Abstract
Rickettsia rickettsii, the causative agent of Rocky Mountain spotted fever, was lethal for the majority of experimentally and transovarially infected Rocky Mountain wood ticks (Dermacentor andersoni). Overall, 94.1% of nymphs infected as larvae by feeding on rickettsemic guinea pigs died during the molt into adults and 88.3% of adult female ticks infected as nymphs died prior to feeding. In contrast, only 2.8% of uninfected larvae failed to develop into adults over two generations. Infected female ticks incubated at 4°C had a lower mortality (80.9%) than did those held at 21°C (96.8%). Rickettsiae were vertically transmitted to 39.0% of offspring, and significantly fewer larvae developed from infected ticks. The lethal effect of R. rickettsii may explain the low prevalence of infected ticks in nature and affect its enzootic maintenance.
Through a series of pioneering investigations conducted in the Bitterroot Valley of western Montana, H. T. Ricketts elucidated the transmission cycle of Rocky Mountain spotted fever (RMSF) (47–50). This disease is an acutely febrile and potentially deadly illness caused by infection with Rickettsia rickettsii (Proteobacteria, alpha subdivision, family Rickettsiaceae), which is transmitted throughout the New World primarily by the bite of an infected tick (35, 50, 55, 56). RMSF is the most common fatal human tick-borne disease in the United States, with a minimal average of 351 confirmed human cases (minimum case/fatality ratio = 4.0%) occurring annually and undoubtedly many more going unreported (11). The Rocky Mountain wood tick (Dermacentor andersoni) is the principal vector in the western United States, while the American dog tick (D. variabilis) transmits the bacterium in the midwestern and eastern United States (7, 9, 30).
Multiple factors may influence the maintenance of R. rickettsii in nature (16, 29). Small mammals such as chipmunks (Tamias spp.), voles (Microtus spp.), ground squirrels (Spermophilus spp.), and rabbits (Sylvilagus spp.) are common blood meal sources for immature wood ticks, are highly susceptible to rickettsial infection, and are apparent R. rickettsii-amplifying hosts (3, 4, 9, 56). Rickettsial infection rates in ticks may be further enhanced by transmission between cofeeding ticks (38, 43) and by transovarial transmission into 30 to 100% of offspring (5, 6, 42, 43). Taken together, one would anticipate that R. rickettsii would attain high field infection rates in wood ticks. However, the bacterium seems relegated to a narrow ecological niche. For example, over the past century in the Bitterroot Valley, virulent R. rickettsii has been limited to the western slopes, where it infects fewer than 1% of wood ticks despite a widespread abundance of arthropod and vertebrate hosts (18, 35, 38, 39, 50).
To better understand the low prevalence of infected ticks, we investigated the maintenance and cycling of R. rickettsii. In this report, we evaluate the deleterious impact of this bacterium on wood tick fitness and speculate on the relevance of our findings to the RMSF enzootic cycle.
MATERIALS AND METHODS
Ticks and rickettsial strains.
Adult ticks (D. andersoni) were collected by flagging in Ravalli County, Mont., from Como Lake (CL) and Blodgett Canyon (BL) on the western slopes of the Bitterroot Valley and from Skalkaho Mountain (SK) on the eastern slopes during each May from 1994 to 1997. A group of second-filial-generation (F-2) SK uninfected ticks originating from a single wild-type (F-0) female and six groups of F-1 BL uninfected ticks originating from six F-0 females were established in the laboratory (25, 27). The strains, origin, and passage history of the rickettsiae used to experimentally infect these tick groups are listed in Table 1. These rickettsial strains include Como-96, an isolate purified from F-0 CL ticks during this study. In addition, several F-O CL ticks naturally infected with R. bellii and R. rhipicephali and F-0 SK ticks infected with R. peacockii were fed for fecundity studies.
TABLE 1.
Summary of rickettsial strains used to infect D. andersoni ticks during feeding
| Rickettsia | Strain | Source | Location and date collecteda | Passage historyb |
|---|---|---|---|---|
| R. rickettsii | Como-96 | D. andersoni | Como Lake, 1996 | 2VCc/1M/1GP |
| R. rickettsii | Wachsmuth | D. andersonid | Tin Cup, 1974 | 1GP/6CE/2VC |
| R. rickettsii | R | D. andersoni | Blodgett, 1945 | 4GP/53CE/1PCEc/5CE/6VC |
| R. rickettsii | Sawtooth | D. andersoni | Sawtooth, 1961 | 8T/1GP/17CE/8VCc |
| R. rickettsii | Swan | Human (fatal) | Bass Creek, 1975 | 10CE |
| R. montana | M/5-6 | Microtus | Broadus, Mont. 1963 | 7CE/4VC |
All R. rickettsii strains originated from the western slopes of the Bitterroot Valley, Mont.
Abbreviations: VC, Vero cells; M, Microtus; GP, guinea pig; CE, chicken yolk sac; PCE, primary chicken embryonic cells; T, wood tick generations.
Plaque purified (59).
Partially engorged adult female tick collected from a human with RMSF; other tick sources collected by flagging.
