Abstract
In this study we evaluated specific and nonspecific toxic effects of aeration and trichloroethylene (TCE) oxidation on methanotrophic bacteria grown with different nitrogen sources (nitrate, ammonia, and molecular nitrogen). The specific toxic effects, exerted directly on soluble methane monooxygenase (sMMO), were evaluated by comparing changes in methane uptake rates and naphthalene oxidation rates following aeration and/or TCE oxidation. Nonspecific toxic effects, defined as general cellular damage, were examined by using a combination of epifluorescent cellular stains to measure viable cell numbers based on respiratory activity and measuring formate oxidation activities following aeration and TCE transformation. Our results suggest that aeration damages predominantly sMMO rather than other general cellular components, whereas TCE oxidation exerts a broad range of toxic effects that damage both specific and nonspecific cellular functions. TCE oxidation caused sMMO-catalyzed activity and respiratory activity to decrease linearly with the amount of substrate degraded. Severe TCE oxidation toxicity resulted in total cessation of the methane, naphthalene, and formate oxidation activities and a 95% decrease in the respiratory activity of methanotrophs. The failure of cells to recover even after 7 days of incubation with methane suggests that cellular recovery following severe TCE product toxicity is not always possible. Our evidence suggests that generation of greater amounts of sMMO per cell due to nitrogen fixation may be responsible for enhanced TCE oxidation activities of nitrogen-fixing methanotrophs rather than enzymatic protection mechanisms associated with the nitrogenase enzymes.
Trichloroethylene (TCE) is a suspected human carcinogen that is also a frequently detected subsurface contaminant. A number of studies have confirmed that TCE can be transformed by methane-oxidizing bacteria via cometabolic oxidation, a reaction that requires the presence of methane for production of the necessary enzymes. The enzymes responsible for rapid cometabolic degradation of TCE by methane oxidizers are nonspecific soluble methane monooxygenases (sMMO). sMMO catalyzes the reaction of TCE with oxygen and reducing equivalents in the form of NADH + H+ to produce intermediates, such as TCE epoxide (13, 33), that are then quickly hydrolyzed to harmless end products, such as CO2 and chloride ions (12, 23, 36).
Although TCE can be rapidly degraded by methane-oxidizing cultures, undesired product toxic effects occur as a result of this reaction. The observed toxic effects on methane-oxidizing cultures include decreased methane oxidation rates, decreased methanol-stimulated oxygen consumption rates, and decreased TCE degradation rates (1, 5, 20, 25). A recent study reported that TCE oxidation caused an exponential decrease in the viability of Methylosinus trichosporium OB3b, as determined by a plate count method (32). Toxic effects associated with TCE oxidation have also been observed in pure-enzyme studies conducted with sMMO, in which specific protein components of the enzyme were damaged due to TCE product toxicity (13). Although some short-lived, highly reactive intermediates, such as TCE epoxide, have been detected and postulated to be responsible for the toxic effects (13, 33), important issues with respect to the nature of the product toxicity and cell viability have not been thoroughly investigated.
Toxic effects of aeration have been also observed with methane-oxidizing cultures (1, 10, 14, 15, 21, 29, 30). For example, when cells were shaken in air in the absence of methane, significant decreases in methane and TCE oxidation rates were observed (1). A number of workers have postulated that the formation of active oxidized compounds resulting from sMMO activity with molecular oxygen might be responsible for the toxic effects of aeration (10, 14, 15, 21, 29, 30). Nevertheless, little is known about the specific toxic effects of aeration on methane-oxidizing cells.
Previous studies (8, 9) have shown that pure and mixed cultures of nitrogen-fixing methane oxidizers exhibit lower product toxicities following TCE oxidation than cells supplied with nitrate or ammonia exhibit. Given that aeration also has toxic effects on methane oxidizers and given that nitrogen fixation is promoted under low-oxygen conditions, there might be a correlation among oxygen-sensitive sMMO activity, nitrogenase activity, and TCE product toxicity under nitrogen-fixing conditions. However, the linkage and fundamental basis of this phenomenon remain unclear.
5-Cyano-2,3-ditolyl tetrazolium chloride (CTC) is a tetrazolium salt that can be used to quantify metabolically active microorganisms in bodies of water and soils (26, 28, 31, 37). CTC functions by serving as an electron acceptor which scavenges electrons from active electron transport systems of active microorganisms. Once CTC is reduced, an insoluble end product, CTC-formazan, accumulates intracellularly and becomes fluorescent when it is excited with UV light. Bacteria containing CTC-formazan can then be detected and counted with a fluorescence microscope. Another fluorescent but nonspecific cell wall stain, 5-(4,6-dichlorotriazinyl)-amino fluorescein (DTAF), can be used along with CTC as a counterstain for total-cell enumeration (4). Accordingly, it is possible to combine these two stains and use them as a tool based on respiratory activity to quantify the viability of cells experiencing toxicity due to aeration or TCE oxidation.
This study was conducted to evaluate the nature of TCE product and the toxic effects of aeration on two pure strains of methane-oxidizing bacteria (Methylosinus trichosporium OB3b and strain CAC-2 [8]) grown with three different nitrogen sources: nitrate, ammonia, and molecular nitrogen. A series of experiments was conducted to measure both the specific toxic effects on sMMO and the nonspecific toxic effects on general biochemical pathways. The specific toxic effects on sMMO were evaluated by measuring methane uptake rates and naphthalene oxidation rates, while the nonspecific toxic effects on cells were determined by monitoring formate oxidation activity and respiratory activity. Since oxidation of formate is the last step in the methane oxidation pathway and does not involve sMMO, formate oxidation activity was used along with respiratory activity as a general indicator of the biochemical integrity of methanotrophs.
MATERIALS AND METHODS
Chemicals.
TCE (> 99% pure), naphthalene crystals, 1- and 2- naphthols (> 99% pure, scintillation grade) were purchased from Aldrich Chemical Co. (Milwaukee, Wis.). Tetrazotized o-dianisidine was purchased from Fluka Chemical Corp. (Ronkonkoma, N.Y.). TCE-saturated water and naphthalene-saturated water were maintained in vials capped with Mininert valves and Teflon liners, respectively. The stock solutions were prepared by adding excess TCE or naphthalene to vials containing deionized water as described previously (9).
