Abstract
Allergic asthma affects more women than men. It is mediated partially by IL-4/IL-13-driven polarization of monocyte-derived macrophages in the lung. We tested whether sex differences in asthma are due to differential IL-4 responsiveness and/or chemokine receptor expression in monocytes and monocyte-derived macrophages from healthy and allergic asthmatic men and women. We found female cells expressed M2 genes more robustly following IL-4 stimulation than male cells, as did cells from asthmatics than those from healthy controls. This likely resulted from increased expression of γC, part of the type I IL-4 receptor, and reduced IL-4–induced SOCS1, a negative regulator of IL-4 signaling, in asthmatic compared to healthy macrophages. Monocytes from asthmatic women expressed more CX3CR1, which enhances macrophage survival. Our findings highlight how sex differences in IL-4 responsiveness and chemokine receptor expression may affect monocyte recruitment and macrophage polarization in asthma, potentially leading to new sex-specific therapies to manage the disease.
Keywords: Asthma, Allergic lung inflammation, Sex differences, Monocytes, Alveolar macrophages, Hormones, IL-4, Chemokine, Cell recruitment
1. Introduction
Allergic asthma is a common chronic inflammatory disease of the lung that affects 235 million people worldwide. Asthma prevalence shows a sex difference that changes with age. More boys than girls are diagnosed before puberty [1] but, in adulthood, women are twice as likely as men to have asthma. Asthmatic women frequently experience premenstrual exacerbations, have greater disease severity, and require more corticosteroid treatment than men [2]. Sex hormones appear to play an essential role in asthma affecting the function of multiple cell types, such as ILC2s [3,4], B-cells/IgE [5,6], mast cells [7,8], and neutrophils [9]. However, the cellular and molecular mechanisms in human cells that underpin the sex differences in asthma are poorly understood.
Monocytes and macrophages are important contributors to asthma pathogenesis. The presence of M2-polarized macrophages is associated with more severe allergic inflammation and poor lung function in humans and animal models [10,11]. Furthermore, M2-polarized macrophages are more abundant in the airways of female asthmatics than in males [12]. M2 macrophages in the lung differentiate either from resident alveolar macrophages [13,14] or from recruited monocytes [15,16] in the presence of the Th2-cytokines, IL-4 or IL-13. M2 macrophages contribute to allergic lung inflammation by producing eosinophil and Th2-cell chemoattractants (e.g., CCL17 and CCL22 [17–19]) and molecules that cause tissue remodeling (e.g., MMP12 [20], TGM2 [21], and CD200R [22]). In addition, we and others have shown previously that, compared to macrophages from male mice, macrophages from female mice express more M2 genes in vitro upon IL-4 stimulation and in vivo after allergen challenge [11,23,24].
Although M2 macrophages are more abundant in the lungs of asthmatic women than in asthmatic men [12], it is not known whether this is due to more monocyte recruitment to the lungs and/or greater propensity to polarize into M2 phenotype upon stimulation with IL-4 and IL-13 in monocytes and macrophages from asthmatic women compared to asthmatic men. How these responses differ from those in monocytes and macrophages from healthy men and women has not been studied. Therefore, we hypothesized that sex differences in mechanisms of monocyte recruitment and M2 polarization result in more M2-polarized MDMs in the lungs of asthmatic women than of asthmatic men, and of healthy men and women without the disease. To understand how sex differences and asthma contribute to human monocyte-macrophage recruitment and IL-4-induced M2 polarization, we conducted an analysis of chemokine and IL-4 receptor expression in peripheral blood monocytes and MDMs from asthmatic men and women. We also measured M2 gene expression in response to IL-4 and IL-13 in both cell types. We then compared these readouts to same cells from healthy men and women without asthma.
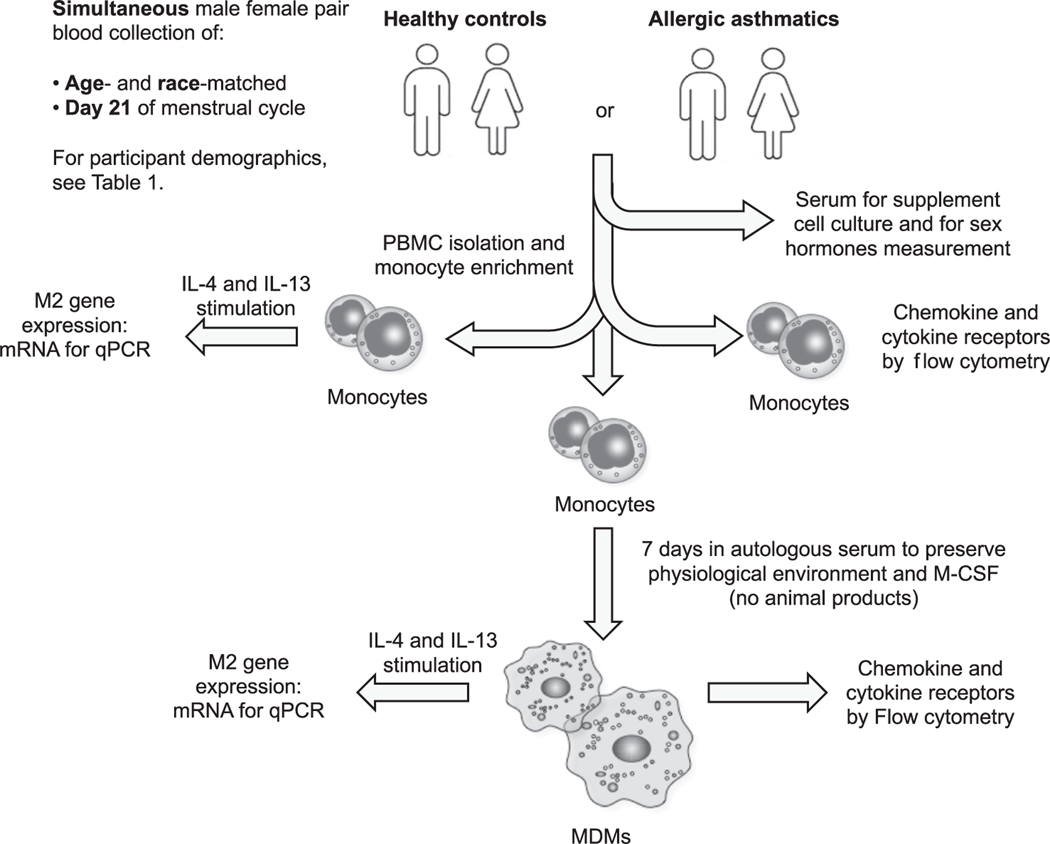
Our interest in the contribution of sex to monocyte-macrophage responses in asthma led us to adopt several unique approaches in our study. We accomplished these comparisons in a highly controlled manner that accounts for age, race, and sex hormone fluctuations across the menstrual cycle in women, by matching pairs of male and female donors for disease status, age, and race. Also, we drew blood from the matched pairs simultaneously on the morning of day 21 of the woman’s menstrual cycle to avoid sex hormone fluctuations. Monocytes and MDMs from the same donor were cultured in autologous serum to maintain the identical sex hormone environment during the experiments. These painstaking approaches sought to avoid the variability found in human populations and allow new insights into how sex differences in monocytes and macrophages affect the outcome of allergic asthma.
