Abstract
A 2.5-year-old cat presented with progressive ataxia and lethargy. Magnetic resonance imaging (MRI) showed enlargement of the cerebellum and herniation of cerebellar vermis. Postmortem examination confirmed the MRI findings, and histopathology showed numerous large dysplastic neurons populating and displacing the Purkinje cell layer and extending into the molecular and granular layers of the cerebellum. The lesion was diagnosed as dysplastic gangliocytoma of the cerebellum. In humans, this tumor is often associated with Cowden syndrome, a genetic disorder characterized by multiple hamartomas and an increased risk of developing certain neoplasms, known to be linked to a germline mutation of the phosphatase and tensin homolog (PTEN) gene. Reduction in PTEN nuclear and cytoplasmic immunohistochemical labeling of dysplastic neurons in this case suggested a possible PTEN mutation involved in the tumorigenesis. This report provides a detailed pathology description of the tumor and the use of neuronal and PTEN markers which will help guide pathologists presented with this rare condition in the future.
Keywords: congenital cerebellar malformations, cats, Cowden syndrome, feline, Lhermitte-Duclos disease, neuropathology, phosphatase and tensin homolog
Dysplastic gangliocytoma of the cerebellum, commonly known as Lhermitte-Duclos disease, is an infrequent benign tumor in humans with only about 220 confirmed cases to date. 8 In veterinary medicine, dysplastic gangliocytoma of the cerebellum has only been described in a horse. 14 In humans, dysplastic gangliocytoma of the cerebellum usually presents as a single unilateral discrete lesion in the cerebellum, and bilateral involvement is rare, with only 4 cases reported to date.2,3,9,18 Similarly, the reported case in a horse was a single unilateral lesion. There are contradictory opinions whether dysplastic gangliocytoma of the cerebellum should be classified as a hamartoma rather than a true neoplasm. 12 Hamartomas are disorganized masses of normal to dysplastic cells which arise at sites where these cells are normally present. 17 The fact that they very often originate from clonal chromosomal aberrations 17 makes their differentiation from benign tumors difficult and maybe even arbitrary. The latest World Health Organization (WHO) central nervous system (CNS) conference graded dysplastic gangliocytoma of the cerebellum as a grade 1 neoplasia of neuronal tumors. 11 The typical onset of dysplastic gangliocytoma of the cerebellum in humans occurs in the third and fourth decade of life, although reports of affected infants 18 and senior patients also exist. 13 The classic macroscopic lesion is a unilateral enlargement of the cerebellum with maintenance of the folia.2,9,18 Microscopic findings are variable and include replacement of the cerebellar granular layer by large, well-differentiated, dysplastic ganglion cells. 3 Dysplastic gangliocytoma of the cerebellum can occur as a sporadic condition, but it has also been associated with Cowden syndrome, an autosomal dominant genetic disorder that is characterized by multiple hamartomas and that leads to an increased risk of developing benign and malignant tumors. Cowden syndrome is associated with germline mutations of the phosphatase and tensin homolog (PTEN) gene, 3 a tumor suppressor gene that consequently leads to activation of the PI3K/AKT signaling pathway and uncontrolled cell proliferation. 17
A 2.5-year-old male neutered domestic shorthair cat was presented to the University Veterinary Hospital Dublin with a 5-month history of progressive ataxia and a 5-day history of lethargy. The cat had no significant medical history, and no abnormalities were detected on general physical examination. Neurological abnormalities included obtundation and profound cerebellovestibular ataxia of all limbs. Proprioceptive positioning was diffusely delayed, hopping was markedly reduced to absent, and extensor postural thrust was absent. There was also positional vertical nystagmus. Clinical signs were consistent with a diffuse encephalopathy, particularly affecting the cerebellovestibular system. Complete blood count and serum biochemistry values were within reference intervals. Serologic testing for feline leukemia virus, feline immunodeficiency virus, and Toxoplasma gondii was negative.
