Abstract
Introduction:
The study aimed to describe daily sleep characteristics for dementia care dyads in the context of adult day services (ADS) use and examine the associations with sleep quality and daytime functioning (fatigue, affect, and behavior problems).
Methods:
Caregivers (CG; N = 173) reported daily bedtime, wake time, and sleep quality for themselves and the persons living with dementia (PLWD) across 8 consecutive days (N = 1,359), where PLWD attended ADS at least 2 days of the week. On each day, caregivers also reported their own fatigue and affect and PLWD’s daytime behavior problems and nighttime sleep problems. Considering the context of ADS use, we compared mean differences in bedtime, wake time, and total time in bed on nights before versus after ADS use. We estimated multilevel models to examine daily sleep-well-being associations.
Results:
On nights before an upcoming ADS day, care dyads went to bed and woke up earlier, and spent less time in bed. Further, PLWD had better sleep quality the night before an upcoming ADS day. Using ADS during the day buffered the negative impact of PLWD’s sleep problems in the previous night, reducing daytime negative affect for caregivers. For caregivers, using ADS yesterday attenuated the association between shorter than typical time in bed and daytime fatigue; it also attenuated the association between PLWD’s nighttime sleep problems and lowered daytime positive affect.
Conclusions:
Regular ADS use may promote earlier sleep timing and protect against the adverse impact of sleep disturbances on daytime functioning for dementia care dyads.
Keywords: Sleep diary, family caregiver, daytime fatigue, affect
Using community-based adult day services (ADS) can reduce daily care-related stressors up to 40% (Zarit, Kim, Femia, Almeida, & Klein, 2014; Zarit et al., 2011), but it is less clear how ADS use may affect daily sleep for persons living with dementia (PLWD) and their family caregivers (CG). Sleep characteristics—such as timing, duration, and quality—may fluctuate daily for dementia care dyads (McCurry, Pike, Vitiello, Logsdon, & Teri, 2008). This is important as sleep is a modifiable health behavior that relates closely to daytime experiences and well-being such as daytime functioning and mood (Li, Kechter, Olmstead, Irwin, & Black, 2018; Mather, Laws, Dixon, Ready, & Akerstedt, 2020; McCrae et al., 2005). ADS use may create fluctuations in daily sleep-wake schedules and other sleep-related characteristics among dementia care dyads, including anticipatory worries the night before ADS use, and effects of activities at ADS on sleep that evening (Zarit et al., 2011).
Differences in stress levels for CG on ADS versus non-ADS days may also affect sleep and other aspects of daytime functioning for care dyads, including daytime fatigue and negative and positive affect for CG. Most prior studies on sleep have typically considered either PLWD or CG rather than both members of the care dyad. Prior work has also examined mean levels of sleep characteristics using composite scores, rather than examining multiple dimensions of sleep; specifically, prior work has not considered variability in dimensions of sleep across days or how daytime activities might affect sleep. The current study extends the literature by describing daily sleep characteristics over multiple days, some of which the PLWD attends ADS and some where they are at home with the CG. We consider variability of multiple dimensions of sleep over 8 days, how ADS use affects daily sleep, and how sleep and ADS use are associated with daytime fatigue, affect, and behavior problems of the care dyads.
Sleep Characteristics of Dementia Care Dyads and ADS Use
Older adults experience age-related deterioration in sleep, which is likely to be exacerbated by dementia or providing care to a family member with dementia. Older adults with dementia may have more sleep disturbances, insomnia, and other sleep-related issues than average older adults their age who do not have dementia (Frohnhofen & Hermann, 2021; Tractenberg, Singer, & Kaye, 2005). Providing care for PLWD can pose considerable challenges to the sleep of the CG, especially when CG live with the PLWD. Reviews found that 50–70% of CG report sleep complaints (Byun, Lerdal, Gay, & Lee, 2016; Peng & Chang, 2013); often these complaints stem from sleep disturbances related to their caregiving responsibilities (McCurry, Song, & Martin, 2015). The most frequent sleep issues among CG include poor sleep quality (Chiu, 2014) and inadequate sleep time (Peng & Chang, 2013). A recent meta-analysis with more than 3,000 older dementia CG showed that compared with age-matched non-CG, CG may lose up to 4 hours of sleep per week due to care demands, in addition to having poorer sleep quality (Gao, Chapagain, & Scullin, 2019).
ADS may affect the sleep of care dyads on both the night prior to and after ADS use. Expecting an upcoming ADS day may entail a more structured sleep-wake schedule, with an earlier bedtime on the previous night, and an earlier wake time on the ADS day. Later sleep timing and bedtime are generally associated with adverse health outcomes, including poorer cognitive function and higher risks for depression, cognitive decline, and falls (Chaput et al., 2020). Additionally, physical and social activities are consistently related to more favorable sleep patterns for PLWD, including adequate sleep duration adjusted by age and reduced nighttime awakenings (Bartfay, Stewart, Bartfay, & Papaconstantinou, 2019; Eggermont & Scherder, 2006). Commuting to an ADS center and ADS activities and programming may engage the PLWD in ways that benefit sleep; but when the PLWD stays at home on non-ADS days, they may become less physically and socially active in ways that hinder sleep (Woodhead, Zarit, Braungart, Rovine, & Femia, 2005). Indeed, ADS use has been associated with fewer CG-reported sleep problems among PLWD (Femia, Zarit, Stephens, & Greene, 2007). Further, as ADS is effective in reducing care-related stressor exposures, an ADS day is likely to be a lower stress day for CG compared with a non-ADS day (Zarit et al., 2011). ADS use may also provide opportunities for CG to engage in more leisure activities, which have been shown to benefit CG sleep (Lee, Xu, Kim, & Chen, 2020; Moore et al., 2011), especially for those prone to stress-related sleep problems.
Sleep Characteristics and Associations with Daytime Functioning Among Dementia Care Dyads
Sleep problems may have consequences such as tiredness or fatigue during the day, which could ultimately lead to other health problems for CG themselves and may even diminish the quality of caregiving and pose safety risks for PLWD. Chiu and colleagues (2014) found that almost all participating CG in their sample reported feeling tired and sleepy during the day due to sleep disturbances. The findings were consistent with an earlier study where two-thirds of the CG reported feeling sleepy during the day due to interrupted sleep (Tractenberg et al., 2005). More generally, sleep problems as measured by the Pittsburgh Sleep Quality Index were associated with worse daytime functioning for CG, including having trouble staying awake while engaging in social activities, and less enthusiasm to get things done (Peng, Lorenz, & Chang, 2019).
Furthermore, PLWD who have sleep interruptions at night often feel tired throughout the day and fall asleep intermittently. Less daytime napping is a general goal of sleep interventions for PLWD with sleep disturbances (McCurry, Gibbons, Logsdon, Vitiello, & Teri, 2003) for at least two reasons. First, PLWD who are inactive during the day are more likely to wake CG up at night (McCurry et al., 1999). Second, excessive daytime napping of the PLWD is related to maladaptive circadian rhythms of activity and sleep (Ancoli-Israel et al., 1997), along with physiological dysregulation (Woods, Kim, & Yefimova, 2011), worse cognitive functioning (Basta et al., 2020), and increased functional impairment (Tractenberg et al., 2005).
Further, CG experience multiple sources of care- and non-care related stressors (Zarit et al., 2014) that can produce sleep and circadian disorders (Kalmbach, Anderson, & Drake, 2018). Similarly, CG’s affective reactivity to daily stressors can vary depending on sleep patterns, where better sleep quality may be associated with lower negative affect for CG (Difrancesco et al., 2021). Prior studies have suggested that sleep health is closely linked to depression and anxiety (Hagen, Barnet, Sprecher, & Peppard, 2020), and CG sleep problems may mediate the association between behavior problems of the PLWD and CG emotional well-being (Jiménez-Gonzalo et al., 2021). Although CG and PLWD consistently show more disturbed sleep characteristics and associations with impaired daily functioning and reduced mental health, it remains unknown how ADS use moderates such associations at the daily level. This is critical as ADS may be a key intervention to enhance both dyadic sleep patterns and daily well-being.
