Abstract
Background
Some, but not all, large-scale randomized controlled trials (RCTs) investigating the effects of marine omega-3 fatty acids supplementation on cardiovascular outcomes have reported increased risks of atrial fibrillation (AF). The potential reasons for disparate findings may be dose related.
Methods
The MEDLINE and Embase databases were searched for articles and abstracts published between January 1, 2012 and December 31, 2020 in addition to a meta-analysis of large cardiovascular RCTs published in 2019. RCTs of cardiovascular outcomes of marine omega-3 fatty acids that reported results for AF, either as pre-specified outcome, adverse event, or a cause for hospitalization, with a minimum sample size of 500 patients and a median follow-up of at least one year were included. RCTs specifically examining shorter term effects of omega-3 fatty acids on recurrent AF in patients with established AF or post-operative AF were not included. The hazard ratio (HR) for the reported AF outcomes within each trial was meta-analyzed using random-effects model with Knapp-Hartung adjustment and evaluated a dose-response relationship with a meta-regression model.
Results
Of 4049 screened records, seven studies were included in the meta-analysis. Of those, five were already detected in a previous meta-analysis of cardiovascular RCTs. Among the 81,210 patients from 7 trials, 58,939 (72.6%) were enrolled in trials testing ≤1gram per day (g/d) and 22,271 (27.4%) in trials testing >1g/d of omega-3 fatty acids. The mean age was 65 years and 31,842 (39%) were female. The weighted average follow-up was 4.9 years. In meta-analysis, the use of marine omega-3 fatty acid supplements was associated with an increased risk of AF (n=2,905; HR 1.25, 95%CI 1.07–1.46, P=0.013). In analyses stratified by dose, the HR was greater in the trials testing >1g/d (HR 1.49, 95%CI 1.04–2.15, P=0.042) as compared with those testing ≤1 g/d (HR 1.12, 95%CI 1.03–1.22, P=0.024, P for interaction<0.001). In meta-regression, the HR for AF increased per 1 gr increase of omega-3 fatty acids dosage (HR 1.11, 95%CI 1.06–1.15, P=0.001).
Conclusion
In RCTs examining cardiovascular outcomes, marine omega-3 supplementation was associated with an increased risk of AF. The risk appeared to be greater in trials testing >1g/d.
Keywords: fatty acids, clinical trials, meta-analysis, atrial fibrillation, omega-3 fatty acid supplements
Brief Summary
Marine omega-3 fatty acid supplementation is associated with an elevated risk of AF in meta-analysis of RCTs involving 81,210 patients.
Introduction
Marine omega-3 fatty acids supplements may have a beneficial effect on the risk of atherosclerotic cardiovascular events 1–4; however, concerns have also been raised regarding potential off target adverse effects on atrial fibrillation (AF) risk within these trials. The Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) reported a decrease of 25% in major cardiovascular events with the use of 4 g of icosapent ethyl over a median follow-up of 4.9 years.2 Based on these results, the 2019 European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) of dyslipidemia guidelines recommended the use of 4g of icosapent ethyl in patients with established cardiovascular disease with triglycerides between 135–499 mg despite statin treatment.5 However, REDUCE-IT also reported an increase in a pre-specified tertiary outcome of AF hospitalization in those randomized to active treatment as compared with placebo (3.1% vs. 2.1%, p= 0.004).2 Subsequently, several randomized controlled trials (RCTs) of marine omega-3 fatty acid supplementation have reported results for AF, but AF case numbers have generally been small.6, 7 The study with the largest number of AF events, the VITAL Rhythm Study, did not find a significant increased risk of incident AF with 1gram per day of marine omega-3 fatty acids (460 mg of eicosapentaenoic acid [EPA] and 380 mg of docosahexaenoic acid [DHA]) compared with placebo (HR 1.09, 95%CI 0.96–1.24, P=0.19).8 In light of these conflicting findings, there is a need to summarize the overall effect of marine omega-3 fatty acid supplements on AF across these studies and explore whether marine omega-3 fatty acid dose might account for the seemingly disparate results.
Methods
Selection criteria and search strategy
This meta-analysis was registered to PROSPERO (CRD42021234291). The PRISMA guidelines was followed for this systematic review and meta-analysis.9 All the data from the original articles that were extracted for this meta-analysis are publicly available. The authors declare that all supporting data are available within the article and its online supplementary files. BG and OTA searched MEDLINE and Embase for all randomized, controlled, double-blind, cardiovascular outcome trials of marine omega-3 fatty acid supplements of more than 500 patients with a minimum follow-up of at least one-year 1, 10 that reported AF events as a primary, secondary, exploratory or safety (adverse events or cause of hospitalization) outcomes. Trials that specifically tested the effect of omega-3 fatty acids on post-operative AF or recurrent AF in patients with established AF were excluded since these hypotheses regarding potential short-term antiarrhythmic effects of omega-3 fatty acids had been addressed in previous meta-analyses.11 12. Based upon the results of these trials, marine omega-3 supplements are not recommended for these indications.5, 13.
The search was began in 2012 after the last systemic review on RCTs specifically examining omega-3 fatty acids and atrial fibrillation11. Records were searched between January 1, 2012 and December 31, 2020, without any language restrictions. Data on available AF outcomes were also extracted from original trials that were screened in a previous meta-analysis of randomized controlled trials of marine omega-3 fatty acids and atherosclerotic cardiovascular outcomes.1 The VITAL Rhythm trial that was presented at the American Heart Association annual meeting in 2020 and published in 2021 by our group was also included.8 BG and OTA screened titles, abstracts and full text of papers identified on this search and assessed the risk of bias using the Cochrane tool. BG and OTA extracted the data for eligible studies using a standardized data form for aggregated study-level and discrepancies were resolved by consensus. Because this meta-analysis was based on data extracted from previously published research, the data and study materials are available in the public domain. For further details on the algorithm used for literature search, see the Supplemental methods.
