Abstract
Background:
Anti-melanoma differentiation-associated gene 5 (anti-MDA5) antibody-positive dermatomyositis (DM) has low survival rate, whereas macrophage activation syndrome (MAS) is a severe and life-threatening syndrome associated with autoimmune diseases. Their coexistence is very rare. This study aimed to describe the prevalence, clinical characteristics, and outcomes of anti-MDA5 antibodies-positive DM patients complicated with MAS.
Methods:
In this retrospective study, we enrolled DM patients with anti-MDA5 antibodies, who were hospitalized between 2016 and 2020 and included patients diagnosed with MAS.
Results:
We identified four (2%) DM patients with anti-MDA5 antibodies. They were females with interstitial lung disease (ILD). The level of aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and ferritin were significantly higher in the MAS group than those without MAS (p < 0.05). Patients with MAS were significantly more likely to develop a dysphagia (p = 0.012). Literature review revealed eight similar cases. Together with the present study, we identified 12 patients complicated with ILD. The median age of disease onset was 52 years with a male to female ratio of 1:6. The median duration between DM onset and MAS diagnosis was 3 months. The mortality of MAS in anti-MDA5 antibody-positive DM was 50%. Patients who died were older than those who survived (56.7 years versus 35.5 years; p = 0.015).
Conclusions:
MAS was rare in anti-MDA5 antibody-positive DM. The higher the level of AST, LDH, and ferritin, the greater the risk of MAS. They were associated with high mortality rates, particularly in older patients.
Keywords: anti-melanoma differentiation-associated gene 5 antibody, dermatomyositis, hemophagocytic syndrome, interstitial lung disease, macrophage activation syndrome, outcomes, prevalence
Introduction
Dermatomyositis (DM) is an idiopathic inflammatory myopathy (IIM), which affects skin, skeletal muscle, lung, and other organs, resulting in various systemic manifestations. The anti-melanoma differentiation-associated gene 5 (anti-MDA5) antibody-positive DM, as a particular type of DM, is a deadly disease characterized by interstitial lung disease (ILD) or rapidly progressive ILD (RP-ILD) and resistance to treatment. 1 Hemophagocytic lymphohistiocytosis (HLH) or hemophagocytic syndrome (HPS) is a life-threatening syndrome related to a dysregulated hyperinflammatory response associated with aberrant activation of lymphocytes and macrophages, resulting in fever, cytopenia, hepatosplenomegaly, and hyperferritinemia. 2 It is classically divided into primary or familial HLH/HPS and secondary HLH/HPS. When HLH/HPS is observed in the context of rheumatologic diseases, secondary HLH/HPS is commonly known as macrophage activation syndrome (MAS) and is frequently found as a complication of systemic juvenile idiopathic arthritis (sJIA), adult-onset Still’s disease (AOSD), and systemic lupus erythematosus (SLE). 3 It has been reported that patients with anti-MDA5 antibody-positive DM have elevated levels of serum ferritin, which may be associated with the disease activity of ILD in DM. 4 In clinical practice, the presence of hyperferritinemia in rheumatic diseases may suggest complications of MAS. However, we sometimes cannot determine whether patients with DM, who have hyperferritinemia, also have MAS, particularly in patients with anti-MDA5 antibody. MAS diagnosis is pivotal in DM patients with anti-MDA5 antibody, because MAS sometimes requires additional treatments.
The aim of the present study was to describe clinical characteristics and follow up anti-MDA5 antibody-positive DM patients complicated by MAS. We also reviewed similar cases in the literature.
Methods
Study cohort and patients
A cohort of 200 Chinese patients, diagnosed with DM with anti-MDA5, were identified at the Rheumatology Department of the China-Japan Friendship Hospital from January 2016 to October 2020. The anti-MDA5 antibody in the serum of patients was detected by the Euroline myositis line-blot assay of Euroimmun (Lübeck, Germany). Clinical manifestations, laboratory data, and radiographic data were extracted from medical records. This study was approved by the Research Review Committee (RRC) and the Ethical Review Committee (ERC) of the China-Japan Friendship Hospital (IRB number is 2016-117). Written informed consent was obtained from all enrolled patients at admission. In this retrospective non-interventional study, all patients’ data were anonymously surveyed.
