Abstract
Background
Obesity is a major risk factor for atherosclerotic cardiovascular disease (ASCVD) and is associated with altered platelet function. The mean platelet volume (MPV) is a rapid measure of platelet activation and a prognostic marker in patients with cardiovascular disease. However, no meta-analysis on the association between MPV and obesity has been conducted, and the value of monitoring the MPV in patients with obesity remains unclear.
Objective
To provide cumulative evidence on whether the mean platelet volume (MPV) is increased in individuals with obesity and to describe associations between the ASCVD-risk factors and the MPV in individuals with obesity.
Methods
This meta-analysis was prepared following the Meta-analysis Of Observational Studies (MOOSE) guidelines. We searched the PubMed and Embase database from inception until the 31st of March 2021. Studies were included when they reported the mean platelet volume in individuals with obesity and provided a suitable non-obese comparator group. The risk of bias was independently assessed by two reviewers using the Newcastle–Ottawa scale. The primary outcome of the meta-analysis was the MPV, while we considered the atherosclerotic risk profiles as a secondary outcome.
Results
We identified 178 citations through the PUBMED and 255 citations through EMBASE database search. In all, 13 studies met the inclusion criteria. Firstly, we report an increased mean platelet volume in individuals with obesity compared to non-obese individuals (MD 0.79; [95%CI: 0.42 to 1.16], I2 = 93.4%). Moreover, the reported increase in the MPV was inversely associated with the body mass index (Coefficient: -0.57, standard error (SE): 0.18, p < 0.001) and directly related to changes in triglyceride levels (Coefficient: 4.99, standard error (SE): 1.14, p < 0.001).
Conclusion
This meta-analysis and meta-regression showed an increased MPV in nondiabetic individuals living with obesity. Moreover, the MPV was associated with hypertriglyceridemia, an independent predictor of atherosclerotic cardiovascular disease. Overall, the findings suggest that MPV may be a valuable rapid marker for the monitoring and risk-stratification of individuals with obesity who may be at risk of developing cardiovascular disease.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40795-022-00541-8.
Introduction
Obesity affects more than a third of the population living in low-to-middle- and high-income countries. It has also emerged as a significant healthcare challenge affecting children and adults [1]. On a global scale, over 650 million adults are obese, with sex and ethnic disparities in the prevalence of obesity [2]. The prevalence of obesity ranges from 3.7% to 7.0% in Asia [1] and as high as 39.2% in the United States of America [3]. Obesity is one of the most prevalent comorbid diseases and an independent risk factor of significant non-communicable diseases like type 2 diabetes, cardiovascular disease, and malignancies. Notably, the prevalence of obesity is commonly estimated using the body mass index (BMI), and several BMI cut-off points exist for children(5–10 years), adolescents(11 to 18 years) and adults (> 18 years) [4]. The WHO BMI cut-off points are known to underestimate the risk of obesity in Asian populations [5]. There are currently three major classification systems used to assess overweight and obesity, and these include the International Obesity Task Force (IOTF)[6], the United States Centers for Disease Control and Prevention [7] and the World Health Organization (WHO) criteria [8].
A previous meta-analysis of Mendelian randomisation studies provided evidence of obesity as an independent predictor of coronary artery disease and showed no association with the risk of strokes [9]. A hypercoagulable state in patients with obesity is primarily due to platelet dysfunction, increased thrombin formation, and impaired fibrinolysis [10]. Activated platelets play a pivotal role in inflammation and the development of atherosclerotic cardiovascular disease (ASCVD). The statistical models used to estimate the 10-year ASCVD-risk of individuals between the ages of 40 to 79 years are based on risk factors which include age, total cholesterol, high-density lipoprotein cholesterol, treated or untreated systolic blood pressure levels, diabetes mellitus status, and their smoking status [11]. The basal platelet activation and reactivity levels predict patient outcomes following antiplatelet therapy [12]. Since the therapeutic adjustment of antiplatelet drug dosing is dependent on weight and platelet responses, in a clinical setting, point of care testing is crucial for optimal patient management [12, 13].
A clinical dilemma arises in patients with obesity, as obesity is associated with impaired platelet responses. The mean platelet volume (MPV) is a convenient measure of platelet activation that is routinely available and can be utilised in an inpatient and outpatient setting [14]. In a previous meta-analysis, Chu et al., showed the potential value of the MPV as a prognostic marker in patients with CVD [14]. The MPV provides rapid platelet activation and thrombotic risk measurement, as the MPV correlates with platelet size, activation, and increased aggregation [15]. Notably, activated and younger pro-thrombotic platelets are associated with a higher MPV [16]. Although the clinical relevance and interpretation of the MPV in individuals with obesity remains uncertain, controversial findings of increased [15, 17–23] and comparable [24] MPV in obese compared to lean individuals, have been reported in observational studies.
No meta-analysis on the MPV in individuals with obesity has been conducted to date. The value of the MPV as a determinant of thrombotic risk in obese patients may offer a practical and cost-effective surrogate of platelet activation in obese patients who may be at risk of developing ASCVD. This meta-regression and meta-analysis were conducted to estimate the MPV in individuals with obesity by pooling data reported in studies evaluating the MPV in obese populations. The emphasis of the current study is to provide cumulative evidence on whether the MPV is increased in individuals with obesity and to describe associations between the ASCVD-risk factors and the MPV in individuals with obesity.
Methods
The study protocol was not registered, and therefore, no registration number has been allocated. This systematic review and meta-analysis was reported in accordance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [25]. All data searches and collection took place between February 2020 and April 2021. There was no patient-level data collected, and the protocol required no informed consent or institutional approval as only publicly available data was accessed. The search strategy was designed to retrieve published studies that aim to address the following research questions;
Is the mean platelet volume increased in individuals with obesity?
Are there any associations between the atherosclerotic cardiovascular-risk factors and the MPV?