Annual event calculations
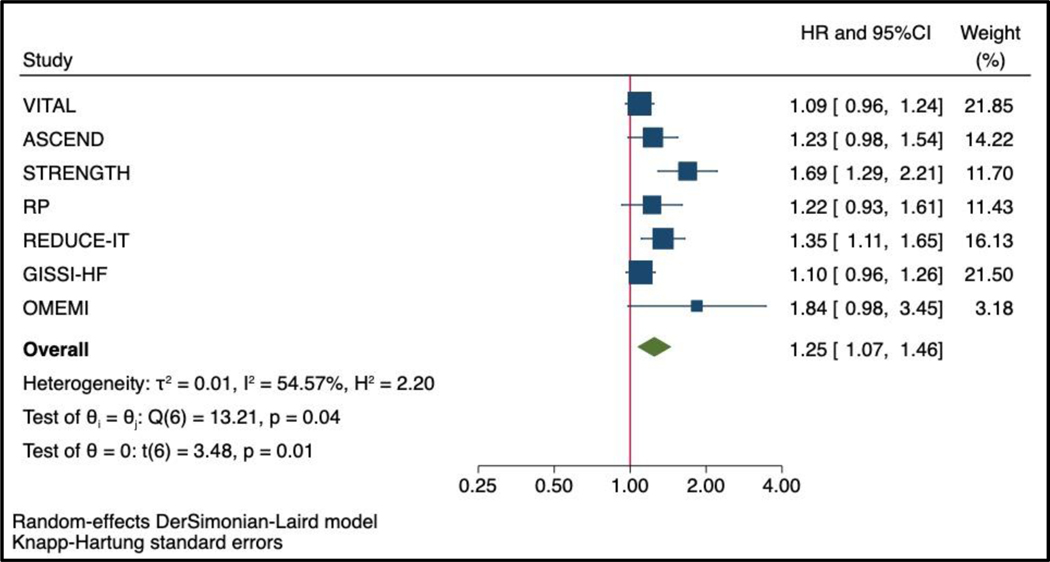
The annual event rate was estimated by dividing the total number of events by the number of persons*median follow-up duration in each of the overall trial population. In one trial (Omega-3 fatty acids in Elderly with Myocardial Infarction [OMEMI]), the median follow-up was not provided, and the maximal follow-up duration completed by 97.8% of patients was used.7 The annual rate across all included trials was estimated by weighting the annual event rate calculated in each trial according to the weight assigned in the primary random-effect model (Figure 1).14
Figure 1: Effect of marine omega-3 fatty acids supplements on the risk of AF events using Knapp-Hartung adjustment for random effect model.
Abbreviations: AF, atrial fibrillation; ASCEND, A Study of Cardiovascular Events in Diabetes; DHA, docosahexaenoic acid; HR, hazard ratio; GISSI-HF, Gruppo Italiano per lo Studio della Sopravvi- venza nell’Insufficienza Cardiaca; OMEMI, Omega-3 fatty acids in Elderly with Myocardial Infarction; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial; RP, The Risk and Prevention Study; STRENGTH, Long-Term Outcomes Study to Assess STatin Residual Risk Reduction With EpaNova in HiGh Cardiovascular Risk PatienTs With Hypertriglyceridemia; VITAL, The Vitamin D and Omega-3 Trial.
Data analysis
Outcomes from each trial were selected to target the definition of AF events used within each trial. The hazard ratio (HR) or risk ratio (RR) were extracted and 95% confidence intervals (95%CI) as reported in original articles. When the original HR or RR was not available, the RR was calculated using the cumulative incidence in each group (csi stata command). A random-effects DerSimonian-Laird meta-analysis was used for the primary analysis to account for heterogeneity across included trials that may be due to marine omega-3 fatty acids dosage, follow-up duration and study population (primary vs. secondary prevention, general population vs. elderly with myocardial infarction, and varying prevalence of preexisting AF). The Knapp-Hartung adjustment was used to account for uncertainty in the between-study variance estimate.15 The heterogeneity was assessed using Cochran’s Q statistic, and Higgins and Thompson’s I2, as well as average dispersion in effect sizes τ2. Because the Knapp-Hartung estimates is conservative when the heterogeneity is low with a small number of studies, 16, 17 a fixed effects model was used when no heterogeneity was observed (I2=0%) as a sensitivity analysis. The risk of bias was assessed according to the Cochrane tool for assessing risk of bias in randomized clinical trials. Publication bias was assessed with a funnel plot,18 as well as the trim and fill method.19 Because the test for a publication is not advised for fewer than 10 studies, Egger’s test was not performed.20
In a secondary analysis, RCTs were stratified by low dose (≤1gr per day) vs. high dose (>1gr per day) of marine omega-3 supplements.15 Exploratory analyses examining the linear association between the dosage of omega-3 fatty acids and the hazard ratio for AF were performed using meta-regression where the intercept was set at zero to reflect the clinical assumption that as the dose goes to zero, the lipid effect goes to zero and the treatment effect goes to one (neutral effect) assuming linearity.21 In a sensitivity analysis a constant term was included. The corresponding HR with 95%CI and p values per 1 gr increase in omega-3 fatty dosage acids are reported. In addition to the dose analyses, four additional sensitivity analysis stratified RCTs by whether AF was a pre-specified outcome, baseline AF was excluded, hospitalizations for AF was the only AF outcome, and DHA was included in the study intervention.1, 10 Statistical analysis was done with Stata 16.0 using the meta suite commands.