2. Material and methods
2.1. Study populations
Men and women (21–45 years old) with and without (control) allergic asthma were recruited according to guidelines of the Johns Hopkins Institutional Review Board. The demographic and clinical features of the participants are reported in Table 1. All allergic asthmatic subjects reported allergies to multiple environmental agents, experienced mild-to-moderate disease, and used symptom-modifying medications. None used oral systemic steroids during the study or were taking, or had a history of taking, immunotherapy treatment. Approximately 60% of reproductive-age women take hormonal birth control [25], but only 1 of 4 healthy women and 4 of 12 asthmatic women in our study were on hormonal birth control medication. To minimize day-to-day experimental variation in culture conditions, we recruited age- and race-matched subjects in pairs, one female and one male, either healthy or asthmatic, for blood collection and simultaneous peripheral blood mononuclear cell (PBMC) isolation and culture. As 20–40% of women with asthma experience premenstrual exacerbations [26], all samples were collected on day 21 of the menstrual cycle of each female subject between 8:00 AM and 10:00 AM. Serum collected from these subjects was used for measurement of sex hormone concentrations and monocyte-to-MDM culture after heat inactivation. The data shown in the figures represent paired male–female comparisons of samples collected, processed, and quantified in parallel.
Table 1.
Demographic and clinical information of study participants.
| Participant demographics | Healthy men (n = 4) | Healthy women (n = 4) | Asthmatic men (n = 12) | Asthmatic women (n = 12) |
|---|---|---|---|---|
|
| ||||
| Age (y), mean ± SEM | 36.8 ± 4.4 | 40.5 ± 4.1 | 28.1 ± 1.7 | 28.3 ± 2.0 |
| Race, n (%) | ||||
| Caucasian | 4 (100) | 2 (50) | 7 (58.3) | 7 (58.3) |
| African American | 0 | 1 (25) | 1 (8.3) | 1 (8.3) |
| Latino | 0 | 0 | 2 (16.6) | 2 (16.6) |
| Asian | 0 | 1 (25) | 2 (16.6) | 2 (16.6) |
| Two allergic triggers, n (%) | N/A | N/A | 12 (100) | 12 (100) |
| Three or more allergic triggers, n (%) | N/A | N/A | 7 (58.3) | 8 (66.6) |
| Serum sex hormone concentration, mean ± SEM | ||||
| Testosterone, ng/ mL* | N/A | N/A | 3.65 ± 0.45 | 0.27 ± 0.04 |
| Progesterone, ng/ mL† | 0.31 ± 0.03 | 4.73 ± 1.08 | ||
| Estrogen, pg/mL‡ | 114.9 ± 8.8 | 199.6 ± 23.9 | ||
| Oral contraceptive use, n (%) | N/A | 1 (25) | N/A | 4 (33.3) |
| Medication use, n (%) | ||||
| Albuterol inhaler, n (%) | N/A | N/A | 7 (58.3) | 8 (66.6) |
| Over-the-counter antihistamines | N/A | N/A | 7 (58.3) | 10 (83.3) |
| Body mass index, mean ± SEM§ | 27.3 ± 4.1 | 24.1 ± 1.5 | 28.0 ± 1.1 | 27.4 ± 2.8 |
N/A indicates not applicable.
The normal range of serum testosterone is 2–15 ng/mL for men and 0.2–0.8 ng/ mL for women.
The normal range of serum progesterone is 0.25–0.90 ng/mL for men and 1–18 ng/mL for women.
The normal range of serum estrogen is 15–50 pg/mL for men and 60–200 pg/ mL for women.
Normal = 18.5–24.9; overweight = 25.0–29.9.
AMs were obtained from asthmatic and healthy subjects for the analysis of SOCS1 expression in response to IL-4. Bronchoalveolar lavages (BAL) were performed to four asthmatic study participants enrolled in the INHALE 2 study, an interventional study to determine whether removing indoor air pollutants in the homes of asthmatic patients improved asthma morbidity and lung inflammation. These study participants were non-smoking adult men and women, aged 18–50, with a physician diagnosis of asthma. Female participants were not pregnant, and none of the participants had other major pulmonary diseases. 50% of the subjects were taking inhaled prescription asthma medications; the other 50% were not taking any medication. The healthy control macrophages were obtained from six healthy volunteers who were undergoing a clinically indicated bronchoscopy and lavage for diagnostic purposes. Typical reasons for bronchoscopy included possible sarcoidosis (4 donors) and investigation of lung nodules (2 donors). AMs were obtained from BAL fluid from uninvolved lobes. Forced expiratory volume in 1 s (FEV1) as an indicator of lung function was measured on healthy and asthmatic donors. Healthy donors had a FEV1 of 98% ± 2.7% while the asthmatics had a significantly decreased FEV1 of 76% ± 5.2% (**p < 0.01) [27].
2.2. PBMC and AM isolation
Enriched monocytes (>90% pure monocytes; Supplemental Fig. 1) were obtained by adherence of PBMCs isolated from whole blood by gradient separation in Lympholyte H (Cedarlane Laboratories, Ontario, Canada). For qPCR analysis, freshly isolated enriched monocytes plated in serum-free X-VIVO 10 (Lonza, USA) were used immediately for cytokine stimulation and gene expression experiments. Other enriched monocytes were differentiated into MDMs by culture for 7 days in rhM-CSF (25 ng/mL) (R&D Systems) in X-VIVO 10 supplemented with 10% heat-inactivated autologous serum (Fig. 1). To measure chemokine receptors and IL-4 receptor subunits by flow cytometry, we stained PBMCs immediately with antibodies that recognized lineage markers, chemokine receptors, and IL-4 receptor subunits (see Table 1).
Fig. 1.
Schematic of the experimental approach to blood donor characteristics, matching, monocyte and MDMs culture, and stimulation conditions. A peripheral blood sample was simultaneously obtained from age-and race-matched subjects in pairs, one female and one male, either healthy or asthmatic between 8:00 AM and 10:00 AM on day 21 of the menstrual cycle of each female subject. Blood mononuclear cells (PBMC) were isolated by gradient separation and monocytes were enriched by adhesion. Serum from each subject was collected for monocyte-to-MDM culture after heat inactivation (autologous serum). Enriched monocytes were either stimulated with IL-4 or IL-13 for 24 h prior to mRNA collection or cultured in 10% autologous serum and 25 ng/mL of rmM-CSF for 7 days (monocyte-to-MDM culture), MDMs were stimulated with IL-4 or IL-13 for 24 h followed by mRNA collection.
AMs from bronchoscopy samples of asthmatic and healthy subjects were isolated after instillation of 50–100 mL warmed sterile saline. Briefly, BAL samples were filtered through a 100 μm strainer, rinsed with phosphate-buffered saline and 2% penicillin/streptomycin, and counted. BAL cells were plated in serum-free X-VIVO 10 (Lonza, USA) for 2 h. Non-adherent cells were removed. AMs were stimulated with IL-4 (20 ng/mL) for 0, 2, 6, or 24 h in X-VIVO 10 supplemented with 20% Cell-ESS (Essential Pharma, Ewing, NJ).
2.3. Cell culture and real-time RT-PCR
Monocytes and MDMs were stimulated for 24 h with IL-4 (0.1, 0.3, 1.0, 3.0 ng/mL) or with IL-13 (3.0 ng/mL). Total RNA was isolated with the RNeasy kit (Qiagen, Valencia, CA), and complementary DNA (cDNA) was generated with the SuperScript III First-Strand Synthesis System (Invitrogen). Quantitative PCR was carried out with specific primers (Supplemental Table 1) in a 7500 Fast Real-Time PCR instrument from Applied Biosystems (Grand Island, NY). M2 gene expression was determined by using the 2-ΔΔCT method and compared to the amount of mRNA in the unstimulated male sample of each pair.
