Abstract
Background
The association of the use of sodium-glucose cotransporter 2 (SGLT2) inhibitor and incident dementia remains unclear. This study aimed to evaluate the risk of incident dementia with the use of SGLT2 inhibitor.
Methods
This is a population-based cohort study utilizing Taiwan’s National Health Insurance Research Database. Each patient who took SGLT2 inhibitors was assigned to the SGLT2 inhibitor group, whereas 1:1 propensity score-matched randomly selected patients who were nonusers of SGLT2 inhibitors were assigned to the non-SGLT2 inhibitor group. The study outcome was incident dementia.
Results
A total of 976,972 patients newly diagnosed with type 2 diabetes mellitus (DM) between 2011 and 2018 were included in this study. After the patients’ propensity score matching by age, sex, duration of DM, comorbidities and drug index date of the patients, a total of 103,247 patients in the SGLT2 inhibitor group and 103,247 in the non-SGLT2 inhibitor group were enrolled for analysis. The SGLT2 inhibitor group was associated with a lower risk of incident dementia (adjusted hazard ratio: 0.89, 95% confidence interval: 0.82–0.96; p = .0021). Diabetic complications were significantly lower in the SGLT2 inhibitor group compared with the non-SGLT2 group. Sensitivity analysis was also consistent with the main analysis.
Conclusions
Patients with type 2 DM who were prescribed SGLT2 inhibitors were associated with a lower risk of incident dementia compared with those not prescribed SGLT2 inhibitors in real-world practice.
Keywords: Sodium–glucose co-transporter inhibitors, dementia, diabetic complications, type 2 diabetes
Introduction
Dementia is a fast-growing global epidemic and there are more than 50 million people living with dementia in 2016 worldwide. 1 This number is expected to increase to triple by 2050 as life expectancy increases. 2 It is one of the most common causes of disability in the elderly. 3 Many studies demonstrated that diabetes mellitus (DM) is associated with an increased risk of cognitive decline and dementia.4–6 Patients with DM along with concurrent dementia may have an increased economic burden on their families and the health care system in the country. Thus, minimizing the impact of incident dementia (ID) in patient with DM is an important health issue and their families.
Previous studies have shown that good glycemic control and low rate of diabetic complications are associated with a low risk of dementia.7–9 Many studies have also demonstrated that the use of sodium-glucose cotransporter 2 (SGLT2) inhibitor results in good glycemic control and low rate of diabetic complications.10–12 However, the association between SGLT2 inhibitor and ID remains unclear. Therefore, we conducted a retrospective cohort study to explore the relationship between SGLT2 inhibitors and ID in the general Taiwanese population. This investigation aimed to determine whether the risk of ID is associated with SGLT2 inhibitor use in a nationwide cohort study of Taiwanese patients with type 2 DM (T2DM).
Materials and methods
Study design and population
This is a retrospective case-control cohort study. We used the insurance claims data provided by the Taiwanese Bureau of National Health Insurance (TBNHI) from January 2011 to December 2018. SGLT2 inhibitor users were defined as those patients who received SGLT2 inhibitor prescriptions for more than 6 months during the study period and the respective index date was set as the initial SGLT2 inhibitor use by an individual per day. In contrast, non- SGLT2 inhibitor users were designated as those patients who did not receive a SGLT2 inhibitor prescription throughout the study period. This study was approved by ethics committee of Chung Shan Medical University Hospital (CS2-20023). Written consent was not obtained from the study participants as only de-identified data were obtained from the TBNHI, and a waiver of patient consent was provided by the ethics committee for this study.
The data from the NHI program in Taiwan from January 2011 to December 2018 using newly diagnosed T2DM codes based on the International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM) and ICD, 10th revision, CM (ICD-10-CM). The newly diagnosed T2DM was defined as the first time that a T2DM code was available in the outpatient or inpatient claim records between 2011 and 2018. The list of ICD-9 and ICD-10 codes that were used to defined the inclusion of T2DM patients, study events, and comorbidities are presented in Supplemental Table 1.