Role of the funding source
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. All authors had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Results
After screening 4,059 records (Figure I in the Supplement for the PRISMA flowchart), data from seven articles were included in the systematic review and meta-analysis. Of those, five trial were already included in a previous meta-analysis of cardiovascular RCTs.1 All trials included reported obtaining approval from the institutional review committees and that all participants gave informed consent (Table 1).2, 6–8, 22–24 Among the 81,210 patients from seven trials, 58,939 (72.6%) were enrolled in trials testing low dose marine omega 3-fatty acids (≤1 gr per day) and 22,271 (27.4%) in trials testing high dose of marine omega 3-fatty acids (>1 gr per day). Trials testing low dose of marine omega-3 fatty included the Vitamin D and OmegA-3 Trial (VITAL) study,8 A Study of Cardiovascular Events in Diabetes (ASCEND),22, 23 the Gruppo Italiano per lo Studio della Sopravvi- venza nell’Insufficienza Cardiaca (GISSI-HF),24 and the Risk and Prevention Study (RP) (Table 2).25 Trials testing high dose of marine omega-3 fatty acids were REDUCE-IT,2 Long-Term Outcomes Study to Assess STatin Residual Risk Reduction With EpaNova in HiGh Cardiovascular Risk PatienTs With Hypertriglyceridemia (STRENGTH),6 and Omega-3 fatty acids in Elderly with Myocardial Infarction (OMEMI).7 The weighted mean age was 65 years and 31,842 (39.2%) were female (Table 1). The weighted median follow-up was 4.9 years. All the trials met the criteria for low risk of bias according to the Cochrane tool for assessing risk of bias in randomized clinical trial (Table I in the Supplement).
Table 1:
Study Characteristics
| Trials | Sample Size | AF at baseline included in the analysis | Mean age, years | Female Patients, (%) | White Patients, (%) | Intervention Arm | Control Arm | Median follow-up, years | Total AF events (% per year) | Type of AF Event |
|---|---|---|---|---|---|---|---|---|---|---|
| VITAL8 | 25119 | No | 67 | 12757 (51) | 17425 (71) | 1g per day of omega-3 fatty acids (460 mg EPA and 380 mg of DHA) | Olive oil | 5.3 | 900 (0.7%) | New-onset AF |
| ASCEND * 22, 23 | 15480* | Yes | 63 | 5796 (37) | 14935 (96) | 1g per day of omega-3 fatty acids (460 mg EPA and 380 mg of DHA) | Olive oil | 7.4 | 301 (0.3%) | Main paper: patient-reported AF (without excluding preexisting AF). Research letter: New-onset AF |
| STRENGTH 6 | 13078 | Yes | 62.5 | 4568 (35) | 10723 (82) | 4g per day of omega-3 fatty acids (EPA and DHA) | Corn oil | 3.5 | 230 (0.5%) | New-onset AF |
| RP 25 | 12505 | Yes | 64 | 4818 (39) | NA | 1g per day of omega-3 fatty acids (EPA and DHA < 85% in ratio from 0.9:1 to 1.5:1) | Olive oil | 5 | 205 (0.3%) | Hospitalization for AF (without excluding preexisting AF). |
| REDUCE-IT 2 | 8179 | Yes | 64 | 2357 (29) | 7379 (90) | 4g per day of icosapent ethyl (ethyl ester of EPA) | Mineral oil | 4.9 | 374 (0.9%) | New-onset or worsening AF events (without excluding preexisting AF). |
| GISSI-HF 24 | 5835 | No | 66 | 1252 (21) | NA | 1g per day of omega-3 fatty acids (850– 882 mg of EPA DHA esters at an average ratio of 1.2:1. | Olive oil | 3.9 | 852 (3.7%) | New-onset AF |
| OMEMI 7 | 1014** | No | 75 | 294 (29) | 1012 (100) | 1.8g per day of omega-3 fatty acids (930 mg EPA and 660 mg DHA) | Corn oil | 2*** | 43 (2.1%) | New-onset AF |
| Total | 81210 | 65 | 31842 (39) | 4.9 | 2905 (1.3%) |
Patient-reported AF adverse events were extracted from the original publication of ASCEND trial. The post-hoc research letter reporting a more comprehensive assessment of AF (N=1177) based on electronic health records was used as sensitivity analysis.
The total sample size of the trial was 1014, but 759 were included in the AF analysis after excluding those with prevalent AF.
Only total duration of the trial was reported.Abbreviations: AF, atrial fibrillation; ASCEND, A Study of Cardiovascular Events in Diabetes; DHA, docosahexaenoic acid; GISSI-HF, Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca; NA, not available, OMEMI, Omega-3 fatty acids in Elderly with Myocardial Infarction; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial; RP, The Risk and Prevention Study; STRENGTH, Long-Term Outcomes Study to Assess STatin Residual Risk Reduction With EpaNova in HiGh Cardiovascular Risk PatienTs With Hypertriglyceridemia; VITAL, The Vitamin D and Omega-3 Trial.