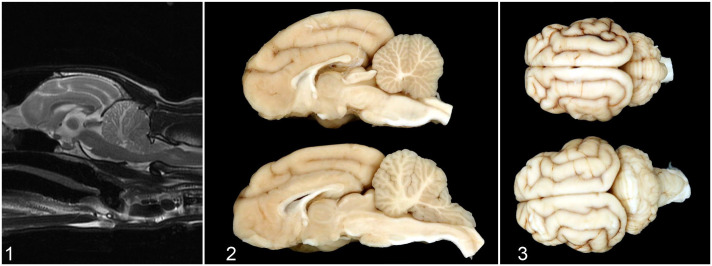
The cat underwent magnetic resonance imaging (MRI) using a 1.5T system (GE SIGNA Artist, Milwaukee, WI). The findings included moderate enlargement of the cerebellum and diffuse marked T2-weighted (T2W) and T2W fluid-attenuated inversion recovery (FLAIR) hyperintensity within the widened white matter tracts of the rostral two-thirds of the cerebellum (Fig. 1). The area was T1-weighted (T1W)-isointense with heterogeneous contrast enhancement and concurrent meningeal enhancement of the rostrodorsal cerebellum. Severe herniation of the cerebellar vermis through the foramen magnum was associated with compression of medulla oblongata and cranial cervical spinal cord. Furthermore, mild bilateral hydrocephalus was present. An additional finding included cervical subcutaneous nodule most consistent with a lipoma.
Figures 1–3.
Dysplastic gangliocytoma, brain, cat. Figure 1. There is diffuse marked T2-weighted hyperintensity within the widened white matter tracts of the rostral two-thirds of the cerebellum. Magnetic resonance imaging. Figure 2. Normal (upper specimen) and dysplastic gangliocytoma (lower specimen). The architecture of the folia is maintained. White matter of the cerebellum is markedly expanded and the vermis is herniated. Figure 3. Normal (upper specimen) and dysplastic gangliocytoma (lower specimen). Markedly enlarged cerebellum with the vermis protruding and extending caudally. The gyri of the cerebrum are flattened and widened.
A poor prognosis was given and the owners elected for euthanasia. In postmortem examination, the cerebellum was notably enlarged with tonsillar herniation through the foramen magnum (Fig. 2, 3). The cerebellar white matter was markedly expanded (Fig. 2), the cerebrum flattened, and the gyri widened (Fig. 3). Thoracic, abdominal, and pelvic organs were unremarkable.
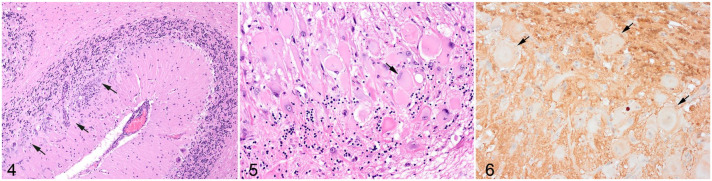
Histologic examination of the cerebellum showed numerous large dysplastic neurons of up to 58 µm in diameter populating the Purkinje cell layer and extending into the molecular and granular layer (Fig. 4, 5). These pleomorphic cells were characterized by scant to abundant pale to brightly eosinophilic cytoplasm. The nuclei were occasionally eccentrically located up to 28 µm in size and varied from large round to oval or reniform, with vesicular chromatin and a prominent nucleolus or with coarsely stippled chromatin (Fig. 5). In the affected areas, the Purkinje cells were shrunken or lost and there was variable to severe depletion of neurons in the granular layer. The neuropil was often rarefied or finely vacuolated (edema) and contained numerous variably sized swollen axons (spheroids). These severe changes alternated frequently with sharply defined areas where normal cerebellar architecture and cell population were maintained (Fig. 4). The white matter was extensively rarefied and contained large numbers of gemistocytes (Supplemental Fig. S1). Examination with luxol fast blue confirmed marked loss of myelin in these affected areas (Supplemental Fig. S2), while no change of myelination was found in the molecular layer. The meninges and perivascular spaces were multifocally infiltrated by low numbers of lymphocytes. There were no abnormal findings at any other level of the brain. The histopathological diagnosis was dysplastic cerebellar gangliocytoma.