The Current Study
The current study had two aims. First, we sought to describe daily sleep characteristics in the context of ADS use, including bedtime, wake time, and total time in bed before and after an ADS versus non-ADS day. Second, we aimed to examine associations of daily sleep characteristics with sleep quality and daytime functioning in the context of daily ADS use (both today’s and yesterday’s ADS use). We hypothesized that more favorable sleep characteristics (i.e., longer than the typical time in bed, earlier bedtime, and fewer PLWD overnight sleep problems) would be associated with better sleep quality reported by CG for themselves and PLWD (H1), less daytime fatigue for CG and fewer daytime behavior problems for PLWD (H2), and lower negative affect and higher positive affect for CG (H3).
Although prior studies have considered the impact of ADS use on daily and long-term health and well-being for CG (Liu, Kim, Almeida, & Zarit, 2015; Liu, Kim, & Zarit, 2015), few have examined the moderating effect of ADS use on the association between daily sleep and well-being. Therefore, we did not make specific hypotheses but evaluated models to explore the main and moderating effect of ADS use today and yesterday in the daily sleep-well-being association for both CG and PLWD.
Methods
Participants and Procedures
Participants were 173 family CG from the Daily Stress and Health (DaSH) study which examined daily caregiving activities and well-being in the context of ADS use (Zarit et al., 2014). By observing CG and PLWD over 8 days, the study approximates a removed treatment and reversal design, in which an intervention (ADS use) is introduced and then removed. Comparison of observations made on treatment and non-treatment days provides a valid comparison of the effects of treatment (Shadish, Cook, & Campbell, 2002). Caregivers were eligible if they were: a) providing primary care to an PLWD who lived in the same household, b) using ADS programs for at least two days a week, and c) the PLWD had a physician’s diagnosis of dementia. CG were initially interviewed in-person at their homes, during which they signed consent forms and completed a set of questionnaires on demographic characteristics and caregiving history. CG then completed 8 consecutive days of daily evening phone calls on their daily caregiving experiences and well-being. All study procedures were reviewed and approved by the Institutional Review Board at the Pennsylvania State University.
Measures
Daily Sleep
CG reported their own and PLWD’s daily bedtime the night before and wake time on the current day. Based on the sleep diary, we calculated daily total time in bed as the difference between self-reported wake time and bedtime for CG and PLWD. Prior studies have demonstrated that a sleep diary is a valid method to quantify time in bed (Mallinson, Kamenetsky, Hagen, & Peppard, 2019). CG rated their sleep quality as well as the PLWD’s sleep quality: “Rate the quality of your/your relative’s sleep last night” on a 5-point scale (1 = poor to 5 = excellent); the single-item measure of sleep quality has been validated in prior studies (McCrae et al., 2016). CG also reported on nighttime sleep problems of the PLWD based on 2 items: “Did your relative have trouble falling asleep last night?” and “Did your relative wake you up during the night” (1 = yes, 0 = no); summed so that higher scores suggested more nighttime sleep problems of PLWD.
ADS Use
Caregivers reported whether they had used ADS that day in each evening call (1 = use, 0 = nonuse). We considered both today’s and yesterday’s ADS use, such that sleep on a night can be in the context of using ADS on both days, or neither day, or only one of the days.
Daytime Functioning
CG Fatigue and Affect.
During each evening telephone interview, CG reported on a 5-point scale (ranging from 1 = none of the day to 5 = all day) how much they had felt tired or fatigued over the past 24 hours. Using the same scale, they also rated daytime frequency of 24 items of emotions adapted from the Non-Specific Psychological Distress Scale (Kessler et al., 2002; Zarit et al., 2014). We performed factor analysis to confirm two domains of negative and positive affect, and subsequently dropped four items because of low factor loadings on either domain. For the remaining 20 items, we calculated the average scores for negative affect (11 items, α = .88; e.g., fidgety, angry, worthless) and positive affect (9 items, α = .92; e.g., in good spirits, calm and peaceful, and full of life), respectively.
Behavior and Psychological Symptoms of Dementia (BPSD).
We used the Daily Record of Behavior (DRB) with 19 items (α = .90) to measure six behavioral categories of the PLWD: resistance to help with activities of daily living (i.e., refused or struggled with bathing, dressing, medication adherence, toileting, and at mealtime), reality problems (i.e., asking to go someplace where they already were, seeing/hearing things that were not there), mood problems (i.e., expressing sadness, talking about feeling worthless, arguing), restless behaviors (i.e., pacing, following the caregiver, leaving the house, exhibiting agitated behavior), disruptive behaviors (i.e., showing physical aggression, acting suspicious or jealous), and memory-related behaviors (i.e., asking questions repeatedly, misplacing items, covering up memory problems) that occurred during the past 24 hours from the time of the call (Zarit et al., 2014). CG reported BPSD occurrence in four periods: waking to 9 am, 9 am to 4 pm, 4 pm to bedtime, and overnight; these time periods corresponded to the modal period between 9 am and 4 pm when the care dyads use ADS. We summed the total number of BPSD occurrence during daytime versus overnight separately in the current study.
Covariates
We considered covariates that are known to be associated with sleep and emotional well-being, including CG age and education (ranging from 1 = less than high school, 2 = some high school, 3 = high school graduate, 4 = some college/trade/vocational, 5 = college graduate, to 6 = post college degree), sex of CG and PLWD (1 = female, 0 = male), whether CG was a spouse or adult child, and duration of care.
Analytical Strategy
Preliminary descriptive analyses were conducted for CG demographic characteristics and all study variables. For the first aim of the study, we ran descriptive analyses on the mean, standard deviation, and range, collapsing all the days across participants. For daily total time in bed and daily bedtime and wake time for both PLWD and CG, we stratified by ADS use today and yesterday. We also ran t-tests to compare the means on ADS versus non-ADS days.
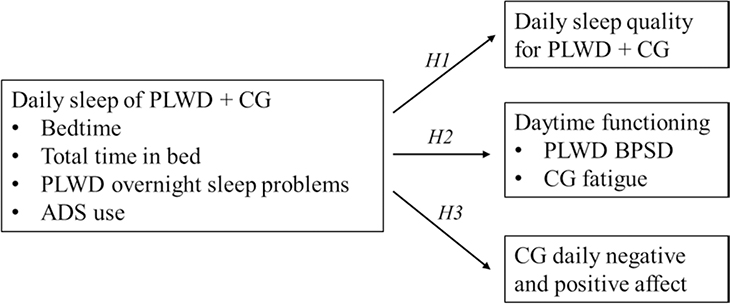
For the second aim, we conducted multilevel analysis to examine the hypotheses on the associations between daily sleep and daytime functioning. To examine H1, we fit separate multilevel models for PLWD and CG with daily sleep quality as the outcome variable. For H2, our multilevel models examined CG daytime fatigue and PLWD daytime BPSD across days as outcomes. Lastly for H3, our multilevel models examined daily negative and positive affect of CG as outcomes. We included key within-person predictors (e.g., daily total time in bed and bedtime, PLWD overnight sleep problems, and ADS use today and yesterday) and between-person predictors (e.g., average total time in bed and bedtime). We considered ADS use today and yesterday in four scenarios—on only yesterday or today, or on both or neither days—and examined how these different ADS use scenarios affected sleep and sleep-health association across days for care dyads. Because both long and short sleep duration are associated with adverse health outcomes (Cappuccio, D’Elia, Strazzullo, & Miller, 2010), we also included a quadratic total time in bed in addition to the linear term as predictors at both within- and between-person levels. We illustrated these hypothesized associations in Figure 1.
Figure 1. Conceptual Model with Hypothesized Associations.
Notes. ADS = adult day services. CG = caregiver. PLWD = person living with dementia.
All the models for hypothesis testing controlled for within-person covariates (i.e., daily BPSD overnight stressors) and between-person covariates (i.e., average time in bed, bedtime, and variations in time in bed and bedtime across days, sex of CG and PLWD, and relationship type). Within-person predictors were centered around the person mean, and between-person predictors were centered around the sample mean. We trimmed off non-significant predictors in the final models for parsimony.
Results
We have reported more detailed sample characteristics previously (Zarit et al., 2014). Briefly, the sample of 173 dementia CG had a mean age of 61.97 (SD = 10.66), 87% were female, 70% were married, and 73% were White. About 58% were adult children CG, 38% were spouses, and 4% were other relationship types. On average, CG first noticed PLWD’s memory problems 3 years ago and had provided care for about two and a half years. The average number of ADS days during the 8-day period was 4.09 (SD = 1.46) days. The average number of daytime BPSD stressors were 4.27 (SD = 5.01).