Table 2:
Summary of AF events outcome assessment and reported treatment effect
| Study | Outcome Assessment | Reported Treatment Effect |
|---|---|---|
| VITAL8 | Incident AF cases were identified through self-reported diagnosis and linkage with claims data for the Centers for Medicare and Medicaid Services. An endpoint committee consisting of cardiologists reviewed medical records and confirmed AF events according to predefined criteria (AF events adjudicated). | 469/12542 in the experimental arm vs. 431/12577 in the control arm. Hazard ratio was 1.09 (95%CI 0.96–1.24, P=0.19). |
| ASCEND (main article) 22 | Patient-reported AF was included as an exploratory vascular outcome, but only primary and secondary outcomes of the trial were adjudicated centrally by clinicians (AF events were not adjudicated). | 166/7740 (2.1%) in the experimental arm vs. 135/7740 (1.7%) in the control arm. Rate ratio was 1.23 (95%CI 0.98–1.54). |
| ASCEND (Research Letter) 23 | More comprehensive assessment of AF events, using additional data extracted from linked electronic health records. AF events were not adjudicated. AF diagnoses in hospital episodes before randomization were used to define previously known AF. Arrhythmia outcomes considered are AF among participants without any previously known AF. | Among the remaining 99%, AF was recorded from either electronic health records or participant reports in 1177 participants, compared with 287 by self-report alone. AF occurred in 7.7% of participants in the experimental arm and in 7.6% in the placebo arm, with a nonsignificant RR of 1.02 (95% CI, 0.91 to 1.15). |
| STRENGTH 6 | New-onset investigator-reported AF was a pre-specified as tertiary outcome, but only the primary and secondary outcomes of the trial were centrally adjudicated by a core laboratory (AF events not adjudicated). | 144/6539 (2.2%) in the experimental arm vs. 86/6539 (1.3%) in the placebo arm. Hazard ratio was 1.69 (95%CI 1.29–2.21, P<0.001). |
| RP 25 | AF reported as a reason for hospital admission for cardiovascular disease, but only the primary outcome was adjudicated (AF events non-adjudicated). | 113/6239 (1.8%) in the experimental arm vs. 92/6266 (1.5%) in the placebo arm. Hazard ratio was 1.22 (95%CI 0.93–1.61, P=0.15). |
| REDUCE-IT 2 | REDUCE-IT that reported the rates for the pre-specified adjudicated tertiary outcome of hospitalization for AF or flutter. The rates were significantly higher in the icosapent ethyl group compared with the placebo group (3.1% vs. 2.1%, P=0.004). Since the required numbers to derive treatment effect were not provided in the manuscript, data were extracted for patient-reported treatment adverse AF events (AF unadjudicated) defined as new-onset or worsening AF since initiation of drug therapy. | 215/4089 (5.3%) AF events in the experimental arm vs. 159/4090 (3.9%) in the placebo am (P=0.003). The calculated risk ratio was 1.35 (95%CI 1.11–1.65, P=0.003) |
| GISSI-HF 24 | Incident AF was reported in a population without baseline AF. New AF events during the trial was defined as follows: AF in the ECGs taken at each visit during the trial, as an event occurring between visits causing or worsening HF/ hospital admission, or as an event occurring while in hospital. The adjudication was not done | 444/2921 (15.2%) new AF events in the experimental arm vs. 408/2914 (14.0%) in the control arm. Hazard ratio was 1.10 (95%CI 0.96–1.25, P=0.19). |
| OMEMI 7 | New AF was a pre-specified secondary outcome, defined as a standard 12-lead ECG recording or a single-lead ECG tracing > 30 showing no discernible repeating P waves and irregular RR intervals. The assessment for AF events was done with clinical records and ECGs taken at study visits. In addition, patients were screened with ambulant single lead rhythm monitoring for 2×30 seconds per day for 14 days All outcomes were adjudicated centrally by an independent endpoint committee of experiences clinicians, blinded to treatment allocation (AF events adjudicated) | 28/387 (7.2%) in the experimental arm vs. 15/372 (4.0%) in the placebo arm restricted in those with no previous AF. Hazard ratio was 1.84 (95%CI 0.98–3.45, P=0.06). |
Four studies reported on new-onset AF events and/or excluded patients with prevalent AF from the analysis; ASCEND trial excluded participants with established cardiovascular disease and/or those treated with anticoagulants, whereas two (REDUCE-IT and RP Study) did not specify whether events were new-onset. A post-hoc research letter from ASCEND trial reported AF events after excluding those preexisting AF at baseline.23 Five studies reported HR and 95% for AF events, whereas REDUCE-IT reported the rates for the pre-specified adjudicated tertiary outcome of hospitalization for AF or flutter and ASCEND trial reported the rate ratio of AF events. In the REDUCE-IT trial, the rates were significantly higher in the icosapent ethyl group compared with the placebo group (3.1% vs. 2.1%, P=0.004); however, the exact numbers were not provided in the original manuscript to derive the effect size. Instead, the published treatment-emergent adverse event rates, defined as an event that first occurs or worsens in severity on or after the date of dispensing study drug, were utilized for AF outcomes in the meta-analysis (215/4089 [5.3%] in the experimental arm vs. 159/4090 [3.9%] in the placebo arm, P=0.003) to calculate the RR in the REDUCE-IT trial (1.35; 95%CI 1.11–1.65, P=0.003). In the Risk and Prevention Study, AF events were listed as a reported reason for cardiovascular disease hospitalization.25 In the GISSI-HF, the analysis reporting new-onset AF events was a post-hoc analysis in an ancillary manuscript.24 In the ASCEND trial, the original publication of the main trial presented patient-reported adverse outcomes due to AF in the overall population with a rate ratio,22 whereas a post-hoc research letter used a more comprehensive review of electronic health records in patients without known AF (N=15374, 99% of the population).23 For the ASCEND trial, data from the original article were used in the primary analysis 22, and data from the research letter were included in a sensitivity analysis.23
The AF outcomes were pre-specified in the methods of the VITAL, OMEMI and STRENGTH trials. For the remaining trials, the assessment of AF events was not pre-specified in the methods of the original article. The extracted AF outcomes were centrally adjudicated by a panel of clinicians in the VITAL and OMEMI trials, whereas in the REDUCE-IT, ASCEND, STENGTH, GISSI-HF and RP trials, the AF outcomes were not.