2.4. Flow cytometry
PBMCs were stained for viability (Zombie Yellow, BioLegend, San Diego, CA) [23]. Anti-Fc receptor and True-stain Monocyte Blocker (BioLegend) were used to reduce nonspecific antibody and fluorophore binding before cells were labeled with antibodies. Non-monocytic cells were excluded with a “dump” channel containing CD3, CD19, CD20, CD56, and CD66b. Staining with CD14 and CD16 identified the two major human monocytic subsets. The antibodies used for IL-4 and chemokine receptor identification are listed in Table 2. Stained cells were acquired with a CytoFLEX flow cytometer (Beckman-Coulter, Brea, CA), courtesy of the Anesthesiology and Critical Care Medicine Flow Cytometry Core. CytEXPERT software v.2.0 (Beckman-Coulter) was used for analysis, and isotype control antibody-labeled cells were used to set negative gates. Data are reported as the change in mean fluorescence intensity (MFI) of the different receptors (MFI target – MFI isotype).
Table 2.
Antibodies used for flow cytometry analysis of monocyte populations and surface receptor.
| Antibody | Fluorophore | Clone |
|---|---|---|
|
| ||
| CD3 | FITC | HIT3a |
| CD19 | FITC | HIB19 |
| CD20 | FITC | 2HT |
| CD56 | FITC | MEM-188 |
| CD66b | FITC | G10F5 |
| CD14 | APC-Cy7 | HCD14 |
| CD16 | PerCP | 3G8 |
| CCR5 | PE | 2D7 |
| CCR1 | PECy7 | 5F10B29 |
| CCR2 | APC | K036C2 |
| CX3CR1 | BV421 | 2A9–1 |
| IL-13Rα1 | PE | 419718 |
| IL-13Rα2 | PE | B-D13 |
| γC | APC | TUGh4 |
| IL-4Rα | APC | 25463 |
2.5. ELISA
Concentrations of testosterone (Immulite 2000 Meditecno), estradiol (MP Biomedicals RIA), and progesterone (ALPCO ELISA) were measured in serum by the University of Virginia Ligand Assay and Analysis Core.
2.6. Statistics
GraphPad Prism software (La Jolla, CA) was used for all graph generation and statistical analysis for IL-4 and chemokine receptor subunits. Data are plotted as the mean ± SEM. Statistical significance was determined by using a parametric Student t-test, and p < 0.05 was considered statistically significant. For M2 gene expression by qPCR, data were analyzed by the Biostatistics Core of the Johns Hopkins Institute for Clinical and Translational Research (ICTR). Non-parametric analyses were used to test for differences because of the small sample sizes and non-normality of the data. Two-group comparisons, i.e., comparing sex, cell type or disease status, were made using Wilcoxon rank sum tests. Doses were compared using a Kruskal-Wallis test. No adjustment was made for multiple comparisons. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC). All tests were two-sided and significance was set at < 0.05.
2.7. Study approval
Both studies were approved by the Johns Hopkins Institutional Review Board, and written informed consent was obtained from each subject.
2.8. Online supplemental material
Supplemental Fig. 1 shows monocyte enrichment by adherence. Supplemental Fig. 2 shows the gating strategy for CD14+CD16− “classical” and CD14−CD16+ “non-classical” monocytes. Supplemental Fig. 3 shows the differential expression of chemokine receptors in monocytes and MDMs from allergic asthmatic donors. Supplemental Fig. 4 shows sex hormone receptor gene expression on monocytes from allergic asthmatic donors. Supplemental Table 1 shows quantitative PCR primer sequences.
3. Results
3.1. Chemokine receptor expression on monocytes from healthy and asthmatic men and women
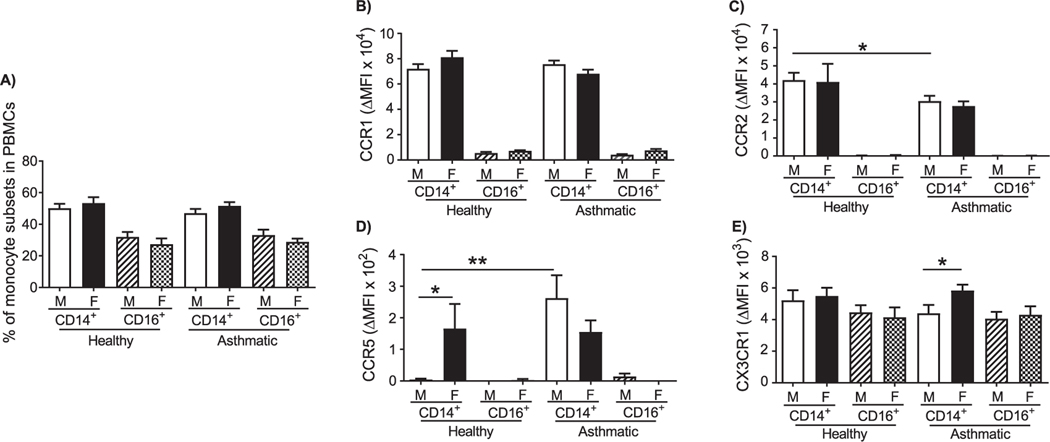
Blood monocyte-derived macrophages recruited to the lung promote allergic lung inflammation [16]. Lung M2 macrophages, that correlate with worse lung function, are more abundant in asthmatic women [12] and in female mice with allergic lung inflammation than in males [23,24]. It is possible that differences in recruitment of monocytes to the allergic lung by chemokines might explain sex differences in the abundance of monocyte-derived M2 macrophage in the allergic lung. Therefore, to determine sex differences in chemokine receptor expression on human monocytes, we measured surface expression of CCR1, CCR2, CCR5, and CX3CR1 on CD14+ “classical,” or inflammatory, monocytes and CD16+ “non-classical,” or “patrolling,” monocytes from asthmatic and healthy men and women by flow cytometry (gating strategy, Supplemental Fig. 2). These receptors participate in monocyte recruitment into inflamed tissues [28–31]. No significant differences were present in the percentages of CD14+ and CD16+ monocytes between men and women (Fig. 2A). However, we observed slight a trend toward a greater percentage of CD14+ monocytes in the PBMCs from women than in those from men, regardless of whether they were healthy or asthmatic. Conversely, men had a trend toward a greater percentage of CD16+ monocytes.
Fig. 2.
Monocyte subpopulations in peripheral blood and monocyte chemokine receptor expression in healthy and allergic asthmatic men and women. PBMCs were isolated from the peripheral blood of healthy and allergic asthmatic study participants, stained with specific antibodies for surface markers of monocyte subsets and chemokine receptors, and analyzed by flow cytometry. Monocytes were gated on live cells, CD45+CD3−CD19−CD20−CD56−CD66b−, and either CD14+ or CD16+. (A) Differential proportions of CD14+CD16− “classical” and CD14−CD16+ “non-classical” monocytes in PBMCs. (B-E) Analysis of (B) CCR1, (C) CCR2, (D) CCR5, and (E) CX3CR1 in classical and non-classical monocytes. The change in mean fluorescence intensity (MFI) of the different chemokine receptors (MFI target – MFI isotype) is shown. The number of donors in each group are healthy male (n = 14), healthy female (n = 11), asthmatic male (n = 12), and asthmatic female (n = 13). *p < 0.05, **p < 0.01.