Information sources and search strategy
We conducted a systematic search using medical subject headings (MesH) on MEDLINE and EMBASE search headings (Emtree) on the EMBASE database, from inception until the 31st of March 2021. The search was independently conducted by two reviewers (BBN and VM) using the PUBMED and OVID interface. The reviewers used the following MeSH terms and text words to retrieve the relevant studies; “Obesity” AND “Blood Platelets” OR “Thrombocytes” OR “Platelet count” OR “Mean Platelet Volume” OR “Plateletcrit” OR “Platelet Distribution Width”. The search strategy was adapted for the ISI Web of Science, the international prospective register of systematic reviews (PROSPERO), Cochrane Collaboration, and the Joanna Briggs Institute (JBI) protocol registry. We restricted the search to human clinical studies with no language restrictions applied. Additional scanning of the bibliographies of the retrieved studies was used to augment the retrieval strategy and ascertain that no relevant citations were inadvertently filtered out through the database search. A detailed description of the search strategy used on the MEDLINE database using the PUBMED search engine is provided on supplementary file 1 (Table 1S), and the Embase data search using the OVID interface is presented in supplementary file 1 (Table 2S).
Eligibility criteria
The studies were included based on the fulfilment of the following eligibility criteria:
Population
For adult participants, we included nondiabetic individuals living with Class I obesity (BMI 30.0-34.9 kg/m2 ) and class II obesity (BMI 35 35.0-39.9 kg/m2). Whereas for Asian populations, adults with a BMI ≥ 25 kg/m2 were considered as living with obesity.
For children and adolescents, we included nondiabetic individuals living with Class 1 obesity (BMI ≥ 95th percentile to <120% of 95th percentile for age and sex) and class II obesity (BMI ≥ 120% to < 140% of 95th percentile or BMI ≥ 35 kg/m2).
Interventions and comparators
There were no specific interventions considered for this study. While the comparator included non-obese individuals (BMI < 25.5).
Outcomes
The primary outcome included the difference in mean platelet volume. The secondary outcome included atherosclerotic cardiovascular disease (ASCVD) risk markers, such as BMI; systolic blood pressure; diastolic blood pressure; high-density lipoprotein and low-density lipoprotein cholesterol.
Study design and selection
In this systematic review and meta-analysis, we included observational studies. We included studies that reported the mean platelet volume as one of the study outcomes. Studies that were case studies, reviews, letters to the editor, animal models or cell lines were excluded.
Data collection process and data items
Two reviewers (BBN and VM) independently extracted detailed study-level participant data using the adapted Cochrane Consumers and Communication Review Group data extraction sheet for included studies template [26]. The data items included the names of the authors; year of publication; sample size; participant age, gender, and BMI; primary outcome measures (MPV) and secondary outcome measures (Systolic blood pressure, diastolic blood pressure, glucose, low-density lipoprotein-c, high-density-lipoprotein-c, triglycerides). A third reviewer (PVD) was consulted for arbitration in instances of discrepancies in the extracted data items.
Assessment of study quality and confidence in the cumulative evidence
The Newcastle–Ottawa tool was used to assess the quality of the included studies [27]. The tool assesses the risk of bias within the following domains; selection of study groups, comparability, and the outcome. Two authors (VM and KM) independently graded the included studies, which were classified as low-quality (score between 0–4), Moderate-quality (score of 5–6), and high-quality (score > 7). Two reviewers (TNM and VM) used the Grading of Recommendations Assessment, development, and Evaluation (GRADE) approach to evaluate the quality of the cumulative evidence [28]. The GRADE tool consists of three domains: consistency, directness, precision, and publication bias. Based on these domains, the certainty of the evidence for each outcome was rated as low, moderate, and high.
Statistical analysis
We computed and estimated the standardised mean difference (SMD) and 95% confidence interval (CI) between obese and non-obese individuals for each included study. The reported probability values (p-values) were also pooled using Edington’s additive method [29]. We then estimated the effect size using the random-effects model when the levels of statistical heterogeneity were substantial (I2 > 50%), and we used a fixed-effects model when the levels of statistical heterogeneity were low (I2 < 40%) [30]. The between-study variance was assessed using the T2 and I2 statistics. Since platelet indices may be affected by geographical variations, a post hoc subgroup analysis was performed based on the reported study location. Moreover, publication bias was assessed using visual inspection of funnel plots and the Egger’s test. All statistical analyses were performed using SATA 16.0 (StataCorp LP, College Station, TX, USA). The risk of bias plots were prepared using the robvis tool [31].
Meta-regression analysis
We performed a random-effects-weighted meta-regression analysis to assess the association between BMI and mean platelet volume. Due to known changes in MPV levels due to age and BMI, a meta-regression was used performed to explore the potential interaction between age and the MPV. The residual maximum likelihood method was used to calculate the between-study component of variance.