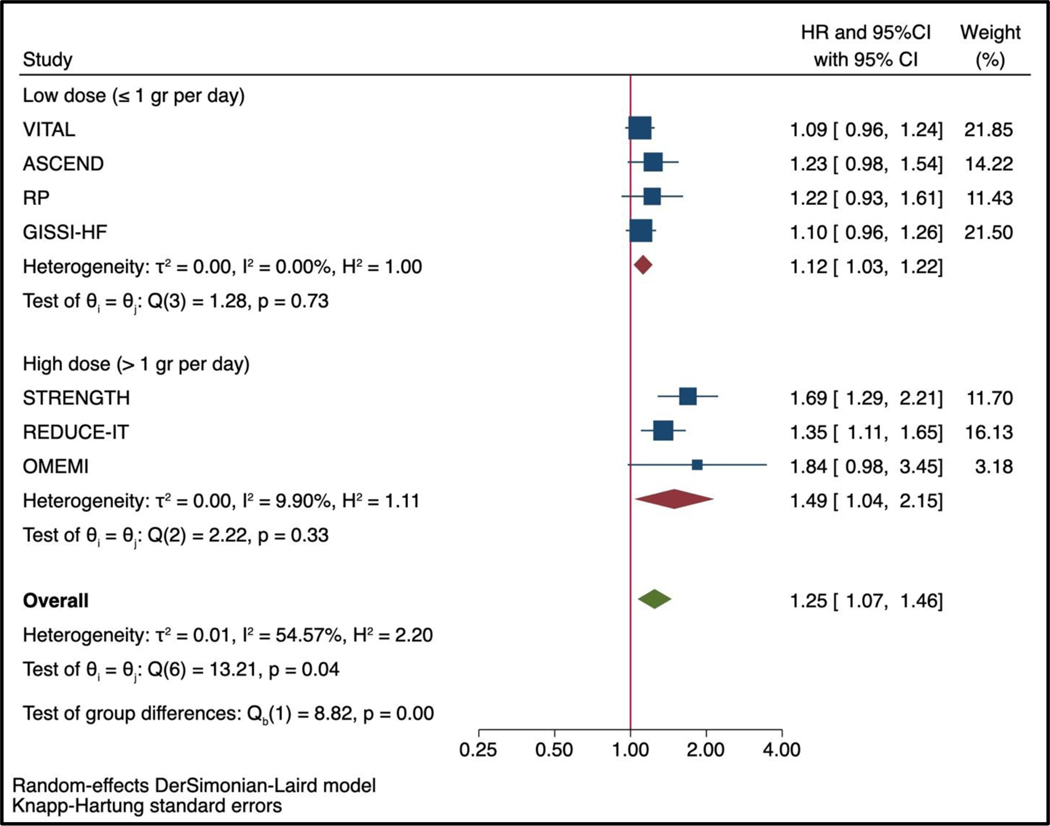
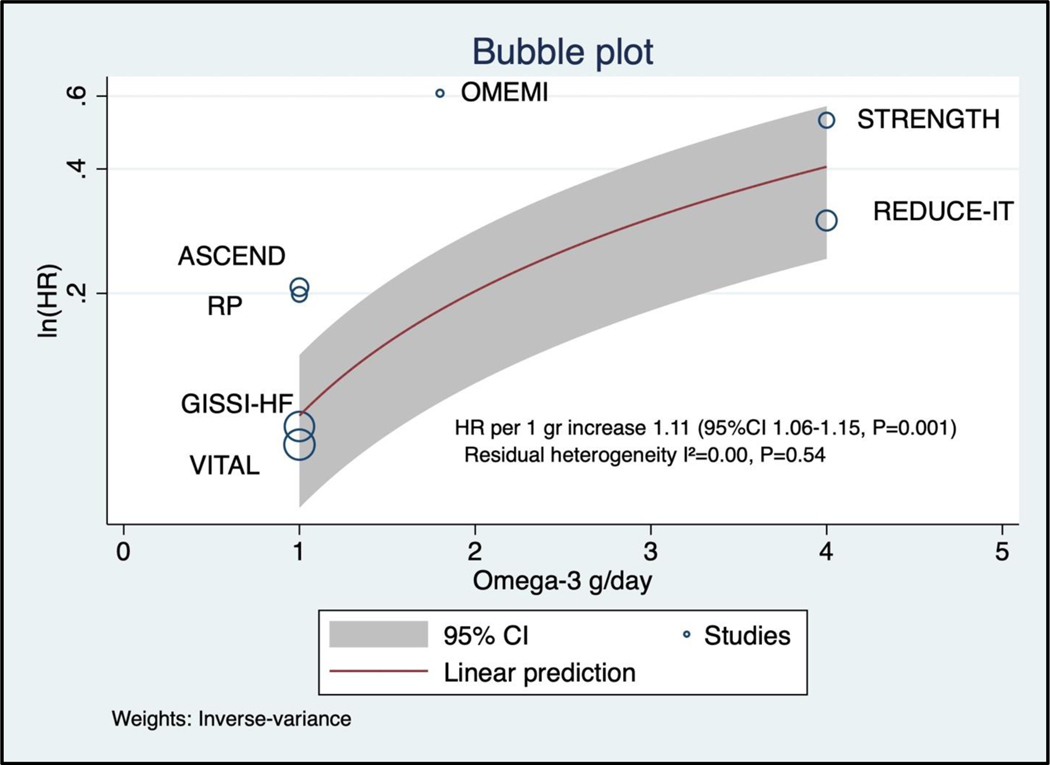
Of the 81,210 participants included in this analysis, 2,905 (3.6%) patients had an AF outcome during the trial period, of which 2,258/2,905 (77.8%) occurred in trials testing low-dose of omega-3 fatty acids supplements and 647/2,905 (22.2%) occurred in trials testing high-dose of omega-3 fatty acids. The pooled hazard ratio for the association between marine omega-3 fatty acids and AF was 1.25 (95%CI 1.07–1.46, P=0.013, Figure 1). The heterogeneity across the studies was moderate (I2= 54.57%). The heterogeneity within the groups decreased substantially in analysis stratified by dose (I2<0.01% for low-dose and I2=9.90% for high-dose marine omega-3 fatty acids). The pooled hazard ratio for AF events compared with placebo was higher in trials testing high-dose (HR 1.49, 95% 1.04–2.15, P=0.042) than in those testing low-dose of marine omega 3 fatty acids (HR 1.12, 95%CI 1.03–1.22, P=0.024, Figure 2; P for interaction <0.001) with Knapp-Hartung adjustment. In the meta-regression model examining the linear association between omega-3 fatty acid dosage and risk of AF events, the HR was 1.11 (95%CI 1.06–1.15, P=0.001, Figure 3) per 1 gr increase of omega-3 fatty acids (residual heterogeneity I2=0.00%, P=0.54) over a range of dosage from 1 gr per day to 4 gr per day. The sensitivity analysis including the constant term gave similar results (HR 1.09, 95%CI 1.01–1.18, P=0.030 per 1 gr increase of omega-3 fatty acids dosage).