For the first aim of the study, we found that care dyads went to bed earlier before an upcoming ADS day and woke up earlier on ADS days. The total time in bed was shorter on the night before an upcoming ADS day for both CG and PLWD. Full descriptive results are presented in Table 1.
Table 1.
Daily Total Time in Bed, Bedtime, Wakeup Time, and Variations for Care Dyads in the Context of ADS Use
| Non-ADS day |
ADS day |
||||
|---|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | Diff | |
|
| |||||
| Today | |||||
| PLWD | 10.89 (2.11) | 5–17 | 9.93 (1.69) | 4.50–16.48 | *** |
| TIB | 10.89 (2.11) | 5–17 | 9.93 (1.69) | 4.50–16.48 | *** |
| TIB variationa | 1.14 (0.61) | 0.06–3.52 | 1.14 (0.60) | 0.06–3.52 | ns |
| Bedtime (HH:MM) | 21:34 (01:29) | 17:00–03:00 | 21:09 (01:22) | 16:45–01:13 | *** |
| Bedtime variationa | 0.64 (0.45) | 0.00–2.55 | 0.62 (0.43) | 0.00–2.55 | ns |
| Wakeup time (HH:MM) | 08:28 (01:43) | 02:00–13:00 | 07:05 (01:08) | 02:00–11:00 | *** |
| Wakeup time variationa | 1.02 (0.60) | 0.00–3.42 | 1.00 (0.60) | 0.00–3.42 | ns |
| CG | |||||
| TIB | 8.10 (1.48) | 2.75–14.50 | 7.41 (1.30) | 2.93–15.00 | *** |
| TIB variationa | 0.99 (0.45) | 0.19–3.26 | 0.99 (0.45) | 0.19–3.26 | ns |
| Bedtime (HH:MM) | 23:11 (01:20) | 18:15–05:14 | 23:02 (01:19) | 19:15–05:00 | * |
| Bedtime variationa | 0.67 (0.43) | 0.00–2.68 | 0.67 (0.41) | 0.00–2.68 | ns |
| Wakeup time (HH:MM) | 07:17 (01:23) | 03:00–12:00 | 06:27 (01:08) | 02:20–11:00 | *** |
| Wakeup time variationa | 0.77 (0.43) | 0.06–2.32 | 0.80 (0.44) | 0.06–2.32 | ns |
| Yesterday | |||||
| PLWD | |||||
| TIB | 10.41 (2.03) | 4.50–17.00 | 10.37 (1.90) | 5.50–16.75 | ns |
| TIB variationa | 1.14 (0.61) | 0.06–3.52 | 1.13 (0.61) | 0.06–3.52 | ns |
| Bedtime (HH:MM) | 21:33 (01:26) | 16:45–01:30 | 21:13 (01:25) | 17:00–03:00 | *** |
| Bedtime variationa | 0.64 (0.44) | 0.00–2.55 | 0.62 (0.43) | 0.00–2.55 | ns |
| Wakeup time (HH:MM) | 07:58 (01:39) | 02:00–13:00 | 07:35 (1:33) | 02:00–13:00 | *** |
| Wakeup time variationa | 1.02 (0.59) | 0.00–3.42 | 1.00 (0.60) | 0.00–3.42 | ns |
| CG | |||||
| TIB | 23:12 (01:18) | 19:15–04:30 | 23:05 (01:19) | 19:30–05:14 | ns |
| TIB variationa | 7.88 (1.46) | 3.88–14.50 | 7.63 (1.38) | 2.93–15.00 | ** |
| Bedtime (HH:MM) | 1.00 (0.46) | 0.19–3.26 | 0.99 (0.45) | 0.19–3.26 | ns |
| Bedtime variationa | 0.68 (0.42) | 0.00–2.68 | 0.66 (0.42) | 0.00–2.68 | ns |
| Wakeup time (HH:MM) | 07:05 (01:22) | 02:20–11:00 | 06:43 (01:18) | 03:00–12:00 | *** |
| Wakeup time variationa | 0.77 (0.42) | 0.06–2.32 | 0.81 (0.45) | 0.45–2.32 | ns |
Notes. Day N = 1,359 (based on 173 caregivers). ADS = adult day services. CG = caregiver. PLWD = person living with dementia. TIB = total time in bed, measured by hours elapsed between self-reported bedtime last night and wakeup time on the day; bedtimes/wakeup times reported in the 24-hour (HH:MM) format; the rest times reported in military time format. Descriptives were calculated by collapsing all days in the sample.
Within-person variability around the mean (i.e., iSD). Group mean comparisons and differences were based on t-tests.
p < .05.
p < .01.
p < .001.
ns = non-significant.
With regard to the second aim of the study, models examining H1 suggested that for CG, either longer or shorter amounts than their typical time in bed (i.e., the quadratic term) was associated with poorer sleep quality (β = −0.044, SE = 0.014, p = .002), while there was no linear association between time in bed and sleep quality (β = 0.044, SE = 0.038, p = .25). For PLWD, longer than their typical time in bed was associated with better CG-reported sleep quality linearly (β = 0.077, SE = 0.024, p = .001). Additionally, better PLWD sleep quality the night before was associated with using ADS the following day (β = 0.142, SE = 0.050, p = .004). Better PLWD sleep quality overnight was associated with a later bedtime on average (β = 0.087, SE = 0.036, p = .015), which was contrary to the hypothesis. Further, PLWD spending a longer time in bed on average across days tended to be associated with better CG-reported sleep quality (β = 0.104, SE = 0.052, p = .049). Lastly, as hypothesized, more PLWD overnight sleep problems were associated with poorer sleep quality for both CG (β = −0.489, SE = 0.057, p < .001) and PLWD (β = −0.716, SE = 0.044, p < .0001). We present model parameter estimates in Table 2.
Table 2.
Sleep Quality and Association with Total Time in Bed, Bedtime, and ADS Use (H1)
| CG sleep quality |
PLWD sleep quality |
|
|---|---|---|
| Variable | Estimate (SE) | Estimate (SE) |
|
| ||
| Intercept | 1.097 (1.151) | 0.644 (1.116) |
| Within-person predictor | ||
| CG daily TIB | 0.044 (0.038) | 0.053 (0.029) |
| CG daily TIB-squared | −0.044 (0.014)** | −0.008 (0.011) |
| CG daily bedtime | 0.020 (0.050) | 0.052 (0.038) |
| PLWD daily TIB | 0.050 (0.031) | 0.077 (0.024)** |
| PLWD daily TIB-squared | −0.006 (0.011) | −0.005 (0.008) |
| PLWD daily bedtime | 0.075 (0.048) | 0.087 (0.036)* |
| PLWD overnight sleep problems | −0.489 (0.057)*** | −0.716 (0.044)*** |
| PLWD overnight BPSD | −0.039 (0.018)* | 0.017 (0.014) |
| ADS use todaya | 0.057 (0.066) | 0.142 (0.050)** |
| ADS use yesterdaya | 0.010 (0.054) | −0.002 (0.041) |
| Between-person predictor | ||
| CG average TIB | 0.102 (0.069) | 0.097 (0.068) |
| CG average bedtime | −0.013 (0.071) | 0.106 (0.069) |
| CG TIB variationb | 0.013 (0.163) | −0.043 (0.157) |
| CG bedtime variationb | 0.077 (0.185) | 0.116 (0.179) |
| PLWD average TIB | 0.087 (0.054) | 0.104 (0.052)* |
| PLWD average bedtime | 0.100 (0.072) | 0.026 (0.070) |
| PLWD TIB variationb | 0.003 (0.110) | 0.048 (0.105) |
| PLWD bedtime variationb | −0.116 (0.147) | −0.325 (0.141)* |
| CG female | −0.294 (0.164) | — |
| PLWD female | — | 0.314 (0.143)* |
| CG adult children | −0.141 (0.116) | −0.413 (0.142)** |
| −2 Log Likelihood | 2,655.2 | 2,151.5 |
| AIC/BIC | 2,659.2/2,665.5 | 2,155.5/2,161.8 |
Notes. Day N = 1,359 (based on 173 caregivers). ADS = adult day services. CG = caregiver. PLWD = person living with dementia. TIB = total time in bed, measured by hours elapsed between self-reported bedtime last night and wakeup time on the day. BPSD = behavior and psychological symptoms of dementia. AIC = Akaike information criterion. BIC = Bayesian information criterion. All daily variables were within-person centered. Non-significant demographic characteristics were trimmed off, including spouse relationship, CG age and education, and duration of care.
