Abstract
Background:
Elevated aggression and impulsivity are implicated in Bipolar Disorder (BD); however, relationships between these behavioral constructs have not been clarified, which can lead to misconceptions with negative consequences including stigma and adverse outcomes including suicide. The study aimed to clarify brain-based distinctions between the two constructs and their associations to risk factors, symptoms and suicide thoughts and behaviors.
Methods:
Self-rated Brown-Goodwin Aggression (BGA) and Barratt Impulsiveness Scale (BIS) scores were compared between adults with BD (n=38, 74% female) and healthy controls (HC, n=29, 64% female). Relationships were examined between BGA and BIS with childhood trauma questionnaire (CTQ), mood, comorbidities, and magnetic resonance imaging gray matter volume (GMV) assessments.
Results:
In BD, BGA and BIS total scores were both elevated and associated with childhood maltreatment (CM), particularly emotional CM, depression, substance use disorders (SUDs) and suicide attempts (SAs). BGA scores were increased by items corresponding to dysregulation of emotional and social behavior and associated with elevated mood states and suicide ideation and GMV decreases in bilateral orbitofrontal cortex and left posterior insula brain regions, previously associated with these behaviors and clinical features. BIS motor impulsiveness scores were associated with GMV decreases in anterior cingulate cortex implicated in mood and behavioral dyscontrol.
Limitations:
modest sample size, self-reports
Conclusions:
The findings suggest separable brain-based domains of dysfunction in BD of motor impulsiveness versus emotionally dysregulated feelings that are primarily self-directed. Both domains are associated with suicide behavior and modifiable risk factors of CM, depression and SUDs that could be targeted for prevention.
Keywords: bipolar disorder, aggression, impulsive behavior, MRI, prefrontal cortex, insula
Introduction
Elevated impulsivity and aggression have previously been implicated in Bipolar Disorder (BD). However, ambiguity surrounds the specific constructs measured by scales designed to assess aggression and how they relate to impulsivity. Misunderstanding of aggression and impulsivity can lead to misconceptions about persons with BD that can have negative consequences, including stigma and missed opportunities for reduction of adverse outcomes of BD including suicide. Studies reporting elevated aggression in BD have used varying measures and often have not distinguished self-directed aggression or have studied impulsive-aggression as a unitary construct. The scales used to measure aggression have assessed many subjective experiences and behaviors, not just violence towards others. Items included on many commonly used scales aimed to measure aggression assess subjective feelings related to emotion dysregulation, self-directed behavior and interpersonal impairment, in addition to outward aggression or violence; however, the contribution of these items, while having quite different implications, is often not identified, risking misattribution of elevated rating to tendency towards violence. When impulsiveness is studied separately, a commonly used measure is the Barratt Impulsiveness Scale (BIS), which instead provides measures of cognitive behavior domains of motor, non-planning, and cognitive-attentional impulsiveness. Relative to aggression, impulsiveness has been more widely studied with BIS elevations detected in adolescents and adults with BD, and independent of mood state, suggesting it may be an early trait feature of BD (Etain et al., 2013; Najt et al., 2007b; Nandagopal et al., 2011; Newman and Meyer, 2014; Peluso et al., 2005; Saddichha and Schuetz, 2014; Swann et al., 2009; Swann et al., 2003).
Potential distinctions between the constructs measured by scales aimed to study aggression and impulsiveness are further suggested by differences in reported brain associations in BD. Scores on scales designed to measure aggression, including the Lifetime History of Aggression (Coccaro et al., 1997) and the Brief Aggression Questionnaire (Webster et al., 2014), have been associated with decreased gray matter volume (GMV) in medial and lateral ventral prefrontal cortices in the general population, and particularly in orbitofrontal cortex (OFC) in psychiatric patients, including persons with BD (Chester et al., 2017; Coccaro et al., 2018; Gansler et al., 2009). The OFC subserves emotional and social behaviors, as well as inhibition of maladaptive responses especially in the context of changing reinforcement contingencies (Rolls, 2019). Decreased OFC GMV has also been associated with BIS scores among non-psychiatric populations by Matsuo and colleagues (Matsuo et al., 2009a). However, in studying persons with BD, they did not detect associations of BIS with decreased OFC GMV, but instead with dorsal and rostral anterior cingulate cortex (ACC) GMV (Cauda et al., 2011), particularly associated with the motor subscale (Matsuo et al., 2009b), suggesting the connection between ACC volume and cognitive motor control in BD. Both aggression and impulsiveness measures have shown associations with childhood maltreatment (CM) (Adigüzel et al., 2019; Garno et al., 2008; Richard-Lepouriel et al., 2019; Song et al., 2020; Tunc and Kose, 2019), mood symptoms (Garno et al., 2008; Strakowski et al., 2009; Swann et al., 2008), substance use disorders (SUDs) (Cassidy et al., 2001; Garno et al., 2008; Grunebaum et al., 2006a; Latalova, 2009; Swann et al., 2004) and suicide behavior in BD (Ekinci et al., 2011; Gilbert et al., 2011; Grunebaum et al., 2006b; Jiménez et al., 2012; Jiménez et al., 2016; Mahon et al., 2012; Michaelis et al., 2004; Oquendo et al., 2004; Oquendo et al., 2000; Reich et al., 2019). Thus, improved understanding of aggression and impulsivity in BD, and the relationship between them with factors, such as CM, SUD, and severe outcomes, could inform prevention strategies.
The current study is one of the first investigations in BD of associations of the self-reported Brown Goodwin Aggression (BGA) and BIS with CM, comorbidity, mood state and symptoms, suicide thoughts and behaviors (STBs), and brain regional GMV. We hypothesized that both BGA and BIS scores would be elevated in BD relative to HC, although BGA score elevations would not exclusively reflect aggression towards others or violent behavior. We anticipated that the BGA and BIS would show similar associations, as identified previously, to risk factors and adverse outcomes including CM, SUDs, mood symptoms and STBs. However, we hypothesized that the BGA and BIS would have different associations with regional GMV in BD, reflecting distinctions in the behavioral constructs they measure.
Methods and Materials
Subjects
Participants included 38 BD subjects (ages 18-58 years; mean age±standard deviation (SD): 35.7±11.5 years; 74% female) and 29 matched HCs (ages 18-57 years; mean±SD: 38.3±11.2 years; 66% female) without any DSM-IV Axis I disorder or first-degree family member with a major mood or psychotic disorder on the Family History Screen for Epidemiological Studies (Lish et al., 1995). Diagnostic groups were matched for age, gender, and socioeconomic status (Hollingshead, 1957) (Table 1).
Table 1.
