-A*32:01 HLA-B*15:01 HLA-B*58:01 |
KVGGNYNYRY | HLA-A*30:02 HLA-B*15:01 HLA-A*01:01 HLA-A*32:01 |
| KVGGNYNYLF | HLA-A*32:01 HLA-B*57:01 HLA-B*58:01 HLA-A*23:01 HLA-A*24:02 |
||
| KVSGNYNYLY | HLA-A*30:02 HLA-A*11:01 HLA-A*01:01 HLA-B*57:01 HLA-A*03:01 HLA-B*58:01 HLA-A*32:01 |
||
| LGAENSVAY | HLA-B*35:01 HLA-B*15:01 HLA-A*30:02 HLA-A*01:01 HLA-B*53:01 HLA-A*26:01 |
LGVENSVAY | HLA-B*35:01 HLA-B*15:01 HLA-A*30:02 HLA-A*01:01 HLA-B*53:01 HLA-A*26:01 |
| LPPAYTNSF | HLA-B*35:01 HLA-B*53:01 HLA-B*51:01 HLA-B*07:02 HLA-B*08:01 HLA-A*26:01 HLA-A*24:02 HLA-A*23:01 |
LPSAYTNSF | HLA-B*35:01 HLA-B*53:01 HLA-B*07:02 HLA-B*51:01 HLA-B*08:01 HLA-A*26:01 |
| NASVVNIQK | HLA-A*68:01 HLA-A*11:01 HLA-A*03:01 |
NASFVNIQK | HLA-A*68:01 HLA-A*11:01 HLA-A*03:01 HLA-A*33:01 |
| NESLIDLQEL | HLA-B*40:01 HLA-B*44:03 HLA-B*44:02 |
NESLIDLQQL | HLA-B*40:01 HLA-B*44:03 HLA-B*44:02 |
| NSASFSTFK | HLA-A*68:01 HLA-A*11:01 HLA-A*03:01 HLA-A*30:01 HLA-A*33:01 |
NLAPFFTFK | HLA-A*68:01 HLA-A*03:01 HLA-A*11:01 HLA-A*33:01 HLA-A*31:01 HLA-A*30:01 |
| NYNYLYRLF | HLA-A*24:02 HLA-A*23:01 |
NYNYLFRLF | HLA-A*24:02 HLA-A*23:01 HLA-A*30:02 HLA-A*32:01 |
| NYNYRYRLF | HLA-A*24:02 HLA-A*23:01 HLA-B*08:01 |
||
| QELGKYEQY | HLA-B*44:03 HLA-B*44:02 HLA-B*40:01 HLA-A*30:02 HLA-B*15:01 HLA-A*01:01 HLA-A*26:01 HLA-B*35:01 |
QQLGKYEQY | HLA-B*15:01 HLA-A*30:02 HLA-B*44:03 HLA-B*44:02 HLA-A*32:01 HLA-A*26:01 HLA-A*01:01 HLA-B*35:01 HLA-A*23:01 HLA-A*24:02 |
| QIAPGQTGK | HLA-A*11:01 HLA-A*03:01 HLA-A*68:01 HLA-A*30:01 |
QIAPGQTGN | – |
| QIAPGQTGT | – | ||
| QTNSPRRAR | HLA-A*31:01 HLA-A*68:01 HLA-A*33:01 HLA-A*30:01 HLA-A*11:01 HLA-A*03:01 |
QTNSRRRAR | HLA-A*31:01 HLA-A*33:01 HLA-A*68:01 HLA-A*30:01 |
| QTKSHRRAR | HLA-A*31:01 HLA-A*33:01 HLA-A*30:01 HLA-A*68:01 |
||
| RASANLAATK | HLA-A*03:01 HLA-A*11:01 HLA-A*30:01 |
RASANLAAIK | HLA-A*03:01 HLA-A*11:01 HLA-A*30:01 |
| SFKEELDKY | HLA-A*30:02 HLA-B*15:01 HLA-A*26:01 HLA-A*01:01 HLA-B*35:01 HLA-A*24:02 HLA-A*23:01 |
LFKEELDKY | HLA-A*30:02 HLA-B*15:01 HLA-A*01:01 HLA-A*26:01 HLA-B*35:01 |
| SIIAYTMSL | HLA-A*02:06 HLA-A*02:01 HLA-A*02:03 HLA-A*68:02 HLA-A*32:01 HLA-B*08:01 HLA-A*26:01 HLA-B*07:02 HLA-B*15:01 HLA-B*35:01 |
SIIAYTMLL | HLA-A*02:06 HLA-A*02:01 HLA-A*68:02 HLA-A*02:03 HLA-A*32:01 HLA-A*26:01 |
| SPRRARSV | HLA-B*07:02 HLA-B*08:01 HLA-B*51:01 |
SRRRARSV | – |
| SHRRARSV | – | ||
| SPRRARSVA | HLA-B*07:02 HLA-B*08:01 |
SRRRARSVA | – |
| SHRRARSVA | HLA-A*30:01 | ||
| SVLNDILSR | HLA-A*11:01 HLA-A*68:01 HLA-A*31:01 HLA-A*03:01 HLA-A*33:01 HLA-A*30:01 HLA-A*26:01 |
SVLNDIFSR | HLA-A*68:01 HLA-A*31:01 HLA-A*11:01 HLA-A*33:01 HLA-A*03:01 HLA-A*30:01 HLA-A*32:01 |
| SWMESEFRVY | HLA-A*30:02 HLA-B*15:01 HLA-A*01:01 HLA-A*24:02 HLA-A*23:01 |
SWMESGVY | HLA-A*30:02 |
| TEKSNIIRGW | HLA-B*44:02 HLA-B*44:03 HLA-B*58:01 |
IEKSNIIRGW | HLA-B*44:02 HLA-B*44:03 HLA-B*57:01 HLA-B*58:01 |
| TPINLVRDL | HLA-B*07:02 HLA-B*51:01 HLA-B*35:01 HLA-B*53:01 HLA-B*08:01 |
TPIIVREPEDL | HLA-B*07:02 |
| VGGNYNYLY | HLA-A*30:02 HLA-A*01:01 HLA-B*58:01 |
VGGNYNYLF | HLA-A*24:02 HLA-B*58:01 HLA-A*23:01 |
| VGGNYNYRY | HLA-A*30:02 HLA-A*01:01 |
||
| VSGNYNYLY | HLA-A*01:01 HLA-A*30:02 HLA-B*58:01 HLA-B*57:01 HLA-A*32:01 HLA-B*35:01 |
||
| VLNDILSRL | HLA-A*02:03 HLA-A*02:01 HLA-A*02:06 HLA-A*32:01 HLA-B*15:01 HLA-A*68:02 HLA-B*08:01 HLA-A*30:02 HLA-A*26:01 |
VLNDIFSRL | HLA-A*02:03 HLA-A*02:01 HLA-A*02:06 HLA-A*32:01 HLA-B*15:01 HLA-B*08:01 HLA-A*68:02 HLA-A*30:02 HLA-A*23:01 HLA-A*24:02 |
| VYDPLQPELDSF | HLA-A*24:02 HLA-A*23:01 |
VYDPLQPELDLF | HLA-A*24:02 HLA-A*23:01 |
| WTAGAAAYY | HLA-A*26:01 HLA-A*01:01 HLA-A*30:02 HLA-A*68:01 HLA-B*35:01 HLA-B*15:01 HLA-B*58:01 HLA-B*53:01 |
LTAGAAAYY | HLA-A*01:01 HLA-A*26:01 HLA-A*30:02 HLA-A*68:01 HLA-B*15:01 HLA-B*35:01 HLA-B*57:01 HLA-B*58:01 HLA-A*11:01 HLA-A*03:01 HLA-A*32:01 HLA-B*53:01 |
| YFQPRTFLL | HLA-A*24:02 HLA-A*23:01 HLA-B*08:01 HLA-A*02:01 HLA-A*02:06 HLA-A*30:02 HLA-A*33:01 HLA-A*32:01 HLA-B*07:02 |
YLQPRTFLL | HLA-A*02:01 HLA-B*08:01 HLA-A*02:06 HLA-A*02:03 HLA-A*32:01 HLA-A*24:02 HLA-A*23:01 HLA-B*15:01 HLA-A*01:01 HLA-A*68:02 HLA-A*30:02 HLA-B*07:02 |
| YGFQPTNGV | HLA-A*68:02 HLA-B*51:01 HLA-A*02:06 HLA-A*02:03 |
YGFQPTYGV | HLA-A*68:02 HLA-A*02:06 HLA-B*51:01 HLA-A*02:01 |
| YSFRPTYGV | HLA-A*68:02 HLA-A*02:06 HLA-A*02:01 HLA-B*51:01 HLA-B*57:01 HLA-A*02:03 HLA-A*32:01 HLA-B*58:01 |
||
| YNYLYRLFR | HLA-A*33:01 HLA-A*31:01 HLA-A*68:01 |
YNYLFRLFR | HLA-A*33:01 HLA-A*31:01 HLA-A*68:01 |
| YNYRYRLFR | HLA-A*31:01 HLA-A*33:01 HLA-A*68:01 |
||
| YQDVNCTEV | HLA-A*02:06 HLA-A*02:01 HLA-A*02:03 HLA-A*01:01 HLA-B*40:01 |
YQGVNCTEV | HLA-A*02:06 HLA-A*02:03 HLA-A*02:01 |
| YTMSLGAENSVAY | HLA-A*01:01 HLA-B*15:01 HLA-B*35:01 HLA-A*30:02 HLA-A*26:01 |
YTMLLGAENSVAY | HLA-A*01:01 HLA-A*26:01 HLA-B*35:01 HLA-A*30:02 |
| YTMSLGVENSVAY | HLA-A*01:01 HLA-A*26:01 HLA-B*35:01 HLA-A*30:02 |
||
| YYHKNNKSW | HLA-A*24:02 HLA-A*23:01 HLA-B*57:01 HLA-B*58:01 HLA-B*53:01 HLA-A*32:01 HLA-B*44:02 HLA-B*44:03 |
DHKNNKSW | – |
Table 5.