CD14+ monocytes expressed an average of 12-fold more CCR1 than did CD16+monocytes. No differences were observed in CCR1 expression between cells from men and women, or between asthmatic and healthy subjects (Fig. 2B). CCR2, a receptor necessary for monocyte egress from bone marrow and recruitment into inflamed tissue [32], was expressed predominantly in CD14+ monocytes. Expression of CCR2 was significantly lower on CD14+ monocytes from asthmatic men than on similar cells from healthy men (Fig. 2C). We did not detect sex differences in CCR2 expression between men and women (Fig. 2C), but we noted that expression of CCR2 decreased after monocyte-to-MDM maturation (Supplemental Fig. 3).
CD14+ monocytes from healthy and asthmatic women, and asthmatic males, expressed similar levels of CCR5, while it was essentially undetectable in similar cells from healthy males (Fig. 2D). Although there were no differences in CX3CR1 expression in monocytes from healthy subjects, CD14+ monocytes from asthmatic women had a modest but significantly higher expression of CX3CR1 than those from asthmatic men (Fig. 2E).
3.2. IL-4-/IL-13-induced M2 gene expression in monocytes and MDMs
IL-4 and IL-13 are major drivers of the M2 polarization program in macrophages [33,34]. To determine sex differences in the degree of M2 polarization of human monocytes and MDMs in response to IL-4 and IL-13 stimulation, we compared expression of five IL-4/IL-13-induced M2 macrophage genes. We measured CCL17, CCL22, TGM2, MMP12, and CD200R by qPCR, as these genes had been described previously as being detrimental to lung function or related to lung fibrosis [17,18,20–22,35]. M2 polarization of monocytes and MDMs from asthmatic and healthy men and women was measured using low concentrations of IL-4 and IL-13 to reveal subtle differences in the response among the different experimental groups. A schematic of the experimental approach is shown in Fig. 1. Thorough statistical analysis was performed by the Biostatistics Core of the Johns Hopkins Institute for Clinical and Translational Research (ICTR), to detect differences between the men and women, disease status (healthy or allergic asthmatic), and the different concentrations of cytokine stimulus (details in section 2.6. Statistics). We were able to detect statistically significant differences in M2 gene expression between the different groups, despite the small sample size. In general, IL-4 induced the mRNAs for the five M2 genes more robustly in cells from asthmatic than from healthy subjects, and from female MDMs more than male MDMs. This is emphasized by the shaded gradient towards the lower right corner in each set of graphs.
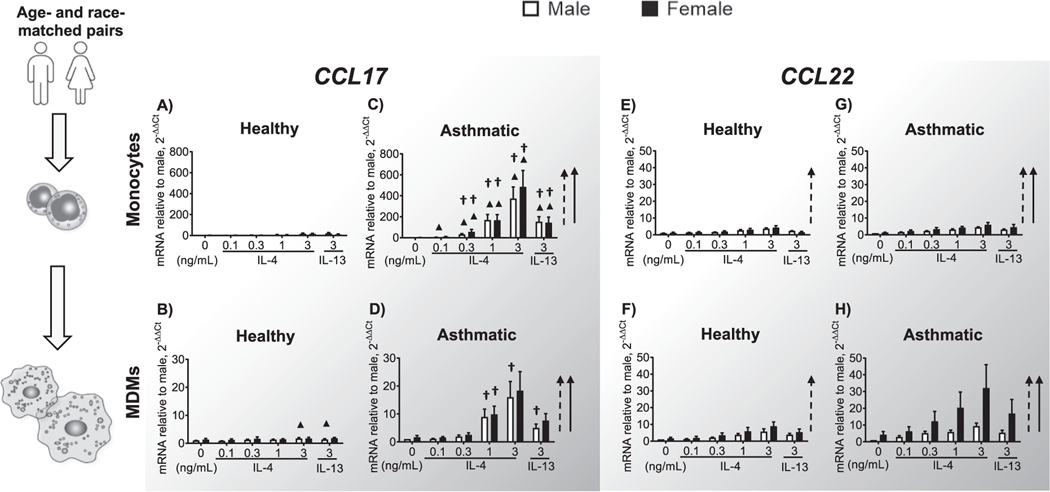
3.3. CCL17 and CCL22 are highly induced in MDMs from asthmatic subjects, with female dominance of CCL22
Monocytes and MDMs from asthmatic men and women expressed significantly more CCL17 in response to IL-4/IL-13, indicated by the vertical arrows to the right of each graph (Fig. 3A–D) compared to same cells from healthy controls. However, these responses were small. CCL17 induction was greater in response to IL-4 than to IL-13 and more robust in monocytes than in MDMs. Monocytes from asthmatic subjects expressed up to 25-fold more CCL17 than did MDMs from the same group. Overall, female cells expressed more CCL17 than males in response to IL-4 but variability within the group prevented statistical significance from being reached. Expression of CCL22 (Fig. 3E–H) in MDMs from asthmatic women was highly induced by IL-4 concentrations as low as 0.3 ng/mL (Fig. 3H) although variability precluded detection of sex differences.
Fig. 3.
CCL17 and CCL22 gene expression in monocytes and MDMs. Monocytes were enriched from PBMCs of healthy and allergic study participants, serum-starved for 2 h, and stimulated with 0.1, 0.3, 1.0, or 3.0 ng/mL IL-4 or 3.0 ng/mL IL-13. Monocytes from the same donor were grown simultaneously into MDMs by culturing them for 7 days in 25 ng/mL rhM-CSF and autologous serum. After the same IL-4/IL-13 stimulation protocol as before, RNA was harvested from the MDMs, and expression of the indicated M2 genes was determined by qPCR using the ΔΔ cycle threshold (CT) method. Amounts of mRNA were compared to those in the male unstimulated sample of each pair. CCL17 expression in (A) monocytes and (B) MDMs from healthy subjects; (C) monocytes and (D) MDMs from asthmatic subjects. CCL22 expression in (E) monocytes and (F) MDMs from healthy subjects; (G) monocytes and (H) MDMs from asthmatic subjects. The number of donors in each group is shown in Table 1. † p < 0.05, comparing asthmatic and healthy samples of the same cell type and sex. ▲ p < 0.05, comparing monocytes and MDMs from participants of the same health status and sex. The dashed vertical arrow indicates a statistically significant response in males, and the solid vertical arrow indicates a statistically significant response in females in the expression of the M2 genes after IL-4 and IL-13 stimulation.
