Abstract
Purpose of review:
The perinatal period is a time of high risk for insomnia and mental health conditions. The purpose of this review is to critically examine the most recent literature on perinatal insomnia, focusing on unique features of this period which may confer specific risk, associations with depression and anxiety, and emerging work on perinatal insomnia treatment.
Recent findings:
A majority of perinatal women experience insomnia, which may persist for years, and is associated with depression and anxiety. Novel risk factors include personality characteristics, nocturnal perinatal-focused rumination, and obesity. Mindfulness and physical activity may be protective. Cognitive-behavioral therapy for insomnia is an effective treatment.
Summary:
Perinatal insomnia is exceedingly common, perhaps due to factors unique to this period. Although closely linked to perinatal mental health, more work is needed to establish causality. Future work is also needed to establish the role of racial disparities, tailor treatments, and determine whether insomnia treatment improves perinatal mental health.
Keywords: insomnia, perinatal, mental health, depression, anxiety
Introduction
Sleep disturbance during the perinatal period has historically been considered a normative feature of pregnancy and postpartum, dismissed as inconsequential by health care providers and unworthy of attention or treatment beyond reassurance. However, over the past two decades, an accumulating body of work has emerged to shed light on the problem of sleep disorders in the perinatal period, including the significant impact of these disorders on perinatal women and their offspring.
Insomnia is broadly defined as difficulty with sleep onset or maintenance. The International Classification of Sleep Disorders (ICSD) outlines the following diagnostic criteria for insomnia disorder: difficulty falling asleep, difficulty staying asleep, and/or difficulty with waking too early despite adequate opportunity and circumstances for sleep, accompanied by daytime consequences or impairment (e.g., fatigue, difficulty with concentration, mood changes, daytime sleepiness, worry about sleep), experienced at least three nights per week [1]. Insomnia disorder is further characterized within the ICSD by duration: short-term (less than three months’ duration) or chronic (three months or longer). Note that within the sleep literature, a distinction is made between insomnia symptoms (i.e., difficulty with sleep) vs. insomnia disorder, which requires daytime dysfunction to be present. An estimated 30% of adults experience insomnia symptoms, and up to 10% of the adult population suffers from insomnia disorder [2]. Women are disproportionately affected by insomnia relative to men, with 12.8% of women experiencing insomnia vs. 9.7% of men [3].
Insomnia one of the most common and consequential perinatal sleep disorders. Vulnerability to insomnia is greatly heightened during times of reproductive transition, including the perinatal period. Pregnant women with insomnia are at elevated risk for myriad perinatal complications including pre-term birth [4], gestational hypertension [5], and maternal psychiatric illness. Indeed, one of the most well-established consequences of insomnia are psychiatric conditions, particularly depression and anxiety [6]. As the perinatal period is a time of significant risk for both sleep disturbance and psychiatric conditions, the purpose of this review is to examine the most recent literature on perinatal insomnia, highlighting unique features of the perinatal period which may confer specific risk, as well as associations with depression and anxiety, and emerging work on treatment of perinatal insomnia. We note that although there is a larger body of literature regarding sleep quality and sleep disturbances, more broadly defined, in the perinatal period, we have elected to maintain a specific focus on insomnia in this review, and therefore we only include those studies which utilized measures of insomnia diagnostic criteria or insomnia symptoms.
Perinatal Insomnia Affects a Majority of Women
Several large cohort studies have demonstrated that the prevalence of insomnia disorder and clinically significant insomnia symptoms are vastly greater in pregnant and postpartum women relative to the general population of women of childbearing age. Sivertsen and colleagues tracked a cohort of 1,480 women in Norway who were studied at late pregnancy, eight weeks postpartum, and two years postpartum. The prevalence of women who met diagnostic criteria for insomnia disorder per DSM-IV-TR criteria was 60% at late pregnancy and eight weeks postpartum, falling to 41% at two years postpartum. Moreover, 39% of women met diagnostic criteria for insomnia at all three time points; 68% who met criteria during pregnancy still had insomnia at eight weeks postpartum, with 50% of those women still experiencing insomnia at two years postpartum [7]. The stability and persistence of insomnia 2 years after childbirth is consistent with a body of literature supporting the chronic nature of insomnia [8]. In contrast, the prevalence of insomnia disorder in the general population of women aged 18–45 years in Norway is approximately 11% [9]. Similar findings were observed in a cohort study of 486 women in Spain who were assessed for insomnia symptoms prior to pregnancy and at each trimester through six months postpartum [10]. In this study, 6.1% of women reported significant insomnia symptoms prior to gestation, which increased to 44.2% in the first trimester, 46.3% in the second trimester, to a peak of 63.7% by the third trimester. At six months postpartum, 33.2% of women were experiencing significant insomnia symptoms.
Unique Characteristics of the Perinatal Period May Increase Vulnerability to Insomnia
Why might rates of insomnia be so incredibly high during the perinatal period? A myriad of physiological and psychosocial changes during the perinatal period contribute to the development and maintenance of insomnia within the framework of the diathesis-stress model of chronic insomnia, which identifies predisposing factors, precipitating events, and perpetuating behaviors (i.e., Spielman’s “Three P Model” [11]) as critical to the evolution of insomnia into chronicity across time (see Figure 1 for the model as applied to the perinatal period).