Approximately half of each tick group was experimentally infected with rickettsial strains, while the remainder were maintained as uninfected controls. Both the infected and uninfected tick groups were divided into two cohorts, which were then incubated without a photoperiod at either 21°C or 27°C between blood meals. The number in each cohort which successfully molted and fed was recorded, as was the number of progeny reared from a portion of infected and uninfected adults. Replete larvae and nymphs which failed to molt within 5 months were considered dead. To compare the mortality and fecundity of infected ticks with those of uninfected siblings, statistical analyses were performed on a portion of the results by using chi-square tests of the observed mean relative to the expected mean with 1 degree of freedom. Salivary glands and ovarial tissues were dissected from adult females postoviposition and stored at −5°C for further testing (32).
To evaluate the impact of low temperatures upon tick health, approximately half of two tick groups (F-2 SK) were infected with R. rickettsii (Como-96) during nymphal feeding. For those which molted, infected adults and their uninfected siblings were both subdivided into two cohorts, which were then incubated at either 4 or 21°C for 8 months. Those surviving the incubation period were allowed to feed for 6 days and then detached. The number of viable, infected ticks which attached was compared with that of uninfected siblings by a chi-square test. Salivary secretions from partially fed ticks were collected with a capillary tube but without application of pilocarpine and stored at −5°C for further testing.
Vertebrate hosts.
A maximum of 500 larvae, 250 nymphs, or 17 adults were fed per guinea pig (12-week-old male Hartley guinea pigs were used). Each tick group was fed on a separate host. The ticks were experimentally infected with rickettsial strains by feeding upon rickettsemic hosts, namely, guinea pigs inoculated intraperitoneally on day 0 with 0.25 ml of a suspension of approximately 105 rickettsiae per ml of brain heart infusion (BHI) broth (40, 41, 43). Larval, nymphal, and adult ticks were attached on days 4, 1, and 0, respectively. Concurrently, uninfected tick controls were fed upon uninfected, normal guinea pigs. Infected adult ticks were fed upon guinea pigs immune to RMSF, enabling them to feed to repletion without the hosts succumbing to illness. Immunity was conferred through two intraperitoneal inoculations of 0.5 ml of heat-killed R. rickettsii suspension (106 rickettsiae/ml of BHI broth) administered 2 weeks apart. Every guinea pig inoculated or fed upon by ticks was monitored daily for RMSF by recording the rectal temperature and signs of clinical illness, namely, scrotal reaction and darkening of extremities. Upon euthanasia, testicular tissue, spleen, and blood were collected from each guinea pig and stored at −5°C for further testing.
PCR assay.
All ticks, their progeny, and their tissues were screened for rickettsiae by a PCR assay. The ticks were surface sterilized by being washed in hydrogen peroxide and then ethanol (32), and all samples were triturated in 0.5 ml of BHI broth with a mortar and pestle. The samples tested included (i) a portion of fed larvae and nymphs from each tick group, (ii) salivary glands and ovarial tissues dissected from adults following oviposition, (iii) a portion of larvae reared from each ovipositing adult, (iv) a portion of nymphs and adults which died, and (v) tick salivary secretions. Similarly, guinea pig tissues and sera were tested. Vero cell cultures of R. rickettsii, uninfected tick tissues, and uninfected Vero cells were also tested as controls.
For the PCR assay, 0.5 ml of each tick triturate, guinea pig tissue, or whole blood was processed with a DNA extraction kit (Stratagene, La Jolla, Calif.) as specified by the manufacturer for whole tissues or blood but with a reduction from the recommended reagent volumes by 95%. Precipitated DNA was pelleted, washed, dried, and resuspended in 50 μl of 10 mM Tris (pH 8.0). Two separate primer sets which detect members of the genus Rickettsia (RpCS.877/1258R) and spotted fever group rickettsiae (Rr190.70/602R) were used (18, 45). Samples generating PCR amplification products with both primer sets were considered infected. To confirm the identity of the rickettsial strain in each group of infected ticks, restriction fragment length polymorphism (RFLP) analysis (18, 32) was performed on PCR products amplified from a tick immediately after a rickettsemic meal, a dead tick, a salivary gland, ovarial tissue, and a larval offspring. For each rickettsial strain, RFLP analysis was performed on PCR products amplified from the testicular tissues and blood of an inoculated guinea pig and one fed on by infected larvae or nymphs.
IFA tests.
To confirm infections, indirect fluorescent-antibody (IFA) tests were selectively performed on tick specimens, including (i) fed larvae and nymphs, (ii) salivary glands and ovarial tissues dissected from adults following oviposition, and (iii) unfed larvae reared from each ovipositing adult. IFA tests were also performed on tick salivary secretions, guinea pig tissues, and the controls listed above. For IFA tests, 10 μl of each triturate sample was fixed to a slide with acetone and stained with anti-R. rickettsii guinea pig serum and then with fluorescein isothiocyanate-labeled rabbit anti-guinea pig serum (32, 34, 37). Rickettsial loads were measured for the following samples: (i) rickettsial seeds used to inoculate guinea pigs, (ii) a portion of replete ticks immediately and 4 weeks following a rickettsemic meal, (iii) a portion of infected ticks immediately following the nymphal molt, and (iv) tick ovarial tissues following oviposition. For each of the infected tick groups tested, their corresponding uninfected siblings were included as controls. As described above, all ticks were surface sterilized and triturated. Rickettsial loads were quantified by both plaque titer determination and direct counting modified for staining triturate filtrates by the IFA test (34, 53, 59).