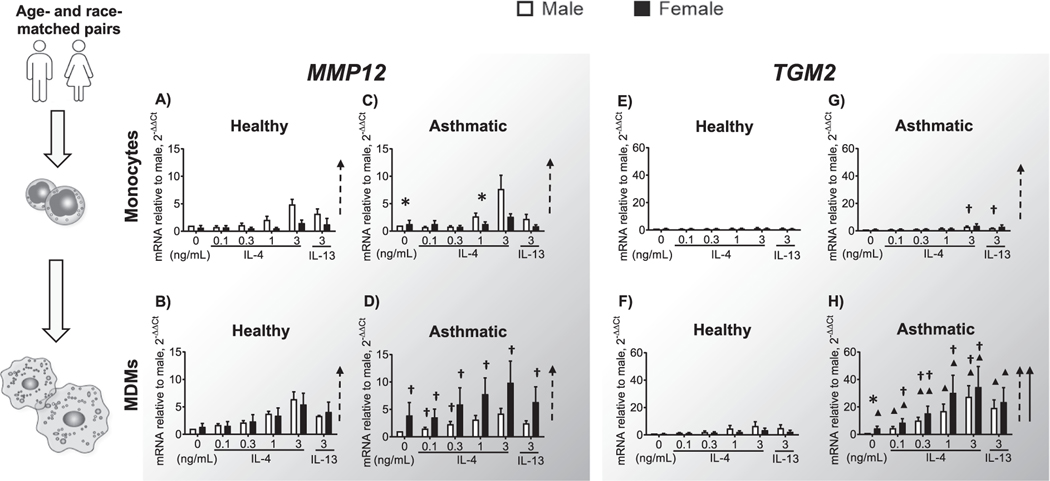
3.4. Tissue remodeling genes MMP12 and TGM2 increase in asthmatic men and women
MMP12 is involved in lung fibrosis and tissue remodeling, and is associated with severe asthma [36,37]. IL-4/IL-13 induced MMP12 expression in all male groups (Fig. 4A–D), yet MDMs from asthmatic women showed greater MMP12 induction after stimulation (Fig. 4D). MMP12 expression was significantly greater in MDMs from asthmatic compared to healthy women (Fig. 4B, D). Similarly, TGM2, a key initiator of airway inflammation [21], was also mainly induced by IL-4/IL-13 in MDMs rather than monocytes (Fig. 4E, G). We saw a clear and robust concentration-dependent induction of TGM2 in MDMs only from asthmatic subjects (Fig. 4H), not from healthy ones (Fig. 4F). Moreover, even at baseline, unstimulated MDMs from asthmatic females expressed higher levels of TGM2 than MDMs from asthmatic males
Fig. 4.
MMP12 and TGM2 gene expression in monocytes and MDMs. Monocytes and MDMs were grown and stimulated as described in Fig. 3. Gene expression was analyzed as in Fig. 3. MMP12 expression in (A) monocytes and (B) MDMs from healthy subjects; (C) monocytes and (D) MDMs from asthmatic subjects. TGM2 expression in (E) monocytes and (F) MDMs from healthy subjects; (G) monocytes and (H) MDMs from asthmatic subjects. The number of donors in each group is shown in Table 1. † p < 0.05, comparing asthmatic and healthy samples of the same cell type and sex. ▲ p < 0.05, comparing monocytes and MDMs from participants of the same health status and sex. Dashed and solid arrows indicate a statistically significant increase in cytokine-dependent M2 gene expression in males and females, respectively. *p < 0.05, comparing men and women of the same health status, cell type, and cytokine stimulation.
3.5. Sex differences in CD200R
CD200R is a marker of M2 macrophage polarization [38] and a negative regulator of AM responses [22]. No CD200R gene induction was observed in monocytes from healthy subjects (Fig. 5A). Induction of CD200R was poor in asthmatic monocytes as well, although statistically significant in some male samples (Fig. 5C). IL-4/IL-13 did not induce CD200R expression on MDMs from healthy men and women, but IL-4 elicited a concentration-dependent increase in CD200R expression in MDMs from asthmatic men (Fig. 5B, D). MDMs from asthmatic men expressed significantly more CD200R than did monocytes from asthmatic men (4C, D) or MDMs from asthmatic women (Fig. 5D) upon IL-13 stimulation.
Fig. 5.
CD200R gene expression in monocytes and MDMs. Monocytes and MDMs were grown and stimulated as described in the legend to Fig. 3. Gene expression was also analyzed as in Fig. 3. CD200R expression in (A) monocytes and (B) MDMs from healthy subjects and (C) monocytes and (D) MDMs from asthmatic subjects. The number of donors in each group is shown in Table 1. † p < 0.05, comparing asthmatic and healthy samples of the same cell type and sex. ▲ P < 0.05, comparing monocytes and MDMs from subjects of the same health status and sex. Dashed and solid arrows indicate a statistically significant increase in cytokine-dependent M2 gene expression in males and females, respectively. *p < 0.05, comparing men and women of the same health status, cell type, and cytokine stimulation.
3.6. Sex differences in IL-4 and IL-13 receptor subunits and SOCS1 in human monocytes and macrophages
We hypothesized that differences in expression of cell surface IL-4 receptor complexes, estrogen or androgen receptors, or regulators of IL-4 signaling such as SOCS1, might explain the differential responsiveness of the cells to IL-4 and IL-13. To test this, we measured the surface expression of all four of the IL-4 and IL-13 receptor subunits on CD14+ and CD16+ monocytes and MDMs by flow cytometry. Expression of γC, which is part of the type I IL-4R complex, was significantly greater on CD14+ monocytes than on CD16+ monocytes (Fig. 6A). CD14+ monocytes from asthmatic subjects expressed more γC than did those from healthy controls, and asthmatic women had significantly more γC than did asthmatic men (Fig. 6A). Similarly, γC expression was greater on MDMs from asthmatics than from healthy subjects (Fig. 6E).
Fig. 6.
IL-4 and IL-13 receptor subunit expression on monocytes and MDMs, and induction of SOCS1 in AMs of healthy and asthmatic men and women. PBMCs were isolated from the peripheral blood of healthy and allergic asthmatic study participants, stained as in Fig. 2 for monocyte subsets and for IL-4 and IL-13 receptor subunits, and analyzed by flow cytometry. Monocytes were gated on live cells, CD45+CD3−CD19−CD20−CD56−CD66b−, and either CD14+ or CD16+. Analysis of (A) gamma C (γC), (B) IL-4Rα, (C) IL-13Rα1, and (D) IL-13Rα2 in “classical” and “non-classical” monocytes. MDMs were identified as live CD45+CD14+CD16+ cells. Analysis of (E) γC, (F) IL-4Rα, (G) IL-13Rα1, and (H) IL-13Rα2 in MDMs. The change in mean fluorescence intensity (MFI) of the different IL-4 and IL-13 receptor subunits (MFI target – MFI isotype) is shown. The number of donors in each group is the same as in Fig. 1. (I) AMs were isolated from the lungs of healthy (n = 6) and asthmatic (n = 4) subjects and stimulated with IL-4 (20 ng/mL) for 0, 2, 6, or 24 h. Expression of SOCS1 was determined by qPCR using the ΔΔ cycle threshold (CT) method. Amounts of mRNA were compared to those in the unstimulated sample of each donor. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Next, we measured IL-4Rα, the IL-4 ligand-binding subunit of type I IL-4 receptors (Fig. 6B). The expression of IL-4Rα on CD14+ monocytes was much greater than that on CD16+ cells, which was very low, and no differences were observed among groups (Fig. 6B). IL-4Rα was more easily measurable on MDMs from asthmatic subjects than on those of healthy controls (Fig. 6F). Expression of IL-13Rα1 was robustly expressed on CD14+ monocytes and very low on CD16+ cells but did not differ between the sexes or the groups (Fig. 6C). IL-13Rα1 was also significantly lower on MDMs than on CD14+ monocytes (Fig. 6G).
Surface expression of IL-13Rα2, the inhibitory or “decoy” receptor for IL-13, was more strongly expressed in CD14+ monocytes than in CD16+ cells across the sexes and groups. Expression of IL-13Rα2 showed a trend to slightly higher expression on CD14+ monocytes from healthy men than on those from healthy women (Fig. 6D) and on MDMs than on monocytes (Fig. 6H).