CTC and DTAF were purchased from Polyscience Co. (Warrington, Pa.) and Molecular Probes Inc. (Eugene, Oreg.), respectively. The CTC staining solution (5 mM) was prepared with nitrate mineral salts (NMS) medium (9), and the DTAF staining solution (1:5, wt/vol) was prepared with 50 mM Na2HPO4 buffer (pH 9). Both the CTC staining solution and the DTAF staining solution were made fresh when they were needed, and they were filtered prior to use. Tris (pH 8.6)-buffered glycerol (1:1, vol/vol) containing 2% 1,4-disobicyclo[2,2,2] octane (Fisher Scientific Co., Pittsburgh, Pa.) was filter sterilized.
Microorganisms and culture conditions.
Pure cultures of two methane-oxidizing strains, M. trichosporium OB3b (= ATCC 35070; referred to below as OB3b) and strain CAC-2 (isolated from a mixed culture [8]), were used in this study. Each strain was enriched by using one of the following three growth media: NMS, AMS, and NFMS media, which contained nitrate, ammonia, and no fixed nitrogen source, respectively (9). The compositions of the three media were identical except that 11.76 mM NaNO3 was added to NMS medium and 5.88 mM (NH4)2SO4 was added to AMS medium. Copper was not added to any of the media. Cells were grown in flasks containing 50 ml of one growth medium, 1 ml of culture inoculum, 10% (initial concentration) CH4, and 20% (initial concentration) O2. For the flasks containing NFMS medium, the headspace was purged with filtered nitrogen gas before 10% CH4 and 5% O2 were added. All inoculated flasks were incubated at 20°C with shaking at 150 rpm. The headspace oxygen was maintained at more than 10% for nitrate- and ammonia-supplied cells and at more than 2% for nitrogen-fixing cells by repeatedly adding oxygen during cell growth. Cell-free flasks were used as negative controls, and the gas losses due to leakage were less than 5%. Cells were harvested during the exponential growth phase for experimental use. Optical densities at 600 nm were correlated with volatile suspended solids (VSS) contents, which were calculated from the changes in weight determined after drying at 105°C overnight and after burning at 550°C for 2 h.
Experimental methods. (i) Cell treatments.
In this study, fresh cells were defined as cells that were freshly harvested from flasks and not exposed to any chemical; the activity of fresh cells was analyzed immediately after the preparation was purged with nitrogen gas (at a flow rate of 300 ml min−1 for 1 min) to remove any residual methane. Acetylene-exposed cells were defined as freshly harvested cells that were exposed to 3% (vol/vol) acetylene (in the headspace) for 10 min before the preparation was purged with nitrogen gas and activity analyses were performed. N2-exposed cells and air-exposed cells were defined as cells that were shaken at 200 rpm with N2 and air, respectively, in the absence of methane for 2 to 24 h before activity was measured. TCE-exposed cells were shaken in the absence of methane at 200 rpm along with air, a given amount of TCE, and 20 mM sodium formate for 90 min or 24 h. For cells exposed to TCE for 90 min, 1.1 μmol of TCE was added to 1.7 to 3.6 mg of VSS. For cells exposed to TCE for 24 h, 15.9 μmol of TCE was added to 9.6 mg of VSS. The transformation capacities (Tc) for cells grown with different nitrogen sources were 1.7, 1.2, and 1.0 μmol of TCE mg of VSS−1 for nitrogen-fixing, nitrate-supplied, and ammonia-supplied OB3b cells, respectively, and 1.2, 1.0, and 0.8 μmol of TCE mg of VSS−1 for nitrogen-fixing, nitrate-supplied, and ammonia-supplied CAC-2 cells, respectively. The Tc were measured as described previously (8). TCE-free cells were used as controls for TCE-exposed cells and were subjected to the same treatments as TCE-exposed cells except that no TCE was added.
(ii) Methane uptake rates.
The methane uptake rates of fresh, acetylene-exposed, TCE-free, TCE-exposed, air-exposed, and N2-exposed cells were measured as described previously (9). Purged cell suspensions (15 to 17 ml) were transferred into 26-ml vials that were sealed with Mininert valves (Alltech Co.) and amended with 0.03 ml of methane. The vials were vigorously shaken by hand for 30 s before the first headspace samples were taken. The methane in the headspaces of the vials was monitored for 1 h. Three or four data points were used to determine the initial concentration slope by linear regression. The slope was then divided by the VSS content to obtain the specific methane uptake rate of cells (mass of CH4 per mass of VSS per unit of time).
(ii) Formate oxidation rates.
The method used for the formate oxidation tests was modified from the method described by Alvarez-Cohen and McCarty (2). Cells were centrifuged and resuspended in growth medium to an absorbance at 600 nm of 0.25. Cell suspensions (10 ml) were transferred into 50-ml polypropylene centrifuge tubes (Corning Inc., Corning, N.Y.) and amended with 1.2 mM formate. The tubes were shaken vigorously by hand for 30 s before the first samples were taken. Then the tubes were incubated on a shaker at 200 rpm, and liquid samples (1.5 ml) were removed from the tubes 1, 2, and 3 h after formate was added. Experiments were performed in duplicate, and cell-free control vials containing formate were analyzed concurrently.
(iii) Naphthalene oxidation rates.
Naphthalene oxidation reactions are commonly used to measure sMMO activity since sMMO is the only enzyme in methanotrophs that can oxidize naphthalene to the dead-end products 1-naphthol and 2-naphthol (6). One milliliter of a cell suspension was incubated with 1 ml of naphthalene-saturated water containing 20 mM sodium formate at 35°C. The specific naphthalene degradation rates were calculated by dividing naphthol measurements obtained at 60 min by the VSS contents. Duplicate vials were used.
(iv) TCE oxidation rates.