RESULTS
Wood ticks originating from the western (BL) and eastern (SK) slopes of the Bitterroot Valley were experimentally infected with six separate rickettsial strains (Tables 1 and 2). For comparison, uninfected sibling, uninfected F-0, and naturally infected F-0 ticks were also studied. For each group of experimental ticks, rickettsial infections were monitored by a PCR assay immediately prior to feeding and after molting, feeding, and oviposition. The infection status was confirmed by IFA tests for all but 17 samples, namely, fed larvae and nymphs. PCR-RFLP analysis confirmed the presence of R. rickettsii in the 17 samples negative by the IFA test. Furthermore, PCR-RFLP analysis confirmed the presence of R. rickettsii in experimentally, naturally, and vertically infected ticks. Direct counts and plaque titer determinations detected fewer than 103 rickettsiae per larva or nymph (20 larvae or nymphs tested) immediately after they fed on rickettsemic guinea pigs (infected with the Como-96 or Wachsmuth strain). Both eastern (SK) and western (BL) slope tick groups were susceptible to and transmitted R. rickettsii (Table 2).
TABLE 2.
Experimentally infected D. andersoni tick groups used to study the effect of R. rickettsii on tick survival and fecundity
| Rickettsial strain | Tick group acquiring infection | No. of ticksb | No. of ticks infected/total no.a
|
|||
|---|---|---|---|---|---|---|
| Immature ticks
|
Adult females
|
|||||
| Fed | Postmolt | Salivary glands | Ovaries | |||
| Como-96 | F-1 BLe larvae | 2,000 (4) | 10/10 | 5/5 | 4/4 | 4/4 |
| Como-96 | F-2 SKe larvae | 2,000 (4) | 10/10 | 5/5 | 2/2 | 2/2 |
| Como-96 | F-2 SK nymphs | 750 (3) | 10/10 | 5/5 | 8/8 | 8/8 |
| Como-96 | F-2 SK nymphsc | 500 (2) | 20/20 | NTe | 10/10 | NT |
| Wachsmuth | F-2 SK larvae | 1,000 (2) | 10/10 | NT | Nonee | None |
| R | F-1 BL nymphs | 500 (2) | 10/10 | 0/10 | 0/12 | 0/12 |
| Sawtooth | F-1 BL larvae | 500 (1) | 10/10 | 0/10 | 0/9 | 0/9 |
| Uninfected | NAe | 7,000 (14) | 0/300d | 0/30 | 0/30 | 0/30 |
Rickettsial infections were determined by PCR-RFLP analysis and IFA tests. Fed immature ticks were tested immediately after a rickettsemic blood meal and after the subsequent molt. Adult female tissues were tested after oviposition.
Approximate number of ticks fed with approximately 500 larvae or 250 nymphs per tick feeding group. The number of tick groups fed on separate guinea pigs is given in parentheses.
These ticks were incubated at 4 or 21°C, and after they underwent partial feeding for 6 days, their salivary secretions were tested for rickettsiae. All other ticks were incubated at 21 or 27°C.
These samples were tested in pools of 10; all others were tested individually.
BL, Blodgett Canyon; SK, Skalkaho Mountain; NT, Not tested; none, ticks all died prior to testing; NA, not applicable.
A notable difference in infectivity for guinea pigs and ticks was observed among several rickettsial strains. The strains (Como-96 and Wachsmuth) grown in chicken embryos or animal tissues were highly virulent. Nonimmune guinea pigs fed upon by ticks infected with these strains developed classic RMSF with high fever, scrotal reaction, and presence of rickettsiae in tissues and blood. The Swan strain also induced lethal RMSF in guinea pigs, while adult ticks acquiring it oviposited nonviable eggs. Rickettsial strains (R and Sawtooth) cultured in Vero cells were of low virulence and induced mild RMSF when inoculated into guinea pigs. Ticks acquiring these strains failed to maintain rickettsiae (Table 2) and remained apparently healthy. Concurrently, no rickettsiae were detected in any uninfected control ticks by the PCR assay, IFA tests, plaque titer determination, or direct counts.
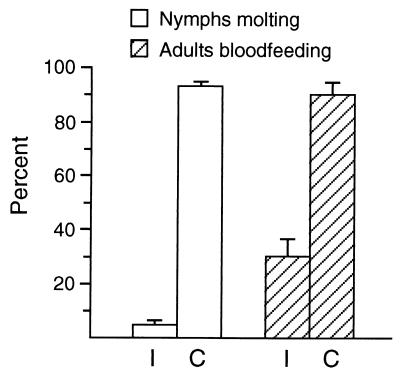
Significant mortality was induced in wood ticks experimentally infected as larvae or nymphs with highly virulent R. rickettsii (Como-96 or Wachsmuth), while the majority of uninfected siblings developed into feeding adults (Fig. 1). Nymphal molting and adult-female feeding success were significantly reduced (P < 0.001) for infected ticks compared with uninfected controls (Fig. 1). No significant difference (P > 0.45) in molting or feeding success occurred between infected ticks held at 21 and 27°C, between two tick strains (BL and SK), and with either rickettsial strain.
FIG. 1.