We previously demonstrated that estrogen and androgen acting through their respective receptors augment M2 polarization of mouse macrophages in vivo and in vitro [23,39]. To determine whether differences in estrogen and androgen receptor expression might contribute to enhanced M2 polarization, we measured expression levels in monocytes from asthmatic men and women by qPCR. Monocytes from women expressed all estrogen receptor-α isoforms (ER α 66, 46, and 33) to a greater degree than did monocytes from men. Surprisingly, women exhibited significantly greater androgen receptor (AR) expression on monocytes than did men (Supplemental Fig. 4), despite having 13.5-fold less circulating testosterone (Table 1). Interestingly, the concentration of estrogen in asthmatic men was elevated above the normal range for healthy men.
We previously reported that SOCS1, a negative regulator of IL-4 signaling and M2 polarization, was poorly induced by IL-4 in peripheral blood monocytes from allergic individuals compared to healthy controls [40]. To determine whether this was true for lung macrophages, we compared SOCS1 induction in BAL macrophages from the lungs of allergic asthmatic and healthy men and women. Although we did not segregate by sex, AMs from asthmatic subjects exhibited less induction of SOCS1 in response to IL-4 than did AMs from healthy controls at 2, 6, and 24 h after stimulation (Fig. 6I).
4. Discussion
In this study, we have shown important differences between monocytes and MDMs from asthmatic and healthy subjects and between men and women. To our knowledge, this is the first study to examine sex differences in human monocyte-macrophage M2 polarization in response to IL-4 and IL-13. Further, we carefully analyzed all subunits of the IL-4/−13 receptor complex and key chemokine receptors. Our study uniquely controls for menstrual cycle as a variable. In summary, we found moderate sex differences in CX3CR1 expression on CD14+ monocytes from asthmatic women and men. This suggests possible enhanced survival of these cells in women although further experiments are needed to support this hypothesis. Also, we found monocytes and MDMs from asthmatic subjects had increased sensitivity to IL-4/IL-13, leading to enhanced M2 polarization, which was greatest in MDMs from asthmatic women. IL-4R subunits from asthmatic subjects, particularly in γC expression, support greater IL-4 responsiveness in female than in male monocytes. We also noted poor induction of SOCS1 that downregulates IL-4 signaling and M2 polarization in AMs from asthmatics, recapitulating our observations in blood monocytes from allergic individuals [40]. Due to the small sample size, we did not segregate SOCS1 expression in AM by sex. When we did, SOCS1 was more highly expressed in AM from both healthy and asthmatic male cells compared to their female counterparts (data not shown), but more samples are required to solidify this finding. Poor induction of SOCS1 in asthmatic AMs supports the idea that lung-infiltrating monocytes that differentiate into AMs lack regulation of IL-4-directed polarization to the M2 phenotype, with female cells being more affected than male cells.
Percentages of CD14+ and CD16+ monocytes in PBMCs were similar across the different groups in our study, but several significant differences were found in the expression of key monocyte-macrophage chemokine receptors. CCR1 has been studied as a monocyte chemoattractant in other diseases [41], but monocyte subset-specific expression has not been conclusively demonstrated [42]. We showed that CCR1 is expressed predominately on CD14+CD16− “classical” monocytes and can be used to differentiate classical from non-classical human blood monocytes. Expression of CCR2 and CCR5 was related to disease status, with greater CCR2 on healthy cells and more CCR5 in asthmatic cells. As CCR2 acts as a “chemokine sink”, CD14+ monocytes from asthmatic subjects with less surface CCR2 may have a reduced ability to clear high concentrations of CCL2 and other CCR2-utilizing chemokines found in the allergic asthmatic lung [43]. CCR5, a marker of monocyte-to-macrophage differentiation [44], was greater on CD14+ monocytes from healthy women than from healthy men. Hence, CD14+ monocytes from women may have a greater propensity to accumulate in inflamed lung and are potentially primed to become pathogenic macrophages. CX3CR1, which promotes monocyte cell survival [29] and M2 macrophage-mediated lung fibrosis [28], was greater on CD14+ monocytes of asthmatic women than on those of asthmatic men. Together, our work suggests that CD14+ monocytes in women may be responsible, in part, for sex differences in asthma-associated allergic inflammation, as they are recruited more easily, survive more efficiently, and have less capacity to down regulate local CCL2 responses than monocytes from men.
Our comparison of IL-4- and IL-13–induced M2 genes in primary human monocytes and MDMs revealed important differences between asthmatic and healthy subjects, monocytes and MDMs, and men and women. Notably, the greater responsiveness of monocytes and MDMs from asthmatic subjects to IL-4 and IL-13 resulted in increased M2 gene expression. Our data suggest that, with monocytes as potential source of CCL17 and MDMs as potential source of CCL22, allergic inflammation would be compounded by increased Th2 cell recruitment in the lungs of asthmatic women. Indeed, blockade of CCL17, CCL22, and their receptor, CCR4, suppresses inflammation in various models [45] and might represent a potential therapy for asthma in women. TGM2, MMP12, and CD200R were dramatically induced by IL-4/13 in MDMs. Moreover, MDMs from asthmatic women expressed MMP12 and TGM2 mRNA to a high degree even in the absence of cytokine. Greater induction of the negative regulator CD200R in monocytes from asthmatic men and MDMs from asthmatic and healthy men suggests that MDMs from men may downregulate activated M2 responses more efficiently than MDMs from women. Future studies will test expression of these and other M2 marker proteins, and the functional consequences of differential chemokine receptor expression on monocyte recruitment and cell survival.
We found that the four IL-4/−13 receptor subunits are expressed predominantly on CD14+ cells. Analysis of all receptor chains is essential because the ratio of subunits that compose functionally competent type I and II IL-4 receptor complexes dictates the outcome of IL-4/−13 responses [46]. In particular, γC expression and type I IL-4 receptor engagement specifically activates IRS-2 signaling and more robust M2 gene expression in macrophages [46]. The elevated expression of γC on CD14+ monocytes from asthmatic subjects, coupled with the higher M2 gene expression observed in women and asthmatic subjects, led us to conclude that CD14+ monocytes from asthmatics and from women have enhanced sensitivity to IL-4. Greater expression of γC likely also affects CD14+ monocyte responses to other asthma-associated cytokines, IL-2, IL-7, IL-9, IL-15, and IL-21, which utilize γC-containing receptor complexes. How sex differences in γC expression on monocytes affect the IL-4/type I receptor-triggered signaling pathways to increase M2 gene expression and responses to other γC cytokines will be the subject of future studies.
Previous reports have highlighted that asthma prevalence and symptoms differ among adults depending on genetic background [47] and age [48]. Here, we carefully matched the male and female blood donor pairs for age and race and sampled subject pairs when the female was in the mid-luteal phase (day 21) of the menstrual cycle. Subsequently, cells were cultured in autologous serum to exclude animal serum proteins or hormones for our M2 gene expression studies, we measured the relative gene expression of our matched subject pairs using the unstimulated male value as the baseline for comparison. This approach was useful for revealing baseline differences in gene expression between men and women, but it added greater variability in values from the female groups. In addition, although subjects taking systemic steroids were excluded from our study, it was impractical to exclude asthmatics using over-the-counter medications, such as antihistamines (Table 1); these compounds also have the potential to impact the monocyte-MDM phenotype and responses. Nonetheless, we were able to detect disease and sex-specific differences in monocytes and MDMs.
Although we found the expected differences in female and male sex hormones and ER α expression between men and women, surprisingly, AR expression was higher in female than in male monocytes. How this expression affects monocyte biology in women that lack significant concentrations of androgen is unknown [49], although both E2 and DHT promote M2 macrophage polarization in vitro [23,39].