Figure 1:
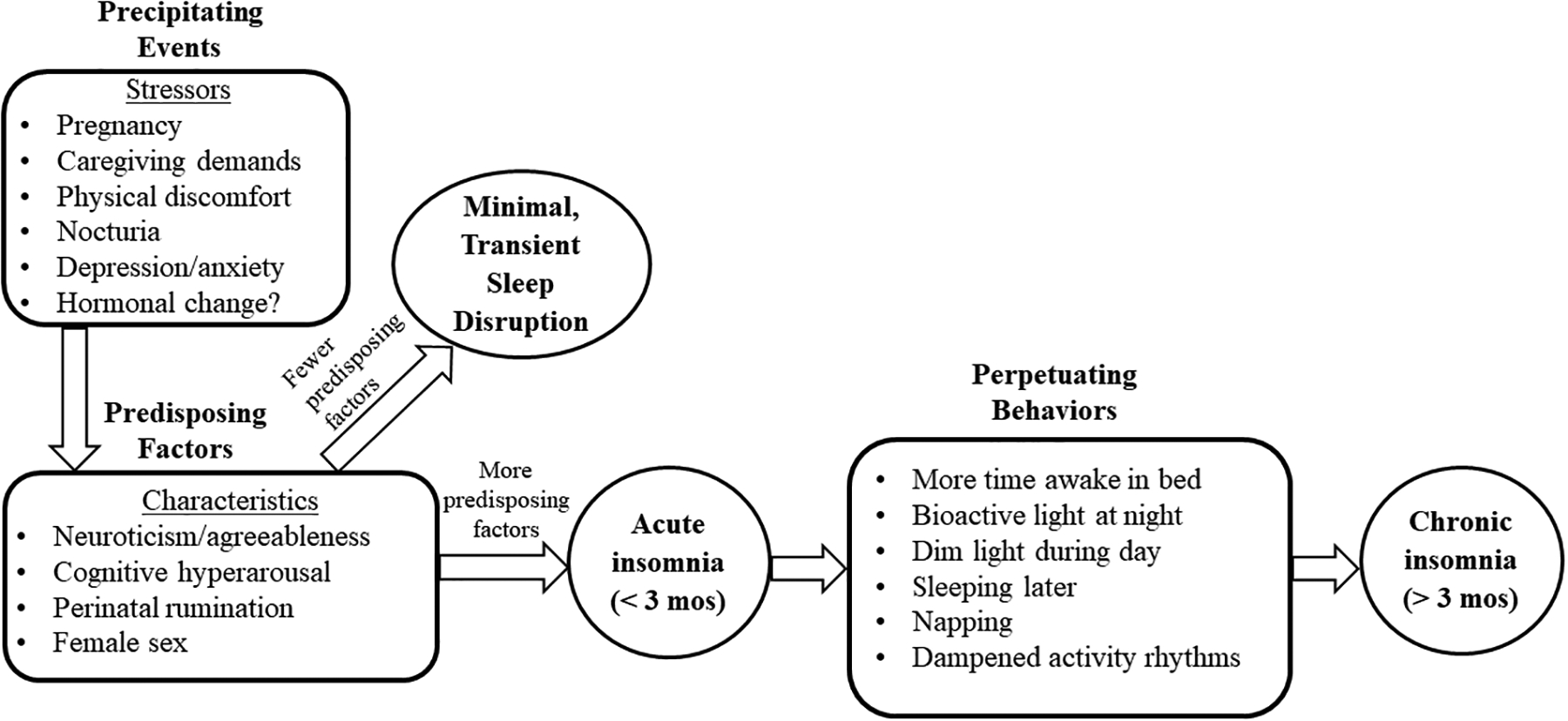
“Three P Model of Insomnia” as Applied to the Perinatal Period (Adapted from Spielman [10])
Perinatal-Specific Predisposing Factors
Predisposing factors are biopsychosocial characteristics which increase vulnerability to insomnia. Insomnia is broadly conceptualized as a disorder of hyperarousal [12, 13], defined as increased physiological (i.e., nervous system) and cognitive activation, and hyperarousal may be a factor which predisposes perinatal women to insomnia. Specifically, there is evidence to suggest that pregnant women experience more cognitive hyperarousal [14] and may be more likely to engage in nocturnal rumination (i.e., repetitive negative thinking at night), relative to non-pregnant women [15], perhaps in part due to hormonal influences [16]. Other possible predisposing factors include personality characteristics and their interaction with hormonal changes; the personality traits of neuroticism (associated with ruminative thought patterns) and agreeableness are concurrently linked to insomnia symptoms during times of reproductive transition in females, including pregnancy [17]. Finally, female sex is a significant predisposing factor for insomnia; women are 1.5 times more likely to experience insomnia relative to men [3].
Pregnancy and Childbirth as Precipitating Events for Insomnia
Acute insomnia is typically precipitated by health/physical changes, stressors (including major life events), and psychiatric conditions [18]; the perinatal period is often accompanied by many, if not all, of these factors. The health and physical changes commonly experienced by perinatal women (physical discomfort, nocturia [19, 20]), as well as caregiving for the infant during the postpartum period [21], may serve as precipitants for insomnia by contributing to nocturnal awakenings and sleep fragmentation. Changes in hormones (i.e., the precipitous drop of estrogen and progesterone after delivery) known to influence the circadian system [22, 23] could also be potential precipitants. Although a recent study did not find a connection between estradiol levels and postpartum insomnia [24], this area is understudied, and more evidence is needed to fully delineate the role of hormones in perinatal insomnia.