Results
Characteristics of included studies
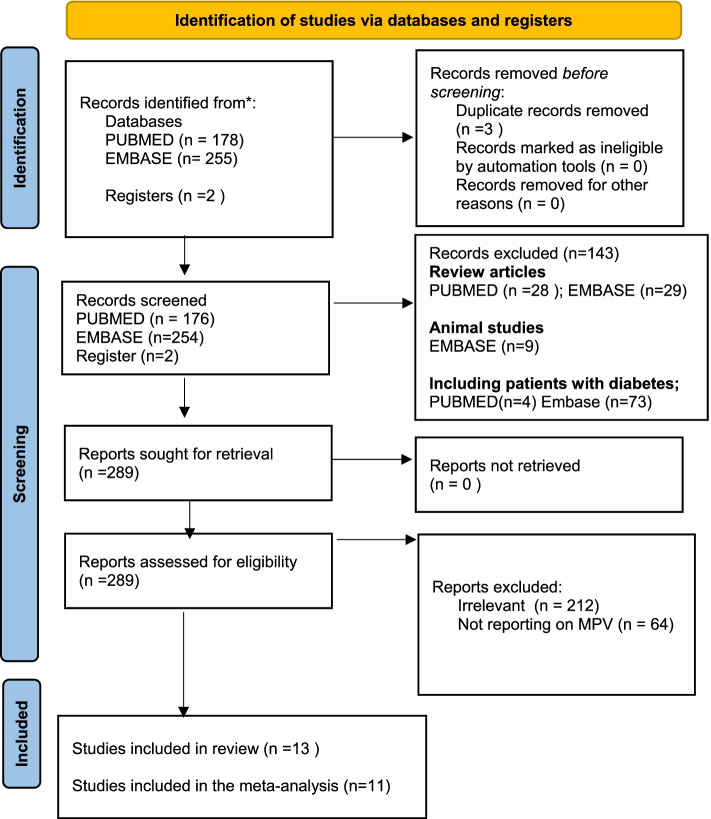
We identified 178 citations through the PUBMED, 255 through EMBASE database search and two citations through searching registries. We screened all retrieved citations, and after excluding duplicates, a total of 430 studies were eligible for full-text screening. In all, 13 studies were included in the qualitative synthesis, whereas eleven studies were included in the meta-analysis (Fig. 1). Notably, the included studies were all cross-sectional studies comprised of studies from 3 different countries, which included; Turkey (n = 9)(16,17,18,19,20,22,23,24), Poland (n = 1) [21], Malaysia (n = 1) [32], China (n = 1) [33], and Brazil (n = 1) [23] (Table 1). The meta-analysis comprised of 814 patients with obesity and 598 non-obese individuals. Only six studies(17,18,19,20,21,22); reported on the mean age and 6 studies [18–23] reported on the mean BMI of obese participants (Table 2). The mean age of the included obese participants was 44.03 ± 18.01, while the mean age of the control participants with normal body weights was 41.67 ± 17.93. Overall, the individuals with obesity were older (mean difference: 1.05; 95%CI: 0.28 to 1.82; p < 0.01) with higher SBP (MD: 6.38; 95%CI: 3.64 to 8.07) and DBP (MD: 2.37; 95%CI: -0.36 to 4.88) when compared to controls. The characteristics of the included studies are presented in Table 1.
Fig. 1.
PRISMA flow diagram illustrating the study selection process
Table 1.
Characteristics of studies reporting on the mean platelet volume in individuals with obesity (n = 13)
| Author | Year | Country | No. participants | Primary aim | Main findings related to platelet indices | Instrument (Reference range) |
|---|---|---|---|---|---|---|
| Arslan [15] | 2010 | Turkey |
47 Non-obese 56 Obese (BMI ≥ 27 kg/m2) |
To investigate the MPV levels in obese adolescents. Moreover, to compare the MPV levels between patients with or without NAFLD and healthy controls |
Obese adolescents had significantly higher MPV levels compared to healthy controls. In addition, the MPV is inversely associated with HDL cholesterol levels and platelet counts |
EDTA, Sysmex XE-500 Reference range: 7.2–11.1 fL |
| Coban [17] | 2005 | Turkey |
100 Non-obese (BMI < 26.6) 100 Obese (BMI ≥ 30 kg/m2) |
To evaluate MPV in patients with obesity compared to non-obese controls | The MPV is increased in individuals with obesity when compared to the control group. In addition, the MPV was positively associated with BMI |
Citrate, Abbot Cell-Dyn 3500 |
| Coban [18] | 2007 | Turkey |
30 Non-obese (BMI < 27.7) 30 Obese women (BMI ≥ 30 kg/m2) |
To evaluate the effect of weight loss on the levels on the levels of MPV in obese patients | The MPV was higher in obese patients. Moreover, the MPV was directly associated with BMI |
Citrate Abbot Cell Dyn 3500 |
| Erdal [19] | 2019 | Turkey |
45 Non-obese < 21 kg/m2) 45 Obese (BMI > 45 kg/m2) |
To compare the complete blood counts, which included PCT and PLR values of healthy subjects with those of morbidly obese individuals in the young population | Platelet counts were increased in individuals with obesity. However, the MPV was comparable between lean and obese | NR |
| Esen [20] | 2015 | Turkey |
204 Non-obese (BMI < 27.2) 290 Obese (BMI ≥ 30 kg/m2) |
To compare the complete blood counts, which included PCT and PLR values of healthy subjects with those of morbidly obese individuals in the young population | The MPV was comparable between obese and non-obese individuals. Although the MPV was inversely correlated with BMI |
EDTA, Sysmex XE-500 (Sysmex Corp, Kobe, Japan) Reference range: 7.2–11.1 fL |
| Furman-Niedzieko [21] | 2014 | Poland |
129 Non-obese (BMI 21.3–25.7 kg/m2) 218 Obese (BMI > 25.1 kg/m2) |
To assess the correlation between the platelet markers, especially the MPV, and the incidence of abdominal obesity in patients with the metabolic syndrome | The MPV was higher in individuals with obesity compared to controls |
NR; SYSMEX XS-1000i |
| Furucouglu [24] | 2016 | Turkey |
73 Non-obese (BMI < 25.53 kg/m2) 74 Obese (BMI ≥ 30–40 kg/m2) |
To aim of our study was to evaluate the effect of weight and smoking status on CBC parameters and biomarkers | The MPV was comparable between obese and individuals with normal body weights. The BMI is positively correlated with platelet indices (Platelet distribution width, plateletcrit and platelet count) other than the MPV | NR |
| Hou [33] | 2015 | China |
3527 Normal (BMI < 24 kg/m2) 899 Obese (BMI ≥ 28 kg/m2) |
To investigate associations between adiposity indices and platelet indices |