Inclusion criteria
We select patients diagnosed with anti-MDA5 antibody-positive DM with MAS, based on revised diagnostic guidelines for HLH/HPS (five out of the eight criteria below) 5 : fever (⩾38.5ºC for ⩾7 days), splenomegaly, cytopenia (affecting two of three lineages in the peripheral blood), hypertriglyceridemia or hypofibrinogenemia, hemophagocytosis in bone marrow or spleen or lymph nodes (no evidence of malignancy), low or absent natural killer (NK) cell activity, ferritin ⩾ 500 mg/l, and soluble CD25 (i.e. soluble IL-2 receptor) ⩾2400 U/ml.
Exclusion criteria
The following patients should be excluded: (1) patients with incomplete clinical data, (2) patients with hematological tumors, and (3) no hemophagocytosis in bone marrow or spleen or lymph nodes.
Literature review
An extensive electronic literature search was conducted, including databases such as PubMed and Embase, starting in 2010, when the first patient with anti-MDA5 antibodies was reported, through 2020. It was performed based on the following key words: dermatomyositis, anti-MDA5 antibody, macrophage activation syndrome, and HPS. We extracted data from patients with anti-MDA5 antibody complicated by MAS or HPS or HLH. All articles were independently reviewed by two authors.
Statistical analysis
Statistical analysis was performed with the IBM SPSS version 21.0 software (IBM Corp., Armonk, NY, USA). Quantitative variables were reported as means and were compared by a nonparametric test. Categorical variables were reported as numbers or percentages and were compared by the chi-square or when appropriate, the Fisher exact test.
Results
Clinical features
Four DM patients with anti-MDA5 antibodies (2%) were complicated with MAS. They were adult females with a mean age of 47.5 years. Their clinical characteristics are listed in Table 1. All of them showed the hallmark cutaneous manifestations of DM, four (100%) patients presented with Heliotrope rash, three (75%) showed Gottron’s and V-neck sign, and one (25%) presented classic features of mechanic’s hands. In addition, muscle weakness was observed in three (75%) patients, one had mild proximal muscle weakness, and one showed cervical flexor weakness. However, only one patient had an elevated level of serum creatine kinase (CK). Furthermore, all patients were diagnosed with ILD by lung imaging. Three (75%) of them developed respiratory failure within 3 months, following onset of respiratory symptoms, thereby categorized as RP-ILD. All patients did not have cancer and were Ro-52 positive. The time from DM onset to MAS diagnosis ranged from 1 to 5 months. When MAS occurred, all patients showed fever, cytopenia, elevated level of serum ferritin, and lactate dehydrogenase (LDH), and decreased count of NK cells.
Table 1.
Patient clinical and laboratory characteristics.
| Patient | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Age, gender | 60-year-old woman | 24-year-old woman | 57-year-old woman | 49-year-old woman |
| Skin lesions | Heliotrope rash, Gottron’s papules, V-neck signs, MH; skin ulcer | Heliotrope rash | Heliotrope and Gottron’s rash, V-neck sign | Heliotrope and Gottron’s rash, V-neck sign |
| Muscle | Mild muscular weakness | No | Muscular weakness | Cervical flexor weakness |
| Fever | Y | Y | Y | Y |
| ILD | Y | Y | Y | Y |
| Blood cells | WBC 4.96 × 109/l, HGB 6.8 g/dl, PLT 18 × 109/l | WBC 2.99 × 109/l, HGB 10.8 g/dl, PLT 90 × 109/l | WBC 13.2 × 109/l, HGB 8.6 g/dl, PLT 36 × 109/l | WBC 1.53 × 109/l, HGB 11.7 g/dl, PLT 121 × 109/l |
| Ferritin (ng/ml) | 14,022 | 1564.9 | 11,430 | 21,876 |
| NK (cell/μl) | 22 | 13 | 13 | 11 |
| ALT (U/l) | 21 | 41 | 240 | 271 |
| AST (U/l) | 62 | 406 | 1134 | 211 |
| LDH (U/l) | 1394 | 286 | 1328 | 876 |
| CK (U/l) | 63 | 185 | 6365 | 35 |
| Ro-52 | + | + | + | – |
| Infection | PCP, CMV | |||
| Time a | 3 months | 3 months | 1 month | 5 months |
| Treatment | MP, CsA, IVIG | MP, RTX, IVIG | MP, CsA, IVIG | MP, CsA, IVIG |
| Outcomes | Fatal | Improve | Fatal | Improve |
+, positive; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CMV, cytomegalovirus; CsA, cyclosporine; DM, dermatomyositis; HGB, hemoglobin; ILD, interstitial lung disease; IVIG, intravenous immunoglobulin; LDH, lactate dehydrogenase; MAS, macrophage activation syndrome; MH, mechanic hands; MP, methylprednisolone; NK, natural killer; PCP, pneumocystis pneumonia; PLT, platelets; RTX, Rituximab; WBC, white blood cells.