Patients who fulfilled any of the following criteria were excluded from the study: (1) prior history of dementia before 1 January 2011 and (2) patients aged <20 years. Given the differences in baseline characteristics and dementia risk between the SGLT2 inhibitor users and non- SGLT2 inhibitor users, we applied propensity score matching with age, sex, duration of DM, comorbidities and drug index date at a ratio of 1:1 for patients with T2DM with and without the use of a SGLT2 inhibitor (Figure 1).
Figure 1.
Patient flow chart.
Outcomes
The drug index date of the SGLT2 inhibitors was defined as the date of the first prescription of SGLT2 inhibitor with the same day of the matched non-SGLT2 inhibitor group. Three types of SGLT2 inhibitors (Empagliflozin, Dapagliflozin, and Canagliflozin) were launched since May 2016 and used till the end of the study (31 December 2018). We followed the SGLT2 inhibitors users since their first prescription of SGLT2 inhibitors, until the occurrence of dementia or the end of the study. In this study, we analyzed the patients’ data according to the group they were originally assigned, regardless of their adherence or duration of usage of a SGLT2 inhibitor. The study outcome was defined by ID based on the ICD-9-CM and ICD-10-CM codes in either an outpatient or inpatient department at least once from 1 May 2016 to 31 December 2018.
Covariables
We considered the following covariates as potential confounders: sex, age, co-medications, and comorbilities. Age was included as a continuous variable and categorized as <40, 40–49, 50–59, 60–69, 70–79, or ≥80. Co-medications included aspirin, beta-blocker, calcium channel blocker, angiotensin converting enzyme inhibitor, angiotensin receptor blocker, other antidiabetic agents, and statins. We defined comorbidities using ICD-9 CM and ICD-10 CM codes, which are listed in Supplemental Table 1. These comorbidities include asthma, hypertension, cardiovascular disease, hyperlipidemia, chronic pulmonary diseases, rheumatic arthritis, diabetic retinopathy, ischemic stroke, hemorrhagic stroke, and liver cirrhosis.
Statistical analysis
Data were presented as valid percentages and mean values with standard deviation. The standardized difference was applied to determine the difference in baseline characteristics between the two study groups. The Cox proportional hazard regression model was used to compare the risk of developing ID between the SGLT2 inhibitor group and the non-SGLT2 inhibitor group. Adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) were calculated, adjusting for important risk factors for developing dementia, which include age, sex, and comorbidities. The risk of study outcomes overtime for the SGLT2 inhibitor group compared with the non-SGLT2 inhibitor group was determined through survival analysis by the Kaplan–Meier method. In additional, sensitivity analyses were conducted to test the robustness of the findings of this study. The patients’ matching (1:2) by age, sex, duration of DM, and drug index date of the patients was used to compare the effect between the two study groups on the study outcome. Finally, subgroup analyses that are stratified by sex and age were conducted at baseline for the outcomes of ID, respectively. All effects were analyzed using an intention-to-treat approach. Statistical significance was defined as p-value < .05. All analyses were performed using SAS 9.3 Statistical Software (SAS Institute Inc, Cary, NC, USA).
Results
Baseline characteristics of all patients
Between January 2011 and December 2018, we identified at total of 103,247 patients with new-onset T2DM who were SGLT2 inhibitor users and 103,247 non-SGLT2 inhibitor users (Figure 1). The baseline characteristics of all patients with T2DM between the SGLT2 inhibitor user group and non-SGLT2 inhibitor user group are presented in Table 1. Most subjects were male (56%) and younger (<60 years of age, 53%).
Table 1.