Comparison of HLA allele restrictions of native and mutant CD8+ T cell epitopes from spike protein of SARS CoV-2.
| Variant | Native | Mutant | No. of alleles that bind native epitope | No. of alleles that bind mutant epitope | No. of common alleles | % of HLA restriction retained for mutant epitope |
|---|---|---|---|---|---|---|
| P.1 | AEHVNNSY | AEYVNNSY | 5 | 5 | 5 | 100.00 |
| QIAPGQTGK | QIAPGQTGT | 4 | 0 | 0 | 0.0 | |
| NASVVNIQK | NASFVNIQK | 3 | 4 | 3 | 100.00 | |
| RASANLAATK | RASANLAAIK | 3 | 3 | 3 | 100.00 | |
| YQDVNCTEV | YQGVNCTEV | 5 | 3 | 3 | 60.00 | |
| LPPAYTNSF | LPSAYTNSF | 8 | 6 | 6 | 75.00 | |
| YGFQPTNGV | YGFQPTYGV | 4 | 4 | 3 | 75.00 | |
| KIADYNYKL | TIADYNYKL | 9 | 6 | 5 | 55.55 | |
| FQFCNDPFL | FQFCNYPFL | 4 | 3 | 3 | 75.00 | |
| YFQPRTFLL | YLQPRTFLL | 9 | 12 | 8 | 88.89 | |
| P.2 | SIIAYTMSL | SIIAYTMLL | 10 | 6 | 6 | 60.00 |
| NASVVNIQK | NASFVNIQK | 3 | 4 | 3 | 100.00 | |
| YQDVNCTEV | YQGVNCTEV | 5 | 3 | 3 | 60.00 | |
| YFQPRTFLL | YLQPRTFLL | 9 | 12 | 8 | 88.89 | |
| YTMSLGAENSVAY | YTMLLGAENSVAY | 5 | 4 | 4 | 80.00 | |
| B.1.617.2.1 | GVYYHKNNK | DVYYHKNNK | 5 | 5 | 3 | 60.00 |
| KIADYNYKL | NIADYNYKL | 9 | 7 | 5 | 55.55 | |
| QIAPGQTGK | QIAPGQTGN | 4 | 0 | 0 | 0.0 | |
| VGGNYNYLY | VGGNYNYRY | 3 | 2 | 2 | 66.67 | |
| NYNYLYRLF | NYNYRYRLF | 2 | 3 | 2 | 100.00 | |
| TEKSNIIRGW | IEKSNIIRGW | 3 | 4 | 3 | 100.00 | |
| GVYFASTEK | GVYFASIEK | 4 | 4 | 4 | 100.00 | |
| KVGGNYNYLY | KVGGNYNYRY | 6 | 4 | 4 | 66.67 | |
| YQDVNCTEV | YQGVNCTEV | 5 | 3 | 3 | 60.00 | |
| DGVYFASTEK | DGVYFASIEK | 3 | 3 | 3 | 100.00 | |
| QTNSPRRAR | QTNSRRRAR | 6 | 4 | 4 | 66.67 | |
| YNYLYRLFR | YNYRYRLFR | 3 | 3 | 3 | 100.00 | |
| SPRRARSV | SRRRARSV | 3 | 0 | 0 | 0.00 | |
| SWMESEFRVY | SWMESGVY | 5 | 1 | 1 | 20.00 | |
| WTAGAAAYY | LTAGAAAYY | 8 | 12 | 8 | 100.00 | |
| SPRRARSVA | SRRRARSVA | 2 | 0 | 0 | 0.00 | |
| YFQPRTFLL | YLQPRTFLL | 9 | 12 | 8 | 88.89 | |
| B.1.1.429 | QELGKYEQY | QQLGKYEQY | 8 | 10 | 7 | 87.50 |
| YQDVNCTEV | YQGVNCTEV | 5 | 3 | 3 | 60.00 | |
| NESLIDLQEL | NESLIDLQQL | 3 | 3 | 3 | 100.00 | |
| YFQPRTFLL | YLQPRTFLL | 9 | 12 | 8 | 88.89 | |
| B.1.1.298 | VGGNYNYLY | VGGNYNYLF | 3 | 3 | 1 | 33.33 |
| NYNYLYRLF | NYNYLFRLF | 2 | 4 | 2 | 100.00 | |
| KVGGNYNYLY | KVGGNYNYRY | 6 | 4 | 4 | 66.67 | |
| YQDVNCTEV | YQGVNCTEV | 5 | 3 | 3 | 60.00 | |
| YNYLYRLFR | YNYLFRLFR | 3 | 3 | 3 | 100.00 | |
| YFQPRTFLL | YLQPRTFLL | 9 | 12 | 8 | 88.89 | |
| VYDPLQPELDSF | VYDPLQPELDLF | 2 | 2 | 2 | 100.00 | |
| SFKEELDKY | LFKEELDKY | 7 | 5 | 5 | 71.43 | |
| KVGGNYNYLY | KVGGNYNYLF | 6 | 5 | 3 | 50.00 | |
| B.1.1.529 | DSKVGGNYNY | DSKVSGNYNY | 4 | 3 | 3 | 75.00 |
| GEVFNATRF | DEVFNATRF | 4 | 3 | 3 | 75.00 | |
| IYKTPPIKDF | IYKTPPIKYF | 3 | 4 | 3 | 100.00 | |
| HVSGTNGTK | HVISGTNGTK | 4 | 4 | 4 | 100.00 | |
| LGAENSVAY | LGVENSVAY | 6 | 6 | 6 | 100.00 | |
| KVGGNYNYLY | KVSGNYNYLY | 6 | 7 | 5 | 83.33 | |
| NSASFSTFK | NLAPFFTFK | 5 | 6 | 5 | 100.00 | |
| QTNSPRRAR | QTKSHRRAR | 6 | 4 | 4 | 66.67 | |
| SPRRARSV | SHRRARSV | 3 | 0 | 0 | 0.00 | |
| SPRRARSVA | SHRRARSVA | 2 | 1 | 0 | 0.00 | |
| SVLNDILSR | SVLNDIFSR | 7 | 7 | 6 | 85.71 | |
| TPINLVRDL | TPIIVREPEDL | 5 | 1 | 1 | 20.00 | |
| VLNDILSRL | VLNDIFSRL | 9 | 10 | 8 | 88.89 | |
| VGGNYNYLY | VSGNYNYLY | 3 | 6 | 3 | 100.00 | |
| YGFQPTNGV | YSFRPTYGV | 4 | 8 | 4 | 100.00 | |
| YYHKNNKSW | DHKNNKSW | 8 | 0 | 0 | 0.00 | |
| YTMSLGAENSVAY | YTMSLGVENSVAY | 5 | 4 | 4 | 80.00 | |
| YQDVNCTEV | YQGVNCTEV | 5 | 3 | 3 | 60.00 | |
| YFQPRTFLL | YLQPRTFLL | 9 | 12 | 8 | 88.89 | |
| TEKSNIIRGW | IEKSNIIRGW | 3 | 4 | 3 | 100.00 | |
| GVYFASTEK | GVYFASIEK | 4 | 4 | 4 | 100.00 | |
| DGVYFASTEK | DGVYFASIEK | 3 | 3 | 3 | 100.00 | |
| AEHVNNSY | AEYVNNSY | 5 | 5 | 5 | 100.00 |
Specifically in the case of Omicron/B.1.1.529 variant analysis, we observed that from the 187 native CD8+ epitopes on the spike protein of SARS-COV-2, 24 were found to be mutated in Omicron/B.1.1.529 (Table 2). One of them (GVYYHKNNK mutating to DHKNNK) was not included in the HLA binding analysis as the mutated epitope is shorter (5 and 7 residues long) than the length (8 to 15 residues long) that can be analyzed by the NetMHCpan 4.1 EL tool. Similarly, SGTNGTK and TVYDPLQPELDLFK were not considered for HLA analysis for the same reason.
From the 52 distinct native-mutated epitope pairs analyzed for HLA restriction (Table 5), 19 (~36%) mutated epitopes retained the HLA restriction of their native counterparts (100% retained HLA restriction) with total 39 (~75%) mutated epitopes retaining more than or equal to 60% HLA restriction. Seven epitopes (~13%) display drastic variation in HLA restriction i.e., no common HLA allele in the restriction sets of native and mutated epitopes, indicated by 0% similarity in Table 5. Overall, results of the analysis reveal that majority (~75%) of the analyzed CD8+ mutated epitopes on the spike protein retain more than 60% HLA restriction compared to their native counterparts.
Taken together, the computational results suggest that along with other variants, the omicron/B.1.1.529 variant too may be capable of eliciting adequate CD8 T cell responses. Calling for further experimental verification of the same, we also suggest possibility of retained efficacy of the currently administered vaccines (originally designed to target the spike protein of Wuhan_Hu_1 strain) against omicron variant.
4. Discussion
SARS-COV-2 will continue to evolve, giving rise to new variants. With emerging variants, it is necessary to design a therapeutic intervention with broad efficacy. Given the complexity of the vaccine design and approval process, a multivalent vaccine is desirable instead of variant-specific vaccines [9]. As local variants of concern (VoC) continue to spread and possess the ability of global dominance, this matter is of international concern and urgency. Considering the exact correlates of protection are still unclear and cellular immunity is most likely one of them, investigating the T cell response variation/conservation across strains can provide insights about the possibility of cellular immune escape, its molecular basis and its effects on vaccine efficacy. Geers et al [29] reported that the variants B.1.1.7 and B.1.351 partially evade humoral immunity induced by SARS-CoV-2 infection or BNT162b2 vaccination but the CD4+ T cell activation against these variants is unchanged compared to the native virus. Tarke et al [30] analyzed the T cell epitope responses against B.1.1.7, B.1.351, P.1, and CAL.20C variants in COVID-19 convalescents and Moderna (mRNA-1273) or Pfizer/BioNTech (BNT162b2) vaccines. Observing a modest decrease (10–22%) in T cell reactivity, they reported that the variants analyzed do not significantly disrupt T cell responses hence it is expected that vaccines won't be rendered completely inefficacious against them. Similarly, there have been few other reports analyzing CD4+ and/or CD8+ T cell responses in clinical settings against some of the SARS-CoV-2 variants including omicron [31], [32], [33], [34], [35], [36].