The nymphal molting and adult-female feeding success of D. andersoni ticks experimentally infected (I) with R. rickettsii were both significantly less (P < 0.001) than those of uninfected controls (C) as determined by chi-square tests of observed mean relative to expected mean with one degree of freedom. Error bars indicate the standard deviation.
For nymphs infected during larval feeding, 94.1% of ticks infected with Como-96 (825 of 877) and 97.7% of those infected with Wachsmuth (252 of 258) died after an uninfected blood meal but prior to molting. In contrast, only 1.4% of uninfected nymphs (14 of 1021) failed to develop into adults. Overall, 96.9% of the dead nymphs from the infected groups (31 of 32) tested positive for R. rickettsii. Each of the surviving ticks (a total of 10 were tested) contained approximately 6.5 × 106 rickettsiae 4 weeks after the nymphs fed. Nearly equal numbers of male and female ticks (27 and 30, respectively) survived the molt, and each contained approximately 1.0 × 107 rickettsiae (a total of 5 were tested). Only 30.0% of infected females (9 of 30) successfully fed, which was significantly lower (P < 0.001) than the 90.7% of uninfected control females (108 of 119) that successfully fed (Fig. 1). Of these, 66.6% of infected (6 of 9) and 81.5% of uninfected (88 of 108) fed ticks oviposited.
High mortality was also observed for ticks infected during nymphal feeding. For these ticks, 34.9% (176 of 504) died during the molt and 88.3% of adult females (189 of 214) failed to feed. For uninfected siblings, 2.1% (12 of 572) died during the molt and 19.2% of adult females (59 of 307) failed to feed. Overall, 100% of the dead ticks from infected groups (40 of 40) tested positive for R. rickettsii.
Ticks infected with Como-96 that were held at 4°C had a significantly greater (P < 0.01) survival and feeding success than those held at 21°C (Table 3), but the success rate was still significantly lower (P < 0.005) than that of uninfected controls. No significant difference (P > 0.45) in survival and feeding success occurred between uninfected ticks at the two temperatures. Only 36.2% of infected ticks (25 of 69) attached (Table 3), and, of these, all the salivary secretions tested were positive for R. rickettsii (a total of 10 ticks were tested). Of the 25 feeding ticks, 8 remained attached only for 3 to 5 days, ingested only a small volume of blood if any, and remained viable for an additional 3 months.
TABLE 3.
Summary of developmental and feeding success of D. andersoni female ticks experimentally infected with R. rickettsii (Como-96) and uninfected controls incubated at 4 or 21°C
| Parameter | Infected ticks
|
Control ticks
|
||
|---|---|---|---|---|
| 4°C | 21°C | 4°C | 21°C | |
| Survivala | 54.2% (64/120) | 32.8% (44/134) | 75.7% (106/140) | 81.0% (111/137) |
| Feedinga | 46.7% (21/45) | 16.7% (4/24) | 80.0% (16/20) | 76.2% (16/21) |
Chi-square tests of observed mean relative to expected mean with one degree of freedom determined that the survival success and feeding success of infected ticks incubated at 4°C were significantly greater (P < 0.01) than those held at 21°C but still significantly lower (P < 0.005) than those of uninfected controls. Results are given as percentages (number showing successful survival or feeding/total number).
Adult ticks experimentally infected as larvae or nymphs with highly virulent R. rickettsii vertically transmitted rickettsiae to 39.0% of offspring (23 of 59 adults) (Table 4). Although only a portion of the progeny inherited rickettsiae, 100% of the 20 adults tested were infected after feeding as nymphs, an observation consistent with transmission between cofeeding ticks (42, 43). Subsequently, 97.5% of infected progeny (235 of 241) died prior to adulthood. Ticks experimentally infected as adults with R. rickettsii and R. montana had ovarial tissues with as many as 2.5 × 107 rickettsiae following oviposition, but they failed to transmit infection vertically to offspring (Table 4), a result consistent with some (38, 43) but not all (6) past studies.
TABLE 4.
Transovarial transmission and fecundity of D. andersoni ticks infected with rickettsiae and uninfected controls
| Rickettsia | Strain | Stage acquiring infection | Proportion of infected offspringb | Mean no. of offspring per ticka (no. of clutches counted) |
|---|---|---|---|---|
| R. rickettsii | Como-96 | Larvae | 14/35 | 45 (5) |
| R. rickettsii | Como-96 | Nymphs | 9/24 | 24 (2) |
| R. rickettsii | Como-96 | Adults | 0/400 | 2,438 (5) |
| R. rickettsii | Wachsmuth | Adults | 0/100 | NAc |
| R. montana | M/5-6 | Adults | 0/100 | 2,166 (3) |
| R. peacockii | Skalkaho | NA | 23/30 | 2,703 (5) |
| Uninfected | NA | NA | 0/500 | 2,811 (5) |
Chi-square tests of observed mean relative to expected mean with one degree of freedom determined that the number of offspring from ticks infected with R. rickettsii as larvae or nymphs was significantly smaller (P < 0.001) than that of uninfected ticks or those infected with R. peacockii.
Progeny were tested as larvae. Those reared from ticks infected while immature were screened individually; all others were tested in pools of 10 or 25. Results are given as number infected/total number.
NA, Not applicable.