TCE oxidation rates were measured as described previously (9) by using 26-ml vials that contained 12- to 14-ml portions of purged cell suspensions, 20 mM sodium formate, and TCE (initial aqueous-phase concentration, 0.8 mg/liter) and were capped with Mininert valves. The TCE-amended vials were then vigorously shaken by hand for 10 s before the first TCE headspace measurement. The vials were incubated on a rotary shaker at 200 rpm, and the disappearance of TCE from the vials over time was monitored by headspace sampling. The specific rates of TCE degradation of the cultures were calculated by performing a linear regression analysis with at least four data points obtained within the first 20 min of the experiments divided by the VSS content. Cell-free controls lost less than 5% of the initial TCE during the experiments. A dimensionless Henry’s constant, 0.3 at 20°C (18), was used to account for aqueous and gaseous partitioning of the total TCE mass in the vials. Duplicate vials were used for TCE oxidation tests.
Analytical methods. (i) Organic compound analysis.
The concentrations of CH4 and TCE were determined by a headspace analysis. Headspace samples (20 or 50 μl) were injected into a Hewlett-Packard model HP 5890A gas chromatograph (GC) equipped with a SUPELCOWAX 10 glass capillary column (0.75 mm [inside diameter] by 30 m; film thickness, 1.0 μm; Supelco Inc., Bellefonte, Pa.) maintained at 85°C along with 11 ml of N2 carrier gas min−1. The injector temperature was 250°C, and the GC was equipped with an electron capture detector maintained at 300°C and a flame ionization detector maintained at 250°C. The electron capture detector was used for TCE analysis, and the flame ionization detector was used for CH4 measurements. TCE calibration curves were obtained by performing a headspace gas analysis with vials containing known amounts of TCE. The vials were prepared by adding different amounts of a TCE-saturated aqueous solution or TCE-methanol mixtures to 26-ml vials containing volumes of deionized water equivalent to the volumes of liquid in sample vials. The TCE-amended vials were then vigorously shaken by hand for 30 s before they were incubated with shaking at 200 rpm at the ambient temperature (20°C). The TCE headspace analysis was performed after 1 h of shaking. Methane calibration curves were obtained by injecting different amounts of pure methane gas into the GC.
(ii) Formate analysis.
Liquid samples were immediately filtered through a 0.2-μm-pore size disk filter to remove the cells. A portion of the filtrate (0.5 ml) was transferred into a 2-ml vial containing 1 ml of deionized water, and the vial was then acidified with 100 μl of 1 N H2SO4. Pretreated samples were stored at 4°C before they were analyzed by liquid chromatography. The concentrations of formate in aqueous samples were measured with a Perkin-Elmer liquid chromatography system which included a model ISS200 LC sample processor, a series 410 LC pump, and a model LC-235 array detector (Perkin-Elmer Corp., Norwalk, Conn.). The system was equipped with an Aminex HPX-87H ion exclusion column (300 by 7.8 mm; Bio-Rad Laboratories, Hercules, Calif.), and 0.016 N H2SO4 was used as the eluent at a rate of 0.9 ml min−1. The sample size was 200 μl. A calibration curve was obtained by analyzing samples containing different sodium formate concentrations before and after each sample analysis.
(iii) Naphthalene oxidation assay.
Naphthalene oxidation assays were conducted as described previously (8). The absorbance at 530 nm of the naphthol-diazo complex formed from the reaction of naphthols with tetrazotized o-dianisidine was measured to quantify naphthalene oxidation. A 100-μl aliquot of 0.2% (wt/vol) tetrazotized o-dianisidine was added to 1 ml of a cell suspension that had been incubated with naphthalene. An extinction coefficient of 38,000 M−1 cm−1 for the naphthol-diazo dye was used for calibration (34). This coefficient was within 5% of the coefficients calculated from standard curves obtained with 1- and 2-naphthols in this study.
(iv) CTC-DTAF staining and epifluorescence microscopy.
The ratio of viable cell counts to total cell counts was determined by using CTC, a specific respiratory activity stain (28), together with DTAF, a nonspecific cell wall stain (3, 4). A cell suspension (5 to 10 μl) was transferred into a 2-ml sterile microcentrifuge tube containing 0.5 ml of a 5 mM CTC staining solution. The tube was incubated with shaking in the dark at 35°C overnight. The mixture was then counterstained with 1 ml of a DTAF staining solution and incubated with shaking in the dark for 30 min. The mixture containing stained cells was then poured into a filtration apparatus containing a 50-ml polysulfone filter funnel (effective filtration area, 2.86 cm2; Gelman Sciences) and a black polycarbonate membrane filter (pore diameter, 0.22 μm; filter diameter, 25 mm; Poretics Corp.) which was supported by a silver membrane diffuser (pore diameter, 5 μm; filter diameter, 25 mm; Poretics Corp.). Na2HPO4 buffer (50 mM) was used to rinse the filter funnel. A few drops of Tris-buffered glycerol were applied to the membrane filter to retard quenching of the fluorescent signal. After it was air dried under a vacuum, the membrane filter was transferred onto a slide, and 1 drop of Tris-buffered glycerol was applied onto the preparation before it was covered with a coverslip. The stained cells retained on the membrane filter were enumerated directly by using an epifluorescence microscope (model BH2-RFC; Olympus Optical Co. Ltd., Lake Success, N.Y.) equipped with polarizing filters (380-nm exciter filter and 510-nm barrier filter) and a 100-W mercury lamp (Chiu Technical Corp., Kings Park, N.Y.). Cells colored with green perimeter fluorescein (DTAF stain) and bright intracellular red formazan (CTC stain) were considered active cells, while cells colored with only green perimeter fluorescein (DTAF stain) were considered inactive cells.
RESULTS
Quantification of toxic effects of aeration and TCE oxidation.