Baseline characteristics of all patients.
| NON- SGLT 2 N = 103247 |
SGLT 2 N = 103247 |
p | |
|---|---|---|---|
| Sex | .4754 | ||
| Male | 57669 (55.86%) | 57830 (56.01%) | |
| Female | 45578 (44.14%) | 45417 (43.99%) | |
| Age | .8957 | ||
| <40 | 6210 (6.01%) | 6176 (5.98%) | |
| 40–49 | 16535 (16.01%) | 16491 (15.97%) | |
| 50–59 | 32086 (31.08%) | 32130 (31.12%) | |
| 60–69 | 34094 (33.02%) | 34039 (32.97%) | |
| 70–79 | 11876 (11.50%) | 11881 (11.51%) | |
| ≥80 | 2446 (2.37%) | 2530 (2.45%) | |
| Comorbidities | |||
| Asthma | 3026 (2.93%) | 3159 (3.06%) | .0860 |
| Hypertension | 63934 (61.92%) | 63587 (61.59%) | .1161 |
| Cardiovascular disease | 15759 (15.26%) | 16044 (15.54%) | .0823 |
| Hyperlipidemia | 70047 (67.84%) | 69629 (67.44%) | .0493 |
| Chronic pulmonary diseases | 3103 (3.01%) | 3353 (3.25%) | .0016 |
| Rheumatic disease | 288 (0.28%) | 341 (0.33%) | .0343 |
| Diabetic retinopathy | 4859 (4.71%) | 4981 (4.82%) | .2076 |
| Ischemic stroke | 4359 (4.22%) | 4528 (4.39%) | .0669 |
| Hemorrhage stroke | 531 (0.51%) | 665 (0.64%) | .0001 |
| Liver cirrhosis | 994 (0.96%) | 1081 (1.05%) | .0549 |
SGLT 2: sodium-glucose cotransporter 2.
Outcomes
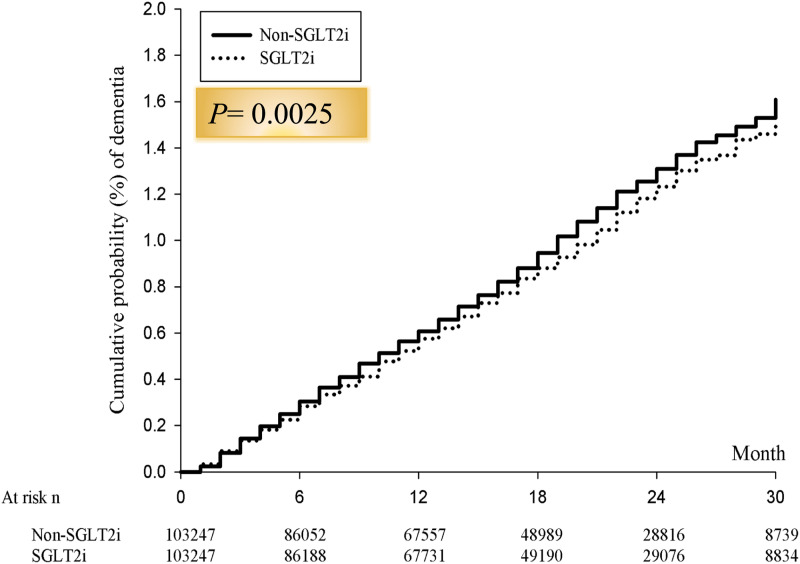
The incidence of ID, the number of IDs for SGLT2 inhibitor and non-SGLT2 inhibitor comparator were 3426 vs 4507, respectively (2.02 vs 2.66 per 1000 person/month; HR: 0.88, 95% CI 0.81–0.97; p = .0015), during the follow-up period (Table 2). Similarly, after adjustment of gender, age and comorbilities, the SGLT2 inhibitor group was associated with a lower risk of ID compared to the non-SGLT2 inhibitor group (aHR: 0.89; 95% CI 0.82–0.96; p = .0021). The Kaplan–Meier curves comparing the cumulative incidence of dementia between the SGLT2 inhibitor group and the non-SGLT2 inhibitor group were consistent with the above findings (Figure 2). In addition, patients with SGLT2 inhibitor use had low rates of diabetic complications than the non-SGLT2 inhibitor users during a 2.7-year follow-up (all p < .0001, Table 3).
Table 2.