In the present study we asked, if and how does the T cell epitope pool vary across the variants delta plus/B.1.617.2.1, gamma/P.1, zeta/P.2, B.1.1.429, Mink cluster 5/B.1.1.298 and Omicron/B.1.1.529 of SARS-COV-2? Can these variants (known to escape humoral immunity) also escape cellular immunity due to mutations in T cell epitopes? If not, what do the uncompromised T cell responses imply in terms of vaccine efficacy against these variants?
As mentioned above, the notion of “retained vaccine efficacy against new SARS-CoV-2 variants due to largely uncompromised T cell responses” has been previously expressed through multiple studies. Arguably, there are a few studies that show mutated epitopes in the variants of SARS CoV-2 lead to a diminished CD4+ and CD8+ T cell responses against them [37], [38], [39], [40]. A previous study by Agerer et al. [37] seems to suggest a diminished CD8+ T cell response due to failure of HLA-I binding of the mutated epitopes of the variants they tested but their study was restricted to only 27 CD8+ T cell epitopes and, only two HLA-alleles of Austrian population were included in the study. Also, a study by Motozono et al. [38] showed that mutants of an immunodominant native epitope of the spike protein “NYNYLYRLF” restricted to HLA-A*24:02, such as NYNYRYRLF having L452R mutation, and NYNYLFRLF having Y453F mutation, harbored by the variants B.1.427/429 and B.1.1.298 respectively, showed a decreased IFN-ϒ expression by CD8+ T-cells in the convalescent individuals with HLA-A*24:02. Likewise, De Silva et al. [39] demonstrated T-cell evasion by showing partial loss of T-cell response with mutants of two immunodominant CD4+ spike protein epitopes. They also observed complete loss of T-cell response to a mutant of one immunodominant native ORF-3a CD8+ epitope and a few mutants of two immunodominant native nucleocapsid CD8+ epitopes [39]. Zhang et al. [40] observed a decreased CD8+ T-cell response when stimulated with mutants of three native spike protein epitopes (from B.1.1.7, B.1.351, P.1 and B.1.617.2) in the PBMCs of 4 different and highly prevalent HLA-I allelic donors of the Chinese population, specific to the epitopes, post 2–3 weeks of vaccination (first immunization) with CoronaVac [40]. However, their study was limited to only three of the spike protein's CD8+ T cell epitopes and one from ORF-1a. Moreover, although their study covers frequent HLA-A alleles of Chinese population, it does not ensure a complete coverage of the diverse HLA population. In an actual infection scenario, many CD8+ T-cell epitopes will be involved from the spike protein alone which may include other immunodominant epitopes that are not found yet, and they could be recognized by CD8+ and CD4+ T cells resulting in immune response. To identify such epitopes, extensive studies are needed that should cover a large pool of epitopes and a diverse HLA-I/II allelic population globally. Also, humans can co-express six different HLA-I alleles and so, the mutants in the studies of Motozono et al. [38], De Silva et al. [39] and Zhang et al. [40] might bind to other HLA-I haplotypes in the same individuals that their parental/native counterparts don't bind, and elicit T-cell responses i.e. the mutants may show a varied HLA-I binding pattern compared to the native epitopes as observed with some epitopes in our predictions (Table 3). Considering such a complexity, the observations of Agerer et al [37], Motozono et al. [38], De Silva et al. [39] and Zhang et al. [40] need not be the case if either the number of epitopes or if the number of HLA allele haplotypes is increased. Our results indicate such a scenario due to the inclusion of a larger pool of CD8+ T cell epitopes as well as HLA-I allele haplotypes (reference set) in our investigation. However, our work is only computational and further investigation in this line is required for better understanding of the immunodominance in spike protein's T-cell epitopes of SARS CoV-2 with respect to HLA restriction and immune escape. It is known that multiple T cell epitopes are recognized by the immune system for initiating T cell response against the virus infections [20]. While a decrease in binding affinity leading to a diminished CD8+ T-cell response for the mutated epitopes of the variants in a HLA specific manner is possible, the conserved native epitopes could still elicit T-cell response. Further research is required to find out the actual number of immunodominant epitopes among the conserved native epitopes of the spike protein across the variants, in a large population with a diverse HLA distribution. Such epitopes need to be carefully monitored for mutations among the circulating and the new emerging variants.
We believe that our study is a non-redundant contribution to the ongoing research because we have performed a comprehensive in silico analysis – analyzing only experimentally verified 97 CD4+ and 187 CD8+ T cell epitopes of spike protein, including delta plus and the omicron variant (considered at present as a variant of concern globally) along with two other variants that are proven to evade humoral immunity (P.1 and P.2). Additionally, we believe that our specific approach (comprehensive analysis/comparison of HLA restriction of all native and mutated T cell epitopes on spike proteins across the unique set of variants to gain insights on vaccine efficacy) is hitherto unexplored.
Our results indicate that native CD4+ T cell epitopes on spike protein are ≥90% conserved across the variants - B.1.617.2.1/delta plus, P.1/gamma, P.2/zeta, B.1.1.429, B.1.1.298/Mink cluster 5, out of which P.1 and P.2 are known to evade humoral response [3]. Also, the native CD4+ T cell epitopes are ~75% conserved in the B.1.1.529/omicron variant. In case of CD8+ T cell epitopes too, ≥ 90% native epitopes on spike protein seem to be conserved. Though the corresponding % of conservation of epitopes are comparatively lower in omicron/B.1.1.529 variant (75% in CD4+ epitopes and 87% in CD8 epitopes) the similar HLA restriction still suggests adequate T cell response as discussed below. These high levels of conservation of T cell epitopes suggest that the cellular responses against the aforementioned variants may remain largely uncompromised. On the other hand, the epitopes that do not undergo any mutations across multiple variants are also of special interest (non-highlighted epitopes in Supplementary Table 1, Supplementary Table 2) as they can be identified as the virus's “vulnerable low-mutating immunogenic regions” to design a broad efficacy intervention.
Apart from the overall mutational changes in T cell epitope landscape, we observed that the majority of variants' spike protein CD8+ T cell epitopes (≥70% for each variant, 100% for P.2 and B.1.1.429) display ≥60% similar HLA allele restriction pattern. This suggests optimism towards largely retained CD8+ T cell responses against the selected variants.
It is clear from our analysis that there are only a lesser number of CD4+ epitopes that have undergone mutations across the tested variants except omicron indicating conserved CD4+ T-cell response against these variants post vaccination. Also, from figure-1, the majority of these mutated epitopes including those of omicron variant were predicted to have similar restriction patterns as their native counterparts indicating their ability to initiate a CD4+ T-cell response. Our analysis is in line with the reports of Geers et al [29] and Tarke et al. [30].
Certain epitopes from our analysis deserve specific attention. The CD4+ T cell epitope HKNNKSWMESEFRVY that mutates to HKNNKSWMESGVY of the delta plus/ B.1.617.2.1 and the mutated epitopes CEFQFCNDPFLD, IVREPEDLPQGFSALEP, KHTPIIVREPEDLPQGF, LDHKNNKSWMES, EIYQAGNKPCNGVAG and PIIVREPEDLPQGFSAL of the omicron variant were predicted not to bind to any of the alleles that their native counterparts bound (figure-1). Similarly, 7 mutated CD8+ epitopes have no common alleles with the native epitopes' HLA restriction sets (Table 3). Six of these mutated epitopes (QIAPGQTGN, QIAPGQTGT, SRRRARSV, SHRRARSV, SRRRARSVA, DHKNNKSW) are predicted to lose the ability to be recognized by MHC class I molecules i.e., all their predicted HLA restriction alleles are non-binders (indicated by their % rank predicted to be higher than the 2% cutoff value). One mutated epitope (SHRRARSVA) is predicted to be restricted to only HLA-A*30:01 – an allele not present in the predicted HLA restriction set of the epitope's native counterpart.
Evidently, these epitopes are not suitable targets while designing a broad efficacy vaccine against SARS-CoV-2. We wonder whether the frequently retained mutations are of special significance in the virus's increased transmission, replication, infectivity and hence evolution, calling for further research in this direction. The present results are only a preliminary and warrant experimental verifications. Further, the conserved epitopes/peptides identified here between native and variant strains could also aid in vitro and in vivo experimental studies.
5. Conclusion
Obsession with humoral immunity and studies arising from the same do not reflect the true spectrum of vaccine induced immune response and aberrations thereof caused by novel variants. Considering that T dependent responses are a major correlate of protection in all forms of vaccine formulations, our analysis suggests that largely conserved CD4+ and CD8+ T cell responses may lead to significantly retained potential of vaccines to fight severity and fatality. Hence, even in case of reduced neutralization by antibodies, variants may not become vaccine resistant. Moving forward in the development/evolution of the vaccine landscape, a balanced approach that leverages all possible correlates of protection (humoral as well as cellular) is required to minimize the burden on our healthcare and socioeconomic systems.
The following are the supplementary data related to this article.
Native spike protein CD4 T cell epitopes.
Native spike protein CD8 T cell epitopes.
Conservation analysis of S protein T cell epitope pools of B.1.1.7 variant sequences.
Mutated epitopes searching algorithm.
Reference allele sets for MHC-I and MHC-II.
Supplementary material 3
Supplementary material 4
Acknowledgement for SARS-COV-2 variants genome sequences sourced from GISAID.
Supplementary material 6
CRediT authorship contribution statement
Sankaranarayanan S: Conceptualization, Methodology, Investigation, Data analysis, Validation, Writing – original draft & editing. Mugdha Mohkhedkar: Conceptualization, Methodology, Investigation, Data analysis, Validation, Writing– original draft & editing. Vani Janakiraman: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Le T.T., Cramer J.P., Chen R., Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:667–668. doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 2.Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021;21:73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Beltran W.F., Lam E.C., St K., Denis A.D., Nitido Z.H., Garcia B.M., Hauser J., Feldman M.N., Pavlovic D.J., Gregory M.C., Poznansky A., Sigal A.G., Schmidt A.J., Iafrate V., Naranbhai A.B.Balazs. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383. doi: 10.1016/j.cell.2021.03.013. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Tracking SARS-CoV-2 variants. 2021. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ Available at: Last Accessed: 21/03/2022.