The number of progeny developing from ticks (F-1 BL and F-2 SK) infected with the highly virulent Como-96 and Wachsmuth strains as larvae or nymphs but not as adults was significantly smaller (P < 0.001) than that developing from uninfected siblings or those naturally infected with symbiotic R. peacockii (Table 4). Further, four separate ticks (F-0 CL) naturally infected with R. bellii, R. montana, or R. rhipicephali exhibited reduced fecundity, with an average of 134 offspring (range, 91 to 185), compared to 2,919 offspring (range, 2,456 to 3,190) per uninfected F-0 tick. In rearing of ticks, either all or no eggs hatched and ovarial tissues dissected from ticks after oviposition retained fewer than 15 unlaid eggs.
DISCUSSION
In this study, we quantified the pernicious effect of R. rickettsii for its tick vector (D. andersoni). Beyond high mortality, far fewer offspring developed from infected ticks than from uninfected siblings. In a previous study on vertical transmission of R. rickettsii by wood ticks, Burgdorfer and Brinton (6) noted reduced survival and fecundity in an undisclosed portion of female ticks after several successive generations. However, the true extent of this adverse impact remained unquantified until now. The lethal effect may limit the natural incidence of R. rickettsii and offer a plausible explanation for the relatively low field infection rates in adult wood ticks. Coupled with 39.0% vertical transmission by ticks infected as larvae or nymphs in this and previous studies (42, 43), our findings imply that horizontal spread of infection must exist for enzootic maintenance. Any fluctuation in the lethal effect may cause RMSF to emerge or disappear in areas where suitable vectors and vertebrate hosts exist. Ecological factors which may influence the lethal effect of R. rickettsii on the tick vector, D. variabilis, in the southeastern United States, a locale with the highest reported incidence of human RMSF cases (11) deserve investigation.
For the natural RMSF transmission cycle to succeed, wood ticks must function as both vectors and overwintering reservoirs. Since any reduction in the survival of infected ticks would have a profoundly negative impact on their vectorial capacity (19, 29, 46), it is imperative that suitable vectors feed more than once to acquire the infection and pass it on. Despite the high mortality of infected ticks, 1,077 larvae acquiring rickettsiae during feeding survived to transmit the infection as nymphs, suggesting that they are a critical link for R. rickettsii to cycle between vertebrates. Meanwhile, few uninfected nymphs ingesting rickettsiae survived to feed as adults, suggesting that they are less critical to perpetuating the RMSF cycle. This may explain the low prevalence of naturally infected adult ticks. The ability of infected adults, and potentially other stages, to attach but only partially feed may contribute to the RMSF enzootic cycle by allowing the same tick stage to remain viable long enough to attach a second time and transmit rickettsiae to a new host.
In spring, adult ticks emerge to feed and transmit R. rickettsii via salivary secretions (17) (see above). The bacterium may then cycle through vertebrates into overlapping tick generations and between cofeeding ticks. Along with RMSF recrudescence comes the opportunity to disseminate and evolve beyond the niche limitations of a single host species of arthropod or vertebrate. In seasons or locales lacking a sufficient number of susceptible vertebrate-amplifying hosts, R. rickettsii may disappear or may rely on transovarial transmission for its maintenance. Since continued vertical passage through successive arthropod generations may lead to avirulence (58), rickettsial strains of moderate or low virulence (39, 41) may arise. Globally, a broad spectrum of Rickettsia spp. has emerged and ranges from arthropod symbiotes to infectious agents of animal and plant disease (12, 44). The ability of avirulent strains or symbiotes to interfere with the transmission of RMSF remains an issue (8, 32, 33, 43). Further, the evolution and perpetuation of novel rickettsial strains in localized tick populations may offer insight into the discrepancy of human mortality rates due to R. rickettsii from year to year or in different geographic areas, as exemplified by a 5% fatality rate in the Snake River basin, Idaho, and an 85% fatality rate in the Bitterroot Valley during the early 1900s (36, 50).
The mechanism responsible for rickettsia-induced tick mortality remains unclear. However, given that infected adult ticks incubated at 4°C survived better than those held at 21 or 27°C, plausible explanations may include reduced rickettsia-carrying capacity, heightened rickettsial virulence, or shorter rickettsial replication periods with increased temperatures. Studies on the poorly understood rickettsial inactivation-reactivation phenomenon, whereby rickettsiae apparently change from an avirulent, overwintering state into an infectious pathogen during tick feeding (35, 42, 43, 57), may provide a better insight into the interaction between rickettsiae and ticks. The factors associated with rickettsial virulence may be addressed through investigations on (i) the inactivation-reactivation phenomenon, (ii) the reduction of rickettsial infectivity for guinea pigs and ticks following Vero cell passage analogous to that described for Borrelia burgdorferi (51), and (iii) reversion of low-virulence R. rickettsii to highly pathogenic strains by passage in chicken yolk sacs (43).
Our findings on the lethal effect of R. rickettsii for its tick vector are consistent with those reported for the cycling of numerous other arthropod-borne microorganisms, including both biologically and mechanically transmitted pathogens. For example, the agents of epidemic typhus (R. prowazekii), bubonic plague (Yersinia pestis), leishmaniasis (Leishmania major and L. infantum), onchocerciasis (Onchocerca spp.), filariasis (Brugia spp. and Dirofilaria immitis), malaria (Plasmodium spp.), mosquito-borne encephalitides, and African swine fever have been reported to reduce the survival, fecundity, or fitness of their vectors (1, 13–15, 20–24, 26, 28, 31, 52, 54). Less clear is the effect of the agents of tularemia (Francisella tularensis) and Lyme disease (B. burgdorferi) on their tick vectors, although pathological effects have been noted (2, 10). Future studies on the transmission dynamics of arthropod-borne microorganisms may be prudent to consider their potential effects on vector health and behavior.