From DM onset to MAS.
Treatment and follow-up
All four anti-MDA5 antibody-positive DM complicated with MAS received a combination therapy, including glucocorticoids plus one or two immunosuppressants (ISAs; Table 1).
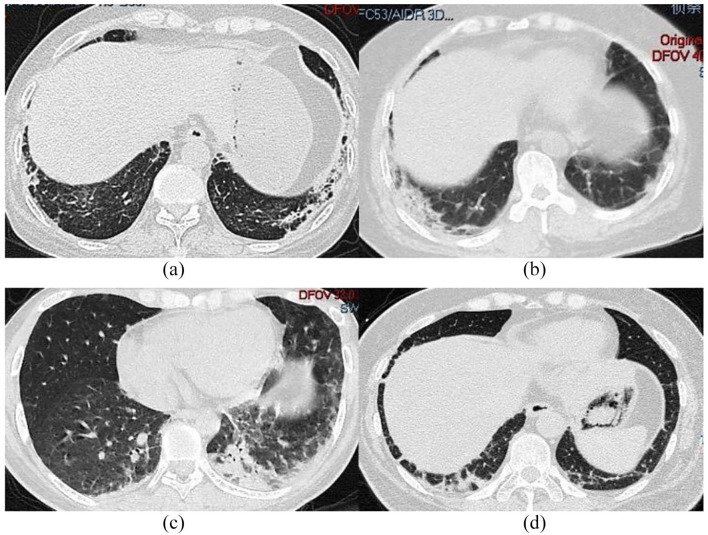
The first patient was a 60-year-old woman, who was admitted to the hospital due to rash and myalgia for 3 months and fever for 3 days. Physical examination (PE) revealed a body temperature of 38.5°C, slight muscle weakness, and skin rash. Laboratory examinations (LEs) revealed normal levels alanine aminotransferase (ALT), whereas LDH and aspartate aminotransferase (AST) levels were elevated. Cytopenias were also observed. Furthermore, patient presented augmented serum ferritin and soluble interleukin-2 receptor (sIL-2R) levels, whereas triglycerides (TG) increased up to 14.58 mmol/l. NK cells and fibrinogens were decreased, whereas anti-MDA5 and Ro-52 antibodies were positive. In addition, hemophagocytosis was observed in bone marrow. ILD, as determined by high-resolution computed tomography (HRCT) (Figure 1(a)). She was daily given 500 mg methylprednisolone (MP) for 3 days, after which it was adjusted to 80 mg a day. Patient was also given 75 mg cyclosporine (CsA) twice a day and 20 g intravenous immunoglobulin (IVIG) for 5 days. Despite active treatment, the cytokine storm caused by MAS resulted in multiple organ failure and death.
Figure 1.
HRCT in different patients at admission: (a) First patient; (b) second patient; (c) third patient; and (d) fourth patient.