Although often a positive event, pregnancy and the birth of a child are major life stressors [25] and identified precipitants for insomnia [26]. Increased stress may contribute to the onset of insomnia via stress-related sleep reactivity [27], associated with insomnia during pregnancy [28]. As a stressor, the perinatal period provides fuel for maladaptive cognitive-emotional regulation strategies, including nocturnal rumination specifically focused on pregnancy and infant/fetal health. Indeed, not only are perinatal women vulnerable to insomnia due to heightened cognitive arousal at night, but recent evidence suggests that pregnant women often specifically worry and ruminate about their pregnancy and fetal/infant health at night, which is linked to perinatal insomnia and depression [15].
In large cohort studies, mental health symptoms, including anxiety (and specifically, fear of childbirth), past history of depression symptoms, and current depression symptoms have been most consistently identified as both concurrent and short-term prospective (i.e., from pregnancy to early postpartum) predictors of perinatal insomnia symptoms [29] and insomnia disorder [30, 31]. However, in contrast, when examined across a longer follow-up period, concurrent maternal depression did not explain the persistence of insomnia disorder through two years postpartum, suggesting that although depression may precipitate the acute episode of insomnia, over time, chronic insomnia may be maintained by factors other than maternal depression [7]. That factors beyond depression may perpetuate chronic insomnia in this population is consistent with Spielman’s “Three P Model” of insomnia.
Perinatal Changes in Sleep Behaviors May Perpetuate Insomnia
When considering perpetuating factors specific to the perinatal period, pregnancy and caring for an infant may elicit many behaviors that are implicated in the transition from an acute, short-term difficulty with sleep to chronic insomnia. Such behaviors include spending long periods awake in bed [32]; sleeping later [33]; and daytime napping [33–35]. Although such behaviors may help to make up for lost sleep and sleep fragmentation in some perinatal women, for others—particularly those who have significant predisposing characteristics—they may serve to maintain the insomnia into chronicity, even as the woman adapts to the initial precipitant, or the precipitant is addressed/treated.
Evidence also implicates perinatal-specific behaviors in the dysregulation of the circadian system, which may perpetuate insomnia due to misalignment between the circadian timing and sleep-wake systems. Such behaviors include little exposure to daytime bright light and exposure to bioactive light at night [36, 37] and dampened circadian and activity rhythmicity [38–40]. Light and physical activity patterns are critical zeitgebers (“time givers”) for the circadian system [41].
The Role of Racial Disparities
Despite the well-established role of racial disparities as a risk factor for poor sleep health among females [42], there is a significant dearth of evidence on racial disparities in perinatal insomnia, as most studies have not enrolled racially/ethnically diverse samples. In a large study of racial disparities in perinatal insomnia symptoms (n > 2,000 pregnant women), black pregnant women were more likely than black non-pregnant women to endorse the insomnia symptom of difficulty falling asleep; this was not observed in other racial/ethnic categories, suggesting that for black women, pregnancy is associated with more sleep disturbance [43]. In a smaller study of pregnant women (n = 267), black women were significantly more likely to have clinically significant insomnia symptoms relative to white women, marked by difficulty falling asleep on ≥ 3 nights per week. Financial status (specifically, poverty) was determined to be the major driver of insomnia symptoms in this study [20]. Overall, the few studies in this area support the critical need for more research on the role of racial disparities and systemic racism on perinatal insomnia.
Potential Protective Factors for Perinatal Insomnia
As most of the work in the arena of perinatal insomnia has emphasized identifying potential risk factors, potential protective factors have been overlooked. Even so, recent evidence suggests that physical activity and mindfulness may protect women against insomnia in the perinatal period. Indeed, physical activity has been associated with a reduced risk of the development of perinatal insomnia symptoms [10], perhaps in part because physical activity reduces obesity, which is a prospective predictor of perinatal insomnia symptoms [10, 30]. Given the potential pathogenicity of rumination for insomnia, perinatal researchers have sought to identify factors that may protect against ruminative thinking. Mindfulness involves intentional and nonjudgmental awareness of internal and external experiences in the present moment and self-compassion. Importantly, ruminative thinking is incompatible with mindfulness. A cross-sectional analysis of 65 pregnant women showed that, although nocturnal rumination was associated with greater symptoms of insomnia and depression, everyday mindfulness was associated with lower insomnia and depressive symptoms. Importantly, women high in mindfulness reported lower levels of rumination relative to those low in mindfulness [44]. This suggests that cultivating mindfulness in pregnancy may reduce ruminative thinking and curb risk for insomnia and depression, but prospective data are needed to investigate this further.
Perinatal Insomnia is Closely Linked to Depression and Anxiety
Depression
Among the most common and debilitating pregnancy complications, up to 19% of perinatal women experience depression [45, 46]. In recognition of the accumulation of research focused on sleep disturbances and depression in the perinatal population, a recent meta-analysis of nine published studies, corresponding to a sample of 1,922 participants, documented a significant, albeit moderate, relationship (r = 0.37), between sleep disturbance and depression. A more robust relationship was observed in cross-sectional studies (r = 0.48) vs. the five longitudinal studies (r = 0.25). Factors such as age, marital status, and breastfeeding did not moderate the depression-sleep disturbance relationship [47].
Insomnia and depression are intricately, bi-directionally linked in a complex relationship [48], and prospective studies are needed to fully understand whether insomnia plays a causal role in perinatal depression. Two recent longitudinal studies have examined the role of prenatal insomnia symptoms during pregnancy in the development of postpartum depression. In a population-based study of nearly 2,000 women, participants were followed from late pregnancy to eight weeks postpartum. Insomnia symptoms in late pregnancy were predictive of postpartum-onset depression symptoms, but only for women who had a past history of depression [29]. In another recent longitudinal study, nearly 600 women participated in clinician-led semi-structured interviews at late pregnancy and again at three months postpartum. Insomnia disorder was found to predict postpartum depression symptoms, but not postpartum depression diagnosis. Adjustment for a past history of depression and affect (negative and positive) also negated the insomnia-depression relationship [49].
