Abstract
Background
Talaromyces marneffei is an opportunistic pathogen that infects immunodeficient and immunocompromised patients. We presented a pediatric patient with a diagnosis of T. marneffei infection who was followed up in the Guangzhou Women and Children’s Medical Centre.
Case presentation
The child was a 5-year-old girl with persistent cough and gasping over 2 months who was confirmed with T. marneffei infection by bronchoalveolar lavage fluid culture and high-throughput sequencing technology. Human immunodeficiency virus (HIV) was negative according to a serum-specific antibody test. She was treated with amphotericin B and itraconazole as antifungal agents, with good clinical response. At follow-up, high-resolution computed tomography showed a mosaic sign in the whole lung field with a diagnosis of post-infectious bronchiolitis obliterans (PIBO) as the sequela. She has a mutated COPA gene with uncertain pathogenic potential on whole-exome sequencing.
Conclusions
Clinicians should consider PIBO as a possible sequela in an HIV-negative paediatric patient with T. marneffei infection.
Keywords: Talaromyces marneffei, Child, Sequelae, Bronchiolitis obliterans
Background
Talaromyces marneffei (formerly known as Penicillium marneffei) is an important dimorphic fungus. It is the only member in the genus that causes systemic mycosis and is more prevalent in South Asia [1]. In adults, T. marneffei infection has been considered to be exclusively associated with acquired immunodeficiency syndrome (AIDS) caused by human immunodeficiency virus (HIV) infection [2], although nowadays the infection rate in non-HIV-infected children has gradually grown [3], paediatric patients with primary immunodeficiency diseases (PIDs) being more susceptible according to previous reports [4, 5]. Here we report a rare case of post-infectious bronchiolitis obliterans (PIBO) as sequela after T. marneffei infection with a mutation in the COPA gene.
Case presentation
In January 2019, a 5-year-old girl was hospitalized with intermittent fever, cough and shortness of breath for two months and she had recurrent lower respiratory tract infection from infancy. There was no family history of PIDs and consanguineous marriage. On admission, she had difficulty breathing. Stridor and moist rales were revealed by auscultation. Rash, lymphadenopathy, and hepatosplenomegaly were all absent. HIV was negative according to a serum-specific antibody test and HIV viral load. Humoral immunoassay showed decreased serum immunoglobulin G (IgG), IgA and IgM, but the serum IgE level was normal. Lymphocyte counts were all in their normal range on admission, including CD4+ subsets, CD8+ subsets, natural killer (NK) cells and CD19+ subsets. The nitroblue tetrazolium test (NBT) was normal (Table 1).
Table 1.
Laboratory findings of the patient on the day of admission
| Laboratory index | Result | Reference range |
|---|---|---|
| WBC (×109/L) | 18.6 | 5–12 |
| N (×109/L) | 13.02 | 2–7.2 |
| Hb (g/dL) | 120.00 | 105–145 |
| PLT (×109/L) | 466.00 | 140–440 |
| CRP (mg/L) | 11.21 | < 8.2 |
| IgG (g/L) | 4.56 | 5.0-10.6 |
| IgA (g/L) | 0.11 | 0.34–1.38 |
| IgM (g/L) | 0.16 | 0.44–1.44 |
| IgE (IU/mL) | 5.00 | 0–60.0 |
| CD3 + 4+ (cells/µL) | 1223.43 | 345–2350 |
| CD3 + 8+ (cells/µL) | 382.35 | 314–2080 |
| CD19+ (cells/µL) | 309.21 | 240–1317 |
| Th/Ts (%) | 2.02 | 0.47–2.05 |
| NK (cells/µL) | 285.62 | 210–1514 |
WBC white blood count, N neutrophils, Hb hemoglobins, PLT platelet count, CRP C-reactive protein, Th helper T cells, Ts inhibited T cells, NK natural killer
High-resolution computed tomography (HRCT) showed small airway obstruction lesions, and bilateral diffuse infiltration and local bronchiectasis in both lungs (Fig. 1A–C). Electronic bronchoscopy showed heavy yellow-white purulent secretion in the airway, and bronchoalveolar lavage fluid (BALF) for culture yielded T. marneffei (Fig. 2). By the same token, high-throughput sequencing detected T. marneffei in BALF. In accordance with pathogenic status, amphotericin B deoxycholate at 20 mg/day was commenced as the primary antifungal therapy for 14 days with good clinical response, and the patient was discharged with oral itraconazole prescribed for 4 weeks.