Demographic, clinical, and behavioral variables by diagnosis
| Variable | BD (n=38) | HC (n=29) |
p value |
|
|---|---|---|---|---|
| Demographic variables | Age (SD) | 35.7 (11.5) | 38.3 (11.2) | .35 |
| Number of females (%) | 28 (74%) | 19 (66%) | .47 | |
| Socioeconomic status (SD) | 40.8 (18.3) | 39.6 (16.8) | .80 | |
| Clinical variables | Hamilton Depression Rating Scale, HDRS (SD) | 12.3 (10.5) | 0.2 (0.7) | <.001 |
| Young Mania Rating Scale, YMRS (SD) | 5.6 (6.4) | 0.3 (0.7) | <.001 | |
| Lifetime suicide attempt (%) | 13 (34%) | NA | NA | |
| Most severe suicide ideation (SD) | 8.0 (9.2) | NA | NA | |
| Mood state (euthymic (%) / depressed (%) / elevated (%)) | 13 (34%) / 10 (26%) / 15 (39%) | NA | NA | |
| Rapid cycling (%) | 12 (32%) | NA | NA | |
| Lifetime psychosis (%) | 15 (40%) | NA | NA | |
| Unmedicated at time of scan (%) | 15 (40%) | NA | NA | |
| Hospitalization (%) | 29 (77%) | NA | NA | |
| Anxiety disorder lifetime (%) | 19 (50%) | NA | NA | |
| Eating disorder lifetime (%) | 3 (8%) | NA | NA | |
| History of alcohol/substance dependence/abuse (%) | 16 (42%) | NA | NA | |
| History of alcohol dependence/abuse (%) | 5 (13%) | NA | NA | |
| History of cocaine dependence/abuse (%) | 6 (16%) | NA | NA | |
| History of cannabis dependence/abuse (%) | 6 (16%) | NA | NA | |
| History of opioid dependence/abuse (%) | 2 (5%) | NA | NA | |
| Total Childhood Trauma Questionnaire (CTQ) (SD) | 58.9 (22.9) | 32.7 (10.1) | <.001 | |
| CTQ emotional abuse subscale (SD) | 14.2 (6.3) | 6.7 (2.6) | <.001 | |
| CTQ physical abuse subscale (SD) | 10.8 (5.9) | 6.5 (3.2) | .003 | |
| CTQ sexual abuse subscale (SD) | 9.6 (7.4) | 5.1 (0.6) | <.001 | |
| CTQ emotional neglect subscale (SD) | 14.9 (7.1) | 8.5 (4.6) | <.001 | |
| CTQ physical neglect subscale (SD) | 9.4 (4.4) | 5.9 (1.5) | <.001 | |
| Behavioral variables | Total Brown-Goodwin Lifetime History of Aggression (BGA) (SD) | 17.3 (7.5) | 11.6 (3.0) | <.001 |
| BGA item 1 (SD) – discipline problem | 1.7 (1.1) | 1.2 (0.6) | .014 | |
| BGA item 2 (SD) – getting along with teachers | 1.5 (1.1) | 1.1 (0.6) | .066 | |
| BGA item 3 (SD) – angry outbursts/temper tantrums | 1.9 (1.2) | 1.0 (0.2) | <.001 | |
| BGA item 4 (SD) – getting along with work supervisor | 1.8 (1.1) | 1.0 (0.0) | <.001 | |
| BGA item 5 (SD) – severe arguments | 2.5 (1.3) | 1.3 (0.8) | <.001 | |
| BGA item 6 (SD) – physical fights | 1.7 (1.1) | 1.3 (0.8) | .11 | |
| BGA item 7 (SD) – destroyed property | 1.6 (1.0) | 1.0 (0.0) | .001 | |
| BGA item 8 (SD) – action against the law but not caught | 1.9 (1.3) | 1.4 (0.7) | .12 | |
| BGA item 9 (SD) – any trouble with police | 1.5 (0.8) | 1.2 (0.5) | .10 | |
| BGA item 10 (SD) – tried to hurt other | 1.3 (0.7) | 1.0 (0.0) | .026 | |
| BGA item 11 (SD) – non suicidal self-harm | 1.5 (0.9) | 1.0 (0.0) | .001 | |
| Total Barratt Impulsiveness Scale (BIS) (SD) | 75.4 (13.0) | 54.6 (7.0) | <.001 | |
| BIS non-planning impulsiveness subscale (SD) | 29.2 (5.2) | 21.1 (3.5) | <.001 | |
| BIS motor impulsiveness subscale (SD) | 26.1 (6.2) | 20.6 (3.0) | <.001 | |
| BIS cognitive-attentional impulsiveness subscale (SD) | 20.1 (4.5) | 13.0 (2.8) | <.001 |
p-values correspond to Mann-Whitney U tests for all continuous variables (except for age, for which an independent-sample t-test was used) and to chi-squared tests for categorical variables. Socioeconomic status not available for 2 BD and 1 HC; CTQ not available for 4 BD and 1 HC; BG item 11 not available for 1 BD.
Psychiatric diagnosis, mood state, rapid cycling, and psychosis history were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1995). Thirteen (34%) BD subjects were euthymic, 10 (26%) depressed, and 15 (39%) in elevated mood states (manic/hypomanic/mixed). Mood symptom severity was assessed with the 29-item Hamilton Depression Rating Scale (Hamilton, 1960) and Young Mania Rating Scale (Young et al., 1978) and most severe lifetime suicide ideation with the Beck Scale for Suicide Ideation (Beck et al., 1979). Thirteen (34%) had an actual SA by the Columbia Suicide History Form (Oquendo et al., 2004). Table 2 lists clinical characteristics of the BD sample.
Table 2.
Correlations between and effects of demographic, clinical, and behavioral variables and comorbidities with Brown-Goodwin Aggression and Barratt Impulsivity Scale total scores within Bipolar Disorder
| BGA mean±SD or r |
p | BIS mean±SD or r |
p | ||
|---|---|---|---|---|---|
| Demographics | Age | −.11 | .52 | −.20 | .22 |
| Sex (female/male) | 18.1±8.1 / 15.0±5.1 | .35 | 76.5±13.5 / 72.2±11.5 | .37 | |
| Socioeconomic status | −.26 | .13 | .06 | .74 | |
| Behavioral | BIS | .40 | .012 | NA | NA |
| BIS non-planning subscale | .18 | .28 | |||
| BIS motor impulsiveness subscale | .44 | .006 | |||
| BIS cognitive-attentional subscale | .36 | .025 | |||
| BGA | NA | NA | .40 | .012 | |
| Clinical | Lifetime suicide attempt (yes/no) | 22.5±8.8 / 14.6±4.9 | .003 | 83.3±13.1 / 71.2±11.1 | .005 |
| Most severe suicide ideation | .50 | .002 | .20 | .24 | |
| Mood state (euthymic / depressed / elevated) | 16.9 ± 8.4 / 13.4 ± 3.9 / 20.3 ± 7.5 | .030 | 73.2±10.4 / 74.8±10.6 / 77.7±16.5 | .65 | |
| Rapid cycling (yes/no) | 17.9 ± 5.5 / 17.0 ± 8.3 | .30 | 80.8±10.9 / 72.9±13.3 | .08 | |
| Lifetime psychosis (yes/no) | 15.1±5.9 / 18.7±8.1 | .16 | 76.4±8.6 / 74.7±15.4 | .71 | |
| Unmedicated at time of scan (yes/no) | 19.0±8.8 / 16.2±6.4 | .28 | 72.8±14.5 / 77.1±12.0 | .33 | |
| Hospitalization (yes/no) | 18.3±7.9 / 14.1±5.0 | .13 | 76.9±13.0 / 70.7±12.6 | .22 | |
| HDRS | .36 | .025 | .37 | .023 | |
| YMRS | .39 | .016 | .21 | .21 | |
| CTQ | .31 | .079 | .34 | .049 | |
| CTQ emotional abuse subscale | .28 | .11 | .57 | <.001 | |
| CTQ physical abuse subscale | .20 | .25 | .32 | .061 | |
| CTQ sexual abuse subscale | .05 | .78 | .02 | .91 | |
| CTQ emotional neglect subscale | .38 | .025 | .35 | .043 | |
| CTQ physical neglect subscale | .21 | .24 | .15 | .41 | |
| Anxiety disorder lifetime (yes/no) | 16.7±5.0 / 18.0±9.4 | .77 | 75.6±13.8 / 75.2±12.5 | .93 | |
| Eating disorder lifetime | 18.0±7.6 / 17.3±7.6 | NA | 74.0±10.6 / 75.5±13.3 | NA | |
| History of alcohol/substance dependence/abuse (yes/no) | 20.8±8.8 / 14.8±5.3 | .021 | 81.6±12.4 / 70.9±11.7 | .010 | |
| History of alcohol dependence/abuse (yes/no) | 19.6±3.5 / 17.0±7.9 | .17 | 75.8±18.1 / 75.3±12.4 | .94 | |
| History of cocaine dependence/abuse (yes/no) | 22.2±13.0 / 16.4±5.8 | .42 | 88.5±7.8 / 72.9±12.3 | .005 | |
| History of cannabis dependence/abuse (yes/no) | 19.5±10.3 / 16.9±6.9 | .70 | 78.8±9.6 / 74.8±13.6 | .49 | |
| History of opioid dependence/abuse (yes/no) | 27.5±2.1 / 16.8±7.2 | NA | 85.0±8.5 / 74.9±13.1 | NA |
p-values correspond to spearman correlations for all continuous variables (except for age, for which a Pearson correlation was used) and to Mann-Whitney U or Kruskal-Wallis H tests for categorical variables. Socioeconomic status not available for 7 BD; Most severe suicide ideation not available for 1 BD; CTQ not available for 4 BD.