The MPV was in lower in obese compared non-obese individuals. The MPV levels were strongly associated with white cell count |
EDTA and Citrate; CELL-DYN 3700, (Abbott, USA); Reference range: 7–11 fL |
| Ozkan [22] | 2015 | Turkey |
48 Non-obese (BMI < 21 kg/m2) 60 Obese (BMI ≥ 23.7 kg/m2) |
To investigate the relation between MPV levels and CIMT measurements | The MPV was increased in obese children with non-alcoholic fatty liver disease | NR |
| Pinto [23] | 2019 | Brazil |
33 Non-obese (< 25 kg/m2) 69 Obese (> 30 kg/m2) |
To verify the influence of EDTA on MPV among normal overweight and obese patients | The MPV was increased in individuals with obesity compared to controls. The BMI and waist circumference were positively associated with the MPV |
K3-EDTA Cell-DYN Ruby analyser |
| Rihayi [32] | 2018 | Malaysia |
56 Non-obese (BMI 18.5–22.9 kg/m2) 28 Obese (BMI 25.5-35 kg/m2) |
To evaluate associations between markers of platelet activation and inflammation in men and women with varying body mass indices | The MPV was elevated in individuals with obesity when compared to controls. Moreover, the MPV was directly associated with the white cell count |
EDTA; Sysmex KX-21 |
| Tavil [34] | 2006 | Turkey |
140 Non-obese 205 Obese (Abdominal obesity: > 102 cm in men; and > 88 cm in women) |
to determine whether MPV values are increased in patients with MS, and secondly to evaluate the relationship between the severity of atherosclerosis and MPV patients with MS | The MPV was significantly increased in individuals with obesity |
K3-EDTA Cell-DYN 3500 (Abbot, IL, USA) Reference range: 7.0–11.00 fL |
| Yilmaz[9] | 2015 | Turkey |
16 Non-obese (BMI < 25 kg/m2) 25 Obese (BMI ≥ 25 kg/m |
To compare hsCRP, MPV and NLR levels in lean and obese PCOS patients | The MPV is increased in individuals with obesity when compared to the control group. In addition, the MPV was positively associated with the BMI | Sysmex XE-2100 |
BMI Body Mass Index, CBC Complete blood count; EDTA ethylenediaminetetraacetic, fL Femtoliters, HDL High-density lipoprotein cholesterol, K3-EDTA tripotassium EDTA, NAFLD Non-Alcoholic fatty Liver Disease(NAFLD), PCT Plateletcrit, PLR Platelet leukocyte Ratio
Table 2.
Cardiovascular-risk profile of included participants
| Effect Measure | Number of studies | Number of participants | Effect Estimate | |||||
|---|---|---|---|---|---|---|---|---|
| Model | MD | SMD | 95% CI | I2, p-value | Z, p-value | |||
| BMI | 10(16, 17, 19–23, 32, 34) | 1902 | RE | 11.15 | – | 8.87 to 13.43 | 98%, p < 0.0001 | 9.59,p < 0.0001 |
| Age | 11(15, 17–23, 32, 34) | 2004 | FE | 1.00 | – | 0.35 to 1.82 | 23%,p = 0.003 | 3.00, p = 0.0031 |
| Platelet count | 9(15–17, 19–24) | 974 | FE | -1.22 | -2.42 to -1.65 | 41.93%,p = 0.09 | -1.98,p = 0.047 | |
| Blood Pressure | ||||||||
| SBP | 4(18, 20, 22, 34) | 1007 | RE | 10.08 | – | -0.22 to 20.37 | 97%, p < 0.0001 | 1.92, p = 0.06 |
| DBP | 3(18, 20, 22, 34) | 1007 | RE | 4.60 | – | -1.29 to 10.48 | 97%,p < 0.0001 | 4.60, p = 0.13 |
| Glucose metabolism | 6(17–20, 22, 34) | 1297 | FE | 4.05 | – | 2.92 to 5.19 | 90%,p < 0.0001 | 7.00,p < 0.00001 |
| Lipid metabolism | ||||||||
| Total cholesterol | 7(16–22, 34) | 1629 | RE | – | 0.19 | 0.00 to 0.38 | 68%,p = 0.005 | 2.00,p = 0.05 |
| HDL Cholesterol | 7(16–22, 34) | 1629 | RE | – | -0.68 | -1.25 to -0.12 | 96%,p < 0.001 | 2.37,p = 0.02 |
| LDL Cholesterol | 7(16–22, 34) | 1629 | RE | – | 0.20 | 0.00 to 0.41 | 71%,p = 0.002 | 2.00,p = 0.05 |
| Triglycerides | 7(16–22, 34) | 1629 | RE | – | 0.60 | 0.20 to 1.01 | 93%,p < 0.001 | 2.90,p = 0.004 |
BMI Body mass index; SBP Systolic Blood Pressure; DBP Diastolic Blood Pressure; HDL high-density lipoprotein; LDL Low-density lipoprotein; RE Random-effects; FE Fixed effects
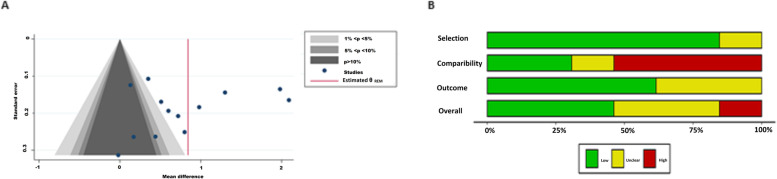
Assessment of publication bias
There were 11 studies included in this meta-analysis, and visual inspection of the funnel plots indicated asymmetry. The contour-enhanced funnel plot was used to distinguish between publication bias and alternative sources of asymmetry. The funnel plot revealed that small studies were not only found in the regions of no statistical significance (p > 10%), but they also fell in the region of statistical significance (p < 5%). This suggests that asymmetry was a result of alternative sources but not solely a result of publication bias. In addition, we performed the Egger's regression test to explore the association between the reported effect size and the study size. In addition, there was no evidence of small study effects (p = 0.34) (Supplementary table 4S) or publication bias by the Egger test (bias: 0.17; p = 0.51).