Figures 4–6.
Dysplastic gangliocytoma, cerebellum, cat. Figure 4. Unaffected cerebellum at right. At left, there are numerous large dysplastic neurons (arrows) populating the Purkinje cell layer and extending into the molecular and granular layer. Hematoxylin and eosin (HE). Figure 5. Dysplastic cells are large, pleomorphic, with pale to brightly eosinophilic cytoplasm, and occasionally eccentric nuclei (arrow) within the Purkinje cell layer and granular layer. Hematoxylin and eosin (HE). Figure 6. Dysplastic cells (arrows) present heterogenous labeling with the vast majority showing the absence of nuclear and reduced cytoplasmic immunolabeling for phosphatase and tensin homolog.
For further investigation, sections of affected cerebellum were immunohistochemically labeled for GFAP (astrocytes), NeuN (neuronal nuclei), NF clone 52 (axonal neurofilament H; heavy), and NF clone 312 (axonal pan-neurofilament). Immunohistochemical labeling of PTEN was also carried out to investigate the possibility of loss of PTEN protein expression as a result of PTEN gene mutation (Supplemental Table S1). Brain tissue from unaffected areas was used as positive control, with the exception of PTEN in which sections of cerebrum from an age-matched control cat were used. There was consistent cell-specific labeling with each of the 5 antibodies (Supplemental Figs. S1, S3-S6). Primary antibodies were replaced by mouse or rabbit IgG as negative controls.
Dysplastic cells were not immunolabeled for GFAP (Supplemental. Fig. S1) or NeuN (Supplemental Fig. S3). Axonal immunolabeling of NF52 and NF312 was maintained in the axons of dysplastic cells (Supplemental Fig. S4, S5). Dysplastic neurons presented a heterogenous labeling pattern for PTEN, 7 with the vast majority showing the absence of nuclear immunolabeling and reduced cytoplasmic immunolabeling (Fig. 6).
This is the first report of dysplastic gangliocytoma of the cerebellum in a cat. Clinically, the cat presented with signs indicating a cerebellovestibular system disease. Differential diagnoses at that time included coronaviral encephalitis (feline infectious peritonitis virus), cerebral toxoplasmosis, meningoencephalitis of unknown origin, Chiari-like malformation, gliomatosis cerebri, and other neoplasms. Serological testing was not suggestive of toxoplasma infection. Following the MRI investigation, Chiari-like malformation and solid neoplasia were ruled out. However, meningoencephalitis, gliomatosis cerebri, and less likely a metabolic encephalopathy such as hepatic encephalopathy or a diffuse infiltrative neoplasia such as lymphoma were considered as possible diagnoses. Nearly pathognomonic MRI findings (both in T1W and in T2W) in humans with dysplastic gangliocytoma of the cerebellum are parallel linear striations on the surface of the mass (so-called tiger stripes) which mirror the expanded cerebellar folia. 3 These findings were absent in the present case, which is most likely due to the bilateral and diffuse presentation and the histological findings of frequent alternations of unaffected and affected areas throughout the entire cerebellum.