- 5.Kannan S.R., Spratt A.N., Cohen A.R., Naqvi S.H., Chand H.S., Quinn T.P., Lorson C.L., Byrareddy S.N., Singh K. Evolutionary analysis of the Delta and Delta plus variants of the SARS-CoV-2 viruses. J. Autoimmun. 2021;124 doi: 10.1016/j.jaut.2021.102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., Schaefer-Babajew D., Cipolla M., Gaebler C., Lieberman J.A., Oliveira T.Y., Yang Z., Abernathy M.E., Huey-Tubman K.E., Hurley A., Turroja M., West K.A., Gordon K., Millard K.G., Ramos V., Silva J.Da, Xu J., Colbert R.A., Patel R., Dizon J., Unson-O’Brien C., Shimeliovich I., Gazumyan A., Caskey M., Bjorkman P.J., Casellas R., Hatziioannou T., Bieniasz P.D., Nussenzweig M.C. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., Prot M., Gallais F., Gantner P., Velay A., Le Guen J., Kassis-Chikhani N., Edriss D., Belec L., Seve A., Courtellemont L., Péré H., Hocqueloux L., Fafi-Kremer S., Prazuck T., Mouquet H., Bruel T., Simon-Lorière E., Rey F.A., Schwartz O. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 8.Cevik M., Grubaugh N.D., Iwasaki A., Openshaw P. COVID-19 vaccines: keeping pace with SARS-CoV-2 variants. Cell. 2021;184:5077–5081. doi: 10.1016/j.cell.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tregoning J.S., Flight K.E., Higham S.L., Wang Z., Pierce B.F. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021;21:626–636. doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson A.C., Humbert M., Buggert M. The known unknowns of T cell immunity to COVID-19. Sci. Immunol. 2020;5 doi: 10.1126/SCIIMMUNOL.ABE8063. [DOI] [PubMed] [Google Scholar]
- 12.Szomolanyi-Tsuda E., Le Q.P., Garcea R.L., Welsh R.M. T-cell-independent immunoglobulin G responses in vivo are elicited by live-virus infection but not by immunization with viral proteins or virus-like particles. J. Virol. 1998;72:6665–6670. doi: 10.1128/jvi.72.8.6665-6670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochsenbein A.F., Pinschewer D.D., Odermatt B., Carroll M.C., Hengartner H., Zinkernagel R.M. Protective T cell-independent antiviral antibody responses are dependent on complement. J. Exp. Med. 1999;190:1165–1174. doi: 10.1084/jem.190.8.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Silva T.I., Liu G., Lindsey B.B., Dong D., Moore S.C., Hsu N.S., Shah D., Wellington D., Mentzer A.J., Angyal A., Brown R., Parker M.D., Ying Z., Yao X., Turtle L., Dunachie S., Maini M.K., Ogg G., Knight J.C., Peng Y., Rowland-Jones S.L., Dong T., Aanensen D.M., Abudahab K., Adams H., Adams A., Afifi S., Aggarwal D., Ahmad S.S.Y., Aigrain L., Alcolea-Medina A., Alikhan N.-F., Allara E., Amato R., Annett T., Aplin S., Ariani C.V., Asad H., Ash A., Ashfield P., Ashford F., Atkinson L., Attwood S.W., Auckland C., Aydin A., Baker D.J., Baker P., Balcazar C.E., Ball J., Barrett J.C., Barrow M., Barton E., Bashton M., Bassett A.R., Batra R., Baxter C., Bayzid N., Beaver C., Beckett A.H., Beckwith S.M., Bedford L., Beer R., Beggs A., Bellis K.L., Berry L., Bertolusso B., Best A., Betteridge E., Bibby D., Bicknell K., Binns D., Birchley A., Bird P.W., Bishop C., Blacow R., Blakey V., Blane B., Bolt F., Bonfield J., Bonner S., Bonsall D., Boswell T., Bosworth A., Bourgeois Y., Boyd O., Bradley D.T., Breen C., Bresner C., Breuer J., Bridgett S., Bronner I.F., Brooks E., Broos A., Brown J.R., Bucca G., Buchan S.L., Buck D., Bull M., Burns P.J., Burton-Fanning S., Byaruhanga T., Byott M., Campbell S., Carabelli A.M., Cargill J.S., Carlile M., Carvalho S.F., Casey A., Castigador A., Catalan J., Chalker V., Chaloner N.J., Chand M., Chappell J.G., Charalampous T., Chatterton W., Chaudhry Y., Churcher C.M., Clark G., Clarke P., Cogger B.J., Cole K., Collins J., Colquhoun R., Connor T.R., Cook K.F., Coombes J., Corden S., Cormie C., Cortes N., Cotic M., Cotton S., Cottrell S., Coupland L., Cox M., Cox A., Craine N., Crawford L., Cross A., Crown M.R., Crudgington D., Cumley N., Curran T., Curran M.D., da Silva Filipe A., Dabrera G., Darby A.C., Davidson R.K., Davies A., Davies R.M., Davis T., de Angelis D., Lacy E.De, de Oliveira Martins L., Debebe J., Denton-Smith R., Dervisevic S., Dewar R., Dey J., Dias J., Dobie D., Dorman M.J., Downing F., Driscoll M., du Plessis L., Duckworth N., Durham J., Eastick K., Easton L.J., Eccles R., Edgeworth J., Edwards S., Bouzidi K.El, Eldirdiri S., Ellaby N., Elliott S., Eltringham G., Ensell L., Erkiert M.J., Zamudio M.E., Essex S., Evans J.M., Evans C., Everson W., Fairley D.J., Fallon K., Fanaie A., Farr B.W., Fearn C., Feltwell T., Ferguson L., Fina L., Flaviani F., Fleming V.M., Forrest S., Foster-Nyarko E., Foulkes B.H., Foulser L., Fragakis M., Frampton D., Francois S., Fraser C., Freeman T.M., Fryer H., Fuchs M., Fuller W., Gajee K., Galai K., Gallagher A., Gallagher E., Gallagher M.D., Gallis M., Gaskin A., Gatica-Wilcox B., Geidelberg L., Gemmell M., Georgana I., George R.P., Gifford L., Gilbert L., Girgis S.T., Glaysher S., Goldstein E.J., Golubchik T., Gomes A.N., Gonçalves S., Goodfellow I.G., Goodwin S., Goudarzi S., Gourtovaia M., Graham C., Graham L., Grant P.R., Green L.R., Green A., Greenaway J., Gregory R., Guest M., Gunson R.N., Gupta R.K., Gutierrez B., Haldenby S.T., Hamilton W.L., Hansford S.E., Haque T., Harris K.A., Harrison I., Harrison E.M., Hart J., Hartley J.A., Harvey W.T., Harvey M., Hassan-Ibrahim M.O., Heaney J., Helmer T., Henderson J.H., Hesketh A.R., Hey J., Heyburn D., Higginson E.E., Hill V., Hill J.D., Hilson R.A., Hilvers E., Holden M.T.G., Hollis A., Holmes C.W., Holmes N., Holmes A.H., Hopes R., Hornsby H.R., Hosmillo M., Houlihan C., Howson-Wells H.C., Hubb J., Huckson H., Hughes W., Hughes J., Hughes M., Hutchings S., Idle G., Illingworth C.J., Impey R., Irish-Tavares D., Iturriza-Gomara M., Izuagbe R., Jackson C., Jackson B., Jackson L.M., Jackson K.A., Jackson D.K., Jahun A.S., James V., James K., Jeanes C., Jeffries A.R., Jeremiah S., Jermy A., John M., Johnson R., Johnson K., Johnston I., Jones O., Jones S., Jones H., Jones C.R., Jones N., Joseph A., Judges S., Kay G.L., Kay S., Keatley J.-P., Keeley A.J., Kenyon A., Kermack L.M., Khakh M., Kidd S.P., Kimuli M., Kirk S., Kitchen C., Kitchman K., Knight B.A., Koshy C., Kraemer M.U.G., Kumziene-Summerhayes S., Kwiatkowski D., Lackenby A., Laing K.G., Lampejo T., Langford C.F., Lavin D., Lawton A.I., Lee J., Lee D., Lensing S.V., Leonard S., Levett L.J., Le-Viet T., Lewis J., Lewis K., Liddle J., Liggett S., Lillie P.J., Lister M.M., Livett R., Lo S., Loman N.J., Loose M.W., Louka S.F., Loveson K.F., Lowdon S., Lowe H., Lowe H.L., Lucaci A.O., Ludden C., Lynch J., Lyons R.A., Lythgoe K., Machin N.W., MacIntyre-Cockett G., Mack A., Macklin B., Maclean A., Macnaughton E., Madona P., Maes M., Maftei L., Mahanama A.I.K., Mahungu T.W., Mair D., Maksimovic J., Malone C.S., Maloney D., Manesis N., Manley R., Mantzouratou A., Marchbank A., Mariappan A., Martincorena I., Nunez R.T.Martinez, Mather A.E., Maxwell P., Mayhew M., Mbisa T., McCann C.M., McCarthy S.A., McCluggage K., McClure P.C., McCrone J.T., McHugh M.P., McKenna J.P., McKerr C., McManus G.M., McMurray C.L., McMurray C., McNally A., Meadows L., Medd N., Megram O., Menegazzo M., Merrick I., Michell S.L., Michelsen M.L., Mirfenderesky M., Mirza J., Miskelly J., Moles-Garcia E., Moll R.J., Molnar Z., Monahan I.M., Mondani M., Mookerjee S., Moore C., Moore J., Moore N., Moore C., Morcrette H., Morgan S., Morgan M., Mori M., Morriss A., Moses S., Mower C., Muir P., Mukaddas A., Munemo F., Munn R., Murray A., Murray L.J., Murray D.R., Mutingwende M., Myers R., Nastouli E., Nebbia G., Nelson A., Nelson C., Nicholls S., Nichols J., Nicodemi R., Nomikou K., O’Grady J., O’Brien S., Odedra M., Ohemeng-Kumi N., Oliver K., Orton R.J., Osman H., Pacchiarini N., Padgett D., Page A.J., Park E.J., Park N.R., Parmar S., Partridge D.G., Pascall D., Patel A., Patel B., Paterson S., Payne B.A.I., Peacock S.J., Pearson C., Pelosi E., Percival B., Perkins J., Perry M., Pinckert M.L., Platt S., Podplomyk O., Pohare M., Pond M., Pope C.F., Poplawski R., Powell J., Poyner J., Prestwood L., Price A., Price