Incidence and risk of dementia.
| NON- SGLT 2 N = 103247 |
SGLT 2 N = 103247 |
p | |
|---|---|---|---|
| Follow up person-month | 1,692,364 | 1,697,582 | |
| Dementia cases | 4507 | 3426 | |
| Incidence rate a | 2.66 (2.59–2.74) | 2.02 (1.95–2.09) | <.0001 |
| Crude HR | Reference | 0.88 (0.81–0.97) | .0015 |
| Adjusted HR | Reference | 0.89 (0.82–0.96) | .0021 |
SGLT 2: sodium-glucose cotransporter 2.
acrude incidence density rate (95% confidence interval) of dementia, per 1000 person-month.
Figure 2.
Cumulative risk of incident dementia for the study cohorts between SGLT2 inhibitor and non-SGLT2 inhibitor group.
Table 3.
Diabetic complications between two groups during 2.7-year follow-up.
| NON- SGLT 2 N = 103247 |
SGLT 2 N = 103247 |
p | |
|---|---|---|---|
| Hypoglycemic events | 2591 (2.51%) | 527 (0.51%) | <.0001 |
| Diabetic retinopathy | 10025 (9.71%) | 9344 (9.05%) | <.0001 |
| Diabetic neuropathy | 5802 (5.62%) | 4491 (4.35%) | <.0001 |
| Diabetic nephropathy | 2117 (2.05%) | 1177 (1.14%) | <.0001 |
SGLT 2: sodium-glucose cotransporter 2.
Sensitivity and subgroup analyses
We performed a sensitivity analysis to investigate the effect of SGLT2 inhibitor on ID. A 1:2 matching by age, sex, duration of DM, and drug index date of the patients was used to match a total of 105,611 patients in the SGLT2 inhibitors group and a total of 211,222 patients in the non-SGLT2 inhibitors group for analysis. Which provided similar results (3541 vs 10237 events, adjusted HR 0.92; 95% CI 0.85–0.99; p = .0460; Table 4).
Table 4.
Sensitivity analysis of incidence and risk of dementia.
| NON- SGLT 2 N = 211222 |
SGLT 2 N = 105611 |
p | |
|---|---|---|---|
| Follow up person-month | 3,450,719 | 1,736,088 | |
| Dementia cases | 10237 | 3541 | |
| Incidence rate a | 2.97 (2.91–3.02) | 2.04 (1.97–2.11) | <.0001 |
| Crude HR | Reference | 0.91 (0.84–0.98) | .0197 |
| Adjusted HR | Reference | 0.92 (0.85–0.99) | .0460 |
HR: hazard ratios; SGLT 2: sodium-glucose cotransporter 2.
acrude incidence density rate (95% confidence interval) of dementia, per 1000 person-month.
Results from the subgroup analyses were partly consistent with the main analyses (Table 5). Similar findings were seen for participants aged 40–49, 50–59, and 60–69 years as well as for male participants (191 vs 435 events, aHR 0.16, 95% CI 0.05–0.52; 523 vs 923 events, aHR 0.81, 95% CI 0.64–0.99; 1101 vs 1525 events, aHR 0.80, 95% CI 0.70–0.92; and 2025 vs 2882 events, aHR 0.82, 95% CI 0.73–0.91; respectively). However, only the trend of treatment effect of ID were seen for participants aged 70–79 and ≥80 years as well as for female participants (1040 vs 1046 events, aHR 0.98, 95% CI 0.84–1.10; 532 vs 463 events, aHR 0.97, 95% CI 0.80–1.18; and 1401 vs 1625 events, aHR 0.91, 95% CI 0.79–1.03; respectively, Table 5).
Table 5.