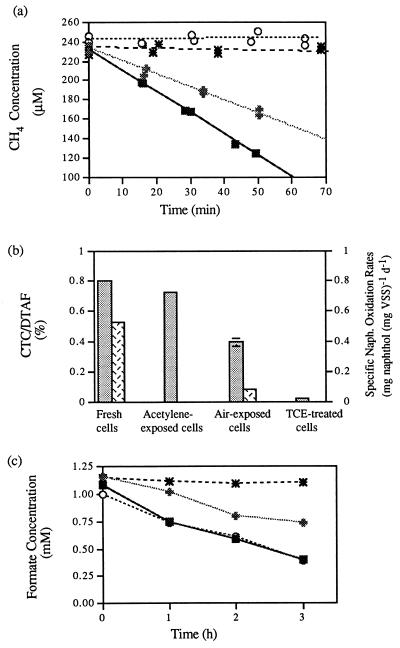
The toxic effects of aeration and TCE oxidation were quantified by comparing the methane uptake, naphthalene oxidation, respiratory, and formate oxidation activities of fresh nitrate-supplied OB3b cells with the activities of acetylene-exposed, air-exposed, and TCE-exposed cells incubated for 24 h (Fig. 1). Fresh cells oxidized methane, naphthalene, and formate and exhibited the expected levels of respiratory activity (CTC/DTAF ratios, ∼80%). Acetylene-exposed cells were not able to take up methane or oxidize naphthalene due to the inhibitory effects of acetylene on sMMO activity (Fig. 1a and b); however, acetylene exerted little effect on the CTC/DTAF ratios of cells as well as on their ability to oxidize formate (Fig. 1b and c), demonstrating that the inhibitory effects of acetylene were specific to sMMO and did not have a significant impact on the general cellular activities.
FIG. 1.
Comparison of methane uptake rates (a), CTC/DTAF and
naphthalene oxidation rates (b), and formate oxidation rates (c)
in nitrate-supplied M. trichosporium OB3b fresh
cells, acetylene-exposed cells, air-exposed cells, and TCE-exposed
cells. The error bars show the range of values obtained with duplicate
vials in panels b and c. (a and c)
 , fresh cells;
, fresh cells;
 , acetylene-exposed cells;
, acetylene-exposed cells;
 , air-exposed cells; ∗,
TCE-exposed cells. (b)
, air-exposed cells; ∗,
TCE-exposed cells. (b)
 , CTC/DTAF ratios;
, CTC/DTAF ratios;
 , naphthalene
oxidation rates. VSS, volatile suspended solids; d, day.
, naphthalene
oxidation rates. VSS, volatile suspended solids; d, day.
The extent of aeration toxicity was evaluated by comparing the activities of fresh cells with the activities of air-exposed cells after 24 h of incubation. With air-exposed cells there was a dramatic decrease in the naphthalene oxidation rate (85% loss) (Fig. 1b), but the decreases in the methane uptake rate (52% loss) (Fig. 1a), the CTC/DTAF ratio (50% loss) (Fig. 1b), and the formate oxidation rate (50% loss) were smaller (Fig. 1c).
Similarly, the extent of the toxic effects of TCE oxidation was examined by comparing the activities of fresh cells with the activities of TCE-exposed cells (Fig. 1) that were incubated with TCE in quantities greater than their Tc for a period of time greater than that required to oxidize the maximum amount of TCE possible (24 h). No oxidation of methane, naphthalene, or formate was detected in TCE-exposed cells following exposure to TCE, and no resumption of methane oxidation activity was observed even after 1 week of incubation with methane. In addition, the CTC/DTAF ratios decreased to less than 5% in TCE-exposed cells.
Nature of aeration toxicity.
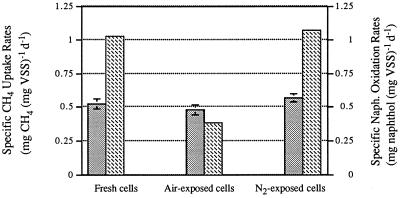
The methane uptake rates and naphthalene oxidation rates of fresh, air-exposed, and N2-exposed cells were compared in order to determine whether oxygen was the actual agent of the so-called aeration toxic effects observed in methane oxidizers (Fig. 2). Exposure to nitrogen gas had no significant effect on either methane uptake rates or naphthalene oxidation rates compared to the rates of fresh cells. However, exposure to air resulted in a naphthalene oxidation rate that was approximately 40% of the rate of fresh cells, while only a slight decrease in the methane uptake rate was observed under the condition. Large decreases in the naphthalene oxidation rate coupled with much smaller decreases in the methane uptake rate following aeration were observed with OB3b and CAC-2 cells grown with each of the three nitrogen sources (data not shown).
FIG. 2.
Methane uptake rates and naphthalene oxidation rates of
nitrogen-fixing M. trichosporium OB3b cells. Fresh cells
were compared with air-exposed and N2-exposed cells after
4 h of incubation. The error bars show the ranges of values
obtained with duplicate vials. ░⃞, CH4 uptake rates;
 ,
naphthalene oxidation rates. d, day.
,
naphthalene oxidation rates. d, day.
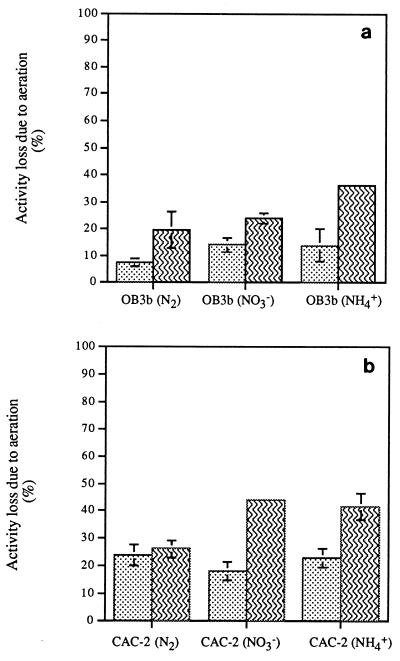
In order to evaluate the potential effects of the nitrogen source on aeration toxicity, the percent losses in the methane uptake and naphthalene oxidation activities following 2 h of aeration of OB3b and CAC-2 cells grown with different nitrogen sources were measured (Fig. 3). In all cases, greater percent losses were observed for the naphthalene oxidation rates (20 to 40% losses for OB3b and CAC-2) than for the methane uptake rates (10 to 20% losses for OB3b and 20 to 25% losses for CAC-2) following exposure to air. The losses in naphthalene oxidation activity were smaller with nitrogen-fixing OB3b and CAC-2 cells than with nitrate- and ammonia-supplied cells. The losses in methane uptake activity were also smaller with nitrogen-fixing OB3b cells than with nitrate- or ammonia-supplied cells, while the losses in methane uptake activity with nitrogen-fixing CAC-2 cells were similar or slightly greater.