1 = use and 0 = nonuse.
Within-person variability around the mean (i.e., iSD).
p < .05.
p < .01.
p < .001.
As hypothesized, models examining H2 suggested that daily total time in bed was the strongest predictor for daytime functioning (see Table 3). Specifically, CG having less than their typical time in bed reported more daytime fatigue (β = −0.172, SE = 0.045, p = .000), whereas PLWD having less than their typical time in bed related to more daytime BPSD as reported by CG (β = −0.250, SE = 0.115, p = .030). Additionally, more PLWD sleep problems overnight were associated with worse daytime fatigue for CG (β = 0.232, SE = 0.057, p < .001) and more daytime BPSD for PLWD (β = 0.865, SE = 0.218, p < .001). As for the moderating effect of ADS use, a significant interaction was found between ADS use yesterday and within-person time in bed for CG (β = 0.234, SE = 0.051, p < .0001). As shown in Figure 2 which was graphed based on parameter estimates and predicted values, this finding suggested that using ADS yesterday ameliorated the negative impact of having shorter than their typical time in bed on daytime fatigue for CG.
Table 3.
Daytime Functioning and Association with Total Time in Bed, Bedtime, and ADS Use (H2)
| CG daytime fatigue |
PLWD daytime BPSD |
|
|---|---|---|
| Variable | Estimate (SE) | Estimate (SE) |
|
| ||
| Intercept | 0.681 (1.491) | −1.794 (6.817) |
| Within-person predictor | ||
| CG daily TIB | −0.172 (0.045)*** | −0.210 (0.140) |
| × ADS use yesterdaya | 0.234 (0.051)*** | — |
| CG daily TIB-squared | 0.020 (0.014) | 0.016 (0.051) |
| CG daily bedtime | −0.041 (0.049) | −0.109 (0.184) |
| PLWD daily TIB | −0.031 (0.031) | −0.250 (0.115)* |
| PLWD daily TIB-squared | 0.000 (0.011) | 0.012 (0.040) |
| PLWD daily bedtime | 0.026 (0.046) | −0.250 (0.174) |
| PLWD overnight sleep problems | 0.232 (0.057)*** | 0.865 (0.218)*** |
| PLWD overnight BPSD | 0.039 (0.019)* | 0.597 (0.073)*** |
| ADS use todaya | 0.037 (0.064) | −1.622 (0.242)*** |
| ADS use yesterdaya | 0.030 (0.053) | 0.083 (0.200) |
| Between-person predictor | ||
| CG average TIB | 0.003 (0.089) | 1.059 (0.406)* |
| CG average bedtime | 0.153 (0.091) | 0.766 (0.419) |
| CG TIB variationb | 0.224 (0.209) | 0.260 (0.956) |
| CG bedtime variationb | −0.210 (0.237) | −0.335 (1.085) |
| PLWD average TIB | −0.028 (0.070) | −0.330 (0.321) |
| PLWD average bedtime | 0.002 (0.093) | −0.791 (0.426) |
| PLWD TIB variationb | 0.029 (0.140) | −0.103 (0.636) |
| PLWD bedtime variationb | −0.075 (0.187) | −0.101 (0.855) |
| −2 Log Likelihood | 2,684.8 | 5,322.1 |
| AIC/BIC | 2,688.8/2,695.1 | 5,326.1/5,332.4 |
Notes. Day N = 1,359 (based on 173 caregivers). ADS = adult day services. CG = caregiver. PLWD = person living with dementia. TIB = total time in bed, measured by hours elapsed between self-reported bedtime last night and wakeup time on the day. BPSD = behavior and psychological symptoms of dementia. AIC = Akaike information criterion. BIC = Bayesian information criterion. All daily variables were within-person centered. Non-significant demographic characteristics were trimmed off, including CG age, gender, spouse and adult children relationship, and education, PLWD gender, and duration of care. Two-way interactions were trimmed, if non-significant.
1 = use and 0 = nonuse.
Within-person variability around the mean (i.e., iSD).
p < .05.
p < .01.
p < .001.
Figure 2. Using ADS During the Day Attenuated the Association Between Shorter Than Typical Time in Bed on the Night and Daytime Fatigue the Following Day.
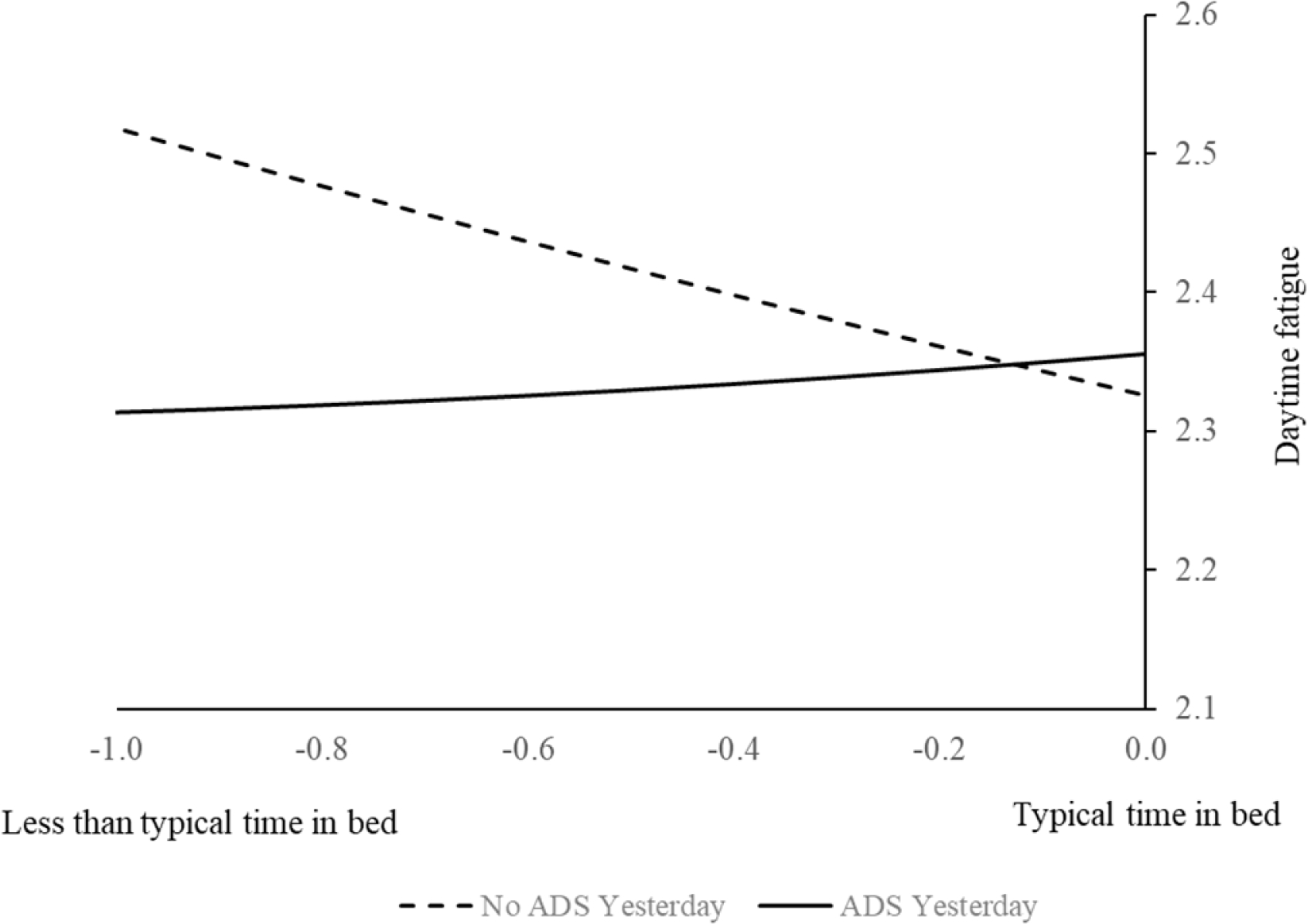
Notes. ADS = adult day services.