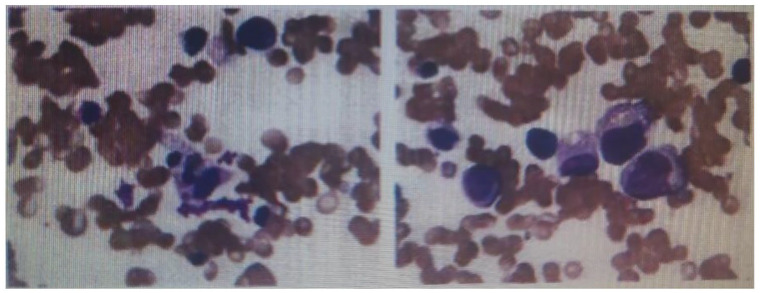
The second patient was a 24-year-old woman. Three months prior to admission, red papules appeared on eyelids and face but with normal muscle strength. HRCT suggested ILD (Figure 1(b)). MP (40 mg/d) was given for 8 days, after which skin lesions improved but she presented dysphagia and dyspnea. On admission, her serum was positive for anti-MDA5 and Ro-52 antibodies and fever appeared (38.8°C) 1 week later. Furthermore, we observed elevated levels of serum ferritin but decreased NK cells count. The transaminase and LDH levels were elevated. Abdominal ultrasound showed splenomegaly, whereas bone marrow aspirate (BMA) revealed hemophagocytosis (Figure 2). She was intravenously administered 500 mg MP for 3 days, after which it was adjusted to 80 mg a day. She was also given 10 g IVIG for 5 days and 600 mg Rituximab (RTX) therapy. Patient’s condition gradually improved and clinical remission was achieved after 3 months of treatment.
Figure 2.
Bone marrow aspirate smears showing hemophagocytosis.
The third patient was a 57-year-old woman, who was admitted to our hospital with joint pain, myalgia, muscle weakness, skin rash, dyspnea, and dysphagia. HRCT revealed ILD, small amount of pleural effusion, and pleural thickening (Figure 1(c)). On admission, PE showed a body temperature of 37.9°C, and skin rash. LE revealed cytopenia and hyperferritinaemia. LDH, AST, ALT, and GGT levels were elevated. Anti-MDA5 and Ro-52 antibodies were positive. Furthermore, pulmonary HRCT showed worsening ILD, whereas BMA revealed hemophagocytosis. Despite intensive combination therapy, including intravenous pulses of MP, CsA, and IVIG was initiated, her shortness of breath progressively worsened, and severe myocardial injury induced by MAS, she ultimately died due to RP-ILD and MAS.
The fourth patient was a 49-year-old woman, who was admitted to our hospital due to skin rash and polyarthritis for more than 4 months and dyspnea for 1 month. HRCT showed double lower lung reticular pattern and patchy shadow (Figure 1(d)). Anti-MDA5 and Ro-52 antibodies were positive. On admission, LE revealed ALT, AST, and LDH levels were elevated, and pancytopenia. She was daily given 40 mg prednisone but 2 weeks later, the patient developed high fever, and dyspnea worsened. Furthermore, HRCT showed progressed ILD. Serum ferritin increased to 21,876 ng/ml, but NK cells and fibrinogens were decreased. BMA revealed hemophagocytosis. She was daily given 80 mg MP, 75 mg CsA twice a day, and 20 g IVIG for 5 days. One week later, her condition improved and by the third month, glucocorticoids were reduced to 5 mg daily. Rash was relieved and ILD improved.
The difference between anti-MDA5 antibody-positive DM patients with and without MAS
The frequency of ILD, skin rash, arthralgia, myalgia, fever, muscular weakness, and Ro-52 antibodies in the MAS group were not different from the group without MAS (p > 0.05). But the occurrence of dysphagia in the MAS group was significantly higher than the group without MAS (p = 0.012). Among these DM patients with anti-MDA5 antibodies, mean levels of AST (453.3 U/L versus 95.6 U/L), LDH (971 U/L versus 336.9 U/L), and ferritin (11,323.2 ng/ml versus 1128.6 ng/ml) in MAS group were higher than those without MAS (all p < 0.05) (see Supplemental Table 1).