As a past history of depression is an established, significant risk factor for postpartum depression, it is critical to identify factors, particularly those which may be modifiable, that influence postpartum depression recurrence. In a prospective study of more than 300 pregnant women who had a history of depression, but were not depressed at the time of enrollment, participants completed a clinician-rated depression measure at the third trimester and again in early postpartum period. Analysis of the specific symptom domains captured in this depression measure identified difficulty falling asleep as one of only three specific symptoms which were significant predictors of postpartum depression recurrence. Specifically, participants who reported nightly trouble falling asleep were nearly four times as likely to experience a recurrence relative to those who experienced difficulty falling asleep occasionally or less often [50].
Taken together, prospective studies in the perinatal population suggest that although insomnia symptoms/disorder play a role in increasing the risk of postpartum depression, it is possible that other factors such as a past history of depression, or negative affect, may be more robustly influential in the causal pathway. However, there is strong evidence in the non-perinatal adult literature showing that insomnia is a prospective predictor of new-onset psychiatric illness (see [6] for a recent review). Although there are likely many reasons for discrepancies between the non-perinatal adult and perinatal literature in this area, we wish to highlight that the methods used in the existing prospective literature on perinatal insomnia and depression are a major limitation. Specifically, these studies have exclusively assessed prenatal insomnia as a predictor for postpartum depression. This research design misses two influential factors. First, significant changes in sleep which occur in the postpartum period, which are likely influential to mood—that is, a woman who has good sleep during pregnancy may develop insomnia in the postpartum period, which may lead her to become depressed, but this would be missed in a design which exclusively focused on prenatal insomnia as the exposure. Second, changes in depression may occur during pregnancy, but such changes would not be captured when depression is exclusively measured as an outcome only in the postpartum period. Thus, studies which examine only the effects of prenatal insomnia on postpartum depression fail to account for the critical sleep changes which occur after childbirth, and miss important prenatal changes in depressive symptoms, thereby limiting the ability to fully detect effects of perinatal insomnia on depression.
Anxiety
Perinatal anxiety, although exceptionally common, is considerably less studied than depression in the context of insomnia. In an early study of 257 perinatal women who presented to an outpatient psychiatric clinic, insomnia symptoms were concurrently associated with generalized anxiety symptoms after controlling for concurrent depression symptoms [51]. More recent studies have used prospective designs with larger sample sizes to more fully elucidate this relationship. A cohort study of more than 500 women followed from mid-pregnancy to early postpartum found that insomnia symptoms at mid-pregnancy were concurrently associated with anxiety during pregnancy, and also predictive of anxiety in early postpartum. A unique feature of this study was assessment of symptoms of obsessive-compulsive disorder (OCD), which is particularly relevant for this population, as the perinatal period is established as a time of greater vulnerability to OCD [52]. Findings showed that insomnia symptoms at mid-pregnancy were predictive of OCD symptoms at eight weeks postpartum [30]. In a similar cohort study completed by the same group, insomnia symptoms during late pregnancy predicted postpartum anxiety, even after accounting for depression [31].
Overall, these findings suggest that insomnia predicts perinatal anxiety, even after controlling for depression. There may be a unique association between insomnia and perinatal anxiety which perinatal depression does not fully explain. As discussed earlier, nocturnal rumination—and more specifically, nocturnal rumination focused on pregnancy and fetal/infant health—is significantly associated with perinatal insomnia [15, 44]. A potentially ripe area of focus for future work is the interplay among perinatal anxiety, perinatal rumination, and insomnia. For example, spending time in bed awake due to acute insomnia may lead to more opportunity for women to engage in ruminative thinking about perinatal-specific anxieties, which in turn may lead insomnia into chronicity via repeated pairing of the bed and negative, ruminative thinking. Such studies could also inform novel interventional targets for perinatal insomnia, such as ruminative thinking.
Cognitive-Behavioral Therapy for Perinatal Insomnia
The recommended first-line treatment for chronic insomnia, cognitive-behavioral therapy for insomnia (CBT-I), is a brief (4–6 sessions), multi-component, non-pharmacological intervention; it targets the factors that perpetuate insomnia [53, 54]. Its efficacy is well-established across numerous randomized controlled trials in non-perinatal adults, which show average treatment response rates between 70–80% [55–57]. Typical remission rates average 50% [58, 59]. Importantly, treatment gains are durable over time, as demonstrated across 2–3 year follow-up periods in several rigorous studies [60–63]. Further, cognitive-behavioral therapy for insomnia also has well-established efficacy for insomnia comorbid with many conditions (including anxiety and depression), with moderate to large effect sizes observed on sleep parameters in a recent meta-analysis [64]. However, the unique features of the perinatal period require separate assessment of CBT-I in this population. Not surprisingly, pregnant women prefer CBT-I over pharmacotherapy or acupuncture [65].
Three recent randomized controlled trials found CBT-I to be effective in both face-to-face [66] and digital formats [67, 68] for insomnia during pregnancy. In the face-to-face trial, 179 participants who were between 18–32 weeks’ gestation and met diagnostic criteria for insomnia were randomly assigned to either CBT-I or a behavioral control condition. Greater reductions in insomnia symptom severity were observed in the CBT-I group vs. control, further bolstered by a higher rates of insomnia remission, which was also achieved faster relative to control [66]. A small, but significant, effect size was observed between groups for reduction in depression symptoms.