FIG. 3.
Decreases in methane uptake and naphthalene oxidation
activities following 2 h of aeration for M.
trichosporium OB3b cells (a) and CAC-2 cells (b). The error bars
show the ranges of values obtained with duplicate vials.
,
CH4 uptake rates;
 , naphthalene
oxidation rates.
, naphthalene
oxidation rates.
Nature of TCE product toxicity.
In order to evaluate the nature of TCE product toxicity on methane-oxidizing cells, the methane uptake rates, naphthalene oxidation rates, and CTC/DTAF ratios of TCE-free cells and TCE-exposed cells were compared (Fig. 4 and 5). In these experiments, TCE-exposed cells were exposed to TCE for 90 min in order to degrade quantities of TCE that were less than the Tc of the cells (36 to 44% of the Tc for OB3b and 30 to 41% of the Tc for CAC-2). In this manner, the toxic effects caused by TCE oxidation could be evaluated in cells that retained a significant amount of activity, which allowed direct comparisons of different activity measurement methods to be made.
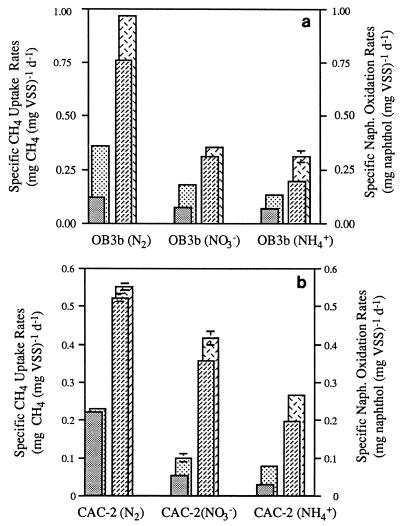
FIG. 4.
Methane uptake rates and naphthalene oxidation rates of
M. trichosporium OB3b (a) and CAC-2 (b) TCE-free cells and
TCE-exposed cells following 90 min of incubation. The error bars show
the ranges of values obtained with duplicate vials.
 , CH4
uptake rates of TCE-free cells;
, CH4
uptake rates of TCE-free cells;
 ,
CH4 uptake rates of TCE-exposed cells;
,
CH4 uptake rates of TCE-exposed cells;
 , naphthalene
oxidation rates of TCE-free cells;
, naphthalene
oxidation rates of TCE-free cells;
 ,
naphthalene oxidation rates of TCE-exposed cells. d, day.
,
naphthalene oxidation rates of TCE-exposed cells. d, day.
FIG. 5.
Decreases in methane uptake and naphthalene oxidation
activities due to TCE product toxic effects on M.
trichosporium OB3b cells (a) and CAC-2 cells (b) following 90 min
of TCE oxidation. The error bars show the ranges of values obtained
with duplicate vials.
 , CH4
uptake rates;
, CH4
uptake rates;  ,
naphthalene oxidation rates; ▩, CTC/DTAF ratios.
,
naphthalene oxidation rates; ▩, CTC/DTAF ratios.
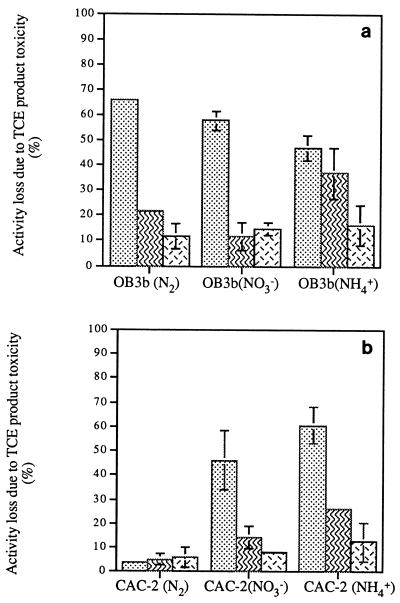
TCE-exposed OB3b and CAC-2 cells grown with each of the three nitrogen sources exhibited decreased methane uptake and naphthalene oxidation rates compared to TCE-free cells (Fig. 4). Nitrogen-fixing OB3b and CAC-2 cells exhibited the highest methane uptake and naphthalene oxidation rates both with and without exposure to TCE. However, when the data were expressed in terms of the percentage of activity lost (Fig. 5), nitrogen-fixing OB3b cells exhibited the greatest decrease in methane uptake of all of the cells tested.
The greatest percent losses due to TCE oxidation were generally observed in methane uptake rates (45 to 65%); there were moderate losses in naphthalene oxidation rates (15 to 40%) and small losses in CTC/DTAF ratios (10 to 20%). Similar trends were exhibited by most of the cultures tested under each type of nitrogen conditions; the only exception was the nitrogen-fixing CAC-2 culture, which exhibited similar small losses (5 to 10%) in methane uptake, naphthalene oxidation, and respiratory activities.
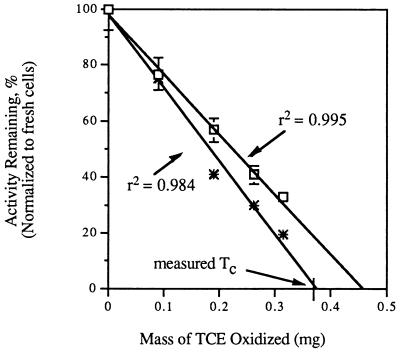
Additional experiments were conducted to evaluate whether TCE oxidation caused a decrease in cell activity that was linear with respect to the amount of TCE degraded and whether naphthalene oxidation rates were suitable for determining the remaining TCE oxidation activity of TCE-exposed cells. TCE oxidation rates and naphthalene oxidation rates were determined for nitrogen-fixing OB3b cells after degradation of a range of TCE masses over time (Fig. 6). The naphthalene oxidation rates and TCE oxidation rates of TCE-exposed cells declined in linear proportion to the mass of TCE degraded. The slopes of the naphthalene oxidation and TCE oxidation lines differed slightly, and the x intercept of the naphthalene oxidation curve was closer to the measured cellular Tc than the x intercept of the TCE oxidation curve was.