Participants were recruited from clinical programs affiliated with the Yale School of Medicine and greater Connecticut community and were without history of medical (except 1 BD with treated hypothyroidism) or neurological disorders/conditions that could affect the central nervous system, including loss of consciousness >5min, substance or alcohol abuse or dependence within 3 months, or positive toxicology screen. Subjects provided written informed consent in accordance with Yale Human Investigation Committee/Institutional Review Board.
Aggression, Impulsiveness and CM Self-Ratings
Lifetime aggression was measured on the BGA by semistructured interview in which subjects reported frequency of specified behaviors in adolescence and adulthood (Brown et al., 1979; Manuck et al., 1998). BGA total score is the sum of scores on ten items, each the maximum of adolescent and adult frequencies (never=1, rarely=2, occasionally=3, often=4) (Coccaro et al., 1996; Manuck et al., 1998). An 11th item assesses non-suicidal self-injury (NSSI). Trait impulsivity was measured using the BIS-11 self-report (Patton et al., 1995) total and three subscale scores: motor, non-planning, and cognitive-attentional impulsiveness. Participants self-reported CM using the Childhood Trauma Questionnaire short form (CTQ) (Bernstein et al., 1994; Bernstein et al., 2003). Analyses were performed for CTQ total and five subscale scores: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect.
Imaging Acquisition
Structural magnetic resonance imaging (sMRI) data were acquired using a single 3-Tesla Siemens Trio MR scanner (Siemens, Erlangen, Germany). Sagittal sMRI images were obtained with a T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence using the following parameters: echo time (TE) = 2.83 ms, repetition time (TR) = 1500 ms, matrix = 256 x 256 matrix, field of view = 256 x 256 mm2, 160 one-mm slices without gaps.
Statistical Analyses
Continuous variables were tested for normality using Kolmogorov-Smirnov tests and normal probability plots. Groups were compared using independent t-tests or Mann-Whitney U tests for continuous, and Chi-square tests for categorical, variables. Spearman correlation coefficients were estimated to examine relationships between behavioral and clinical variables and BGA total and BIS total and subscale scores within HCs and BDs, separately. Mann-Whitney U or Kruskal-Wallis tests (BGA) and independent samples t-tests or ANOVA (BIS total) were used to examine relationships between the scores with categorical demographic and behavioral variables within each group. Comorbidities were analyzed if in ≥10 BD subjects. As these analyses were exploratory, results were considered significant at an uncorrected, two-tailed alpha threshold of 0.05.
Imaging Processing and Analyses
Statistical Parametric Mapping (SPM)-12 was used for image processing, as described previously (Lippard et al., 2019), and for group level analyses. General linear models were constructed to conduct whole-brain voxel-based analyses to assess relationships between GMV and BGA or BIS, within BD and HC groups, corrected for individual intracranial volume (ICV) (Pell et al., 2008). Results were considered significant at voxel threshold p<.005 and cluster k>=20, and if surviving cluster-based correction for multiple comparisons of p<.05 [family-wise error (FWE)-corrected; pfwe-cluster<.05]. Exploratory analyses were performed using partial correlation tests between GMV extracted from significant clusters and each BIS subscale, controlling for the other subscales and corrected for ICV, and results were considered significant at puncorrected<.05. All significant results are reported.
Results
Compared to HC group, the BD group had higher BGA total scores (U=274.5, p<.001), with increases observed for items 1 (discipline), 3 (angry outbursts/temper tantrums), 4 (getting along with supervisor), 5 (severe arguments), 7 (destroyed property), 10 (tried to hurt other) and 11 (NSSI) (Table 2, p’s<.05). Higher BIS total (U=77.0, p<.001) and all subscale (U≥104.0, p’s<.001) scores were observed among BD compared to HCs.
BGA Score Associations Within BD
BGA scores were positively associated with CTQ emotional neglect subscale (rs=.384, p=.025), HDRS (rs=.363, p=.025) and YMRS (rs=.389, p=.016) scores and mood state (H(2)=7.0, p=.030). BGA scores were higher in BD subjects in elevated than depressed states (p=.008); scores in euthymia were intermediate but did not differ significantly from the acute mood state groups. BGA scores were higher in past SAs than non-attempters (NSAs) (U=66.5, p=.003), and associated with most severe lifetime SI (rs,=.497, p=.002) and with a history of alcohol/substance abuse/dependence (U=99.0, p=.021).
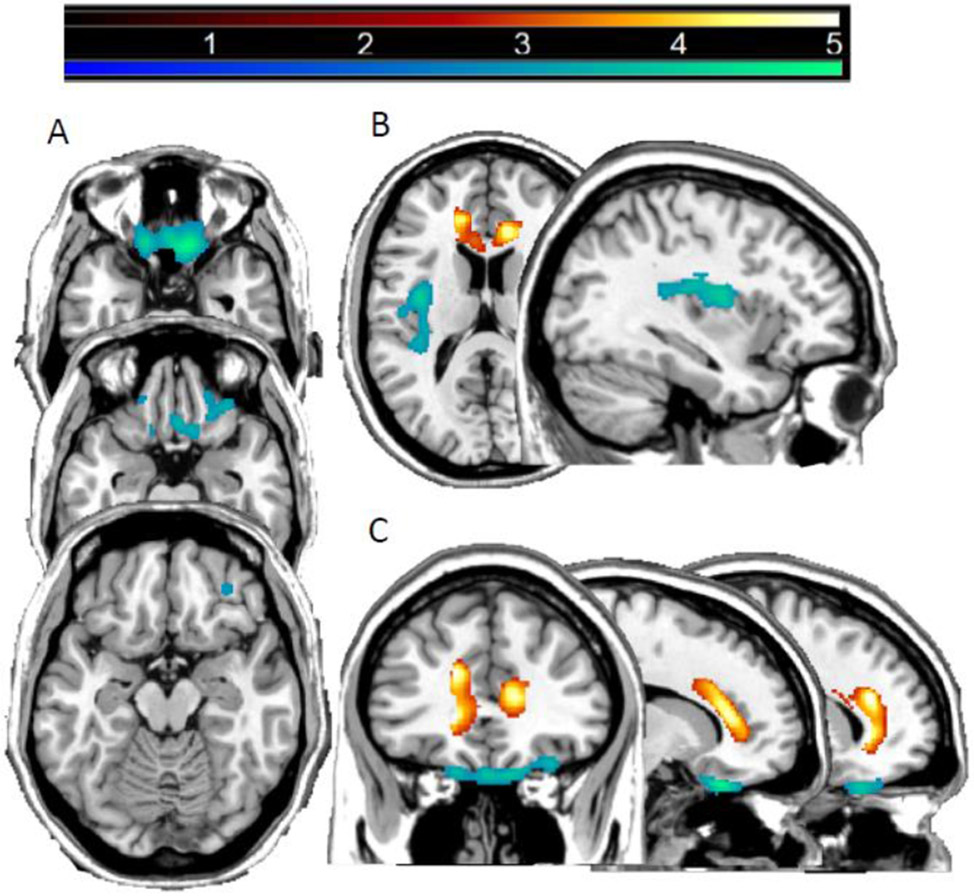
BGA scores correlated negatively with GMV in bilateral OFC (Brodman Areas, BAs, 11/47; peak Montreal Neurological Institute (MNI) coordinates x=12mm, y=28mm, z=−27mm; k=1819 voxels, pfwe-cluster=.001) and left posterior insula (PIns; peak x=−38mm, y=−8mm, z=16mm; k=1066 voxels, pfwe-cluster=.026) (Figure 1).
Figure 1: Regions of decreased gray matter volume associated with Brown-Goodwin Aggression and Barratt Impulsiveness Scale scores in bipolar disorder.
The magnetic resonance imaging T1 images display the regions where gray matter volume showed a significant negatively correlation (pfwe-cluster<0.05) with Brown-Goodwin Aggression (BGA) total scores (blue-green) or Barratt Impulsiveness Scale (BIS) total scores (red-yellow) among individuals with bipolar disorder. For BGA scores, associations were seen with bilateral orbitofrontal cortex and left posterior insula; for BIS scores, associations were seen with anterior cingulate cortex. Slices are shown at Montreal Neurological Institute (A) axial-oblique at z=−27mm, −22mm, −16mm and (B) z=16mm and sagittal at x=−36mm and (C) x=14mm, −16mm and coronal at y=34mm planes. The right sides of the axial-oblique images are the right side of the brain. The color bar shows the range of F values.