Continued investigations on the tick-rickettsia interaction may provide a better understanding of the factors influencing the emergence and distribution of arthropod-transmitted pathogens. Over time, rickettsia-induced vector mortality may alter the host-pathogen relationship to include fluctuations in rickettsial virulence, tick survivorship, and incidence of rickettsioses. The significance of symbiotic and infectious arthropod-borne agents for vectorial capacity deserves greater attention. Such studies on the ecology and evolution of host-pathogen relationships may also ultimately lead to improved detection, prediction, and control of vector-borne infectious diseases.
ACKNOWLEDGMENTS
We express our gratitude to Bob Karstens for insights on tick rearing, Gary Hettrick for graphical contributions, and Ken Gage, Joe Hinnebusch, and an anonymous reviewer for critical evaluation of this work.
REFERENCES
- 1.Bacot A W, Martin C J. Observations on the mechanism of the transmission of plague by fleas. J Hyg Plague Suppl. 1914;3:423–439. [PMC free article] [PubMed] [Google Scholar]
- 2.Balashov Y S. Bloodsucking ticks (Ixodoidea)—vectors of diseases of man and animals. Misc Pub Entomol Soc Am. 1972;8:161–376. [Google Scholar]
- 3.Bozeman F M, Shirai A, Humphries J W, Fuller H S. Ecology of Rocky Mountain spotted fever. II. Natural infection of wild mammals and birds in Virginia and Maryland. Am J Trop Med Hyg. 1967;16:48–59. [PubMed] [Google Scholar]
- 4.Burgdorfer W, Newhouse V F, Pickens E G, Lackman D B. Ecology of Rocky Mountain spotted fever in western Montana. I. Isolation of Rickettsia rickettsii from wild mammals. Am J Hyg. 1962;76:293–301. doi: 10.1093/oxfordjournals.aje.a120284. [DOI] [PubMed] [Google Scholar]
- 5.Burgdorfer W, Varma M G R. Trans-stadial and transovarial development of disease agents in arthropods. Annu Rev Entomol. 1967;12:347–376. doi: 10.1146/annurev.en.12.010167.002023. [DOI] [PubMed] [Google Scholar]
- 6.Burgdorfer W, Brinton L P. Mechanisms of transovarial infection of spotted fever rickettsiae in ticks. Ann N Y Acad Sci. 1975;266:61–72. doi: 10.1111/j.1749-6632.1975.tb35088.x. [DOI] [PubMed] [Google Scholar]
- 7.Burgdorfer W. A review of Rocky Mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. J Med Entomol. 1975;12:269–278. doi: 10.1093/jmedent/12.3.269. [DOI] [PubMed] [Google Scholar]
- 8.Burgdorfer W, Hayes S F, Mavros A J. Non-pathogenic rickettsiae in D. andersoni: a limiting factor for the distribution of Rickettsia rickettsii. In: Burgdorfer W, Anacker R L, editors. Rickettsiae and rickettsial diseases. New York, N.Y: Academic Press, Inc.; 1981. pp. 585–594. [Google Scholar]
- 9.Burgdorfer W. Ecological and epidemiological considerations of Rocky Mountain spotted fever and scrub typhus. In: Walker D H, editor. Biology of rickettsial diseases. Vol. 1. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 33–50. [Google Scholar]
- 10.Burgdorfer, W., S. F. Hayes, and D. Corwin. 1989. Pathophysiology of the Lyme disease spirochete, Borrelia burgdorferi, in ixodid ticks. Rev. Infect. Dis. 11(Suppl. 6):S1442–S1450. [DOI] [PubMed]
- 11.Dalton M J, Clarke M J, Holman R C, Krebs J W, Fishbein D B, Olson J G, Childs J E. National surveillance for Rocky Mountain spotted fever, 1981–1992: epidemiological risk factors for fatal outcome. Am J Trop Med Hyg. 1995;52:405–413. doi: 10.4269/ajtmh.1995.52.405. [DOI] [PubMed] [Google Scholar]
- 12.Davis M J, Ying Z, Brunner B R, Pantoja A, Ferwerda F H. Rickettsial relative associated with papaya bunchy top disease. Curr Microbiol. 1998;36:80–84. doi: 10.1007/s002849900283. [DOI] [PubMed] [Google Scholar]
- 13.Duke B O L. Studies on factors influencing the transmission of onchocerciasis. I. The survival rate of Simulium damnosum under laboratory conditions and the effect upon it of Onchocerca volvulus. Ann Trop Med Parasitol. 1962;56:130–135. [PubMed] [Google Scholar]
- 14.el Sawaf B M, el Sattar S A, Shehata M G, Lane R P, Morsy T A. Reduced longevity and fecundity in Leishmania-infected sand flies. Am J Trop Med Hyg. 1994;51:767–770. doi: 10.4269/ajtmh.1994.51.767. [DOI] [PubMed] [Google Scholar]
- 15.Faran M E, Turell M J, Romoser W S, Routier R G, Gibbs P H, Cannon T L, Bailey C L. Reduced survival of adult Culex pipiens infected with Rift Valley fever virus. Am J Trop Med Hyg. 1987;37:403–409. doi: 10.4269/ajtmh.1987.37.403. [DOI] [PubMed] [Google Scholar]