H3 was partially supported. We found a significant main effect of PLWD overnight sleep problems on higher CG negative affect (β = 0.121, SE = 0.032, p = .001) and lower positive affect (β = −0.186, SE = 0.053, p = .001) that day (see Table 4). ADS use, however, moderated this daily sleep-affect association for CG. Specifically, using ADS today attenuated the impact of PLWD’s overnight sleep problems on greater daytime negative affect for CG (β = −0.111, SE = 0.035, p = .001). The interaction between today and yesterday’s ADS use on negative affect is illustrated in Figure 3 for four different scenarios of ADS use today and yesterday. Additionally, using ADS yesterday buffered the impact of PLWD’s overnight sleep problems on higher daytime negative affect (β = −0.081, SE = 0.035, p = .022) and lower positive affect (β = 0.133, SE = 0.059, p = .024) for CG; we illustrated the effect in Figure 4 for scenarios of ADS use today and yesterday based on parameter estimates and predicted values.
Table 4.
Caregiver Daily Mood and Association with Total Time in Bed, Bedtime, and ADS Use (H3)
| CG negative affect |
CG positive affect |
|
|---|---|---|
| Variable | Estimate (SE) | Estimate (SE) |
|
| ||
| Intercept | 0.599 (0.697) | 4.694 (1.356)*** |
| Within-person predictor | ||
| CG daily TIB | −0.020 (0.015) | 0.018 (0.024) |
| CG daily TIB-squared | 0.003 (0.005) | −0.005 (0.009) |
| CG daily bedtime | −0.010 (0.019) | −0.002 (0.032) |
| PLWD daily TIB | 0.002 (0.012) | −0.011 (0.020) |
| PLWD daily TIB-squared | −0.005 (0.004) | 0.003 (0.007) |
| PLWD daily bedtime | 0.026 (0.018) | 0.006 (0.030) |
| PLWD overnight sleep problems | 0.121 (0.032)*** | −0.186 (0.053)*** |
| × ADS use todaya | −0.111 (0.035)*** | 0.072 (0.057) |
| × ADS use yesterdaya | −0.081 (0.035)* | 0.133 (0.059)* |
| PLWD overnight BPSD | 0.023 (0.008)** | −0.001 (0.013) |
| ADS use todaya | 0.061 (0.036) | −0.064 (0.059) |
| ADS use yesterdaya | 0.100 (0.032)** | −0.113 (0.053)* |
| × ADS use todaya | −0.115 (0.042)** | 0.143 (0.069)* |
| Between-person predictor | ||
| CG average TIB | 0.053 (0.042) | −0.076 (0.081) |
| CG average bedtime | 0.057 (0.043) | −0.022 (0.084) |
| CG TIB variationb | 0.128 (0.098) | 0.008 (0.190) |
| CG bedtime variationb | −0.103 (0.111) | −0.017 (0.216) |
| PLWD average TIB | −0.044 (0.033) | −0.033 (0.064) |
| PLWD average bedtime | 0.005 (0.044) | −0.027 (0.085) |
| PLWD TIB variationb | 0.073 (0.065) | −0.163 (0.126) |
| PLWD bedtime variationb | −0.026 (0.088) | 0.027 (0.171) |
| −2 Log Likelihood | 937.7 | 1,962.8 |
| AIC/BIC | 941.7/948.0 | 1,966.8/1,973.1 |
Notes. Day N = 1,359 (based on 173 caregivers). ADS = adult day services. CG = caregiver. PLWD = person living with dementia. TIB = total time in bed, measured by hours elapsed between self-reported bedtime last night and wakeup time on the day. BPSD = behavior and psychological symptoms of dementia. AIC = Akaike information criterion. BIC = Bayesian information criterion. All daily variables were within-person centered. Non-significant demographic characteristics were trimmed off, including CG age, gender, spouse and adult children relationship, and education, PLWD gender, and duration of care.
1 = use and 0 = nonuse.
Within-person variability around the mean (i.e., iSD).
p < .05.
p < .01.
p < .001.
Figure 3. Using ADS Today and Yesterday Buffered the Impact of PLWD’s Sleep Problems Overnight on Higher Daytime Negative Affect for CG.
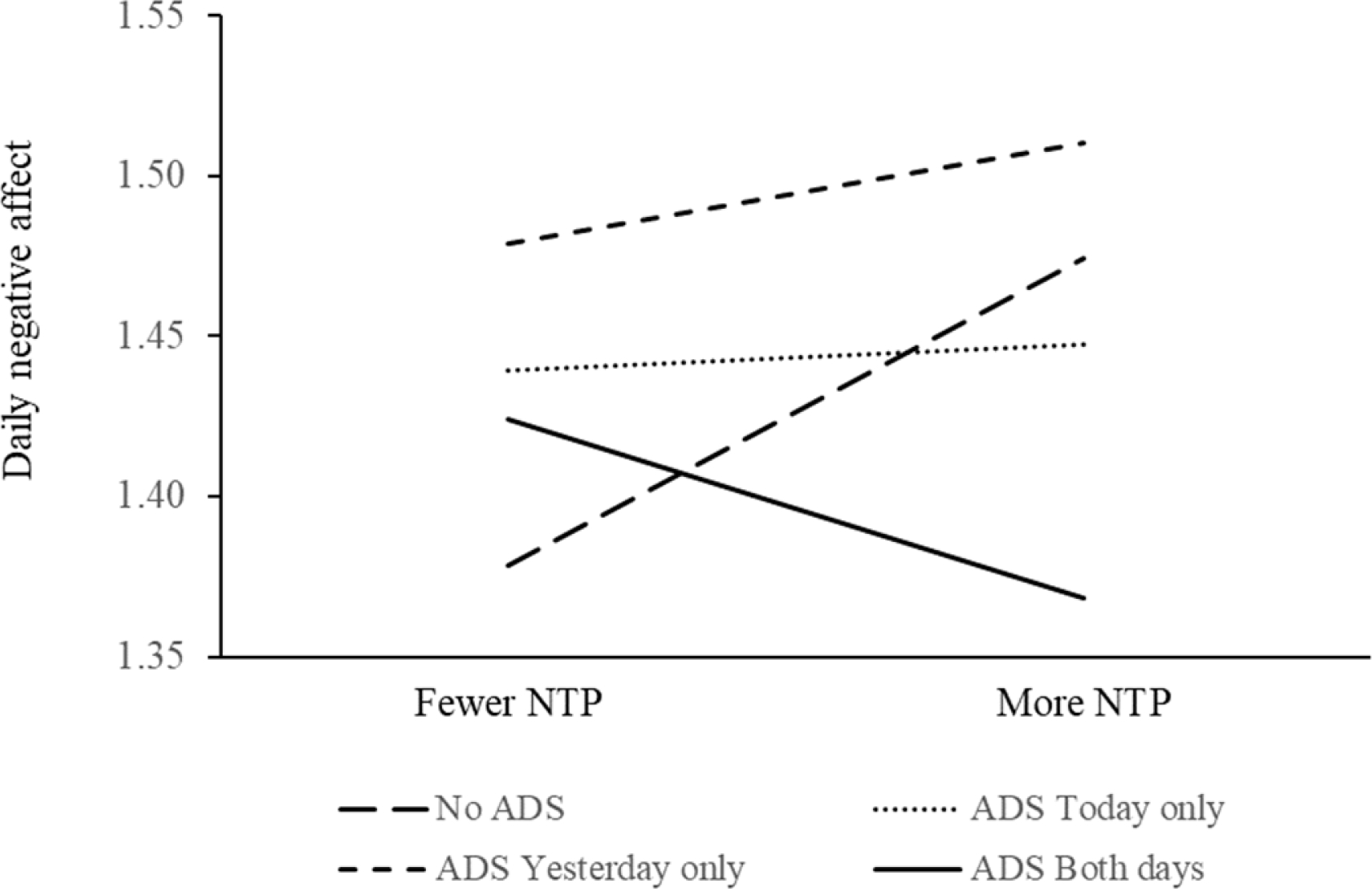
Notes. ADS = adult day services. CG = caregiver. PLWD = person living with dementia. NTP = PLWD nighttime sleep problems.
Figure 4. Using ADS Today and Yesterday Attenuated the Impact of PLWD’s Sleep Problems Overnight on Lowered Daytime Positive Affect for CG.