BIS Total and Subscore Associations Within BD
BIS total scores were positively associated with CTQ total (rs=.340, p=.023), emotional abuse (rs=.567, p<.001) and emotional neglect (rs=.349, p=.043) and HDRS (rs=.369, p=.023) scores. BIS scores were higher in past SAs than NSAs (t(36)=−2.98, p=.005) and among subjects with history of alcohol/substance abuse/dependence (t(36)=−2.73, p=.010). Cannabis and cocaine abuse/dependence were the only substances in sufficient numbers to explore; BIS total scores were higher in those with than without a history of cocaine abuse/dependence (t(36)=−2.96, p=.005).
BIS total scores correlated negatively with GMV in bilateral ACC regions (BAs 32/24; bilaterally the peaks were in dorsal ACC and the clusters extended to rostral ACC and on the left into ventral ACC (x=−16mm, y=34mm, z=22mm; x=14mm, y=33mm, z=12mm, k=3404 voxels; pfwe-cluster<.001) (Figure 1). Controlling for other two subscales, the BIS motor subscale was negatively correlated with bilateral ACC GMV (r=−.476, p=.004).
Discussion
In adults with BD, both BGA and BIS scores were higher in the BD than in the HC group and associated with emotional subtypes of CM, depression, and history of SUDs and SAs. BGA, but not BIS, scores were associated with elevated mood states and lifetime SI severity. BIS, but not BGA, scores were associated with history of cocaine abuse/dependence. In BD, BGA scores were negatively associated with GMV in bilateral OFC and left Pins, while BIS scores were negatively associated with GMV in bilateral ACC.
Higher BGA scores in BD are consistent with prior literature (Ballester et al., 2012; Perroud et al., 2011). However, it is noteworthy that the BGA provides subject’s self-report of a wide array of behaviors, with items ranging from benign outbursts to attempting to hurt others. Six of the ten items were significantly higher in BD. Several indicate dysregulated social interactions but not violent physical aggression, and these were the most frequently reported of the items; for example, being disciplined (item 1), having outbursts (item 3), difficulty getting along with supervisors (item 4), and severe arguments (item 5). While item 10, in which subjects reported having attacked another person with a weapon, was significantly higher in BD, only six participants endorsed this behavior and for two who provided the context it was as self-defense when under physical and sexual assault. Together with associations of BGA scores with SI, suicide attempts and NSSI, the findings are consistent with literature that individuals with psychiatric disorders are more likely to self-harm than harm others and be the victims rather than perpetrators of violence (Trevillion et al., 2012). Future research that carefully parses the specific behavioral domains within the wide range of behavior often subsumed under the term “aggression,” and whether the behavior is directed to self or others, as well as the circumstances, may help to reduce misconceptions and societal stigmatization of those with BD.
The identification of associations of CM and adverse clinical factors in BD with BGA and BIS scores has potential to inform strategies to reduce and prevent aggression and impulsiveness. Consistent with previous reports, both were associated with emotional subtypes of CM (Garno et al., 2008; Tunc and Kose, 2019), suggesting the importance of addressing prevention of not only physical but also emotional CM. Also in agreement with earlier studies, both BGA and BIS were higher in individuals with a history of comorbid SUDs (Grunebaum et al., 2006a; Latalova, 2009; Swann et al., 2004). Though a small subsample had history of cocaine dependence/abuse, the association with elevated BIS score in BD is consistent with prior reports (Moeller et al., 2002; Moeller et al., 2001). While CM and SUDs may be risk factors for aggression and impulsiveness, longitudinal studies are needed to clarify these relationships.
Both BGA and BIS scores were associated with HDRS scores suggesting associations of aggression and impulsiveness with negative affect. In line with prior reports on BGA scores, in addition to being associated with depressive symptoms, scores were also associated with manic symptoms as measured by YMRS (Garno et al., 2008), as well as elevated mood states in BD. These data further suggest the BGA measure’s relation to emotion dysregulation and both depressed and elevated symptoms (Rey et al., 2016; Van Rheenen et al., 2015). While associations to mood symptoms may reflect contributions of emotion dysregulation to aggressive feelings and behavior, it is also possible that mood at time of assessment influenced subjects’ self-reported ratings (Barnett et al., 2011; Vojta et al., 2001). In contrast to BGA, and in line with previous findings, BIS was only associated to depressive, but not manic, symptoms (Strakowski et al., 2010). For example, Strakowski and colleagues found that, in individuals with BD in manic/mixed states, impulsivity was correlated with depressive symptoms but not manic symptoms (Strakowski et al., 2009). Further research is required to elucidate the relationships between affective states with aggression and impulsive behavioral constructs.
In addition to clinical and behavioral manifestations, brain structural correlates were different for BGA and BIS, providing evidence that the two instruments measure different brain-based domains of behavior in BD. While some in the field have considered impulsivity and aggression a unitary construct, conceptualizing the two as distinct but related behavioral domains is supported by many others, who have identified complexity within each construct and in the relationship between the two, while still drawing distinctions between them (Bresin, 2019; Critchfield et al., 2004; Ende et al., 2016; Gokcay and Balcioglu, 2020; Soloff et al., 2017). Further, the findings are in line with a body of work that has identified distinct neurological correlates of the two constructs, both in BD and the general population (Antonucci et al., 2006; Chester et al., 2017; Coccaro et al., 2018; Gansler et al., 2009; Matsuo et al., 2009b). In the BD group, BGA scores were negatively associated with GMV in OFC and PIns. The OFC is involved in the regulation of behavioral responses to negative and positive emotional stimuli (Gyurak et al., 2011; Phillips et al., 2008; Rolls, 2004; Rolls, 2019; Roy et al., 2012), including the management of behavioral responses in the context of changing social reinforcement contingencies (Diekhof et al., 2011), and has shown decreased volume in BD (Dickstein et al., 2005; Najt et al., 2007a). In social situations, individuals with OFC deficits may not be able to inhibit maladaptive responses leading to negative social interactions (Berlin et al., 2004; Grafman et al., 1996; Hornak et al., 2003; Pietrini et al., 2000) that can be similar to ones measured on the BGA. Other research groups have reported reductions and asymmetries in OFC volume in psychiatric populations, including specifically in BD, in association with earlier BGA versions (Antonucci et al., 2006; Gansler et al., 2009). Prior studies by our and other research groups of individuals with BD in elevated mood states have shown attenuated OFC resting state activity and responses during a Stroop task in which prepotent responses need to be inhibited to respond (Blond and Blumberg, 2010; Blumberg et al., 2003; Blumberg et al., 1999). As here we also observed elevated symptoms in association with the BGA, this suggests the OFC may be a common substrate for constructs measured by the BGA and for elevated mood states. Our finding that BGA scores were higher for those in manic states further supports this hypothesis. The PIns is involved in interoception, and sensorimotor and emotion processing and integration (Deen et al., 2010; Grecucci et al., 2015). The left PIns has been implicated as particularly important in the perception of interoceptive stimuli and pain (Duerden et al., 2013; Segerdahl et al., 2015; Tan et al., 2017). Reduced insular volume and connectivity are also increasingly implicated in BD by neuroimaging studies. Reduced GMV in the insula has been associated with BD in adolescents and adults (Lisy et al., 2011; Matsuo et al., 2012; Neves et al., 2015; Selvaraj et al., 2012; Shepherd et al., 2012; Wang et al., 2011; Wang et al., 2018), with some studies specifically reporting left insula (Lisy et al., 2011; Neves et al., 2015). In BD, decreased functional connectivity of the insula has been observed, including of left Pins (Yin et al., 2018). As research of the insula in BD advances, this study suggests consideration of the region’s relationship to behavioral domains measured by the BGA.