J.R., Prieto J.A., Pritchard D.T., Prosolek S.J., Pugh G., Pusok M., Pybus O.G., Pymont H.M., Quail M.A., Quick J., Radulescu C., Raghwani J., Ragonnet-Cronin M., Rainbow L., Rajan D., Rajatileka S., Ramadan N.A., Rambaut A., Ramble J., Randell P.A., Randell P., Ratcliffe L., Raviprakash V., Raza M., Redshaw N.M., Rey S., Reynolds N., Richter A., Robertson D.L., Robinson E., Robson S.C., Rogan F., Rooke S., Rowe W., Roy S., Rudder S., Ruis C., Rushton S., Ryan F., Saeed K., Samaraweera B., Sambles C.M., Sanderson R., Sanderson T., Sang F., Sass T., Scher E., Scott G., Scott C., Sehmi J., Shaaban S., Shah D., Shaw J., Shelest E., Shepherd J.G., Sheridan L.A., Sheriff N., Shirley L., Sillitoe J., Silviera S., Simpson D.A., Singh A., Singleton D., Skvortsov T., Sloan T.J., Sluga G., Smith K., Smith K.S., Smith P., Smith D.L., Smith L., Smith C.P., Smith N., Smollett K.L., Snell L.B., Somassa T., Southgate J., Spellman K., Chapman M.H.Spencer, Spurgin L.G., Spyer M.J., Stanley R., Stanley W., Stanton T.D., Starinskij I., Stockton J., Stonehouse S., Storey N., Studholme D.J., Sudhanva M., Swindells E., Taha Y., Tan N.K., Tang J.W., Tang M., Taylor B.E.W., Taylor J.F., Taylor S., Temperton B., Templeton K.E., O’Toole Xeine, Thomas C., Thomson L., Thomson E.C., Thornton A., Thurston S.A.J., Todd J.A., Tomb R., Tong L., Tonkin-Hill G., Torok M.E., Tovar-Corona J.M., Trebes A., Trotter A.J., Tsatsani I., Turnbull R., Twohig K.A., Umpleby H., Underwood A.P., Vamos E.E., Vasylyeva T.I., Vattipally S., Vernet G., Vipond B.B., Volz E.M., Walsh S., Wang D., Warne B., Warwick-Dugdale J., Wastnedge E., Watkins J., Watson L.K., Waugh S., Webster H.J., Weldon D., Westwick E., Whalley T., Wheeler H., Whitehead M., Whiteley M., Whitwham A., Wierzbicki C., Willford N.J., Williams L.-A., Williams R., Williams C., Williams C., Williams C.A., Williams R.J., Williams T., Williams C., Williamson K.A., Wilson-Davies E., Witele E., Withell K.T., Witney A.A., Wolverson P., Wong N., Workman T., Wright V., Wright D.W., Wyatt T., Wyllie S., Xu-McCrae L., Yavus M., Yaze G., Yeats C.A., Yebra G., Yew W.C., Young G.R., Young J., Zarebski A.E., Zhang P., Baillie J.K., Semple M.G., Openshaw P.J.M., Carson G., Alex B., Andrikopoulos P., Bach B., Barclay W.S., Bogaert D., Chand M., Chechi K., Cooke G.S., da Silva Filipe A., Docherty A.B., Dumas M.-E., Dunning J., Fletcher T., Green C.A., Greenhalf W., Griffin J.L., Gupta R.K., Harrison E.M., Hiscox J.A., Ho A.Y.Wai, Horby P.W., Ijaz S., Khoo S., Klenerman P., Law A., Lewis M.R., Liggi S., Correia G.dos S., Lim W.S., Maslen L., Mentzer A.J., Merson L., Meynert A.M., Noursadeghi M., Olanipekun M., Osagie A., Palmarini M., Palmieri C., Paxton W.A., Pollakis G., Price N., Rambaut A., Robertson D.L., Russell C.D., Sancho-Shimizu V., Sands C.J., Scott J.T., Sigfrid L., Solomon T., Sriskandan S., Stuart D., Summers C., Swann O.V., Takats Z., Takis P., Tedder R.S., Thompson A.A.R., Thomson E.C., Thwaites R.S., Zambon M., Hardwick H., Donohue C., Griffiths F., Oosthuyzen W., Donegan C., Spencer R.G., Dalton J., Girvan M., Saviciute E., Roberts S., Harrison J., Marsh L., Connor M., Halpin S., Jackson C., Gamble C., Plotkin D., Lee J., Leeming G., Law A., Wham M., Clohisey S., Hendry R., Scott-Brown J., Shaw V., McDonald S.E., Keating S., Ahmed K.A., Armstrong J.A., Ashworth M., Asiimwe I.G., Bakshi S., Barlow S.L., Booth L., Brennan B., Bullock K., Catterall B.W.A., Clark J.J., Clarke E.A., Cole S., Cooper L., Cox H., Davis C., Dincarslan O., Dunn C., Dyer P., Elliott A., Evans A., Finch L., Fisher L.W.S., Foster T., Garcia-Dorival I., Gunning P., Hartley C., Jensen R.L., Jones C.B., Jones T.R., Khandaker S., King K., Kiy R.T., Koukorava C., Lake A., Lant S., Latawiec D., Lavelle-Langham L., Lefteri D., Lett L., Livoti L.A., Mancini M., McDonald S., McEvoy L., McLauchlan J., Metelmann S., Miah N.S., Middleton J., Mitchell J., Moore S.C., Murphy E.G., Penrice-Randal R., Pilgrim J., Prince T., Reynolds W., Ridley P.M., Sales D., Shaw V.E., Shears R.K., Small B., Subramaniam K.S., Szemiel A., Taggart A., Tanianis-Hughes J., Thomas J., Trochu E., van Tonder L., Wilcock E., Zhang J.E., Flaherty L., Maziere N., Cass E., Carracedo A.D., Carlucci N., Holmes A., Massey H., Murphy L., Wrobel N., McCafferty S., Morrice K., MacLean A., Adeniji K., Agranoff D., Agwuh K., Ail D., Aldera E.L., Alegria A., Allen S., Angus B., Ashish A., Atkinson D., Bari S., Barlow G., Barnass S., Barrett N., Bassford C., Basude S., Baxter D., Beadsworth M., Bernatoniene J., Berridge J., Berry C., Best N., Bothma P., Chadwick D., Brittain-Long R., Bulteel N., Burden T., Burtenshaw A., Caruth V., Chadwick D., Chambler D., Chee N., Child J., Chukkambotla S., Clark T., Collini P., Cosgrove C., Cupitt J., Cutino-Moguel M.-T., Dark P., Dawson C., Dervisevic S., Donnison P., Douthwaite S., Drummond A., DuRand I., Dushianthan A., Dyer T., Evans C., Eziefula C., Fegan C., Finn A., Fullerton D., Garg S., Garg S., Garg A., Gkrania-Klotsas E., Godden J., Goldsmith A., Graham C., Hardy E., Hartshorn S., Harvey D., Havalda P., Hawcutt D.B., Hobrok M., Hodgson L., Hormis A., Jacobs M., Jain S., Jennings P., Kaliappan A., Kasipandian V., Kegg S., Kelsey M., Kendall J., Kerrison C., Kerslake I., Koch O., Koduri G., Koshy G., Laha S., Laird S., Larkin S., Leiner T., Lillie P., Limb J., Linnett V., Little J., Lyttle M., MacMahon M., MacNaughton E., Mankregod R., Masson H., Matovu E., McCullough K., McEwen R., Meda M., Mills G., Minton J., Mirfenderesky M., Mohandas K., Mok Q., Moon J., Moore E., Morgan P., Morris C., Mortimore K., Moses S., Mpenge M., Mulla R., Murphy M., Nagel M., Nagarajan T., Nelson M., Norris L., O’Shea M.K., Otahal I., Ostermann M., Pais M., Palmieri C., Panchatsharam S., Papakonstantinou D., Paraiso H., Patel B., Pattison N., Pepperell J., Peters M., Phull M., Pintus S., Pooni J.S., Planche T., Post F., Price D., Prout R., Rae N., Reschreiter H., Reynolds T., Richardson N., Roberts M., Roberts D., Rose A., Rousseau G., Ruge B., Ryan B., Saluja T., Schmid M.L., Shah A., Shanmuga P., Sharma A., Shawcross A., Sizer J., Shankar-Hari M., Smith R., Snelson C., Spittle N., Staines N., Stambach T., Stewart R., Subudhi P., Szakmany T., Tatham K., Thomas J., Thompson C., Thompson R., Tridente A., Tupper-Carey D., Twagira M., Vallotton N., Vancheeswaran R., Vincent-Smith L., Visuvanathan S., Vuylsteke A., Waddy S., Wake R., Walden A., Welters I., Whitehouse T., Whittaker P., Whittington A., Papineni P., Wijesinghe M., Williams M., Wilson L., Cole S., Winchester S., Wiselka M., Wolverson A., Wootton D.G., Workman A., Yates B., Young P. The impact of viral mutations on recognition by SARS-CoV-2 specific T cells. IScience. 2021;24 doi: 10.1016/j.isci.2021.103353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riou C., Keeton R., Moyo-Gwete T., Hermanus T., Kgagudi P., Baguma R., Valley-Omar Z., Smith M., Tegally H., Doolabh D., Iranzadeh A., Tyers L., Mutavhatsindi H., Tincho M.B., Benede N., Marais G., Chinhoyi L.R., Mennen M., Skelem S., du Bruyn E., Stek C., South African cellular immunity network, de Oliveira T., Williamson C., Moore P.L., Wilkinson R.J., Ntusi N.A.B., Burgers W.A. Escape from recognition of SARS-CoV-2 variant spike epitopes but overall preservation of T cell immunity. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abj6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng X., Ouyang J., Isnard S., Lin J., Fombuena B., Zhu B., Routy J.P. Sharing CD4+ T cell loss: when COVID-19 and HIV collide on immune system. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.596631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., Liu J., Peter L., Atyeo C., Zhu A., Bondzie E.A., Dagotto G., Gebre M.S., Jacob-Dolan C., Li Z., Nampanya F., Patel S., Pessaint L., Van Ry A., Blade K., Yalley-Ogunro J., Cabus M., Brown R., Cook A., Teow E., Andersen H., Lewis M.G., Lauffenburger D.A., Alter G., Barouch D.H. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulien I., Kemming J., Oberhardt V., Wild K., Seidel L.M., Killmer S., Sagar F.Daul, Lago M.Salvat, Decker A., Luxenburger H., Binder B., Bettinger D., Sogukpinar O., Rieg S., Panning M., Huzly D., Schwemmle M., Kochs G., Waller C.F., Nieters A., Duerschmied D., Emmerich F., Mei H.E., Schulz A.R., Llewellyn-Lacey S., Price D.A., Boettler T., Bengsch B., Thimme R., Hofmann M., Neumann-Haefelin C. Characterization of pre-existing and induced SARS-CoV-2-specific CD8+ T cells. Nat. Med. 2021;27:78–85. doi: 10.1038/s41591-020-01143-2. [DOI] [PubMed] [Google Scholar]