Fig. 1.
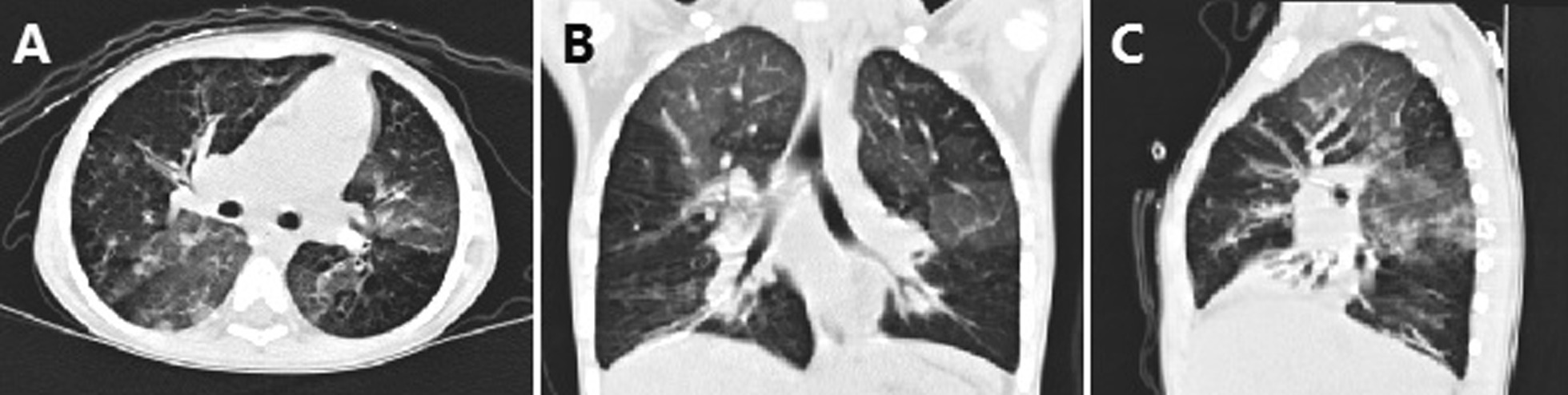
High-resolution computed tomography (CT) scan of the chest revealing small airway obstruction lesions with double pneumonia, insufficiency of the right middle and left lower lung segments, and local bronchiectasis in both lungs on first hospitalization
Fig. 2.
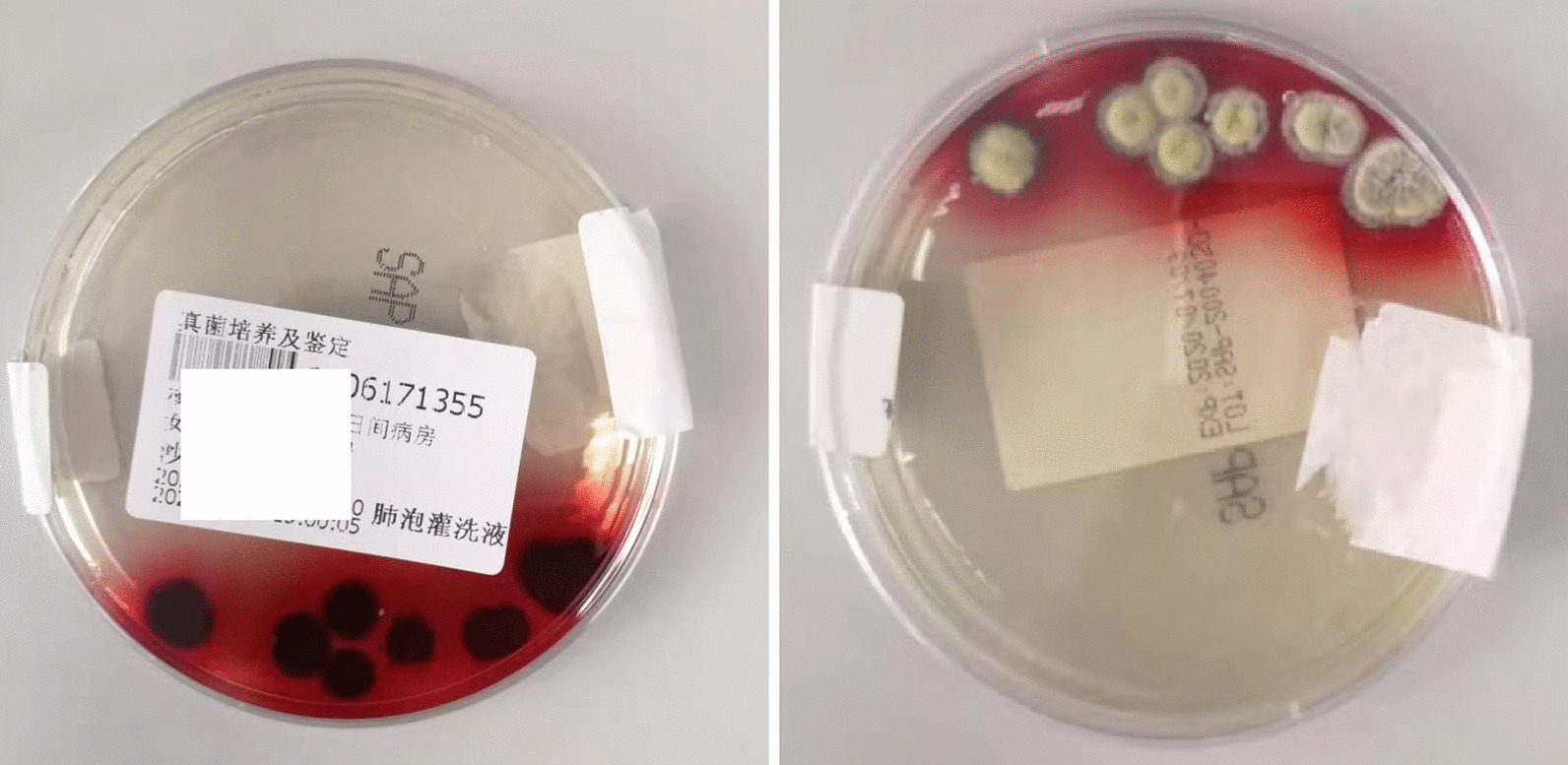
Granular colony of T. marneffei with a characteristic soluble red pigment that diffused into the agar after 7 days of incubation at 25 °C in BALF
One month after discharge, she presented to the emergency department with shortness of breath and oedema of eyelids and limbs. HRCT showed multiple patchy ground-glass opacities that manifested as mosaic attenuation (Fig. 3A–C). Culture of T. marneffei was negative in BALF and blood during this hospitalization. A restrictive abnormality with reduction of diffusion capacity was mainly found in pulmonary function. She was treated with intravenous Ig (400 mg/kg/day) for 3 days as well as aerosol inhalation of budesonide. After 10 days of treatment, the dyspnoea was relieved and she was discharged with recommended continued use of a Symbicort Turbuhaler.
Fig. 3.