- 16.Friedhoff K T. Interaction between parasite and vector. Int J Parasitol. 1990;20:525–535. doi: 10.1016/0020-7519(90)90200-7. [DOI] [PubMed] [Google Scholar]
- 17.Gage K L, Gilmore R D, Karstens R K, Schwan T G. Detection of Rickettsia rickettsii in saliva, hemolymph and triturated tissues of infected Dermacentor andersoni ticks by polymerase chain reaction. Mol Cell Probes. 1992;6:333–341. doi: 10.1016/0890-8508(92)90010-u. [DOI] [PubMed] [Google Scholar]
- 18.Gage K L, Schrumpf M E, Karstens R K, Burgdorfer W, Schwan T G. DNA typing of rickettsiae in naturally infected ticks using a polymerase chain reaction/restriction fragment length polymorphism system. Am J Trop Med Hyg. 1994;50:247–260. doi: 10.4269/ajtmh.1994.50.247. [DOI] [PubMed] [Google Scholar]
- 19.Hardy J C, Houk E J, Kramer L D, Reeves W C. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 20.Hess W R, Endris R G, Haslett T M, Monahan M J, McCoy J P. Potential arthropod vectors of African swine fever virus in North America and the Caribbean basin. Vet Parasitol. 1987;26:145–155. doi: 10.1016/0304-4017(87)90084-7. [DOI] [PubMed] [Google Scholar]
- 21.Hess W R, Endris R G, Lousa A, Caiado J M. Clearance of African swine fever virus from infected tick (Acari) colonies. J Med Entomol. 1989;26:314–317. doi: 10.1093/jmedent/26.4.314. [DOI] [PubMed] [Google Scholar]
- 22.Hogg J C, Hurd H. Malaria-induced reduction of fecundity during the first gonotrophic cycle of Anopheles stephensi mosquitoes. Med Vet Entomol. 1995;9:176–180. doi: 10.1111/j.1365-2915.1995.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 23.Hurd H, Hogg J C, Renshaw M. Interactions between blood-feeding, fecundity, and infection in mosquitoes. Parasitol Today. 1995;11:411–416. [Google Scholar]
- 24.Husain A, Kershaw W E. The effect of filariasis on the ability of a vector mosquito to fly and feed and to transmit the infection. Trans R Soc Trop Med Hyg. 1971;65:617–619. doi: 10.1016/0035-9203(71)90045-9. [DOI] [PubMed] [Google Scholar]
- 25.Jellison W L, Philip C B. Technique for routine and experimental feeding of certain ixodid ticks on guinea pigs and rabbits. Public Health Rep. 1933;48:1081–1082. [Google Scholar]
- 26.Kershaw M E, Lavoipierre M M J, Chalmers T A. Studies on the intake of microfilariae by their insect vectors, their survival, and their effect on the survival of their vectors. I. Dirofilaria immitis and Aedes aegypti. Ann Trop Med Parasitol. 1953;47:207–224. doi: 10.1080/00034983.1953.11685561. [DOI] [PubMed] [Google Scholar]
- 27.Kohls G M. Tick rearing methods with special reference to the Rocky Mountain wood tick, Dermacentor andersoni. In: Galtsoff P S, Lutz F E, Welch P S, Needham J G, editors. Culture methods for invertebrate animals. Ithaca, N.Y: Comstock Publishing Co.; 1937. pp. 246–256. [Google Scholar]
- 28.Maier W A, Becker-Feldman H, Seitz H M. Pathology in malaria infected mosquitoes. Parasitol Today. 1987;3:216–218. doi: 10.1016/0169-4758(87)90063-9. [DOI] [PubMed] [Google Scholar]
- 29.Mather T N, Ginsberg H S. Vector-host-pathogen relationships: transmission dynamics of tick-borne infections. In: Sonenshine D E, Mather T N, editors. Ecological dynamics of tick-borne zoonoses. New York, N.Y: Oxford University Press; 1994. pp. 68–90. [Google Scholar]
- 30.McDade J E, Newhouse V F. Natural history of Rickettsia rickettsii. Annu Rev Microbiol. 1986;40:287–309. doi: 10.1146/annurev.mi.40.100186.001443. [DOI] [PubMed] [Google Scholar]
- 31.McGaw, M. M., L. J. Chandler, L. P. Wasieloski, C. D. Blair, and B. J. Beaty. Effect of La Crosse virus infection on overwintering Aedes triseriatus. Am. J. Trop. Med. Hyg. 58:168–175. [DOI] [PubMed]
- 32.Niebylski M L, Schrumpf M E, Burgdorfer W, Fischer E R, Gage K L, Schwan T G. Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int J Syst Bacteriol. 1997;47:446–452. doi: 10.1099/00207713-47-2-446. [DOI] [PubMed] [Google Scholar]
- 33.Niebylski M L, Peacock M G, Fischer E R, Porcella S F, Schwan T G. Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Appl Environ Microbiol. 1997;63:3933–3940. doi: 10.1128/aem.63.10.3933-3940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ormsbee R, Peacock M, Gerloff R, Tallent G, Wike D. Limits of rickettsial infectivity. Infect Immun. 1978;19:239–245. doi: 10.1128/iai.19.1.239-245.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker R R, Spencer R R. Rocky Mountain spotted fever: a study of the relationship between the presence of rickettsia-like organisms in tick smears and infectiveness of the same ticks. Public Health Rep. 1926;41:461–469. [Google Scholar]