- 19.Ewer K.J., Barrett J.R., Belij-Rammerstorfer S., Sharpe H., Makinson R., Morter R., Flaxman A., Wright D., Bellamy D., Bittaye M., Dold C., Provine N.M., Aboagye J., Fowler J., Silk S.E., Alderson J., Aley P.K., Angus B., Berrie E., Bibi S., Cicconi P., Clutterbuck E.A., Chelysheva I., Folegatti P.M., Fuskova M., Green C.M., Jenkin D., Kerridge S., Lawrie A., Minassian A.M., Moore M., Mujadidi Y., Plested E., Poulton I., Ramasamy M.N., Robinson H., Song R., Snape M.D., Tarrant R., Voysey M., Watson M.E.E., Douglas A.D., Hill A.V.S., Gilbert S.C., Pollard A.J., Lambe T., Ali A., Allen E., Baker M., Barnes E., Borthwick N., Boyd A., Brown-O’Sullivan C., Burgoyne J., Byard N., Puig I.C., Cappuccini F., Cho J.S., Cicconi P., Clark E., Crocker W.E.M., Datoo M.S., Davies H., Dunachie S.J., Edwards N.J., Elias S.C., Furze J., Gilbride C., Harris S.A., Hodgson S.H.C., Hou M.M., Jackson S., Jones K., Kailath R., King L., Larkworthy C.W., Li Y., Lias A.M., Linder A., Lipworth S., Ramon R.L., Madhavan M., Marlow E., Marshall J.L., Mentzer A.J., Morrison H., Noé A., Pipini D., Pulido-Gomez D., Lopez F.R., Ritchie A.J., Rudiansyah I., Sanders H., Shea A., Silk S., Spencer A.J., Tanner R., Themistocleous Y., Thomas M., Tran N., Truby A., Turner C., Turner N., Ulaszewska M., Worth A.T., Kingham-Page L., Alvarez M.P.P., Anslow R., Bates L., Beadon K., Beckley R., Beveridge A., Bijker E.M., Blackwell L., Burbage J., Camara S., Carr M., Colin-Jones R., Cooper R., Cunningham C.J., Demissie T., Maso C.Di, Douglas N., Drake-Brockman R., Drury R.E., Emary K.R.W., Felle S., Feng S., Ford K.J., Francis E., Gracie L., Hamlyn J., Hanumunthadu B., Harrison D., Hart T.C., Hawkins S., Hill J., Howe E., Howell N., Jones E., Keen J., Kelly S., Kerr D., Khan L., Kinch J., Koleva S., Lees E.A., Lelliott A., Liu X., Marinou S., McEwan J., Morey E., Morshead G., Muller J., Munro C., Murphy S., Mweu P., Nuthall E., O’Brien K., O’Connor D., O’Reilly P.J., Oguti B., Osborne P., Owino N., Parker K., Pfafferott K., Provstgaard-Morys S., Ratcliffe H., Rawlinson T., Rhead S., Roberts H., Sanders K., Silva-Reyes L., Smith C.C., Smith D.J., Szigeti A., Thomas T.M., Thompson A., Tonks S., Varughes R., Vichos I., Walker L., White C., White R., Yao X.L., Conlon C.P., Frater J., Cifuentes L., Baleanu I., Bolam E., Boland E., Brenner T., Damratoski B.E., Datta C., Muhanna O.El, Fisher R., Galian-Rubio P., Hodges G., Jackson F., Liu S., Loew L., Morgans R., Morris S.J., Olchawski V., Oliveria C., Parracho H., Pabon E.R., Tahiri-Alaoui A., Taylor K., Williams P., Zizi D., Arbe-Barnes E.H., Baker P., Batten A., Downing C., Drake J., English M.R., Henry J.A., Iveson P., Killen A., King T.B., Larwood J.P.J., Mallett G., Mansatta K., Mirtorabi N., Patrick-Smith M., Perring J., Radia K., Roche S., Schofield E., Naude R.te W., Towner J., Baker N., Bewley K.R., Brunt E., Buttigieg K.R., Charlton S., Coombes N.S., Elmore M.J., Godwin K., Hallis B., Knott D., McInroy L., Shaik I., Thomas K., Tree J.A., Blundell C.L., Cao M., Kelly D., Skelly D.T., Themistocleous A., Dong T., Field S., Hamilton E., Kelly E., Klenerman P., Knight J.C., Lie Y., Petropoulos C., Sedik C., Wrin T., Meddaugh G., Peng Y., Screaton G., Stafford E. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021;27:270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 20.Nelde A., Bilich T., Heitmann J.S., Maringer Y., Salih H.R., Roerden M., Lübke M., Bauer J., Rieth J., Wacker M., Peter A., Hörber S., Traenkle B., Kaiser P.D., Rothbauer U., Becker M., Junker D., Krause G., Strengert M., Schneiderhan-Marra N., Templin M.F., Joos T.O., Kowalewski D.J., Stos-Zweifel V., Fehr M., Rabsteyn A., Mirakaj V., Karbach J., Jäger E., Graf M., Gruber L.C., Rachfalski D., Preuß B., Hagelstein I., Märklin M., Bakchoul T., Gouttefangeas C., Kohlbacher O., Klein R., Stevanović S., Rammensee H.G., Walz J.S. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2021;22:74–85. doi: 10.1038/s41590-020-00808-x. [DOI] [PubMed] [Google Scholar]
- 21.Shu Y., McCauley J. GISAID: global initiative on sharing all influenza data – from vision to reality. Eurosurveillance. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarke A., Sidney J., Kidd C.K., Dan J.M., Ramirez S.I., Yu E.D., Mateus J., da Silva Antunes R., Moore E., Rubiro P., Methot N., Phillips E., Mallal S., Frazier A., Rawlings S.A., Greenbaum J.A., Peters B., Smith D.M., Crotty S., Weiskopf D., Grifoni A., Sette A. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med. 2021;2 doi: 10.1016/j.xcrm.2021.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saini S.K., Hersby D.S., Tamhane T., Povlsen H.R., Amaya Hernandez S.P., Nielsen M., Gang A.O., Hadrup S.R. SARS-CoV-2 genome-wide T cell epitope mapping reveals immunodominance and substantial CD8+ T cell activation in COVID-19 patients. Sci. Immunol. 2021;6 doi: 10.1126/sciimmunol.abf7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quadeer A.A., Ahmed S.F., McKay M.R. Landscape of epitopes targeted by T cells in 852 individuals recovered from COVID-19: meta-analysis, immunoprevalence, and web platform. Cell Rep. Med. 2021;2 doi: 10.1016/j.xcrm.2021.100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickett B.E., Sadat E.L., Zhang Y., Noronha J.M., Squires R.B., Hunt V., Liu M., Kumar S., Zaremba S., Gu Z., Zhou L., Larson C.N., Dietrich J., Klem E.B., Scheuermann R.H. ViPR: an open bioinformatics database and analysis resource for virology research. Nucleic Acids Res. 2012;40 doi: 10.1093/nar/gkr859. D593-D598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynisson B., Alvarez B., Paul S., Peters B., Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2021;48 doi: 10.1093/NAR/GKAA379. W449-W454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Southwood S., Sidney J., Kondo A., del Guercio M.F., Appella E., Hoffman S., Kubo R.T., Chesnut R.W., Grey H.M., Sette A. Several common HLA-DR types share largely overlapping peptide binding repertoires. J. Immunol. 1998;160:3363–3373. http://www.ncbi.nlm.nih.gov/pubmed/9531296 [PubMed] [Google Scholar]
- 28.Fleri W., Paul S., Dhanda S.K., Mahajan S., Xu X., Peters B., Sette A. The immune epitope database and analysis resource in epitope discovery and synthetic vaccine design. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geers D., Shamier M.C., Bogers S., den Hartog G., Gommers L., Nieuwkoop N.N., Schmitz K.S., Rijsbergen L.C., van Osch J.A.T., Dijkhuizen E., Smits G., Comvalius A., van Mourik D., Caniels T.G., van Gils M.J., Sanders R.W., Oude Munnink B.B., Molenkamp R., de Jager H.J., Haagmans B.L., de Swart R.L., Koopmans M.P.G., van Binnendijk R.S., de Vries R.D., GeurtsvanKessel C.H. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarke A., Sidney J., Methot N., Yu E.D., Zhang Y., Dan J.M., Goodwin B., Rubiro P., Sutherland A., Wang E., Frazier A., Ramirez S.I., Rawlings S.A., Smith D.M., da Silva Antunes R., Peters B., Scheuermann R.H., Weiskopf D., Crotty S., Grifoni A., Sette A. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021;2 doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucas C., Vogels C., Yildirim I. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity. Nature. 2021 doi: 10.1038/s41586-021-04085-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni L., Ye F., Cheng M.L., Feng Y., Deng Y.Q., Zhao H., Wei P., Ge J., Gou M., Li X., Sun L., Cao T., Wang P., Zhou C., Zhang R., Liang P., Guo H., Wang X., Qin C.F., Chen F., Dong C. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977. doi: 10.1016/j.immuni.2020.04.023. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasan A., Al-Ozairi E., Al-Baqsumi Z., Ahmad R., Al-Mulla F. Cellular and humoral immune responses in Covid-19 and immunotherapeutic approaches. ImmunoTargets Ther. 2021;10:63–85. doi: 10.2147/itt.s280706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cremoni M., Ruetsch C., Zorzi K., Fernandez C., Boyer-Suavet S., Benzaken S., Demonchy E., Dellamonica J., Ichai C., Esnault V., Brglez V., Seitz-Polski B. Humoral and cellular response of frontline health care workers infected by SARS-CoV-2 in Nice, France: a prospective single-center cohort study. Front. Med. 2021;7 doi: 10.3389/fmed.2020.608804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J., Chandrashekar A., Sellers D., Barrett J., Jacob-Dolan C., Lifton M., McMahan K., Sciacca M., VanWyk H., Wu C., Yu J., Collier A.Y., Barouch D.H. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 omicron. Nature. 2022 doi: 10.1038/s41586-022-04465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keeton R., Tincho M.B., Ngomti A., Baguma R., Benede N., Suzuki A., Khan K., Cele S., Bernstein M., Karim F., Madzorera S.V., Moyo-Gwete T., Mennen M., Skelem S., Adriaanse M., Mutithu D., Aremu O., Stek C., du Bruyn E., Van Der Mescht M.A., de Beer Z., de Villiers T.R., Bodenstein A., van den Berg G., Mendes A., Strydom A., Venter M., Giandhari J., Naidoo Y., Pillay S., Tegally H., Grifoni A., Weiskopf D., Sette A., Wilkinson R.J., de Oliveira T., Bekker L.G., Gray G., Ueckermann V., Rossouw T., Boswell M.T., Bihman J., Moore P.L., Sigal A., Ntusi N.A.B., Burgers W.A., Riou C. T cell responses to SARS-CoV-2 spike cross-recognize omicron. Nature. 2022 doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agerer B., Koblischke M., Gudipati V., Montaño-Gutierrez L.F., Smyth M., Popa A., Genger J.W., Endler L., Florian D.M., Mühlgrabner V., Graninger M., Aberle S.W., Husa A.M., Shaw L.E., Lercher A., Gattinger P., Torralba-Gombau R., Trapin D., Penz T., Barreca D., Fae I., Wenda S., Traugott M., Walder G., Pickl W.F., Thiel V., Allerberger F., Stockinger H., Puchhammer-Stöckl E., Weninger W., Fischer G., Hoepler W., Pawelka E., Zoufaly A., Valenta R., Bock C., Paster W., Geyeregger R., Farlik M., Halbritter F., Huppa J.B., Aberle J.H., Bergthaler A. SARS-CoV-2 mutations in MHC-I-restricted epitopes evade CD8+ T cell responses. Sci. Immunol. 2021;6 doi: 10.1126/SCIIMMUNOL.ABG6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motozono C., Toyoda M., Zahradnik J., Saito A., Nasser H., Tan T.S., Ngare I., Kimura I., Uriu K., Kosugi Y., Yue Y., Shimizu R., Ito J., Torii S., Yonekawa A., Shimono N., Nagasaki Y., Minami R., Toya T., Sekiya N., Fukuhara T., Matsuura Y., Schreiber G., Ikeda T., Nakagawa S., Ueno T., Sato K. SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. 2021;29:1124–1136. doi: 10.1016/j.chom.2021.06.006. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Silva T.I., Liu G., Lindsey B.B., Dong D., Moore S.C., Hsu N.S., Shah D., Wellington D., Mentzer A.J., Angyal A., Brown R., Parker M.D., Ying Z., Yao X., Turtle L., Dunachie S., Maini M.K., Ogg G., Knight J.C., Peng Y., Rowland-Jones S.L., Dong T., Aanensen D.M., Abudahab K., Adams H., Adams A., Afifi S., Aggarwal D., Ahmad S.S.Y., Aigrain L., Alcolea-Medina A., Alikhan N.-F., Allara E., Amato R., Annett T., Aplin S., Ariani C.V., Asad H., Ash A., Ashfield P., Ashford F., Atkinson L., Attwood S.W., Auckland C., Aydin A., Baker D.J., Baker P., Balcazar C.E., Ball J., Barrett J.C., Barrow M., Barton E., Bashton M., Bassett A.R., Batra R., Baxter C., Bayzid N., Beaver C., Beckett A.H., Beckwith S.M., Bedford L., Beer R., Beggs A., Bellis K.L., Berry L., Bertolusso B., Best A., Betteridge E., Bibby D., Bicknell K., Binns D., Birchley A., Bird P.W., Bishop C., Blacow R., Blakey V., Blane B., Bolt F., Bonfield J., Bonner S., Bonsall D., Boswell T., Bosworth A., Bourgeois Y., Boyd O., Bradley D.T., Breen C., Bresner C., Breuer J., Bridgett S., Bronner I.F., Brooks E., Broos A., Brown J.R., Bucca G., Buchan S.L., Buck D., Bull M., Burns P.J., Burton-Fanning S., Byaruhanga T., Byott M., Campbell S., Carabelli A.M., Cargill J.S., Carlile M., Carvalho S.F., Casey A., Castigador A., Catalan J., Chalker V., Chaloner N.J., Chand M., Chappell J.G., Charalampous T., Chatterton W., Chaudhry Y., Churcher C.M., Clark G., Clarke P., Cogger B.J., Cole K., Collins J., Colquhoun R., Connor T.R., Cook K.F., Coombes J., Corden S., Cormie C., Cortes N., Cotic M., Cotton S., Cottrell S., Coupland L., Cox M., Cox A., Craine N., Crawford L., Cross A., Crown M.R., Crudgington D., Cumley N., Curran T., Curran M.D., da Silva Filipe A., Dabrera G., Darby A.C., Davidson R.K., Davies A., Davies R.M., Davis T., de Angelis D., Lacy E.De, de Oliveira Martins L., Debebe J., Denton-Smith R., Dervisevic S., Dewar R., Dey J., Dias J., Dobie D., Dorman M.J., Downing F., Driscoll M., du Plessis L., Duckworth N., Durham J., Eastick K., Easton L.J., Eccles R., Edgeworth J., Edwards S., Bouzidi K.El, Eldirdiri S., Ellaby N., Elliott S., Eltringham G., Ensell L., Erkiert M.J., Zamudio M.E., Essex S., Evans J.M., Evans C., Everson W., Fairley D.J., Fallon K., Fanaie A., Farr B.W., Fearn C., Feltwell T., Ferguson L., Fina L., Flaviani F., Fleming V.M., Forrest S., Foster-Nyarko E., Foulkes B.H., Foulser L., Fragakis M., Frampton D., Francois S., Fraser C., Freeman T.M., Fryer H., Fuchs M., Fuller W., Gajee K., Galai K., Gallagher A., Gallagher E., Gallagher M.D., Gallis M., Gaskin A., Gatica-Wilcox B., Geidelberg L., Gemmell M., Georgana I., George R.P., Gifford L., Gilbert L., Girgis S.T., Glaysher S., Goldstein E.J., Golubchik T., Gomes A.N., Gonçalves S., Goodfellow I.G., Goodwin S., Goudarzi S., Gourtovaia M., Graham C., Graham L., Grant P.R., Green L.R., Green A., Greenaway J., Gregory R., Guest M., Gunson R.N., Gupta R.K., Gutierrez B., Haldenby S.T., Hamilton W.L., Hansford S.E., Haque T., Harris K.A., Harrison I., Harrison E.M., Hart J., Hartley J.A., Harvey W.T., Harvey M., Hassan-Ibrahim M.O., Heaney J., Helmer T., Henderson J.H., Hesketh A.R., Hey J., Heyburn D., Higginson E.E., Hill V., Hill J.D., Hilson R.A., Hilvers E., Holden M.T.G., Hollis A., Holmes C.W., Holmes N., Holmes A.H., Hopes R., Hornsby H.R., Hosmillo M., Houlihan C., Howson-Wells H.C., Hubb J., Huckson H., Hughes W., Hughes J., Hughes M., Hutchings S., Idle G., Illingworth C.J., Impey R., Irish-Tavares D., Iturriza-Gomara M., Izuagbe R., Jackson C., Jackson B., Jackson L.M., Jackson K.A., Jackson D.K., Jahun A.S., James V., James K., Jeanes C., Jeffries A.R., Jeremiah S., Jermy A., John M., Johnson R., Johnson K., Johnston I., Jones O., Jones S., Jones H., Jones C.R., Jones N., Joseph A., Judges S., Kay G.L., Kay S., Keatley J.-P., Keeley A.J., Kenyon A., Kermack L.M., Khakh M., Kidd S.P., Kimuli M., Kirk S., Kitchen C., Kitchman K., Knight B.A., Koshy C., Kraemer M.U.G., Kumziene-Summerhayes S., Kwiatkowski D., Lackenby A., Laing K.G., Lampejo T., Langford C.F., Lavin D., Lawton A.I., Lee J., Lee D., Lensing S.V., Leonard S., Levett L.J., Le-Viet T., Lewis J., Lewis K., Liddle J., Liggett S., Lillie P.J., Lister M.M., Livett R., Lo S., Loman N.J., Loose M.W., Louka S.F., Loveson K.F., Lowdon S., Lowe H., Lowe H.L., Lucaci A.O., Ludden C., Lynch J., Lyons R.A., Lythgoe K., Machin N.W., MacIntyre-Cockett G., Mack A., Macklin B., Maclean A., Macnaughton E., Madona P., Maes M., Maftei L., Mahanama A.I.K., Mahungu T.W., Mair D., Maksimovic J., Malone C.S., Maloney D., Manesis N., Manley R., Mantzouratou A., Marchbank A., Mariappan A., Martincorena I., Nunez R.T.Martinez, Mather A.E., Maxwell P., Mayhew M., Mbisa T., McCann C.M., McCarthy S.A., McCluggage K., McClure P.C., McCrone J.T., McHugh M.P., McKenna J.P., McKerr C., McManus G.M., McMurray C.L., McMurray C., McNally A., Meadows L., Medd N., Megram O., Menegazzo M., Merrick I., Michell S.L., Michelsen M.L., Mirfenderesky M., Mirza J., Miskelly J., Moles-Garcia E., Moll R.J., Molnar Z., Monahan I.M., Mondani M., Mookerjee S., Moore C., Moore J., Moore N., Moore C., Morcrette H., Morgan S., Morgan M., Mori M., Morriss A., Moses S., Mower