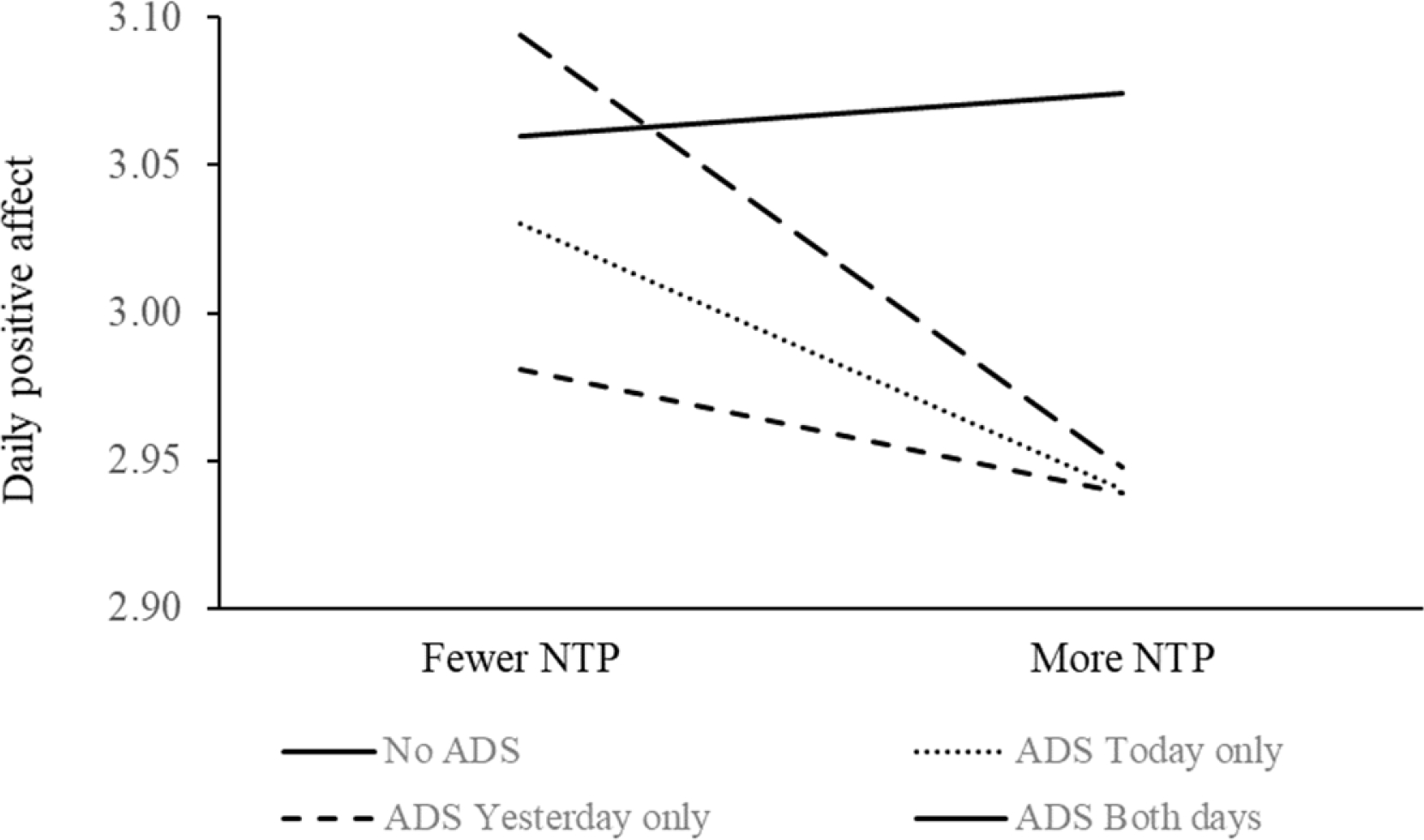
Notes. ADS = adult day services. CG = caregiver. PLWD = person living with dementia. NTP = PLWD nighttime sleep problems.
Discussion
Utilizing an 8-day daily diary design, we described some of the key characteristics of sleep of both CG and PLWD across days in the context of ADS use. We considered four scenarios of daily ADS use—on only yesterday or today, or on both or neither days—and examined the association between daily sleep and daytime functioning of the care dyads. Our findings suggested that anticipating ADS use the next day was associated with earlier bedtime and wake time for care dyads, and CG reported better sleep quality for PLWD. Further, using ADS today reduced the association between PLWD’s sleep problems overnight and daytime negative affect for CG. Additionally, using ADS yesterday attenuated the associations between a) CG’s own typical time in bed overnight and daytime fatigue, and b) PLWD’s sleep problems overnight and CG’s lower daytime positive affect. The current study extended the literature a) by examining the daily experiences of sleep from a dyadic perspective for PLWD and their care partners and b) by examining the contextual effect of ADS use across days on the within-person association between sleep and daytime functioning for the care dyads.
ADS Use, Daily Sleep, and Well-Being for Care Dyads
Our findings confirmed the hypothesis that using ADS the next day was associated with earlier sleep timing; compared to non-ADS days, care dyads spent less amount of time in bed before an upcoming ADS day. A systematic review found that earlier sleep timing and regularity in bedtime and wakeup time are associated with better health outcomes in adults (Chaput et al., 2020), whereas greater intraindividual variability in sleep and wake patterns were associated with more chronic health conditions and poorer mental health (Bei, Wiley, Trinder, & Manber, 2016). Studies have shown that free-schedule days with essentially unstructured sleep were associated with greater night-to-night variability in sleep timing and duration, and worse alignment between sleep onset and circadian phase (McMahon et al., 2020)—both of which are crucial factors for mood and mental health problems (Bei, Manber, Allen, Trinder, & Wiley, 2017). Thus, regular ADS use may help regulate bedtime schedules, which may benefit care dyads’ health and well-being.
It is not clear why using ADS was associated with a shorter amount of time in bed for care dyads, but CG still reported better sleep quality for PLWD. It is possible that ADS might have helped with consistency in bedtime scheduling, which helped with sleep quality. Or, anticipating an easier day helped lower CG distress (Klein et al., 2016), thus helping CG sleep better. Therefore, CG may have reported better sleep for PLWD since they were able to sleep better themselves. This finding suggested that attending ADS may affect sleep of both CG and PLWD. Besides earlier sleep timing for the dyads, using ADS may lower CG’s anticipatory or actual stress or by helping PLWD become more physically and socially active; thus the dyads tended to have better sleep.
As illustrated in Figures 2 and 3, regular daily ADS use seemed to be most beneficial in ameliorating the negative impact of PLWD’s overnight sleep problems on CG’s greater daytime negative affect and lower positive affect. Using ADS during the day may promote physical and social activities and manage related sleep issues for care dyads. Activities are closely related to sleep health in general (Mead, Baron, Sorby, & Irish, 2019; Mesas, Hagen, & Peppard, 2018), and particularly for dementia care dyads. For PLWD, being able to engage in favorite activities and social interactions is associated with greater functional independence and lower levels of depressive symptoms (Regier, Parisi, Perrin, & Gitlin, 2021), and higher levels of positive mood (Beerens et al., 2018). Engaging in activity may also help promote agency of PLWD, which improves their well-being (Chung, Ellis-Hill, & Coleman, 2017). For caregivers, they may be less likely to ruminate about general life worries at night when they are able to take care of business while the PLWD is at ADS (Sladek, Doane, & Breitenstein, 2020). Beside, caregivers’ activity levels and patterns were associated with depressive symptoms (Smagula et al., 2019), whereas restriction in social and recreational activities tended to be associated with poorer sleep and daytime dysfunction (Moore et al., 2011). For care dyads, being physically active may contribute to fewer BPSD in PLWD and may also attenuate caregiving burden for CG (Christofoletti et al., 2011).
Limitations and Conclusions
The current study has some important limitations. Due to the use of secondary data, it was impossible to examine whether improved activity levels were the actual mechanism for the direct association between sleep and well-being for care dyads, or to control for contextual factors such as daily alcohol use and whether CG and PLWD shared the same bed or room. We did collect information at baseline on CG sleep medication use, but did not control for it in the hypothesis testing because we did not have that information for each of the observation days and also because of the low prevalence rate (i.e., 12.5% caregivers reported having prescription sleep medication). We did not have any objective sleep measures based on actigraphy besides the caregiver-reported sleep diary, and future studies need to incorporate both actigraphy-based sleep measures and sleep diaries in trying to replicate the current findings.