Figure 2: Effect of marine omega-3 fatty acids supplements on the risk of AF events stratified by low dose (≤1 gr per day) vs. high dose (>1 gr per day) using Knapp-Hartung adjustment for random effect model..
See Figure 1 for abbreviation
Figure 3: Regression of omega-3 fatty acids dosage and risk for AF events in 7 randomized controlled trials using Knapp-Hartung adjustment for random effect model.
See Figure 1 for abbreviation.
In sensitivity analysis using data from the post-hoc publication of the ASCEND trial, the risk of AF persisted in the pooled association between marine omega-3 fatty acids and AF events (HR 1.20, 95%CI 1.01–1.43, P=0.039 Figure II in the Supplement). The estimates remained statistically significant with Knapp-Hartung adjustment among trials testing high dose of omega-3 fatty acids (>1 gr per day) with an HR of 1.49 (95%CI 1.04–2.15, P=0.042), but not in trials testing low-dose (≤1 gr per day) with an HR of 1.08 (95%CI 0.99–1.17, P=0.077, Figure III in the Supplement). Since the heterogeneity was absent in the subgroup of trials with low-dose marine omega-3 fatty acids, the application of the fixed effects model yielded a marginally significant increased risk of AF (HR 1.075, 95%CI 1.003–1.153, P=0.042). In the meta-regression model for the association of omega-3 fatty acid dosage with the risk of AF events, the HR was 1.10 (95%CI 1.05–1.14, P=0.002, Figure IV in the Supplement) per 1 gr increase of omega-3 fatty acids dosage (residual heterogeneity I2=0.00%, P=0.46).
No significant interaction (P=0.36, Figure V in the Supplement) was observed between studies that prespecified AF outcomes (HR 1.42, 95%CI 0.70–2.90) vs. studies that did not prespecify AF outcomes (HR 1.19, 95%CI 1.02–1.38). Sensitivity analysis with studies that excluded baseline AF or qualified AF events as new-onset yielded similar results (HR 1.26, 95%CI 0.85–1.87) compared with studies that did not exclude baseline AF (HR 1.28, 95%CI 1.11–1.48) with no significant interaction (Figure VI in the Supplement, P=0.92). The effect of marine omega-3 fatty acid supplements on the risk of AF events after excluding REDUCE-IT (the only trial without DHA) remained significant (HR 1.23, 95%CI 1.02–1.49, P=0.038, Figure VII in the Supplement). Additional sensitivity analysis excluding the RP trial, which only reported AF hospitalizations, yielded similar results (HR 1.26, 95%CI 1.04–1.52, P=0.028) with no significant interaction (P=0.85). A funnel plot suggested the presence of a slight publication bias possibly related to the dosage of omega-3 (Figure VIII in the Supplement), because studies with higher dosage were more likely to be on the right side (greater treatment effect). The ‘trim and fill’ method imputed one study on the left side of the funnel plot (Figure IX in the Supplement ) and the effect size after imputation (HR 1.23, 95%CI 1.10–1.39) was similar to the observed effect size (HR 1.25, 95%CI 1.11–1.40) using the DerSimonian-Laird approach.
Discussion
This meta-analysis adds new evidence regarding the risk of AF in patients taking marine omega-3 fatty acid supplements. The risk of AF was significantly more pronounced in trials testing high doses of marine omega-3 fatty supplements (> 1gr daily) compared with placebo vs. low dose of marine omega-3 fatty supplements (≤1gr daily) vs. placebo. The association appeared to have a dose relationship with a 10–11% higher relative risk of AF events per 1 gr increased in daily supplementation. The stratification of the trials by the dosage significantly attenuated the heterogeneity across trials; however, no trial has directly compared different dosages (e.g. high vs. low vs. placebo) of marine omega-3 supplements on the risk of AF events.