Quality assessment of the included studies
The Newcastle–Ottawa scale was used to assess the risk of bias in the included studies (Fig. 2B). In all, 42% of the included studies [15, 17, 18, 22, 34] scored as high-quality (scores > 7), and an equal proportion of 42% of the studies [16, 19, 20, 23, 32] were of moderate-quality (scores ranging from 5–6). While, only 16% were considered as having a low-quality [21, 24] and had scored lower than 5 (Table 2S).
Fig. 2.
Publication and risk of bias assessment. A shows the funnel plots, and. B demonstrates the overall risk of bias
Primary findings
A total of 7 studies reported increased MPV levels in children [15, 22] and adults with obesity [16–18, 21, 23]. Only three studies reported findings suggesting that the MPV remains unchanged in obesity [20, 24, 35]. Notably, the included studies reported an inverse [20] and a direct association [16–18, 23] between BMI levels and the MPV in individuals with obesity. Moreover, the MPV is also inversely associated with HDL cholesterol levels and platelet counts in individuals with obesity. The primary outcome of this meta-analysis included changes in the mean platelet volume in 814 individuals with obesity reported in 10 studies [15–20, 22–24, 36]. After pooling the effect estimates, we showed that the mean platelet volume significantly increased in individuals with obesity compared to non-obese individuals (MD 0.79; (95%CI: 0.42 to 1.16). However, the levels of statistical heterogeneity were high (I2 = 93.44%), which were unexplained by the test for subgroup analysis based on the risk of bias in the included studies (Fig. 2).
Secondary findings
In assessing the secondary outcome of the meta-analysis which focused on the ASCVD-risk in individuals with obesity. Only 5 (50%) of the included studies reported on fasting blood glucose levels [17–20, 22], and 6 studies [16–22] lipid profiles in individuals with obesity. As expected, our meta-analysis showed that individuals with obesity had higher; fasting blood glucose levels (MD:2.75; 95%CI, 1.55 to 3.94; I2 = 76%,p = 0.02); total cholesterol (SMD: 0.14 [95%CI: 0.03 to 0.25], I2 = 72%, p = 0.04); LDL cholesterol (SMD: 0.22[95%CI: -0.03 to 0.47], I2 = 76%, p = 0.009) and triglycerides (SMD: 0.43[95%CI:0.18 to 0.68], I2 = 75%, p = 0.0007) (Table 2).
Subgroup and sensitivity analysis
There were substantial levels of statistical heterogeneity in the reported effect estimates. We, therefore, performed a subgroup analysis to explore the sources of heterogeneity based on the differences in the risk of bias in the included studies (Fig. 3). Although the small number of studies and covariance because of an unequal number of studies in each subgroup, the test for subgroup effect showed a significant subgroup effect (p = 0.19). This suggests that the reported differences in MPV may be influenced by the risk of bias in the included studies.
Fig. 3.
Forest plot showing the Random-effects pooled mean difference of the mean platelet volume in obese and non-obese individuals
Notably, the forest plot (Fig. 3) demonstrates that the studies with high-quality showed a more significant mean difference (MD) in the MPV of individuals with obesity (MD: 1.01 [0.39 to 1.62]], I2 = 94.1%) when compared to studies with a moderate-quality (MD: 0.44 [0.19 to 0.70], I2 = 56.9%). In comparison, studies with a low-quality showed the highest increase in MPV in individuals with obesity compared to controls (MD: 1.22 [-0.49 to 2.92], I2 = 93.3%). Notably, amongst the studies reporting on a cohort of individuals with obesity from the Middle East, all studies were conducted in Turkey, and only 28.6%(n = 2) of the studies were moderate-quality. While the majority, 71% (n = 5) of the studies had a low-quality of bias (Table 1). Unlike the studies from Brazil and Poland, which were both of moderate-quality. The sensitivity analysis showed that studies with a low-quality overestimated the effect size of the primary outcome (0.98[0.33 to 1.62], I2 = 92.15%, p < 0.001) when compared to studies with a moderate-quality of bias (0.25[0.01 to 0.48], I2 = 62.06%, p < 0.041]. We also explored whether the variations in the included participants' age modified the effect estimate of the primary outcome (supplementary table 2S). Two of the included studies reported on the MPV of children (< 18 years of age) [15, 22], while the majority of the studies reported on cohorts comprised of adults (> 18 years of age) [16–21, 23, 24].
Meta-regression analysis
We explored the associations between the markers of ASCVD-risk reported pooled mean platelet volume estimates. In the reported markers, collinearity existed between the BMI, systolic blood pressure, diastolic blood pressure and fasting blood glucose levels. So, the explanatory variables selected for the meta-regression model included Age, BMI, platelet counts, cholesterol and Triglyceride levels. Notably, the age of the study participants was reported in 8 studies [17–22], and BMI levels were reported in 7 studies [6–12]. Whereas only six studies [16–22] reported on the total cholesterol levels (Table 2).
In the meta-analysis, the levels of MPV were significantly elevated in obesity with moderate levels of certainty (Table 3). Interestingly, the meta-regression showed that age, BMI, and total cholesterol levels are significant confounders of the reported differences in changes in MPV in individuals with obesity (Table 2). While the inverse association between the BMI and MPV (Coefficient: -0.57, standard error (SE): 0.18, p < 0.001) is congruent with the previous study by Esen et al. [20], these contradict the positive associations reported in four of the included studies [16, 17, 22, 23]. Furthermore, the meta-regression showed a significant direct association between the differences in triglyceride and MPV levels (Coefficient: 4.99, standard error (SE): 1.14, p < 0.001).
Table 3.