BIS, on the other hand, consistent with the report of Matsuo and colleagues, was associated with reduction in GMV in the bilateral ACC in the BD participants. The peak was in the dorsal ACC (dACC), part of the cognitive division of the ACC (Bush et al., 2000) that modulates one’s response in challenging scenarios via conflict detection and resolution; dACC deficits were previously associated with past suicidal behavior and disruptions in its conflict-related functions were postulated to be potential mechanisms contributing to STBs (Carter et al., 1999; Gasquoine, 2013; Heilbronner and Hayden, 2016; Hung et al., 2018; Minzenberg et al., 2016; Mohanty et al., 2007). The cluster of findings extended to the rostral ACC bilaterally and extended to the ventral ACC on the left, part of the affective division of the ACC (Bush et al., 2000; Kaufman et al., 2003). Rostral and ventral ACC structural and functional abnormalities have been repeatedly observed in BD (Blond et al., 2012; Blumberg et al., 2005; Liu et al., 2012; Lochhead et al., 2004; Sassi et al., 2004; Wang et al., 2009), although dorsal ACC findings have varied (Adler et al., 2005; López-Larson et al., 2002) which could relate at least in part to heterogeneity in the magnitude of impulsiveness (Matsuo et al., 2009b), as well as its associated clinical features. For example, cocaine-associated comorbid SUDs—associated here and in prior studies with greater impulsivity—have also been associated with dACC abnormalities (Minzenberg et al., 2016; Yip et al., 2018). Further, our exploratory analysis suggested that the motor subscale drove the correlation between ACC GMV and impulsivity, as also found by Matsuo et al (Matsuo et al., 2009b). The results of the present study, along with the literature, suggest that, in BD, ACC deficits are related to a tendency of acting upon impulses.
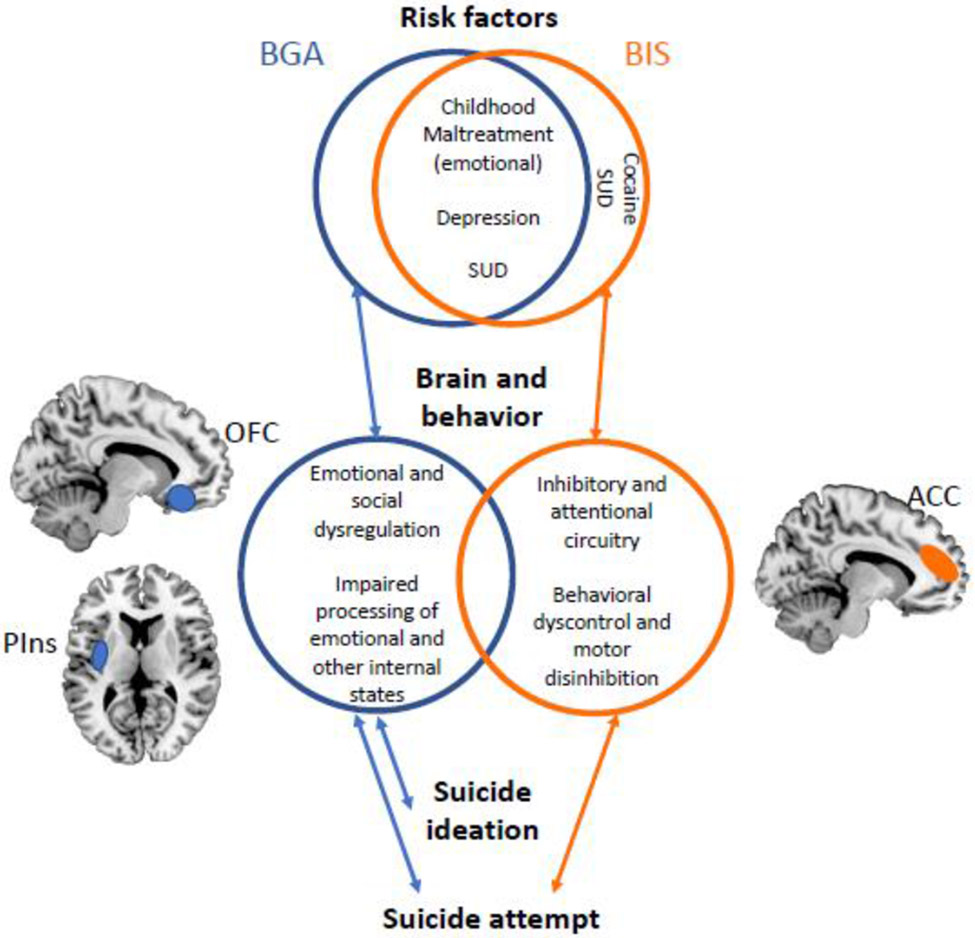
Aggression and impulsivity have both long been associated with SAs (Ekinci et al., 2011; Gilbert et al., 2011; Grunebaum et al., 2006b; Jiménez et al., 2012; Michaelis et al., 2004; Oquendo et al., 2004; Oquendo et al., 2000; Reich et al., 2019; Swann et al., 2005). Consistent with this, the findings of this study showed that both aggression and impulsivity were significantly higher in those with history of SAs than those without. Further, we found that BGA, but not BIS, was significantly positively associated with most severe past SI. This finding reinforces our conceptualization of BGA, more so than BIS, reflecting internal emotional processes (Law et al., 2015) and suggests this may contribute to SI. Prior work showed elevated BIS, especially motor impulsivity, in attempters compared to individuals without prior suicide behavior even if they had ideation (Jiménez et al., 2012). Both scales may capture constructs important in the transition to suicide behavior, but the BGA may capture ones associated with the emergence of suicidal ideation. Given the distinct regions associated with BGA and BIS in those with BD, we propose that the two measures may reflect differing, though potentially interacting, brain-based behavioral pathways to SAs in BD: one in which emotional dysregulation, and the other behavioral dyscontrol, play a particularly predominant role (Figure 2).
Figure 2: A proposed model of risk, clinical, and brain circuitry features of “aggression” and “impulsivity” in bipolar disorder.
The figure illustrates common and differing risk, brain and clinical associations found to the Brown Goodwin Aggression (BGA) and Barratt Impulsiveness Scale (BIS) scores, and relationships observed among them. Abbreviations: SUD, substance abuse disorder; OFC, orbitofrontal cortex; Pins, posterior insula; ACC, anterior cingulate cortex.
Limitations
The findings of the present study should be interpreted in the context of the following limitations. Scores on the BGA and BIS were dependent on participants’ self-reports which could be influenced by many factors. No causal conclusions can be drawn from our results; longitudinal experiments are needed in order to address causal links between aggression and impulsivity, with CM, clinical and brain factors in BD. The modest sample size may have limited our ability to identify relationships among the factors studied. Excluding participants with recent SUDs may have decreased generalizability, particularly to BD populations with SAs who often have high rates of SUDs. While previous studies have investigated psychotropic medications as potential treatments for aggressive (Aguglia et al., 2018; Barzman et al., 2006; Hafeman et al., 2020; Saxena et al., 2006; Swann, 1999) or impulsive behavior (Akingbala et al., 2006; Reddy et al., 2014) in BD with varying results, the present study is unable to draw conclusion on the effect of medication given the lack of systematic study. That GMV reductions associated with BGA or BIS were not detected in the HC group may be due to underlying differences in BD or to the smaller variance in HC groups. Finally, studies of BGA and BIS in other psychopathologies are needed to assess whether associations are transdiagnostic or specific to BD.
Highlights.
“Aggression” in bipolar disorder is more likely to be directed to self than others
Aggression and impulsivity reflect two pathways to suicide risk in bipolar disorder
MRI shows distinct brain circuits in bipolar disorder aggression and impulsivity
Acknowledgments:
We thank the research subjects for their participation.
Role of the Funding:
This work was supported by grants from AIM for Youth Mental Health and Klingenstein Third Generation Foundation (Dr. Sankar), the National Center for Advancing Translational Science (grant number UL1TR000142), the National Institute of Mental Health (grant numbers R01MH69747, R01MH070902), American Foundation for Suicide Prevention, Brain and Behavior Foundation, International Bipolar Foundation, For the Love of Travis Foundation and the John and Hope Furth Endowment (Dr. Blumberg).