Related studies
We analyzed eight patients identified from the literature.6–11 Their characteristics are summarized in Tables 2 and 3. Together with our cohort, 12 patients were identified, including one juvenile DM (JDM). The median age of disease onset was 52.0 (range = 4–63 years) years, and the male to female ratio was 1:6. The median duration between DM onset and MAS diagnosis was 3 months (range = 1–5 months). The main DM symptoms were cutaneous lesions (100%), muscular symptoms (75%, n = 9), and ILD (100%, n = 12). At the onset of MAS, most of the patients (91.7%, n = 11) had fever, 7/12 (58.3%) had leukopenia and anemia, and 9/12 (75%) had thrombocytopenia. All patients (100%, n = 10) had elevated serum ferritin. Ro antibodies were detected in 7/9 (77.8%) patients. All patients received glucocorticoids and ISAs, such as CsA, cyclophosphamide (CYC), and tacrolimus (TAC), and additional treatments, such as IVIG, plasma exchange, and RTX. Six (50%) patients recovered. However, three patients died of MAS and complications of comorbidities. Plasma exchange was performed in five patients but only two patients treated with RTX survived. In the analysis, older patients (56.7 years versus 35.5 years, p = 0.015) died. In these patients, higher serum ferritin (17,217.2 ng/ml) levels were observed, as compared with the surviving group (6892.6 ng/ml) but the difference was not significant (p > 0.05).
Table 2.
Clinical features of anti-MDA5 antibody-positive DM and MAS, as described in the literature.
| Studies | Year | Patient (no.)/sex/age | Skin lesions | Muscle | Fever | ILD | From DM onset to MAS | Treatment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Wakiguchi et al. 6 | 2015 | 1/F/4 | Gottron’s papules, heliotrope and butterfly like rash | Myalgia, muscle weakness | Y | Y | 3 months | CS, CYC, CsA | Improve |
| Yoshimatsu et al. 7 | 2015 | 1/M/51 | skin eruptions, MH Gottron’s and heliotrope rash | Mild muscle weakness | Y | Y | 1 month | PSL, CYC, CsA, IVIG | Improve |
| Fujita et al. 8 | 2018 | 1/F/56 | skin eruptions, Gottron’s sign, heliotrope and shawl sign, nailfold bleeding, periungual erythema | Myalgia, muscle weakness | Y | Y | 2 months | MP, CYC, TAC, Plasma exchange | Improve |
| Honda et al. 9 | 2019 | 1/F/63, 1/F/57, 1/M/50 |
Gottron’s papules in 1/3; heliotrope eruption, shawl, and V-neck signs in 1/3; MH in 2/3 | Muscle weakness in 2/3 patients | Fever in 2 patients | ILD in 3/3 | ND | MP, CYC, CNI, Plasma exchange | Fatal |
| Kishida et al. 10 | 2020 | 1/F/29 | Heliotrope rash, Gottron’s and palmar papules, facial erythema, MH, skin eruptions | Myalgia, muscle weakness | Y | Y | 1 month | MP, CYC, CNIs, RTX, Plasma exchange |
Improve |
| Paul et al. 11 | 2020 | 1/F/53 | Heliotrope rash, Gottron’s patches, and Shawl sign | Normal | Y | Y | 3 months | CS | Fatal |
CNI, calcineurin inhibitor; CS, corticosteroid; CsA, cyclosporine; CYC, cyclophosphamide; DM, dermatomyositis; F, female; ILD, interstitial lung disease; IVIG, intravenous immunoglobulin; M, male; MAS, macrophage activation syndrome; anti-MDA5, anti-melanoma differentiation-associated gene 5; MH, mechanic hands; MP, methylprednisolone; ND, not described; PSL, prednisolone; RTX, Rituximab; TAC, tacrolimus.
Table 3.
Laboratory characteristics of DM patients with MDA5 antibody and MAS in the literature.
| Studies | Year | ALT (U/l) | AST (U/l) | LDH (U/l) | CK (U/l) | Ferritin (ng/ml) | Ro Abs | Blood cells |
|---|---|---|---|---|---|---|---|---|
| Wakiguchi et al. 6 | 2015 | 596 | 1154 | 2267 | 40 | 8062 | ND | WBC 2.56 × 109/l, HGB 13.4 g/dl, PLT 119 × 109/l |
| Yoshimatsu et al. 7 | 2015 | 94 | 108 | 451 | 1483 | 2929 | ND | WBC 5.6 × 109/l, HGB 13.0 g/dl, PLT 100 × 109/l |
| Fujita et al. 8 | 2018 | 1435 | 1439 | 710 | 746 | 5953 | ND | WBC 3.0 × 109/l, HGB 15.1 g/dl, PLT 74 × 109/l |
| Honda et al. 9 | 2019 | ND | ND | ND | 8430, 32, 277 | 59,877, 11,498, 4876 | + | Cytopenia |
| Kishida et al. 10 | 2020 | 38 | 72 | 499 | 1120 | 971 | – | WBC 1.55 × 109/l, HGB 10.2 g/dl, PLT 39 × 109/l |
| Paul et al. 11 | 2020 | ND | ND | 490 | 279 | 1600 | – | Thrombocytopenia and anemia |
Abs, antibodies; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; DM, dermatomyositis; HGB, hemoglobin; LDH, lactate dehydrogenase; MAS, macrophage activation syndrome; MDA5, melanoma differentiation-associated gene 5; ND, not described; PLT, platelets; WBC, white blood cells.