Summary of findings
| Mean platelet volume in individuals with obesity compared to non-obese individuals | ||||||
|---|---|---|---|---|---|---|
|
Patient or population: Class I and Class II Obesity Setting: Outpatient Intervention: None Comparison: Individuals with Normal Body weights | ||||||
| Outcomes | Absolute effects* (95% CI) |
Relative effect (95% CI) |
№ of participants (studies) |
Certainty of the evidence (GRADE) |
Comments | |
| Basal | SMD | |||||
| Mean Platelet Volume (MPV) Scale from: 6.5 to 12.0 |
8.5 (7.49–9.51) |
0.79 (0.42–1.16) |
Higher (0.42 higher to 1.16 higher) - |
1577 (9 observational studies) |
⨁⨁⨁◯ MODERATEa |
The MPV is increased in individuals with obesity when compared to non-obese individuals |
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
CI Confidence interval; SMD Standardised mean difference
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI)
Discussion
This meta-regression and meta-analysis is the first to estimate the MPV in individuals with obesity by pooling data reported in studies with a primary or secondary aim of evaluating the MPV in obese populations. The risk of bias in included studies was low to moderate, with only 15% of the studies deemed high-risk. In addition, the quality of the evidence was moderate for the primary outcome aimed at providing an estimate of the MPV in individuals with obesity. In comparison, the quality of evidence regarding the secondary outcome of ASCVD-risk in individuals with obesity was high. The MPV remains a cost-effective measure of platelet activation, which is under-utilised in the clinical setting. The primary reasons include methodological reasons and a lack of definitive reference intervals associated with clinical significance or outcomes. In our study, the majority of the included studies reported on EDTA collected blood (46%)[15, 20, 23, 32–34], while only 15% reported on citrated whole blood samples [17, 18] and 23% of the included studies failed to report on the anticoagulant used [19, 22, 24]. Although geographic and gender-specific differences exist for several haematological parameters, the included studies reported on similar MPV reference intervals ranging from 7.0- 11.1 fL [15, 20, 33, 34]. This may further support the comparability of the clinical significance of MPV associated findings across the included studies.
Our primary findings demonstrate the MPV is moderately higher (MD: 0.79) in individuals with obesity. Notably, substantial levels of statistical heterogeneity remained unexplained following a subgroup analysis based on the levels of potential bias and sensitivity analysis based on the age of the included participants. The secondary outcome of this study was addressed by performing a meta-regression, aimed at assessing the associations between the MPV and ASCVD-risk in individuals with obesity. The reported pooled estimates in this meta-analysis are congruent with previous findings of elevated MPV in individuals with obesity [15, 17–19, 21–23, 32, 34]. Our findings support the well-described levels of platelet activation in obesity and mechanisms that involve morphological and functional changes as a consequence of low-grade inflammation in obesity.
Upon activation, platelets undergo degranulation and change shape from quiescent disks to spread spheres with an increased diameter which correlates to the measured MPV. Larger platelets are metabolically active and densely express fibrinogen receptors, increasing their functional capability and affinity to forming aggregates [37]. Increased platelet activation and aggregation levels are strongly associated with cardiovascular complications, and the MPV is a well-establish independent risk factor for myocardial infarcts [38, 39]. Since obesity is a significant risk factor for cardiovascular disease, the routine monitoring of the MPV of individuals with obesity may offer is a cost-effective valuable marker used in the thrombotic-risk stratification of individuals at an increased risk of ASCVD. Moreover, the MPV has been associated with insulin resistance and coronary artery disease in non-obese individuals, with lifestyle modifications attenuating insulin resistance and MPV levels in these individuals [40].
Our secondary findings from the meta-regression analysis aimed at evaluating the associations between the ASCVD-risk factors and the MPV showed a strong association between MPV and the differences in the ASCVD-risk profiles of individuals with obesity compared to controls. An elevated MPV is associated with coronary artery disease in patients with the metabolic syndrome [34]. Notably, the confounders that modify the reported MPV estimates in this meta-analysis included age, BMI, platelet count, total cholesterol, and triglycerides. The pooled ASCVD-risk estimates showed that the included studies reported on individuals with hallmark features of the metabolic syndrome (MetS). This was illustrated by the basal characteristics of elevated fasting blood glucose levels, low levels of high-density lipoproteins (HDL) and elevated triglycerides. In all, our findings may be applicable in the context of individuals with obesity and MetS. In that context, an inverse relationship between BMI and MPV was previously reported in large cross-sectional studies comprising 290 individuals with obesity [20] and 3827 Korean adults [41]. However, in smaller studies, contradictory findings of a direct [16–18] and no association between BMI and MPV have been reported [24]. Moreover, in adolescents with obesity, elevated MPV levels were associated with increased HDL levels and platelet counts [15].
In our meta-regression analysis, the mean differences in age were associated with a decreased MPV (Table 2). Unlike in our meta-analysis, which included obese adults with a mean age of 44 years, in elderly patients (≥ 75 years), age is an independent predictor of larger MPV and median values above 10.85 fl were associated with increased prevalence of coronary artery disease. In our meta-analysis, the total cholesterol levels were slightly higher (SMD: 0.19) in individuals with obesity and these inversely correlated with the MPV. In comparison, the triglyceride levels were moderately increased in individuals with obesity (p = 0.004) (Table 4). In addition, elevated triglyceride levels were associated with increased MPV in individuals with obesity. In contrast, Park et al. showed that the MPV was inversely associated with fasting triglyceride levels in non-obese individuals [42].
Table 4.
Meta-regression of potential modifiers of the reported differences in MPV
| Parameter | Coefficient | SE | Z-value | p-value | 95%CI |
|---|---|---|---|---|---|
| Age | -6.51 | 1.15 | -5.68 | < 0.0001 | -8.76 to -4.26 |
| BMI | -0.57 | 0.18 | -3.12 | 0.002 | -0.93 to -0.21 |
| Platelet count | -3.83 | 1.01 | 3.81 | < 0.0001 | -5.80 to-1.86 |
| TC | -4.52 | 1.06 | -4.24 | < 0.0001 | -6.61 to -2.43 |
| TG | 4.99 | 1.14 | 4.36 | < 0.0001 | 2.75 to 7.24 |
BMI Body Mass Index; SE Standard Error; TC Total Cholesterol; TG Triglyceride
The strengths of the meta-analysis and meta-regression include the comprehensive search and levels of certainty with minimal risk of bias in the reported findings. Although the interpretation of these findings should be taken with caution, the geographic distribution of the included participants was largely restricted to European countries, with mainly studies reporting on individuals with obesity in Turkey. Hence the potential influence of geographic settings on the reported effect estimates could not be determined using a subgroup analysis based on geographic regions due to the few studies from South America and Asia included in this meta-analysis. This would result in misleading subgroup effects due to covariance because of an unequal number of studies in each subgroup. Lastly, all the studies included in the meta-analysis and meta-regression were cross-sectional studies, and these are known to overestimate the overall effect estimate. Future studies should focus on determining cut-off MPV values associated with primary cardiovascular events in individuals with obesity who may have variable responses to antiplatelet therapy. The MPV may be useful in predicting patients at risk of residual platelet reactivity due to increased levels of circulating larger and hyperactive platelets.