Although face-to-face CBT-I was clearly efficacious for insomnia during pregnancy, the small number of providers who are trained in CBT-I may limit its widespread utility. Therefore, studies evaluating more accessible options, such as digital CBT-I, are important. To date, the two randomized controlled trials evaluating digital CBT-I in perinatal women have delivered digital CBT-I using the established online program Sleepio (Big Health). Sleepio is a standardized, fully automated online CBT-I program; the program is entirely self-guided and does not involve any interaction with a therapist. In the first randomized controlled trial of digital CBT-I, delivered using Sleepio, 208 women were enrolled up to 28 weeks’ gestation [67]. Of note, some participants were eligible on the basis of insomnia symptoms, not insomnia disorder per se. Participants were randomized to either a full course of Sleepio or “standard care” (which encompassed their routine clinical care, including medications, alternative treatments, psychotherapy, etc). Sleepio was associated with medium-to-large effects on insomnia symptom parameters, which were found to be maintained 2-months postpartum. A small, but significant effect was observed on depression symptoms. In the second randomized controlled trial of digital CBT-I, 91 participants with clinically significant insomnia symptoms were enrolled around the 3rd trimester and randomly assigned to either Sleepio or a digital sleep education control condition [68]. Participants were followed through 6 weeks postpartum. Digital CBT-I was associated with significant improvements in insomnia symptoms relative to control; participants in this group also had better sleep and fewer insomnia symptoms at 6 weeks postpartum. However, there were no differences on depression symptoms between the groups, and CBT-I did not significantly reduce cognitive hyperarousal symptoms.
To date, there is only one trial of CBT-I in postpartum women, which showed that postpartum women with comorbid insomnia and depression report significant reductions in insomnia and depressive symptoms after face-to-face CBT-I Importantly, this trial showed that postpartum women are capable of engaging in behavioral sleep treatment, despite challenges such as disrupted sleep due to infant waking and nighttime feedings. While this trial is limited by an uncontrolled design, these results suggest that CBT-I may significantly benefit postpartum women with insomnia and depression [69].
As a whole, the results of these trials suggest that CBT-I should be a recommended treatment for insomnia during pregnancy and early postpartum. The findings on depression, however, are limited because these trials did not enroll women who were specifically experiencing depression.
The Future of Perinatal Insomnia Treatments
As discussed above, several unique perinatal factors can trigger and perpetuate perinatal insomnia, including pregnancy/maternal-specific worries, physical discomfort and pain, and caregiving for the infant. Combined, these factors can lead to significant changes in maternal sleep patterns. Sleep treatments for perinatal women may benefit from providing education and normalization of these experiences, in addition to behavioral and cognitive strategies to manage these sleep-interfering challenges.
Perinatal women have difficulty adhering to rigid behavioral sleep strategies. These adherence difficulties necessitate the development of behavioral sleep strategies that improve sleep efficiency and stabilize sleep-wake patterns, while permitting flexibility to compensate for sleep deprivation (e.g., due to nighttime feedings or dysregulated infant sleep) and childcare. Prior CBT-I trials for perinatal insomnia added 30 minutes to habitual time-in-bed when prescribing sleep schedules, never reduced the sleep window to less than 6 hours, and provided patients with bed- and wake-time windows (30–60-min) to accommodate variable infant sleep patterns [66, 69]. Notably, flexibility in bed/wake-times does not reduce treatment efficacy [70].
Specific to the postpartum period, infant sleep problems and nighttime feedings must be navigated in treatment. Education about typical infant sleep patterns in the first few months of life may help women to have realistic expectations about normative postpartum sleep fragmentation. Encouraging equitable nocturnal caregiving with partners when available may enhance CBT-I efficacy, which was an important augmentation utilized previously in a study of CBT-I for postpartum insomnia [69]. As sleep begins to consolidate by 6 months for most infants, postpartum maternal sleep treatment may include teaching women behavioral strategies to promote infant sleep.
Conclusions
Over the past 20 years, scientific discoveries regarding the ubiquity and consequences of perinatal insomnia have elevated it from dismissible complaint to serious perinatal complication in need of treatment. Insomnia increases risk for perinatal depression, anxiety, cardiometabolic disorders, and childbirth complications. To maximally reduce risk for perinatal complications, the field must better understand both risk and protective factors for perinatal insomnia in an effort to curb risk before an insomnia disorder becomes fully established in pregnancy or postpartum. In recent years, nocturnal rumination (particularly focused on pregnancy and fetal/infant health concerns) and obesity have been identified as novel, potentially modifiable risk factors, whereas mindfulness and physical activity may serve as protective factors against insomnia development. As behavioral factors which may contribute to circadian dysregulation are common among perinatal women, the circadian system and its role in perinatal insomnia is another area ripe for further study. An overarching, major limitation in the existing body of work in perinatal insomnia is the significant homogeneity of study samples with respect to race and ethnicity. Racial and ethnic disparities and the role of systemic racism in perinatal insomnia remain critically important areas for future investigation.
To fully understand the role of perinatal insomnia as a risk factor for depression, more prospective studies are needed, particularly work which examines new-onset depression in both pregnancy and postpartum, as well as studies which are designed to allow for consideration of both sleep and depression throughout the entire perinatal period. In just the past two years, the first randomized, controlled insomnia therapy trials were conducted in pregnant women. While the current state of the science supports the use of traditional CBT-I to improve perinatal insomnia, future investigations of strategies to tailor the treatment for this population to increase adherence and address nocturnal caregiving may optimize treatment outcomes. Other important next steps in the field are to test whether treatment of perinatal insomnia is a viable avenue for addressing comorbid depression, and whether such treatments reduce risk for the development of perinatal depression.