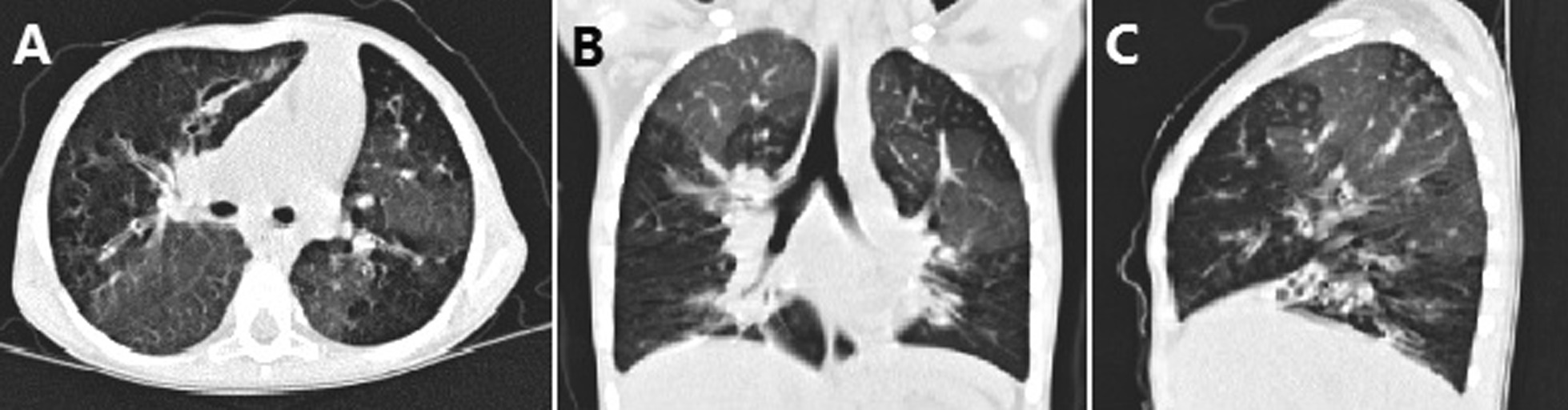
High-resolution CT scan of the chest revealing both lungs scattered widely with ground-glass-like shadows, while focal areas of increased transmittance show “mosaic” changes. Bronchiectasis in lower lobe of both lungs and middle lobe of right lung on second hospitalization
Follow-up and gene report
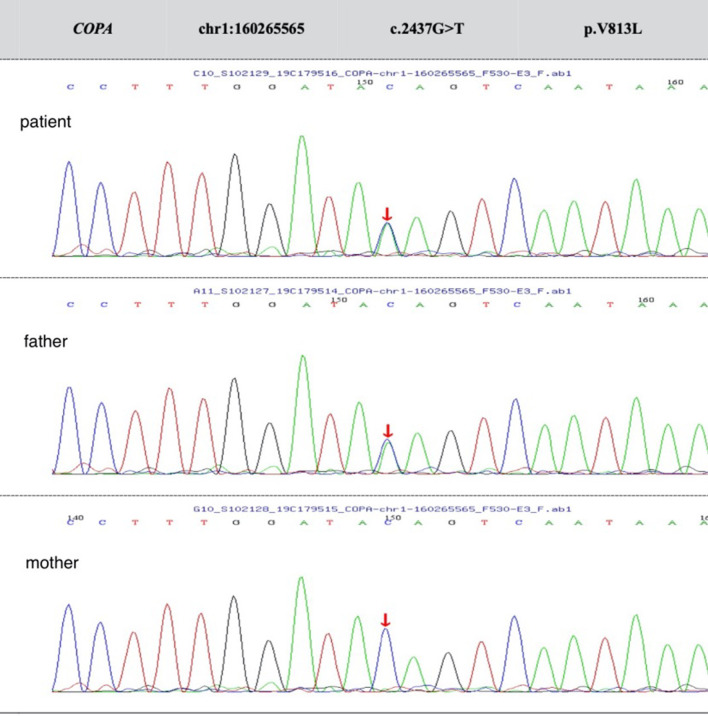
Cough and yellow phlegm were reduced, but intermittent wheezing symptoms still persisted after the patient left hospital. In addition, whole-exome sequencing identified a de novo missense mutation c.2437G > T(p.V813L) in the COPA gene (Fig. 4), but the mutation was predicted to be uncertain based on the American Center for Medical Genetics and Genomics guidelines. During the follow-up, the child still wheezed intermittently and did not show any positive symptoms of kidney or autoimmune inflammatory arthritis problems.
Fig. 4.