Additionally, contrary to H1, a later bedtime for PLWD was associated with better sleep quality reported by CG. As CG went to bed later than PLWD (as suggested by Table 1), it is possible that CG were more tired on a day with a later bedtime, so they slept better–and reported better sleep quality for PLWD as well. Without daytime nap data for PLWD or objectively measured sleep quality, it is beyond the scope of the current study to examine why we have observed these findings. One possibility is that sleep timing may interact with homeostatic sleep drive to affect sleep quality (Deboer, 2018). Future studies utilizing objective sleep measures on quality, timing, and daytime naps collected from both CG and PLWD can help clarify this unexpected finding. Further, as illustrated in Figure 2, although ADS use yesterday statistically ameliorated the negative impact of having a shorter amount of time in bed than their average on higher levels of daytime fatigue for CG, the difference seemed quite small and may have no practical value. How much of an effect of sleep on daytime fatigue is large enough and meaningful for CG is open to debate.
In conclusion, findings from the current study suggested that regular daily ADS use may benefit sleep health for older dementia care dyads by encouraging earlier and more regular sleep timing. Further, regular ADS use may also attenuate the negative impact of daily sleep disturbance of the PLWD on CG’s daytime functioning, including fatigue and greater negative affect and lower positive affect. The patterns of findings were largely the same regardless of the CG-PLWD relationship types, which was somewhat surprising because it would be expected that spouses who are likely to share the same bed or sleep in the same room as the PLWD would be more directly impacted by the PLWD’s sleep patterns. However, the present finding indicated that ADS may have similar benefits across different relationship types and the broad applicability of this approach to improve sleep outcomes for dementia care dyads. Such daily and micro processes, if unattended, may otherwise cumulate and contribute to more serious health problems and poor mental health of CG over time, and ultimately lowering quality of life for the care dyads. Long-term care policies need to consider stronger support for community-based caregiving respite utilization, making family caregiving more sustainable, and delaying or avoiding early institutionalization of PLWD.
Acknowledgement
This study was supported by the National Institute on Aging (NIA) at the National Institutes of Health (grant number R01 AG031758 awarded to Dr. Steven H. Zarit). Drs. Amanda N. Leggett and Courtney A. Polenick were funded by the National Institute on Aging (NIA) at the National Institutes of Health (grant numbers K01 AG056557; K01 AG059829).
Footnotes
Disclosure statement on competing interests:
Authors do not have potential competing interest.
References
- Ancoli-Israel S, Klauber MR, Jones DW, Kripke DF, Martin J, Mason W, . . . Fell R. (1997). Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep, 20(1), 18–23. doi: 10.1093/sleep/20.1.18 [DOI] [PubMed] [Google Scholar]
- Bartfay E, Stewart P, Bartfay W, & Papaconstantinou E (2019). Is there an association between physical activity and sleep in community-dwelling persons with dementia: An exploratory study using self-reported measures? Healthcare, 7(1), 6. doi: 10.3390/healthcare7010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta M, Koutentaki E, Vgontzas A, Zaganas I, Vogiatzi E, Gouna G, . . . Simos P. (2020). Objective daytime napping is associated with disease severity and inflammation in patients with mild to moderate dementia. Journal of Alzheimer’s Disease, 74(3), 803–815. doi: 10.3233/JAD-190483 [DOI] [PubMed] [Google Scholar]
- Beerens HC, Zwakhalen SMG, Verbeek H, Tan ES, F., Jolani S, Downs M, . . . Hamers JPH. (2018). The relation between mood, activity, and interaction in long-term dementia care. Aging & Mental Health, 22(1), 26–32. doi: 10.1080/13607863.2016.1227766 [DOI] [PubMed] [Google Scholar]
- Bei B, Manber R, Allen NB, Trinder J, & Wiley JF (2017). Too long, too short, or too variable? Sleep intraindividual variability and its associations with perceived sleep quality and mood in adolescents during naturalistically unconstrained sleep. Sleep, 40(2). doi: 10.1093/sleep/zsw067 [DOI] [PubMed] [Google Scholar]
- Bei B, Wiley JF, Trinder J, & Manber R (2016). Beyond the mean: A systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Medicine Reviews, 28, 108–124. doi: 10.1016/j.smrv.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Byun E, Lerdal A, Gay CL, & Lee KA (2016). How adult caregiving impacts sleep: A systematic review. Current Sleep Medicine Reports, 2(4), 191–205. doi: 10.1007/s40675-016-0058-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, & Miller MA (2010). Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep, 33(5), 585–592. doi: 10.1093/sleep/33.5.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput J-P, Dutil C, Featherstone R, Ross R, Giangregorio L, Saunders TJ, . . . Carrier J. (2020). Sleep timing, sleep consistency, and health in adults: A systematic review. Applied Physiology, Nutrition, and Metabolism, 45(10 (Suppl. 2)), S232–S247. doi: 10.1139/apnm-2020-0032 [DOI] [PubMed] [Google Scholar]
- Chiu Y-C, Lee Y-N, Wang P-C, Chang T-H, Li C-L, Hsu W-C, & Lee S-H (2014). Family caregivers’ sleep disturbance and its associations with multilevel stressors when caring for patients with dementia. Aging & Mental Health, 18(1), 92–101. doi: 10.1080/13607863.2013.837141 [DOI] [PubMed] [Google Scholar]
- Christofoletti G, Oliani MM, Bucken-Gobbi LT, Gobbi S, Beinotti F, & Stella F (2011). Physical activity attenuates neuropsychiatric disturbances and caregiver burden in patients with dementia. Clinics, 66(4), 613–618. doi: 10.1590/S1807-59322011000400015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung PYF, Ellis-Hill C, & Coleman P (2017). Supporting activity engagement by family carers at home: Maintenance of agency and personhood in dementia. International Journal of Qualitative Studies on Health and Well-being, 12(1), 1267316. doi: 10.1080/17482631.2016.1267316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer T (2018). Sleep homeostasis and the circadian clock: Do the circadian pacemaker and the sleep homeostat influence each other’s functioning? Neurobiology of Sleep and Circadian Rhythms, 5, 68–77. doi: 10.1016/j.nbscr.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difrancesco S, Penninx BWJH, Antypa N, van Hemert AM, Riese H, & Lamers F (2021). The day-to-day bidirectional longitudinal association between objective and self-reported sleep and affect: An ambulatory assessment study. Journal of Affective Disorders, 283, 165–171. doi: 10.1016/j.jad.2021.01.052 [DOI] [PubMed] [Google Scholar]
- Eggermont LHP, & Scherder EJA (2006). Physical activity and behaviour in dementia: A review of the literature and implications for psychosocial intervention in primary care. Dementia, 5(3), 411–428. doi: 10.1177/1471301206067115 [DOI] [Google Scholar]
- Femia EE, Zarit SH, Stephens MAP, & Greene R (2007). Impact of adult day services on behavioral and psychological symptoms of dementia. The Gerontologist, 47(6), 775–788. doi: 10.1093/geront/47.6.775 [DOI] [PubMed] [Google Scholar]
- Frohnhofen H, & Hermann DM (2021). Sleep in elderly adults and in subjects with dementia. In DelRosso LM& Ferri R (Eds.), Sleep Neurology: A Comprehensive Guide to Basic and Clinical Aspects (pp. 289–300). Cham: Springer International Publishing. [Google Scholar]
- Gao C, Chapagain NY, & Scullin MK (2019). Sleep duration and sleep quality in caregivers of patients with dementia: A systematic review and meta-analysis. JAMA Network Open, 2(8), e199891. doi: 10.1001/jamanetworkopen.2019.9891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen EW, Barnet JH, Sprecher KE, & Peppard PE (2020). Midlife sleep health is associated with later-life depression and anxiety. Paper presented at the Virtual Sleep 2020, the 34th Annual Meeting of the Associated Professional Sleep Societies. [Google Scholar]
- Jiménez-Gonzalo L, Romero-Moreno R, Pedroso-Chaparro MDS, Fernandes-Pires JA, Barrera-Caballero S, Olazarán J, & Losada-Baltar A (2021). The role of caregivers’ sleep problems in the association between behavioral symptoms of dementia and caregiving depression and anxiety. Behavioral Sleep Medicine, 19(5), 640–651. doi: 10.1080/15402002.2020.1835662 [DOI] [PubMed] [Google Scholar]