Footnotes
Publisher's Disclaimer: This version of the article has been accepted for publication, after peer review (when applicable) and is subject to Springer Nature’s AM terms of use, but is not the Version of Record and does not reflect post-acceptance improvements, or any corrections. The Version of Record is available online at: http://dx.doi.org/10.1007/s11920-020-01198-5
Human and Animal Rights. This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd Edition. Darien, IL: American Academy of Sleep Medicine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7–10. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. [DOI] [PubMed] [Google Scholar]
- 4.Felder JN, Baer RJ, Rand L, Jelliffe-Pawlowski LL, Prather AA. Sleep disorder diagnosis during pregnancy and risk of preterm birth. Obstet Gynecol. 2017;130(3):573–81. doi: 10.1097/aog.0000000000002132. [DOI] [PubMed] [Google Scholar]
- 5.Okun ML, O’Brien LM. Concurrent insomnia and habitual snoring are associated with adverse pregnancy outcomes. Sleep Med. 2018;46:12–9. doi: 10.1016/j.sleep.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Pigeon WR, Bishop TM, Krueger KM. Insomnia as a precipitating factor in new onset mental illness: a systematic review of recent findings. Curr Psychiatry Rep. 2017;19(8):44. doi: 10.1007/s11920-017-0802-x. [DOI] [PubMed] [Google Scholar]
- 7. ••.Sivertsen B, Hysing M, Dorheim SK, Eberhard-Gran M. Trajectories of maternal sleep problems before and after childbirth: a longitudinal population-based study. BMC Pregnancy Childbirth. 2015;15:129. doi: 10.1186/s12884-015-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; This longitdinal study followed perinatal women from pregnancy through the longest period to date (two years postpartum) to establish the typical course of perinatal insomnia disorder using a population-based sample.
- 8.Morin CM, Belanger L, LeBlanc M, Ivers H, Savard J, Espie CA, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169(5):447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 9.Pallesen S, Sivertsen B, Nordhus IH, Bjorvatn B. A 10-year trend of insomnia prevalence in the adult Norwegian population. Sleep Med. 2014;15(2):173–9. doi: 10.1016/j.sleep.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Román-Gálvez RM, Amezcua-Prieto C, Salcedo-Bellido I, Martínez-Galiano JM, Khan KS, Bueno-Cavanillas A. Factors associated with insomnia in pregnancy: A prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2018;221:70–5. doi: 10.1016/j.ejogrb.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541–53. [PubMed] [Google Scholar]
- 12.Bonnet MH, Arand DL. Hyperarousal and insomnia: State of the science. Sleep Med Rev. 2010;14(1):9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Michopoulos V, Rothbaum AO, Corwin E, Bradley B, Ressler KJ, Jovanovic T. Psychophysiology and posttraumatic stress disorder symptom profile in pregnant African-American women with trauma exposure. Arch Womens Ment Health. 2015;18(4):639–48. doi: 10.1007/s00737-014-0467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalmbach DA, Cheng P, Ong JC, Ciesla JA, Kingsberg SA, Sangha R, et al. Depression and suicidal ideation in pregnancy: exploring relationships with insomnia, short sleep, and nocturnal rumination. Sleep Med. 2020;65:62–73. doi: 10.1016/j.sleep.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. 2005;84(3):701–10. doi: 10.1016/j.fertnstert.2005.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dørheim SK, Garthus-Niegel S, Bjorvatn B, Eberhard-Gran M. Personality and perinatal maternal insomnia: A study across childbirth. Behav Sleep Med. 2016;14(1):34–48. doi: 10.1080/15402002.2014.941063. [DOI] [PubMed] [Google Scholar]
- 18.LeBlanc M, Merette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32(8):1027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mindell JA, Jacobson BJ. Sleep disturbances during pregnancy. J Obstet Gynecol Neonatal Nurs. 2000;29(6):590–7. [DOI] [PubMed] [Google Scholar]
- 20.Kalmbach DA, Cheng P, Sangha R, O’Brien LM, Swanson LM, Palagini L, et al. Insomnia, short sleep, and snoring in mid-to-late pregnancy: Disparities related to poverty, race, and obesity. Nat Sci Sleep. 2019;11:301–15. doi: 10.2147/NSS.S226291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery-Downs H, Insana S, Clegg-Kraynok M, Mancini L. Normative longitudinal maternal sleep: the first 4 postpartum months. Am J Obstet Gynecol. 2010;203(5):465.e1–.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leibenluft E Do gonadal steroids regulate circadian rhythms in humans? J Affect Disord. 1993;29(2–3):175–81. [DOI] [PubMed] [Google Scholar]
- 23.Lord C, Sekerovic Z, Carrier J. Sleep regulation and sex hormones exposure in men and women across adulthood. Pathol Biol (Paris). 2014;62(5):302–10. doi: 10.1016/j.patbio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Drozdowicz-Jastrzębska E, Skalski M, Gdańska P, Mach A, Januszko P, Nowak RJ, et al. Insomnia, postpartum depression and estradiol in women after delivery. Metab Brain Dis. 2017;32(6):1913–8. doi: 10.1007/s11011-017-0079-0. [DOI] [PubMed] [Google Scholar]