Conclusion
This meta-analysis and meta-regression provide comprehensive evidence on increased MPV levels associated with increased triglyceride levels and inversely associated with BMI in individuals with obesity. Overall, the findings suggest that MPV may be a valuable rapid marker for the monitoring and risk-stratification of individuals with obesity who may be at risk of developing cardiovascular disease associated with an elevated MPV and hypertriglyceridemia.
Supplementary Information
Additional file 1. Reporting checklist for meta-analysis of observational studies.
Additional file 2: Table S1. Search strategy used on the Medline database using the PubMed search engine. Table S2. Search strategy used on the Embase database using OVID interface. Table S3. Assessment of risk of bias using the Newcastle-Ottawa Scale. Table S4. Analysis of publication bias and of small-study effect in the included studies. Table S5. Sensitivity analysis of studies included in meta-analysis of studies reporting on the MPV in obese individuals
Acknowledgements
Not applicable
Authors' contributions
BBN, PVD conceptualised, designed and drafted this manuscript. All authors including VM, and TMN wrote and approved the final manuscript. BBN is the guarantor of this manuscript.
Funding
The current study is partially funded by the National Research Foundation (NRF) of South Africa (Grant Number: 107519 awarded to BB Nkambule) and the South African Medical Research Council (SAMRC) (Grant number 9894 awarded to BB Nkambule). BBN is also a University of KwaZulu-Natal (UKZN) Developing Research Innovation, Localisation and Leadership in South Africa (DRILL) fellow. DRILL is an NIH D43 grant (D43TW010131) awarded to UKZN in 2015 to support a research training and induction programme for early-career academics. PV Dludla was partially supported as a Post-Doctoral Fellow by funding from the Research Capacity Division of the South African Medical Research Council (SAMRC) through its division of Research Capacity Development under the Intra-mural Postdoctoral Fellowship Programme from funding received from the South African Treasury. The content hereof is the sole responsibility of the authors and do not necessarily present the official views of SAMRC or the funders.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests associated with this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bongani Brian Nkambule, Email: nkambuleb@ukzn.ac.za.
Vuyolwethu Mxinwa, Email: 218081787@stu.ukzn.ac.za.
Tawanda Maurice Nyambuya, Email: mnyambuya@nust.na.
Phiwayinkosi Vusi Dludla, Email: pdludla@mrc.ac.za.
References
- 1.OECD/European Observatory on Health Systems and Policies Obesity Update 2017. Diabetologe. 2017;13:331–341. doi: 10.1007/s11428-017-0241-7. [DOI] [Google Scholar]
- 2.Organisation WH, others. WHO fact sheet on overweight and obesity. Updated October 2017. 2019.
- 3.Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021;143:e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020;360:1–8. [PubMed]
- 5.Anon. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England) 2004;363:157–163. [DOI] [PubMed]
- 6.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7:284–294. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 7.Fryar CD, Kruszon-Moran D, Gu Q, Ogden CL. Mean Body Weight, Height, Waist Circumference, and Body Mass Index Among Adults: United States, 1999–2000 Through 2015–2016. Natl Health Stat Report. 2018;122:1–16. [PubMed]
- 8.Anon. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. Switzerland; 2000. [PubMed]
- 9.Riaz H, Khan MS, Siddiqi TJ, et al. Association between obesity and cardiovascular outcomes: a systematic review and meta-analysis of Mendelian randomisation studies. JAMA Netw open. 2018;1:e183788–e183788. doi: 10.1001/jamanetworkopen.2018.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood, J Am Soc Hematol. 2013;122:3415–3422. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. Circulation. 2019;139:e1162–e1177. doi: 10.1161/CIR.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 12.Hackeng CM. Monitoring antiplatelet therapy with point-of-care platelet function assays : a review of the evidence. 2008;4:33–55. [DOI] [PubMed]
- 13.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular Risk Factors and Venous Thromboembolism. 2008:93–103. [DOI] [PubMed]
- 14.Chu SG, Becker RC, Berger PB. Mean platelet volume as a predictor of cardiovascular risk : a systematic review and meta-analysis. 2010:148–156. [DOI] [PMC free article] [PubMed]
- 15.Arslan N, Makay B. Mean Platelet Volume in Obese Adolescents with Nonalcoholic Fatty Liver Disease. 2010;i:807–813. [DOI] [PubMed]
- 16.Yilmaz MA, Duran C, Basaran M. The mean platelet volume and neutrophil to lymphocyte ratio in obese and lean patients with polycystic ovary syndrome. J Endocrinol Invest. 2016;39:45–53. doi: 10.1007/s40618-015-0335-2. [DOI] [PubMed] [Google Scholar]
- 17.Coban E, Ozdogan M, Yazicioglu G, Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59:981–982. doi: 10.1111/j.1742-1241.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 18.Coban E, Yilmaz A, Sari R. The effect of weight loss on the mean platelet volume in obese patients. 2007;i:212–216. [DOI] [PubMed]
- 19.Erdal E, Inanir M. Platelet-to-lymphocyte ratio (PLR) and Plateletcrit (PCT) in young patients with morbid obesity. Rev Assoc Med Bras. 2019;65:1182–1187. doi: 10.1590/1806-9282.65.9.1182. [DOI] [PubMed] [Google Scholar]