- 36.Parker R R. Certain phases of the problem of Rocky Mountain spotted fever. Arch Pathol. 1933;15:398–429. [Google Scholar]
- 37.Peacock M G, Burgdorfer W, Ormsbee R A. Rapid fluorescent-antibody conjugation procedure. Infect Immun. 1971;3:355–357. doi: 10.1128/iai.3.2.355-357.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philip C B. Some epidemiological considerations in Rocky Mountain spotted fever. Public Health Rep. 1959;74:595–600. [PMC free article] [PubMed] [Google Scholar]
- 39.Philip R N, Casper E A. Serotypes of spotted fever group rickettsiae isolated from Dermacentor andersoni (Stiles) ticks in western Montana. Am J Trop Med Hyg. 1981;30:230–238. doi: 10.4269/ajtmh.1981.30.230. [DOI] [PubMed] [Google Scholar]
- 40.Price W H. Interference phenomenon in animal infections with rickettsiae of Rocky Mountain spotted fever. Proc Soc Exp Biol Med. 1953;82:180–184. doi: 10.3181/00379727-82-20060. [DOI] [PubMed] [Google Scholar]
- 41.Price W H. The epidemiology of Rocky Mountain spotted fever. I. The characterization of strain virulence of Rickettsia rickettsii. Am J Hyg. 1953;58:248–268. doi: 10.1093/oxfordjournals.aje.a119604. [DOI] [PubMed] [Google Scholar]
- 42.Price W H. The epidemiology of Rocky Mountain spotted fever. II. Studies on the biological survival mechanism of Rickettsia rickettsii. Am J Hyg. 1954;60:292–319. doi: 10.1093/oxfordjournals.aje.a119723. [DOI] [PubMed] [Google Scholar]
- 43.Price W H. Variation in virulence of “Rickettsia rickettsii” under natural and experimental conditions. In: Hartman F W, Horsfall F L Jr, Kidd J G, editors. The dynamics of virus and rickettsial infections. New York, N.Y: The Blakiston Co.; 1954. pp. 164–183. [Google Scholar]
- 44.Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719. doi: 10.1128/cmr.10.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regnery R L, Spruill C L, Plikaytis B D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiter P. Weather, vector biology, and arboviral recrudescence. In: Monath T P, editor. The arboviruses. Vol. 1. Boca Raton, Fla: CRC Press, Inc.; 1986. pp. 245–256. [Google Scholar]
- 47.Ricketts H T. The transmission of Rocky Mountain spotted fever by the bite of the wood tick (Dermacentor occidentalis) JAMA. 1906;47:358. [Google Scholar]
- 48.Ricketts H T. Further experiments with the wood tick in relation to Rocky Mountain spotted fever. JAMA. 1907;49:1278–1281. [Google Scholar]
- 49.Ricketts H T. Observations on the virus and means of transmission of Rocky Mountain spotted fever. J Infect Dis. 1907;4:141–153. [Google Scholar]
- 50.Ricketts H T. Some aspects of Rocky Mountain spotted fever as shown by recent investigations. Med Rec. 1909;76:843–855. doi: 10.1093/clinids/13.6.1227. [DOI] [PubMed] [Google Scholar]
- 51.Schwan T G, Burgdorfer W, Garon C F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott T W, Lorenz L H. Reduction of Culiseta melanura fitness by eastern equine encephalomyelitis virus. Am J Trop Med Hyg. 1998;59:341–346. doi: 10.4269/ajtmh.1998.59.341. [DOI] [PubMed] [Google Scholar]
- 53.Silberman R, Fiset P. Methods for counting rickettsiae and chlamydiae in purified suspensions. J Bacteriol. 1968;95:259–261. doi: 10.1128/jb.95.1.259-261.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snyder J C, Wheeler C M. The experimental infection of the human body louse, Pediculus humanus corporis, with murine and epidemic louse-borne typhus strains. J Exp Med. 1945;82:1–20. doi: 10.1084/jem.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spencer R R, Parker R R. Rocky Mountain spotted fever: experimental studies on tick virus. Public Health Rep. 1924;39:3027–3040. [Google Scholar]
- 56.Spencer R R. Rocky Mountain spotted fever. J Infect Dis. 1929;44:257–276. [Google Scholar]
- 57.Spencer R R, Parker R R. Rocky Mountain spotted fever: infectivity of fasting and recently fed ticks. Public Health Rep. 1924;38:333–339. [Google Scholar]
- 58.Werren J H. Wolbachia run amok. Proc Natl Acad Sci USA. 1997;94:11154–11155. doi: 10.1073/pnas.94.21.11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wike D A, Burgdorfer W. Plaque formation in tissue culture by Rickettsia rickettsii isolated from whole blood and tick hemolymph. Infect Immun. 1972;6:736–738. doi: 10.1128/iai.6.5.736-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]