- 25.Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res. 1967;11(2):213–8. [DOI] [PubMed] [Google Scholar]
- 26.Bastien C, Vallires A, Morin C. Precipitating factors of insomnia. Behav Sleep Med. 2004;2(1):50–62. [DOI] [PubMed] [Google Scholar]
- 27.Drake CL, Kalmbach DA, Arnedt JT, Cheng P, Tonnu CV, Cuamatzi-Castelan A, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2). doi: 10.1093/sleep/zsy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palagini L, Cipollone G, Masci I, Novi M, Caruso D, Kalmbach DA, et al. Stress-related sleep reactivity is associated with insomnia, psychopathology and suicidality in pregnant women: preliminary results. Sleep Med. 2019;56:145–50. doi: 10.1016/j.sleep.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Dorheim SK, Bjorvatn B, Eberhard-Gran M. Can insomnia in pregnancy predict postpartum depression? A longitudinal, population-based study. PloS one. 2014;9(4):e94674. doi: 10.1371/journal.pone.0094674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osnes RS, Eberhard-Gran M, Follestad T, Kallestad H, Morken G, Roaldset JO. Mid-pregnancy insomnia is associated with concurrent and postpartum maternal anxiety and obsessive-compulsive symptoms: A prospective cohort study. J Affect Disord. 2020;266:319–26. doi: 10.1016/j.jad.2020.01.140. [DOI] [PubMed] [Google Scholar]
- 31.Osnes RS, Roaldset JO, Follestad T, Eberhard-Gran M. Insomnia late in pregnancy is associated with perinatal anxiety: A longitudinal cohort study. J Affect Disord. 2019;248:155–65. doi: 10.1016/j.jad.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Montgomery-Downs HE, Clawges HM, Santy EE. Infant feeding methods and maternal sleep and daytime functioning. Pediatrics. 2010;126(6):e1562–8. doi: 10.1542/peds.2010-1269. [DOI] [PubMed] [Google Scholar]
- 33.Swain AM, O’Hara MW, Starr KR, Gorman LL. A prospective study of sleep, mood, and cognitive function in postpartum and nonpostpartum women. Obstet Gynecol. 1997;90(3):381–6. [DOI] [PubMed] [Google Scholar]
- 34.Lillis TA, Hamilton NA, Pressman SD, Khou CS. The association of daytime maternal napping and exercise with nighttime sleep in first-time mothers between 3 and 6 months postpartum. Behav Sleep Med. 2018;16(6):527–41. doi: 10.1080/15402002.2016.1239580. [DOI] [PubMed] [Google Scholar]
- 35.Signal TL, Gander PH, Sangalli MR, Travier N, Firestone RT, Tuohy JF. Sleep duration and quality in healthy nulliparous and multiparous women across pregnancy and post-partum. Aust N Z J Obstet Gynaecol. 2007;47(1):16–22. doi: 10.1111/j.1479-828X.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 36.Tsai S-Y, Barnard K, Lentz M, Thomas K. Twenty-four hours light exposure experiences in postpartum women and their 2–10-week-old infants: an intensive within-subject design pilot study. Int J Nurs Stud. 2009;46(2):181–8. [DOI] [PubMed] [Google Scholar]
- 37.McBean AL, Montgomery-Downs HE. What are postpartum women doing while the rest of the world is asleep? J Sleep Res. 2015;24(3):270–8. doi: 10.1111/jsr.12265. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto K, Shinkoda H, Kang MJ, Seo YJ. Longitudinal study of mothers’ sleep-wake behaviors and circadian time patterns from late pregnancy to postpartum – monitoring of wrist actigraphy and sleep logs. Biol Rhythm Res. 2003;34(3):265–78. doi: 10.1076/brhm.34.3.265.18812. [DOI] [Google Scholar]
- 39.Thomas KA, Burr RL, Spieker S, Lee J, Chen J. Mother-infant circadian rhythm: Development of individual patterns and dyadic synchrony. Early Hum Dev. 2014;90(12):885–90. doi: 10.1016/j.earlhumdev.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin-Fairey CA, Zhao P, Wan L, Roenneberg T, Fay J, Ma X, et al. Pregnancy induces an earlier chronotype in both mice and women. J Biol Rhythms. 2019;34(3):323–31. doi: 10.1177/0748730419844650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quante M, Mariani S, Weng J, Marinac CR, Kaplan ER, Rueschman M, et al. Zeitgebers and their association with rest-activity patterns. Chronobiol Int. 2019;36(2):203–13. doi: 10.1080/07420528.2018.1527347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson CL, Powell-Wiley TM, Gaston SA, Andrews MR, Tamura K, Ramos A. Racial/ethnic disparities in sleep health and potential interventions among women in the United States. J Womens Health. (2002). 2020;29(3):435–42. doi: 10.1089/jwh.2020.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. •.Feinstein L, McWhorter KL, Gaston SA, Troxel WM, Sharkey KM, Jackson CL. Racial/ethnic disparities in sleep duration and sleep disturbances among pregnant and non-pregnant women in the United States. J Sleep Res. :e13000. doi: 10.1111/jsr.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the largest study to compare racial and ethnic disparities in sleep among pregnant and non-pregnant women.