Sequencing analysis of COPA in the patient and her father revealed the heterozygous substitution [c.2437G > T, p.V813L ] in chr1:160265565 as a de novo mutation (arrow), and her mother was negative for this mutation. The mutation of nucleotide 2437 from guanine to thymine resulted in the missense mutation of amino acid 813 from valine to leucine
Discussion and conclusion
T. marneffei is the only temperature-biphasic pathogenic fungus in Penicillium, and is endemic in Southeast Asia [6]. In adults, most T. marneffei infections occur in AIDS patients, especially HIV infected, while varying among children. We present the case of a child with sequelae of PIBO arising from T. marneffei infection without HIV and accompanied by mutations in the COPA gene.
In many aspects, the clinical manifestations of paediatric patients with T. marneffei infection are not typical, which is a potential reason for misdiagnosis of T. marneffei infection [7]. Our patient presented with fever, cough, and dyspnoea but there was no manifestation of disseminated T. marneffei infection, including rash, weight loss, lymphadenopathy, and hepatosplenomegaly in this patient as in the previous reports [8, 9]. Although the clinical history spanned 2 months, the diagnosis of T. marneffei infection was not confirmed until she was hospitalized in our centre. Positive culture and high-throughput sequencing of BALF were the most important criteria in the final diagnosis of T. marneffei infection in this child, suggesting that BALF can be used for the early diagnosis of such an infection.
PIDs that commonly manifest some degree of hypogammaglobulinemia include selective IgA deficiency, common variable immunodeficiency, and congenital agammaglobulinaemias. Less common causes include agammaglobulinaemia with thymoma (Good syndrome) and X-linked lymphoproliferative syndrome [10]. In addition, concomitant opportunistic infections in this child should raise suspicion of a cellular defect that also affects antibody production, such as nuclear factor κB essential modulator (NEMO; also called IKK-γ) or CD40 ligand (CD154) deficiencies [10, 11]. Because the exact kind of PIDs may be difficult to determine based on the peripheral immunological results alone, genetic testing was carried out. The patient was identified with a de novo missense mutation at exon 17 (c.2437G > T, p.V813L) in the COPA gene. Patients with COPA mutations typically have normal numbers and percentages of lymphocytes and lymphocyte subsets along with unremarkable Ig levels and intact production of specific antibodies [4, 12]. However, the child had markedly decreased Ig with normal numbers of lymphocytes. The exact mechanism by which COPA gene mutation causes T. marneffei infection is currently unknown.
Pulmonary fungal infections complicated by PIBO sequelae are very rare. Recent research suggests that pulmonary colonization with Aspergillus species has been implicated as a potential risk factor in the development of PIBO [13]. However, T. marneffei infection with secondary PIBO had not been previously reported. According to her repeated dyspnoea and wheezing over a period of longer than 2 months and mosaic signs on HRCT, despite lung biopsy being essential for the diagnosis of PIBO this procedure was not performed in this patient because of her tender age, although PIBO was also considered in the differential diagnosis. Interestingly, lung involvement is usually in the form of interstitial lung disease in patients with COPA gene mutation [14] and, as such, the mechanism of PIBO might be a combination of T. marneffei infection and COPA gene mutation.
In conclusion, it must be stressed that while T. marneffei infection with PIBO is very rare, this patient also showed a de novo missense mutation in the COPA gene. Evidence from this report suggests that all clinicians must consider PIBO as a possible sequela in an HIV-negative paediatric patient with T. marneffei infection. Moreover, the role of COPA in T. marneffei infection is worthy of further study.
Acknowledgements
We are very appreciative to the child and her families.
Abbreviations
- AIDS
Immunodeficiency syndrome
- ACMG
American Center for Medical Genetics and Genomics
- BALF
Bronchoalveolar lavage fluid
- HIV
Human immunodeficiency virus
- HRCT
High-resolution computed tomography
- NBT
Nitroblue tetrazolium test
- NK
Natural killer
- PIBO
Post-infectious bronchiolitis obliterans
- PID
Primary immunodeficiency disease
Authors’ contributions
L.L. and H.F.F analyzed data and wrote the paper. L.L. and D.W.Z. collected patients’ clinical data and modified the paper. G.L communicated with the parents and supervised the whole writing process. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All data and materials of this article are included in the manuscript and are thus available to the reader.