Footnotes
Disclosures
Dr. Oquendo receives royalties from the Research Foundation for Mental Hygiene for the commercial use of the Columbia Suicide Severity Rating Scale and owns shares in Mantra, Inc. She serves as an advisor to Alkermes, Otsuka, ATAI, St. George’s University and Fundacion Jimenez Diaz. Her family owns stock in Bristol Myers Squibb. All other authors report no financial relationships with commercial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adigüzel V, özdemir N, Şahin ŞK, 2019. Childhood traumas in euthymic bipolar disorder patients in Eastern Turkey and its relations with suicide risk and aggression. Nordic journal of psychiatry 73, 490–496. [DOI] [PubMed] [Google Scholar]
- Adler CM, Levine AD, DelBello MP, Strakowski SM, 2005. Changes in Gray Matter Volume in Patients with Bipolar Disorder. Biological Psychiatry 58, 151–157. [DOI] [PubMed] [Google Scholar]
- Aguglia A, Mineo L, Rodolico A, Signorelli MS, Aguglia E, 2018. Asenapine in the management of impulsivity and aggressiveness in bipolar disorder and comorbid borderline personality disorder: an open-label uncontrolled study. International clinical psychopharmacology 33, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akingbala F, Dhanani N, Brown ES, 2006. Impulsivity in Patients with Bipolar Disorder and Cocaine or Amphetamine Dependence Given Lamotrigine. Journal of Dual Diagnosis 2, 73–83. [Google Scholar]
- Antonucci AS, Gansler DA, Tan S, Bhadelia R, Patz S, Fulwiler C, 2006. Orbitofrontal correlates of aggression and impulsivity in psychiatric patients. Psychiatry Research: Neuroimaging 147, 213–220. [DOI] [PubMed] [Google Scholar]
- Ballester J, Goldstein T, Goldstein B, Obreja M, Axelson D, Monk K, Hickey M, Iyengar S, Farchione T, Kupfer DJ, Brent D, Birmaher B, 2012. Is bipolar disorder specifically associated with aggression? Bipolar Disorders 14, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J, Huang J, Perlis R, Young M, Rosenbaum J, Nierenberg A, Sachs G, Nimgaonkar V, Miklowitz D, Smoller J, 2011. Personality and bipolar disorder: dissecting state and trait associations between mood and personality. Psychological medicine 41, 1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzman DH, DelBello MP, Adler CM, Stanford KE, Strakowski SM, 2006. The efficacy and tolerability of quetiapine versus divalproex for the treatment of impulsivity and reactive aggression in adolescents with co-occurring bipolar disorder and disruptive behavior disorder (s). Journal of Child & Adolescent Psychopharmacology 16, 665–670. [DOI] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A, 1979. Assessment of suicidal intention: The Scale for Suicide Ideation. Journal of Consulting and Clinical Psychology 47, 343–352. [DOI] [PubMed] [Google Scholar]
- Berlin H, Rolls E, Kischka U, 2004. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain 127, 1108–1126. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J, 1994. Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American journal of psychiatry. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W, 2003. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect 27, 169–190. [DOI] [PubMed] [Google Scholar]
- Blond BN, Blumberg HP, 2010. Functional neuroimaging research in bipolar disorder. Behavioral Neurobiology of Bipolar Disorder and its Treatment, 227–245. [DOI] [PubMed] [Google Scholar]
- Blond BN, Fredericks CA, Blumberg HP, 2012. Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala–anterior paralimbic neural system. Bipolar disorders 14, 340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, Gueorguieva R, Fulbright RK, McGlashan TH, Gore JC, Krystal JH, 2005. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology 183, 308–313. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Leung H-C, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS, 2003. A Functional Magnetic Resonance Imaging Study of Bipolar Disorder: State- and Trait-Related Dysfunction in Ventral Prefrontal Cortices. Archives of General Psychiatry 60, 601–609. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, White T, Epstein J, Isenberg N, McBride PA, Kemperman I, Emmerich S, Dhawan V, Eidelberg D, Kocsis JH, Silbersweig DA, 1999. Rostral and Orbital Prefrontal Cortex Dysfunction in the Manic State of Bipolar Disorder. American Journal of Psychiatry 156, 1986–1988. [DOI] [PubMed] [Google Scholar]
- Bresin K, 2019. Impulsivity and aggression: A meta-analysis using the UPPS model of impulsivity. Aggression and Violent Behavior 48, 124–140. [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF, 1979. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry research 1, 131–139. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI, 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences 4, 215–222. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD, 1999. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the Neurosciences 10, 49–58. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Ahearn EP, Carroll BJ, 2001. Substance abuse in bipolar disorder. Bipolar Disorders 3, 181–188. [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A, 2011. Functional connectivity of the insula in the resting brain. NeuroImage 55, 8–23. [DOI] [PubMed] [Google Scholar]
- Chester DS, Lynam DR, Milich R, DeWall CN, 2017. Physical aggressiveness and gray matter deficits in ventromedial prefrontal cortex. Cortex 97, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Berman ME, Kavoussi RJ, 1997. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry research 73, 147–157. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Cremers H, Fanning J, Nosal E, Lee R, Keedy S, Jacobson KC, 2018. Reduced frontal grey matter, life history of aggression, and underlying genetic influence. Psychiatry Research: Neuroimaging 271, 126–134. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Sheline YI, Lish JD, Csernansky JG, 1996. Impulsive aggression in personality disorder correlates with tritiated paroxetine binding in the platelet. Archives of General Psychiatry 53, 531–536. [DOI] [PubMed] [Google Scholar]
- Critchfield KL, Levy KN, Clarkin JF, 2004. The Relationship Between Impulsivity, Aggression, and Impulsive-Aggression in Borderline Personality Disorder: An Empirical Analysis of Self-Report Measures. Journal of Personality Disorders 18, 555–570. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA, 2010. Three Systems of Insular Functional Connectivity Identified with Cluster Analysis. Cerebral Cortex 21, 1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, Leibenluft E, 2005. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Archives of General Psychiatry 62, 734–741. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O, 2011. Fear is only as deep as the mind allows: A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage 58, 275–285. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Arsalidou M, Lee M, Taylor MJ, 2013. Lateralization of affective processing in the insula. NeuroImage 78, 159–175. [DOI] [PubMed] [Google Scholar]
- Ekinci O, Albayrak Y, Ekinci AE, Caykoylu A, 2011. Relationship of trait impulsivity with clinical presentation in euthymic bipolar disorder patients. Psychiatry Research 190, 259–264. [DOI] [PubMed] [Google Scholar]
- Ende G, Cackowski S, Van Eijk J, Sack M, Demirakca T, Kleindienst N, Bohus M, Sobanski E, Krause-Utz A, Schmahl C, 2016. Impulsivity and Aggression in Female BPD and ADHD Patients: Association with ACC Glutamate and GABA Concentrations. Neuropsychopharmacology 41, 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etain B, Mathieu F, Liquet S, Raust A, Cochet B, Richard J, Gard S, Zanouy L, Kahn J-P, Cohen R, 2013. Clinical features associated with trait-impulsiveness in euthymic bipolar disorder patients. Journal of Affective Disorders 144, 240–247. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J, 1995. Structured Clinical Interview for DSM-IV Axis I & Axis II Disorders (Version 2.0). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Gansler DA, McLaughlin NC, Iguchi L, Jerram M, Moore DW, Bhadelia R, Fulwiler C, 2009. A multivariate approach to aggression and the orbital frontal cortex in psychiatric patients. Psychiatry Research: Neuroimaging 171, 145–154. [DOI] [PubMed] [Google Scholar]
- Garno JL, Gunawardane N, Goldberg JF, 2008. Predictors of trait aggression in bipolar disorder. Bipolar disorders 10, 285–292. [DOI] [PubMed] [Google Scholar]
- Gasquoine PG, 2013. Localization of function in anterior cingulate cortex: From psychosurgery to functional neuroimaging. Neuroscience & Biobehavioral Reviews 37, 340–348. [DOI] [PubMed] [Google Scholar]