Discussion
As MAS is a rare complication, we possess limited information about its frequency in DM. This study aimed to show anti-MDA5 antibody-positive DM patients associated with MAS by clinical symptoms and BMA, and review the literature to include similar cases. We present four patients with anti-MDA5 antibody-positive DM complicated by MAS. Thus, 12 of such cases have been reported to date. According to the research of MAS in each autoimmune disease, the proportion of underlying diseases were from 0.9% to 4.6%, 7% to 13%, and 6.9% of SLE, sJIA, and DM, respectively.12–14 Our study first described only 2% prevalence of MAS in DM patients with anti-MDA5. Regarding the temporal relation with DM onset, most of the patients developed MAS within 3 months. Thus, MAS should not be neglected when anti-MDA5 antibody-positive DM patients present with a high fever, cytopenia, and hypofibrinogenemia within several months of DM onset.
In the present four cases, fever, decreased NK cells, hypertriglyceridemia, hypofibrinogenemia, hyperferritinemia with cytopenia, and elevated transaminase suggested MAS as a complication. Anti-MDA5 antibody-positive DM alone did not explain these laboratory findings. Therefore, MAS was confirmed by BMA. Although serum ferritin is sometimes associated with anti-MDA5 antibody-positive DM, hyperferritinemia may be caused by concomitant MAS. It is vital not to overlook concomitant MAS in findings associated with hyperferritinemia.
Regarding MAS in other autoimmune diseases, 9.5% of patients with AOSD and 4.9% of patients with SLE had fatal conditions.15,16 In this review, combined with our cohort, 50% of patients had fatal conditions due to MAS or its complications. These results support the findings of very high mortality in anti-MDA5 antibody-positive DM patients complicated with MAS. DM patients with MDA5 antibody with MAS had higher levels of AST, LDH, and ferritin than patients without MAS by comparative analysis. In addition, the frequency of dysphagia was higher in patients with MAS. These results suggested that if the patient had high levels of transaminase and ferritin, accompanied by dysphagia, we need to be alert to the occurrence of MAS. In the analysis, older patients died and possessed higher serum ferritin levels compared with improving patients. This may suggest that the risk of death correlates with age of onset and high serum ferritin levels. These results needed to be confirmed by further research.
In the literature review, one case of JDM improved after dexamethasone and CsA, without high doses of glucocorticoids. Recently, a systematic review about MAS/HPS in JDM was reported. 17 It suggested that MAS/HPS in JDM may not be rare. However, it was difficult to treat because MP pulse therapy alone was insufficient in most cases. Despite aggressive combination therapy, 2 of the 12 cases of JDM died. Dai Kishida reviewed adult DM complicated with MAS/HPS. Seven of the 18 patients (38.9%) had deadly conditions due to MAS/HPS or its complications. It can be seen that adult DM prognosis, particularly involving anti-MDA5 antibody complicated by MAS is worse than that of JDM and other subtypes of DM.
Patients with anti-MDA5 had significantly higher levels of serum soluble CD163 (sCD163) than other DM patients without such antibody. 18 CD163 is an exclusive marker of cells of the monocyte/macrophage lineage. It is usually expressed on macrophages, and elevated levels of serum sCD163 have been reported as a marker of macrophage activation in various diseases and conditions including MAS,19,20 which may indicate that DM patients with anti-MDA5 antibody are prone to occur with MAS.