FIG. 6.
Decreases in TCE and naphthalene oxidation activities of nitrogen-fixing OB3b cells due to oxidation of different amounts of TCE. The mass of TCE that corresponded to the measured Tc of the cells is indicated. The error bars show the ranges of values obtained with duplicate vials. □, TCE oxidation rates, ∗, naphthalene oxidation rates. The lines are the linear regression lines for the data.
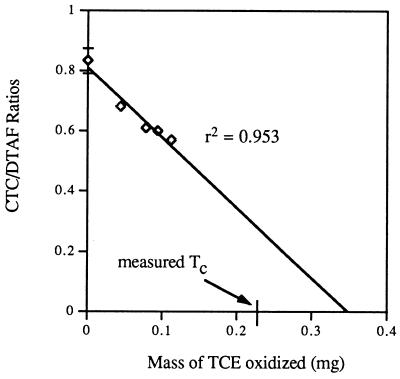
An experiment was also conducted to evaluate the relationship between respiratory activity and TCE product toxicity; in this experiment we determined the CTC/DTAF ratios of cells after oxidation of different masses of TCE in a given time (Fig. 7). Respiratory activity decreased linearly in proportion to the amount of TCE degraded. The x-intercept value of the linear regression significantly exceeded the measured Tc of the cells.
FIG. 7.
Decreases in respiratory activity as measured by CTC/DTAF ratios of nitrogen-fixing OB3b cells following oxidation of different amounts of TCE during a 4-h incubation. The mass of TCE that corresponded to the measured Tc of the cells is indicated. The error bars show the ranges of values obtained with duplicate vials. The data points are CTC/DTAF ratios, and the line is the linear regression line for the data.
DISCUSSION
The extent and nature of aeration toxicity exerted on methanotrophic cultures were evaluated in this study. The dramatic decrease in naphthalene oxidation rates and the moderate decreases in formate oxidation and respiratory activities observed in cells following prolonged aeration suggest that aeration had a more significant and direct impact on sMMO than on general cellular functions. The much smaller losses in methane uptake rates (52% in Fig. 1a; 10 to 15% for OB3b and 20 to 25% for CAC-2 in Fig. 3) than in naphthalene oxidation rates (85% in Fig. 1b; 20 to 40% for OB3b and 25 to 45% for CAC-2 in Fig. 3) might be explained by changes in enzyme affinity toward the substrates after starvation, a phenomenon that has been reported in a number of other microorganisms (11, 16, 17, 22, 24). Experimental results indicated that oxygen was responsible for the aeration toxic effects. These results are consistent with previous reports of greater losses in methane oxidation rates of air-exposed cells than of N2-exposed cells (29, 30). It is possible that the observed oxygen-induced toxic effects were due to generation of active oxygen species by sMMO (14), which in the absence of reduced substrate may have caused enzyme damage.
In contrast to the aeration effects, TCE oxidation resulted in much greater losses in methane uptake activity than in naphthalene oxidation activity. These results are difficult to interpret given that both of these reactions are catalyzed by the sMMO. Regardless of the reasons for the difference in the losses in the methane uptake and naphthalene oxidation activities, the significant losses observed in both specific sMMO-catalyzed reaction activities and general cellular functions, such as formate oxidation and respiratory activities, indicate that TCE oxidation exerts toxic effects that damage sMMO specifically, as well as cause general cellular damage. This broad type of toxicity damage was observed for both cultures of methanotrophs grown under all three nitrogen conditions (nitrogen-fixing, nitrate-supplied, and ammonia-supplied conditions). It is likely that the epoxide and other reactive transient degradation products of sMMO-catalyzed TCE oxidation reactions are responsible for the observed enzymatic and cellular damage (1, 14, 20, 25, 32).
One of the most significant findings of this study was the extent of the damage caused by severe TCE oxidation toxicity. When cells oxidized quantities of TCE that corresponded to the Tc of the cells, methane, naphthalene, and formate oxidation activities ceased entirely, while the respiratory activity decreased to less than 5% of the original activity, suggesting that energy generation for recovery was limited or even impossible. In fact, cell recovery following severe TCE oxidation toxicity did not occur even after 7 days of incubation with methane.
The viability of TCE-exposed cells is a practical issue with respect to whether to discard or recycle TCE-exposed methane-oxidizing cultures in reactor configurations. As shown in Fig. 6 and 7, methanotrophic activities, as measured by TCE oxidation rates, naphthalene oxidation rates, and respiratory activities, exhibited a linear decay relationship with the amount of TCE degraded, suggesting that there is a window for recycling TCE-exposed cells if the cells have not exceeded their TCE Tc. In addition, these observations supported the previous modeling assumption that the amount of biomass inactivated is proportional to the amount of TCE degraded (2, 7). However, for respiratory activity, the x-intercept value of the linear regression significantly exceeded the measured Tc of the cells, suggesting that cells may be capable of some residual respiration after the TCE oxidation activity is exhausted. This conclusion was supported by the observation that cells irreversibly damaged by severe TCE oxidation toxicity retained a small amount of respiratory activity.
The linear decay in cell activity with TCE product toxicity observed in this study contradicts the results of a recent study that described an exponential decay in the viability of TCE-exposed OB3b cells (as measured by plate counts) that was much greater than the measured linear decay in TCE oxidation activity (32). Using plate counts to measure the viability of methanotrophic cells following exposure to TCE might be problematic; that is, methanotrophs do not grow well on agar plates (19, 27, 35), and therefore it is possible that the stress conditions produced by toxicity may cause otherwise viable methanotrophic cells to become incapable of colony growth on plates, resulting in artificially low viability counts.
The major implication of the loss of cell viability due to TCE oxidation is that although cell recycling based on methane-induced activity regeneration may be suitable for maintaining biomass that has experienced small amounts of TCE oxidation toxicity, cell regrowth rather than simply cell recovery is required to counter toxic effects and to promote prolonged TCE oxidation.