- Gilbert AM, Garno JL, Braga RJ, Shaya Y, Goldberg TE, Malhotra AK, Burdick KE, 2011. Clinical and cognitive correlates of suicide attempts in bipolar disorder: is suicide predictable? The Journal of clinical psychiatry 72, 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokcay H, Balcioglu YH, 2020. Neurobiology of impulsivity and aggression as substrates of suicidal behavior: A narrative focus on the involvement of serum lipids. [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown H, Salazar AM, 1996. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology 46, 1231–1231. [DOI] [PubMed] [Google Scholar]
- Grecucci A, Theuninck A, Frederickson J, Job R, 2015. Mechanisms of social emotion regulation: From neuroscience to psychotherapy. Emotion regulation: Processes, cognitive effects and social consequences, 57–84. [Google Scholar]
- Grunebaum MF, Galfalvy HC, Nichols CM, Caldeira NA, Sher L, Dervic K, Burke AK, Mann JJ, Oquendo MA, 2006a. Aggression and substance abuse in bipolar disorder. Bipolar Disorders 8, 496–502. [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Ramsay SR, Galfalvy HC, Ellis SP, Burke AK, Sher L, Printz DJ, Kahn DA, John Mann J, Oquendo MA, 2006b. Correlates of suicide attempt history in bipolar disorder: a stress-diathesis perspective. Bipolar disorders 8, 551–557. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A, 2011. Explicit and implicit emotion regulation: A dual-process framework. Cognition and Emotion 25, 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM, Rooks B, Merranko J, Liao F, Gill MK, Goldstein TR, Diler R, Ryan N, Goldstein BI, Axelson DA, 2020. Lithium Versus Other Mood-Stabilizing Medications in a Longitudinal Study of Youth Diagnosed With Bipolar Disorder. Journal of the American Academy of Child and Adolescent Psychiatry 59, 1146–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry 23, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Hayden BY, 2016. Dorsal Anterior Cingulate Cortex: A Bottom-Up View. Annual Review of Neuroscience 39, 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB, 1957. Two factor index of social position. [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock P, Polkey C, 2003. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain 126, 1691–1712. [DOI] [PubMed] [Google Scholar]
- Hung Y, Gaillard SL, Yarmak P, Arsalidou M, 2018. Dissociations of cognitive inhibition, response inhibition, and emotional interference: Voxelwise ALE meta-analyses of fMRI studies. Human Brain Mapping 39, 4065–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez E, Arias B, Castellví P, Goikolea JM, Rosa A, Fananas L, Vieta E, Benabarre A, 2012. Impulsivity and functional impairment in bipolar disorder. Journal of affective disorders 136, 491–497. [DOI] [PubMed] [Google Scholar]
- Jiménez E, Arias B, Mitjans M, Goikolea JM, Ruíz V, Brat M, Sáiz PA, García-Portilla MP, Burón P, Bobes J, Oquendo MA, Vieta E, Benabarre A, 2016. Clinical features, impulsivity, temperament and functioning and their role in suicidality in patients with bipolar disorder. Acta Psychiatrica Scandinavica 133, 266–276. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H, 2003. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience 23, 7839–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latalova K, 2009. Bipolar disorder and aggression. International Journal of Clinical Practice 63, 889–899. [DOI] [PubMed] [Google Scholar]
- Law KC, Khazem LR, Anestis MD, 2015. The role of emotion dysregulation in suicide as considered through the ideation to action framework. Current Opinion in Psychology 3, 30–35. [Google Scholar]
- Lippard ETC, Johnston JA, Spencer L, Quatrano S, Fan S, Sankar A, Weathers J, Pittman B, Oquendo MA, Blumberg HP, 2019. Preliminary examination of gray and white matter structure and longitudinal structural changes in frontal systems associated with future suicide attempts in adolescents and young adults with mood disorders. Journal of affective disorders 245, 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lish JD, Weissman MM, Adams PB, Hoven CW, Bird H, 1995. Family psychiatric screening instrument for epidemiologic studies: pilot testing and validation. Psychiatry research 57, 169–180. [DOI] [PubMed] [Google Scholar]
- Lisy ME, Jarvis KB, DelBello MP, Mills NP, Weber WA, Fleck D, Strakowski SM, Adler CM, 2011. Progressive neurostructural changes in adolescent and adult patients with bipolar disorder. Bipolar Disorders 13, 396–405. [DOI] [PubMed] [Google Scholar]
- Liu J, Blond BN, van Dyck LI, Spencer L, Wang F, Blumberg HP, 2012. Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disorders 14, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead RA, Parsey RV, Oquendo MA, Mann JJ, 2004. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biological Psychiatry 55, 1154–1162. [DOI] [PubMed] [Google Scholar]
- López-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM, 2002. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biological psychiatry 52, 93–100. [DOI] [PubMed] [Google Scholar]
- Mahon K, Burdick KE, Wu J, Ardekani BA, Szeszko PR, 2012. Relationship between suicidality and impulsivity in bipolar I disorder: a diffusion tensor imaging study. Bipolar Disorders 14, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF, 1998. Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology 19, 287–299. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Kopecek M, Nicoletti M, Hatch J, Watanabe Y, Nery F, Zunta-Soares G, Soares J, 2012. New structural brain imaging endophenotype in bipolar disorder. Molecular psychiatry 17, 412–420. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MAM, Nery FG, Soares JC, 2009a. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Human Brain Mapping 30, 1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti MA, Peluso MAM, Hatch JP, Nemoto K, Watanabe Y, Nery FG, Monkul ES, Zunta-Soares GB, Bowden CL, Soares JC, 2009b. Anterior cingulate volumes associated with trait impulsivity in individuals with bipolar disorder. Bipolar Disorders 11, 628–636. [DOI] [PubMed] [Google Scholar]
- Michaelis BH, Goldberg JF, Davis GP, Singer TM, Garno JL, Wenze SJ, 2004. Dimensions of impulsivity and aggression associated with suicide attempts among bipolar patients: a preliminary study. Suicide and Life-Threatening Behavior 34, 172–176. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Lesh TA, Niendam TA, Cheng Y, Carter CS, 2016. Conflict-Related Anterior Cingulate Functional Connectivity Is Associated With Past Suicidal Ideation and Behavior in Recent-Onset Psychotic Major Mood Disorders. J Neuropsychiatry Clin Neurosci 28, 299–305. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Harper RA, Swann AC, 2002. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug and alcohol dependence 68, 105–111. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J, 2001. The impact of impulsivity on cocaine use and retention in treatment. Journal of substance abuse treatment 21, 193–198. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ringo Ho M-H, Banich MT, Webb AG, Warren SL, Miller GA, 2007. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology 44, 343–351. [DOI] [PubMed] [Google Scholar]
- Najt P, Nicoletti M, Chen HH, Hatch JP, Caetano SC, Sassi RB, Axelson D, Brambilla P, Keshavan MS, Ryan ND, 2007a. Anatomical measurements of the orbitofrontal cortex in child and adolescent patients with bipolar disorder. Neuroscience letters 413, 183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najt P, Perez J, Sanches M, Peluso M, Glahn D, Soares JC, 2007b. Impulsivity and bipolar disorder. European Neuropsychopharmacology 17, 313–320. [DOI] [PubMed] [Google Scholar]
- Nandagopal JJ, Fleck DE, Adler CM, Mills NP, Strakowski SM, DelBello MP, 2011. Impulsivity in adolescents with bipolar disorder and/or attention-deficit/hyperactivity disorder and healthy controls as measured by the Barratt Impulsiveness Scale. Journal of child and adolescent psychopharmacology 21, 465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves M.d.C.L., Albuquerque MR, Malloy-Diniz L, Nicolato R, Silva Neves F, de Souza-Duran FL, Busatto G, Corrêa H, 2015. A voxel-based morphometry study of gray matter correlates of facial emotion recognition in bipolar disorder. Psychiatry Research: Neuroimaging 233, 158–164. [DOI] [PubMed] [Google Scholar]