This meta-analysis of large cardiovascular trials with different doses and formulations of omega-3 fatty acid supplementations (Icosapent Ethyl, carboxylic acid formulations, regular EPA/DHA combinations) with over 2900 AF events provided adequate power to assess small-to-moderate risk of AF and an examination of dose effect relationship at the study-level. Prior to the publication of the REDUCE-IT trial, no safety concerns were reported with omega-3 fatty acid supplements. The results suggesting an increased risk for AF hospitalizations and adverse events observed in REDUCE-IT were then replicated with other AF outcomes in two other studies. In the STRENGTH trial, 4 gr of marine omega-3 fatty acids was associated with an increased risk of AF events (2.2% vs 1.3%, HR 1.69, 95%CI 1.29–2.21<P<0.001).6 In the OMEMI trial, older patients with recent myocardial infarction (≥ 75 years) who were randomized to 1.8 gr supplements also had a higher risk of AF events detected clinically or by electrocardiographic monitoring when compared with placebo, although the result did not reach statistical significance (7.2% vs. 4.0%, HR 1.94, 95%CI 0.98–3.45, P=0.06). In the VITAL trial, the largest AF primary prevention randomized trial that tested 1 gr of omega-fatty acids, the findings were neutral (HR 1.09, 95%CI 0.96–1.24, P=0.19) and did not support the use of supplemental for the primary prevention of AF. Although the formulations across trials were different (Icosapent Ethyl, carboxylic acid formulations, regular EPA/DHA combinations), these results were consistent when REDUCE-IT trial, which tested only EPA, was excluded. However, these data do not exclude the possibility that specific omega-3 formulations used across trials may have differing impacts on AF risk.
The 2019 ESC guidelines for dyslipidemia have now integrated icosapent ethyl as second line treatment in addition to statin for high-risk patients with high triglyceride values.5 According to the 2012 National Health Interview Survey, fish oil supplements are the natural product most commonly taken by adults. About 7.8% of patients reported using marine omega-3 fatty supplements, corresponding to approximately 18.8 million people in the US (https://www.nccih.nih.gov/health/omega3-supplements-in-depth). These results suggest that both the benefits and risks of marine omega-3 supplementation should be discussed with the patients, especially when prescribing a higher dosage. The risk-benefit ratio may not only vary according to dose and/or formulation but may also differ according to the patient characteristics. In the OMEMI trial, the absolute risk difference for developing AF (>3%) was the highest among those 75 years or older. However, the investigators of the OMEMI trial also used monitoring to capture AF events; and thus it is likely that more asymptomatic, subclinical cases were detected.7 Since this meta-analysis pooled aggregate-level trial data, and not individual participant data, this report is unable to undertake subgroup analysis by age or other patient level characteristics. To better understand the risk-benefit ratio in some high-risk subgroups (e.g. elderly, patients with cardiac morbidity), future trials testing omega-3 fatty acid supplements will need to include systematic, pre-specified ascertainment and adjudication of AF outcomes, which can be facilitated with ECG monitoring via new devices (e.g. smartphone) and adjudication by independent clinicians.
This comprehensive meta-analysis encompasses recently published large-scale trials of omega-3 fatty supplementation; however, there are limitations to be considered. First, differences exist in the AF outcome assessment between trials. In the ASCEND (main paper), REDUCE-IT and RP trials, participants with preexisting AF were not systematically excluded from the analysis, while in other trials, the analysis reported new-onset AF events and/or excluded participants with preexisting AF. Sensitivity analysis with studies that excluded baseline AF yielded similar results compared with studies that did not exclude baseline AF with no significant interaction. Second, the HR with 95%CI were provided in each trial, except REDUCE-IT where the RR was calculated based on the number of patients with AF in each of the treatment arms. The ASCEND trial reported only rate ratio. Since the incidence rate of AF was quite uncommon, the use of RR is an appropriate proxy of HR and this limitation should not affect the results. Third, not every large cardiovascular clinical trial testing omega-3 fatty acid supplementation reported AF outcomes (e.g. JELIS and ORIGIN). This limitation should not bias the current conclusions, because trials that hypothesized the superiority of omega-3 fatty acid supplements on atherosclerotic CV outcomes are unlikely to have intentionally underreported a neutral safety outcomes for AF events. Of note, the quality of the trials included in this meta-analysis was high with a low risk of bias. Fourth, the lack of variability in dosage among trials, particularly at the lower dose range, limited the ability to definitive test the linearity of the relationship with meta regression. Fifth, participants who are included in clinical trials might not be representative of those seen in everyday practice.
In conclusion, in this meta-analysis incorporating data from 7 large scale RCTs, omega-3 fatty acid supplementation was associated with a risk of AF, especially in trials testing higher dose of omega-3 fatty acids. Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AF should be balanced against the benefit on atherosclerotic cardiovascular outcomes.
Supplementary Material
Clinical Perspectives.
What is new?
In this updated meta-analysis of seven randomized controlled trials including 81,200 patients, marine omega-3 fatty acid supplementation was associated with a significant increased risk of AF compared with placebo.
The incremental risk of AF associated with omega-3 fatty acid appeared to be greater in trials testing >1gram per day of omega-3 fatty acid supplementation.
What are the clinical implications?
The potential risk of developing AF should be discussed with the patients when prescribing marine omega-3 supplementation, especially when prescribing a higher dosage.
Post-marketing surveillance for AF along with systematic ascertainment of AF outcomes in future trials of marine omega-3 supplementation will be needed to better define the risk-benefit ratio across omega-3 formulations.
Acknowledgments
Conflict of Interest Disclosures
Dr Djousse reported receiving grants from the National Institutes of Health and received investigator initiated award from Novartis. Dr Albert reported receipt of grants from St Jude Medical, Abbott, and Roche Diagnostics. Dr Manson reported receiving grants from Mars Symbioscience. No other disclosures were reported.