Another practical question addressed in this study was whether naphthalene oxidation rates are appropriate indicators for evaluating the remaining TCE degradation ability of TCE-exposed cells. The good correlation between TCE oxidation rates and naphthalene oxidation rates shown in Fig. 6, along with the close match between the x-intercept value and the measured TCE Tc, suggests that naphthalene oxidation rates may indeed be useful indicators when direct measurement of TCE activity is not possible.
Previous studies on the effects of the nitrogen source on toxicity due to TCE oxidation have reported that nitrogen-fixing methane-oxidizing cultures exhibited lower TCE product toxicity and higher TCE Tc than nitrate- and ammonia-supplied cells (8, 9). Two suggested explanations for these observations were that nitrogen-fixing conditions result in the generation of greater amounts of sMMO per cell and/or that enzymatic protection mechanisms associated with the oxygen-sensitive nitrogenase enzymes or the nitrogenases themselves may help protect microorganisms from TCE product toxicity. In this study, nitrogen source effects on toxicity due to aeration and TCE oxidation were examined further. The slightly smaller loss in naphthalene oxidation activity following aeration observed in nitrogen-fixing cultures than in other cultures suggests that nitrogen-fixing cultures might indeed have some protective mechanisms that help sMMO survive aeration. However, the evidence that there are mechanisms that protect against TCE oxidation toxicity was inconsistent. Although the percents of loss in the methane and naphthalene activities following TCE oxidation were significantly smaller with nitrogen-fixing CAC-2 cells than with nitrate- or ammonia-supplied cells, the losses were greater with nitrogen-fixing OB3b cells than with nitrate-supplied cells. In addition, the losses in respiratory activity due to TCE oxidation were similar for both cultures under all three nitrogen conditions, further suggesting that enzymatic protective mechanisms may not play a significant defensive role.
On the other hand, although nitrogen-fixing OB3b cells did not exhibit the smallest percent losses in methane or naphthalene activity due to TCE oxidation, nitrogen-fixing cells of both cultures exhibited the highest specific methane uptake and naphthalene oxidation activities following exposure to TCE (Fig. 4). These results suggest that nitrogen-fixing conditions may result in the generation of greater amounts of sMMO per cell. However, due to the inherent complexities of the nitrogenase enzyme system, specific resolution of this issue is not yet possible.
In summary, in this study we confirmed that aeration and TCE oxidation exert both specific toxic effects on sMMO and nonspecific cellular toxic effects and demonstrated the nature of these toxic effects. TCE product toxicity caused methanotrophic activity to decrease in proportion to the mass of TCE degraded, and cells were not able to recover following severe TCE product toxicity. The results of this study also suggested that nitrogen fixation by methanotrophs may result in greater amounts of sMMO per cell, leading to enhanced TCE degradation. These results should be particularly useful in designing more economic and efficient engineered systems for both in situ and aboveground bioremediation applications. In particular, the linear cell inactivation that was observed can be used to design a feeding strategy that promotes sustained TCE degradation in bioreactors. Furthermore, growth of nitrogen-fixing methane-oxidizing cultures could be promoted under low oxygen partial pressures in order to obtain greater TCE degradation ability and lower TCE product toxicity.
ACKNOWLEDGMENTS
This work was supported in part by a fellowship from the University of California Toxic Substance Research and Teaching Program, in part by grant P42-ES04705 from the National Institute of Environmental Health Sciences, and by National Science Foundation Young Investigator Award BES-9457246.
REFERENCES
- 1.Alvarez-Cohen L, McCarty P L. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl Environ Microbiol. 1991;57:228–235. doi: 10.1128/aem.57.1.228-235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Cohen L, McCarty P L. Product toxicity and cometabolic competitive inhibition modeling of chloroform and trichloroethylene transformation by methanotrophic resting cells. Appl Environ Microbiol. 1991;57:1031–1037. doi: 10.1128/aem.57.4.1031-1037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhupathiraju, V., M. Hernandez, D. Landfear, and L. Alvarez-Cohen. Application of a tetrazolium dye as an indicator of viability in anaerobic bacteria. Submitted for publication. [DOI] [PubMed]
- 4.Bloem J, Veninga M, Shepherd J. Fully automatic determination of soil bacterium numbers, cell volumes, and frequencies of dividing cells by confocal laser scanning microscopy and image analysis. Appl Environ Microbiol. 1995;61:926–936. doi: 10.1128/aem.61.3.926-936.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broholm K, Jensen B K, Christensen T H, Olsen L. Toxicity of 1,1,1-trichloroethane and trichloroethene on a mixed culture of methane-oxidizing bacteria. Appl Environ Microbiol. 1990;56:2488–2493. doi: 10.1128/aem.56.8.2488-2493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brusseau G A, Tsien H C, Hanson R S, Wackett L P. Optimization of trichloroethylene oxidation by methanotrophs and the use of a colorimetric assay to detect soluble methane monooxygenase activity. Biodegradation. 1990;1:19–29. doi: 10.1007/BF00117048. [DOI] [PubMed] [Google Scholar]
- 7.Chang H L, Alvarez-Cohen L. Model for the cometabolic biodegradation of chlorinated organics. Environ Sci Technol. 1995;29:2357–2367. doi: 10.1021/es00009a031. [DOI] [PubMed] [Google Scholar]
- 8.Chu K H, Alvarez-Cohen L. Effect of nitrogen source on growth and TCE degradation by methane-oxidizing bacteria. Appl Environ Microbiol. 1998;64:3451–3457. doi: 10.1128/aem.64.9.3451-3457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu K H, Alvarez-Cohen L. Trichloroethylene degradation by methane-oxidizing cultures grown with various nitrogen sources. Water Environ Res. 1996;68:76–82. [Google Scholar]
- 10.Dalton H, Wilkins P C, Jiang Y. Structure and mechanism of action of the hydroxylase of soluble methane monooxygenase. In: Murrell J C, Kelley D P, editors. Microbial growth on C1 compounds. Andover, United Kingdom: Intercept; 1993. pp. 65–80. [Google Scholar]