- Newman AL, Meyer TD, 2014. Impulsivity: present during euthymia in bipolar disorder?-a systematic review. International journal of bipolar disorders 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Lizardi D, Greenwald S, Weissman MM, Mann JJ, 2004. Rates of lifetime suicide attempt and rates of lifetime major depression in different ethnic groups in the United States. Acta Psychiatrica Scandinavica 110, 446–451. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Waternaux C, Brodsky B, Parsons B, Haas GL, Malone KM, Mann JJ, 2000. Suicidal behavior in bipolar mood disorder: clinical characteristics of attempters and nonattempters. Journal of Affective Disorders 59, 107–117. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Pell GS, Briellmann RS, Chan CHP, Pardoe H, Abbott DF, Jackson GD, 2008. Selection of the control group for VBM analysis: influence of covariates, matching and sample size. Neuroimage 41, 1324–1335. [DOI] [PubMed] [Google Scholar]
- Peluso M, Hatch J, Glahn D, Monkul S, Sanches M, Najt P, Kaur S, Bowden C, Barratt E, Soares J, 2005. Impulsivity and hostility in mood disorders. Biological Psychiatry 57, 200S–200S. [Google Scholar]
- Perroud N, Baud P, Mouthon D, Courtet P, Malafosse A, 2011. Impulsivity, aggression and suicidal behavior in unipolar and bipolar disorders. Journal of affective disorders 134, 112–118. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC, 2008. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry 13, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini P, Guazzelli M, Basso G, Jaffe K, Grafman J, 2000. Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. American Journal of Psychiatry 157, 1772–1781. [DOI] [PubMed] [Google Scholar]
- Reddy LF, Lee J, Davis MC, Altshuler L, Glahn DC, Miklowitz DJ, Green MF, 2014. Impulsivity and Risk Taking in Bipolar Disorder and Schizophrenia. Neuropsychopharmacology 39, 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich R, Gilbert A, Clari R, Burdick KE, Szeszko PR, 2019. A preliminary investigation of impulsivity, aggression and white matter in patients with bipolar disorder and a suicide attempt history. Journal of affective disorders 247, 88–96. [DOI] [PubMed] [Google Scholar]
- Rey G, Piguet C, Benders A, Favre S, Eickhoff SB, Aubry J-M, Vuilleumier P, 2016. Resting-state functional connectivity of emotion regulation networks in euthymic and non-euthymic bipolar disorder patients. European psychiatry 34, 56–63. [DOI] [PubMed] [Google Scholar]
- Richard-Lepouriel H, Kung A-L, Hasler R, Bellivier F, Prada P, Gard S, Ardu S, Kahn J-P, Dayer A, Henry C, 2019. Impulsivity and its association with childhood trauma experiences across bipolar disorder, attention deficit hyperactivity disorder and borderline personality disorder. Journal of Affective Disorders 244, 33–41. [DOI] [PubMed] [Google Scholar]
- Rolls ET, 2004. The functions of the orbitofrontal cortex. Brain and Cognition 55, 11–29. [DOI] [PubMed] [Google Scholar]
- Rolls ET, 2019. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia 128, 14–43. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD, 2012. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences 16, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddichha S, Schuetz C, 2014. Is impulsivity in remitted bipolar disorder a stable trait? A meta-analytic review. Comprehensive psychiatry 55, 1479–1484. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC, 2004. Reduced left anterior cingulate volumes in untreated bipolar patients. Biological Psychiatry 56, 467–475. [DOI] [PubMed] [Google Scholar]
- Saxena K, Chang K, Steiner H, 2006. Treatment of aggression with risperidone in children and adolescents with bipolar disorder: a case series. Bipolar Disorders 8, 405–410. [DOI] [PubMed] [Google Scholar]
- Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I, 2015. The dorsal posterior insula subserves a fundamental role in human pain. Nature Neuroscience 18, 499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Arnone D, Job D, Stanfield A, Farrow TF, Nugent AC, Scherk H, Gruber O, Chen X, Sachdev PS, Dickstein DP, Malhi GS, Ha TH, Ha K, Phillips ML, McIntosh AM, 2012. Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disorders 14, 135–145. [DOI] [PubMed] [Google Scholar]
- Shepherd AM, Matheson SL, Laurens KR, Carr VJ, Green MJ, 2012. Systematic Meta-Analysis of Insula Volume in Schizophrenia. Biological Psychiatry 72, 775–784. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Abraham K, Burgess A, Ramaseshan K, Chowdury A, Diwadkar VA, 2017. Impulsivity and aggression mediate regional brain responses in Borderline Personality Disorder: An fMRI study. Psychiatry Research: Neuroimaging 260, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Chon M-W, Ryu V, Yu R, Lee D-K, Lee H, Lee W, Lee JH, Park DY, 2020. Cortical Volumetric Correlates of Childhood Trauma, Anxiety, and Impulsivity in Bipolar Disorder. Psychiatry investigation 17, 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Fleck DE, DelBello MP, Adler CM, Shear PK, Kotwal R, Arndt S, 2010. Impulsivity across the course of bipolar disorder. Bipolar disorders 12, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Fleck DE, DelBello MP, Adler CM, Shear PK, McElroy SL, Keck PE Jr, Moss Q, Cerullo MA, Kotwal R, 2009. Characterizing impulsivity in mania. Bipolar Disorders 11, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, 1999. Treatment of aggression in patients with bipolar disorder. The Journal of clinical psychiatry 60, 25–28. [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Moeller FG, 2004. Impulsivity: a link between bipolar disorder and substance abuse. Bipolar disorders 6, 204–212. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG, 2005. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. American Journal of Psychiatry 162, 1680–1687. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG, 2009. Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disorders 11, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG, 2003. Impulsivity and phase of illness in bipolar disorder. Journal of affective disorders 73, 105–111. [DOI] [PubMed] [Google Scholar]
- Swann AC, Steinberg JL, Lijffijt M, Moeller FG, 2008. Impulsivity: Differential relationship to depression and mania in bipolar disorder. Journal of Affective Disorders 106, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LL, Pelzer P, Heinl C, Tang W, Gangadharan V, Flor H, Sprengel R, Kuner T, Kuner R, 2017. A pathway from midcingulate cortex to posterior insula gates nociceptive hypersensitivity. Nature neuroscience 20, 1591. [DOI] [PubMed] [Google Scholar]
- Trevillion K, Oram S, Feder G, Howard LM, 2012. Experiences of domestic violence and mental disorders: a systematic review and meta-analysis. PloS one 7, e51740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc S, Kose S, 2019. The Effect of Childhood Trauma on Impulsivity in Patients with Bipolar Disorder. [Google Scholar]
- Van Rheenen TE, Murray G, Rossell SL, 2015. Emotion regulation in bipolar disorder: profile and utility in predicting trait mania and depression propensity. Psychiatry research 225, 425–432. [DOI] [PubMed] [Google Scholar]
- Vojta C, Kinosian B, Glick H, Altshuler L, Bauer MS, 2001. Self-reported quality of life across mood states in bipolar disorder. Comprehensive Psychiatry 42, 190–195. [DOI] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, Tie K, Gong G, Shah MP, Jones M, Uderman J, Constable RT, Blumberg HP, 2009. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 66, 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, Womer FY, Edmiston EE, Chepenik LG, Chen R, Spencer L, Blumberg HP, 2011. Olfactocentric paralimbic cortex morphology in adolescents with bipolar disorder. Brain 134, 2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tian F, Wang S, Cheng B, Qiu L, He M, Wang H, Duan M, Dai J, Jia Z, 2018. Gray matter bases of psychotic features in adult bipolar disorder: A systematic review and voxel-based meta-analysis of neuroimaging studies. Human Brain Mapping 39, 4707–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GD, DeWall CN, Pond RS Jr, Deckman T, Jonason PK, Le BM, Nichols AL, Schember TO, Crysel LC, Crosier BS, 2014. The brief aggression questionnaire: Psychometric and behavioral evidence for an efficient measure of trait aggression. Aggressive behavior 40, 120–139. [DOI] [PubMed] [Google Scholar]
- Yin Z, Chang M, Wei S, Jiang X, Zhou Y, Cui L, Lv J, Wang F, Tang Y, 2018. Decreased Functional Connectivity in Insular Subregions in Depressive Episodes of Bipolar Disorder and Major Depressive Disorder. Frontiers in Neuroscience 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Worhunsky PD, Xu J, Morie KP, Constable RT, Malison RT, Carroll KM, Potenza MN, 2018. Gray-matter relationships to diagnostic and transdiagnostic features of drug and behavioral addictions. Addict Biol 23, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: Reliability, validity and sensitivity. The British Journal of Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]