- 20.Esen B, Atay AE, Gunoz N, et al. The relation of mean platelet volume with microalbuminuria and glomerular filtration rate in obese individuals without other metabolic risk factors: The role of platelets on renal functions. Clin Nephrol. 2015;83:322–330. doi: 10.5414/CN108534. [DOI] [PubMed] [Google Scholar]
- 21.Furman-Niedziejko A, Rostoff P, Rychlak R, et al. Relationship between abdominal obesity, platelet blood count and mean platelet volume in patients with metabolic syndrome. Folia Med Cracov. 2014;54:55–64. [PubMed] [Google Scholar]
- 22.Özkan EA, Khosroshahi HE, Serin H, et al. The evaluation of carotid intima-media thickness and mean platelet volume values and correlation with cardiac functions in obese children. Int J Clin Exp Med. 2015;8:22557–22563. [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto RVL, Rodrigues G, Simões RL, Porto LC. Analysis of Post-Sample Collection EDTA Effects on Mean Platelet Volume Values in Relation to Overweight and Obese Patient Status. Acta Haematol. 2019;142:149–153. doi: 10.1159/000499101. [DOI] [PubMed] [Google Scholar]
- 24.Furuncuoǧlu Y, Tulgar S, Dogan AN, Cakar S, Tulgar YK, Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: A retrospective study. Eur Rev Med Pharmacol Sci. 2016;20:1300–1306. [PubMed] [Google Scholar]
- 25.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 26.Ryan R, Synnot A, Prictor M HS. Cochrane Consumers and Communication Group Data extraction template for included studies. CCCG. La Trobe Univ Melbourne Publ N Approv (S Hill) Novemb 29th 2016.
- 27.Peterson J, Welch V, Losos M, Tugwell PJ. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. 2011;2(1):1–2.
- 28.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–406. [DOI] [PubMed]
- 29.Edgington ES. An additive method for combining probability values from independent experiments. J Psychol. 1972;80:351–363. doi: 10.1080/00223980.1972.9924813. [DOI] [Google Scholar]
- 30.Higgins JPT, Thomas J, Chandler J, et al.Cochrane handbook for systematic reviews of interventions. 2nd Edition. Chichester (UK): Wiley; 2019.
- 31.McGuinness LA. robvis: An R package and web application for visualising risk-of-bias assessments. 2019. [DOI] [PubMed]
- 32.Riyahi N, Tohit ERM, Thambiah SC, Ibrahim Z. Platelet-related cytokines among normal body mass index, overweight, and obese Malaysians. Asia Pac J Clin Nutr. 2018;27:182–188. doi: 10.6133/apjcn.032017.01. [DOI] [PubMed] [Google Scholar]
- 33.Hou J, Liu C, Yao P, et al. Association of adiposity indices with platelet distribution width and mean platelet volume in Chinese adults. PLoS ONE. 2015;10:1–13. doi: 10.1371/journal.pone.0129677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tavil Y, Sen N, Yazici HU, Hizal F, Abaci A, Cengel A. Mean platelet volume in patients with metabolic syndrome and its relationship with coronary artery disease. Thromb Res. 2007;120:245–250. doi: 10.1016/j.thromres.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Eisele G, Schwedhelm E, Schieffer B, Tsikas D, Böger RH. Acetylsalicylic acid inhibits monocyte adhesion to endothelial cells by an antioxidative mechanism. J Cardiovasc Pharmacol. 2004;43:514–521. doi: 10.1097/00005344-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Kutlucan A, Bulur S, Kr S, et al. The relationship between mean platelet volume with metabolic syndrome in obese individuals. Blood Coagul Fibrinolysis. 2012;23:388–390. doi: 10.1097/MBC.0b013e328352e8fa. [DOI] [PubMed] [Google Scholar]
- 37.Schoene NW. Design criteria: tests used to assess platelet function. Am J Clin Nutr. 1997;65:1665S–1668S. doi: 10.1093/ajcn/65.5.1665S. [DOI] [PubMed] [Google Scholar]
- 38.Jagroop IA, Mikhailidis DP. Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol. 2003;120:169–170. doi: 10.1046/j.1365-2141.2003.03983_4.x. [DOI] [PubMed] [Google Scholar]
- 39.Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb. 2003;9:177–190. doi: 10.1177/107602960300900301. [DOI] [PubMed] [Google Scholar]
- 40.Varol E, Akcay S, Ozaydin M, Erdogan D, Dogan A, Altinbas A. Mean platelet volume is associated with insulin resistance in non-obese, nondiabetic patients with coronary artery disease. J Cardiol. 2010;56:154–158. doi: 10.1016/j.jjcc.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Park B-J, Shim J-Y, Lee H-R, Jung D-H, Lee J-H, Lee Y-J. The relationship of platelet count, mean platelet volume with metabolic syndrome according to the criteria of the American Association of Clinical Endocrinologists: a focus on gender differences. Platelets. 2012;23:45–50. doi: 10.3109/09537104.2011.589014. [DOI] [PubMed] [Google Scholar]
- 42.Volkov P, Bacos K, Ofori JK, et al. Whole-Genome Bisulfite Sequencing of Human Pancreatic Islets Reveals Novel Differentially Methylated Regions in Type 2 Diabetes Pathogenesis. Diabetes. 2017;66:1074–1085. doi: 10.2337/db16-0996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Reporting checklist for meta-analysis of observational studies.
Additional file 2: Table S1. Search strategy used on the Medline database using the PubMed search engine. Table S2. Search strategy used on the Embase database using OVID interface. Table S3. Assessment of risk of bias using the Newcastle-Ottawa Scale. Table S4. Analysis of publication bias and of small-study effect in the included studies. Table S5. Sensitivity analysis of studies included in meta-analysis of studies reporting on the MPV in obese individuals
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary files.