- 11.Davis C L, Robb F T. Maintenance of different mannitol uptake systems during starvation in oxidative and fermentative marine bacteria. Appl Environ Microbiol. 1985;50:743–748. doi: 10.1128/aem.50.4.743-748.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogel M M, Taddeo A R, Fogel S. Biodegradation of chlorinated ethenes by a methane-utilizing mixed culture. Appl Environ Microbiol. 1986;51:720–724. doi: 10.1128/aem.51.4.720-724.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox B G, Borneman J G, Wackett L P, Lipscomb J D. Haloalkene oxidation by the soluble methane monooxygenase from Methylosinus trichosporiumOB3b—mechanistic and environmental implications. Biochemistry. 1990;29:6419–6427. doi: 10.1021/bi00479a013. [DOI] [PubMed] [Google Scholar]
- 14.Fox B G, Froland W A, Dege J E, Lipscomb J D. Methane monooxygenase from Methylosinus trichosporiumOB3b. J Biol Chem. 1989;264:10023–10033. [PubMed] [Google Scholar]
- 15.Froland W A, Andersson K K, Lee S K, Liu Y, Lipscomb J D. The catalytic cycle of methane monooxygenase and the novel roles played by protein component complexes during turnover. In: Murrell J C, Kelley D P, editors. Microbial growth on C1 compounds. Andover, United Kingdom: Intercept; 1993. pp. 81–92. [Google Scholar]
- 16.Geesey G G, Morita R Y. Capture of arginine at low concentrations by a marine psychrophilic bacterium. Appl Environ Microbiol. 1979;38:1092–1097. doi: 10.1128/aem.38.6.1092-1097.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glick M A. Substrate capture, uptake, and utilization of some amino acids by starved cells of a psychrophilic marine Vibrio. M.S. thesis. Corvallis: Oregon State University; 1980. [Google Scholar]
- 18.Gossett J M. Measurement of Henry’s law constants for C1 and C2chlorinated hydrocarbons. Environ Sci Technol. 1987;21:202–208. [Google Scholar]
- 19.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry S M, Grbic-Galic D. Inhibition of trichloroethylene oxidation by the transformation intermediate carbon monoxide. Appl Environ Microbiol. 1991;57:1770–1776. doi: 10.1128/aem.57.6.1770-1776.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins I J, Best D J, Turner A P, Jezequel S G, Hill H A. Applied aspects of methylotrophy: bioelectrochemical applications, purification of methanol dehydrogenase, and mechanism of methane monooxygenase. In: Crawford R L, Hanson R S, editors. Microbial growth on C1 compounds. Washington, D.C: American Society for Microbiology; 1984. pp. 297–305. [Google Scholar]
- 22.Kurth G. Some physiological bases for survival of marine bacteria during nutrient starvation. M.S. thesis. Corvallis: Oregon State University; 1980. [Google Scholar]
- 23.Little C D, Palumbo A V, Herbes S E, Lidstrom M E, Tyndall R L, Gilmer P J. Trichloroethylene biodegradation by a methane-oxidizing bacterium. Appl Environ Microbiol. 1988;54:951–956. doi: 10.1128/aem.54.4.951-956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morita R Y. Bioavailability of energy and its relationship to growth and starvation survival in nature. Can J Microbiol. 1988;34:436–441. [Google Scholar]
- 25.Oldenhuis R, Oedzes J Y, Vanderwaarde J J, Janssen D B. Kinetics of chlorinated hydrocarbon degradation by Methylosinus trichosporiumOB3b and toxicity of trichloroethylene. Appl Environ Microbiol. 1991;57:7–14. doi: 10.1128/aem.57.1.7-14.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pyle B H, Broadaway S C, McFeters G A. A rapid, direct method for enumerating respiring enterohemorrhagic Escherichia coliO157:H7 in water. Appl Environ Microbiol. 1995;61:2614–2619. doi: 10.1128/aem.61.7.2614-2619.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed W N, Dugan P R. Isolation and characterization of the facultative methylotroph MycobacteriumIDY. J Gen Microbiol. 1987;113:1389–1396. doi: 10.1099/00221287-133-5-1389. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez G G, Phipps D, Ishiguro K, Ridgway H F. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992;58:1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roslev P, King G M. Aerobic and anaerobic starvation metabolism in methanotrophic bacteria. Appl Environ Microbiol. 1995;61:1563–1570. doi: 10.1128/aem.61.4.1563-1570.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roslev P, King G M. Survival and recovery of methanotrophic bacteria starved under oxic and anoxic conditions. Appl Environ Microbiol. 1994;60:2602–2608. doi: 10.1128/aem.60.7.2602-2608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaule G, Flemming H C, Ridgway H F. Use of 5-cyano-2,3-ditolyl tetrazolium chloride for quantifying planktonic and sessile respiring bacteria in drinking water. Appl Environ Microbiol. 1993;59:3850–3857. doi: 10.1128/aem.59.11.3850-3857.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hylckama Vlieg J E T, de Koning W, Janssen D B. Effect of chlorinated ethene conversion on viability and activity of Methylosinus trichosporiumOB3b. Appl Environ Microbiol. 1997;63:4961–4964. doi: 10.1128/aem.63.12.4961-4964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Hylckama Vlieg J E T, de Koning W, Janssen D B. Transformation kinetics of chlorinated ethenes by Methylosinus trichosporiumOB3b and detection of unstable epoxides by on-line gas chromatography. Appl Environ Microbiol. 1996;62:3304–3312. doi: 10.1128/aem.62.9.3304-3312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wackett L P, Gibson D T. Rapid method for detection and quantitation of hydroxylated aromatic intermediates produced by microorganisms. Appl Environ Microbiol. 1983;45:1144–1147. doi: 10.1128/aem.45.3.1144-1147.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whittenbury R, Phillips K C, Wilkinson J F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970;61:205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- 36.Wilson J T, Wilson B H. Biotransformation of trichloroethylene in soil. Appl Environ Microbiol. 1985;49:242–243. doi: 10.1128/aem.49.1.242-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winding A, Binnerup S J, Sorensen J. Viability of indigenous soil bacteria assayed by respiratory activity and growth. Appl Environ Microbiol. 1994;60:2869–2875. doi: 10.1128/aem.60.8.2869-2875.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]