5. Conclusions
Here, we have shown that monocytes and MDMs from asthmatic and healthy women and men differed in their responsiveness to IL-4/−13 by sex that could impact the outcome of allergic asthma. In general, MDMs expressed IL-4-induced M2 genes more robustly than monocytes. Similarly, asthmatic MDMs were more responsive than MDMs from healthy men and women. This reveals the importance of MDMs as mediators of inflammation in asthma and suggests why women suffer more severe asthma in general than men. Analysis of cell surface IL-4 receptor complexes and chemokine receptors revealed that the four IL-4/−13 receptor subunits are expressed predominantly on CD14+ cells compared to CD16+, that γC expression was greater on MDMs from asthmatics than from healthy subjects, and that asthmatic women had significantly more γC than did asthmatic men. Also, interesting differences were found in chemokine receptor expression on monocytes. CD14+ cells from asthmatic women may be responsible, in part, for sex differences in asthma-associated allergic inflammation as might have a decreased regulatory profile (lower expression of CCR2), accelerated maturation into macrophages (increased CCR5), and increased survival (increased CX3CR1) when compared to monocytes from men.
In summary, we have shown important differences between monocytes and MDMs from asthmatic and healthy subjects and from men and women. Our results suggest that targeting recruitment, cytokine responsivity, and hormone responses have the potential to be exploited to treat sex-related asthma exacerbations.
Supplementary Material
Acknowledgments
The authors extend their gratitude to the University of Virginia Center for Research in Reproduction’s Ligand Assay and Analysis Core for serum sex hormone measurements. Support for statistical analysis was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by grant number UL1 TR003098 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH, and NIH Roadmap for Medical Research. We thank Claire Levine, ELS, and Melina V. Jones, Ph. D., for help in editing the manuscript.
Funding
This research was supported by funding from National Institutes of Health Grant R01 HL124477 (to N.M.H.).
Abbreviations:
- AMs
alveolar macrophages
- MDMs
monocyte-derived macrophages
- M-CSF
macrophage colony-stimulating factor
- ELISA
Enzyme-linked immunosorbent assay
- SOCS
Suppressor of cytokine signaling
- PBMC
peripheral blood mononuclear cell
- BAL
bronchoalveolar lavage
- FEV1
Forced expiratory volume in 1s
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cellimm.2020.104252.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Kynyk JA, Mastronarde JG, McCallister JW, Asthma, the sex difference, Curr. Opin. Pulm. Med. 17 (2011) 6–11. [DOI] [PubMed] [Google Scholar]
- [2].Koper I, Hufnagl K, Ehmann R, Gender aspects and influence of hormones on bronchial asthma - secondary publication and update, World Allergy Organ. J. 10 (2017) 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bartemes K, Chen CC, Iijima K, Drake L, Kita H, IL-33-responsive group 2 innate lymphoid cells are regulated by female sex hormones in the uterus, J. Immunol. 200 (2018) 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Laffont S, Blanquart E, Savignac M, Cenac C, Laverny G, Metzger D, Girard JP, Belz GT, Pelletier L, Seillet C, Guery JC, Androgen signaling negatively controls group 2 innate lymphoid cells, J. Exp. Med. 214 (2017) 1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vellutini M, Viegi G, Parrini D, Pedreschi M, Baldacci S, Modena P, Biavati P, Simoni M, Carrozzi L, Giuntini C, Serum immunoglobulins E are related to menstrual cycle, Eur. J. Epidemiol. 13 (1997) 931–935. [DOI] [PubMed] [Google Scholar]
- [6].Di Lorenzo G, Mansueto P, Melluso M, Morici G, Norrito F, Esposito Pellitteri M, Di Salvo A, Colombo A, Candore G, Caruso C, Non-specific airway hyperresponsiveness in mono-sensitive Sicilian patients with allergic rhinitis. Its relationship to total serum IgE levels and blood eosinophils during and out of the pollen season, Clin. Exp. Allergy 27 (1997) 1052–1059. [DOI] [PubMed] [Google Scholar]
- [7].Narita S, Goldblum RM, Watson CS, Brooks EG, Estes DM, Curran EM, Midoro-Horiuti T, Environmental estrogens induce mast cell degranulation and enhance IgE-mediated release of allergic mediators, Environ. Health Perspect. 115 (2007) 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zaitsu M, Narita S, Lambert KC, Grady JJ, Estes DM, Curran EM, Brooks EG, Watson CS, Goldblum RM, Midoro-Horiuti T, Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx, Mol. Immunol. 44 (2007) 1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Koziol-White CJ, Goncharova EA, Cao G, Johnson M, Krymskaya VP, Panettieri RA Jr., DHEA-S inhibits human neutrophil and human airway smooth muscle migration, BBA 2012 (1822) 1638–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Melgert BN, ten Hacken NH, Rutgers B, Timens W, Postma DS, Hylkema MN, More alternative activation of macrophages in lungs of asthmatic patients, J. Allergy Clin. Immunol. 127 (2011) 831–833. [DOI] [PubMed] [Google Scholar]
- [11].Bang BR, Chun E, Shim EJ, Lee HS, Lee SY, Cho SH, Min KU, Kim YY, Park HW, Alveolar macrophages modulate allergic inflammation in a murine model of asthma, Exp. Mol. Med. 43 (2011) 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Draijer C, Boorsma CE, Robbe P, Timens W, Hylkema MN, Ten Hacken NH, van den Berge M, Postma DS, Melgert BN, Human asthma is characterized by more IRF5+ M1 and CD206+ M2 macrophages and less IL-10+ M2-like macrophages around airways compared with healthy airways, J. Allergy Clin. Immunol. 140 (2017) 280–283 e283. [DOI] [PubMed] [Google Scholar]
- [13].Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV, T-helper type 2-driven inflammation defines major subphenotypes of asthma, Am. J. Respir. Crit. Care Med. 180 (2009) 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choy DF, Modrek B, Abbas AR, Kummerfeld S, Clark HF, Wu LC, Fedorowicz G, Modrusan Z, Fahy JV, Woodruff PG, Arron JR, Gene expression patterns of Th2 inflammation and intercellular communication in asthmatic airways, J. Immunol. 186 (2011) 1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, Chen CI, Anekalla KR, Joshi N, Williams KJN, Abdala-Valencia TJ Yacoub M. Chi, Chiu S, Gonzalez-Gonzalez FJ, Gates K, Lam AP, Nicholson TT, Homan PJ, Soberanes S, Dominguez S, Morgan VK, Saber R, Shaffer A, Hinchcliff M, Marshall SA, Bharat A, Berdnikovs S, Bhorade SM, Bartom ET, Morimoto RI, Balch WE, Sznajder JI, Chandel NS, Mutlu GM, Jain M, Gottardi CJ, Singer BD, Ridge KM, Bagheri N, Shilatifard A, Budinger GRS, Perlman H, Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span, J. Exp. Med. 214 (2017) 2387–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zaslona Z, Przybranowski S, Wilke C, van Rooijen N, Teitz-Tennenbaum S, Osterholzer JJ, Wilkinson JE, Moore BB, Peters-Golden M, Resident alveolar macrophages suppress, whereas recruited monocytes promote, allergic lung inflammation in murine models of asthma, J. Immunol. 193 (2014) 4245–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M, The chemokine system in diverse forms of macrophage activation and polarization, Trends Immunol. 25 (2004) 677–686. [DOI] [PubMed] [Google Scholar]