Although there is no generally acceptable protocol for MAS treatment, controlling inflammation of the underlying disease is essential. Chemo-immunotherapy, including the administration of etoposide, glucocorticoid, IVIG, and CsA has been recommended for MAS. 5 Except for one case without description about the treatment, all reported cases of DM complicated by MAS required glucocorticoids and additional treatments, including CYC, IVIG, plasma exchange, CsA, TAC, and RTX therapy. These results demonstrated that glucocorticoids are the main medication required in the treatment of MAS. However, it is insufficient as a monotherapy, similar to the management of primary HLH. An intensive therapy would facilitate the improvement of MAS prognosis.
In the present study, one female patient refractory to large doses of glucocorticoids, gradually improved after RTX. Another study showed a female DM patient with anti-MDA5 antibody complicated by MAS, who was initially refractory to a combination therapy such as MP, CYC, and CsA. TAC, plasma change, and RTX were then administrated, resulting in an excellent improvement of her condition. 10 Other studies also have demonstrated that MAS secondary to SLE responded well to RTX. 21 Based on these findings, RTX may be a useful remedy in anti-MDA5 antibodies-positive DM patients with refractory MAS.
Plasma change combination with ISAs was effective for MAS second to SLE or AOSD. 22 Fujita et al. 8 reported a DM patient with anti-MDA5 antibody complicated by MAS that was successfully treated with aggressive immunosuppressive therapy and plasma change for her DM and MAS. However, the effect of plasma change needs to be further verified. Etoposide is one of the alternative therapies for HLH. None of the patients received etoposide. Therefore, it is impossible to evaluate whether etoposide is effective in treating MAS and anti-MDA5 antibody-positive DM.
While it is known that MAS is a possible complication of MDA5-DM, it is still unclear why this occurs in certain cases. The similarities and differences between the pathogenesis of MAS caused by MDA5-DM and other immune diseases need to be further studied in the future. This study has several limitations. Only a relatively small number of patients was included and it results in challenging to speculate risk factors for MAS. Furthermore, ILD may rapidly progress, leading to respiratory failure, and BMA cannot be performed in some patients. Therefore, the incidence of MAS may be underestimated. Finally, due to the small number of cases, it is difficult to find the most effective treatment.
Conclusion
MAS tends to occur in the early phase of anti-MDA5 antibody-positive DM. Its mortality was higher, particularly in elderly patients. These findings suggest that clinicians should monitor the development of MAS. The higher the serum ferritin, the greater risk of MAS and the worse prognosis. In addition, a monotherapy of glucocorticoids was insufficient to control the disease, and additional treatments including RTX may be required.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221098128 for Anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis complicated with macrophage activation syndrome by Yukang Ding and Yongpeng Ge in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Footnotes
Author contributions: Yukang Ding: Conceptualization; Data curation; Investigation; Writing – original draft.
Yongpeng Ge: Conceptualization; Methodology; Writing – review & editing.
Availability of data and materials: The original contributions generated for the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics approval and consent to participate: This study was approved by the Research Review Committee (RRC) and the Ethical Review Committee (ERC) of the China-Japan Friendship Hospital (IRB number 2016-117).
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Yongpeng Ge  https://orcid.org/0000-0003-3674-9307
https://orcid.org/0000-0003-3674-9307
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yukang Ding, Department of Rheumatology, The Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, China.
Yongpeng Ge, Department of Rheumatology, Key Laboratory of Myositis, China-Japan Friendship Hospital, Yinghua East Road, Chaoyang District, 100029 Beijing, China.