The histopathological presentation of dysplastic gangliocytoma of the cerebellum in humans is defined by the following components: (1) variable replacement of the cerebellar internal granular layer by dysplastic ganglion cells, (2) abnormal myelinization of the molecular layer, (3) reduced number of Purkinje cells, (4) large, bizarre neurons, and (5) vacuolization of the cerebellar white matter. 3 All were identified in the current case except for abnormal myelinization of the molecular layer. Interestingly, the current case posed a bilateral presentation, which is rarely described in humans.2,9,18 The mild inflammation, which was not considered to be of clinical significance, most likely occurred secondary to decreased cerebrospinal flow as a result of cerebellar enlargement and herniation. 4
Immunohistochemical results confirmed that the dysplastic cells were of neuronal origin; axonal neurofilament immunolabelling was present, although NeuN immunolabelling was not. Similar results have been reported in gangliocytomas in humans. 15
Cowden syndrome is a multiple hamartoma syndrome associated with PTEN mutation in 80% of human cases 3 of dysplastic gangliocytoma of the cerebellum, and clinical diagnosis is made by the presence of a combination of characteristic criteria including mucocutaneous lesions and a wide variety of benign and malignant tumors. 3 Major and minor diagnostic criteria for Cowden syndrome in humans include adult onset of dysplastic gangliocytoma of the cerebellum and benign lipomas.5,9 The additional finding of a lipoma in the current case is similar to what has been reported for Cowden syndrome in humans. A single case of Cowden-like syndrome has been described in the veterinary literature involving a Great Dane puppy that had colorectal hamartomatous polyposis, ganglioneuromatosis, and an associated PTEN mutation. 1 PTEN mutation (ie, downregulation/ dysfunction) is also described in several veterinary publications and is suspected to be part of tumorigenesis in cats and dogs.10,16 Immunohistochemical investigations for PTEN mutation were performed in this case. The majority of the dysplastic neurons showed loss of nuclear PTEN immunolabeling and reduced cytoplasmic immunolabeling. This may suggest that PTEN mutation contributed to the tumorigenesis of the dysplastic gangliocytoma of the cerebellum similar to what has been reported for Cowden syndrome in humans. The gold standard for germline mutations like PTEN would be Sanger sequencing. However, several studies evaluated IHC detection of PTEN mutations as partially equal or even superior to sequencing as it also addresses the epigenetic aspects that contribute to the loss of PTEN function. 6
This is the first reported case of dysplastic gangliocytoma of the cerebellum in a cat and highlights the rare bilateral hemispheric growth with an uncommon MRI presentation and suggests impaired PTEN function as a pathway of tumorigenesis.
Supplemental Material
Supplemental material, sj-pdf-1-vet-10.1177_03009858221075594 for Dysplastic gangliocytoma of the cerebellum in a cat by Michelle Imlau, Mamoun Saeed, Jane Cryan, Seamus Hoey, Myles McKenna, Hanne Jahns and Pamela Kelly in Veterinary Pathology
Acknowledgments
The authors would like to thank Mr Brian Cloak (School of Veterinary Medicine, University College Dublin, Ireland) for taking the images and preparing the figures, and the Laboratory Services of the Department of Pathology, Brigham & Women’s Hospital of the Harvard Medical School, USA for the immunohistology (PTEN).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Michelle Imlau  https://orcid.org/0000-0002-2565-8314
https://orcid.org/0000-0002-2565-8314
Myles McKenna  https://orcid.org/0000-0002-9099-9254
https://orcid.org/0000-0002-9099-9254
Hanne Jahns  https://orcid.org/0000-0001-6944-154X
https://orcid.org/0000-0001-6944-154X
Pamela Kelly  https://orcid.org/0000-0002-3047-8465
https://orcid.org/0000-0002-3047-8465
Supplemental material for this article is available online.