- [18].Kawasaki S, Takizawa H, Yoneyama H, Nakayama T, Fujisawa R, Izumizaki M, Imai T, Yoshie O, Homma I, Yamamoto K, Matsushima K, Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice, J. Immunol. 166 (2001) 2055–2062. [DOI] [PubMed] [Google Scholar]
- [19].Staples KJ, Hinks TSC, Ward JA, Gunn V, Smith C, Djukanovi R ć, Phenotypic characterization of lung macrophages in asthmatic patients: overexpression of CCL17, J. Allergy Clin. Immunol. 130 (6) (2012) 1404–1412.e7, 10.1016/j.jaci.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Madala SK, Pesce JT, Ramalingam TR, Wilson MS, Minnicozzi S, Cheever AW, Thompson RW, Mentink-Kane MM, Wynn TA, Matrix metalloproteinase 12-deficiency augments extracellular matrix degrading metalloproteinases and attenuates IL-13-dependent fibrosis, J. Immunol. 184 (2010) 3955–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hallstrand TS, Wurfel MM, Lai Y, Ni Z, Gelb MH, Altemeier WA, Beyer RP, Aitken ML, Henderson WR, Transglutaminase 2, a novel regulator of eicosanoid production in asthma revealed by genome-wide expression profiling of distinct asthma phenotypes, PLoS ONE 5 (2010) e8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Holt PG, Strickland DH, The CD200-CD200R axis in local control of lung inflammation, Nat. Immunol. 9 (2008) 1011–1013. [DOI] [PubMed] [Google Scholar]
- [23].Keselman A, Fang X, White PB, Heller NM, Estrogen signaling contributes to sex differences in macrophage polarization during asthma, J. Immunol. 199 (2017) 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M, Hylkema MN, Ray A, Macrophages: regulators of sex differences in asthma? Am. J. Respir. Cell Mol. Biol. 42 (2010) 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kavanaugh ML, Jerman J, Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014, Contraception, 97 (2018) 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Graziottin A, Serafini A, Perimenstrual asthma: from pathophysiology to treatment strategies, Multidiscip. Respir. Med. 11 (2016) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Konig P, Ner Z, Acton JD, Ge B, Hewett J, Is an FEV1 of 80% predicted a normal spirometry in cystic fibrosis children and adults? Clin. Respir. J. 12 (2018) 2397–2403. [DOI] [PubMed] [Google Scholar]
- [28].Ishida Y, Kimura A, Nosaka M, Kuninaka Y, Hemmi H, Sasaki I, Kaisho T, Mukaida N, Kondo T, Essential involvement of the CX3CL1-CX3CR1 axis in bleomycin-induced pulmonary fibrosis via regulation of fibrocyte and M2 macrophage migration, Sci. Rep. 7 (2017) 16833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S, CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival, Blood 113 (2009) 963–972. [DOI] [PubMed] [Google Scholar]
- [30].Weber C, Weber KS, Klier C, Gu S, Wank R, Horuk R, Nelson PJ, Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and T(H)1-like/CD45RO(+) T cells, Blood 97 (2001) 1144–1146. [DOI] [PubMed] [Google Scholar]
- [31].Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV Jr., Broxmeyer HE, Charo IF, Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice, J. Clin. Invest. 100 (1997) 2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF, Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites, J. Clin. Invest. 117 (2007) 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stein M, Keshav S, Harris N, Gordon S, Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation, J. Exp. Med. 176 (1992) 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Van Dyken SJ, Locksley RM, Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease, Annu. Rev. Immunol. 31 (2013) 317–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, Powers CA, Proudfoot AE, Coyle AJ, Gearing D, Gutierrez-Ramos JC, Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation, J. Immunol. 163 (1999) 403–411. [PubMed] [Google Scholar]
- [36].Hinks TSC, Brown T, Lau LCK, Rupani H, Barber C, Elliott S, Ward JA, Ono J, Ohta S, Izuhara K, Djukanovic R, Kurukulaaratchy RJ, Chauhan A, Howarth PH, Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1, J. Allergy Clin. Immunol. 138 (2016) 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mukhopadhyay S, Sypek J, Tavendale R, Gartner U, Winter J, Li W, Page K, Fleming M, Brady J, O’Toole M, Macgregor DF, Goldman S, Tam S, Abraham W, Williams C, Miller DK, Palmer CN, Matrix metalloproteinase-12 is a therapeutic target for asthma in children and young adults, J. Allergy Clin. Immunol. 126 (2010) 70–76 e16. [DOI] [PubMed] [Google Scholar]
- [38].Warren KJ, Fang X, Gowda NM, Thompson JJ, Heller NM, The TORC1-activated proteins, p70S6K and GRB10, regulate IL-4 signaling and M2 macrophage polarization by modulating phosphorylation of insulin receptor substrate-2, J. Biol. Chem. 291 (2016) 24922–24930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Becerra-Diaz M, Strickland AB, Keselman A, Heller NM, Androgen and androgen receptor as enhancers of M2 macrophage polarization in allergic lung inflammation, J. Immunol. 201 (2018) 2923–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McCormick SM, Gowda N, Fang JX, Heller NM, Suppressor of cytokine signaling (SOCS)1 regulates interleukin-4 (IL-4)-activated insulin receptor substrate (IRS)-2 tyrosine phosphorylation in monocytes and macrophages via the proteasome, J. Biol. Chem. 291 (2016) 20574–20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fox JM, Kausar F, Day A, Osborne M, Hussain K, Mueller A, Lin J, Tsuchiya T, Kanegasaki S, Pease JE, CXCL4/Platelet Factor 4 is an agonist of CCR1 and drives human monocyte migration, Sci. Rep. 8 (2018) 9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kawanaka N, Yamamura M, Aita T, Morita Y, Okamoto A, Kawashima M, Iwahashi M, Ueno A, Ohmoto Y, Makino H, CD14+, CD16+ blood monocytes and joint inflammation in rheumatoid arthritis, Arthritis Rheum. 46 (2002) 2578–2586. [DOI] [PubMed] [Google Scholar]
- [43].Tylaska LA, Boring L, Weng W, Aiello R, Charo IF, Rollins BJ, Gladue RP, Ccr2 regulates the level of MCP-1/CCL2 in vitro and at inflammatory sites and controls T cell activation in response to alloantigen, Cytokine 18 (2002) 184–190. [DOI] [PubMed] [Google Scholar]
- [44].Tuttle DL, Harrison JK, Anders C, Sleasman JW, Goodenow MM, Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1, J. Virol. 72 (1998) 4962–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Perros F, Hoogsteden HC, Coyle AJ, Lambrecht BN, Hammad H, Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation, Allergy 64 (2009) 995–1002. [DOI] [PubMed] [Google Scholar]
- [46].Heller NM, Qi X, Junttila IS, Shirey KA, Vogel SN, Paul WE, Keegan AD, Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages, Sci. Signal, 1 (2008) ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ejebe IH, Jacobs EA, Wisk LE, Persistent differences in asthma self-efficacy by race, ethnicity, and income in adults with asthma, J. Asthma 52 (2015) 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dunn RM, Lehman E, Chinchilli VM, Martin RJ, Boushey HA, Israel E, Kraft M, Lazarus SC, Lemanske RF, Lugogo NL, Peters SP, Sorkness CA, Szefler S, Wechsler ME, Network NACR, Impact of age and sex on response to asthma therapy, Am. J. Respir. Crit. Care Med. 192 (2015) 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Becerra-Diaz M, Song M, Heller N, Androgen and androgen receptors as regulators of monocyte and macrophage biology in the healthy and diseased lung, Front. Immunol. 11 (2020) 1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.