References
- 1. Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005; 52: 1571–1576. [DOI] [PubMed] [Google Scholar]
- 2. Esteban YM, de Jong JLO, Tesher MS. An overview of hemophagocytic lymphohistiocytosis. Pediatr Ann 2017; 46: e309–e313. [DOI] [PubMed] [Google Scholar]
- 3. Sen ES, Clarke SL, Ramanan AV. Macrophage activation syndrome. Indian J Pediatr 2016; 83: 248–253. [DOI] [PubMed] [Google Scholar]
- 4. Gono T, Kawaguchi Y, Ozeki E, et al. Serum ferritin correlates with activity of anti-MDA5 antibody-associated acute interstitial lung disease as a complication of dermatomyositis. Mod Rheumatol 2011; 21: 223–227. [DOI] [PubMed] [Google Scholar]
- 5. Henter J-I, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007; 48: 124–131. [DOI] [PubMed] [Google Scholar]
- 6. Wakiguchi H, Hasegawa S, Hirano R, et al. Successful control of juvenile dermatomyositis-associated macrophage activation syndrome and interstitial pneumonia: distinct kinetics of interleukin-6 and -18 levels. Pediatr Rheumatol Online J 2015; 13: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshimatsu Y, Kotani T, Fujiki Y, et al. Successful treatment with intravenous high-dose immunoglobulin for cardiomyopathy in dermatomyositis complicated with rapid progressive interstitial pneumonia. Int J Rheum Dis 2015; 22: 321–324. [DOI] [PubMed] [Google Scholar]
- 8. Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med 2018; 57: 3473–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune-associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol 2020; 49: 244–246. [DOI] [PubMed] [Google Scholar]
- 10. Kishida D, Sakaguchi N, Ueno KI, et al. Macrophage activation syndrome in adult dermatomyositis: a case-based review. Rheumatol Int 2020; 40: 1151–1162. [DOI] [PubMed] [Google Scholar]
- 11. Paul N, Avalos C, Estifan E, et al. Interstitial lung disease in dermatomyositis complicated by right ventricular thrombus secondary to macrophage activation syndrome – a case report. AME Case Rep 2020; 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vilaiyuk S, Sirachainan N, Wanitkun S, et al. Recurrent macrophage activation syndrome as the primary manifestation in systemic lupus erythematosus and the benefit of serial ferritin measurements: a case-based review. Clin Rheumatol 2013; 32: 899–904. [DOI] [PubMed] [Google Scholar]
- 13. Boom V, Anton J, Lahdenne P, et al. Evidenced-based diagnosis and treatment of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Pediatr Rheumatol Online 2015; 13: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumakura S, Murakawa Y. Clinical characteristics and treatment outcomes of autoimmune-associated hemophagocytic syndrome in adults. Arthritis Rheumatol 2014; 66: 2297–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bae CB, Jung JY, Kim HA, et al. Reactive hemophagocytic syndrome in adult-onset Still disease: clinical features, predictive factors, and prognosis in 21 patients. Medicine 2015; 94: e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gavand PE, Serio I, Arnaud L, et al. Clinical spectrum and therapeutic management of systemic lupus erythematosus-associated macrophage activation syndrome: a study of 103 episodes in 89 adult patients. Autoimmun Rev 2017; 16: 743–749. [DOI] [PubMed] [Google Scholar]
- 17. Poddighe D, Dauyey K. Macrophage activation syndrome in juvenile dermatomyositis: a systematic review. Rheumatol Int 2020; 40: 695–702. [DOI] [PubMed] [Google Scholar]
- 18. Kawasumi W, Katsumata Y, Nishino A, et al. Association of serum soluble CD163 with polymyositis and dermatomyositis, especially in antiMDA5 antibody-positive cases. J Rheumatol 2018; 45: 947–955. [DOI] [PubMed] [Google Scholar]
- 19. Coca A, Bundy KW, Marston B, et al. Macrophage activation syndrome: serological markers and treatment with anti-thymocyte globulin. Clin Immunol 2009; 132: 10–18. [DOI] [PubMed] [Google Scholar]
- 20. Peng QL, Zhang YL, Shu XM, et al. Elevated serum levels of soluble CD163 in polymyositis and dermatomyositis: associated with macrophage infiltration in muscle tissue. J Rheumatol 2015; 42: 979–987. [DOI] [PubMed] [Google Scholar]
- 21. Junga Z, Stitt R, Tracy C, et al. Novel use of rituximab in macrophage activation syndrome secondary to systemic lupus erythematosus. BMJ Case Rep 2017; 2017: bcr2017221347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lorenz G, Schul L, Schraml F, et al. Adult macrophage activation syndrome–haemophagocytic lymphohistiocytosis: ‘of plasma exchange and immunosuppressive escalation strategies’ – a single centre reflection. Lupus 2020; 29: 324–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221098128 for Anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis complicated with macrophage activation syndrome by Yukang Ding and Yongpeng Ge in Therapeutic Advances in Chronic Disease