Subgroup analysis.
| Non- SGLT 2 | SGLT 2 | Adjusted hazard ratios (95% CI) | |||
|---|---|---|---|---|---|
| Event | Incidence rate a | Event | Incidence rate a | ||
| Sex | |||||
| Male | 2882 | 3.43 (3.34–3.51) | 2025 | 2.17 (2.08–2.27) | 0.82 (0.72–0.92) |
| Female | 1625 | 2.40 (2.32–2.48) | 1401 | 1.87 (1.78–1.97) | 0.91 (0.79–1.03) |
| Age | |||||
| <40 | 115 | 1.12 (0.99–1.26) | 39 | 0.34 (0.25–0.46) | Not estimated |
| 40–49 | 435 | 1.70 (1.60–1.81) | 191 | 0.67 (0.58–0.77) | 0.16 (0.05–0.52) |
| 50–59 | 923 | 1.91 (1.83–2.00) | 523 | 0.98 (0.90–1.07) | 0.81 (0.64–0.99) |
| 60–69 | 1525 | 3.14 (3.04–3.25) | 1101 | 2.06 (1.94–2.18) | 0.80 (0.70–0.92) |
| 70–79 | 1046 | 6.51 (6.25–6.77) | 1040 | 5.88 (5.54–6.24) | 0.98 (0.84–1.10) |
| >=80 | 463 | 14.42 (13.58–15.32) | 532 | 15.07 (13.86–16.38) | 0.97 (0.80–1.18) |
SGLT 2: sodium-glucose cotransporter 2.
acrude incidence density rate (95% confidence interval) of dementia, per 1000 person-month.
Discussion
In this population-based cohort study, the users of SGLT2 inhibitors with low rate of diabetic complications had a significantly lower risk of ID than the non-users of SGLT2 inhibitors. The trends of results from sensitivity and subgroup analyses were consistent with the main analysis. Specifically, we also found the lower risk of ID associated with male gender in the subgroup analyses.
DM is an important risk factor for dementia and, particularly, patients with type 2 DM seem to have an increased risk of dementia.13,14 This risk may be caused by a complex pathophysiology of diabetes and dementia, which involves hyperglycemia, hyperinsulinemia, oxidative stress, vascular effects, inflammation, increased cerebral amyloid-β peptides, brain insulin resistance, and formation of advanced glycation end-products.14–17 This has instigated the interest of exploring antidiabetic medications that can reduce the risk of dementia in patients with diabetes. Previous studies have demonstrated that the administration of metformin was associated with a decreased risk of dementia as compared with the non-user patients.18–20 The mechanism underlying the association between metformin and dementia is likely to be multifactorial, with evidence supporting the involvement of the reduced formation of advanced glycation end-products 21 as well as inflammation and oxidative stress.22,23
SGLT2 inhibitor is a new drug class that reduces plasma glucose levels by blocking the renal reabsorption of glucose.24,25 When combined with a healthy lifestyle, SGLT2 inhibitors are efficacious as monotherapy and add-on therapy for patients with T2DM uncontrolled by other antihyperglycemic drugs. The effective glycemic control alleviates the risk of T2DM-related complications.10–12,24 In this study, the users of SGLT2 inhibitors were protected from ID. Till now, data on the therapeutic strategies to improve the prognosis in these patients are scarce. Many animal studies have suggested that SGLT2 inhibitors may prevent cognitive decline by reducing hyperglycemia, hyperinsulinemia, oxidative stress, and inflammation and improving brain mitochondrial function in the hippocampus in patients with T2DM.26–28 Only one study has shown that the use of SGLT2 inhibitors in patients with T2DM is associated with significantly lower odds of dementia, which is consistent with our finding. 29 However, given the small number of patients with T2DM and the short follow-up period, it is not completely clear whether SGLT2 inhibitors are associated with ID when compared with the non-users.
In the previous clinical observation study, the users of metformin, glitazones, dipeptidyl peptidase-4 inhibitors, and glucagon-like peptide-1 analog were negatively associated with dementia while insulin was positively associated with dementia.30,31 Similarly, the users of SGLT2 inhibitors had a significantly lower risk of ID than the nonusers of SGLT2 inhibitors in our study. Furthermore, SGLT2 inhibitors also improve renal outcomes and reduce all-cause and cardiovascular death in patients with heart failure with reduced ejection fraction in two independent trials.32,33 These findings emphasize the value of SGLT2 inhibitors in clinical practice. SGLT2 inhibitors will represent an important class of compounds to be further evaluated in the clinical trials for dementia treatment.