- Kalmbach DA, Anderson JR, & Drake CL (2018). The impact of stress on sleep: Pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. Journal of Sleep Research, 27(6), e12710. doi: 10.1111/jsr.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SLT, . . . Zaslavsky AM. (2002). Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychological Medicine, 32(6), 959–976. doi: 10.1017/S0033291702006074 [DOI] [PubMed] [Google Scholar]
- Klein LC, Kim K, Almeida DM, Femia EE, Rovine MJ, & Zarit SH (2016). Anticipating an easier day: Effects of adult day services on daily cortisol and stress. The Gerontologist, 56(2), 303–312. doi: 10.1093/geront/gnu060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Xu L, Kim BJ, & Chen L (2020). Leisure activity, gender and depressive symptoms among dementia caregivers: Findings from the REACH II. Aging & Mental Health, 24(11), 1886–1893. doi: 10.1080/13607863.2019.1660853 [DOI] [PubMed] [Google Scholar]
- Li MJ, Kechter A, Olmstead RE, Irwin MR, & Black DS (2018). Sleep and mood in older adults: Coinciding changes in insomnia and depression symptoms. International Psychogeriatrics, 30(3), 431–435. doi: 10.1017/S1041610217001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kim K, Almeida DM, & Zarit SH (2015). Daily fluctuation in negative affect for family caregivers of individuals with dementia. Health Psychology, 34(7), 729–740. doi: 10.1037/hea0000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kim K, & Zarit SH (2015). Health trajectories of family caregivers: Associations with care transitions and adult day service use. Journal of Aging and Health, 27(4), 686–710. doi: 10.1177/0898264314555319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallinson DC, Kamenetsky ME, Hagen EW, & Peppard PE (2019). Subjective sleep measurement: Comparing sleep diary to questionnaire. Nature and Science of Sleep, 11, 197–206. doi: 10.2147/NSS.S217867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather MA, Laws HB, Dixon JS, Ready RE, & Akerstedt AM (2020). Sleep behaviors in persons with Alzheimer’s disease: Associations with caregiver sleep and affect. Journal of Applied Gerontology. Online first publication. doi: 10.1177/0733464820979244 [DOI] [PubMed] [Google Scholar]
- McCrae CS, Dzierzewski JM, McNamara JPH, Vatthauer KE, Roth AJ, & Rowe MA (2016). Changes in sleep predict changes in affect in older caregivers of individuals with Alzheimer’s dementia: A multilevel model approach. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 71(3), 458–462. doi: 10.1093/geronb/gbu162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae CS, Rowe MA, Tierney CG, Dautovich ND, DeFinis AL, & McNamara JPH (2005). Sleep complaints, subjective and objective sleep patterns, health, psychological adjustment, and daytime functioning in community-dwelling older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 60(4), P182–P189. doi: 10.1093/geronb/60.4.P182 [DOI] [PubMed] [Google Scholar]
- McCurry SM, Gibbons LE, Logsdon RG, Vitiello M, & Teri L (2003). Training caregivers to change the sleep hygiene practices of patients with dementia: The NITE-AD project. Journal of the American Geriatrics Society, 51(10), 1455–1460. doi: 10.1046/j.1532-5415.2003.51466.x [DOI] [PubMed] [Google Scholar]
- McCurry SM, Logsdon RG, Teri L, Gibbons LE, Kukull WA, Bowen JD, . . . Larson EB. (1999). Characteristics of sleep disturbance in community-dwelling Alzheimer’s disease patients. Journal of Geriatric Psychiatry and Neurology, 12(2), 53–59. doi: 10.1177/089198879901200203 [DOI] [PubMed] [Google Scholar]
- McCurry SM, Pike KC, Vitiello MV, Logsdon RG, & Teri L (2008). Factors associated with concordance and variability of sleep quality in persons with Alzheimer’s disease and their caregivers. Sleep, 31(5), 741–748. doi: 10.1093/sleep/31.5.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry SM, Song Y, & Martin JL (2015). Sleep in caregivers: What we know and what we need to learn. Current Opinion in Psychiatry, 28(6), 497–503. doi: 10.1097/YCO.0000000000000205 [DOI] [PubMed] [Google Scholar]
- McMahon WR, Ftouni S, Phillips AJK, Beatty C, Lockley SW, Rajaratnam SMW, . . . Anderson C. (2020). The impact of structured sleep schedules prior to an in-laboratory study: Individual differences in sleep and circadian timing. PLoS ONE, 15(8), e0236566. doi: 10.1371/journal.pone.0236566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead MP, Baron K, Sorby M, & Irish LA (2019). Daily associations between sleep and physical activity. International Journal of Behavioral Medicine, 26(5), 562–568. doi: 10.1007/s12529-019-09810-6 [DOI] [PubMed] [Google Scholar]
- Mesas AE, Hagen EW, & Peppard PE (2018). The bidirectional association between physical activity and sleep in middle-aged and older adults: A prospective study based on polysomnography. Sleep, 41(9). doi: 10.1093/sleep/zsy114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RC, Harmell AL, Chattillion E, Ancoli-Israel S, Grant I, & Mausbach BT (2011). PEAR model and sleep outcomes in dementia caregivers: Influence of activity restriction and pleasant events on sleep disturbances. International Psychogeriatrics, 23(9), 1462–1469. doi: 10.1017/S1041610211000512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H-L, & Chang YP (2013). Sleep disturbance in family caregivers of individuals with dementia: A review of the literature. Perspectives in psychiatric care, 49(2), 135–146. doi: 10.1111/ppc.12005 [DOI] [PubMed] [Google Scholar]
- Peng H-L, Lorenz RA, & Chang Y-P (2019). Factors associated with sleep in family caregivers of individuals with dementia. Perspectives in psychiatric care, 55(1), 95–102. doi: 10.1111/ppc.12307 [DOI] [PubMed] [Google Scholar]
- Regier NG, Parisi JM, Perrin N, & Gitlin LN (2021). Engagement in favorite activity and implications for cognition, mental health, and function in persons living with and without dementia. Journal of Applied Gerontology. Online first publication. doi: 10.1177/0733464821999199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadish WR, Cook TD, & Campbell DT (2002). Experimental and quasi-experimental designs for generalized causal inference (2nd ed.): Houghton. [Google Scholar]
- Sladek MR, Doane LD, & Breitenstein RS (2020). Daily rumination about stress, sleep, and diurnal cortisol activity. Cognition and Emotion, 34(2), 188–200. doi: 10.1080/02699931.2019.1601617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagula SF, Hasler BP, Schulz R, Graves JL, Reynolds CF, Aizenstein HJ, . . . Hall MH. (2019). Activity patterns related to depression symptoms in stressed dementia caregivers. International Psychogeriatrics. Online first publication. doi: 10.1017/S1041610219001601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tractenberg RE, Singer CM, & Kaye JA (2005). Symptoms of sleep disturbance in persons with Alzheimer’s disease and normal elderly. Journal of Sleep Research, 14(2), 177–185. doi: 10.1111/j.1365-2869.2005.00445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhead EL, Zarit SH, Braungart ER, Rovine MR, & Femia EE (2005). Behavioral and psychological symptoms of dementia: The effects of physical activity at adult day service centers. American Journal of Alzheimer’s Disease and Other Dementias, 20(3), 171–179. doi: 10.1177/153331750502000314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DL, Kim H, & Yefimova M (2011). To nap or not to nap: Excessive daytime napping is associated with elevated evening cortisol in nursing home residents with dementia. Biological Research For Nursing, 15(2), 185–190. doi: 10.1177/1099800411420861 [DOI] [PubMed] [Google Scholar]
- Zarit SH, Kim K, Femia EE, Almeida DM, & Klein LC (2014). The effects of adult day services on family caregivers’ daily stress, affect, and health: Outcomes from the Daily Stress and Health (DaSH) study. The Gerontologist, 54(4), 570–579. doi: 10.1093/geront/gnt045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarit SH, Kim K, Femia EE, Almeida DM, Savla J, & Molenaar PCM (2011). Effects of adult day care on daily stress of caregivers: A within-person approach. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(5), 538–546. doi: 10.1093/geronb/gbr030 [DOI] [PMC free article] [PubMed] [Google Scholar]