- 44.Kalmbach DA, Roth T, Cheng P, Ong JC, Rosenbaum E, Drake CL. Mindfulness and nocturnal rumination are independently associated with symptoms of insomnia and depression during pregnancy. Sleep Health. 2020;6(2):185–91. doi: 10.1016/j.sleh.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–83. [DOI] [PubMed] [Google Scholar]
- 46.O’Hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry. 1996;8(1):37–54. doi: doi: 10.3109/09540269609037816. [DOI] [Google Scholar]
- 47.Emamian F, Khazaie H, Okun ML, Tahmasian M, Sepehry AA. Link between insomnia and perinatal depressive symptoms: A meta-analysis. J Sleep Res. 2019:e12858. doi: 10.1111/jsr.12858. [DOI] [PubMed] [Google Scholar]
- 48.Manber R, Chambers AS. Insomnia and depression: a multifaceted interplay. Curr Psychiatry Rep. 2009;11(6):437–42. [DOI] [PubMed] [Google Scholar]
- 49.Marques M, Bos S, Soares MJ, Maia B, Pereira AT, Valente J, et al. Is insomnia in late pregnancy a risk factor for postpartum depression/depressive symptomatology? Psychiatry Res. 2011;186(2–3):272–80. doi: 10.1016/j.psychres.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 50.Suri R, Stowe ZN, Cohen LS, Newport DJ, Burt VK, Aquino-Elias AR, et al. Prospective longitudinal study of predictors of postpartum-onset depression in women with a history of major depressive disorder. J Clin Psychiatry. 2017;78(8):1110–6. doi: 10.4088/JCP.15m10427. [DOI] [PubMed] [Google Scholar]
- 51.Swanson LM, Pickett SM, Flynn H, Armitage R. Relationships among depression, anxiety, and insomnia symptoms in perinatal women seeking mental health treatment. J Women’s Health (2002). 2011;20(4):553–8. doi: 10.1089/jwh.2010.2371. [DOI] [PubMed] [Google Scholar]
- 52.Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377–85. doi: 10.4088/JCP.12r07917. [DOI] [PubMed] [Google Scholar]
- 53.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–33. doi: 10.7326/m15-2175. [DOI] [PubMed] [Google Scholar]
- 54.Morgenthaler TT. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep (New York, NY). 2006;29(11):1415–9. [PubMed] [Google Scholar]
- 55.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151(8):1172–80. [DOI] [PubMed] [Google Scholar]
- 56.Murtagh DRR, Greenwood KM. Identifying effective psychological treatments for insomnia: A meta-analysis. J Consult Clin Psychol. 1995;63(1):79–89. [DOI] [PubMed] [Google Scholar]
- 57.Smith MT, Perlis ML, Park A, Smith MS, Pennington J, Giles DE, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5–11. [DOI] [PubMed] [Google Scholar]
- 58.Morin CM, Vallieres A, Guay B, Ivers H, Savard J, Merette C, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. JAMA. 2009;301(19):2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–95. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991–9. [DOI] [PubMed] [Google Scholar]
- 61.Backhaus J, Hohagen F, Voderholzer U, Riemann D. Long-term effectiveness of a short-term cognitive-behavioral group treatment for primary insomnia. Eur Arch Psychiatry Clin Neurosci. 2001;251(1):35–41. [DOI] [PubMed] [Google Scholar]
- 62.Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: A randomized controlled trial. JAMA. 2006;295(24):2851–8. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 63.Blom K, Jernelov S, Ruck C, Lindefors N, Kaldo V. Three-year follow-up comparing cognitive behavioral therapy for depression to cognitive behavioral therapy for insomnia, for patients with both diagnoses. Sleep. 2017;40(8). doi: 10.1093/sleep/zsx108. [DOI] [PubMed] [Google Scholar]
- 64.Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: A meta-analysis. JAMA Intern Med. 2015;175(9):1461–72. doi: 10.1001/jamainternmed.2015.3006. [DOI] [PubMed] [Google Scholar]
- 65.Sedov ID, Goodman SH, Tomfohr-Madsen LM. Insomnia treatment preferences during pregnancy. J Obstet Gynecol Neonatal Nurs. 2017;46(3):e95–e104. doi: 10.1016/j.jogn.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 66. ••.Manber R, Bei B, Simpson N, Asarnow L, Rangel E, Sit A, et al. Cognitive behavioral therapy for prenatal insomnia: A randomized controlled trial. Obstet Gynecol. 2019;133(5):911–9. doi: 10.1097/aog.0000000000003216. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first published randomized, controlled trial of cognitive-behavioral therapy for insomnia in pregnancy; the study sample was also diverse with respect to race/ethnicity.
- 67.Felder JN, Epel ES, Neuhaus J, Krystal AD, Prather AA. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: A randomized clinical trial. JAMA Psychiatry. 2020. doi: 10.1001/jamapsychiatry.2019.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalmbach DA, Cheng P, O’Brien LM, Swanson LM, Sangha R, Sen S, et al. A randomized controlled trial of digital cognitive behavioral therapy for insomnia in pregnant women. Sleep Med. 2020;72:82–92. doi: 10.1016/j.sleep.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swanson LM, Flynn H, Adams-Mundy JD, Armitage R, Arnedt JT. An open pilot of cognitive-behavioral therapy for insomnia in women with postpartum depression. Behav Sleep Med. 2013;11(4):297–307. doi: 10.1080/15402002.2012.683902. [DOI] [PubMed] [Google Scholar]
- 70.Shaffer KM, Hedeker D, Morin CM, Ingersoll K, Thorndike F, Ritterband LM. Intra-Individual Variability in Sleep Schedule: Effects of an Internet-Based Cognitive-Behavioral Therapy for Insomnia Program and its Relation with Symptom Remission. Sleep. 2020. doi: 10.1093/sleep/zsaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]