C., Muir P., Mukaddas A., Munemo F., Munn R., Murray A., Murray L.J., Murray D.R., Mutingwende M., Myers R., Nastouli E., Nebbia G., Nelson A., Nelson C., Nicholls S., Nichols J., Nicodemi R., Nomikou K., O’Grady J., O’Brien S., Odedra M., Ohemeng-Kumi N., Oliver K., Orton R.J., Osman H., Pacchiarini N., Padgett D., Page A.J., Park E.J., Park N.R., Parmar S., Partridge D.G., Pascall D., Patel A., Patel B., Paterson S., Payne B.A.I., Peacock S.J., Pearson C., Pelosi E., Percival B., Perkins J., Perry M., Pinckert M.L., Platt S., Podplomyk O., Pohare M., Pond M., Pope C.F., Poplawski R., Powell J., Poyner J., Prestwood L., Price A., Price J.R., Prieto J.A., Pritchard D.T., Prosolek S.J., Pugh G., Pusok M., Pybus O.G., Pymont H.M., Quail M.A., Quick J., Radulescu C., Raghwani J., Ragonnet-Cronin M., Rainbow L., Rajan D., Rajatileka S., Ramadan N.A., Rambaut A., Ramble J., Randell P.A., Randell P., Ratcliffe L., Raviprakash V., Raza M., Redshaw N.M., Rey S., Reynolds N., Richter A., Robertson D.L., Robinson E., Robson S.C., Rogan F., Rooke S., Rowe W., Roy S., Rudder S., Ruis C., Rushton S., Ryan F., Saeed K., Samaraweera B., Sambles C.M., Sanderson R., Sanderson T., Sang F., Sass T., Scher E., Scott G., Scott C., Sehmi J., Shaaban S., Shah D., Shaw J., Shelest E., Shepherd J.G., Sheridan L.A., Sheriff N., Shirley L., Sillitoe J., Silviera S., Simpson D.A., Singh A., Singleton D., Skvortsov T., Sloan T.J., Sluga G., Smith K., Smith K.S., Smith P., O’Toole Xeine, Smith D.L., Smith L., Smith C.P., Smith N., Smollett K.L., Snell L.B., Somassa T., Southgate J., Spellman K., Chapman M.H.Spencer, Spurgin L.G., Spyer M.J., Stanley R., Stanley W., Stanton T.D., Starinskij I., Stockton J., Stonehouse S., Storey N., Studholme D.J., Sudhanva M., Swindells E., Taha Y., Tan N.K., Tang J.W., Tang M., Taylor B.E.W., Taylor J.F., Taylor S., Temperton B., Templeton K.E., Thomas C., Thomson L., Thomson E.C., Thornton A., Thurston S.A.J., Todd J.A., Tomb R., Tong L., Tonkin-Hill G., Torok M.E., Tovar-Corona J.M., Trebes A., Trotter A.J., Tsatsani I., Turnbull R., Twohig K.A., Umpleby H., Underwood A.P., Vamos E.E., Vasylyeva T.I., Vattipally S., Vernet G., Vipond B.B., Volz E.M., Walsh S., Wang D., Warne B., Warwick-Dugdale J., Wastnedge E., Watkins J., Watson L.K., Waugh S., Webster H.J., Weldon D., Westwick E., Whalley T., Wheeler H., Whitehead M., Whiteley M., Whitwham A., Wierzbicki C., Willford N.J., Williams L.-A., Williams R., Williams C., Williams C., Williams C.A., Williams R.J., Williams T., Williams C., Williamson K.A., Wilson-Davies E., Witele E., Withell K.T., Witney A.A., Wolverson P., Wong N., Workman T., Wright V., Wright D.W., Wyatt T., Wyllie S., Xu-McCrae L., Yavus M., Yaze G., Yeats C.A., Yebra G., Yew W.C., Young G.R., Young J., Zarebski A.E., Zhang P., Baillie J.K., Semple M.G., Openshaw P.J.M., Carson G., Alex B., Andrikopoulos P., Bach B., Barclay W.S., Bogaert D., Chand M., Chechi K., Cooke G.S., da Silva Filipe A., Docherty A.B., Dumas M.-E., Dunning J., Fletcher T., Green C.A., Greenhalf W., Griffin J.L., Gupta R.K., Harrison E.M., Hiscox J.A., Ho A.Y.Wai, Horby P.W., Ijaz S., Khoo S., Klenerman P., Law A., Lewis M.R., Liggi S., Lim W.S., Maslen L., Mentzer A.J., Merson L., Meynert A.M., Noursadeghi M., Olanipekun M., Osagie A., Palmarini M., Palmieri C., Paxton W.A., Pollakis G., Price N., Rambaut A., Robertson D.L., Russell C.D., Sancho-Shimizu V., Sands C.J., Scott J.T., Sigfrid L., Solomon T., Sriskandan S., Stuart D., Summers C., Swann O.V., Takats Z., Takis P., Tedder R.S., Thompson A.A.R., Thomson E.C., Thwaites R.S., Zambon M., Hardwick H., Donohue C., Griffiths F., Oosthuyzen W., Donegan C., Spencer R.G., Dalton J., Girvan M., Saviciute E., Roberts S., Harrison J., Marsh L., Connor M., Halpin S., Jackson C., Gamble C., Plotkin D., Lee J., Leeming G., Law A., Wham M., Clohisey S., Hendry R., Scott-Brown J., Shaw V., McDonald S.E., Keating S., Ahmed K.A., Armstrong J.A., Ashworth M., Asiimwe I.G., Bakshi S., Barlow S.L., Booth L., Brennan B., Bullock K., Catterall B.W.A., Clark J.J., Clarke E.A., Cole S., Cooper L., Cox H., Davis C., Dincarslan O., Dunn C., Dyer P., Elliott A., Evans A., Finch L., Fisher L.W.S., Foster T., Garcia-Dorival I., Gunning P., Hartley C., Jensen R.L., Jones C.B., Jones T.R., Khandaker S., King K., Kiy R.T., Koukorava C., Lake A., Lant S., Latawiec D., Lavelle-Langham L., Lefteri D., Lett L., Livoti L.A., Mancini M., McDonald S., McEvoy L., McLauchlan J., Metelmann S., Miah N.S., Middleton J., Mitchell J., Moore S.C., Murphy E.G., Penrice-Randal R., Pilgrim J., Prince T., Reynolds W., Ridley P.M., Sales D., Shaw V.E., Shears R.K., Small B., Subramaniam K.S., Szemiel A., Taggart A., Tanianis-Hughes J., Thomas J., Trochu E., van Tonder L., Wilcock E., Zhang J.E., Flaherty L., Maziere N., Cass E., Carracedo A.D., Carlucci N., Holmes A., Massey H., Murphy L., Wrobel N., McCafferty S., Morrice K., MacLean A., Adeniji K., Agranoff D., Agwuh K., Ail D., Aldera E.L., Alegria A., Allen S., Angus B., Ashish A., Atkinson D., Bari S., Barlow G., Barnass S., Barrett N., Bassford C., Basude S., Baxter D., Beadsworth M., Bernatoniene J., Berridge J., Berry C., Best N., Bothma P., Chadwick D., Brittain-Long R., Bulteel N., Burden T., Burtenshaw A., Caruth V., Chadwick D., Chambler D., Chee N., Child J., Chukkambotla S., Clark T., Collini P., Cosgrove C., Cupitt J., Cutino-Moguel M.-T., Dark P., Dawson C., Dervisevic S., Donnison P., Douthwaite S., Drummond A., DuRand I., Dushianthan A., Dyer T., Evans C., Eziefula C., Fegan C., Finn A., Fullerton D., Garg S., Garg S., Garg A., Gkrania-Klotsas E., Godden J., Goldsmith A., Graham C., Hardy E., Hartshorn S., Harvey D., Havalda P., Hawcutt D.B., Hobrok M., Hodgson L., Hormis A., Jacobs M., Jain S., Jennings P., Kaliappan A., Kasipandian V., Kegg S., Kelsey M., Kendall J., Kerrison C., Kerslake I., Koch O., Koduri G., Koshy G., Laha S., Laird S., Larkin S., Leiner T., Lillie P., Limb J., Linnett V., Little J., Lyttle M., MacMahon M., MacNaughton E., Mankregod R., Masson H., Matovu E., McCullough K., McEwen R., Meda M., Mills G., Minton J., Mirfenderesky M., Mohandas K., Mok Q., Moon J., Moore E., Morgan P., Morris C., Mortimore K., Moses S., Mpenge M., Mulla R., Murphy M., Nagel M., Nagarajan T., Nelson M., Norris L., O’Shea M.K., Otahal I., Ostermann M., Pais M., Palmieri C., Panchatsharam S., Papakonstantinou D., Paraiso H., Patel B., Pattison N., Pepperell J., Peters M., Phull M., Pintus S., Pooni J.S., Planche T., Post F., Price D., Prout R., Rae N., Reschreiter H., Reynolds T., Richardson N., Roberts M., Roberts D., Rose A., Rousseau G., Ruge B., Ryan B., Saluja T., Schmid M.L., Shah A., Shanmuga P., Sharma A., Shawcross A., Sizer J., Shankar-Hari M., Smith R., Snelson C., Spittle N., Staines N., Stambach T., Stewart R., Subudhi P., Szakmany T., Tatham K., Thomas J., Thompson C., Thompson R., Tridente A., Tupper-Carey D., Twagira M., Vallotton N., Vancheeswaran R., Vincent-Smith L., Visuvanathan S., Vuylsteke A., Waddy S., Wake R., Walden A., Welters I., Whitehouse T., Whittaker P., Whittington A., Papineni P., Wijesinghe M., Williams M., Wilson L., Cole S., Winchester S., Wiselka M., Wolverson A., Wootton D.G., Workman A., Yates B., Young P., Correia G.dos S. The impact of viral mutations on recognition by SARS-CoV-2 specific T cells. IScience. 2021;24 doi: 10.1016/j.isci.2021.103353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H., Deng S., Ren L., Zheng P., Hu X., Jin T., Tan X. Profiling CD8+ T cell epitopes of COVID-19 convalescents reveals reduced cellular immune responses to SARS-CoV-2 variants. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Native spike protein CD4 T cell epitopes.
Native spike protein CD8 T cell epitopes.
Conservation analysis of S protein T cell epitope pools of B.1.1.7 variant sequences.
Mutated epitopes searching algorithm.
Reference allele sets for MHC-I and MHC-II.
Supplementary material 3
Supplementary material 4
Acknowledgement for SARS-COV-2 variants genome sequences sourced from GISAID.
Supplementary material 6