References
- 1. Bemelmans I, Küry S, Albaric O, et al. Colorectal hamartomatous polyposis and ganglioneuromatosis in a dog. Vet Pathol. 2011;48(5):1012–1015. Accessed January 14, 2022. https://pubmed.ncbi.nlm.nih.gov/20952721/. [DOI] [PubMed] [Google Scholar]
- 2. Borni M, Kammoun B, Kolsi F, et al. The Lhermitte-Duclos disease: a rare bilateral cerebellar location of a rare pathology. Pan Afr Med J. 2019;33:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brat DJ, Perry A. Pattern Recognition Series: Practical Surgical Neuropathology: A Diagnostic Approach. 2nd ed. Philadelphia, PA: Elsevier; 2018. [Google Scholar]
- 4. Deren KE, Packer M, Forsyth J, et al. Reactive astrocytosis, microgliosis and inflammation in rats with neonatal hydrocephalus. Exp Neurol. 2010;22 6(1):110–119. Accessed January 14, 2022. https://www.sciencedirect.com/science/article/pii/S001448861000292X. [DOI] [PubMed] [Google Scholar]
- 5. Derrey S, Proust F, Debono B, et al. Association between Cowden syndrome and Lhermitte-Duclos disease. Surg Neurol. 2004;61(5):447–454. Accessed January 14, 2022. http://www.sciencedirect.com/science/article/pii/S0090301903005767. [DOI] [PubMed] [Google Scholar]
- 6. Djordjevic B, Hennessy BT, Li J, et al. Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing. Mod Pathol. 2012;25(5):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg K, Broaddus RR, Soslow RA, et al. Pathologic scoring of PTEN immunohistochemistry in endometrial carcinoma is highly reproducible. Int J Gynecol Pathol. 2012;31(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joo G, Doumanian J. Radiographic findings of dysplastic cerebellar gangliocytoma (Lhermitte-Duclos Disease) in a woman with Cowden syndrome: a case study and literature review. J Radiol Case Rep. 2020;14(3):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khandpur U, Huntoon K, Smith-Cohn M, et al. Bilateral recurrent dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) in Cowden syndrome: a case report and literature review. World Neurosurg. 2019;127: 319–325. [DOI] [PubMed] [Google Scholar]
- 10. Levine RA, Forest T, Smith C. Tumor suppressor PTEN is mutated in canine osteosarcoma cell lines and tumors. Vet Pathol. 2002;39(3):372–378. [DOI] [PubMed] [Google Scholar]
- 11. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820. [DOI] [PubMed] [Google Scholar]
- 12. Nowak DA, Trost HA. Lhermitte-Duclos disease (dysplastic cerebellar gangliocytoma): a malformation, hamartoma or neoplasm? Acta Neurol Scand. 2002;105(3):137–145. [DOI] [PubMed] [Google Scholar]
- 13. Önder E, Arikök AT, Türkoğlu E, et al. Lhermitte-Duclos Disease: a rare lesion with variable presentations and obscure histopathology. Turk Patoloji Der. 2018;34(1):92–99. [DOI] [PubMed] [Google Scholar]
- 14. Poss M, Young S. Dysplastic disease of the cerebellum of an adult horse. Acta Neuropathol. 1987;75(2):209–211. [DOI] [PubMed] [Google Scholar]
- 15. Rainov NG, Holzhausen H-J, Burkert W. Dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos disease). Clin Neurol Neurosurg. 1995;97(2):175–180. Accessed January 14, 2022. https://www.sciencedirect.com/science/article/pii/030384679500017E. [DOI] [PubMed] [Google Scholar]
- 16. Ressel L, Millanta F, Caleri E, et al. Reduced PTEN protein expression and its prognostic implications in canine and feline mammary tumors. Vet Pathol. 2009;46(5):860–868. [DOI] [PubMed] [Google Scholar]
- 17. Robbins SL, Cotran RS, Kumar V, et al. Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Saunders Elsevier; op. 2015. Student consult. [Google Scholar]
- 18. Zak M, Ledbetter M, Maertens P. Infantile Lhermitte-Duclos disease treated successfully with rapamycin. J Child Neurol. 2017;32(3):322–326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vet-10.1177_03009858221075594 for Dysplastic gangliocytoma of the cerebellum in a cat by Michelle Imlau, Mamoun Saeed, Jane Cryan, Seamus Hoey, Myles McKenna, Hanne Jahns and Pamela Kelly in Veterinary Pathology