Our study suggests that in patients with T2DM who are on SGLT2 inhibitors, the risk of dementia development is 11% lower when compared with the non-users, which may be the possible cause for the increase in its use. 34 These findings signify the need for conducting clinical trials to prove the role of the drug in preventing dementia in the future.
This study has several limitations. First, we ascertained that the exposure to SGLT2 inhibitors in the cohort is real and supported by the claims data, which include medication prescription. However, treatment adherence was not available from these secondary data. Second, the laboratory data such as blood sugar levels, hemoglobin A1c levels, renal function, and liver function were not available from these secondary data. This is an important limitation. However, because the data we used were population-based data, we assumed that there were no differences between the two groups. Further randomized clinical trial is needed to confirm our result. Third, the present study was based on the Taiwan NHI program and claims data sets, which may limit generalizability of the result for other countries.
In summary, patients with T2DM taking SGLT2 inhibitors are associated with a lower risk of ID and low rate of diabetic complications compared with those without SGLT2 inhibitors prescription in real-world practice. The findings give rationale for conducting clinical trials to prove such a benefit for prevention of dementia in future.
Supplemental Material
Supplemental Material for The association between sodium-glucose cotransporter 2 inhibitors and incident dementia: A nationwide population-based longitudinal cohort study by Wun-Zhih Siao, Tsung-Kun Lin, Jing-Yang Huang, Chin-Feng Tsai and Gwo-Ping Jong in Diabetes & Vascular Disease Research
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the grant from Chung Shan Medical University Hospital (CSH-2020-C-001).
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Gwo-Ping Jong https://orcid.org/0000-0002-7786-5497
References
- 1.World Health Organization . The epidemiology and impavct of dementia. Geneva, Switzerland: World Health Organization, 2019. [Google Scholar]
- 2.Prince MJ. World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London, UK: Alzheimer’s Disease International (ADI), 2015. [Google Scholar]
- 3.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016; 388(10053): 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luchsinger JA. Type 2 diabetes, related conditions, in relation and dementia: an opportunity for prevention? J Alzheimers Dis 2010; 20(3): 723–736. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Huang E. An update on type 2 diabetes mellitus as a risk factor for dementia. J Alzheimers Dis 2016; 53(2): 393–402. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016; 39(2): 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng B, Su B, Price G, et al. Glycemic control, diabetic complications, and risk of dementia in patients with diabetes: results from a large U.K. cohort study. Diabetes Care 2021; 44(7): 1556–1563. [DOI] [PubMed] [Google Scholar]
- 8.Sheen Y-J, Sheu WHH. Association between hypoglycemia and dementia in patients with type 2 diabetes. Diabetes Res Clin Pract 2016; 116: 279–287. [DOI] [PubMed] [Google Scholar]
- 9.Gul CB, Oz Gul O, Cander S, et al. Relationship between glycemic control, microalbuminuria and cognitive functions in elderly type 2 diabetic patients. Ren Fail 2014; 36(8): 1258–1262. [DOI] [PubMed] [Google Scholar]
- 10.Handelsman Y. Rationale for the early use of sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes. Adv Ther 2019; 36(10): 2567–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whalen K, Miller S, Onge ES. The role of sodium-glucose co-transporter 2 inhibitors in the treatment of type 2 diabetes. Clin Ther 2015; 37(6): 1150–1166. [DOI] [PubMed] [Google Scholar]
- 12.Katz PM, Leiter LA. The role of the kidney and SGLT2 inhibitors in type 2 diabetes. Can J Diabetes 2015; 39(Suppl 5): S167–S175. [DOI] [PubMed] [Google Scholar]
- 13.Ninomiya T. Diabetes mellitus and dementia. Curr Diab Rep 2014; 14(5): 487. [DOI] [PubMed] [Google Scholar]
- 14.Hanyu H. Diabetes-related dementia. Adv Exp Med Biol 2019; 1128: 147–160. [DOI] [PubMed] [Google Scholar]