Declarations
Ethics approval and consent to participate
This study protocol was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Guangzhou Women and Children’s Medical Centre of Guangzhou Medical University. Written informed consents were signed during hospitalization. The data used in this study were anonymised before its use.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lin Lin, Email: lyhn_lin@163.com.
Huifeng Fan, Email: sonny-000@163.com.
Dongwei Zhang, Email: zhangdongwei625@163.com.
Gen Lu, Email: lugen5663330@sina.com.
References
- 1.Ying RS, Le T, Cai WP, et al. Clinical epidemiology and outcome of HIV-associated talaromycosis in Guangdong, China, during 2011–2017. HIV Med. 2020;21(11):729–38. doi: 10.1111/hiv.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanittanakom N, Cooper CR, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi XH, Xue YM, Wang QS, et al. Penicillium marneffei Diagnosis and treatment of diffusible in human immunodeficiency virus-negative patients: a challenge for the physician. Indian J Med Microbiol. 2017;35(4):617–620. doi: 10.4103/ijmm.IJMM_15_418. [DOI] [PubMed] [Google Scholar]
- 4.Qiang Zeng Y, Jin G, Yin, et al. Peripheral immune profile of children with Talaromyces marneffei infections: a retrospective analysis of 21 cases. BMC Infect Dis. 2021;21:287–94. doi: 10.1186/s12879-021-05978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan M, Qiu Y, Zeng W, et al. Disseminated Talaromyces marneffei infection presenting as multiple intestinal perforations and diffuse hepatic granulomatous inflammation in an infant with STAT3 mutation: a case report. BMC Infect Dis. 2020;20(1):394–400. doi: 10.1186/s12879-020-05113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo LN, Yu SY, Wang Y, et al. Species distribution and antifungal susceptibilities of clinical isolates of Penicillium and Talaromyces species in China. Int J Antimicrob Agents. 2021 doi: 10.1016/j.ijantimicag.2021.106349. [DOI] [PubMed] [Google Scholar]
- 7.Qiu Y, Lu DC, Zhang J, et al. Treatment of disseminated talaromyces marneffei with tracheal infection: two case reports. Mycopathologia. 2015;180(3–4):245–9. doi: 10.1007/s11046-015-9891-4. [DOI] [PubMed] [Google Scholar]
- 8.Guo J, Li BK, Li TM, et al. Characteristics and prognosis of talaromyces marneffei infection in non-HIV- infected children in southern China. Mycopathologia. 2019;184(6):735–45. doi: 10.1007/s11046-019-00373-4. [DOI] [PubMed] [Google Scholar]
- 9.Ding X, Huang H, Zhong L, et al. Talaromyces marneffei Disseminated Infection in a Non-HIV Infant With a Homozygous Private Variant of. Front Cell Infect Microbiol. 2021 doi: 10.3389/fcimb.2021.605589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira JB, Fleisher TA. Laboratory evaluation of primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2 Suppl 2):297–305. doi: 10.1016/j.jaci.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson EP, Monaco-Shawver L, Solt LA, Madge LA, Banerjee PP, May MJ, Orange JS. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J Allergy Clin Immunol. 2008;122(6):1169–117716. doi: 10.1016/j.jaci.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefano Volpi J, Tsui, Mariani M, et al. Type I interferon pathway activation in Copa syndrome. Clin Immunol 2018. doi:10.1016/j.clim.2017.10.001. [DOI] [PubMed]
- 13.Weigt SS, Copeland CAF, Derhovanessian A, et al. Colonization with small conidia Aspergillus species is associated with bronchiolitis obliterans syndrome: a two-center validation study. Am J Transplant. 2013;13:919–27. doi: 10.1111/ajt.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vece TJ, Watkin LB, Nicholas S, et al. Copa syndrome: a novel autosomal dominant immune dysregulatory disease. J Clin Immunol. 2016;36:377–87. doi: 10.1007/s10875-016-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials of this article are included in the manuscript and are thus available to the reader.