Source of Funding
The VITAL Rhythm Study was supported by grant R01HL116690 from the National Heart, Lung, and Blood Institute and the VITAL trial was supported by grants U01 CA138962 and R01 CA138962, which included support from the National Cancer Institute, National Heart, Lung and Blood Institute, Office of Dietary Supplements, National Institute of Neurological Disorders and Stroke, and National Center for Complementary and Integrative Health.
Non-standard Abbreviations and Acronyms
- AF
Atrial Fibrillation
- ASCEND
A Study of Cardiovascular Events in Diabetes
- DHA
Docosahexaenoic Acid
- EAS
European Atherosclerosis Society
- EPA
Eicosapentaenoic Acid
- ESC
European Society of Cardiology
- GISSI-HF
Gruppo Italiano per lo Studio della Sopravvi- venza nell’Insufficienza Cardiaca
- HR
Hazard Ratio
- OMEMI
Omega-3 fatty acids in Elderly with Myocardial Infarction
- RCT
Randomized Controlled Trial
- REDUCE-IT
Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial
- RP
Risk and Prevention Study
- RR
Risk Ratio
- STRENGTH
Long-Term Outcomes Study to Assess STatin Residual Risk Reduction With EpaNova in HiGh Cardiovascular Risk PatienTs With Hypertriglyceridemia
- VITAL
Vitamin D and Omega-3 Trial
References
- 1.Hu Y, Hu FB and Manson JE. Marine Omega-3 Supplementation and Cardiovascular Disease: An Updated Meta-Analysis of 13 Randomized Controlled Trials Involving 127 477 Participants. J Am Heart Assoc. 2019;8:e013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CMand Investigators R-I. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 3.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 4.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K and Japan EPAlisI. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8. [DOI] [PubMed] [Google Scholar]
- 5.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O and Group ESCSD. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 6.Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Ridker PM, Ray KK, Katona BG, Himmelmann A, Loss LE, Rensfeldt M, Lundström T, Agrawal R, Menon V, Wolski K and Nissen SE. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. Jama. 2020;324:2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, Nilsen DWT, Tveit A, Fagerland MW, Solheim S, Seljeflot I, Arnesen H and investigators O. Effects of n-3 Fatty Acid Supplements in Elderly Patients after Myocardial Infarction: A Randomized Controlled Trial. Circulation. 2021. Feb 9;143(6):528–539. [DOI] [PubMed] [Google Scholar]
- 8.Albert CM, Cook NR, Pester J, Moorthy MV, Ridge C, Danik JS, Gencer B, Siddiqi HK, Ng C, Gibson H, Mora S, Buring JE and Manson JE. Effect of Marine Omega-3 Fatty Acid and Vitamin D Supplementation on Incident Atrial Fibrillation: A Randomized Clinical Trial. JAMA. 2021;325:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG and Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 10.Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, Chew EY, Bosch J, Collins R, Lewington S, Armitage J, Clarke R and Omega-3 Treatment Trialists C. Associations of Omega-3 Fatty Acid Supplement Use With Cardiovascular Disease Risks: Meta-analysis of 10 Trials Involving 77917 Individuals. JAMA Cardiol. 2018;3:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariani J, Doval HC, Nul D, Varini S, Grancelli H, Ferrante D, Tognoni G and Macchia A. N-3 polyunsaturated fatty acids to prevent atrial fibrillation: updated systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2013;2:e005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Wu JH, de Oliveira Otto MC, Sandesara CM, Metcalf RG, Latini R, Libby P, Lombardi F, O’Gara PT, Page RL, Silletta MG, Tavazzi L and Marchioli R. Fish oil and post-operative atrial fibrillation: a meta-analysis of randomized controlled trials. Journal of the American College of Cardiology. 2013;61:2194–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr., Sperling L, Virani SS and Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gencer B, Marston NA, Im K, Cannon CP, Sever P, Keech A, Braunwald E, Giugliano RP and Sabatine MS. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2020;396:1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartung J and Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20:3875–89. [DOI] [PubMed] [Google Scholar]
- 16.Bender R, Friede T, Koch A, Kuss O, Schlattmann P, Schwarzer G and Skipka G. Methods for evidence synthesis in the case of very few studies. Res Synth Methods. 2018;9:382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson D, Law M, Rucker G and Schwarzer G. The Hartung-Knapp modification for random-effects meta-analysis: A useful refinement but are there any residual concerns? Stat Med. 2017;36:3923–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duval S and Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rucker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D and Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 21.Marston NA, Giugliano RP, Im K, Silverman MG, O’Donoghue ML, Wiviott SD, Ference BA and Sabatine MS. Association Between Triglyceride Lowering and Reduction of Cardiovascular Risk Across Multiple Lipid-Lowering Therapeutic Classes: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Circulation. 2019;140:1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Group ASC, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S and Armitage J. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. The New England journal of medicine. 2018;379:1540–1550. [DOI] [PubMed] [Google Scholar]
- 23.Parish S, Mafham M, Offer A, Barton J, Wallendszus K, Stevens W, Buck G, Haynes R, Collins R, Bowman L, Armitage J and Group ASC. Effects of Omega-3 Fatty Acid Supplements on Arrhythmias. Circulation. 2020;141:331–333. [DOI] [PubMed] [Google Scholar]
- 24.Aleksova A, Masson S, Maggioni AP, Lucci D, Fabbri G, Beretta L, Mos L, Paino AM, Nicolosi GL, Marchioli R, Tognoni G, Tavazzi L, Sinagra G, Latini R and Investigators GI- HF. n-3 polyunsaturated fatty acids and atrial fibrillation in patients with chronic heart failure: the GISSI-HF trial. Eur J Heart Fail. 2013;15:1289–95. [DOI] [PubMed] [Google Scholar]
- 25.Risk, Prevention Study Collaborative G, Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, Tognoni G and Marchioli R. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.