- 15.Jayaraj RL, Azimullah S, Beiram R. Diabetes as a risk factor for Alzheimer’s disease in the Middle East and its shared pathological mediators. Saudi J Biol Sci 2020; 27(2): 736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albai O, Frandes M, Timar R, et al. Risk factors for developing dementia in type 2 diabetes mellitus patients with mild cognitive impairment. Neuropsychiatr Dis Treat 2019; 15: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nisar O, Pervez H, Mandalia B, et al. Type 3 diabetes mellitus: a link between Alzheimer’s disease and type 2 diabetes mellitus. Cureus 2020; 12(11): e11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samaras K, Makkar S, Crawford JD, et al. Metformin use is associated with slowed cognitive decline and reduced incident dementia in older adults with type 2 diabetes: the Sydney memory and ageing study. Diabetes Care 2020; 43(11): 2691–2701. [DOI] [PubMed] [Google Scholar]
- 19.Scherrer JF, Salas J, Floyd JS, et al. Metformin and sulfonylurea use and risk of incident dementia. Mayo Clin Proc 2019; 94(8): 1444–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell JM, Stephenson MD, de Courten B, et al. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J Alzheimers Dis 2018; 65(4): 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beisswenger P, Ruggiero-Lopez D. Metformin inhibition of glycation processes. Diabetes Metab 2003; 29(4 Pt 2): 6S95–6S103. [DOI] [PubMed] [Google Scholar]
- 22.Cameron AR, Morrison VL, Levin D, et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res 2016; 119: 652–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng C-H. Metformin and the risk of dementia in type 2 diabetes patients. Aging Dis 2020; 11(3): 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab 2019; 21(5): 1237–1250. [DOI] [PubMed] [Google Scholar]
- 25.Kluger AY, Tecson KM, Lee AY, et al. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc Diabetol 2019; 18(1): 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaikh S, Rizvi SM, Shakil S, et al. Forxiga (dapagliflozin): plausible role in the treatment of diabetes-associated neurological disorders. Biotechnol Appl Biochem 2016; 63(1): 145–150. [DOI] [PubMed] [Google Scholar]
- 27.Rizvi SM, Shakil S, Biswas D, et al. Invokana (canagliflozin) as a dual inhibitor of acetylcholinesterase and sodium glucose co-transporter 2: advancement in Alzheimer’s disease-diabetes type 2 linkage via an enzoinformatics study. CNS Neurol Disord Drug Targets 2014; 13(3): 447–451. [DOI] [PubMed] [Google Scholar]
- 28.Lin B, Koibuchi N, Hasegawa Y, et al. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovas Diabetol 2014; 13: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wium-Andersen IK, Osler M, Jørgensen MB, et al. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case-control study. Eur J Endocrinol 2019; 181(5): 499–507. [DOI] [PubMed] [Google Scholar]
- 30.Bohlken J, Jacob L, Kostev K. Association between the use of antihyperglycemic drugs and dementia risk: a case-control study. J Alzheimers Dis 2018; 66: 725–732. [DOI] [PubMed] [Google Scholar]
- 31.Akimoto H, Negishi A, Oshima S, et al. Antidiabetic drugs for the risk of Alzheimer disease in patients with type 2 DM using FAERS. Am J Alzheimers Dis Other Demen 2020; 35: 1533317519899546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon SD, Jhund PS, Claggett BL, et al. Effect of dapagliflozin in patients with HFrEF treated with sacubitril/valsartan: the DAPA-HF trial. JACC Heart Fail 2020; 8: 811–818. [DOI] [PubMed] [Google Scholar]
- 33.Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation 2021; 143: 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Secnik J, Xu H, Schwertner E, et al. Dementia diagnosis is associated with changes in antidiabetic drug prescription: an open-cohort study of ∼130,000 Swedish subjects over 14 years. J Alzheimers Dis 2020; 76(4): 1581–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The association between sodium-glucose cotransporter 2 inhibitors and incident dementia: A nationwide population-based longitudinal cohort study by Wun-Zhih Siao, Tsung-Kun Lin, Jing-Yang Huang, Chin-Feng Tsai and Gwo-Ping Jong in Diabetes & Vascular Disease Research